Abstract
Pharmaceutical interventions are urgently needed to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and transmission. As SARS-CoV-2 infects and spreads via the nasopharyngeal airways, we analyzed the antiviral effect of selected nasal and oral sprays on virus infection in vitro. Two nose sprays showed virucidal activity but were cytotoxic precluding further analysis in cell culture. One nasal and one mouth spray suppressed SARS-CoV-2 infection of TMPRSS2-expressing Vero E6 cells and primary differentiated human airway epithelial cultures. The antiviral activity in both sprays could be attributed to polyanionic ι- and κ-carrageenans. Thus, application of carrageenan-containing nasal and mouth sprays may reduce the risk of acquiring SARS-CoV-2 infection and may limit viral spread, warranting further clinical evaluation.
Keywords: carrageenan, sulfated polysaccharides, virucidal, virus inhibition, virus transmission
INTRODUCTION
The coronavirus disease 2019 (COVID-19)-causing agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged at the end of 2019 and quickly spread within the human population around the globe (1). Manifestations range from mild common cold symptoms to severe lung injury, multiorgan dysfunctions, and eventually death, especially in the elderly or patients suffering from comorbidities (2). Measures to confine the spread of the virus include lockdown strategies, which severely affect socioeconomic structures. SARS-CoV-2 is mainly transmitted via respiratory droplets and aerosols exhaled from infected individuals and subsequent exposure of the respiratory mucosa of an uninfected individual (3–5). Agents reducing viral loads in the throat and nasal cavity or protecting mucosal tissue from initial infection may prevent infection and reduce virus spread between individuals (6, 7). Sprays applied to the nasal and oral mucosa to soothe symptoms, reduce disease duration, and increase viral clearance of respiratory infections caused by viruses such as rhino-, influenza-, or common cold coronaviruses have been approved and are available as over-the-counter medicine. Some contain decongestant compounds like xylometazoline (8), tramazoline, or oxymetazoline (9) to reduce symptoms of nasal congestion (10). This effect is supported by moisturizing or gel-forming mucoprotective substances such as dexpanthenol (9) and hydroxypropyl methylcellulose (11, 12). In addition, sulfated polysaccharides such as carrageenans are included as broad-spectrum antiviral agents (13–17).
As SARS-CoV-2 infects the nasopharyngeal airways, we here analyzed one oral and five nasal sprays (Table 1) for their virucidal and antiviral activity against SARS-CoV-2. All sprays are commercially available and do not require prescription. Two of the sprays exert direct virucidal activity at high concentrations but also elicited cytotoxic effects. Two carrageenan-containing sprays inhibited SARS-CoV-2 infection of immortalized cells and, more importantly, fully differentiated human airway epithelial cells resembling a crucial entry portal of the virus, with little to no effect on cell viability. Thus, application of these sprays may help to prevent from acquiring SARS-CoV-2 or suppress viral replication in the nasal epithelia in infected individuals, which may result in attenuated disease and reduced transmission rates. Further evaluation of antiviral nose sprays in clinical studies is warranted.
Table 1.
Overview and composition of tested products A–F
| Product | Trade Name | Active Agent | Additives |
|---|---|---|---|
| A | Viruseptin (nasal) | ι- and κ-carrageenan (1.2 and 0.4 mg/mL) | Sodium chloride |
| B | Viruseptin (oral) | ι-carrageenan (1.2 mg/mL) | Sodium chloride, xylitol, cherry flavor |
| C | Nasic (nasal) | Xylometazoline hydrochloride (0.1%), dexpanthenol (5%) | Benzalkonium chloride, monopotassium phosphate, disodium phosphate dodecahydrate |
| D | Rhinospray (nasal) | Tramazoline hydrochloride (1.264 mg/mL) | Sodium chloride, citric acid, benzalkonium chloride, menthol, cineol, camphor racemic, sodium hydroxide, magnesium sulfate, magnesium chloride, calcium chloride, sodium hydrogen carbonate, povidone-iodine glycerol 85%, hypromellose |
| E | Wick Erste Abwehr (nasal) | Hydroxypropyl methylcellulose | Succinic acid, disodium succinate, pyroglutamic acid |
| F | Wick Sinex Avera (nasal) | Oxymetazoline hydrochloride (0.5 mg/mL) | Sorbitol, trisodium citrate, polysorbate 80, benzyl alcohol, citric acid, benzalkonium chloride, acesulfame potassium, menthol, cineol, sodium edetate, aloe dry extract, l-carvone |
MATERIALS AND METHODS
Reagents
Viruseptin nasal and oral sprays were obtained from Hälsa Pharma GmbH, Nasic from Klosterfrau Berlin GmbH, Rhinospray from Sanofi-Aventis, and Wick Erste Abwehr and Wick Sinex Avera from Wick Pharma, Procter & Gamble GmbH. ι- and κ-carrageenan were purchased from Sigma.
Cell Culture
All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. Vero E6 (Cercopithecus aethiops derived epithelial kidney) medium was supplemented with 2.5% heat-inactivated fetal calf serum (FCS), 1 mM sodium pyruvate, and 1× nonessential amino acids. Caco-2 (human epithelial colorectal adenocarcinoma) cells (kindly provided by Holger Barth, Ulm University) were supplemented with 10% FCS. TMPRSS2-expressing Vero E6 cells [kindly provided by the National Institute for Biological Standards and Control (NIBSC), No. 100978] were supplemented with 10% FCS and 1 mg/mL geneticin.
Generation of Air-Liquid Interface Cultures of Human Airway Epithelial Cells
Differentiated air-liquid interface (ALI) cultures of human airway epithelial cells (HAECs) were generated from primary human basal cells isolated from airway epithelia, as recently described (18). Cells were isolated from tissue obtained from a male and a female donor in the age range of 25–50 yr. All experiments were performed with approval of the ethics committee of Medical School Hannover (Project No. 2701-2015). In short, 3.5 × 104 cells were seeded onto the apical side of collagen-coated, 6.5-mm Transwell filters (Corning Costar) in 200 µL of apical and 600 µL of basolateral growth medium. After 48 h, the apical medium was replaced, and after 72–96 h, upon confluency, it was completely removed (air lifting). Then, the basolateral medium was replaced by differentiation medium, consisting of DMEM-H and LHC Basal (1:1) (Thermo Fisher) supplemented with Airway Epithelial Cell Growth Medium Supplement Pack, and was replaced every 2 days. Air lifting defined day 0 of ALI culture, and experiments were performed at days 25–28. To avoid mucus accumulation on the apical side, cells were washed apically with PBS for 30 min every 3 days from day 14 onward.
Virus Strain and Virus Propagation
Viral isolate BetaCoV/France/IDF0372/2020 (No. 014 V-03890) was obtained through the European Virus Archive global. Virus was propagated by inoculation of 70% confluent Caco-2 cells in 75-cm2 cell culture flasks in medium containing 15 mM HEPES. Three days after inoculation, when a strong cytopathic effect (CPE) was visible, supernatants were harvested. Supernatants were centrifuged for 5 min at 1,000 g to remove cellular debris, aliquoted, and stored at −80°C. Infectious virus titer was determined as plaque-forming units, as previously described (19).
TCID50 Endpoint Titration
To determine the tissue culture infectious dose 50 (TCID50), 20,000 Vero E6 cells were seeded per 96 wells. 10 µL of SARS-CoV-2 was mixed with 90 µL of PBS or compound and incubated for 30 min at room temperature. Then, the mixture was titrated fivefold, and 18 μL of each dilution was used for inoculation in triplicates in a total volume of 180 µL. Cells were incubated for 6 days and monitored for CPE. TCID50/mL was calculated according to Reed and Muench, and detection limits were determined by minimal applied virus dilution or cytotoxicity of the present compound.
SARS-CoV-2 Infection Assay
To assess infection rate, virus-induced cell death was determined by quantifying cell viability via MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. To this end, 18,000 TMPRSS2-expressing Vero E6 cells were seeded in 96-well plates. The next day, the respective compound of interest was added, and the cells were inoculated with the desired multiplicity of infection (MOI) of SARS-CoV-2 in a total volume of 180 μL. After 2.5 days, when CPE was visible, 36 µL of CellTiter 96 AQueous One Solution Reagent (Promega G3580) was added to the medium and incubated for 3 h at 37°C. Then, optical density (OD) was recorded at 620 nm using an Asys Expert 96 UV microplate reader (Biochrom). All values were corrected for the background signal derived from uninfected cells, and untreated controls were set to 100% infection.
Cell Viability Assay
Cytotoxicity of the compounds was assessed using a cell viability assay measuring ATP levels in cell lysates with a commercially available kit (CellTiter-Glo, Promega). Experiments were performed corresponding to the respective infection assays in the absence of virus.
Effect of Products A and B on SARS-CoV-2 Infection of HAECs
Immediately before infection, the apical surface of HAECs was washed three times with 200 µL of PBS to remove accumulated mucus. Next, 50 µL of PBS or product and 50 µL of SARS-CoV-2 (MOI 0.07) were added to the apical surface and incubated for 2 h at 37°C before inoculum was removed and cells were washed three times with PBS. After 1, 2, and 3 days, cells were fixed for 30 min in 4% paraformaldehyde in PBS and permeabilized for 10 min with 0.2% saponin and 10% FCS in PBS (perm/staining buffer). Cells were washed twice with PBS and stained for SARS-CoV-2 spike protein (ab252690, Abcam) diluted 1:300 in staining buffer overnight at 4°C. After two PBS washes, cells were stained with AlexaFluor488-labeled anti-rabbit anti-rat secondary antibody (1:500; Thermo Scientific) and DAPI + phalloidin AF 405 (1:5,000; Thermo Scientific) for 1 h at room temperature. Images were taken on an inverted confocal microscope (Leica TCS SP5) using a ×40 lens (Leica HC PL APO CS2 40x1.25 OIL). Images for the blue (DAPI) and green (AlexaFluor488) channel were taken using appropriate excitation and emission settings that were kept constant for all the acquisitions. For quantification, randomly chosen locations in each filter were selected and z-stacks acquired. A maximum z-projection was performed, and anti-SARS-CoV-2-positive cells per area (0.15 mm2) were visually identified and counted.
RESULTS
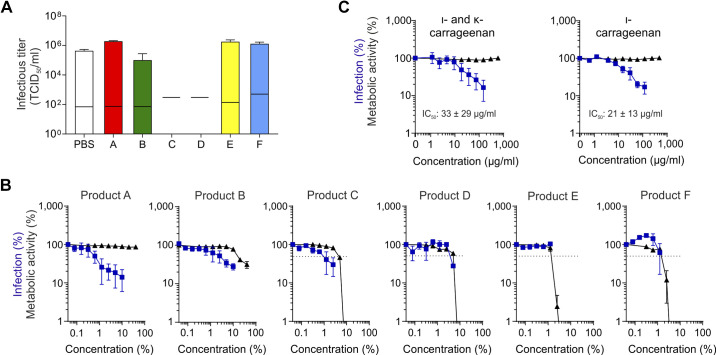
To address whether commercially available, topically applied pharmaceuticals affect SARS-CoV-2, we first determined the virucidal activity of five nasal sprays (products A, C–F) and one oral spray (product B) (Table 1). To this end, high titers of the SARS-CoV-2 isolate France/IDF0372 were incubated for 30 min in 90% (v/v) PBS or products A–F. Remaining infectivity was determined by measuring the tissue culture infectious dose 50 (TCID50) on Vero E6 cells. Incubation with products A, B, E, and F resulted in similar infectious titers as incubation in PBS, showing that these sprays have no direct virucidal activity (Fig. 1A). Products C and D inactivated SARS-CoV-2 infectivity entirely; however, they also affected cell viability (observed by light microscopy), so that the detection limit increased to 2 × 103 TCID50/mL (Fig. 1A, black lines), corresponding to a reduction of the viral titer by at least 99.5%.
Figure 1.
Effect of nasal and oral sprays as well as carrageenans on SARS-CoV-2. A: SARS-CoV-2 was incubated for 30 min in 90% PBS or products A–F. The remaining infectious titer was determined by TCID50 analysis on Vero E6 cells. Values shown are means ± SD derived from three independent experiments (n = 3), each performed in technical triplicates. Black lines indicate detection limits that increase upon cytotoxicity of the respective compound that was observed by light microscopy. B, C: TMPRSS2-expressing Vero E6 cells were treated with indicated concentrations of products A–F (B) or carrageenans (C) and infected with SARS-CoV-2. Infection rates were determined 2.5 days later by MTS assay (blue squares). For determination of toxicity, cells were treated with indicated concentrations of compounds in the absence of virus, and cellular ATP was measured by the CellTiter-Glo assay 2.5 days later (black triangles). Values shown in B and C are means ± SEM derived from two (products C, D, E, and F) or three (products A and B, ι- and κ-carrageenan, and ι-carrageenan) independent experiments (n = 2–3), each performed in technical triplicates. TCID50, tissue culture infectious dose 50.
We next explored whether the sprays may inhibit SARS-CoV-2 infection. For this, the products were titrated on TMPRSS2-expressing Vero E6 cells that were subsequently infected with SARS-CoV-2. Viral infection was determined 2.5 days later by MTS assay (20). Simultaneously, cell viability in the presence of the products but absence of virus was determined by quantifying intracellular ATP concentrations. Final cell culture concentrations of products D–F that exceeded ∼2%–5% (v/v) resulted in massive cell death precluding any reliable conclusion regarding antiviral activity (Fig. 1B). Product C, which was virucidal (Fig. 1A), was also cytotoxic (half-maximal cytotoxic concentration, CC50 ∼ 4.4 ± 0.15%) but reduced viral infection with a half-maximal inhibitory concentration (IC50) value of 1.3 ± 0.7%, corresponding to a selectivity index (SI) of 3.3. The nonvirucidal products A (a nasal spray) and B (a mouth spray) inhibited SARS-CoV-2 infection with IC50 values of ∼1.3 ± 0.8% (v/v; corresponding to a ∼77-fold dilution of product A) and ∼3.1 ± 1.7% (v/v, corresponding to ∼32-fold dilution of product B), respectively. Product A did not affect Vero E6 cell viability at concentrations up to 50% (2-fold dilution), whereas product B reduced cell viability with a CC50 value of ∼ 19.3% (∼5-fold dilution, SI ∼6.2).
Products A and B contain carrageenans (Table 1), which are sulfated polysaccharides isolated from red seaweeds previously shown to exert antiviral activity (14, 21–25). Product A contains ι-carrageenan (1.2 mg/mL) and κ-carrageenan (0.4 mg/mL), and product B contains ι-carrageenan only (1.2 mg/mL). To evaluate whether these polyanions exert antiviral activity against SARS-CoV-2, we analyzed purified ι- and κ-carrageenan as well as ι-carrageenan only, without the additives of the products (Fig. 1C). Both carrageenan solutions reduced SARS-CoV-2 infection with IC50 values of 21 ± 13 µg/mL and 33 ± 28 µg/mL and did not affect cell viability at concentrations up to 160 µg/mL and 120 µg/mL, respectively (Fig. 1C). The antiviral activities of both carrageenan preparations are similar to those of products A and B with calculated IC50 values of 20 ± 13 µg/mL and 37 ± 20 µg/mL, respectively. Thus, ι- and κ-carrageenans inhibit SARS-CoV-2 infection and are responsible for the antiviral activity in products A and B.
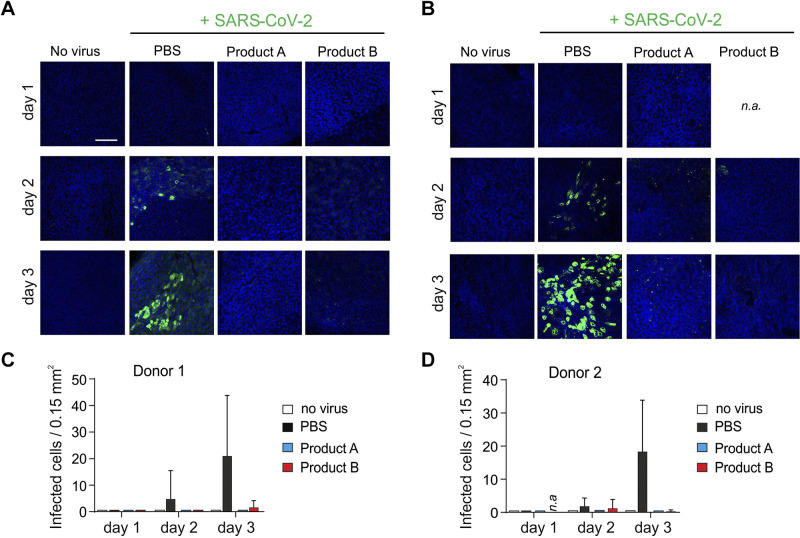
We next tested whether products A and B may also prevent SARS-CoV-2 infection of physiologically relevant target cells. For this, we generated from two donors, fully differentiated human airway epithelial cultures (HAECs) that morphologically and functionally resemble the entry site for SARS-CoV-2 (21, 26). Cultures were exposed at the air-liquid interface to either PBS or a twofold dilution [50% (v/v)] of products A or B and were then inoculated with SARS-CoV-2. Then, 1, 2, and 3 days later, cultures were stained for nuclei (DAPI) and SARS-CoV-2 spike protein, as described (27), and then imaged by confocal microscopy (Fig. 2). At day 2, infected HAECs from both donors stained clearly positive for viral spike protein when treated with PBS. The signal intensities (Fig. 2, A and B) and the number of infected cells (Fig. 2, C and D) further increased at day 3, demonstrating productive infection. Products A and B blocked SARS-CoV-2 infection entirely (Fig. 2, A and C) in HAECs from donor 1, whereas a few spike-positive cells could be detected in HAECs from donor 2 (Fig. 2, B and D). Thus, SARS-CoV-2 infection of fully differentiated airway epithelial cell cultures can be efficiently reduced by carrageenan-containing nasal and mouth sprays.
Figure 2.
Products A and B inhibit SARS-CoV-2 infection of primary human airway epithelial cultures (HAECs). A and B: HAECs derived from donor 1 (A) and donor 2 (B) were exposed to PBS or 50% (v/v) of product A or B and then infected with SARS-CoV-2. After 2 h, virus and compound mixture were removed, and cells were washed in PBS to restore the air-liquid interface. After 1, 2, and 3 days, filters were fixed and stained for SARS-CoV-2 spike protein (green) and cell nuclei (blue) and imaged by confocal microscopy (n = 1 per donor). Shown are merged images. Scale bars represent 100 µm. n.a., not available. C and D: number of infected cells per area was obtained by counting SARS-CoV-2-infected cells within microscopic images. Data represent analysis of 3–5 images per timepoint and condition and are means ± SD.
DISCUSSION
As SARS-CoV-2 primarily enters the human body via infection of nasal epithelial cells (5, 28), we here evaluated whether nasal sprays may exert antiviral activity against this novel pathogen. We found that carrageenan-containing products A (a nose spray) and B (a mouth spray) inhibit SARS-CoV-2 infection of human airway epithelial cultures, which represent a physiologically relevant entry site for SARS-CoV-2. Both over-the-counter products were applied as twofold dilution at the air-liquid interface of the epithelia, and at these concentrations, both products efficiently blocked SARS-CoV-2 infection of HAECs derived from two donors. The limited availability of these primary epithelia did not allow for testing of further dilutions of the sprays and hence to determine IC50 values. However, dose-response inhibition studies performed in a cell line showed that a ∼77-fold dilution of product A suppressed SARS-CoV-2 half-maximally and a 10- to 20-fold dilution suppressed SARS-CoV-2 by more than 80%, suggesting that application of the spray into the nostrils might reach local concentrations on nasal epithelia that are sufficient to block SARS-CoV-2 infection.
Products C and D showed virucidal effects upon incubation of virus in 90% (v/v) of the compounds. This virucidal effect is likely mediated by the ingredients xylometazoline hydrochloride and dexpanthenol (29) (present in product D) or the additive benzalkonium (30) (present in products C and D), all of which have previously been described as virucidal (29, 30). Similar antiviral activities against SARS-CoV-2 were also reported for povidone-iodine-containing sprays (present in product D), probably because of the disinfectant properties (31). Upon application of diluted nose sprays, antiviral activity was lost for product D but not for product C, which showed an IC50 value of 1.3 ± 0.7% (v/v). However, both sprays diminished cell viability at concentrations exceeding 5% (v/v) in cell culture, possibly due to the ingredient benzalkonium, a known cytotoxic preservative in both sprays (32–34). Also, the microgel-containing products E and F were cytotoxic under conditions tested, precluding any conclusions regarding a possible anti-SARS-CoV-2 effect. It should be mentioned, however, that the cytotoxic effects of products C–F obtained in our in vitro cell cultures assays do not reflect toxicity in vivo, since all sprays analyzed are tested for safety in humans. Furthermore, we emphasize that a repeated administration of nasal sprays (or respective drops) containing decongestants may have harmful effects on the mucosa, which may inadvertently foster infection (35–37).
Carrageenan-containing products A and B inhibited SARS-CoV-2 infection of Vero E6 cells with IC50 values of 1.3 ± 0.8% (corresponding to 20 ± 13 µg/mL of ι-/κ-carrageenan) and 3.1 ± 1.7% (corresponding to 37 ± 20 µg/mL), respectively. The anti-SARS-CoV-2 activity of purified ι-/κ-carrageenans was in the same range, showing that these polymers are the responsible antiviral factors in products A and B. Carrageenans have previously been reported to have broad antiviral activity against, e.g., influenza A, dengue, hepatitis A, and rhino- and common cold coronaviruses in cell culture and some clinical studies (16, 22, 24, 38), and application of carrageenan-containing nose sprays to combat SARS-CoV-2 has been suggested (39–41). Four preprint articles support our findings and show that a mixture of gellan and λ-carrageenan (42) or ι-carrageenan inhibits SARS-CoV-2 infection (25, 43, 44). The antiviral effect of carrageenans is most likely based on decreased viral attachment to and entry into target cells. ι-carrageenan has been shown to interfere with papilloma or rhinovirus binding and entry due to its sulphated polysaccharide characteristics that mimic cellular heparan sulfates or aggregates of viral particles (21, 23). Viral binding by ι-carrageenan has also been shown for influenza A and human coronavirus OC43 (38, 45). Thus, ι- and κ-carrageenans, which only differ in the number and location of sulfate moieties on the hexose scaffolds, potentially inhibit SARS-CoV-2 by a similar mechanism. This is supported by a recent study that confirmed inhibition and suggested SARS-CoV-2 aggregation by ι-carrageenan (46).
Carrageenan-containing products A and B do not contain potentially harmful decongestants. Furthermore, clinical trials showed that ι-carrageenan-containing sprays have a good safety profile and resulted in symptomatic benefits, reduced duration of symptoms, and reduced viral loads in adult and pediatric patients with common cold symptoms (13–17). Thus, application of product A may be advisable as a prophylactic agent to protect from acquiring SARS-CoV-2, or at the very early stage of viral infection, because it may reduce viral spread and viral loads in the nasal cavity. Notably, development of severe COVID-19 is always associated with viral dissemination from the upper into the lower respiratory tract. Thus, reducing viral infectivity in the nasal cavity by antiviral nasal sprays or in the oral cavity by oral sprays and rinses (47) early in infection may attenuate disease outcome (38), viral spread, or transmission. It has to be considered that sprays applied to the nasal or oral cavity will not be evenly distributed as a protective film but are instead confined to some areas (48–50). Moreover, the deposited substance will be cleared by mucociliary (11, 49, 51) or salivary clearance (52–54). Thus, the protective effect might be temporally restricted and will not replace the effect of wearing a protective mask. Nonetheless, while providing only some protection, application of the sprays on already infected areas might prevent local spread of the virus, potentially reducing viral loads and thus symptoms or transmission to another individuum.
In conclusion, ι-/κ-carrageenan-containing sprays might be useful repurposed pharmaceuticals for prevention and treatment of SARS-CoV-2/COVID-19, and animal and clinical studies are urgently required to evaluate efficacy in both settings. Finally, it should also be considered to improve the current formulations by combination of carrageenans with other anti-SARS-CoV-2 agents, e.g. gelating agents (42), molecular tweezers (55), peptides (26, 56), or neutralizing antibodies (8, 57).
DATA AVAILABILITY
All data are available upon request to the qualified researcher.
GRANTS
This project has received funding through a Collaborative Research Centre Grant of the German Research Foundation (316249678 – SFB 1279) and from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No. 101003555 (Fight-nCoV) to J.M. and A.N.Z. J.A.M. is indebted to the Baden-Württemberg Stiftung for the financial support of this research project by the Elite Program for Postdocs. D.S., C.C., T.W., L.W., and R.G. are a part of and R.G. is funded by a scholarship from the International Graduate School in Molecular Medicine Ulm. J.M. and M.F. further acknowledge funding by the Ministry for Science, Research and the Arts of Baden-Württemberg, Germany, and the German Research Foundation (458685747 – Fokus-Förderung COVID-19).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., C.C., R.G., S.S., A.Z., J.A.M., and J.M. conceived and designed research; D.S., C.C., G.F., R.G., T.W., L.W., S.S., A.Z., T.K.H., and J.A.M. performed experiments; D.S., C.C., G.F., R.G., T.W., L.W., S.S., A.Z., T.K.H., and J.A.M. analyzed data; D.S., C.C., G.F., R.G., T.W., L.W., S.S., A.Z., T.K.H., and J.A.M. interpreted results of experiments; D.S., C.C., T.W., and J.A.M. prepared figures; D.S., C.C., S.S., M.F., J.A.M., and J.M. drafted manuscript; D.S., C.C., G.F., R.G., T.W., L.W., S.S., A.Z., T.K.H., M.F., J.A.M., and J.M. edited and revised manuscript; D.S., C.C., G.F., R.G., T.W., L.W., S.S., A.Z., T.K.H., M.F., J.A.M., and J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Daniela Krnavek and Nicola Schrott for technical assistance.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, DinnonKH , et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446.e14, 2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. N Engl J Med 382: 2063–2063, 2020. doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469, 2020. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Little P, Read RC, Amlôt R, Chadborn T, Rice C, Bostock J, Yardley L. Reducing risks from coronavirus transmission in the home—the role of viral load. BMJ 369: m1728, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha NK, Marco Canosa F, Nowacki AS, Procop GW, Vogel S, Fraser TG, Erzurum SC, Terpeluk P, Gordon SM. Distribution of transmission potential during nonsevere COVID-19 illness. Clin Infect Dis 71: 2927–2932, 2020. doi: 10.1093/cid/ciaa886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graf C, Bernkop-Schnürch A, Egyed A, Koller C, Prieschl-Grassauer E, Morokutti-Kurz M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int J Gen Med 11: 275–283, 2018. doi: 10.2147/IJGM.S167123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mösges R, Shah-Hosseini K, Hucke H-P, Joisten M-J. Dexpanthenol: an overview of its contribution to symptom relief in acute rhinitis treated with decongestant nasal sprays. Adv Ther 34: 1850–1858, 2017. doi: 10.1007/s12325-017-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckx L, De Sutter AI, Guo L, Mir NA, van Driel ML. Nasal decongestants in monotherapy for the common cold. Cochrane Database Syst Rev 10: CD009612, 2016. doi: 10.1002/14651858.CD009612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennington AK, Ratcliffe JH, Wilson CG, Hardy JG. The influence of solution viscosity on nasal spray deposition and clearance. Int J Pharm 43: 221–224, 1988. doi: 10.1016/0378-5173(88)90277-3. [DOI] [Google Scholar]
- 12.Valerieva A, Popov TA, Staevska M, Kralimarkova T, Petkova E, Valerieva E, Mustakov T, Lazarova T, Dimitrov V, Church MK. Effect of micronized cellulose powder on the efficacy of topical oxymetazoline in allergic rhinitis. Allergy Asthma Proc 36: e134–e139, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res 11: 108, 2010. doi: 10.1186/1465-9921-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eccles R, Winther B, Johnston SL, Robinson P, Trampisch M, Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir Res 16: 121, 2015. doi: 10.1186/s12931-015-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazekas T, Eickhoff P, Pruckner N, Vollnhofer G, Fischmeister G, Diakos C, Rauch M, Verdianz M, Zoubek A, Gadner H, Lion T. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med 12: 147, 2012. doi: 10.1186/1472-6882-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenighofer M, Lion T, Bodenteich A, Prieschl-Grassauer E, Grassauer A, Unger H, Mueller CA, Fazekas T. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Respir Med 9: 57, 2014. doi: 10.1186/2049-6958-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig M, Enzenhofer E, Schneider S, Rauch M, Bodenteich A, Neumann K, Prieschl-Grassauer E, Grassauer A, Lion T, Mueller CA. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir Res 14: 124, 2013. doi: 10.1186/1465-9921-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkelmann VE, Thompson KE, Neuland K, Jaramillo AM, Fois G, Schmidt H, Wittekindt OH, Han W, Tuvim MJ, Dickey BF, Dietl P, Frick M. Inflammation-induced upregulation of P2X 4 expression augments mucin secretion in airway epithelia. Am J Physiol Lung Cell Mol Physiol 316: L58–L70, 2019. doi: 10.1152/ajplung.00157.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conzelmann C, Gilg A, Groß R, Schütz D, Preising N, Ständker L, Jahrsdörfer B, Schrezenmeier H, Sparrer KMJ, Stamminger T, Stenger S, Münch J, Müller JA. An enzyme-based immunodetection assay to quantify SARS-CoV-2 infection. Antiviral Res 181: 104882, 2020. doi: 10.1016/j.antiviral.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matza-Porges S, Eisen K, Ibrahim H, Haberman A, Fridlender B, Joseph G. A new antiviral screening method that simultaneously detects viral replication, cell viability, and cell toxicity. J Virol Methods 208: 138–143, 2014. doi: 10.1016/j.jviromet.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Buck CB, Thompson CD, Roberts JN, Müller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog 2: e69, 2006. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girond S, Crance JM, Van Cuyck-Gandre H, Renaudet J, Deloince R. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol 142: 261–270, 1991. doi: 10.1016/0923-2516(91)90011-Q. [DOI] [PubMed] [Google Scholar]
- 23.Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J 5: 107, 2008. doi: 10.1186/1743-422X-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S-H, Lin Y-S, Wu C-W, Wu C-J. Assessment of the inhibition of Dengue virus infection by carrageenan via real-time monitoring of cellular oxygen consumption rates within a microfluidic device. Biomicrofluidics 8: 024110, 2014. doi: 10.1063/1.4870772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang Y, Shin H, Lee MK, Kwon OS, Shin JS, Kim Y, Kim M. Antiviral activity of lambda-carrageenan against influenza viruses in mice and severe acute respiratory syndrome coronavirus 2. Sci Rep 11: 821, 2021. doi: 10.1038/s41598-020-80896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30: 343–355, 2020. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller JA, Groß R, Conzelmann C, Krüger J, Koepke L, Steinhart J, WeilT , et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab 3: 149–165, 2021. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 28.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26: 681–687, 2020. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rheinbaben F. V, Köhnlein J, Naujox K, Werner S. Zur Wirksamkeit der Kombination von Xylometazolinhydrochlorid und Dexpanthenol gegen Bakterien, Hefen und Viren. Krankenhaus-Hygiene + Infekt 39: 36–39, 2017. doi: 10.1016/j.khinf.2017.03.002. [DOI] [Google Scholar]
- 30.Armstrong JA, Froelich EJ. Inactivation of viruses by benzalkonium chloride. Appl Microbiol 12: 132–137, 1964. doi: 10.1128/AM.12.2.132-137.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson DE, Sivalingam V, Kang AEZ, Ananthanarayanan A, Arumugam H, Jenkins TM, Hadjiat Y, Eggers M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther 9: 669–675, 2020. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi H-Y, Lee Y-H, Lim C-H, Kim Y-S, Lee I-S, Jo J-M, Lee H-Y, Cha H-G, Woo HJ, Seo D-S. Assessment of respiratory and systemic toxicity of Benzalkonium chloride following a 14-day inhalation study in rats. Part Fibre Toxicol 17: 5, 2020. doi: 10.1186/s12989-020-0339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci 40: 619–630, 1999. [PubMed] [Google Scholar]
- 34.Johnson NF. Pulmonary toxicity of benzalkonium chloride. J Aerosol Med Pulm Drug Deliv 31: 1–17, 2018. doi: 10.1089/jamp.2017.1390. [DOI] [PubMed] [Google Scholar]
- 35.Jiao J, Zhang L. Influence of intranasal drugs on human nasal mucociliary clearance and ciliary beat frequency. Allergy Asthma Immunol Res 11: 306–319, 2019. doi: 10.4168/aair.2019.11.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passàli D, Salerni L, Passàli GC, Passàli FM, Bellussi L. Nasal decongestants in the treatment of chronic nasal obstruction: efficacy and safety of use. Expert Opin Drug Saf 5: 783–790, 2006. doi: 10.1517/14740338.5.6.783. [DOI] [PubMed] [Google Scholar]
- 37.Quadir M, Zia H, Needham TE. Toxicological implications of nasal formulations. Drug Deliv 6: 227–242, 1999. doi: 10.1080/107175499266823. [DOI] [Google Scholar]
- 38.Leibbrandt A, Meier C, König-Schuster M, Weinmüllner R, Kalthoff D, Pflugfelder B, Graf P, Frank-Gehrke B, Beer M, Fazekas T, Unger H, Prieschl-Grassauer E, Grassauer A. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS One 5: e14320–e14320, 2010. doi: 10.1371/journal.pone.0014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hui K. Povidone-iodine and carrageenan are candidates for SARS-CoV-2 infection control. Hong Kong Med J 26: 464, 2020. doi: 10.12809/hkmj208889. [DOI] [PubMed] [Google Scholar]
- 40.Pereira L, Critchley AT. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J Appl Phycol 32: 1875–1877, 2020. doi: 10.1007/s10811-020-02143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satpati GG. Algal sulfated polysaccharides: potent immunomodulators against COVID-19 in pandemic 2020. Biosci Biotechnol Res Asia 17: 601–605, 2020. doi: 10.13005/bbra/2863. [DOI] [Google Scholar]
- 42.Moakes RJA, Davies SP, Stamataki Z, Grover LM. Formulation of a composite nasal spray enabling enhanced surface coverage and prophylaxis of SARS-CoV-2 (Preprint). bioRxiv2020. doi: 10.1101/2020.11.18.388645. [DOI] [PMC free article] [PubMed]
- 43.Bansal S, Jonsson CB, Taylor SL, Figueroa JM, Dugour AV, Palacios C, César VJ. Iota-carrageenan and Xylitol inhibit SARS-CoV-2 in cell culture (Preprint). bioRxiv 2020. doi: 10.1101/2020.08.19.225854. [DOI] [PMC free article] [PubMed]
- 44.Morokutti-Kurz M, Graf P, Grassauer A, Prieschl-Grassauer E. SARS-CoV-2 in-vitro neutralization assay reveals inhibition of virus entry by iota-carrageenan (Preprint). bioRxiv 2020. doi: 10.1101/2020.07.28.224733. [DOI]
- 45.Morokutti-Kurz M, Graf C, Prieschl-Grassauer E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int J Gen Med 10: 53–60, 2017. doi: 10.2147/IJGM.S120665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song S, Peng H, Wang Q, Liu Z, Dong X, Wen C, Ai C, Zhang Y, Wang Z, Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct 11: 7415–7420, 2020. doi: 10.1039/D0FO02017F. [DOI] [PubMed] [Google Scholar]
- 47.Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, Münch J, Krawczyk A, Steinmann J, Steinmann J, Pfaender S, Steinmann E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis 222: 1289–1292, 2020. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djupesland PG, Skretting A. Nasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray pump. J Aerosol Med Pulm Drug Deliv 25: 280–289, 2012. doi: 10.1089/jamp.2011.0924. [DOI] [PubMed] [Google Scholar]
- 49.Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release 87: 187–198, 2003. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 50.Kublik H, Vidgren MT. Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev 29: 157–177, 1998. doi: 10.1016/S0169-409X(97)00067-7. [DOI] [PubMed] [Google Scholar]
- 51.Sahin-Yilmaz A, Naclerio RM. Anatomy and physiology of the upper airway. Proc Am Thorac Soc 8: 31–39, 2011. doi: 10.1513/pats.201007-050RN. [DOI] [PubMed] [Google Scholar]
- 52.Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc 139: 18S–24S, 2008. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]
- 53.Hunter L. Saliva and oral health, 4th edition. Br Dent J 214: 425–425, 2013. doi: 10.1038/sj.bdj.2013.421. [DOI] [Google Scholar]
- 54.Lynge Pedersen AM, Belstrøm D. The role of natural salivary defences in maintaining a healthy oral microbiota. J Dent 80: S3–S12, 2019. doi: 10.1016/j.jdent.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Weil T, Groß R, Röcker A, Bravo-Rodriguez K, Heid C, SowislokA , et al. Supramolecular mechanism of viral envelope disruption by molecular tweezers. J Am Chem Soc 142: 17024–17038, 2020. doi: 10.1021/jacs.0c06400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia S, Yan L, Xu W, Agrawal AS, Algaissi A, Tseng C-TK, Wang Q, Du L, Tan W, Wilson IA, Jiang S, Yang B, Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv 5: eaav4580, 2019. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Detalle L, Stohr T, Palomo C, Piedra PA, Gilbert BE, Mas V, Millar A, Power UF, Stortelers C, Allosery K, Melero JA, Depla E. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother 60: 6–13, 2016. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request to the qualified researcher.