Abstract
The outbreak of severe acute respiratory syndrome coronavirus 2 that first emerged in Wuhan in December 2019 has resulted in the devastating pandemic of coronavirus disease 2019, creating an emerging need for knowledge sharing. Meanwhile, myocardial infarction is and will probably remain the foremost cause of death in the Western world throughout the coming decades. Severe deregulation of the immune system can unnecessarily expand the inflammatory response and participate in target and multiple organ failure, in infection but also in critical illness. Indeed, the course and fate of inflammatory cells observed in severe ST-elevation myocardial infarction (neutrophilia, monocytosis, and lymphopenia) almost perfectly mirror those recently reported in severe coronavirus disease 2019. A pleiotropic proinflammatory imbalance hampers adaptive immunity in favor of uncontrolled innate immunity and is associated with poorer structural and clinical outcomes. The goal of the present review is to gain greater insight into the cellular and molecular mechanisms underlying this canonical activation and downregulation of the two arms of the immune response in both entities, to better understand their pathophysiology and to open the door to innovative therapeutic options. Knowledge sharing can pave the way for therapies with the potential to significantly reduce mortality in both infectious and noninfectious scenarios.
Keywords: coronavirus disease 2019, immunity, myocardial infarction
INTRODUCTION
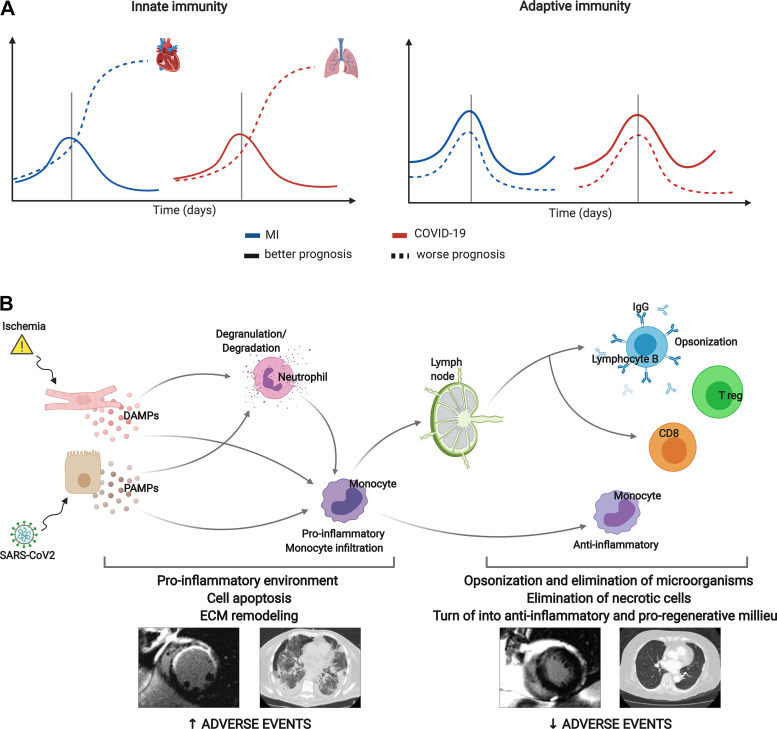
ST-segment elevation myocardial infarction (STEMI) represents the most critical presentation of ischemic heart disease. Despite the extraordinary number of deaths saved by early reperfusion, up to 10%–15% of patients die within the first weeks and severe structural consequences in survivors can result in heart failure and death during the following months and years (1). Based on detailed analyses of blood cell subtypes and myocardial samples in both humans and experiments (Fig. 1A), a deregulated immune system is becoming recognized as a decisive factor in unnecessarily extended myocardial and microvascular damage and thus worse clinical outcomes (2–5).
Figure 1.
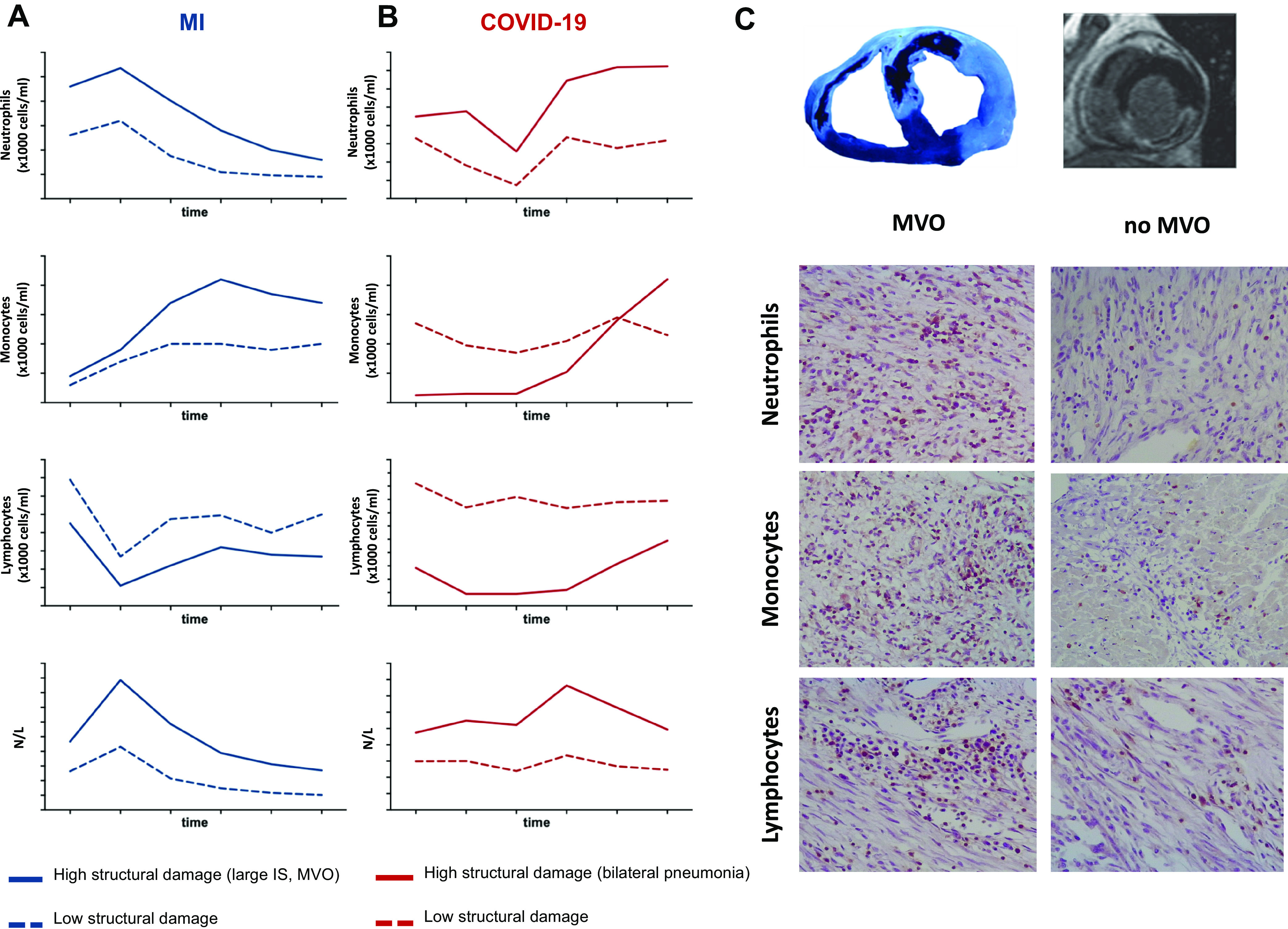
Temporal evolution of circulating white blood cells after MI and COVID-19. In a cohort of 650 reperfused ST-segment elevation patients with MI (A) and 103 patients with COVID-19 (B), this figure shows the dynamics of neutrophils (top), monocytes (top middle), lymphocytes (bottom middle), and neutrophil-to-lymphocyte ratio (bottom) (×1,000 cells/mL) according to the extent of structural damage (larger IS and MVO) in case of MI or bilateral pneumonia in case of COVID-19. Patients with a more damaged structure display higher neutrophils, monocytes, and total leukocyte counts and a lower number of lymphocytes. C: in a controlled experimental model of reperfused MI, the infiltration of neutrophils (top), monocytes (middle), and lymphocytes (bottom) in myocardial tissue with MVO is higher than in those regions without MVO. COVID-19, coronavirus disease 2019; IS, infarct size; MI, myocardial infarction; MVO, microvascular obstruction.
Since 2001, we have recorded white blood cell subtype counts (within the first 96 h) in a large ongoing prospective registry of 650 reperfused patients with STEMI with sequential structural assessment using cardiac magnetic resonance (CMR) at predischarge and at 6 mo. Characteristically, the post-STEMI course of circulating inflammatory cell subsets seems to display two different patterns: a rapid rise after ischemia onset and a fall after reperfusion in the case of lymphocytes, and a more progressive and sustained increase in neutrophils and monocytes (Fig. 1A) (2–5). Patients with cardiac events, large infarctions, and microvascular obstruction (MVO) display significantly higher neutrophil and monocyte counts and severe decay in lymphocytes. This leads to a marked boost in the neutrophil-to-lymphocyte ratio (NLR), as a marker of the proinflammatory imbalance of innate over adaptive immunity (Fig. 1A) (2, 4, 5). In fact, it has been recently demonstrated in data from 60,087 patients (from five clinical trials) that NLR significantly predicts all-cause mortality and cardiovascular risk (6).
Interestingly, in the midst of the coronavirus disease 2019 (COVID-19) pandemic, preliminary data from our institution (2), along with other findings, reflect a parallel between the dynamics of inflammatory cells in patients with severe lung damage and complicated clinical course, and those already detected in patients with large infarctions and other critical infectious and noninfectious situations. Indeed, patients with bilateral pneumonia need intensive care unit (ICU) admission or death display higher neutrophil and monocyte counts, fewer circulating lymphocytes, and consequently a disproportionate predominance of innate over adaptive immunity characterized by a raised NLR (Fig. 1B and Fig. 2) (2, 7, 8).
Figure 2.
Deregulation of the innate and adaptive immune system correlates with poorer outcomes after MI and COVID-19. A: in both entities, analyses of blood cell subtypes indicate that excessive activation of innate immunity (principally due to neutrophilia and monocytosis) and uncontrolled decline in adaptive immunity (reflected by a marked lymphopenia) are associated with massive injury in target organs (lung and heart) and worse clinical outcomes. However, a controlled immune reaction is observed in patients with better prognosis and less structural tissue damage. B: DAMPs secreted by ischemic cardiomyocytes and PAMPs after SARS-CoV-2 infection lead to neutrophil degranulation and proinflammatory monocyte activation and main cellular components of innate immunity. Both neutrophilia and monocytosis are demonstrated to be associated with a more structural damage and higher rate of adverse events after STEMI and COVID-19, probably due to massive cell apoptosis and adverse ECM remodeling. Afterward, proinflammatory situation turns into anti-inflammatory and proregenerative milieu, mainly driven by lymphocytes, key players in adaptive immunity. Clinical studies have demonstrated a clear association between lymphopenia (lower activation of adaptive immunity) and more structural damage and worse prognosis in both COVID-19 and STEMI. COVID-19, coronavirus disease 2019; DAMP, damage-associated molecular pattern; ECM, extracellular matrix; Ig, immunoglobulin; MI, myocardial infarction; PAMP, pathogen-associated molecular pattern; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STEMI, ST-segment elevation MI; Treg, regulatory T cells. Created with Biorender.com and published with permission.
The immune system is a complex network whose various synergistic and antagonistic roles, classified into the innate and adaptive arms, should work in a complementary and cooperative way for an intact and fully effective response. Critical illnesses such as STEMI or COVID-19 can challenge this balance, hindering adaptive in favor of uncontrolled innate immunity with potentially damaging structural effects in the target (heart and lungs) and other organs. Figure 1A reflects the mere tip of the iceberg of a delicate complex of mechanisms, but strongly suggest that a canonical immune response takes place after both STEMI and COVID-19 (Fig. 1).
Based on previous clinical data reporting that a deregulation of the immune response (excessive innate or depressed adaptive response) exerts a negative impact in organ structure and prognosis following STEMI and COVID-19, this study aims to dissect the common cellular and molecular mechanisms implicated in the deregulation of the immune response after these both entities. Back- and forth-sharing of knowledge pertaining to the entity that causes the highest morbimortality in Western countries (MI) and to the new global threat (COVID-19) seems crucial. After describing the shared mechanisms underlying the immune response in STEMI and COVID-19, based on the similarities found we will address therapeutic alternatives that could in due course be effective in reducing unnecessary inflammatory tissue damage.
THE IMMUNE SYSTEM IS A COMPLEX NETWORK
The immune system is a multiscale dynamic system made up of a hierarchically organized set of molecular, cellular, and tissue networks that work together to promote effective host defense (9). In this review, we aim to highlight that, although completely different initial threats (viral versus sterile inflammation), a canonical deregulation of the immune response (neutrophilia, monocytosis, and lymphopenia) occurs in both entities. Consequently, by explaining and comparing the sequential steps and mechanisms occurring in the activation and attenuation of the immune reaction following STEMI and COVID-19 can help develop therapeutic options to take the immune reaction under control.
When our organism is attacked (by either endogenous or exogenous agents), two types of defense mechanism are activated: innate and adaptive immunity (10). Although we tend to consider these two systems as separate, they are cooperative and interconnected responses. Innate immunity represents the first line of defense and contributes toward subsequent activation of adaptive immunity, but precise regulation of innate immunity via adaptive immunity is also crucial for an appropriate inflammatory response. These two mechanisms differ fundamentally in the cellular and molecular pathways involved, but synergy and cross talk between them are essential for an intact and fully effective immune response.
Initiation of the Inflammatory Cascade after an Exogenous or Endogenous Threat: Role of Antigen-Presenting Cells
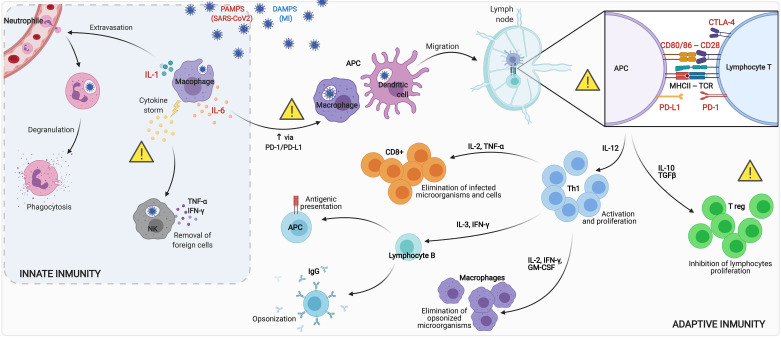
The innate immune system is triggered by recognition of pathogen-associated molecular patterns in infections (i.e., viral fragments in the case of COVID-19) and endogenous damage-associated molecular patterns in sterile situations (i.e., necrotic cells and damaged extracellular matrix elements after MI) by pattern recognition receptors (Fig. 3) (10–12). Among them, toll-like receptors (TLRs) have the widest spectrum of pathogen recognition and are implicated in recognition of self- and non-self-antigens, detection of invasive pathogens, regulation of cytokine production, proliferation, and survival of immune cells (13). TLRs are usually expressed on sentinel cells like macrophages and dendritic cells (DCs), also known as antigen-presenting cells (APCs), one of whose main functions is to recognize, engulf, and digest (phagocytosis) invading microorganisms as well as necrotic and damaged cells and present the antigen to naïve lymphocytes.
Figure 3.
Pathophysiology of the innate and adaptive immune reaction after SARS-CoV-2 infection and MI. The massive arrival of PAMPs after infections (viral fragments in case of COVID-19) and DAMPs in sterile situations (necrotic cells and damaged extracellular matrix elements after infarction) is recognized by toll-like receptors in APCs (mainly macrophages and dendritic cells). Activated APCs secrete high amounts of proinflammatory cytokines (e.g., TNF-α, IL-1, and IL-6) in a so-called cytokine storm. As a consequence, transmigration of neutrophils as well as NK cells takes place to remove necrotic cells (in case of MI) and viruses (in case of COVID-19). Acting as a link between innate and adaptive immunity, APCs capture, process, and present extracellular and intracellular antigens to naive T lymphocytes in lymph node. For T-cell activation, two principal interactions are needed: First, TCR binds to the antigen presented in the peptide-binding groove of MHC I, and second, costimulatory molecule CD28 on T cells binds to CD80 or CD86 on the APC. However, the interaction of CTLA-4 to CD80/CD86 provokes inhibition of T lymphocytes, resulting in apoptosis. Moreover, binding of PD-1 on T lymphocytes to PD-L1 on the surface of APC, upregulated by IL-6 secretion, contributes to T-cell apoptosis. As a result of this double interaction, APCs secrete high amounts of IL-12 to promote Th1 lymphocytes. Activation of Th1 cells promotes cell-mediated inflammatory responses by inducing macrophages to eliminate opsonized microorganisms, B cells to secrete IgG to induce opsonization and become APC, and CD8+ cells to eliminate infected microorganisms and cells. An uncontrolled immune reaction can result in deleterious effects. Therefore, modulating several steps in the inflammatory cascade, including proinflammatory cytokines (IL-1 and IL-6), immune checkpoints (PD-1 and CTLA-4) as well as regulatory T cells, is a key to regulate the immune response after MI and SARS-CoV-2 infection. APC, antigen-presenting cell; COVID-19, coronavirus disease 2019; CTLA-4, cytotoxic T lymphocyte antigen 4; DAMP, damage-associated molecular pattern; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; Ig, immunoglobulin; IL, interleukin; MHC, major histocompatibility complex; MI, myocardial infarction; NK, natural killer; PAMP, pathogen-associated molecular pattern; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCR, T cell receptor; TFGβ, transforming growth factor-β; TNF-α, tumor necrosis factor-α. Th1:, lymphocyte T helper-1; Treg, regulatory T cell. Created with Biorender.com and published with permission.
Monocyte-macrophage system, originating from myeloid progenitors in the bone marrow and entering into the peripheral blood, represents only between 4% and 10% of nucleated cells. Discrete populations of monocytes were first identified by morphology and differential expression of CD14 and CD16 in humans (14). Three principal human monocyte subsets have been defined: CD14+CD16− (known as classical monocytes and representing 80%–90% of the monocyte pool), CD14+CD16+ (intermediate), and CD14LowCD16+ (nonclassical monocytes). Monocytes migrate into target tissues by adhering to endothelial cells using adhesion molecules expressed in response to damage (15). Once in the tissue, they differentiate into macrophages following exposure to local growth factors, cytokines, chemokines, and microbial products (12, 16).
Activated macrophages secrete large amounts of pro-inflammatory molecules [interferon (IFN)-γ, interleukin (IL)-6, IL-1, IL-12, and tumor necrosis factor (TNF)-α] in a so-called cytokine storm, with the aim of controlling both viral workload and tissue damage (Fig. 3) (11, 16, 17). Macrophages are essential for clearance of infections (such as COVID-19), also driving repair and healing in apoptotic cells and in damaged extracellular matrix in sterile inflammation (MI). However, when out of control they can contribute to unnecessary tissue damage and organ failure by triggering an excessive inflammatory response.
In our prospective registry of patients with STEMI, we detected a steady increase in monocyte count over the first 96 h. Overall, higher peripheral monocyte counts are related to large CMR-derived infarctions, MVO, depressed left ventricular ejection fraction, and more cardiac events (Fig. 1A) (2). A similar time course occurred in a highly controlled experimental model of reperfused MI in swine. We observed that 2–3 days after MI, once the neutrophil-driven hyper-inflammatory phase was complete, monocytes predominated and paved the way for myofibroblast differentiation and repair (18). In a similar fashion, clinical studies performed in patients with COVID-19, including our data, have reported elevated monocyte counts, especially in cases with bilateral pneumonia and worse outcomes (Fig. 1B) (2, 7). Indeed, patient necropsies with COVID-19 revealed elevated macrophage presence in the pulmonary parenchyma (19). Consequently, although different initial threats, monocytosis exerts deleterious effects in both scenarios.
Along with monocytes, DCs are also responsible for recognizing and responding to pathogen-associated (infections) and danger-associated (sterile inflammation) signals, thus heightening the acute immune response. Even though all DCs are professional APCs, they are composed of heterogeneous subtypes that vary in their response to specific pathogens and interaction with different T cells and display distinct surface markers. Myeloid cDC1 (CD141) shows high intrinsic capacity to cross-present antigens via major histocompatibility complex (MHC) class I, thus activate CD8+ T cells, and promote T helper type 1 (Th1) and natural killer (NK) responses through IL-12 (20, 21). Myeloid cDC2 (CD11b+) is excellent APC that presents antigens via MHC class II and produces IL-12 (at higher levels even than cDC1), IL-23, IL-1, TNF-α, IL-8, and IL-10, whereas secretion of type III IFN is relatively low (20, 22, 23). Plasmacytoid DCs are specialized in sensing and responding to viral infection through several mechanisms via rapid production of high quantities of type I and type III IFN and cytokines (24, 25). Overall, DCs play a significant role in the entire inflammatory cascade, not only as APCs but also as regulators of the whole process.
Environments associated with massive antigen release, either pathogens (such as COVID-9) or endogenous threats (such as MI), can leave DCs overwhelmed. In fact, after STEMI, a dramatic reduction of circulating DC cell precursors has been reported (26), probably due to recruitment into the injured myocardium (as revealed by immunohistological analysis in postmortem infarcted heart) (27).
Activation of Innate Immunity
Massive monocyte and DC activation boosts the pro-inflammatory milieu, leading subsequently to higher recruitment of proinflammatory leukocytes (mainly monocytes and neutrophils) to the site of injury (Fig. 3).
Though neutrophils have traditionally been considered unsophisticated cells whose movements and actions are mainly determined by the stimuli of cell adaptive immunity, it is now recognized that (via release of granule contents) they can orchestrate later adaptive T-cell immunity, by their influence on DC priming of these cells and directly on Th17 cells via chemokine (C-C motif) ligand 2 and 20 (28). Indeed, as we will see throughout the review, this yin and yang dualism is one of the main characteristics of immunity independently on whether the response is against necrotic cardiomyocytes of viral agents, both as a whole and separately within each of its cell subtypes.
Neutrophils are the most abundant leukocytes in peripheral blood and aggressively infiltrate tissues within minutes of sterile injury (STEMI) (4, 5, 29) or shortly after infection (COVID-19) (2, 7, 30, 31). Neutrophils play a pivotal role in heightening the inflammatory response, either by secreting proteolytic enzymes or by recruiting more proinflammatory cells (12, 32). On the one hand, they secrete a group of proteases, collagenases, and elastases as well as reactive oxygen species, which help clear the damaged region of necrotic cells and detrimental extracellular matrix (in the case of MI) and of pathogens (in COVID-19). In addition, neutrophils promote tissue cleaning by favoring targeted recruitment of inflammatory (but not reparatory) monocytes and by releasing granule components such as proteoglycans and cathepsin G.
Moreover, the neutrophil release of tangled DNA filaments and proteins, referred to as neutrophil extracellular traps (NETs), aggravates tissue injury and may lead to death in several disease conditions (33). Recent studies analyzing coronary thrombi from patients with STEMI have found that the level of NETs in thrombi positively correlated with infarct size and negatively with ST-segment resolution (34, 35). NETs have been demonstrated to promote vessel occlusion and thrombus formation by providing a scaffold for platelets and procoagulant molecules (36). Consistent with these observations, patients with severe COVID-19 have elevated serum markers of neutrophil activation and NET formation (37). Interestingly, histopathologic analysis of lung biopsies from patients with COVID-19 exhibited characteristics of microvascular occlusion with thrombi containing increased neutrophils and NETs formation (38).
It is unsurprising that as with monocytes, neutrophilia and a higher NLR (2, 6, 29) are common findings in reperfused STEMI (Fig. 1A), but interestingly the same trend also occurs in patients with COVID-19 (Fig. 1B) (2, 7, 30, 31). Neutrophil count and NLR peak early (a few hours) after revascularization in STEMI, whereas there is a delay of up to several days before the same can be observed in COVID-19. Exacerbation of this dynamic, as a marker of the predominance of uncontrolled innate immunity over depressed adaptive immune cells, correlated with worse clinical course, larger infarctions, and MVO in STEMI (Fig. 1A and Fig. 2) (2, 6, 29). In COVID-19 scenario, it relates to more severe lung damage, need of intensive care resources, and death (Fig. 1B and Fig. 2) (2, 7, 30, 31).
Apart from neutrophils, eosinophils are circulating and tissue-resident leukocytes participating in the innate immunity that are markedly altered in some inflammatory scenarios (e.g., parasitic infection, asthma, and allergic reactions) (39, 40). Eosinophils normally account for 1%–3% of circulating leukocytes and quickly degranulate and release potent factors after activation to promote protective immunity, coagulation, and platelet aggregation (39, 40). After STEMI, eosinophils were seen to dramatically decay after reperfusion and this drop was more pronounced in those patients displaying worse outcomes and extensive structural damage (2, 3). This early drop could be caused by their migration into the infarcted heart, as reflected by their presence in myocardial samples isolated from experimental models and autopsies (3). In line with these results, eosinophil counts were also diminished in patients with COVID-19 and associated with worse outcomes (2, 41).
It could be speculated that innate immunity hyper-activation in patients with severe STEMI and COVID-19 is purely the result of more extensive organ damage and that these findings are casual rather than causal. On the other hand, it could also be legitimately argued that unnecessary stimulation of this potent arm of the immune response may directly result in increased organ failure and worse patient outcomes in both situations. The potential therapeutic implications of this viewpoint will be considered in therapeutic options to keep the inflammatory response under control after discussion of the physiological mechanisms aimed at attenuating this excessive innate immune response through adaptive immunity.
Cross Talk between Innate and Adaptive Immunity
Whereas for prognostic purposes clinicians have traditionally focused on the tip of the iceberg of a complex chain of mechanisms (such as an increase of white blood cells as a whole, neutrophils, or downstream products), little attention has been paid to lymphocytes, the true strategists of the immune system. Furthermore, neither pro- and anti-inflammatory T lymphocytes nor regulatory T cells have been sufficiently analyzed in clinical settings.
Although neutrophils live only for hours and generate no memory of their engagement, which is carried out through inherited receptors similar in all hosts, lymphocytes are long-lived cells that can survive for decades and the lymphocyte repertoire is tailored to each individual (42). When mobilized in immune responses, lymphocytes undergo clonal burst, differentiate into distinct types of effector cells, and memorize information about the antigen (42, 43). Genetics, age, sex, infections, and environmental exposure mold the individual’s lymphocyte pool (42, 44).
After phagocytosis by APCs (including DCs, macrophages, and B cells), extracellular and intracellular antigens are processed and converted into peptides which are presented on MHC to naive T lymphocytes in lymph nodes. In this way, APCs act as a bridge between innate and adaptive immune systems (Fig. 3).
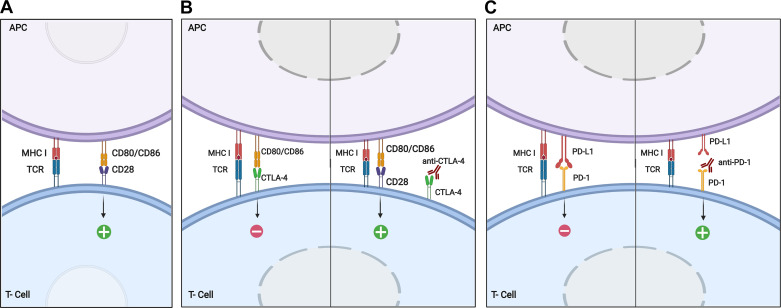
The two main MHC classes implicated in immune response are MHC class I, expressed in all nucleated cells and recognized by CD8+ cells, and MHC class II, exclusively expressed by specialized APC and recognized by CD4+ cells (45). After lymphocyte activation (Fig. 4A), APCs secrete high amounts of IL-12 to stimulate production of IFN-γ in Th1 cells (46).
Figure 4.
Mechanism of T-cell activation and implication of immune checkpoints CTLA-4 and PD-1. A: for T cell activation, two principal interactions are needed: first, TCR binds to the antigen presented in the peptide-binding groove of MHC I, and second, costimulatory molecule CD28 on T cells binds to CD80 or CD86 on the APC. As a result of this double interaction, APCs secrete high amounts of IL-12 to activate T lymphocytes. B: CTLA-4, structurally similar to CD28, binds with greater affinity and avidity than CD28 to CD80/CD86. CTLA-4 induces an inhibitory signal to T cell, thus reducing lymphocyte activation and minimizing the inflammatory response. The administration of anti-CTLA-4 neutralizing antibody favors the interaction of CD28 to CD80/CD86, thus boosting T lymphocyte activation and cytotoxic T cell proliferation. C: PD-1 to PD-L1 binding on the surface of APC induces T-cell apoptosis. Administration of anti-PD-1 antibodies avoids PD-1 interaction to PD-L1, and thus lymphocyte activation occurs. APC, antigen-presenting cell; CTLA-4, cytotoxic T lymphocyte antigen 4; IL, interleukin; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor. Created with Biorender.com and published with permission.
Th1 cells promote cell-mediated inflammatory responses by inducing activation of macrophages, NK cells, B cells, and CD8+ Th cells. Th1 cells also regulate macrophage function through upregulation of various molecules including granulocyte-macrophage colony-stimulating factor (for activating production of macrophage precursors in bone marrow) and reactive oxygen species expression (46, 47). Indeed, IFN-γ secreted by Th1 cells plays a major role in overexpressing MHC class I, MHC class II (48), and costimulatory molecules on macrophage to induce their activation, enhance microbicidal activity via nitric oxide upregulation, and promote humoral responses in B cells to mediate the switch to IgG1 isotype, leading to opsonization. Finally, IFN-γ together with IL-2 (both secreted by Th1) participates in CD8+ T-cell differentiation into cytotoxic effector as well as in the development of CD8+ memory T cells, consequently ensuring a robust secondary immune response.
Acute deregulation of the adaptive immune system has been observed in patients with MI, seemingly bringing about deleterious consequences. In a group of these patients, Cheng et al. (50) showed that an excessive proinflammatory Th1 imbalance (in comparison with a control group) was associated with left ventricular dilation, systolic dysfunction, and a worse functional class. Moreover, we have reported that reperfusion of the occluded artery with a pro-inflammatory milieu induces endothelial cell apoptosis (49), and this can in turn facilitate massive edema in the risk area and contribute via neutrophil plugging to the deleterious effects associated with MVO. Therefore, a pro-Th1 imbalance is capable of boosting a pleiotropic and harmful inflammatory response.
An accurate and balanced immune response is of utmost importance if infectious agents (COVID-19) or sterile antigens (MI) are to be removed without exerting excessive and detrimental damage in target organs (lungs and heart, respectively) (Fig. 2). It has been widely acknowledged that patients with heightened circulating neutrophil and monocyte counts (excessive innate reaction partly mediated by an initial boost of adaptive immunity) exhibit worse clinical outcomes and severe structural damage, as reflected by extensive infarct size and depressed systolic function after STEMI (4, 5) or bilateral pneumonia and ICU admission in case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2, 7, 8) (Fig. 1B and Fig. 2).
Contrary to the trend described in innate immunity cells, an increased incidence of events has been reported in acute diseases with lymphopenia-mediated immunosuppression. Events such as acquired immunodeficiency syndrome, post-transplant-induced immunosuppression, or major burns relate to a significant decrease in CD4 cells, and patient survival is linked to lymphocyte count recovery (51, 52). This evidence has fueled the notion that loss of lymphocytes could play a role in critical illness outcomes. Indeed, patients with severe STEMI and COVID-19 (Fig. 1) exhibited lymphopenia but not lymphocytosis (2, 4, 8) and a higher NLR owing to excessively low lymphocyte counts. As a more in-depth understanding of the significance and mechanisms underlying reactive adaptive immune system downregulation in both illnesses is therefore essential, we are going to mention potential cellular and molecular mechanisms implicated in avoiding massive proinflammatory immune response after both entities.
ATTENUATION OF THE IMMUNE REACTION TO AVOID EXCESSIVE TISSUE DAMAGE AND RESTORE HEMOSTASIS
Cellular Mechanisms Implicated in Controlling the Immune Response
The theory that hyperinflammation is the body’s immune response only in critical illness has been challenged. Without downregulation, physiological processes run a high risk of becoming pathological. An uncontrolled and exacerbated response during the acute inflammatory phase or timely extended reactivity could cause organ dysfunction and compromise patient survival, supporting a vital role for the endogenous cellular and molecular mechanisms involved in accurately maintaining inflammation under control. However, this salutary attempt to control excessive and unnecessary immune responses can eventually become deregulated. Indeed, depression of adaptive immunity could be a decisive player in organ failure, in which the main underlying mechanism seems to be lymphocyte apoptosis, maybe as a phylogenetically inherited self-protective response to an overshoot of proinflammatory cytokines.
We performed a translational study using a porcine reperfusion model of STEMI and analysis of lymphocyte subsets in patients with STEMI undergoing percutaneous coronary intervention (4). In this model, circulating T lymphocytes exhibited rapid increase during the brief 60-min period of coronary occlusion, followed by a sharp drop within the first hours after reperfusion (4). This probably suggests a rapid lymphocyte response to heart damage, followed by an immediate, potent attempt at attenuation. Notably, increased apoptosis of circulating lymphocytes and massive inflammatory infiltration including proinflammatory Th1 lymphocytes in the heart, mainly in the core of the area with MVO (Fig. 1C), were observed after reperfusion. Similarly, in patients with STEMI, a sharp drop in circulating T lymphocyte subsets occurred within the first 24 h postreperfusion. Interestingly, the early increase in lymphocytes observed during acute ischemia in swine cannot be detected in patients who arrive at hospital several hours after ischemia onset, as generally occurs. CMR studies of these patients revealed an inverse association between 24-h circulating T lymphocyte numbers and infarction size at 1 wk and 6 mo after revascularization (4). In addition, we have also reported a strong association between severe lymphopenia postreperfusion and the presence of extensive CMR-derived MVO in patients with STEMI (53).
As previously pointed out, almost all cell components of the inflammatory cascade can play a yin and yang role, acting as promoters or inhibitors of inflammation depending on the specific subpopulation or milieu created; these include regulatory T cells, M2-like macrophages, regulatory cytokines (e.g., IL-10 and transforming growth factor-β), and immune checkpoints (54–56) (Fig. 3). Even neutrophils, during phagocytosis, can release products that end up attenuating proinflammatory lymphocytes (57).
Regarding cellular mechanisms, regulatory T lymphocytes are CD4+CD25+ cells, which constitutively express surface receptors, cytotoxic T lymphocyte-associated protein (CTLA)-4, and glucocorticoid-induced TNF receptor, and use transcription factor Foxp3. They may suppress target T cells via direct interaction by cell-cell contact or indirectly by releasing suppressive factors and competing for essential metabolites such as tryptophan (56).
CD4+CD25+ regulatory T cell, centrally involved in maintaining self-tolerance and suppressing aberrant or excessive immune responses, can affect tryptophan metabolism via the indoleamine 2,3-dyoxygenase enzyme in DC resulting in tryptophan deficiency. As tryptophan is an essential proliferative stimulus for effector T cells, these cells undergo apoptosis (56). This could be one of the mechanisms involved in the severe lymphopenia occurring within the acute phase of MI and COVID-19, and in this way regulatory T cells would protect the organism from a boost in effector T cell-derived proinflammatory cytokines. The relationship between CD4+CD25+ T cells and DC seems to be bidirectional. As a professional APC, DC can induce and expand regulatory T cells, suggesting that accumulation of this T-cell subtype depends on continuous peripheral stimulation, perhaps via tissue antigens presented by resident APC. Besides influencing DC, CD4+CD25+ T cells can convey suppressor activity to conventional CD4+ T effector cells; this suppressor function is contact-dependent and partially mediated by transforming growth factor-β and IL-10 (58).
Another cellular mechanism to minimize unnecessary tissue damage is driven by anti-inflammatory M2 macrophages polarized by anti-inflammatory Th2 cytokines (such as IL-4, IL-10, IL-13, IL-21, or IL-33). M2 macrophages have an anti-inflammatory cytokine reservoir, including IL-10 and transforming growth factor-ß. Functionally, M2 macrophages exert phagocytosis, scavenge debris and apoptotic cells, and promote tissue repair through proangiogenic and profibrotic effects (16, 59, 60).
Again, although these signals are extremely necessary to avoid an overshoot of aggressive proinflammatory lymphocytes, if uncontrolled they can induce massive lymphocyte loss that can ultimately lead to unselected loss of lymphocyte subpopulations; as previously indicated, this relates to more severe clinical and structural outcomes, in both COVID-19 and STEMI (2). In fact, generalized loss of lymphocyte subsets including regulatory T cells occurred in our series of patients with STEMI after reperfusion. Undoubtedly, this may further facilitate the predominance of uncontrolled innate immunity over a depressed adaptive response and in turn unnecessarily expand inflammation and organ failure.
Immune Checkpoints as Master Molecular Mechanisms Downregulating the Immune System
As previously mentioned, clinical studies have demonstrated a clear association between lymphopenia and more structural damage and worse prognosis in both COVID-19 and STEMI. T-cell depletion or dysfunction, also known as lymphocyte exhaustion, is characterized by reduced production of IL-2 and proinflammatory cytokines and is considered a key mechanism to limit T cell damage against endogenous healthy tissue (Fig. 3) (55). Understanding the implications of these mechanisms in critical illness can be helpful to explore promising therapeutic alternatives aimed at regulating the immune reaction in infectious and sterile inflammatory scenarios.
Immune checkpoint, including programmed death (PD)-1, CTLA-4, T-cell immunoreceptor with Ig and ITIM domains, and T-cell immunoglobulin mucin-3, are one of the most important molecular pathways to promote T-cell apoptosis. Augmented expression of these key checkpoints downregulates T-cell function and number by activating various molecular pathways leading to lymphocyte apoptosis (55, 61, 62). Immune checkpoints have been widely addressed in oncology, as their upregulation leads to decreased lymphocyte activation, thus reducing cellular response against neoplastic cells and compromising patient prognosis (55, 62). In fact, blockade of immune checkpoints (principally PD-1 and CTLA-4) exerts beneficial effects in terms of minimizing tumor growth and patient life expectancy by potentiating the adaptive immune response against malignant cells. Given that their potential involvement after STEMI and COVID-19 is not well established, we chose to focus on PD-1 and CTLA-4 pathways.
CTLA-4, constitutively expressed on the surface of regulatory T cells and internally in activated T lymphocytes, regulates the extent of T-cell activation at early stages. The inhibitory molecule CTLA-4 is structurally similar to CD28 and can also bind to CD80/CD86 with higher affinity than CD28, consequently interfering with T-cell activation and thus hampering the immune response (63, 64) (Fig. 4B).
PD-1 is also a negative costimulatory molecule, which reduces the inflammatory response when binding to PD-ligand 1(PD-L1). PD-1 is expressed on T cells, B cells, and certain myeloid cells, whereas PD-L1 is widely found in peripheral tissues (lung, heart, spleen, or lymph nodes) and APCs (61).
Under physiological conditions, PD-1/PD-L1 axis downregulates T-cell response by inhibiting: 1) proliferation and differentiation of naive to effector T lymphocytes and 2) activation, proliferation, and function of effector T lymphocytes (65, 66) (Fig. 4C).
IL-6 is one of the most important proinflammatory cytokines and is massively released by APCs in the hyper-acute phase of COVID-19 and STEMI upon pathogen- or danger-associated molecular pattern detection (67). This product plays a decisive role in inducing immediate (and sometimes excessive) innate immunity response, but on the other hand it activates PD-L1 expression of APCs (68). If unregulated, this dual mechanism can end up with disproportionate predominance of hyper-activated innate immunity over adaptive immunity depressed by lymphocyte apoptosis. Therefore, the PD-1/PD-L1 axis may participate decisively in tissue damage, triggered by infection in COVID-19 and by sterile inflammation in STEMI.
A previous study from our group including clinical and experimental STEMI models demonstrated that PD-1 expression increases significantly in mononuclear leukocytes during ischemia, followed by a sudden drop a few hours after reperfusion. This decay in PD-1 expression is more pronounced in patients with extensive infarct size determined by the gold-standard noninvasive technique (CMR) (69). The early and excessive activation of the PD-1 pathway during acute ischemia, probably as a self-regulatory mechanism aimed at blocking aggressive lymphocyte subsets, participates in the massive lymphocyte loss via apoptosis observed soon after reperfusion.
The implications of immune checkpoints have also been addressed in the context of COVID-19. Studies recently found heightened expression of PD-1, T-cell immunoglobulin mucin-3 (70), and T cell immunoreceptor with Ig and ITIM domains (71) in patients compared to controls, which was greater in patients requiring ICU admission. Consequently, as with patients with STEMI, immune checkpoint upregulation might exert a central role in diminished lymphocyte survivorship, which could lead to hampered defenses against specific viral agents, uncontrolled innate immunity, and more severe pulmonary damage.
AUGMENTED INFLAMMATORY RESPONSE INDUCES MICROVASCULAR DYSFUNCTION AND THROMBOSIS IN STEMI AND COVID-19
Ischemia reperfusion injury and infection disturb hemostasis and activate systemic inflammatory signals. Extensive cross talk exists between inflammation and coagulation cascades, and the hyper-acute immune reaction provokes global disruption of vascular hemostasis, owing principally to a damaged endothelial barrier (72). Under physiological conditions, endothelial cells exert paracrine, endocrine, and autocrine functions to keep coagulation, inflammation, and platelet activation under control. However, oxidative stress and a proinflammatory milieu after MI (12) and endothelitis produced by severe acute respiratory syndrome (SARS)-CoV-2 infection (73) break endothelial integrity, leading to a more procoagulant and prothrombotic environment. This phenomenon is known as thromboinflammation, in which not only endothelial cells, but also activated immune cells (i.e., neutrophils and mast cells) and platelets participate (72, 74). In this regard, it is worth restating that excessive neutrophil activity could provoke massive release of NETs, leading ultimately to coagulation cascade activation and microvascular thrombi formation (33, 34). Clinical studies recently reported the presence of NETs in the context of MVO after STEMI (33, 34) and in lung autopsies from patients with COVID-19 (37, 38) and their correlation with adverse effects. Collectively, the higher the inflammatory response, the more intense the thromboinflammation phenomena taking place. Similar to the dynamics of immune cell types, prothrombotic situation is also canonical in both entities. Therefore, sharing knowledge about these two situations can be useful for researchers to develop promising therapeutic options.
In STEMI, just one century since James B. Herrick reported the first human evidence on the role of thrombotic obstruction of coronary arteries in the pathophysiology of MI (75), the generalized use of timely coronary reperfusion has brought about a dramatic improvement in outcomes. Unfortunately, up to 10%–15% of patients die within the first weeks after reperfused MI and the deterioration of myocardial perfusion that occurs in as many as 50%–60% of cases plays a decisive role in this finding (76, 77). This phenomenon is known as MVO, and CMR represents the most reliable noninvasive imaging technique for its detection (Fig. 1C). A variety of factors participates in the pathophysiology, including edema, hemorrhage, vasospasm, increased endothelial permeability, microthrombi formation, and neutrophil plugging (77). Highly controlled in vitro and experimental models have contributed to a more detailed understanding of its dynamics and pathological characteristics. In the setting of ischemia-reperfusion injury, we have reported that loss of coronary endothelial cells, decrease in microvascular density, and massive inflammatory infiltration in the core of the infarcted area play a decisive role in the development of MVO (78). This abnormality is now generally accepted as a major predictor of adverse ventricular remodeling and an increased risk of future cardiovascular events (77, 79). In both experimental models and humans, a spontaneous and salutary tendency can be found toward reparation of MVO within the first weeks and months following reperfusion, mediated by angiogenic stimuli that start early in the course of the disease.
In the COVID-19 scenario, SARS-CoV-2 seems to adhere to angiotensin-converting enzyme-2 receptor on endothelial cells (73). Autopsies in patients with COVID-19 revealed the presence of SARS-CoV-2 inclusion bodies in pulmonary endothelial cells (19, 73). As the intracellular replication of SARS-CoV-2 induces host cells to undergo apoptosis, damage on endothelial cells will induce exposure of subendothelial space to platelets and leukocytes, finally leading to thrombi formation (80). In the end, with different triggers, this mechanism mirrors the previously described apoptotic loss of endothelial coronary cells mediated by ischemia-reperfusion injury in STEMI. There is increasing evidence of venous and arterial thromboembolic events in patients with severe COVID-19. Zhang et al. recently demonstrated that 46% of patients with COVID-19 developed deep vein thrombosis, which correlated with worse prognosis (81). In a study comprising 183 patients, elevated levels of D-dimer and fibrin degradation product, longer prothrombin time, and activated partial thromboplastin time at admission correlated significantly with higher mortality rate (82). Based on these clinical data, it is now widely accepted that the inflammatory response following SARS-CoV-2 infection results in a higher rate of thrombotic events.
Thus, a precise and controlled immune response following both STEMI and COVID-19 seems crucial, not only to lessen damage in the target organ (myocardium or lung) but also to avoid thromboembolic events, which can result in further unwanted consequences.
THERAPEUTIC OPTIONS TO KEEP THE INFLAMMATORY RESPONSE UNDER CONTROL
Having reviewed the deleterious structural, prothrombotic, and prognostic repercussions mediated by a canonical type of uncontrolled immune reaction shared by MI and COVID-19, both prevalent and worrisome critical illnesses, we turn now to suggest common potential therapeutic opportunities that could ultimately exert beneficial effects for keeping immune reaction under control.
Prevention
Primary prevention undoubtedly represents the first-line defense with the most effective results in terms of epidemiology and absolute number of lives saved. In the case of STEMI, an exquisite control of cardiovascular risk factors can dramatically diminish the prevalence of ischemic heart disease. In contrast, uncontrolled obesity, diabetes, smoking, or dyslipidemia provokes baseline activation of the immune system mediated by exogenous stimuli such as fat, incipient atheroma plaques, or tobacco toxins (83). This acts on the local inflammatory milieu provoking destabilization of coronary plaques, partly mediated by augmented Th1 cells and proinflammatory macrophages, diminished regulatory T cells, and progressive thinning of the smooth muscle layer in the coronary artery wall. Immediately after plaque rupture and onset of STEMI, an exacerbated release of damage-associated molecular patterns will further disturb the already uncontrolled immune system exponentially, thus magnifying cardiac damage. As is extensively reported and recommended, but not widely followed by Western populations, an excellent control of cardiovascular risk factors via lifestyle (83) and if needed via therapies such as statins (84) can be decisive in controlling the prevalence of ischemic heart disease and improving reperfusion outcomes after STEMI.
In the case of COVID-19, prevention including distancing, frequent handwashing, and wearing masks, and massive testing and tracing are currently the most effective measures to prevent the spread of the pandemic. The long-awaited vaccine, once obtained, available, and demonstrated to be successful in all subsets, will represent a decisive step in the primary prevention of the disease.
However, even if the most optimistic predictions are correct, both patients with STEMI and probably COVID-19 with severe presentation will continue to be admitted, in cases where preventive measures failed. At this point, therapies designed to combat the ultimate cause of the disease take over from prevention. Timely revascularization in STEMI, one of the pivotal medical breakthroughs of the last century, effectively tackles the cause of the disease (acute coronary occlusion) and has been associated with dramatically decreased mortality in this scenario (76). In the case of COVID-19, as early as a few months after the first outbreak of the pandemic therapies had already been developed to directly treat the cause of the disease (SARS-CoV-2), such as remdesivir. Originally developed to treat Ebola and Marburg viruses, this adenosine prodrug can reduce mortality in up to 30% of cases in patients with the most severe presentations (85).
In both STEMI and COVID-19, however, once patients require hospital admission and despite the state-of-the-art medical management, mortality within the first days and weeks is now in the range of 10%–15% (76). This clearly suggests that if we are to further decrease these excessive death tolls, complementary treatments directly targeted at regulating the uncontrolled inflammatory reaction need to be explored. Again, knowledge sharing can be an opportunity to briefly reflect on the crucial Wh-questions regarding these therapeutic opportunities (why, who, when, where, and which).
Wh-Questions on anti-Inflammatory Therapies
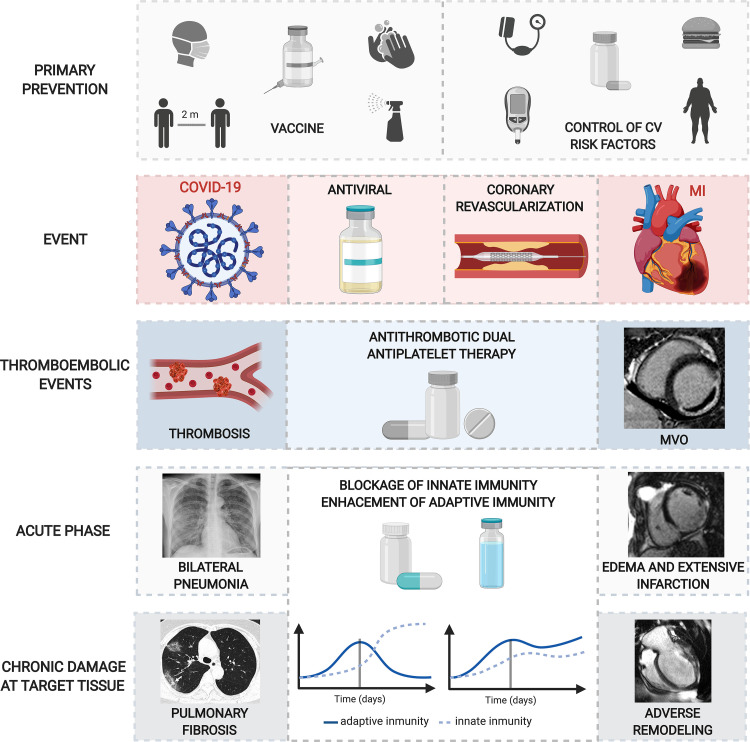
The answer to the “why” question lies in the fact that acute deregulation of the immune system, as indicated throughout this review, is one of the main mediators of poor outcomes of patients with STEMI and COVID-19 with severest presentation. In both scenarios, exacerbated innate and decreased adaptive immune response is observed in cases with the worst structural and clinical outcomes, whereas the opposite occurs if immune response can be controlled (Fig. 2). It is therefore vital to explore therapies aimed at accurate modulation in time and intensity of the innate and adaptive arms of immunity in the acute phase of patients with STEMI and COVID-19 (Fig. 5).
Figure 5.
Potential therapeutic options after COVID-19 (left) and MI (right). Top. As primary prevention, vaccine, social distancing, frequent handwashing, wearing masks (in COVID-19), and gaining control over cardiovascular risk factors (obesity, diabetes, smoking, dyslipidemia, atherosclerosis) (in STEMI) are the first-line defense mechanisms before the main event. Top central: antivirals soon after SARS-CoV-2 infection and coronary revascularization, mainly by primary angioplasty, once the thrombotic occlusion takes places are the gold standard approaches to diminish structural damage and improve prognosis. Bottom central: in both scenarios, thrombotic events (such as distal thrombi in COVID-19 and MVO in MI) occur. Antithrombotic drugs and dual antiplatelet therapy are essential to reduce the incidence of microvascular events. Bottom: following COVID-19 and MI, uncontrolled immune reaction results in adverse events at acute (bilateral pneumonia and edema and extensive infarction) and chronic (pulmonary fibrosis and adverse remodeling) phases in target tissues. Therapeutic alternatives to reduce innate immunity (via neutralization of the proinflammatory cytokines IL-6 and IL-1) and potentiate adaptive immune cells (by blocking the immune checkpoints CTLA-4 and PD-1 to reduce lymphocyte apoptosis) using specific monoclonal antibodies could potentially promote a controlled immune response, thus minimizing target organ damage. COVID-19, coronavirus disease 2019; CTLA-4, cytotoxic T lymphocyte antigen 4; CV, cardiovascular; IL, interleukin; MI, myocardial infarction; MVO, microvascular obstruction; PD-1, programmed death-1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STEMI, ST-segment elevation MI. Created with Biorender.com and published with permission.
Regarding the question of “who,” indiscriminate use of complementary therapies in all patients is, for now at least, unnecessary. As regards STEMI, a considerable number of cases (>50%) are revascularized promptly and display a preserved or nearly normal left ventricular ejection fraction (86). The event rate of these patients is very low (86), and the effectiveness of therapies other than ones already recommended by current guidelines is uncertain. Likewise, the vast majority of patients with COVID-19 (at least 80%) have a benign course with symptomatic treatment, which is a generally unfavorable setting for implementing innovative therapies. On an individualized level, nonetheless, a considerable number of patients are present with features suggesting a complicated course. In the case of STEMI, the presence of clinical instability, extensive EKG changes, or large ischemic burden strongly predicts a high risk of massive myocardial loss. Turning to COVID-19, symptoms, signs, and radiological data associated with respiratory insufficiency upon presentation pinpoint a patient subset with risk of ventilatory support requirement and death. High neutrophil/low lymphocyte counts and increased NLR as simple markers of uncontrolled inflammation can also be helpful to detect patients who will receive future benefit from innovative drugs that are proven effective in regulating inflammation, in both STEMI and COVID-19.
As for the question of when, the desired regulatory effect of any co-adjuvant therapy requires a timely response. Based on the curves displaying the dynamics of white blood cell subtypes in patients with STEMI and COVID-19 with higher risk of events and organ damage, a significant imbalance of innate over adaptive immunity can already be detected at the first sample upon patient arrival (2, 4, 7) (Figs. 1 and 2). In patients with COVID-19, low-dose glucocorticoid therapy is recommended to start at least five days after onset of symptoms. In this stage, we allowed the activation of adaptive immunity and avoided the appearance of severe symptoms before immunity neutralization via glucocorticoids (87). In line with this statement, when progression of thoracic CT, elevation of proinflammatory markers (IL-6, D-dimer, ferritin, or C-reactive protein) or decreased lymphocyte counts, tocilizumab (anti-IL-6 antibody) administration is described to be beneficial to diminish cytokine storm (88).
In the case of STEMI, superselective release of tested products into the area at risk (where its effect is needed) can easily be achieved by administering potential therapies through the lumen of one of the angioplasty balloons used for primary angioplasty immediately upon patient arrival at the cath laboratory at the time of reperfusion. Alternative approaches have also been tested, but differences exist depending on the route of administration. For instance, two clinical studies demonstrated that intracoronary administration of platelet aggregation inhibitors (i.e., abciximab or tirofiban) when compared to intravenous route leads to higher beneficial effects (89, 90). Our group has demonstrated an effective release of the anti-inflammatory IL-10 in the risk area by delivering genetic therapy through catheters positioned at the coronary sinus (91); this option could be effective in providing localized, time-restricted treatment, to limit excessive inflammation in the infarcted area. In the case of COVID-19, for now patients have to receive drugs orally or intravenously. However, a clinical trial is currently undergoing to evaluate the efficacy of intranasal dexamethasone as an adjuvant in patients with COVID-19.
Which Therapy?
Unfortunately, the most important question, “which,” remains unanswered, and research addressed at combating the acute deregulation of the immune system underlying the pathophysiology of severe STEMI and COVID-19 cases has not resulted yet in consistent alternative therapies. However, by understanding the canonical cellular and molecular mechanisms implicated in the deregulation of the immune response (excessive innate or depressed adaptive response) following both entities, researchers could test different therapeutic approaches.
High-dose corticosteroids could be the standard treatment to downregulate the immune system indiscriminately. Even though effective results were obtained in animal models of nonreperfused STEMI, its translation to clinic was controversial due to the limited attenuation of myocardial injury and multiple systemic adverse effects (92). These studies used potent and unselective corticoid therapy and were undertaken several decades ago, before the generalized use of reperfusion therapies. These findings probably warrant revisiting, using new approaches in terms of patient management, drugs tested, and administration routes.
Promising mortality reduction outcomes have been obtained in patients with severe COVID-19 treated with dexamethasone (93). This constitutes a proof of concept demonstrating the plausibility and efficacy of anti-inflammatory therapies to ameliorate outcomes, although certain controversy exists regarding the risk of secondary infections as well as delayed virus elimination in patients receiving corticoids (94).
Following the capture of sterile antigens in STEMI and of viruses in SARS-CoV-2 infection by APCs, a massive release of proinflammatory cytokines (the so-called cytokine storm) takes place (11, 16, 17), in which IL-1 and IL-6 increase rapidly and drive pleiotropic proinflammatory effects. Pharmacological blockade using tocilizumab (anti-IL-6) or canakinumab (anti-IL-1β) has been proposed to attenuate the deleterious consequences of these products at hyper-acute phases.
Conversely, the “Cardiovascular Risk Reduction Study (Reduction in Recurrent Major CV Disease Events)” (CANTOS trial) studying ischemic heart disease included more than 10,000 patients with a prior MI and concluded that neutralizing innate immunity via canakinumab administration results in significantly lower incidence of nonfatal MI, stroke, or cardiovascular death. In fact, the reduction in NLR due to canakinumab has been recently demonstrated (6). This study represents the first proof of concept showing that complementary therapies targeted at downregulating inflammation can be effective to improve outcomes in patients with post-MI. In addition, in a murine model, Kobara et al. (95) suggested that IL-6 receptor-targeted therapy decreased inflammation and attenuated left ventricular remodeling post-MI. Hence, tocilizumab could induce beneficial effects on infarct size and cardiac remodeling. The “ASSessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction” (ASSAIL-MI trial) is currently underway to evaluate the effects of tocilizumab in myocardial salvage after STEMI (96).
Regarding COVID-19, the effects of canakinumab are still under research. Tocilizumab was initially considered a potential therapeutic option due the surge in IL-6 levels detected in patients. A retrospective study of 1,351 patients concluded that IL-6 neutralization via tocilizumab administration leads to lower risk of invasive mechanical ventilation or death (97).
Whereas the therapies aim to modulate the first steps of uncontrolled innate immunity, options addressed at regulating the massive loss of lymphocytes and the severe depression of adaptive immunity described in critical illnesses might exert similar or even additional beneficial effects. Lessons learned over the past few decades teach us that patient survival goes hand in hand with lymphocyte count and adaptive cell immunity recovery. The course of T cells in human immunodeficiency virus-positive patients successfully treated with antiretroviral drugs or in survivors of shock, sepsis, burns, and severe STEMI illustrates this point.
Indeed, lymphocyte apoptosis can be lessened via neutralization of hyper-activated immune checkpoints (e.g., CTLA-4 or PD-1) at subacute stages. The success story represented by controlled immune potentiation in certain cancers reflects (in a different scenario) the potential of individualized modulation of the immune system in terms of survivorship (55, 62). As far as we know, no clinical trials have tested the efficacy of this group of drugs in patients with STEMI, whereas several international clinical trials are underway evaluating their role in COVID-19.
A combined approach aimed at 1) blocking key proinflammatory cytokines that trigger innate immunity (by either anti-IL-1β or anti-IL-6) and 2) neutralizing the immune checkpoints that mediate adaptive immunity depression (by either anti-PD-1 or anti-CTLA-4) may attenuate the expansion or tissue damage driven by an overstimulated inflammatory response (98) (Fig. 5). However, this proposal is merely speculative and experimental and clinical research should be undertaken before translating these and other pathophysiologically based initiatives into our daily therapeutic armamentarium.
Lastly, as microvascular damage and thrombotic events are shared by STEMI and COVID-19, complementary approaches to control these phenomena are crucial. In STEMI, current guidelines recommend use of heparin during percutaneous revascularization and dual antiplatelet therapy after discharge (99). In COVID-19, anticoagulation seems to be associated with improved outcomes (100). However, the cross talk between inflammation and thrombosis is a multifaceted, complex phenomenon and further research is first needed for greater insight into and correct management of the unwanted prothrombotic events that complicate STEMI and COVID-19.
CONCLUSIONS
STEMI and COVID-19 can bring about a pleiotropic pro-inflammatory imbalance that hampers adaptive in favor of uncontrolled innate immunity, with deleterious effects in terms of structural damage and clinical outcomes. Unraveling the thread of this canonical course of inflammation in critical illness will lead to a better understanding of pathophysiology and will guide the exploration of innovative therapeutic options. In our view, the first steps must be: 1) recognizing that the immune response plays a definitive role in the pathophysiology and clinical outcomes of critical illness, 2) admitting that much research is still needed to understand its basic mechanisms in more depth, and 3) acknowledging that we do not currently know the ideal specific treatment nor the direction or timing it should follow (toward immune potentiation or suppression). Knowledge sharing has the potential to inspire therapies, which could bring unprecedented mortality reduction in infectious and noninfectious scenarios.
GRANTS
This study was funded by Instituto de Salud Carlos III and Fondos Europeos de Desarrollo Regional (FEDER) Research Grants PI17/01836, PI20/00637, and CIBERCV16/11/00486 (to V.B.) and a postgraduate contract FI18/00320 (to C.R.-N.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.-N. and V.B. conceived and designed research; C.R.-N. and E.d.D. prepared figures; C.R.-N., E.d.D., and V.B. drafted manuscript; C.R.-N., E.d.D., M.J.F., and V.B. edited and revised manuscript; C.R.-N., E.d.D., M.J.F., and V.B. approved final version of manuscript.
REFERENCES
- 1.Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail 6: 179–186, 2018. doi: 10.1016/j.jchf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 2.de Dios E, Rios-Navarro C, Perez-Sole N, Gavara J, Marcos-Garces V, Rodríguez E, Carratala A, Forner MJ, Navarro J, Blasco ML, Bondia E, Signes-Costa J, Vila JM, Forteza MJ, Chorro FJ, Bodi V. Similar clinical course and significance of circulating innate and adaptive immune cell counts in STEMI and COVID-19. J Clin Med 9: 3484, 2020. doi: 10.3390/jcm9113484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rios-Navarro C, Gavara J, Vidal V, Bonanad C, Racugno P, Bayes-Genis A, Minana G, Husser O, Oltra R, Nunez J, Chorro FJ, Bodi V, Ruiz-Sauri A. Characterization and implications of the dynamics of eosinophils in blood and in the infarcted myocardium after coronary reperfusion. PLoS One 13: e0206344, 2018. doi: 10.1371/journal.pone.0206344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forteza MJ, Trapero I, Hervas A, de Dios E, Ruiz-Sauri A, Minana G, Bonanad C, Gomez C, Oltra R, Rios-Navarro C, Ketelhuth DFJ, Nunez J, Chorro FJ, Bodi V. Apoptosis and mobilization of lymphocytes to cardiac tissue is associated with myocardial infarction in a reperfused porcine model and infarct size in patients. Oxid Med Cell Longev 2018: 1–9, 2018. doi: 10.1155/2018/1975167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodi V, Sanchis J, Nunez J, Mainar L, Minana G, Benet I, Solano C, Chorro FJ, Llacer A. Uncontrolled immune response in acute myocardial infarction; unraveling the thread. Am Heart J 156: 1065–1073, 2008. doi: 10.1016/j.ahj.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, Tabas IA, Mehta NN, Ridker PM. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analysis from five contemporary randomized trials. Eur Heart J 42: 896–903, 2021. doi: 10.1093/eurheartj/ehaa1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian D-S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71: 762–768, 2020. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang Y-Q, Wang Q, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 5: 33, 2002. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian N, Torabi-Parizi P, Gottschalk RA, Germain RN, Dutta B. Network representations of immune system complexity. Wiley Interdiscip Rev Syst Biol Med 7: 13–38, 2015. doi: 10.1002/wsbm.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 125: S3–S23, 2010. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The Trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. The inflammatory response in myocardial injury, repair and remodeling. Nat Rev Cardiol 11: 255–265, 2014. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidya MK, Kumar VG, Sejian V, Bagath M, Krishnan G, Bhatta R. Toll-like receptors: significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol 37: 20–36, 2018. doi: 10.1080/08830185.2017.1380200. [DOI] [PubMed] [Google Scholar]
- 14.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74: 2527–2534, 1989. [PubMed] [Google Scholar]
- 15.Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity 49: 595–613, 2018. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233: 6425–6440, 2018. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair. Circulation 121: 2437–2445, 2010. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20: 1135–1140, 2020. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 154: 3–20, 2018. doi: 10.1111/imm.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang X-N, Malinarich F, Malleret B, Larbi A, Tan P, Zhao H, Poidinger M, Pagan S, Cookson S, Dickinson R, Dimmick I, Jarrett RF, Renia L, Tam J, Song C, Connolly J, Chan JKY, Gehring A, Bertoletti A, Collin M, Ginhoux F. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37: 60–73, 2012. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sittig SP, Bakdash G, Weiden J, Sköld AE, Tel J, Figdor CG, de Vries IJM, Schreibelt G. A comparative study of the T cell stimulatory and polarizing capacity of human primary blood dendritic cell subsets. Mediators Inflamm 2016: 3605643, 2016. doi: 10.1155/2016/3605643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nizzoli G, Larghi P, Paroni M, Crosti MC, Moro M, Neddermann P, Caprioli F, Pagani M, De Francesco R, Abrignani S, Geginat J. IL-10 promotes homeostatic proliferation of human CD8+ memory T cells and, when produced by CD1c+ DCs, shapes naive CD8+ T-cell priming. Eur J Immunol 46: 1622–1632, 2016. doi: 10.1002/eji.201546136. [DOI] [PubMed] [Google Scholar]
- 24.Bao M, Liu YJ. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 4: 40–52, 2013. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15: 471–485, 2015. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christ A, Temmerman L, Legein B, Daemen MJAP, Biessen EAL. Dendritic cells in cardiovascular diseases. Epiphenomenon, contributor, or therapeutic opportunity. Circulation 128: 2603–2613, 2013. doi: 10.1161/CIRCULATIONAHA.113.003364. [DOI] [PubMed] [Google Scholar]
- 27.Nagai T, Honda S, Sugano Y, Matsuyama T-A, Ohta-Ogo K, Asaumi Y, Ikeda Y, Kusano K, Ishihara M, Yasuda S, Ogawa H, Ishibashi-Ueda H, Anzai T. Decreased myocardial dendritic cells is associated with impaired reparative fibrosis and development of cardiac rupture after myocardial infarction in humans. J Am Heart Assoc 3: e000839, 2014. doi: 10.1161/JAHA.114.000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115: 335–343, 2010. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Diao J, Qi C, Jin J, Li L, Gao X, Gong L, Wu W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord 18: 75, 2018. doi: 10.1186/s12872-018-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Du X, Chen J. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81: e6-12, 2020. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana COVID-19 patients. Shock 54: 652–658, 2020. doi: 10.1097/SHK.0000000000001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epelman S, Mann DL. Communication in the heart: The role of the innate immune system in coordinating cellular responses to ischemic injury. J Cardiovasc Transl Res 5: 827–836, 2012. doi: 10.1007/s12265-012-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 34.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter M-P, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 116: 1182–1192, 2015. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 35.Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM, Adriaenssens T, Guagliumi G, Godschalk TC, Neumann F-J, Trenk D, Feldman LJ, Steg PG, Desmet W, Alfonso F, Goodall AH, Wojdyla R, Dudek D, Philippi V, Opinaldo S, Titova A, Malik N, Cotton J, Jhagroe DA, Heestermans AACM, Sinnaeve P, Vermeersch P, Valina C, Schulz C, Kastrati A, Massberg S; Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 37: 1538–1549, 2016. doi: 10.1093/eurheartj/ehv419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol 39: 1724–1738, 2019. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight 5: e138999, 2020. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, DaBler-Plenker J, Guerci P, Huynh C, Knight JS, Loda M, Looney MR, McAllister F, Rayes R, Renaud S, Rousseau S, Salvatore S, Schwartz RE, Spicer JD, Yost CC, Weber A, Zuo Y, Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med 217: e20200652, 2020. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Possa SS, Leick EA, Prado CM, Martins MA, Tiberio IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol 4: 46, 2013.doi: 10.3389/fphar.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diny NL, Hou X, Barin JG, Chen G, Talor MV, Schaub J, Russell SD, Klingel K, Rose NR, Cihakova D. Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to heart. Eur J Immunol 46: 2749–2760, 2016. doi: 10.1002/eji.201646557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophils counts in COVID-19 patients. Allergy 6: 471–482, 2020. doi: 10.1111/all.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goronzy JJ, Weyand CM. Immunosuppression in atherosclerosis. Circulation 114: 1901–1904, 2006. doi: 10.1161/CIRCULATIONAHA.106.656751. [DOI] [PubMed] [Google Scholar]
- 43.Caligiuri G, Nicoletti A. Lymphocytes responses in acute coronary syndromes: lack of regulation spawns deviant behavior. Eur Heart J 27: 2485–2486, 2006. doi: 10.1093/eurheartj/ehl284. [DOI] [PubMed] [Google Scholar]
- 44.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 45.Malissen B, Bongrand P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu Rev Immunol 33: 539–561, 2015. doi: 10.1146/annurev-immunol-032414-112158. [DOI] [PubMed] [Google Scholar]
- 46.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol 2012: 925135, 2012. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grifka-Walk HM, Giles DA, Segal BM. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur J Immunol 45: 2780–2786, 2015. doi: 10.1002/eji.201545800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitra S, Leonard WJ. Biology of IL-2 and its therapeutic modulation: mechanisms and strategies. J Leukoc Biol 103: 643–655, 2018. doi: 10.1002/JLB.2RI0717-278R. [DOI] [PubMed] [Google Scholar]
- 49.Forteza MJ, Novella S, Trapero I, Hermenegildo C, Ruiz-Sauri A, Chaustre F, Bonanad C, Oltra R, Palacios L, O’Connor JE, Chorro FJ, Bodi V. Dynamics of serum-induced endothelial cell apoptosis in patients with myocardial infarction. Eur J Clin Invest 44: 46–53, 2014. doi: 10.1111/eci.12189. [DOI] [PubMed] [Google Scholar]
- 50.Cheng X, Liao Y-H, Ge H, Li B, Zhang J, Yuan J, Wang M, Liu Y, Guo Z, Chen J, Zhang J, Zhang L. TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J Clin Immunol 25: 246–253, 2005[Erratum inJ Clin Immunol34: 748-9, 2014] doi: 10.1007/s10875-005-4088-0. [DOI] [PubMed] [Google Scholar]
- 51.Ducloux D, Courivaud C, Bamoulid J, Vivet B, Chabroux A, Deschamps M, Rebibou J-M, Ferrand C, Chalopin J-M, Tiberghien P, Saas P. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol 21: 868–875, 2010. doi: 10.1681/ASN.2009090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Girardot T, Rimmelé T, Venet F, Monneret G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 22: 295–305, 2017. doi: 10.1007/s10495-016-1325-3. [DOI] [PubMed] [Google Scholar]
- 53.Bodí V, Sanchis J, Núñez J, Rumiz E, Mainar L, López-Lereu MP, Monmeneu JV, Oltra R, Forteza MJ, Chorro FJ, Llácer À. Post-reperfusion lymphopenia and microvascular obstruction in ST-segment elevation acute myocardial infarction. Rev Esp Cardiol 62: 1109–1117, 2009. doi: 10.1016/S1885-5857(09)73325-0. [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204–7218, 2018. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol 47: 765–779, 2017. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 56.Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 17: 823–844, 2018. doi: 10.1038/nrd.2018.148. [DOI] [PubMed] [Google Scholar]
- 57.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol 9: 113, 2018. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, Kabat J, Matsumura R, Dorward DW, Glass DD, Shevach EM. Regulatory T cells mediate specific suppression by depleting peptide–MHC class II from dendritic cells. Nat Immunol 20: 218–231, 2019. doi: 10.1038/s41590-018-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MPJ, Donners MMPC. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17: 109–118, 2014. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 60.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 183: 6469–6477, 2009. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 61.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 27: 195–201, 2006. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol 11: 31, 2018. doi: 10.1186/s13045-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grabie N, Lichtman AH, Padera R. T cell checkpoint regulators in the heart. Cardiovasc Res 115: 869–877, 2019. doi: 10.1093/cvr/cvz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood 131: 58–67, 2018. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677–704, 2008. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236: 219–242, 2010. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 17: 395–412, 2018. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, Chen Y, Jiang R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer 8: e000285, 2020. doi: 10.1136/jitc-2019-000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forteza MJ, Hervas A, de Dios E, Trapero I, Ruiz-Sauri A, Minana G, Bonanad C, Gomez C, Husser O, Nunez J, Chorro FJ, Bodi V. Programmed death-1 (PD-1): a novel mechanism for understanding the acute immune deregulation in ST-segment elevation myocardial infarction. Int J Cardiol 177: 8–10, 2014. doi: 10.1016/j.ijcard.2014.09.114. [DOI] [PubMed] [Google Scholar]
- 70.Diao B, Wang C, Tan Y. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 11: 827, 2020. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Huang Z, Yin G, Zhang X, Ye W, Hu Z, Hu C, Wei H, Zheng Y, Chi Y, Cheng C, Lin F, Lu H, Xiao L, Song Y, Wang C, Yi Y, Dong L. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. Lancet Respir Med 2020. doi: 10.2139/ssrn.3555267. [DOI] [Google Scholar]
- 72.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 133: 906–918, 2019. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 73.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endothelitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo L, Rondina MT. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol 10: 2204, 2019. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrick JB. Clinical features of sudden obstruction of the coronary arteries. JAMA 250: 1757–1765, 1983. doi: 10.1001/jama.1983.03340130075039. [DOI] [PubMed] [Google Scholar]
- 76.Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 37: 1024–1033, 2016. doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 77.Rios-Navarro C, Marcos-Garces V, Bayes-Genis A, Husser O, Núñez J, Bodi V. Microvascular obstruction in ST-segment elevation myocardial infarction: looking back to move forward. Focus on CMR. J Clin Med 8: 1805, 2019. doi: 10.3390/jcm8111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ríos-Navarro C, Hueso L, Miñana G, Núñez J, Ruiz-Saurí A, Sanz MJ, Cànoves J, Chorro FJ, Piqueras L, Bodí V. Coronary serum obtained after myocardial infarction induces angiogenesis and microvascular obstruction repair. Role of hypoxia-inducible factor-1A. Rev Esp Cardiol (Engl Ed) 71: 440–449, 2018. doi: 10.1016/j.recesp.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 79.Bodi V, Monmeneu JV, Ortiz-Perez JT, Lopez-Lereu MP, Bonanad C, Husser O, Minana G, Gomez C, Nunez J, Forteza MJ, Hervas A, de Dios E, Moratal D, Bosch X, Chorro FJ. Prediction of reverse remodeling at cardiac MR imaging soon after first ST-segment-elevation myocardial infarction: results of a large prospective registry. Radiology 278: 54–63, 2016. doi: 10.1148/radiol.2015142674. [DOI] [PubMed] [Google Scholar]
- 80.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, , et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 75: 2950–2973, 2020. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, Liao J, Jia H, Pang X, Song Y, Tian Y, Wang B, Wu C, Yuan H, Zhang Y, Li Y, Sun W, Zhang Y, Zhu S, Wang S, Xie Y, Ge S, Zhang L, Hu Y, Xie M. Deep vein thrombosis in hospitalized patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation 142: 114–128, 2020. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 82.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18: 844–847, 2020. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barton P, Andronis L, Briggs A, McPherson K, Capewell S. Effectiveness and cost effectiveness of cardiovascular disease prevention in whole populations: modelling study. BMJ 343: d4044, 2011. doi: 10.1136/bmj.d4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US preventive services task force. JAMA 316: 2008–2024, 2016. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 85.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, , et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382: 2327–2336, 2020. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng VG, Lansky AJ, Meller S, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie B, Shah R, Mehran R, Stone GW. The prognostic importance of left ventricular function in patients with ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 3: 67–77, 2014. doi: 10.1177/2048872613507149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cárdenas G, Torres-García D, Cervantes-Torres J, Rosales-Mendoza S, Fleury A, Fragoso G, Laclette JP, Sciutto E. Role of systemic and nasal glucocorticoid treatment in the regulation of the inflammatory response in patients with SARS-Cov-2 infection. Arch Med Res 52: 143–150, 2021. doi: 10.1016/j.arcmed.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keske Ş, Tekin S, Sait B, İrkören P, Kapmaz M, Çimen C, Ugur S, Celebi I, Bakir VO, Palaoglu E, Senturk E, Caglayan B, Cakar N, Tabak L, Ergonul O. Appropriate use of tocilizumab in COVID-19 infection. Int J Infect Dis 99: 338–343, 2020. doi: 10.1016/j.ijid.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osman M, Yassen I, Elhefny EHAB. Comparison between intracoronary versus intravenous bolus injection of tirofiban on infarct size during primary PCI in patients with acute anterior ST segment elevation myocardial infarction. Eur Heart J 41: ehaa946–1437, 2020. doi: 10.1093/ehjci/ehaa946.1437. [DOI] [Google Scholar]
- 90.Gu YL, Kampinga MA, Wieringa WG, Fokkema ML, Nijsten MW, Hillege HL, van den Heuvel AFM, Tan E-S, Pundziute G, van der Werf R, Guyomi SH, van der Horst ICC, Zijlstra F, de Smet BJGL. Intracoronary versus intravenous administration of abciximab in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with thrombus aspiration: the comparison of intracoronary versus intravenous abciximab administration during emergency reperfusion of ST-segment elevation myocardial infarction (CICERO) trial. Circulation 122: 2709–2717, 2010. doi: 10.1161/CIRCULATIONAHA.110.002741. [DOI] [PubMed] [Google Scholar]
- 91.Aliño SF, José Herrero M, Bodi V, Noguera I, Mainar L, Dasí F, Sempere A, Sanchez M, Diaz A, Sabater L, Lledo S. Naked DNA delivery to whole pig cardiac tissue by coronary sinus retrograde injection employing non-invasive catheterization. J Gene Med 12: 920–926, 2010. doi: 10.1002/jgm.1510. [DOI] [PubMed] [Google Scholar]
- 92.Giugliano GR, Giugliano RP, Gibson CM, Kuntz RE. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol 91: 1055–1059, 2003. doi: 10.1016/S0002-9149(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 93.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MV, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Gedealvares FS Jr, Morais DC, Zung S, Machado FR, Azevedo LCP; COALITION COVID-19 Brazil III Investigators. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 324: 1307, 2020. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zheng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 214: 108393, 2020. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobara M, Noda K, Kitamura M, Okamoto A, Shiraishi T, Toba H, Matsubara H, Nakata T. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res 87: 424–430, 2010. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- 96.Anstensrud AK, Woxholt S, Sharma K, Broch K, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damas JK, Hopp E, Kleveland O, Stensaeth KH, Opdahl A, Klow N-E, Seljeflot I, Anderson GO, Wiseth R, Aukrust P, Gullestad L. Rationale for the ASSAIL-MI-trial: a randomised controlled trial designed to assess the effect of tocilizumab on myocardial salvage in patients with acute ST-elevation myocardial infarction (STEMI). Open Heart 6: e001108, 2019. doi: 10.1136/openhrt-2019-001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo C, Orlando G, Borghi V, Santoro A, Gaetano MD, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbi L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2: e474–e484, 2020. doi: 10.1016/s2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res 78: 5011–5022, 2018. doi: 10.1158/0008-5472.CAN-18-0118. [DOI] [PubMed] [Google Scholar]
- 99.Ibañez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 39: 119–177, 2017. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 100.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 76: 122–124, 2020. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]