Abstract
The prevalence of gastric cancer (GC) differs among regions worldwide, with the highest occurrence in east Asia. Thus, its etiology, with respect to ethnic background, environmental factors, and lifestyles, is also thought to differ essentially. In addition, etiology of GC is speculated to be changing due to the recent decrease in the Helicobacter pylori (H. pylori) infection in Japan. State-of-the-art somatic/germline cancer genomics has clarified the etiologies of gastric carcinogenesis. In this review article, we summarize past and present milestones in our understanding of GC achieved through genomic approaches, including a recent report that revealed higher-than-expected frequencies of GCs attributed to east Asian-specific germline variants in ALDH2 or CDH1 in combination with lifestyles. Based on this updated knowledge, we also discuss the possible impact of and high-risk approaches for GCs in the upcoming “H. pylori-negative era.”
Subject terms: Gastric cancer, Cancer genetics
Introduction
Gastric cancer (GC) is the fifth most frequently diagnosed malignancy and the third leading cause of cancer mortality worldwide [1]. However, the incidence of GC varies substantially around the globe and is the highest in east Asian countries including Japan, Korea, and China [1, 2]. In recent statistics in Japan, GC was reported to be the second and fourth most common cancer in men and women, respectively (https://ganjoho.jp/reg_stat/statistics/stat/summary.html). The most popular etiology of GC is Helicobacter pylori (H. pylori) infection. However, in Japan, the prevalence of H. pylori infection in younger individuals has been declining at an accelerating rate [3] and eradication therapies for H. pylori have been approved by the national health insurance in 2013; thus, the incidence of GC has been gradually decreasing [4–6].
Recent advancements in cancer genome sequencing have revealed the comprehensive somatic mutation profiles in GCs. The Cancer Genome Atlas (TCGA) group has reported that GC can be genetically/etiologically classified into four subgroups: genetically stable, chromosomal instability, microsatellite instability, and EBV-associated GCs [7]. Large-scale sequencing studies of GC identified and confirmed frequent gene amplifications in genes encoding receptor tyrosine kinases (e.g., ERBB2, ERBB3, EGFR, FGFR2, MET, and VEGFR) and somatic mutations in p53, ARID1A, PIK3CA, SMAD4, CDH1 (E-Cadherin), and RHOA genes, with high frequencies in the last two specifically among diffuse-type GC (DGC) [7–12]. Somatic gene fusion of CLDN18/ARHGAP has also been reported as a frequent event in DGC [7, 13]. These somatic genetics have clarified the molecularly defined subtypes of GC and their driver events, which could be candidate therapeutic targets [14, 15].
Germline variations in the personal human genome are also known to play important roles in carcinogenesis in various organs, including the stomach [16]. Investigation of the cancer incidences among Japanese migrants in Hawaii showed that the rates of GC had decreased in the first- and then second-generation immigrants but were still higher than the rate in the local populations in Hawaii [17], suggesting that individuals of east Asian ethnicity harbor genetic traits predisposing them to GC. Large-scale genome-wide association studies (GWAS) of sporadic GCs have been conducted in east Asian populations and have identified several common germline variations (described later) [18–21]. As to rare variants with large effect sizes, well-known germline variations among hereditary DGC (HDGC) families have been found in CDH1 worldwide [22] and other variants have also been reported in genes encoding DNA repair machineries (BRCA1/2, PALB2, RAD51, MSH2, ATR, NBN, and RECQL5) [23, 24]. However, hereditary GCs are clinically difficult to identify in east Asian countries with considerably high incidences of sporadic GCs. Therefore, in these regions, the precise frequency of hereditary GC and the predisposing germline genetics have not been fully elucidated.
Since the early 1960s, statistical epidemiology of cancer patients has revealed various links of lifestyles, occupational environments, and dietary factors to the development of cancers [25, 26]. Investigation of the epidemiological factors that predispose individuals to malignancies have long been warranted in the view of preventive medicine. However, cancers typically arise because of complexed combinations of epidemiological factors, and carcinogenesis occurs over a long duration of time, during which the past epidemiological information of patients may become obscure. Therefore, precise enumeration and evaluation of the epidemiological links between lifestyles and cancer in a scientifically robust manner has been challenging. Genetic analysis so-called “mutational signatures” in the cancer genome has rapidly emerged as a solution to this concern, which would make it feasible to evaluate so-far missing links between epidemiology and carcinogenesis on a genetic basis [27–31].
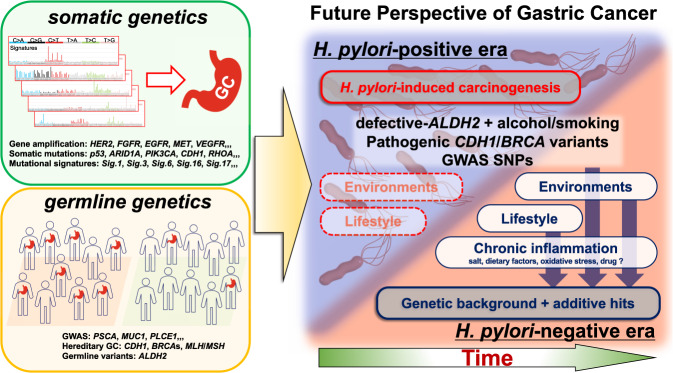
In this review, we first summarize the recent findings regarding the links between lifestyles and somatic/germline genetics in GC patients among east Asian populations, with special focuses on alcohol intake and smoking habits. We also summarize the germline variations that predispose affected individuals to GC, including a recent report of a higher-than-expected frequency of CDH1 germline variants among Japanese patients with GCs, most of which were clinically considered as sporadic cases [32]. Finally, we discuss the future perspectives of GC and its prevention in the upcoming era of H. pylori-negative background (Fig. 1).
Fig. 1.
Future perspectives for gastric cancer (GC) in east Asia in the upcoming H. pylori-negative era. Schematic summary of this review article. State-of-the-art somatic and germline genetic analyses have clarified the precise molecular pathology of gastric carcinogenesis (left). Based on such knowledge of the genetics of GC in the current era, the future perspectives for new types of GC in the H. pylori-negative era are speculated (right). Graphs of the mutational signatures are derived from COSMIC website (Mutational Signatures v2, https://cancer.sanger.ac.uk/cosmic/signatures_v2.tt)
Epidemiology of H. pylori and lifestyles in GC
The best-known extrinsic risk factor for GC is H. pylori infection worldwide [33, 34]. Although the exact proportion of H. pylori-positive GCs including those with past infections is a matter of debate, as is how H. pylori-positivity should be evaluated serologically, histologically, and endoscopically [35–39], the incidence of H. pylori-positive GC is considered to be extremely high in east Asian countries, such as 96.0% in Korea [35, 36] and 94.6% or higher in Japan [37–39]. A trans-ethnic meta-analysis of 12 studies of 1,228 GCs showed a statistical link between H. pylori seropositivity and non-cardia GCs, with an odds ratio (OR) of 3.0 (95% confidence interval [CI], 2.3–3.8), and an even stronger correlation (OR, 5.9; 95% CI, 3.4–10.3) when the duration between blood data for H. pylori infection and GC diagnosis was longer than 10 years [40]. Another trans-ethnic meta-analysis of 19 studies with 2,491 GCs also showed an epidemiological link between H. pylori seropositivity and GCs of any kinds with an OR of 1.92 (95% CI, 1.32–2.78) [41]. Genetic variants of H. pylori strains, specifically those in CagA and VacA genes, are also known to be linked to differential risks for GC [34, 42, 43]. Dr Hatakeyama’s group reported that east Asian CagA has higher binding affinity (by two orders of magnitude) for the N-SH2 domain of SHP2 than type I western CagA, and such strong binding makes the structure of the N-SH2 more relaxed and more efficiently activates SHP2, leading to the neoplastic transformation of gastric epithelial cells [44]. Given the strong correlation between H. pylori and GC, a substantially large portion of GCs has been attributed to H. pylori infection and the subsequent pathological inflammation/regeneration of gastric mucosa [33, 34, 42]. However, the prevalence of H. pylori infection is predicted to be decreasing in Japan. A meta-analysis showed that the multivariable adjusted prevalence of H. pylori infection has been drastically decreasing among younger individuals [3]; the predicted prevalence among populations born in 1920, 1930, 1990, and 2000 was 65.9% (95% CI, 63.9–67.9), 67.4% (95% CI, 66.0–68.7), 15.6% (95% CI, 14.0–17.3), and 6.6% (95% CI, 4.8–8.9), respectively. In addition, the Japanese government broadened the application of H. pylori eradication therapy for national health insurance in 2013, and ~1.5 million people in Japan undergo the eradication of H. pylori each year [5]. Presumably due to the combinations of such decreasing prevalence of H. pylori infection and increase of the eradication therapy as well as the early detection surveillance of GC [45], the incidence of GC in Japan has been gradually decreasing [4–6], and deaths from GC have fallen from 48,427 in 2013 to 45,509 in 2016 [5]. Importantly, this continuously decreasing trend in H. pylori-related GC implies the emergence of an era of GCs with H. pylori-negative background in the coming decades. Therefore, investigations of the etiology and carcinogenesis of H. pylori-negative GC are warranted.
Regarding our daily lifestyles, it has been shown that the intake of salty and smoked foods is related to the development of GC [46]. Like other human malignancies, epidemiological studies have suggested that GC is attributed to alcohol intake and smoking habits [47–49]. A large-scale investigation of 54,682 Japanese population with a 13.4-year follow up revealed that alcohol intake was significantly associated with an increased risk of GC among men, with hazard ratio (HR) as high as 1.85 (95% CI, 1.35–2.53) compared to nondrinkers [47]. This study also showed that every 10-g increase in alcohol intake led a HR of 1.07 (95% CI, 1.04–1.10) for GC in men. Trans-ethnic meta-analyses of the risks of alcohol intake for GC showed that alcohol elevated the risk of GC with an OR of 1.39 (95% CI, 1.20–1.61) (in 19,302 individuals from ten studies) [48] and a risk ratio of 1.17 (95% CI, 1.00–1.34) (in 5,886,792 individuals from 23 studies) [49]. For smoking, multiple meta-analyses confirmed a risk for GC among populations from various ethnic backgrounds [50, 51]. The Stomach cancer Pooling Project, which included 10,290 patients with GC and 26,145 controls, showed that, when compared to never smokers, the OR of current smokers was as high as 1.25 (95% CI, 1.11–1.40), and the OR of individuals with a smoking history longer than 40 years was elevated to 1.33 (95% CI, 1.14–1.54) [50].
Mutational signatures in the cancer genome
The cancer genome harbors numerous somatic mutations resulting from DNA damages and erroneous repairs caused by various endogenous and exogenous processes, including mutagenic chemical exposures, physical destruction of DNA, and defective DNA repair pathways, among others. Single-nucleotide substitutions (C>A, C>G, C>T, T>A, T>C, and T>G) can be classified into 96 types according to their neighboring two nucleotides (both 5′ and 3′), and somatic mutations in the cancer genome can be defined as a set of those 96 types of substitutions. Interestingly, analysis of the whole-genome or whole-exome sequences of large cohorts of cancers has revealed that cancer genome mutations can be mathematically factorized into a mixture of patterns based on the combinations of these 96 substitution types (Fig. 2). These patterns of somatic mutations are called mutational signatures (or mutational spectra) [27–31]. A cancer genome is composed of additive (nonnegative) accumulations of the source patterns of the mutational signatures; thus, nonnegative matrix factorization is utilized to compute contribution scores of the mutational signatures in each of the cancer genome [29]. The Catalog of Somatic Mutations in Cancer (COSMIC) group categorized such mutational signatures into 30 types (Mutational Signature v2, March 2015) (https://cancer.sanger.ac.uk/cosmic/signatures_v2.tt). Some of these mutational signatures have been suggested to be epidemiologically linked to specific carcinogenic factors, such as smoking (Sig.4), ultraviolet exposure (Sig.7), alkylating agents (Sig.11), aristolochic acid (Sig.22), aflatoxin (Sig.24), and tobacco chewing habit (Sig.29), while others are etiologically linked to intrinsic factors like ageing (Sig.1), altered activation of AID/APOBEC cytidine deaminases (Sigs.2, 9, and 13), germline/somatic BRCA mutations (Sig.3), defective mismatch repair (Sigs.6, 15, 20, and 26), and altered activity of the error-prone polymerase POLE (Sig.10) (Mutational Signature v2, COSMIC). Recently, COSMIC has further extended these signatures into more than 60, including those of possible sequence artefacts (Mutational Signature v3.1, Aug 2020).
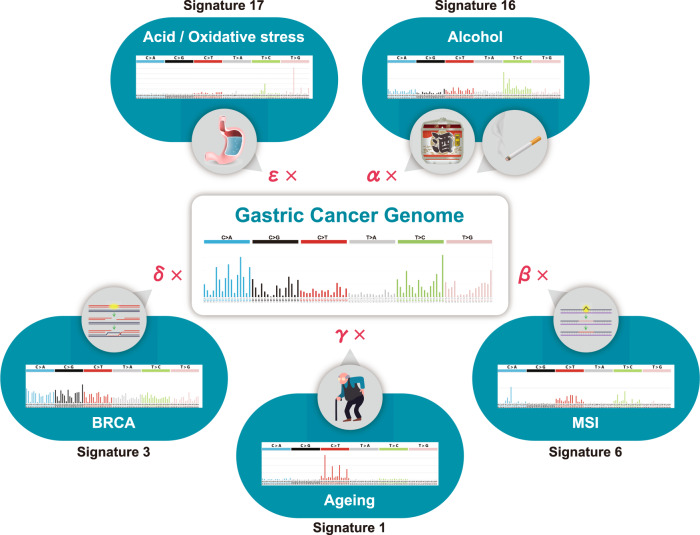
Fig. 2.
Mutational signatures in the cancer genome. The somatic mutation profile in an individual gastric cancer genome (center) can be mathematically factorized into cumulative combinations of mutational signatures (outer graphs). To date, more than 60 mutational signatures have been proposed by COSMIC, several of which are linked to specific carcinogenic factors (smoking, alcohol use, ultraviolet exposure, ageing, etc.). By calculating the contribution score for each mutational signature (α, β, γ, etc.), the relative contributions of the causative factors to carcinogenesis can precisely be estimated. Graphs of the mutational signatures are derived from COSMIC website (Mutational Signatures v2, https://cancer.sanger.ac.uk/cosmic/signatures_v2.tt)
By analyzing the proportions of these mutational signatures in the cancer genome, it can be feasible to mathematically enumerate the relative contributions of each factor to carcinogenesis [27–31] (Fig. 2).
Identification of an east Asian-specific subtype of GC linked by mutational signatures, germline factors, and lifestyles
In a recent report, in which a large cohort of trans-ethnic 531 GCs was classified based on the patterns of the mutational signatures in the cancer genome, a GC subgroup was shown to have a high contribution of Sig.16 [32]. This GC subgroup was characteristic for its Asian ethnicity and inactive ALDH2 allele (rs671 AA or AG). In a focused investigation of 243-case Japanese GCs, it was revealed that 6.6% (16/243) of cases were classified in a cluster with a high contribution of Sig.16; furthermore, 68.8% (11/16) of these cases were alcohol consumers with inactive ALDH2 allele (rs671 AA or AG) [32]. In a detailed analysis of the frequencies of the Sig.16 mutations found in each of 243 Japanese GC, patients with both an alcohol use habit and an inactive ALDH2 allele showed synergistically increased numbers of Sig.16 somatic mutations (11.1-fold) compared to other GC patients. The link between Sig.16 and alcohol use has also been reported for other malignancies [52–54]. Although the molecular mechanism linking alcohol intake and Sig.16 mutations remains to be investigated, various acetaldehyde-derived DNA adducts have been identified [55], which could be a cause of the frequent T>C transitions observed in Sig.16. An intriguing additive effect of smoking on the accumulation of Sig.16 in GCs was also identified, in which the Sig.16 somatic mutations were synergistically increased among overall GC patients with the triple combination of alcohol intake, smoking, and a defective ALDH2 allele (p = 0.0339) [32]. This phenomenon is highly east Asian-specific, based on the specificity of the defective ALDH2 allele among Asian populations [56]. As discussed above, epidemiology showed that alcohol intake and smoking had significant but only mild associations with GCs [47–51]; however, an integrated investigation of mutational signatures, germline genetics, and lifestyle information showed that these risk factors are apparently more obvious among specific east Asian populations with a defined germline variant. It will also be important to identify any characteristic mutational signatures specifically among non-Asian populations.
Germline variations that predispose affected individuals to GC
Large-scale GWAS of single-nucleotide polymorphisms (SNPs) have also been conducted to identify susceptibility loci in GC. These studies have been conducted almost exclusively in east Asian populations such as Japanese and Chinese. Comprehensive GWAS by Dr Hirohashi’s and Dr Matsuda’s groups in Japan identified SNPs that were significantly associated with GC. SNPs of prostate stem cell antigen (PSCA) (8q24.3) and Mucin1 (MUC1) (1q22) were shown to be associated with DGC [18–20] (Table 1). In addition, SNPs on 12q24.11-12 (cut like homeobox 2 [CUX2]), 20q11.21 (a gene cluster of the defensin beta family), and 9q34.2 (the ABO locus) were also associated with GC [20] (Table 1), and were more strongly associated with DGC than intestinal-type GCs, although it was not statistically significant. The high-risk SNPs of PSCA and MUC1 identified among Japanese populations were also confirmed among Korean populations [18, 19] (Table 1). A GWAS of Chinese GCs showed that multiple variants on 10q23 were significantly correlated with GC, and a notable value was found for rs2274223 in phospholipase C epsilon 1 (PLCE1) [21] (Table 1).
Table 1.
Representative GC-associated germline variants identified by GWAS
| Gene | Chromosome | SNP | Ethnicity | Risk | Association with histology or anatomy | Reference |
|---|---|---|---|---|---|---|
| PSCA | 8q24.3 | rs2976392 | Japanese | OR, 1.62 (95% CI, 1.38–1.89) effect allele: A | Stronger association with DGC | # [18] |
| Korean | OR, 1.90 (95% CI, 1.56–2.33) effect allele: A | Stronger association with DGC | # [18] | |||
| European descent | OR, 1.88 (95% CI, 1.47–2.43) effect allele: A | Significant in both DGC/IGC | # [61] | |||
| rs2294008 | Japanese | OR, 1.58 (95% CI, 1.35–1.85) effect allele: T | Stronger association with DGC | # [18] | ||
| Korean | OR, 1.91 (95% CI, 1.57–2.33) effect allele: T | Stronger association with DGC | # [18] | |||
| Chinese | OR, 1.20 (95% CI, 1.15–1.28) effect allele: T | Analysis of non-cardia GC | # [58] | |||
| European descent | OR, 1.88 (95% CI, 2.42–7.70) effect allele: T | Significant in both DGC/IGC | # [61] | |||
| Caucasian | OR, 1.42 (95% CI, 1.23–1.66) effect allele: T | Particularly in non-cardia GC | # [62] | |||
| Latin American | OR, 2.34 (95% CI, 1.36–4.01) effect allele: T | Significant in both cardia/non-cardia GC significant in both DGC/IGC | # [63] | |||
| MUC1 | 1q22 | rs2070803 | Japanese/Korean | OR, 1.71 (95% CI, 1.48–1.98) effect allele: G | Stronger association with DGC | # [19] |
| rs4072037 | Japanese/Korean | OR, 1.66 (95% CI, 1.44–1.93) effect allele: A | Stronger association with DGC | # [19] | ||
| Chinese | OR, 0.75 (95% CI, 0.67–0.84) effect allele: G | Significant in both cardia/non-cardia GC | # [21] | |||
| Chinese | OR, 0.75 (95% CI, 0.69–0.79) effect allele: G | Analysis of non-cardia GC | # [58] | |||
| Chinese | OR, 1.33 (95% CI, 1.22–1.45) effect allele: A | – | # [59] | |||
| Korean | OR, 0.82 (95% CI, 0.72–0.94) effect allele: G | Not different between DGC/IGC | # [65] | |||
| East Asian/Chinese/Korean | OR, 0.76 (95% CI, 0.69–0.84) effect allele: G | Significant in both cardia/non-cardia GC | # [60] | |||
| European descent | OR, 0.64 (95% CI, 0.49–0.81) effect allele: G | Significant in both DGC/IGC | # [61] | |||
| CUX2 | 12q24.11-12 | rs6490061 | Japanese | OR 0.905 (P = 3.20 × 10−8) effect allele: T | Not statistically different between DGC/IGC | # [20] |
| DEFB cluster | 20q11.21 | rs2376549 | Japanese | OR 1.109 (P = 8.11 × 10−10) effect allele: C | Not statistically different between DGC/IGC | # [20] |
| ABO locus | 9q34.2 | rs7849280 | Japanese | OR 1.148 (P = 2.64 × 10−13) effect allele: G | Not statistically different between DGC/IGC | # [20] |
| PLCE1 | 10q23 | rs2274223 | Chinese | OR 1.31 (P = 8.40 × 10−9) effect allele: G | Significantly associated with cardia GC | # [21] |
| Korean | OR, 0.96 (95% CI, 0.87–1.06) effect allele: G | # [65] | ||||
| Japanese | Not significant | # [20] | ||||
| Caucasian | Not significant | # [64] | ||||
| European descent | Not significant | # [61] |
The molecular biological functions of these SNPs have been investigated, and the cancer-related functional disturbances they presumably cause have been described. For instance, PSCA is confirmed to be expressed in gastric epithelial cells, and substitution of a C allele with the high-risk T allele at rs2294008 in the 1st exon reduced its transcriptional activity [18]. It is also noteworthy that the alleles of this SNP of PSCA have opposing effects in GC and duodenal ulcers [57]. The T allele, which results in a longer membranous PSCA, has growth-promoting effects in inflamed gastric epithelia, making the T allele a high-risk factor for GC. In contrast, the C allele, which results in a shorter cytosolic PSCA, may enhance the immune reaction and might accelerate pathological inflammation in the duodenum as well as induce antitumor immunity during gastric carcinogenesis [57]. It has also been reported that the rs6490061 SNP in the CUX2 significantly reduced its mRNA expression in response to H. pylori infection [20]. Additional cell biological experiments investigating the effects of the identified SNPs through gene editing using CRISPR or other techniques would help reveal precise molecular mechanisms underlying the significant predispositions to GCs in affected individuals, which may reveal novel modality of preventive strategies against GCs.
These polymorphisms, which were initially identified in Japanese and/or Chinese populations, have been confirmed in other studies of not only other east Asians [58–60] but also western populations, including Caucasians [61–63] (Table 1). However, at the same time, discrepancies have also been reported in the significance of these SNPs among populations of different ethnic backgrounds [64]. For instance, the significant association of the PLCE1 polymorphism with GC identified among Chinese populations has not been confirmed among Caucasian populations [64], and in fact, an inverted trend of correlation was observed in a Korean population, although not statistically significant [65] (Table 1). The abovementioned GWAS from Japan did not find the PLCE1 SNP to be significant [18–20]. PLCE1 is a phospholipase enzyme that connects signals from small GTPases to various pathways, including MAP kinase cascades [66–68]; thus, it may regulate cancer-related processes, such as cell growth and differentiation. This PLCE1 SNP (rs2274223) was initially identified as a common risk allele for both esophageal and GCs [21], and a meta-analysis of Chinese populations reported that it is associated with cardia GC [69]. The prevalence of cardia and non-cardia GCs is known to differ substantially between ethnicities, and in Japan, non-cardia GC has been characteristically prevalent [70, 71]; thus, the inconsistency in the significance of the PLCE1 SNP between Chinese and Japanese GCs might reflect the difference in the preferred location of GC as well as its background environmental factors.
From the viewpoint of preventive medicine, with the relatively low ORs and small effect sizes of the common germline variants in GCs identified thus far, genotyping of these SNPs alone is not sufficient to stratify individuals for the prediction of gastric carcinogenesis. Thus, a high-risk approach using a small number of common variants may be insufficient to effectively prevent GC.
Unexpectedly high incidence of germline variants in CDH1 among east Asians with GC
In addition to the germline genetics described above, HDGC is a well-known GC-predisposing syndrome that is attributed to germline variants of causative genes such as CDH1 [22]. CDH1 is a member of cadherin superfamily consisting of a precursor domain, five extracellular cadherin domains, a transmembrane domain, and a cytoplasmic domain (Fig. 3) and plays an important role in the epithelial cell-to-cell adhesion [72]. Its loss-of-function is known to contribute to the disseminative and invasive phenotypes of cancer cells by affecting various molecular pathways [72]. Germline variants in other genes have also been found in DNA repair machinery (BRCA1/2, PALB2, RAD51, MSH2, ATR, NBN, and RECQL5) [23, 24], as described later. The CDH1 germline variants among HDGC was first discovered in Maori kindred in New Zealand in 1998 [73]. Thus far, at least 155 germline CDH1 variants have been identified worldwide [74–76], and recently, the International Gastric Cancer Linkage Consortium (IGCLC) updated the diagnostic criteria and clinical practice guidelines for HDGC (Aug 2020) (https://hereditarydiffusegastriccancer.org/) [22].
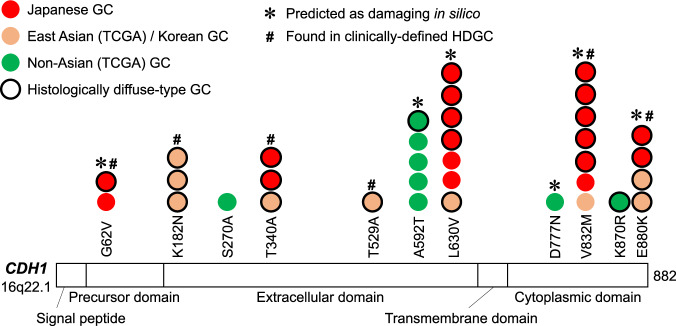
Fig. 3.
Germline variants in CDH1 gene identified in GC patients. A histogram of non-silent germline variants of CDH1 gene identified in a recent trans-ethnic study [32]. Colors of the circles represent ethnicities of the patients as indicated. Circles with black rims represent cases of DGC. * and # represent CDH1 variants that were predicted as damaging in silico and found in clinical HDGC families, respectively
However, most of the CDH1 variations were reported among non-Asian populations. As high as 40% of detection rates of germline CDH1 variants have been reported among non-Asian HDGC families [77–80], although one report of European familial GC identified no germline variants in CDH1 [81]. In contrast, the results of several representative studies of germline CDH1 variants in cases of possible HDGC or familial GC in Japan [82–86] suggested that the detection rates of CDH1 variants among east Asian populations are relatively rarer (Table 2). Thus, determining the frequency of germline CDH1 variants in familial GCs among east Asians has remained elusive, due in part to the paucity of comprehensive studies with large cohorts of HDGC families as well as the difficulty in identifying genuine cases of HDGC among east Asians because of the high incidence of sporadic GC.
Table 2.
Representative studies of germline CDH1 variants in familial GCs among trans-ethnic populations
| Ethnicity/country | Frequency of germline CDH1 variants | Criteria of familial GC | Mutaiton | Reference | ||
|---|---|---|---|---|---|---|
| Northern European desscents, and Spanish, Haida, French-Canadian, and Italian descents, including unknown origin | 31.0% | 13/42 families |
Two or more DGC in first-degree relatives, with at least one diagnosed before 50 Two or more GC, with at least one DGC diagnosed before 50 Three or more DGC in first-degree relatives Three or more GC with at least one DGC Individuals diagnosed with DGC at younger than 45 Individuals diagnosed with both DGC and LBC One family member diagnosed DGC and another with LBC One family member diagnosed DGC and another with colon cancer |
382delC | Frameshift | [77] |
| G892A | A298T | |||||
| 1064insT | Frameshift | |||||
| 1134 del8, ins5 | Deletion | |||||
| 1212delC | N405Ifs | |||||
| T1226C | W409R | |||||
| 1476delAG | Frameshift | |||||
| 1779insC | Frameshift | |||||
| 2061delTG | Frameshift | |||||
| G2195A | R732A | |||||
| 2310delC | Frameshift | |||||
| IVS5(+1) G>A | Splice site | |||||
| IVS11(+5) G>A | Splice site | |||||
| English, Irish, Spanish, Columbian, Filipino, Swedish/Norwagian, including unknown origin | 39.5% | 15/38 families |
at least 2 GCs with 1 DGC younger than 50 either 1 DGC younger than 35 or multiple DGCs older than 50 |
283C>T | Q95X | [78] |
| 715G>A | G239R | |||||
| 1137G>A splicing | Splicing site | |||||
| 1397-1398delTC | Frameshift | |||||
| 1682-1683insA | Frameshift | |||||
| 1901C>T | A634V | |||||
| 1913G>A | W638X | |||||
| 2064-2065delTG | Frameshift | |||||
| 2164+5G>A splicing | Splicing site | |||||
| 2195G>A | R732Q | |||||
| 2245C>T | R749W | |||||
| 2343A>T | E781D | |||||
| 2398delC | Frameshift | |||||
| Caucasian, Hispanic, Maori, Chinese (one family) | 29.2% | 7/24 families | IGCLC criteria | 49-2A>C | Splice site | [79] |
| 353c>G | T118R | |||||
| 715G>A | G239R | |||||
| 1107delC | Frameshift | |||||
| 1137G>A | Splice site | |||||
| 1391_1392delTC | Frameshift | |||||
| 1901C>T | A634V | |||||
| 2095C>T | Q699X | |||||
| 2440-6C>G | Splice site | |||||
| Netherlands, Portugal, Germany, Italy, Poland | 0.0% | 0/53 families |
Diagnosed below 35 families with two GCs at or below 60 Families with three GCs at or below 70 |
[81] | ||
| Japanese | 0.0% | 0/14 families |
At least three relatives had GCs with at least one first-degree relative of the other two At least two successive generations had GCs |
[84] | ||
| Japanese | 2.6% | 2/78 families |
Three and more GCs in a family At least two successive generations had GCs GCs diagnosed younger than 50 in one of the relatives |
1243A>C | I415L | [85] |
| Japanese | 11.8% | 2/17 individuals | At least one sibling diagnosed with GC | IVS+6T>C | Splice site | [86] |
| 2494G>A | V832M | |||||
| Japanese | 15.4% | 2/13 families | IGCLC criteria | 1212delC | N405IfsX12 | [82] |
| 164-?_387+?del | V55GfsX38 | |||||
Representative studies, which investigated more than 10 kindreds of HDGC or familial GCs, are listed
The disease penetrance of the germline CDH1 variants had been estimated to be substantially high among non-Asian populations, and one study concluded that the cumulative risk of GC by 80 years of ages was 67% (95% CI, 39–99) and 83% (95% CI, 12–84) in men and women, respectively [87], and another estimated these risks as 40% (95% CI, 12%–91%) and 63% (95% CI, 19%–99%) by the age of 75 years for men and women, respectively [78]. However, the disease penetrance among east Asian populations has not been established to date.
To evaluate the actual germline contributions to unselected GCs in east Asian populations, the germline genetics of the abovementioned 243-case Japanese GC cohort were investigated [32]. Analysis of 624 cancer-related genes revealed that the CDH1 gene had the highest density of germline rare variants (ratio of variant frequency to the length of the gene) [32]. In total, 18 out of the 243 Japanese GCs (7.4%) harbored germline variations in CDH1. All the non-silent germline variations identified in the study are listed in Table 3 and shown in Fig. 3. Most of the Japanese GCs with CDH1 variations (77.8%, 14/18 cases) were diagnosed as DGC (Table 3) [32], which is consistent with the molecular dysfunction of CDH1 specifically in DGC [88] and strongly suggests pathogenic roles for these variants in the gastric carcinogenesis. For DGC, the frequency of CDH1 variants in this cohort (13.3%, 14/105 cases) was 4.1-fold higher than that in non-Asian GCs of TCGA (3.2%, 2/62 cases) [32]. Distributions of the variants in CDH1 gene among Japanese GCs showed that they were enriched at five positions that were shared with Korean populations with GC [89] but were clearly different from those of non-Asians (Table 3) (Fig. 3). Four of the five have previously been reported in early onset GCs in Korea [89] and families with GCs in Japan and Brazil, including p.G62V in a family of Japanese ethnicity [83, 86, 90]. Two of them, T340A and V832M, were suggested to be pathogenic based on in vitro experiments [91, 92]. Molecular links between the CDH1 variants and gastric carcinogenesis specifically in relation to the differences in ethnic backgrounds should be investigated further in the future.
Table 3.
Germline CDH1 variants identified in the 243-case Japanse and trans-ethnic GCs in a recent report
| CDH1 variants | Exon | Domain | HDGCa | ClinVar | Polyphen2 (HumDiv) | Japanese 243 GCsb | Korean populationc | TCGA non-Asian 212 GCs | Japanese 105 DGCs | TCGA non-Asian 62 DGCs | DGC: Japanese vs. non-Asian | Japanese ToMMo 1070 individuals | Japanese DGC vs. ToMMo | 1000 Genomes EAS 504 individuals | 1000 Genomes EUR 503 individuals | 1000 Genome EAS vs. EUR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Lauren’s classification | Family history of cancers | Age | Lauren’s classification | ||||||||||||||||
| p.G62V | Exon 3 | Precursor region | ◯ | Ref. # [83] | Uncertain significance | Probably damaging | 80s | IGC | Yes | 1 | 3 | |||||||||
| 60s | DGC | No | ||||||||||||||||||
| p.K182N | Exon 5 | Extracellular domain | ◯ | Ref. # [89] | Conflicting interpretations of pathogenicity | benign | 30s | DGC | 1 | 1 | ||||||||||
| 40s | DGC | |||||||||||||||||||
| 50s | DGC | |||||||||||||||||||
| p.S270A | Exon 6 | Extracellular domain | Conflicting interpretations of pathogenicity | benign | 1 | |||||||||||||||
| p.T340A | Exon 8 | Extracellular domain | ◯ | Ref. # [89, 90, 92] | Benign | benign | 80s | DGC | Yes | 2 | 6 | 1 | ||||||||
| 60s | DGC | No | ||||||||||||||||||
| 30s | DGC | |||||||||||||||||||
| p.T529A | Exon 11 | Extracellular domain | ◯ | Ref. # [89] | Conflicting interpretations of pathogenicity | benign | 30s | DGC | ||||||||||||
| p.A592T | Exon 12 | Extracellular domain | Benign | Probably damaging | 5 | 1 | 2 | |||||||||||||
| p.L630V | Exon 12 | Extracellular domain | Benign | Probably damaging | 70s | DGC | Yes | 4 | 9 | 6 | ||||||||||
| 70s | DGC | No | ||||||||||||||||||
| 80s | IGC | Yes | ||||||||||||||||||
| 60s | DGC | No | ||||||||||||||||||
| 80s | DGC | Yes | ||||||||||||||||||
| 60s | IGC | Yes # | ||||||||||||||||||
| 60s | DGC | |||||||||||||||||||
| p.D777N | Exon 15 | Cytoplasmic domain | Conflicting interpretations of pathogenicity | Probably damaging | 1 | |||||||||||||||
| p.V832M | Exon 16 | Cytoplasmic domain | ◯ | Ref. # [86, 92] | Benign | Probably damaging | 90s | DGC | No | 6 | 10 | 2 | ||||||||
| 50s | DGC | Yes | ||||||||||||||||||
| 50s | DGC | Yes | ||||||||||||||||||
| 60s | IGC | No | ||||||||||||||||||
| 60s | DGC | No | ||||||||||||||||||
| 50s | DGC | Yes # | ||||||||||||||||||
| 60s | DGC | Yes | ||||||||||||||||||
| 70s | IGC | |||||||||||||||||||
| p.K870R | Exon 16 | Cytoplasmic domain | Not found | benign | 1 | 1 | ||||||||||||||
| p.E880K | Exon 16 | Cytoplasmic domain | ◯ | Ref. # [89] | Conflicting interpretations of pathogenicity | Probably damaging | 30s | DGC | Yes | 1 | 7 | 2 | ||||||||
| 30s | DGC | |||||||||||||||||||
| 30s | DGC | |||||||||||||||||||
| 18 (7.41%) | 8 (3.77%) | 14 (13.33%) | 2 (3.23%) | 4.13-fold | 36 (3.36%) | 3.96-fold | 12 (2.38%) | 2 (0.40%) | 5.99-fold | |||||||||||
Germline CDH1 variants discovered in a recent report [Ref. #32] are listed. Mixed type GCs in the Lauren classification were categorized as DGC
DGC diffuse-type GC, IGC intestinal-type GC, EAS east Asian, EUR Europian populations
Lauren’s classification (if DGC), family history (if Yes), and GC case numbers among east Asians are highlighted as bold.
aReported in GC cases that met clinical criteria of HDGC such as strong femilial aggregation and/or extremely early onsets [Ref. #83, 86, 89, 90, 92]
bData from a recent study of large-scale trans-ethnic GCs [Ref. #32]
cCombined data from TCGA (Korean) and a large-scale Korean study of early onset GCs [Ref. #89]
It should be noted that the combined frequency of CDH1 rare variants among Japanese DGCs is 3.96-fold higher than that of general Japanese population (3.4%, 36/1,070). Furthermore, in the first place, it was firstly documented that as high as 3.4% of the general Japanese population harbors pathogenic germline CDH1 variants [32], which has significant impacts in clinical fields, as discussed below. However, this observation needs to be confirmed by an independent analysis with a larger cohort. As to V832M, a negative study was recently reported in a Korean population [93].
These germline CDH1 variants had not been identified as pathogenic in any previous GWAS or other large-scale genetic studies [18–21, 89]. One possible reason for this is the low minor allele frequency of the variants. The frequency of even the most common germline CDH1 variant found in the ToMMo database, V832M, is only 0.93%; thus, the CDH1 rare variants had probably been omitted from previous GWAS due to their insufficient statistical power. Recent statistical methods to analyze rare variants such as SKAT (SNP-set (Sequence) Kernel Association Test) where sets of rare/common variants, for instance in a gene or a region, can be evaluated integratively [94, 95] might make it feasible to discover significant rare variants among GC including those of CDH1. Another reason could be related to the ways of curation in the discovery of pathogenic rare germline variants; germline CDH1 variants were not intensively focused unless they were clearly annotated by the ClinVar database [96] or if they existed at measurable frequencies in general populations. None of the CDH1 variants in Table 3 are currently annotated as “pathogenic” in the ClinVar database.
Four out of the five condensed germline variants of CDH1 specifically among Japanese GCs were also shared in Korean populations with GC [32, 89] (Table 3), indicating that these germline variants of CDH1 are specifically and widely distributed among east Asian populations. Thus, it is hypothesized that common ancestral events in multiple loci of the CDH1 gene can explain the increased incidence of GC in east Asia; however, their evolutional significance, including any possible benefit, is still unclear. Interestingly, one of the oldest modern humans, a 45,000-year-old male individual found in Ust’-Ishim in Siberia [97], harbored a heterozygous V832M (rs35572355 G>A) germline variant in CDH1 (https://bioinf.eva.mpg.de/jbrowse). The Ust’-Ishim genome shared more alleles with modern east Asian populations among non-African populations [97]; thus, germline CDH1 variants can be assumed to be rooted at least in that era.
Germline variants of BRCA family genes and GC
Germline variations in the genes encoding double-strand break repair machinery, such as BRCA1/2, PALB2, and RAD51, have been shown to be causative factors for familial GCs [23]. Variations in other DNA repair genes, such as ATR, NBN, and RECQL5, and the mismatch repair gene MSH2 have also been reported in cases of HDGC without CDH1 variations [24]. It is generally difficult to extract pathogenic rare variants from many other nonpathogenic backgrounds because a statistical approach is not usually effective. In addition, unlike breast and gynecological cancers, there have been few reports of systematic germline surveys in large-scale GC cohorts. In the study of a Japanese GC cohort mentioned above, 9.1% of the Japanese GC patients (22/243 cases) had probably pathogenic germline variants with functional defects in BRCA pathways, i.e., cases with the BRCA-related somatic mutational signature (Sig.3) [32]. This frequency is comparable to that among non-Asian GCs in the TCGA data (9.0%, 19/212 cases) [32]. These frequencies differ slightly from those reported in other large-scale genetic studies. For instance, the prevalence of pathogenic variants in BRCA pathway and TP53 genes were reported to be 5.7% in Japanese patients with breast cancer [98], and pathogenic BRCA1/2 variants were detected in 12.1% and 12.7% of western European and Asian patients with breast and ovarian cancers, respectively [99]; however, GCs were not investigated in these studies and different criteria were utilized for mutation detection. It has been proposed that malignancies with the BRCA-related mutational signature are good candidates for PARP inhibitors in combination with platinum-induced DNA damage [100], which might be clinically applicable for GC patients.
Clinical intervention for hereditary GC
It is of worth noting that, in the abovementioned study [32], the germline CDH1 variants found in east Asian populations exhibited mild disease penetrance in affected individuals. Out of the 18 Japanese GC individuals with germline CDH1 variants, 11 had family histories of cancers (Table 3), and one had a lobular breast carcinoma, consistent with a germline variant of CDH1 [22]. However, only one individual fulfilled the IGCLC criteria for HDGC, as a DGC was diagnosed in the 30s of age [32]. The seven other individuals did not have any family history of malignancy. Thus, Japanese individuals even with possibly pathogenic CDH1 variants do not always develop GC. The reason why the disease penetrance of the CDH1 variants among Japanese is lower is not scientifically obvious to date. A possible reason for this might be because gastric carcinogenesis requires additional etiological hits that may be missing in the current lifestyles of east Asians. A recent case report from Japan investigated familial GC in two siblings; one sibling was infected with H. pylori and had an advanced GC, while the other was free from H. pylori and had an early-stage GC [101]. Although additional larger studies are necessary to draw a conclusion, this suggests that H. pylori infection plays a significantly additive role in gastric carcinogenesis, even in individuals with pathogenic germline CDH1 variants. It is also speculated that the disease penetrance of HDGC in east Asian populations is going to be modified due to yet undefined and ever-changing lifestyle factors, even without H. pylori infection in the upcoming H. pylori-negative era.
The updated ICGLC guidelines (Aug 2020) for HDGC has recommended prophylactic gastrectomy for individuals with pathogenic CDH1 variants [22]. However, as discussed above, the disease penetrance and behaviors of CDH1 variants are substantially different between Asian and non-Asian ethnic backgrounds. Therefore, more careful consideration of prophylactic surgery is recommended for individuals of east Asian ethnicity who are just uncertainly suspected as familial GC than for Caucasians [22, 102–104]. A more specific and practical definition of HDGC among east Asian populations, with evaluations of the pathogenicity and influence of lifestyle/environmental factors on the penetrance of CDH1 variants, including their future trends, is needed.
As has also been discussed by the IGCLC group [22], routine endoscopic surveillance of the stomach is another possible method to prevent the development of advanced GC in the affected individuals. In fact, histologically detectable cancer foci have been discovered by endoscopic multiple sampling in a large portion of individuals with germline CDH1 variants [105–107], and one study succeeded in identifying HDGC based on endoscopic observations [108]. Advancements in technologies for high-definition endoscopy, including fluorescent techniques, Raman spectrometry, and artificial intelligence [109–113], will hopefully provide increasing evidence that periodic endoscopic surveillance of pathogenic CDH1 variant carriers is an adequate preventive option for those at risk of GC. Recent advancements in liquid biopsy such as detecting cell-free DNA and circulating tumor cells would also help identify and monitor early-stage GC [114–116]. Thus, prophylactic surgeries would be considered only when genuine high-risk individuals could be identified based on the findings of future research on HDGC.
Future perspectives
In accordance with the changes in our daily lifestyles and environmental factors in the coming decades, the incidence and epidemiology of GCs in east Asia will also be gradually changing. The recent decrease in H. pylori infection among populations such as in Japan [3–6] will greatly impact the epidemiology of GC. It is scientifically hard to speculate the precise molecular pathology of future GCs. As a hypothesis, we postulate that GCs in the H. pylori-negative era will be found only among individuals with specific genetic backgrounds combined with risky lifestyles. Individuals with pathogenic germline variants may have risks of developing GC, even without H. pylori infection, possibly due to causative lifestyle and dietary factors that induce chronic gastritis, such as intake of salty and smoky foods, smoking, oxidative stresses, and chemical agents that modulate host immunity, as well as other yet unidentified factors [117–120]. Currently, with the high prevalence of H. pylori-induced GC, the etiological effects of other lifestyle/dietary factors have been masked in epidemiological studies. However, in the upcoming H. pylori-negative era, previously undefined lifestyle and environmental factors related to GC, combined with germline backgrounds, might be manifested more clearly (Fig. 1).
Based on our current knowledge of the genetics of the GC development, the relative frequency of GC with high Sig.16 contributions among individuals with alcohol use/smoking habits with an inactive ALDH2 allele will likely increase among east Asian populations. The risk of GC among such populations is substantially lower compared to the risk of esophageal cancers, in which smoking and alcohol use combined with ALDH2/ADH1B risk alleles has an OR of 189.26 (95% CI, 95.1–376.6) [121]. However, when considering a high-risk approach for the prevention of future GC, it is epidemiologically important to reduce the risky daily habits of alcohol use and smoking, especially for east Asian individuals with germline variants of ALDH2.
It should be underscored that a higher-than-expected frequency (3.4%) of germline CDH1 variations among the general Japanese population was recently documented; moreover, enrichment of these variants among Japanese patients with DGC was essentially higher (13.3%) [32]. Thus far, the relative contribution of the germline CDH1 variants to the overall prevalence of GC in east Asians had been considered to be lower than that in other ethnic groups. However, the frequencies of such GCs among east Asians with genetic background will probably rise in the coming era of H. pylori-negative GC in combinations with changes in yet undefined lifestyles that would function as additive hits in gastric carcinogenesis. Thus, in the next H. pylori-negative era, it can be predicted that genetic predispositions of GC would be revealed more clearly, with the aid of the advancements in statistical methods. In clinical fields, it is necessary to consider the possibility of germline variants in patients with GC in daily practice, even when they are clinically considered sporadic cases. Establishment of practical endoscopic and liquid biopsy strategies to discover early-stage HDGCs are also needed.
The definitive clinical features of H. pylori-negative GCs among east Asian populations have not been established due to the difficulties in identifying truly H. pylori-negative cases in east Asian countries. Previous studies have suggested some characteristics of H. pylori-negative GCs in east Asia, including early diagnosis (often under 60 years of age); more frequently located in the cardia (although controversial); more advanced TNM stage and poorer prognosis; and a higher proportion of DGC and signet-ring cell carcinoma, although these were not always statistically significant [35, 36, 39]. As to TCGA classification of GCs [7], MSI and EBV GCs might arise by etiologies independent of H. pylori, although it is worth noting that EBV is known to activate H. pylori CagA via inhibition of host SHP1 [122]. Thus, these GCs may be more prevalent among H. pylori-negative GCs; however, they will benefit from immune checkpoint inhibitors [123], due to the higher neo-antigen or viral antigen burdens.
In this decade, it is necessary for researchers to extensively characterize H. pylori-negative GCs in preparation for its global impact in the upcoming era. Through precise investigations of the somatic and germline genetics of GCs, along with stratifications according to patients’ lifestyles, as shown in this review, it should be feasible to clarify robust, personalized molecular mechanisms of both the current and novel types of GC in the upcoming H. pylori-negative era.
Acknowledgements
This work was supported by AMED P-CREATE (JP21cm0106551) to SI, and KAKENHI Grant-in-Aid for Scientific Research (A) (19H01032) to HK.
Author contributions
S.I. directed the scopes and perspectives of this review article, and H.K. and S.I. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Gastroenterol Rev. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Nishiyama T, Kikuchi S, Inoue M, Sawada N, Tsugane S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908–2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep. 2017;7:15491. doi: 10.1038/s41598-017-15490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaoka Y. How to eliminate gastric cancer-related death worldwide? Nat Rev Clin Oncol. 2018;15:407–8. doi: 10.1038/s41571-018-0029-8. [DOI] [PubMed] [Google Scholar]
- 5.Tsuda M, Asaka M, Kato M, Matsushima R, Fujimori K, Akino K, et al. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22:e12415. doi: 10.1111/hel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asaka M, Kobayashi M, Kudo T, Akino K, Asaka Y, Fujimori K, et al. Gastric cancer deaths by age group in Japan: Outlook on preventive measures for elderly adults. Cancer Sci. 2020;111:3845–53. doi: 10.1111/cas.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Cancer Genome Atlas Research Network, The Cancer Genome Atlas, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583–7. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–82. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 10.Ushiku T, Ishikawa S, Kakiuchi M, Tanaka A, Katoh H, Aburatani H, et al. RHOA mutation in diffuse-type gastric cancer: a comparative clinicopathology analysis of 87 cases. Gastric Cancer. 2016;19:403–11. doi: 10.1007/s10120-015-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rokutan H, Hosoda F, Hama N, Nakamura H, Totoki Y, Furukawa E, et al. Comprehensive mutation profiling of mucinous gastric carcinoma. J Pathol. 2016;240:137–48. doi: 10.1002/path.4761. [DOI] [PubMed] [Google Scholar]
- 12.Katoh H, Ishikawa S. Genomic pathobiology of gastric carcinoma. Pathol Int. 2017;67:63–71. doi: 10.1111/pin.12493. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka A, Ishikawa S, Ushiku T, Yamazawa S, Katoh H, Hayashi A, et al. Frequent CLDN18-ARHGAP fusion in highly metastatic diffuse-type gastric cancer with relatively early onset. Oncotarget. 2018;9:29336–50. doi: 10.18632/oncotarget.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishizawa T, Nakano K, Harada A, Kakiuchi M, Funahashi SI, Suzuki M, et al. DGC-specific RHOA mutations maintained cancer cell survival and promoted cell migration via ROCK inactivation. Oncotarget. 2018;9:23198–207. doi: 10.18632/oncotarget.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa T, Nakano K, Fujii E, Komura D, Kuroiwa Y, Ishimaru C, et al. In vivo effects of mutant RHOA on tumor formation in an orthotopic inoculation model. Oncol Rep. 2019;42:1745–54. doi: 10.3892/or.2019.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sud A, Kinnersley B, Houlston RS. Genome-wide association studies of cancer: current insights and future perspectives. Nat Rev Cancer. 2017;17:692–704. doi: 10.1038/nrc.2017.82. [DOI] [PubMed] [Google Scholar]
- 17.Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle and cancer risk. Nat Rev Cancer. 2004;4:519–27. doi: 10.1038/nrc1389. [DOI] [PubMed] [Google Scholar]
- 18.Study Group of Millennium Genome Project for C. Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer. Nat Genet. 2008;40:730–40. doi: 10.1038/ng.152. [DOI] [PubMed] [Google Scholar]
- 19.Saeki N, Saito A, Choi IJ, Matsuo K, Ohnami S, Totsuka H, et al. A functional single nucleotide polymorphism in Mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology. 2011;140:892–902. doi: 10.1053/j.gastro.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Tanikawa C, Kamatani Y, Toyoshima O, Sakamoto H, Ito H, Takahashi A, et al. Genome‐wide association study identifies gastric cancer susceptibility loci at 12q24.11‐12 and 20q11.21. Cancer Sci. 2018;109:4015–24. doi: 10.1111/cas.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–7. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair VR, McLeod M, Carneiro F, Coit DG, D'Addario JL, van Dieren JM, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol. 2020;21:e386–97. doi: 10.1016/S1470-2045(20)30219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahasrabudhe R, Lott P, Bohorquez M, Toal T, Estrada AP, Suarez JJ, et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology. 2017;152:983–6.e6. doi: 10.1053/j.gastro.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fewings E, Larionov A, Redman J, Goldgraben MA, Scarth J, Richardson S, et al. Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole-exome sequencing study. Lancet Gastroenterol Hepatol. 2018;3:489–98. doi: 10.1016/S2468-1253(18)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411:390–5. doi: 10.1038/35077256. [DOI] [PubMed] [Google Scholar]
- 26.Colditz GA, Sellers TA, Trapido E. Epidemiology—identifying the causes and preventability of cancer? Nat Rev Cancer. 2006;6:75–83. doi: 10.1038/nrc1784. [DOI] [PubMed] [Google Scholar]
- 27.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–93. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 2013;3:246–59. doi: 10.1016/j.celrep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexandrov LB, Stratton MR. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev. 2014;24:52–60. doi: 10.1016/j.gde.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15:585–98. doi: 10.1038/nrg3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki A, Katoh H, Komura D, Kakiuchi M, Tagashira A, Yamamoto S, et al. Defined lifestyle and germline factors predispose Asian populations to gastric cancer. Sci Adv. 2020;6:eaav9778. doi: 10.1126/sciadv.aav9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306–16. doi: 10.1016/j.chom.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Cheung DY. Must-have knowledge about the helicobacter pylori-negative gastric cancer. Gut Liver. 2016;10:157–9. doi: 10.5009/gnl16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Kim N, Yoon H, Choi YJ, Lee JY, Kwon YH, et al. Comparison between resectable helicobacter pylori-negative and -positive gastric cancers. Gut Liver. 2016;10:212–9. doi: 10.5009/gnl14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415–9. doi: 10.1111/j.1523-5378.2011.00889.x. [DOI] [PubMed] [Google Scholar]
- 38.Ono S, Kato M, Suzuki M, Ishigaki S, Takahashi M, Haneda M, et al. Frequency of helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion. 2012;86:59–65. doi: 10.1159/000339176. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Fujisaki J, Omae M, Hirasawa T, Igarashi M. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endosc. 2015;27:551–61. doi: 10.1111/den.12471. [DOI] [PubMed] [Google Scholar]
- 40.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang J-Q, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–79. doi: 10.1016/S0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 42.Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44:239–48. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 43.López-Vidal Y, Ponce-de-León S, Castillo-Rojas G, Barreto-Zúñiga R, Torre-Delgadillo A. High diversity of vacA and cagA helicobacter pylori genotypes in patients with and without gastric cancer. PLoS ONE. 2008;3:e3849. doi: 10.1371/journal.pone.0003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi T, Senda M, Suzuki N, Nishikawa H, Ben C, Tang C, et al. Differential mechanisms for SHP2 binding and activation are exploited by geographically distinct helicobacter pylori CagA oncoproteins. Cell Rep. 2017;20:2876–90. doi: 10.1016/j.celrep.2017.08.080. [DOI] [PubMed] [Google Scholar]
- 45.Sasako M. Progress in the treatment of gastric cancer in Japan over the last 50 years. Ann Gastroenterol Surg. 2020;4:21–29. doi: 10.1002/ags3.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomark Prev. 2014;23:700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Eshak ES, Shirai K, Liu K, Dong JY, Iso H, et al. Alcohol consumption and risk of gastric cancer: the Japan Collaborative Cohort Study. J Epidemiol. 2021;31:30–36. doi: 10.2188/jea.JE20190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma K, Baloch Z, He T-T, Xia X. Alcohol consumption and gastric cancer risk: a meta-analysis. Med Sci Monit. 2017;23:238–46. doi: 10.12659/MSM.899423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han X, Xiao L, Yu Y, Chen Y, Shu HH. Alcohol consumption and gastric cancer risk: a meta-analysis of prospective cohort studies. Oncotarget. 2017;8:83237–45. doi: 10.18632/oncotarget.19177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev. 2018;27:124–33. doi: 10.1097/CEJ.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 51.Li W-Y, Han Y, Xu H-M, Wang ZN, Xu YY, Song YX, et al. Smoking status and subsequent gastric cancer risk in men compared with women: a meta-analysis of prospective observational studies. BMC Cancer. 2019;19:377. doi: 10.1186/s12885-019-5601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garaycoechea JI, Crossan GP, Langevin F, Mulderrig L, Louzada S, Yang F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553:171–7. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Letouzé E, Shinde J, Renault V, Couchy G, Blanc JF, Tubacher E, et al. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis. Nat Commun. 2017;8:1315. doi: 10.1038/s41467-017-01358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang J, Tan W, Ling Z, Xi R, Shao M, Chen M, et al. Genomic analysis of oesophageal squamous-cell carcinoma identifies alcohol drinking-related mutation signature and genomic alterations. Nat Commun. 2017;8:15290. doi: 10.1038/ncomms15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Borinskaya S, Yoshimura K, Kal'ina N, Marusin A, Stepanov VA, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–45. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanikawa C, Urabe Y, Matsuo K, Kubo M, Takahashi A, Ito H, et al. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat Genet. 2012;44:430–4. doi: 10.1038/ng.1109. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Dai J, Hu N, Miao X, Abnet CC, Yang M, et al. Identification of new susceptibility loci for gastric non-cardia adenocarcinoma: pooled results from two Chinese genome-wide association studies. Gut. 2017;66:581–7. doi: 10.1136/gutjnl-2015-310612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan C, Zhu M, Ding Y, Yang M, Wang M, Li G, et al. Meta-analysis of genome-wide association studies and functional assays decipher susceptibility genes for gastric cancer in Chinese populations. Gut. 2020;69:641–51. doi: 10.1136/gutjnl-2019-318760. [DOI] [PubMed] [Google Scholar]
- 60.Hu N, Wang Z, Song X, Wei L, Kim BS, Freedman ND, et al. Genome-wide association study of gastric adenocarcinoma in Asia: a comparison of associations between cardia and non-cardia tumours. Gut. 2016;65:1611–8. doi: 10.1136/gutjnl-2015-309340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kupcinskas J, Wex T, Link A, Bartuseviciute R, Dedelaite M, Kevalaite G, et al. PSCA and MUC1 gene polymorphisms are associated with gastric cancer and pre-malignant gastric conditions. Anticancer Res. 2014;34:7167–75. [PubMed] [Google Scholar]
- 62.Sala N, Muñoz X, Travier N, Agudo A, Duell EJ, Moreno V, et al. Prostate stem-cell antigen gene is associated with diffuse and intestinal gastric cancer in Caucasians: results from the EPIC-EURGAST study. Int J Cancer. 2012;130:2417–27. doi: 10.1002/ijc.26243. [DOI] [PubMed] [Google Scholar]
- 63.Rizzato C, Kato I, Plummer M, Muñoz N, Canzian F. Genetic variation in PSCA and risk of gastric advanced preneoplastic lesions and cancer in relation to helicobacter pylori infection. PLoS ONE. 2013;8:e73100. doi: 10.1371/journal.pone.0073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer AJ, Lochhead P, Hold GL, Rabkin CS, Chow WH, Lissowska J, et al. Genetic variation in C20orf54, PLCE1 and MUC1 and the risk of upper gastrointestinal cancers in Caucasian populations. Eur J Cancer Prev. 2012;21:541–4. doi: 10.1097/CEJ.0b013e3283529b79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song H-R, Kim HN, Kweon S-S, Choi JS, Shim HJ, Cho SH, et al. Common genetic variants at 1q22 and 10q23 and gastric cancer susceptibility in a Korean population. Tumor Biol. 2014;35:3133–7. doi: 10.1007/s13277-013-1409-4. [DOI] [PubMed] [Google Scholar]
- 66.Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, et al. Phospholipase C is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci. 2007;104:15543–8. doi: 10.1073/pnas.0702943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu S, Choi W-I, Choi YJ, Kim HY, Hildebrandt F, Gee HY. PLCE1 regulates the migration, proliferation, and differentiation of podocytes. Exp Mol Med. 2020;52:594–603. doi: 10.1038/s12276-020-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelley GG. Phospholipase Cepsilon: a novel Ras effector. EMBO J. 2001;20:743–54. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan J, Li Y, Tian T, Li N, Zhu Y, Zou J, et al. Risk prediction for early-onset gastric carcinoma: a case-control study of polygenic gastric cancer in Han Chinese with hereditary background. Oncotarget. 2016;7:33608–15. doi: 10.18632/oncotarget.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honda M, Wong SL, Healy MA, Nakajima T, Watanabe M, Fukuma S, et al. Long-term trends in primary sites of gastric adenocarcinoma in Japan and the United States. J Cancer. 2017;8:1935–42. doi: 10.7150/jca.19174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–24. doi: 10.1136/pgmj.2004.029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Roy F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer. Nat Rev Cancer. 2014;14:121–34. doi: 10.1038/nrc3647. [DOI] [PubMed] [Google Scholar]
- 73.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 74.Figueiredo J, Melo S, Carneiro P, Moreira AM, Fernandes MS, Ribeiro AS, et al. Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J Med Genet. 2019;56:199–208. doi: 10.1136/jmedgenet-2018-105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary diffuse gastric cancer syndrome. JAMA Oncol. 2015;1:23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 77.Brooks-Wilson AR. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–17. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaurah P, MacMillan A, Boyd N, Senz J, De Luca A, Chun N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297:2360–72. doi: 10.1001/jama.297.21.2360. [DOI] [PubMed] [Google Scholar]
- 79.More H, Humar B, Weber W, Ward R, Christian A, Lintott C, et al. Identification of seven novel germline mutations in the human E-cadherin (CDH1) Gene. Hum Mutat. 2007;28:203–203. doi: 10.1002/humu.9473. [DOI] [PubMed] [Google Scholar]
- 80.Keller G. Germline mutations of the E-cadherin(CDH1) and TP53 genes, rather than of RUNX3 and HPP1, contribute to genetic predisposition in German gastric cancer patients. J Med Genet. 2004;41:e89. doi: 10.1136/jmg.2003.015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vogelaar IP, van der Post RS, van Krieken JHJ, Spruijt L, van Zelst-Stams WA, Kets CM, et al. Unraveling genetic predisposition to familial or early onset gastric cancer using germline whole-exome sequencing. Eur J Hum Genet. 2017;25:1246–52. doi: 10.1038/ejhg.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamada H, Shinmura K, Ito H, Kasami M, Sasaki N, Shima H, et al. Germline alterations in the CDH1 gene in familial gastric cancer in the Japanese population. Cancer Sci. 2011;102:1782–8. doi: 10.1111/j.1349-7006.2011.02038.x. [DOI] [PubMed] [Google Scholar]
- 83.Shinmura K, Kohno T, Takahashi M, Sasaki A, Ochiai A, Guilford P, et al. Familial gastric cancer: clinicopathological characteristics, RER phenotype and germline p53 and E-cadherin mutations. Carcinogenesis. 1999;20:1127–31. doi: 10.1093/carcin/20.6.1127. [DOI] [PubMed] [Google Scholar]
- 84.Iida S, Akiyama Y, Ichikawa W, Yamashita T, Nomizu T, Nihei Z, et al. Infrequent germ-line mutation of the E-cadherin gene in Japanese familial gastric cancer kindreds. Clin Cancer Res. 1999;5:1445–7. [PubMed] [Google Scholar]
- 85.Wang Y, Song JP, Ikeda M, Shinmura K, Yokota J, Sugimura H. Ile-Leu substitution (I415L) in germline E-cadherin gene (CDH1) in Japanese familial gastric cancer. Jpn J Clin Oncol. 2003;33:17–20. doi: 10.1093/jjco/hyg002. [DOI] [PubMed] [Google Scholar]
- 86.Yabuta T, Shinmura K, Tani M, Yamaguchi S, Yoshimura K, Katai H, et al. E-cadherin gene variants in gastric cancer families whose probands are diagnosed with diffuse gastric cancer. Int J Cancer. 2002;101:434–41. doi: 10.1002/ijc.10633. [DOI] [PubMed] [Google Scholar]
- 87.Pharoah PDP, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121:1348–53. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 88.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–5. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 89.Cho SY, Park JW, Liu Y, Park YS, Kim JH, Yang H, et al. Sporadic early-onset diffuse gastric cancers have high frequency of somatic CDH1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology. 2017;153:536–49.e26. doi: 10.1053/j.gastro.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreira-Nunes CA, Barros MBL, do Nascimento Borges B, Montenegro RC, Lamarão LM, Ribeiro HF, et al. Genetic screening analysis of patients with hereditary diffuse gastric cancer from northern and northeastern Brazil. Hered Cancer Clin Pr. 2014;12:18. doi: 10.1186/1897-4287-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mateus AR, Simões-Correia J, Figueiredo J, Heindl S, Alves CC, Suriano G, et al. E-cadherin mutations and cell motility: a genotype–phenotype correlation. Exp Cell Res. 2009;315:1393–402. doi: 10.1016/j.yexcr.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 92.Suriano G, Mulholland D, de Wever O, Ferreira P, Mateus AR, Bruyneel E, et al. The intracellular E-cadherin germline mutation V832 M lacks the ability to mediate cell–cell adhesion and to suppress invasion. Oncogene. 2003;22:5716–9. doi: 10.1038/sj.onc.1206672. [DOI] [PubMed] [Google Scholar]
- 93.Shin S, Kim Y, Lee JK, Lee KA. Frequency and clinical characteristics of unselected Korean gastric cancer patients with a germline CDH1 V832M mutation. J Cancer. 2020;11:208–12. doi: 10.7150/jca.36513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brito LA, Yamamoto GL, Melo S, Malcher C, Ferreira SG, Figueiredo J, et al. Rare variants in the epithelial Cadherin gene underlying the genetic etiology of nonsyndromic cleft lip with or without cleft palate. Hum Mutat. 2015;36:1029–33. doi: 10.1002/humu.22827. [DOI] [PubMed] [Google Scholar]
- 96.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–5. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu Q, Li H, Moorjani P, Jay F, Slepchenko SM, Bondarev AA, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–9. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Momozawa Y, Iwasaki Y, Parsons MT, Kamatani Y, Takahashi A, Tamura C, et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat Commun. 2018;9:4083. doi: 10.1038/s41467-018-06581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–33. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pilié PG, Gay CM, Byers LA, O'Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25:3759–71. doi: 10.1158/1078-0432.CCR-18-0968. [DOI] [PubMed] [Google Scholar]
- 101.Funakoshi T, Miyamoto S, Kakiuchi N, Nikaido M, Setoyama T, Yokoyama A, et al. Genetic analysis of a case of Helicobacter pylori-uninfected intramucosal gastric cancer in a family with hereditary diffuse gastric cancer. Gastric Cancer. 2019;22:892–8. doi: 10.1007/s10120-018-00912-w. [DOI] [PubMed] [Google Scholar]
- 102.Pilonis ND, Tischkowitz M, Fitzgerald RC, di Pietro M. Hereditary diffuse gastric cancer: approaches to screening, surveillance, and treatment. Annu Rev Med. 2021;72:263–80. doi: 10.1146/annurev-med-051019-103216. [DOI] [PubMed] [Google Scholar]
- 103.Guilford P, Humar B, Blair V. Hereditary diffuse gastric cancer: translation of CDH1 germline mutations into clinical practice. Gastric Cancer. 2010;13:1–10. doi: 10.1007/s10120-009-0531-x. [DOI] [PubMed] [Google Scholar]
- 104.Vos EL, Salo-Mullen EE, Tang LH, Schattner M, Yoon SS, Gerdes H, et al. Indications for total gastrectomy in CDH1 mutation carriers and outcomes of risk-reducing minimally invasive and open gastrectomies. JAMA Surg. 2020;155:1050–7. doi: 10.1001/jamasurg.2020.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mi EZ, Mi EZ, di Pietro M, O'Donovan M, Hardwick RH, Richardson S, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87:408–18. doi: 10.1016/j.gie.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim YC, di Pietro M, O'donovan M, Richardson S, Debiram I, Dwerryhouse S, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc. 2014;80:78–87. doi: 10.1016/j.gie.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 107.Namikawa K, Kawachi H, Tsugeno Y, Nakajima T, Fujisaki J. Detection of multiple intramucosal signet-ring cell carcinomas by white-light endoscopy and magnifying endoscopy with narrow-band imaging in a hereditary diffuse gastric cancer patient with a CDH1 germline mutation. VideoGIE. 2021:1–4. 10.1016/j.vgie.2020.11.020. [DOI] [PMC free article] [PubMed]
- 108.Iwaizumi M, Yamada H, Fukue M, Maruyama Y, Sonoda A, Sugimoto M, et al. Two independent families with strongly suspected hereditary diffuse gastric cancer based on the probands’ endoscopic findings. Clin J Gastroenterol. 2020;13:754–8. doi: 10.1007/s12328-020-01163-y. [DOI] [PubMed] [Google Scholar]
- 109.Tang Y, Anandasabapathy S, Richards‐Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2020;2020:1878-0261.12792. doi: 10.1002/1878-0261.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gulati S, Patel M, Emmanuel A, Haji A, Hayee B, Neumann H. The future of endoscopy: advances in endoscopic image innovations. Dig Endosc. 2020;32:512–22. doi: 10.1111/den.13481. [DOI] [PubMed] [Google Scholar]
- 111.Graham DG, Banks MR. Advances in upper gastrointestinal endoscopy. F1000Research. 2015;4:1457. doi: 10.12688/f1000research.6961.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teh J-L, Shabbir A, Yuen S, So JB. Recent advances in diagnostic upper endoscopy. World J Gastroenterol. 2020;26:433–47. doi: 10.3748/wjg.v26.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Onoyama H, Kamiya M, Kuriki Y, Komatsu T, Abe H, Tsuji Y, et al. Rapid and sensitive detection of early esophageal squamous cell carcinoma with fluorescence probe targeting dipeptidylpeptidase IV. Sci Rep. 2016;6:26399. doi: 10.1038/srep26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leal A, van Grieken NCT, Palsgrove DN, Phallen J, Medina JE, Hruban C, et al. White blood cell and cell-free DNA analyses for detection of residual disease in gastric cancer. Nat Commun. 2020;11:525. doi: 10.1038/s41467-020-14310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu C, Zhang J, Li H, Xu W, Zhang X. The potential of liquid biopsies in gastrointestinal cancer. Clin Biochem. 2020;84:1–12. doi: 10.1016/j.clinbiochem.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26:1859–64. doi: 10.1038/s41591-020-1063-5. [DOI] [PubMed] [Google Scholar]
- 117.Song JH, Kim YS, Heo NJ, Lim JH, Yang SY, Chung GE, et al. High salt intake is associated with atrophic gastritis with intestinal metaplasia. Cancer Epidemiol Biomark Prev. 2017;26:1133–8. doi: 10.1158/1055-9965.EPI-16-1024. [DOI] [PubMed] [Google Scholar]
- 118.Li Y, Li W, Wang X, Ding C, Liu J, Li Y, et al. High‐salt diet‐induced gastritis in C57BL/6 mice is associated with microbial dysbiosis and alleviated by a buckwheat diet. Mol Nutr Food Res. 2020;64:1900965. doi: 10.1002/mnfr.201900965. [DOI] [PubMed] [Google Scholar]
- 119.Lin S, Gao T, Sun C, Jia M, Liu C, Ma X, et al. Association of dietary patterns and endoscopic gastric mucosal atrophy in an adult Chinese population. Sci Rep. 2019;9:16567. doi: 10.1038/s41598-019-52951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–75. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 122.Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S, et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein–Barr virus. Nat Microbiol. 2016;1:16026. doi: 10.1038/nmicrobiol.2016.26. [DOI] [PubMed] [Google Scholar]
- 123.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]