Abstract
Nanomedicine research is an active field that produces thousands of studies every year. However, translation of nanotherapeutics to the clinic has yet to catch up with such a vast output. In recent years, the need to better understand nanomedicines’ in vivo behavior has been identified as one of the major challenges for efficient clinical translation. In this context, non-invasive imaging offers attractive solutions to provide valuable information about nanomedicine biodistribution, pharmacokinetics, stability or therapeutic efficacy. Here, we review the latest imaging approaches employed in the development of therapeutic nanomedicines, discuss why these strategies bring added value along the translational pipeline, and give a perspective on future advances in the field.
1. Introduction
Nanomedicine, the application of nanotechnology to disease prevention and treatment, initially sparked great excitement as a promising avenue to improve drug delivery. Indeed, preclinical nanomedicine research is still a blooming field that produces thousands of studies every year. To date, however, the number of nanoformulations approved for clinical use is comparatively low [1,2]. Clinical nanomedicine applications are so far mostly limited to cancer, but promising preclinical studies indicate that its use in other scenarios like cardiovascular disease [3,4], organ transplantation [5] or autoimmune disorders [6] could be beneficial. These applications, which exploit nanoparticle formulations to modulate the immune system in a so-called nanoimmunotherapeutic fashion, are producing exciting results and instilling new vigor to the field.
The paucity in nanomedicine translation can be attributed to a range of challenges, which include production scalability and regulatory issues, but mainly relate to deficient understanding of nanoformulations’ in vivo behavior and a lack of patient selection methods [1,7]. In this setting, the value of imaging is increasingly recognized at different steps along the nanomedicine development pathway as it can provide critical information in a non-invasive fashion. In this review, we discuss recent advances in the use of imaging strategies applied to therapeutic nanomedicine development over the past two years, and provide an outlook on the field’s future directions.
2. Imaging techniques
Imaging techniques like X-ray computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET) have revolutionized medical care. These techniques allow non-invasive in vivo visualization of anatomical structures and biological processes to diagnose and prognose disease. However, no standalone imaging technique is perfect, as all have their own strengths and weaknesses. While a detailed discussion about their limitations is out of the scope of this article, a brief summary of their features is included in Table 1. In the context of nanomedicine development, nuclear (PET and single-photon emission computed tomography [SPECT]), anatomical (MRI and CT), as well as optical imaging techniques (such as fluorescence molecular tomography [FMT], fluorescence imaging, or intravital microscopy) are the most widely used to study nanoformulations in vivo (and ex vivo). Given the limitations of standalone imaging techniques, a combination of two or more of them is increasingly preferred in order to attain optimal characterization. Typically, multimodal imaging combines two techniques that bring together complementary information, such as functional and anatomical data as in PET/CT or PET/MRI. Using hybrid scanners both acquisitions can be performed in a single session. However, multimodal imaging can also be approached asynchronously, by acquiring images at different times in different scanners.
Table 1.
Features, advantages and disadvantages, and examples of imaging agents of techniques commonly used in the context of nanomedicine development [8,53,54].
| Technique | Imaging agent |
Spatial resolution |
Sensitivity | Penetration in tissue |
Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Anatomical | CT | Au, Iodine | <0.2 mm [P] 0.5–1 mm [C] |
mM | No limit | Fast; high spatial resolution | Ionizing radiation; low contrast sensitivity |
| MRI | Iron oxide, Gd, Mn | <0.1 mm [P] 1–2 mm [C] |
μM-mM | No limit | High spatial resolution; soft tissue contrast | Low contrast sensitivity; time-consuming | |
| Nuclear | PET | 18F, 64Cu, 68Ga, 89Zr | 1–2 mm [P] 6–10 mm [C] |
fM | No limit | High sensitivity; quantitative | Ionizing radiation; expensive; limited spatial resolution |
| SPECT | 99mTc, 111In, 123I, 125I | 0.5–2 mm [P] 7–15 mm [C] |
<pM | No limit | |||
| Optical | FI | Fluorophores, quantum dots | 1–5 mm [P] | nM | mm-cm | High sensitivity; multiplexing; inexpensive | Low penetration depth |
| FMT | Fluorophores, quantum dots | <1 mm [P] | pM | cm | High sensitivity | Signal attenuation; limited penetration | |
| IVM | Fluorophores, quantum dots | <1 μm [P] 1 μm [C] |
<nM | <mm | Cell-level resolution; real time imaging | Complex setup; limited field of view | |
[P]: preclinical; [C]: clinical; FI: fluorescence imaging; FMT: fluorescence molecular tomography; IVM: intravital microscopy.
3. Imaging applications in nanomedicine development
Therapeutic nanomedicine development can greatly benefit from the use of different imaging modalities. These nanomedicines may intrinsically contain contrast-generating or imaging agents, or may be modified to do so, which allows their in vivo tracking by the corresponding imaging technique. In this section, we summarize the most recent, innovative and relevant examples of imaging-based strategies applied to different aspects and stages of a nanoformulation’s development.
3.1. Evaluation of in vivo behavior.
One of the greatest challenges facing nanomedicine translation is the lack of understanding of their in vivo performance in terms of stability, pharmacokinetics, as well as tissue and cellular distribution. These features can be investigated using anatomical, nuclear and optical imaging methods. However, not all techniques are equally suited for the purpose. While CT, MRI and optical imaging have been used to track nanoparticles in vivo, only PET and SPECT can afford truly quantitative information [8]. Moreover, due to their high sensitivity, nuclear imaging techniques allow minimal modification of the nanomaterials to introduce the required radioactive tag. On the other hand, nuclear imaging’s low spatial resolution limits its use to whole-body and tissue level distribution, leaving cellular (and subcellular) evaluation to optical imaging techniques.
3.1.1. Biodistribution and pharmacokinetics.
Nanotherapeutics with inorganic cores, such as iron oxide or gold, can be tracked in vivo by MRI [9-11] and CT [10,12,13], respectively. The generated contrast can be quantified to derive information about tissue distribution and clearance kinetics. On the other hand, nanomedicines labeled with fluorophores or carrying a fluorescent nanocrystal core can be visualized using fluorescence imaging methods [14,15]. While these optical methods have issues related to tissue absorption or penetration in vivo, this is an affordable semi-quantitative approach that can be further complemented by ex vivo microscopy. For example, a recent work used X-ray- and fluorescence-based imaging methods to study nanoparticle dynamics associated with different pulmonary delivery methods [16].
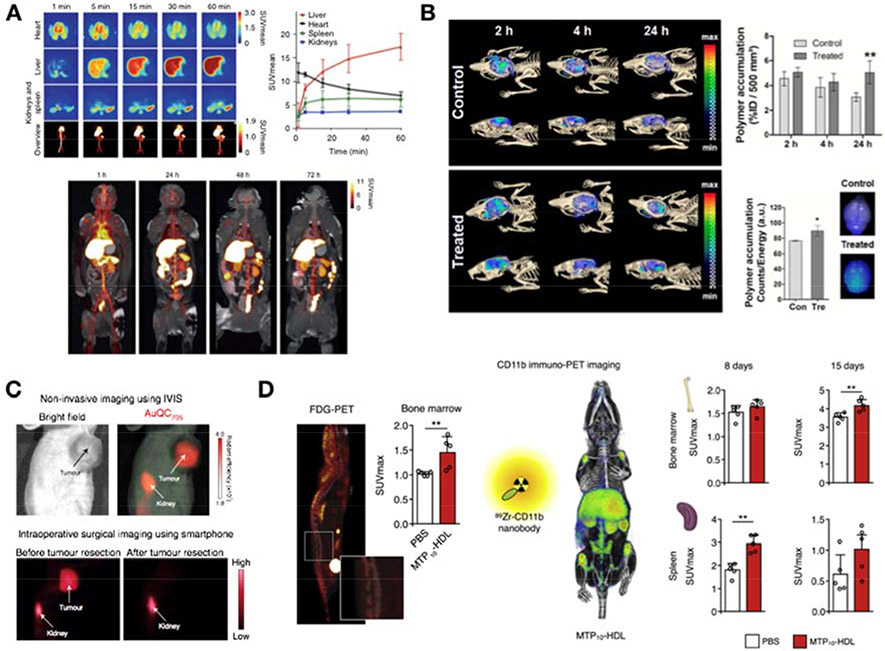
In recent years, however, the multimodal combination of highly sensitive and quantitative PET or SPECT with anatomical CT imaging is being increasingly favored for the assessment of nanomedicines’ biodistribution and pharmacokinetics [8]. With the advent of hybrid PET/MRI scanners, anatomical reference with high soft-tissue contrast is available for a more accurate localization of PET hot spots. Nuclear imaging approaches allow dynamic and longitudinal evaluation of a radiolabeled nanomedicine and in vivo comparison of its biodistribution in different species (Figure 1A) [3,4,17]. Importantly, the imaging results can be validated ex vivo by radioactivity counting in tissues of interest, and by autoradiography, which provides information about regional distribution in tissues with sub-millimeter resolution.
Figure 1. Non-invasive imaging applications in nanomedicine development.
A) Biodistribution and pharmacokinetics. The tissue distribution and clearance kinetics of a TRAF6 inhibitor-loaded nanobiologic was evaluated in non-human primates by PET/MRI. The formulation was radiolabeled with 89Zr and monitored dynamically for the first hour after administration (top left). Quantitative data could be derived from selected tissues (top right). Subsequently, static scans were performed at 24, 48 and 72 hours post injection (bottom). Adapted from Lameijer et al. [4]. B) Targeting efficiency. A sonoporation treatment to enhance delivery across the BBB was evaluated by in vivo FMT/CT imaging using fluorophore-labeled nanoparticles. A significant increase in the florescent signal was measured 24 hours post-administration in treated animals compared to controls (top right). The results were corroborated ex vivo by fluorescence reflectance imaging of explanted brains (bottom right). Adapted from May et al. [19]. C) Imaging-guided therapy. An ultra-small gold quantum cluster nanoparticle (AuQC705), detectable by near-infrared fluorescence, CT and MRI, was successfully employed to guide tumor resection by fluorescence imaging using a portable smartphone imaging system prototype. Adapted from Yang et al. [39]. D) Treatment monitoring. Non-invasive imaging can be used to monitor nanomedicine treatment efficacy and its underlying mechanisms of action. A trained-immunity promoting nanobiologic (MTP10-HDL) was developed as a novel anticancer therapy. Its effects on immune response activation were monitored by PET imaging of metabolic activation in the bone marrow using 18F-FDG (FDG-PET, left) and myelopoiesis in bone marrow and spleen using a radiolabeled nanobody (CD11b immuno-PET, right). Adapted from Priem at al. [17].
3.1.2. Targeting.
Non-invasive imaging can be used to assess the ability of nanotherapeutics to reach their targets, e.g. a tumor or inflammatory lesions, such as atherosclerotic plaques. Again, while MRI and CT can do this if a nanoformulation contains appropriate contrast-generating agents, PET and SPECT yield more accurate information due to their high sensitivity and truly quantitative nature. However, these techniques require auxiliary anatomical reference, typically in combination with CT. A more precise localization of accumulation spots can be achieved by combination with MRI due to this technique’s excellent soft tissue contrast. This is particularly critical for nanomedicines targeting small regions of interest such as the atherosclerotic vessel wall [18].
Imaging-based strategies additionally enable evaluation of the efficiency of delivery-enhancing interventions. This is of great value in the development of nanodrugs that need to cross impassable biological barriers such as the blood-brain barrier (BBB). Two recent studies made use of FMT/CT [19] or MRI [20] in combination with other optical imaging methods to evaluate the effect of ultrasound-mediated permeation of the BBB on the accumulation of polymeric and liposomal nanoparticles. In both studies, the use of fluorophores allowed the authors to validate the in vivo imaging results using fluorescence microscopy ex vivo (Figure 1B). A similar approach was implemented to assess magnetic targeting of an iron oxide-gold core-shell photothermal nanotherapy formulation by MRI [9].
Targeting evaluation at the cellular level, however, requires the use of optical imaging methods. Sofias et al. employed a combination of PET and intravital microscopy to investigate the fate of αvβ3-integrin targeted nanoformulations [21]. The information thus gathered revealed significant differences between targeted and untargeted nanoformulations at tissue level, and a new mechanism of nanoparticle accumulation via phagocyte “hitchhiking”. Importantly, the use of fluorescent labels allows further investigation of a nanomedicine’s fate at the cellular level by ex vivo fluorescence microscopy or fluorescence-activated cell sorting. This is particularly important in the development of nanoimmunotherapies that work by selectively targeting a given immune cell population [17].
3.1.3. Nanoformulation integrity.
Most nanomedicines are composite materials, typically made of a nanocarrier and a drug. Generally, their evaluation focuses on the nanocarrier, and it is assumed that the nanoconstruct remains stable over time after administration. However, early release of the cargo is an issue with most formulations, and this can be investigated by non-invasive imaging. SPECT, for instance, allows multiplexing by using two isotopes with different gamma photon emission energies, which can be used to label different components of a nanomedicine. This approach was adopted by Llop et al. to investigate the biodistribution and pharmacokinetics of a composite iron oxide-polymeric nanoformulation in vivo by SPECT [22]. A slightly different approach was followed by Lamichhane et al., who used 111In-labeled liposomes encapsulating a 18F-labeled carboplatin derivative [23]. Analysis of the 111In signal by SPECT and 18F by PET using a multimodal preclinical PET/SPECT/CT system showed similar tissue distribution, suggesting a stable integration of both components. Analogous approaches can be implemented using optical methods, which also allow multiplexing , and more specifically exploiting Förster resonance energy transfer [24,25]. We anticipate these multiplexing strategies to gain traction in early nanomedicine evaluation as they can provide critical information about a nanoconstruct’s in vivo stability.
3.2. Imaging-guided therapy.
The use of imaging to improve therapeutic outcome in a personalized manner has become a feasible goal. These imaging-guided approaches rely on labeled nanomaterials to generate a trackable in vivo signal to detect –and quantify– their accumulation in tissues in a so-called theranostic fashion. Down the line, these strategies could evolve to enable patient selection and dose adjustment protocols. In recent years, numerous studies have reported on inorganic core nanoparticles with intrinsic contrast-generating or imaging properties engineered for therapeutic purposes. Wang et al., for instance, developed a polymeric nanocapsule containing iron-based magnetic nanocrystals, indocyanine green and doxorubicin for imaging-guided dual photodynamic and chemodynamic therapy [26]. The magnetic and fluorescent properties were exploited for thorough characterization of the nanocapsules’ in vivo tumor accumulation by MRI and fluorescence imaging, respectively, whereas thermal infrared imaging was used to monitor photodynamic therapy [26]. Jing et al. pursued an analogous strategy using a composite nanomaterial containing Fe3O4 superparamagnetic nanoclusters, MnO2 nanosheets, the anticancer drug curcumin and the photosensitizer chlorin e6 for dual chemotherapy and photodynamic therapy guided by MRI and fluorescence imaging [11].
Indeed, nanoparticles for photothermal or photodynamic therapy have intrinsic imaging capabilities, and this feature is exploited for guiding treatment [12,27-30]. Moreover, these nanoformulations are frequently engineered to incorporate MRI [9,31-33] and/or CT [32-35] contrast agents in order to visualize their distribution at the whole-body level. For instance, Sharma et al. developed gold nanorods for imaging-guided photothermal therapy, reliant upon the MRI, X-ray, and optical imaging properties of the nanomaterial [36] Another such example is the combination of bismuth nanoparticles with up-converting nanophosphors in core-shell multimodal nanoparticles for CT and up-conversion luminescence imaging-guided photothermal therapy [37]. In a similar fashion, a photothermal nanotherapeutic that activates its imaging and therapeutic features in the presence of tumor-overexpressed β-gallactosidase was recently reported [38]. The combination of anatomical imaging contrast agents and fluorophores has another interesting oncological application. Firstly, the nanomaterials can be located at whole-body level using the anatomical technique and subsequently used for precise tumor resection using fluorescence guidance. Yang et al. report on an α-lactalbumin-stabilized ultra-small gold quantum cluster with intrinsic detectability by near-infrared fluorescence imaging, CT and MRI, and therapeutic activity through inhibition of the MAPK and PI3K–AKT pathways. The authors prove that the nanomaterial is renally cleared due to its small size and can be used for intraoperative surgical imaging (Figure 1C) [39].
3.3. Treatment monitoring.
Finally, non-invasive imaging can be used to evaluate the therapeutic efficacy of a nanomedicine. This approach allows direct investigation of the formulation’s therapeutic effect as well as its mechanism of action. For instance, we implemented one such strategy to translate a statin-loaded nanobiologic for the treatment of atherosclerosis from mice to large animals. Using a multimodal imaging approach to longitudinally monitor treatment in rabbits and pigs, combining PET- and MRI-based readouts, we were able to assess the anti-inflammatory effect of the nanobiologic formulation using restricted group sizes [3]. Similarly, the effect of a trained-immunity promoting nanobiologic was imaged by both 18F-fluorodeoxyglucose (18F-FDG) PET, to measure metabolic activation in the bone marrow, and by immunoPET using a CD11b-targeted nanobody to quantify the expected increased myelopoiesis (Figure 1D) [17]. In clinical trials, imaging-based readouts could be included to directly assess treatment response [40], potentially eliminating the need for large patient cohorts and long follow-up periods, as well as the associated high costs.
4. Perspective and conclusion
Non-invasive imaging strategies like the ones summarized here can be integrated into nanoformulation screening procedures to speed up early-stage development. Formulation libraries can be generated and thoroughly characterized for biodistribution, pharmacokinetics, targeting or cell specificity using complementary imaging techniques as discussed above [17,41,42]. The wealth of imaging data generated in these screenings demands high-throughput analyses, which will greatly benefit from integration of artificial intelligence (AI) into the development pipeline [43,44]. Furthermore, mathematical modelling of these data can greatly help to understand the nanomaterial’s in vivo performance as well as biological aspects influencing its behavior [45,46]. Thus, those formulations with poor in vivo behavior can be easily identified and discarded before further evaluation and waste of resources.
On the other hand, the excessive reliance on mouse studies has been traditionally blamed for the limited translational success of nanomedicine. This is partly due to a comparatively low number of available large animal models of disease, although in recent years there has been increasing interest in the development of this type of valuable research tools [47,48]. In this context, we envision that large animal studies will be increasingly relevant. Choosing multiple robust non-invasive imaging readouts to longitudinally evaluate a nanoformulation’s efficacy in these translational studies allows the use of a limited number of animals [3]. This approach generates large amounts of data on independent markers of therapeutic efficacy and exploits the statistical power of longitudinal assessments, thereby increasing the likelihood of finding a statistically significant and biologically relevant effect.
One key challenge in nanomedicine translation remains the identification of patients that would benefit most from a nanotherapeutic intervention. In most cases, inter- and intra-patient disease heterogeneity is difficult to assess by common testing and therefore non-invasive imaging can greatly aid in this process [49]. Development of imaging-based patient selection protocols to homogenize cohorts in clinical trials would de-risk nanomedicine translation to the clinic by increasing the likelihood of detecting a response in amenable subjects. While most theranostic approaches have seemingly very little translational potential, simpler surrogate “nanoreporter” PET imaging [50,51], or analogous strategies using MRI [52], have yielded promising results. Ultimately, these patient selection protocols would be performed not just in trials but also in the clinic before the start of treatment, where they could additionally help to tailor the therapeutic regimen in an intrinsically personalized manner.
In conclusion, non-invasive imaging is being increasingly integrated at different stages of the nanomedicine development pipeline. We believe that the discussed strategies provide extremely valuable in vivo data and could at last help to bridge the translational gap between bench and bedside.
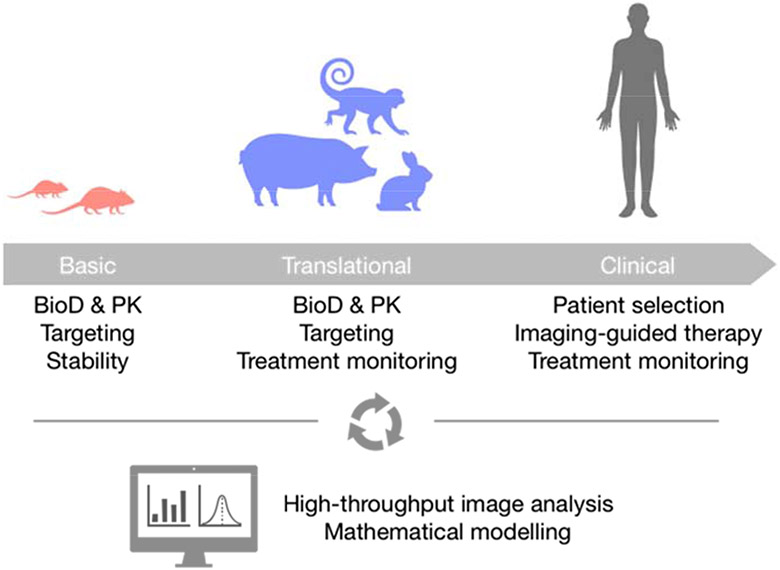
Figure 2. Integration of imaging along the translational pipeline.
Non-invasive imaging can be integrated at different stages of a nanoformulation’s development. At the early stages, imaging-based screening of promising candidates can be performed to elucidate their in vivo behavior in terms of biodistribution (BioD), pharmacokinetics (PK), targeting or stability. In addition to assessing in vivo behavior, translational studies in large animal models can benefit from the integration of non-invasive imaging to longitudinally investigate treatment response using limited group sizes. Finally, in the clinic, imaging-based protocols can aid in selecting amenable patients, guiding therapy and monitoring response. At all stages, AI-based image analyses will be of paramount importance to generate quality data and facilitate mathematical modelling in order to understand and possibly predict nanomedicines’ performance.
Acknowledgements
The work of AB and CPM is supported by Comunidad Autónoma de Madrid's Programa de Atracción de Talento (2018 T1/BMD10758). WJMM and CPM are supported by National Institutes of Health (NIH) grant R01 CA220234. WJMM is also supported by NIH grants R01 HL144072, P01 HL131478, and NWO/ZonMW Vici 91818622.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Germain M, Caputo F, Metcalfe S, Tosi G, Spring K, Åslund AKO, Pottier A, Schiffelers R, Ceccaldi A, Schmid R: Delivering the power of nanomedicine to patients today. J Control Release 2020, 326:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anselmo AC, Mitragotri S: Nanoparticles in the clinic: An update. Bioeng Transl Med 2019, 4:e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Binderup T, Duivenvoorden R, Fay F, van Leent MMT, Malkus J, Baxter S, Ishino S, Zhao Y, Sanchez-Gaytan B, Teunissen AJP, et al. : Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci Transl Med 2019, 11:eaaw7736. (••) In this study, we implemented an imaging-based approach to translate a nanoimmunotherapy from small to large animals that included evaluation of in vivo behavior by PET/CT and PET/MRI, as well as treatment monitoring by PET/MRI.
- 4.Lameijer M, Binderup T, Van Leent MMT, Senders ML, Fay F, Malkus J, Sanchez-Gaytan BL, Teunissen AJP, Karakatsanis N, Robson P, et al. : Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates. Nat Biomed Eng 2018, 2:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braza MS, van Leent MMT, Lameijer M, Sanchez-Gaytan BL, Arts RJW, Pérez-Medina C, Conde P, Garcia MR, Gonzalez-Perez M, Brahmachary M, et al. : Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity 2018, 49:819–828.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. : Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016, 530:434–440. (•) Beautiful study demonstrating nanomedicine's potential to modulate the immune system in the context of autoimmune diseases such as diabetes and multiple sclerosis.
- 7.Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST: Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev 2017, 108:25–38. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Medina C, Teunissen AJP, Kluza E, Mulder WJM, van der Meel R: Nuclear imaging approaches facilitating nanomedicine translation. Adv Drug Deliv Rev 2020, doi: 10.1016/j.addr.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 9. Abed Z, Beik J, Laurent S, Eslahi N, Khani T, Davani ES, Ghaznavi H, Shakeri-Zadeh A: Iron oxide-gold core–shell nano–theranostic for magnetically targeted photothermal therapy under magnetic resonance imaging guidance. J Cancer Res Clin Oncol 2019, 145:1213–1219. (•) The authors use MRI to guide and monitor an iron oxide-based nano-photothermal therapy that is directed to the targeted site by a magnetic field.
- 10.Rajaee A, Wang S, Zhao L, Wang D, Liu Y, Wang J, Ying K: Multifunction bismuth gadolinium oxide nanoparticles as radiosensitizer in radiation therapy and imaging. Phys Med Biol 2019, 64. [DOI] [PubMed] [Google Scholar]
- 11.Jing X, Xu Y, Liu D, Wu Y, Zhou N, Wang D, Yan K, Meng L: Intelligent nanoflowers: A full tumor microenvironment-responsive multimodal cancer theranostic nanoplatform. Nanoscale 2019, 11:15508–15518. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Wang J, Liu L, Sun Q, You Q, Cheng Y, Wang Y, Wang S, Tan F, Li N: One-Pot Synthesis of a Bismuth Selenide Hexagon Nanodish Complex for Multimodal Imaging-Guided Combined Antitumor Phototherapy. Mol Pharm 2018, 15:1941–1953. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Hu Y, Miao Z, Xu H, Li C, Zhao Y, Li Z, Chang M, Ma Z, Sun Y, et al. : Dual-Stimuli Responsive Bismuth Nanoraspberries for Multimodal Imaging and Combined Cancer Therapy. Nano Lett 2018, 18:6778–6788. [DOI] [PubMed] [Google Scholar]
- 14.Tan H, Hou N, Liu Y, Liu B, Cao W, Zheng D, Li W, Liu Y, Xu B, Wang Z, et al. : CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine Nanotechnology, Biol Med 2020, 27:102192. [DOI] [PubMed] [Google Scholar]
- 15.Biancacci I, Sun Q, Möckel D, Gremse F, Rosenhain S, Kiessling F, Bartneck M, Hu Q, Thewissen M, Storm G, et al. : Optical imaging of the whole-body to cellular biodistribution of clinical-stage PEG-b-pHPMA-based core-crosslinked polymeric micelles. J Control Release 2020, doi: 10.1016/j.jconrel.2020.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Gradl R, Dierolf M, Möller W, Kutschke D, Feuchtinger A, Hehn L, Donnelley M, Günther B, Achterhold K, et al. : Multimodal Precision Imaging of Pulmonary Nanoparticle Delivery in Mice: Dynamics of Application, Spatial Distribution, and Dosimetry. Small 2019, 15:e1904112. [DOI] [PubMed] [Google Scholar]
- 17.Priem B, van Leent MMT, Teunissen AJP, Sofias AM, Mourits VP, Willemsen L, Klein ED, Oosterwijk RS, Meerwaldt AE, Munitz J, et al. : Trained Immunity-Promoting Nanobiologic Therapy Suppresses Tumor Growth and Potentiates Checkpoint Inhibition. Cell 2020, 183:786–801.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobatto ME, Binderup T, Robson PM, Giesen LFP, Calcagno C, Witjes J, Fay F, Baxter S, Wessel CH, Eldib M, et al. : Multimodal Positron Emission Tomography Imaging to Quantify Uptake of 89Zr-Labeled Liposomes in the Atherosclerotic Vessel Wall. Bioconjug Chem 2020, 31:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May JN, Golombek SK, Baues M, Dasgupta A, Drude N, Rix A, Rommel D, Von Stillfried S, Appold L, Pola R, et al. : Multimodal and multiscale optical imaging of nanomedicine delivery across the blood-brain barrier upon sonopermeation. Theranostics 2020, 10:1948–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aryal M, Papademetriou I, Zhang YZ, Power C, McDannold N, Porter T: MRI Monitoring and Quantification of Ultrasound-Mediated Delivery of Liposomes Dually Labeled with Gadolinium and Fluorophore through the Blood-Brain Barrier. Ultrasound Med Biol 2019, 45:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sofias AM, Toner YC, Meerwaldt AE, van Leent MMT, Soultanidis G, Elschot M, Gonai H, Grendstad K, Flobak Å, Neckmann U, et al. : Tumor Targeting by αvβ3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano 2020, 14:7832–7846. (•) The use of intravital microscopy to investigate nanoformulations' in vivo fate at the cellular level is demonstrated in this study, in combination with whole-body biodistribution and pharmacokinetics assessment by PET/CT.
- 22. Llop J, Jiang P, Marradi M, Gómez-Vallejo V, Echeverría M, Yu S, Puigivila M, Baz Z, Szczupak B, Pérez-Campaña C, et al. : Visualisation of dual radiolabelled poly(lactide-co-glycolide) nanoparticle degradation in vivo using energy-discriminant SPECT. J Mater Chem B 2015, 3:6293–6300. (•) A nice example of how energy-discriminating SPECT can be used to assess the in vivo stability of a nanoformulation.
- 23.Lamichhane N, Dewkar GK, Sundaresan G, Mahon RN, Zweit J: [18F]-Fluorinated Carboplatin and [111In]-Liposome for Image-Guided Drug Delivery. Int J Mol Sci 2017, 18:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skajaa T, Zhao Y, Van Den Heuvel DJ, Gerritsen HC, Cormode DP, Koole R, Van Schooneveld MM, Post JA, Fisher E a., Fayad Z a., et al. : Quantum dot and Cy5.5 labeled nanoparticles to investigate lipoprotein biointeractions via Forster resonance energy transfer. Nano Lett 2010, 10:5131–5138. (••) In this study, the authors use fluorescent nanoparticles to evaluate lipoprotein dynamics in vivo by FRET, a strategy that can be advantageously exploited to assess nanoformulation integrity.
- 25.Teunissen AJP, Pérez-Medina C, Meijerink A, Mulder WJM: Investigating supramolecular systems using Förster resonance energy transfer. Chem Soc Rev 2018, 47:7027–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Ju Y, Ali Z, Yin H, Sheng F, Lin J, Wang B, Hou Y: Near-infrared light and tumor microenvironment dual responsive size-switchable nanocapsules for multimodal tumor theranostics. Nat Commun 2019, 10:4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang L, Sun X, Liu N, Zhou Z, Yu F, Zhang X, Sun X, Chen X: Radiolabeled Angiogenesis-Targeting Croconaine Nanoparticles for Trimodality Imaging Guided Photothermal Therapy of Glioma. ACS Appl Nano Mater 2018, 1:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Lin L, Zhang Y, Sheng S, Wang Y, Xu C, Tian H, Chen X: Two-dimensional nanosheets with high curcumin loading content for multimodal imaging-guided combined chemo-photothermal therapy. Biomaterials 2019, 223:119470. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Chen J, Li J, Xia B, Xu J, Wang Q, Xie C, Fan Q, Huang W: Single nanoparticles as versatile phototheranostics for tri-modal imaging-guided photothermal therapy. Biomater Sci 2019, 7:3609–3613. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Shi Y, Zhang S, Huang X, Zhang J, Zhang Y, Si W, Dong X: Hydrogen Peroxide Responsive Iron–Based Nanoplatform for Multimodal Imaging–Guided Cancer Therapy. Small 2019, 15:e1803791. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Tang Y, Hu Y, Lu F, Lu X, Wang Y, Li J, Li Y, Ji Y, Wang W, et al. : Gadolinium-chelated conjugated polymer-based nanotheranostics for photoacoustic/magnetic resonance/NIR-II fluorescence imaging-guided cancer photothermal therapy. Theranostics 2019, 9:4168–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv X, Wang X, Li T, Wei C, Tang Y, Yang T, Wang Q, Yang X, Chen H, Shen J, et al. : Rationally Designed Monodisperse Gd2O3/Bi2S3 Hybrid Nanodots for Efficient Cancer Theranostics. Small 2018, 14:e1802904. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Lu T, Wang Y, Song Y, Wang S, Lu Q, Yang L, Tan F, Li J, Li N: Glutathione-Mediated Clearable Nanoparticles Based on Ultrasmall Gd2O3 for MSOT/CT/MR Imaging Guided Photothermal/Radio Combination Cancer Therapy. Mol Pharm 2019, 16:3489–3501. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Hou M, Zhang L, Qian D, Yang Q, Xu Z, Kang Y, Xue P: PEGylated mesoporous Bi 2 S 3 nanostars loaded with chlorin e6 and doxorubicin for fluorescence/CT imaging-guided multimodal therapy of cancer. Nanomedicine Nanotechnology, Biol Med 2019, 17:1–12. [DOI] [PubMed] [Google Scholar]
- 35.Zhang DY, Zheng Y, Zhang H, Yang GG, Tan CP, He L, Ji LN, Mao ZW: Folate receptor-targeted theranostic IrS:X nanoparticles for multimodal imaging-guided combined chemo-photothermal therapy. Nanoscale 2018, 10:22252–22262. [DOI] [PubMed] [Google Scholar]
- 36.Sharma G, Jagtap JM, Parchur AK, Gogineni VR, Ran S, Bergom C, White SB, Flister MJ, Joshi A: Heritable modifiers of the tumor microenvironment influence nanoparticle uptake, distribution and response to photothermal therapy. Theranostics 2020, 10:5368–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S, Tian R, Shao B, Feng Y, Yuan S, Dong L, Zhang L, Liu K, Wang Z, You H: Designing of UCNPs@Bi@SiO 2 Hybrid Theranostic Nanoplatforms for Simultaneous Multimodal Imaging and Photothermal Therapy. ACS Appl Mater Interfaces 2019, 11:394–402. [DOI] [PubMed] [Google Scholar]
- 38.Zhen X, Zhang J, Huang J, Xie C, Miao Q, Pu K: Macrotheranostic Probe with Disease-Activated Near-Infrared Fluorescence, Photoacoustic, and Photothermal Signals for Imaging-Guided Therapy. Angew Chemie - Int Ed 2018, 57:7804–7808. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Wang T, Zhao L, Rajasekhar VK, Joshi S, Andreou C, Pal S, Hsu H ting, Zhang H, Cohen IJ, et al. : Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat Biomed Eng 2020, 4:686–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif J-C, et al. : Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011, 378:1547–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang J, Baxter S, Menon A, Alaarg A, Sanchez-Gaytan BL, Fay F, Zhao Y, Ouimet M, Braza MS, Longo VA, et al. : Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci 2016, 113:E6731–E6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alaarg A, Senders ML, Varela-Moreira A, Pérez-Medina C, Zhao Y, Tang J, Fay F, Reiner T, Fayad ZA, Hennink WE, et al. : A systematic comparison of clinically viable nanomedicines targeting HMG-CoA reductase in inflammatory atherosclerosis. J Control Release 2017, 262:47–57. [DOI] [PubMed] [Google Scholar]
- 43.Yamankurt G, Berns EJ, Xue A, Lee A, Bagheri N, Mrksich M, Mirkin CA: Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat Biomed Eng 2019, 3:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adir O, Poley M, Chen G, Froim S, Krinsky N, Shklover J, Shainsky-Roitman J, Lammers T, Schroeder A: Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv Mater [date unknown], 32:1901989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng TSC, Garlin MA, Weissleder R, Miller MA: Improving nanotherapy delivery and action through image-guided systems pharmacology. Theranostics 2020, 10:968–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dogra P, Butner JD, Nizzero S, Ruiz Ramírez J, Noureddine A, Pelaez MJ, Elganainy D, Yang Z, Le AD, Goel S, et al. : Image-guided mathematical modeling for pharmacological evaluation of nanomaterials and monoclonal antibodies. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 2020, 12:e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shim J, Al-Mashhadi RH, Sørensen CB, Bentzon JF: Large animal models of atherosclerosis - New tools for persistent problems in cardiovascular medicine. J Pathol 2016, 238:257–266. [DOI] [PubMed] [Google Scholar]
- 48.Schachtschneider KM, Schwind RM, Newson J, Kinachtchouk N, Rizko M, Mendoza-Elias N, Grippo P, Principe DR, Park A, Overgaard NH, et al. : The oncopig cancer model: An innovative large animal translational oncology platform. Front Oncol 2017, 7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Maar JS, Sofias AM, Siegel TP, Vreeken RJ, Moonen C, Bos C, Deckers R: Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics 2020, 10:1884–1909. (••) A comprehensive review highlighting the value of different imaging techniques to understand nanotherapeutics' in vivo behavior as well as disease heterogeneity, and how this information can be exploited to increase nanomedicine translational and clinical success.
- 50.Pérez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJM, Reiner T: Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun 2016, 7:11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Gaddy D, Ventura M, Bernards N, de Souza R, Kirpotin D, Wickham T, Fitzgerald J, Zheng J, Hendriks BS: Companion Diagnostic 64 Cu-Liposome Positron Emission Tomography Enables Characterization of Drug Delivery to Tumors and Predicts Response to Cancer Nanomedicines. Theranostics 2018, 8:2300–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramanathan RK, Korn RL, Raghunand N, Sachdev JC, Newbold RG, Jameson G, Fetterly GJ, Prey J, Klinz SG, Kim J, et al. : Correlation between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients with Advanced Solid Tumors: A Pilot Study. Clin Cancer Res 2017, 23:3638–3648. [DOI] [PubMed] [Google Scholar]
- 53.Lu F-M, Yuan Z: PET/SPECT molecular imaging in clinical neuroscience: recent advances in the investigation of CNS diseases. Quant Imaging Med Surg 2015, 5:433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choo YW, Jeong J, Jung K: Recent advances in intravital microscopy for investigation of dynamic cellular behavior in vivo. BMB Rep 2020, 53:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]