Abstract
The EXIT-CJS (N = 1005) multisite open-label randomized controlled trial will compare retention and effectiveness of extended-release buprenorphine (XR-B) vs. extended-release naltrexone (XR-NTX) to treat opioid use disorder (OUD) among criminal justice system (CJS)-involved adults in six U.S. locales (New Jersey, New York City, Delaware, Oregon, Connecticut, and New Hampshire). With a pragmatic, noninferiority design, this study hypothesizes that XR-B (n = 335) will be noninferior to XR-NTX (n = 335) in retention-in-study-medication treatment (the primary outcome), self-reported opioid use, opioid-positive urine samples, opioid overdose events, and CJS recidivism. In addition, persons with OUD not eligible or interested in the RCT will be recruited into an enhanced treatment as usual arm (n = 335) to examine usual care outcomes in a quasi-experimental observational cohort.
Keywords: Opioid use disorder, Medication treatment, Buprenorphine, Naltrexone, Injection, Criminal justice
1. Introduction
Effective interventions for criminal justice–involved adults with opioid use disorders (OUD) are urgently needed. One-third of persons who use heroin cycle through correctional institutions annually; and more are under community supervision (parole, probation, drug courts) (Rich, Wakeman, & Dickman, 2011). U.S. jails and prisons offer a unique opportunity to identify this large flow of persons with OUD and engage them in medications for opioid use disorders (MOUD) (Friedmann et al., 2012).
This protocol paper describes the design of EXIT-CJS, a multiple principal investigator, multisite, randomized controlled, noninferiority trial comparing monthly injectable extended-release buprenorphine (XR-B) to monthly injectable extended-release naltrexone (XR-NTX). EXIT-CJS is funded by and conducted within the National Institute on Drug Abuse’s Justice and Community Opioid Innovation Network (JCOIN).
Previous studies of XR-NTX initiated in jails or prisons indicate XR-NTX’s effectiveness vs. usual care, (Lee et al., 2016) and superiority vs. placebo (S.A. Springer et al., 2018) among persons not interested in or able to access agonist MOUD, buprenorphine, and methadone. A pilot RCT of XR-NTX immediately postrelease from a NYC jail vs. usual care with no medication (Lee et al., 2015) found less return to use and less opioid use, findings which were then duplicated in a larger RCT at the same site (Lee et al., 2018). A randomized double blind placebo-controlled trial using XR-NTX conducted among persons in prisons and jails living with HIV and OUD showed superiority in improving or maintaining HIV viral suppression and reduced opioid use, six months postrelease (S.A. Springer et al., 2018). None of these trial designs compared naltrexone directly to buprenorphine or methadone. An RCT in the county jail of Albuquerque showed no differences in return to use for XR-NTX vs. usual care (Farabee, Condon, Hallgren, & McCrady, 2020).
Buprenorphine has been effective in reducing opioid craving and use and improving additional HIV viral suppression (S.A. Springer, Qiu, Saber-Tehrani, & Altice, 2012) in justice-involved populations. Two large RCTs in community OUD populations compared daily sublingual buprenorphine to monthly XR-NTX; a U.S. trial demonstrated superior effects on return to use for buprenorphine due to higher rates of induction (Lee et al., 2016, 2018), while a Norwegian protocol found similar and noninferior rates of successful induction and retention in treatment for both medications (Tanum et al., 2017). Large randomized trials evaluating buprenorphine’s effectiveness in CJS populations and at re-entry do not exist in great number.
An industry trial of XR-B (Sublocade, Indivior) demonstrated superiority over placebo (Haight et al., 2019). A competing monthly formulation of XR-B was compared to sublingual buprenorphine in separate industry trials and was noninferior for treatment retention (Lofwall et al., 2018). We piloted XR-B vs. standard sublingual buprenorphine in NYC jails and at release, and found the medication acceptable to incarcerated individuals, easy to implement in a large urban jail, and comparable to SL buprenorphine for postrelease treatment retention and opioid risk reduction (J.D. Lee et al., 2020). Studies have not otherwise evaluated XR-B in criminal justice system (CJS) settings or populations or compared to XR-NTX agonist treatment. This trial seeks to provide definitive comparative effectiveness data for the two FDA-approved monthly long-acting MOUD options.
2. Methods
2.1. Study design
EXIT-CJS (N = 1005) is a multisite, open-label, head-to-head randomized controlled noninferiority trial of XR-B vs. XR-NTX in CJS-involved adult volunteers seeking medication treatment for a diagnosis of OUD (Fig. 1). Six hundred and seventy adults will be randomized 1:1 to XR-B and XR-NTX prior to release and treated for 24 weeks following release from incarceration or upon entry into a community-based program. Participants will be referred to appropriate community MOUD treatment options at week 24. Final follow-up occurs at week 48. The primary outcome is retention-in-study medication-treatment during weeks 1–24 (six scheduled monthly injections), using a noninferiority comparison. Secondary measures include urine drug tests and self-reported opioid use.
Fig. 1.
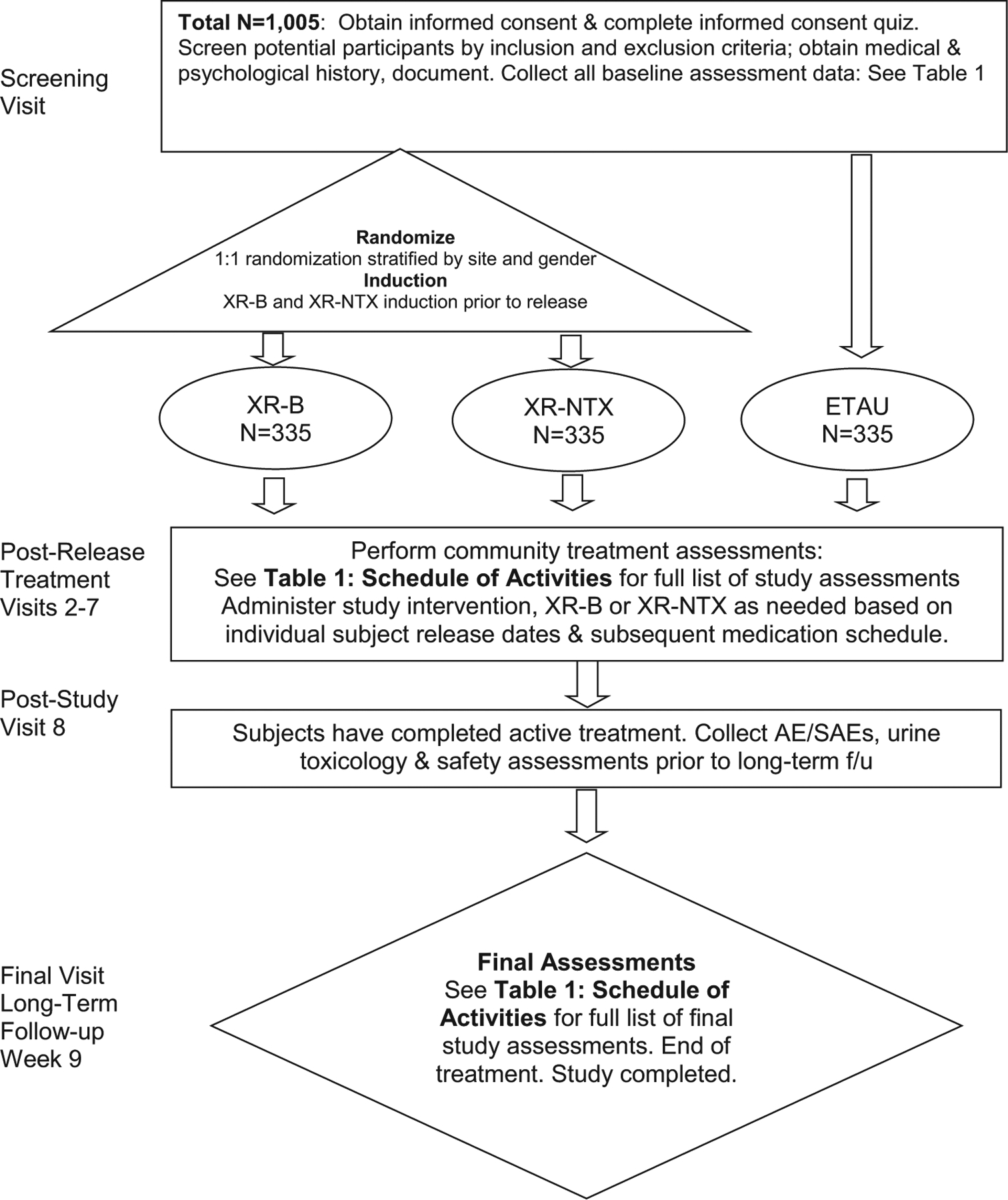
Study flow chart.
A third nonrandomized, observational arm of persons with OUD (n = 335) will be able to enroll in an enhanced treatment-as-usual (ETAU) group. ETAU allows the observational study of usual nonstudy OUD treatment pathways, which vary by site. Research staff will encourage ETAU to access nonstudy MOUDs and the study will follow this group for the same outcomes but it will not receive study treatment.
The New York University Single Institutional Review Board (sIRB) has approved the protocol; the study has received OHRP authorization for research involving prisoners, obtained a certificate of confidentiality, registered at clinicaltrials.gov (NCT04219540), and is conducted under a Food and Drug Administration Investigator New Drug application allowing for certain off-label use of XR-B (Sublocade).
2.2. Study population
Recruitment and follow-up visits take place across multiple CJS and community sites affiliated with the multiple academic partners: (1) New Jersey Department of Corrections and Rutgers NJ Medical School in Newark (NYU-Rutgers); (2) Bellevue Hospital (NYU); (3) Connecticut DOC, Community Health Center Inc., and Ledge Light Health District in New London and Hartford Counties (Yale); (4) Clackamas and Washington County Jails, CODA Inc., and Clackamas County Health Centers (Oregon Health & Science University); (5) New Hampshire Department of Corrections and ROAD to a Better Life (Dartmouth College); and (6) Delaware DOC and Connections Community Support Programs (Friends Research Institute).
All community providers currently prescribe or can refer to any form of MOUD treatment and will directly provide XR-B and XR-NTX in this trial. Among CJS sites, all offer daily sublingual buprenorphine-naloxone and XR-NTX treatment, some prescribe XR-B (NH and NJ DOC), and some routinely continue and/or offer induction onto methadone (CT, DE, NJ, both OR jails).
Primary inclusion criteria for the RCT are:
Adult volunteer aged 18 years or older;
Current CJS incarceration or community CJS-involvement;
Current or history of moderate-to-severe opioid use disorder (OUD, DSM-5);
Not planning to move out of state or to new location within 6-months post-release; and
Willing to accept XR-B or XR-NTX.
Fig. 2 presents complete inclusion and exclusion criteria for the RCT and ETAU arms.
Fig. 2.
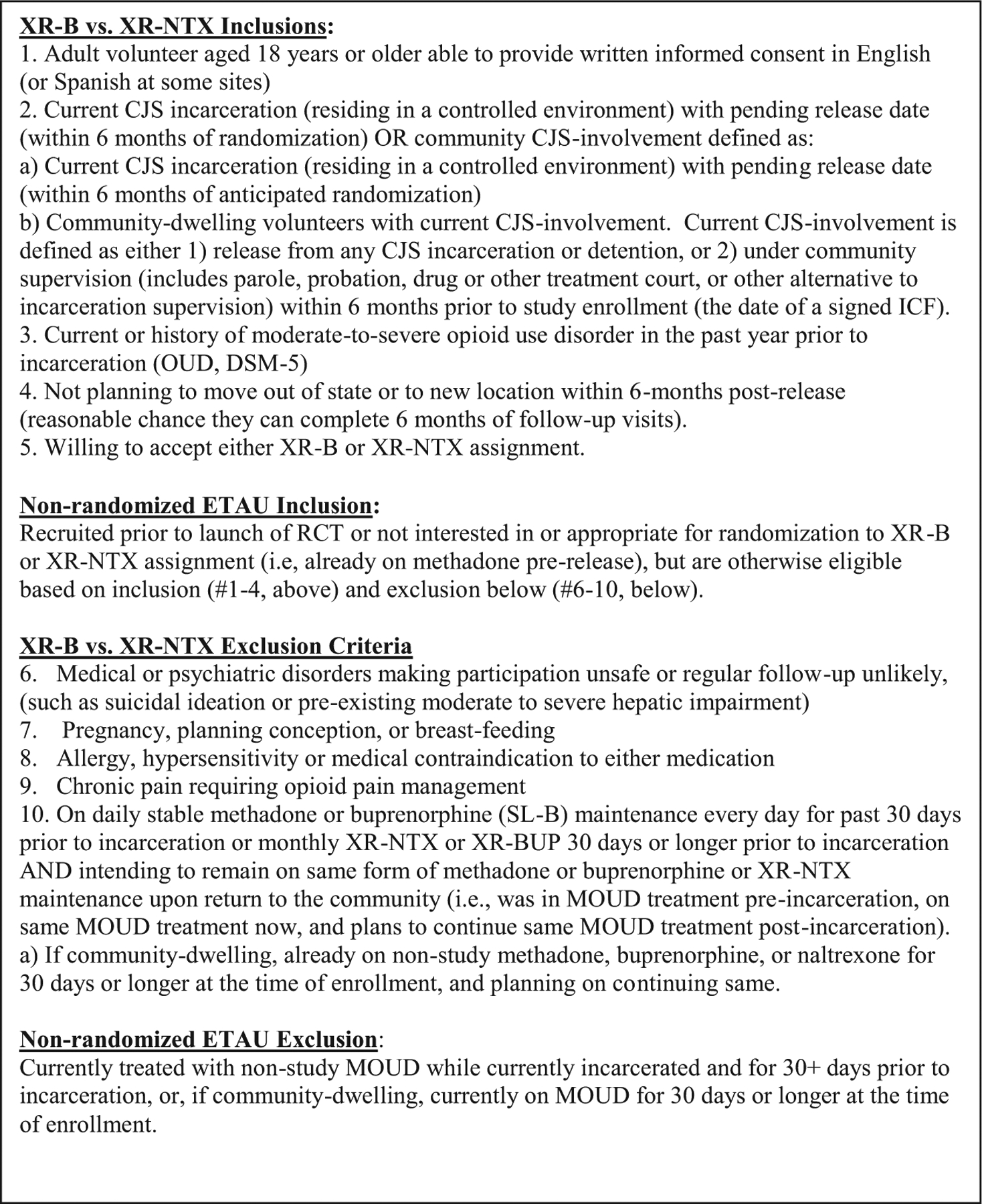
Detailed inclusion and exclusion criteria.
2.3. Recruitment, informed consent, and eligibility assessment
The informed consent form and recruitment strategy were directly informed by our work on the NIDA CTN-0050 X:BOT trial (PMID: 29150198), which randomized between XR-NTX and SL buprenorphine-naloxone film. Research staff, and corrections and community treatment partners will collaborate to identify potentially eligible study candidates. Research staff will introduce the study to potential participants through a scripted “pre-screen” conversation that includes a brief eligibility screener. This conversation precedes completion of informed consent and formal eligibility assessment by study clinicians. The informed consent form explains crucial differences between agonist and antagonist medications, and study clinicians explain these differences further when study candidates meet with study clinicians for eligibility assessments. Study clinicians review medical records and check LFTs when indicated (Saxon, Ling, Hillhouse, et al., 2013). We exclude persons with significant medical and psychiatric co-morbidities that may prevent safe study participation. This includes history of end-stage liver disease or current hepatic impairment (e.g., acute hepatitis). We estimate accrual of N = 1005 over a 39-month period; a maximum accrual ceiling is N = 1505.
2.4. Study interventions
XR-B (Sublocade™; Indivior) is a long-acting buprenorphine formulation indicated for moderate-to-severe OUD and opioid maintenance. XR-NTX (Vivitrol®; Alkermes) is a long-acting naltrexone formulation for OUD relapse prevention following opioid detoxification. Both are FDA-approved monthly injectables, donated here in-kind by the manufacturers.
Sites are expected to use and adapt the two medications within local CJS and community treatment provider standards. This adaption will include potentially wide site variability in terms of XR-NTX detoxification and induction approaches, sublingual buprenorphine maintenance prior to XR-B, adjustments to monthly injection schedules for either medication, XR-B dose levels, monitoring of liver function tests (LFT), and follow-up of medication-related adverse events. Sites and providers are encouraged to counsel and treat participants consistent with an OUD medical management model, which emphasizes medication adherence, harm reduction, and improved function. Additional counseling availability and access will vary by site but is not required. Weekly multisite clinician calls will discuss cases and troubleshoot protocol, treatment, or safety concerns.
2.5. Dosing and administration
Both medications are continued as monthly injections administered every 28 days postrelease and in the community. RCT participants will start study medication prior to release whenever possible followed by 5 or more injections in the community. Medication induction can also occur postrelease and anytime over the 24-week postrelease treatment phase if pre-release induction does not occur.
XR-buprenorphine induction: XR-B (Sublocade) will be delivered as a pre-filled 1.5 cc subcutaneous monthly injection, using a 300 mg or 100 mg, 0.5 cc starting dose. XR-B consists of a depot injectable formulation in polymeric solution to the abdomen and releases buprenorphine over 28 days (4 weeks) by diffusion as the polymer biodegrades. Prior to an initial injection, guidelines generally recommend that the patient be inducted onto 7 days or longer of sublingual buprenorphine (SLB) at doses of 8 mg/day or higher. Individuals not currently on agonist medications will be inducted using sublingual buprenorphine per a, “start low, go slow,” approach to buprenorphine induction of opioid naïve persons (S.A. Springer et al., 2012; Vocci et al., 2015).
XR-buprenorphine maintenance: Participants will receive at least one XR-B monthly injection prior to release, which we anticipate to be the 300 mg dose. Some participants may be recruited earlier during their incarceration or experience delayed release dates; XR-B will be continued monthly from the time of induction to the day of release. XR-B is available in two doses, 300 mg and 100 mg. Study clinicians are encouraged to follow package labeling and administer two months of 300 mg doses followed by maintenance doses of 100 mg monthly for four months.
The maintenance dose may be increased to 300 mg monthly for patients who do not tolerate the 100 mg dose or do not demonstrate a satisfactory clinical response, as evidenced by self-reported illicit opioid use or urine drug screens positive for illicit opioid use. We project the proportion of participants maintained on 300 mg vs. 100 mg monthly beyond 12 weeks of study treatment to be less than half of the XR-B arm, and will follow and report on this emerging clinical outcome. We note the product labeling allowing for this dose flexibility and the recommendation for LFT monitoring of patients on Sublocade, particularly those maintained on 300 mg monthly (Indivior, 2017). The intensity and frequency of LFT monitoring in this trial will be at the discretion of the individual sites and not mandated by the study protocol or tracked as study data. We note a higher proportion of 300 mg maintained individuals in the pivotal trial had elevated LFT, but not higher rates of hepatic serious adverse events, and LFT elevations reportedly did not result in any dose reductions or discontinuation of XR-B in the 300 mg maintenance arm (Haight et al., 2019). We note LFT monitoring is a labeled recommendation for all buprenorphine OUD formulations, but in practice can be a costly barrier to treatment and is not routinely performed during SL BUP maintenance at our participating sites.
XR-B dosing intervals and missed doses: XR-B is administered in rotating abdominal quadrants every 26–35 days. In the event of a missed visit, the dose can be delivered within 28 days (up to 8 weeks since the prior dose). Longer missed dose windows will require re-establishing a SL-B lead-in week prior to the next XR-B injection.
XR-NTX induction and maintenance: XR-NTX is delivered as a 380 mg (4 cc) intramuscular injection to the upper outer gluteus (buttock). Participants who still require opioid detoxification at the time of randomization will access standard detox protocols available at each site. A standard naloxone and/or oral naltrexone challenge will be optional, as most individuals are expected to have been opioid-free at randomization and induction. XR-NTX is packaged as a refrigerated kit, warmed to room temperature, and mixed and shaken just prior to administration. XR-NTX is a single monthly 380 mg dose that bio-degrades over 4–6 weeks.
XR-NTX dosing intervals and missed doses: XR-NTX is recommended every 4 weeks. Participants reporting increased cravings or with evidence of opioid use, particularly toward the end of the 4-week interval, can be offered earlier injections more frequently (every 21 days). Participants 36 or more days since the prior injection are evaluated for recent opioid use and opioid tolerance and may require detoxification and XR-NTX re-induction.
2.6. Study procedures
Table 1 provides a full schedule of assessments and procedures. JCOIN has harmonized instruments and assessments across the sites, and this study adopts the JCOIN core and recommended measures for demographics, health, mental health, drug use histories and current use, criminal justice outcomes, social function, quality of life, and cost measures. Once consented, the study will randomize participants in the RCT portion 1:1 to XR-NTX or XR-B. Induction onto study drug occurs after random assignment and prior to release. Institutional or study medical staff will initiate treatment, with variation by site. The study will follow RCT participants for 24 weeks postrelease in active study treatment (beginning the week of release as week 1, or 2 weeks post-randomization if community recruitment), with a post-treatment visit at 28 weeks and a final follow-up visit six months post-treatment at week 48 to conclude study participation. In addition to screening and baseline assessments, the study will assess participants in monthly treatment visits (weeks 4, 8 12, 16, 20, & 24), and again at week 28 & 48. The study will compensate all participants equally for time, travel, and data collection, independent of study arm or retention on study drug. Incentive amounts vary by visit number and by site, ranging from $25 to $100 per visit; payment is increased for follow-ups at weeks 24 and 48.
Table 1.
Schedule of study activities.
| Research assessments | Wk 0 | Wk 1 | Wk 4 | Wk 8 | Wk 12 | Wk 16 | Wk 20 | Wk 24 | Wk 28 | Wk 48 |
|---|---|---|---|---|---|---|---|---|---|---|
| Visit 0 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8b | Visit 9 | |
| Informed consent and quiz | X | |||||||||
| Inclusion/exclusion criteria | X | |||||||||
| Rapid opioid evaluation (OUD screen) (Wickersham, Azar, Cannon, Altice, & Springer, 2015) | X | |||||||||
| Demographics | X | |||||||||
| Non-study medical & other services | X | X | X | X | X | X | X | X | ||
| MOUD treatment history | X | X | X | X | X | X | X | X | ||
| Crime and legal history | X | X | X | X | X | X | X | X | ||
| PROMIS PROPr (quality of life) (Hanmer et al, 2018) | X | X | X | X | X | X | X | X | ||
| HIV risk assessment battery (Navaline et al., 1994) | X | X | ||||||||
| OUD treatment preference | X | |||||||||
| Drug and alcohol use | X | X | X | X | X | X | X | X | ||
| Opioids timeline follow back calendar (Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutigliano, 2000) | X | X | X | X | X | X | X | X | X | |
| Motivation for participation | X | |||||||||
| Controlled environment status | X | X | X | X | X | X | X | X | ||
| OUD DSM-5 checklist (American Psychiatric Association, 2013) | X | |||||||||
| Overdose recall form | X | X | X | X | X | X | X | X | X | |
| Opioid craving, visual analog scale (VAS) | X | X | X | X | X | X | X | X | ||
| Randomization treatment assignment | X | |||||||||
| Medical assessments and procedures | ||||||||||
| Urine toxicology point-of-care | X | X | X | X | X | X | X | X | X | X |
| Pregnancy point-of-care | X | X | X | X | X | X | X | |||
| HIV, HCV status (EMR and self-report)a | X | |||||||||
| Medical/psychiatric history | X | |||||||||
| Naloxone challengea,b | X | |||||||||
| XR-NTX injection logb | X | X | X | X | X | X | ||||
| SL-B dosingb | X | |||||||||
| XR-B injection logb | X | X | X | X | X | X | ||||
| AE/SAE | X | X | X | X | X | X | X | X | X | |
| Administrative forms | ||||||||||
| Locator forms | X | X | X | X | X | X | X | X | X | X |
| Study clinician note | X | X | X | X | X | X | X | X | X | |
| RA/RC progress note | X | X | X | X | X | X | X | X | X | |
| Payment vouchers | X | X | X | X | X | X | X | X | X | X |
Optional measures/procedures.
XR-B; XR-NTX Arms Only.
2.7. Outcomes
The primary outcome is retention on study medication. The study will derive this outcome from study medication logs for consecutive monthly injections received, which will range from 0 to 6. Retention was an optimal primary outcome as it is real-world, based on universally available administrative data (medication logs), and studies have shown it to drive improved OUD outcomes in the case of either medication (Biondi, Zheng, Frank, Petrakis, & Springer, 2020).
Secondary outcomes are rates of self-reported opioid use (days per month), urine nonstudy opioid results (rates of negative vs. positive or missing), opioid craving ratings, overdose events (fatal and nonfatal); serious adverse events; new criminal charges, housing stability, employment, new arrests, re-incarceration episodes and re-incarceration days; quality of life; depression; and HIV and HCV (prevalence and risk).
2.8. Statistical analyses
The primary analytic sample will be a modified intent-to-treat sample consisting of participants consented, enrolled, and randomized, and released to the community. This removes participants whose release dates change and are then incarcerated indefinitely or for much longer than anticipated. We expect this to occur among only 1–5% of all those randomized. Secondary analyses will examine all randomized participants (ITT) and participants both inducted and released as planned (per protocol).
2.8.1. Power analysis
Assuming that the average participant in the RCT receives around 3–4 monthly doses, we simulated 95% confidence intervals under different sample size scenarios. A sample size of 400+ randomized provided acceptable margins, which translated to a standard probabilistic noninferiority margin of ~6%. We then increased the target sample to 600+, allowing a narrower noninferiority margin, accounting for possibly larger than expected attrition or under-recruitment, and taking into account key OUD and CJS secondary outcomes and planned multiple comparisons.
2.8.2. Analysis of the primary aim(s)
We will evaluate if XR-B is at least as effective as XR-NTX using a noninferiority approach. The primary outcome measure is retention on assigned study medication treatment defined as the number of injections received during the 24-week post-release treatment phase, range 0–6. The study will base the comparison of the two arms on the log-odds ratio of the injection rate for participants randomized to XR-B vs. XR-NTX. We will estimate treatment effects using a generalized linear regression model with a binomial distribution and a log-odds link and compare the two with a noninferiority margin of ~4%.
2.8.3. Analysis of the secondary outcomes
Secondary outcome measures include other opioid treatment outcomes including: rates of self-reported opioid use (days per month), urine nonstudy opioid results (negative vs. positive or missing, monthly), overdose events (fatal and nonfatal); serious adverse events; new criminal charges, new arrests, re-incarceration episodes, and re-incarceration days; quality of life; depression; and HIV and HCV prevalence and risk behaviors. The study will assess differences for all the secondary outcomes for the two treatment arms using linear or generalized linear models depending on the nature of the outcome. In the case where there are repeated measurements for each individual, the study will use mixed effect models with participant-level random effects.
2.9. Adaptations to the study design and protocol in response to COVID-19
COVID-19 has significantly affected implementation of this study. To date, we have responded as follows.
Potential impact on incarceration rates: CJS collaborators have projected that both COVID-19 and state-level CJS reforms (in Oregon, Ballot Measrue 110; in NY and NJ, cash bail reforms; in all states potential decarceration related to COVID-19) will reduce the number of jail and prison admissions in the coming year(s), particularly those sentenced for low-level drug-related offenses. We will now also recruit community-dwelling adults with OUD and current or recent CJS involvement (i.e., probationers, parolees). To better understand COVID-era OUD program and population changes, we are administering a detailed site survey regarding MOUD policies, practices, and scope of services for all participating corrections and community treatment sites.
COVID-19 in jails and prisons: See Table 2 for a summary of mitigation strategies designed for protection of study participants and staff as rates of COVID-19 fluctuate across all participating sites.
Table 2.
Sample COVID-19 mitigation strategies that EXIT-CJS sites adopted prior to launch.
| Strategy | Setting | ||
|---|---|---|---|
| Jail/prison | Community | ||
| Rapid ramp down | We have designed our human research plan so that we have multiple methods to conduct our research so that we can change our research conduct as needed. This will allow us to be nimble and be able to conduct our study interviews via telephone. We will request to remain active as essential research because we will be providing study medication for opioid use disorder. If needed, recruitment and enrollment can be limited and those already enrolled will be maintained on their study medication. | X | X |
| Remote study visits | Study interviews will be done over the telephone when possible, using HIPAA-compliant telehealth or telephone systems | X | X |
| Recruitment will be performed electronically as is feasible. | X | X | |
| Research staff will meet with participants to conduct the Screening, Informed Consent, and Baseline research visits using either the NH DOC telehealth system, or using tablets provided to residents in the NH DOC | X | ||
| To facilitate access to a HIPAA-compliant telehealth system, all participants in NH will be provided a study cell phone with 12 months of unlimited data upon release from the NH DOC sites. Research staff will set up HIPPA-compliant Zoom on each smartphone and provide technology support to help participants learn to use their smartphone and Zoom. | X | ||
| Remote urine toxicology | To gather toxicology data, NH participants will either be mailed or pick-up a package with assessment materials, including the urine drug screen. Research staff will instruct the participant on collecting and discarding the urine specimen while conducting telehealth visits. Whenever possible, the participant will show the results card to research staff through HIPAA-compliant Zoom. Participants will also take a screenshot of the results and upload this to a secure server. | X | |
| Limit visits to Research Spaces | Every effort will be made to schedule the study drug injection on the same day as the interview to limit exposure. | X | |
| Dedensification | When possible, no more than 1 to 3 participants at the visit space per day. | X | |
| RAs will conduct interviews from a safe distance (minimum of 6 ft). There will be no other people in the waiting area other than the research staff person and the participant. The seating in the area is hard plastic which will be amenable to decontamination. | X | ||
| Symptom screening | RAs will call participants the week prior and the day prior to their appointments to screen for potential Covid-19 symptoms. If the screen is positive, direct the participant to outpatient testing and follow-up with the participant in 24 to 48 h to determine test outcome and adjust follow-up research visit will occur based on timeline recommendations for quarantine or isolation. | X | |
| If a participant does not have face protection, a mask will be provided before they are allowed to enter the research space. Temperature will be taken with digital thermometer upon arrival. | X | ||
| PPE | All participants must be informed before arrival that they will need to wear a cloth or droplet mask to their research visit. If they do not have one, the research team can arrange to provide | X | X |
| All research staff should wear their designated mask | X | X | |
| Universal precautions (gloves, face shield, gown, lab coat) should be implemented in addition to mask use during sample collection | X | X | |
| Research staff will sanitize the seats in waiting area and the meeting/exam rooms as well as all surfaces using medical grade disinfectant before and immediately after each study visit. | X | X | |
| Sanitize study spaces | Hand sanitizer will be readily available to anybody sitting in the waiting area. | X | |
| Participants will be instructed to not leave the immediate area in which they are seated. Research staff will disinfect the touch surfaces in the bathroom including faucet handles, light switch, door handle, and flush handle. | X | X | |
| Health and Symptom monitoring of research personnel | All personnel will self-monitor and stay at home if they are experiencing any health issues. If an individual experiences fever, cough, or difficulty breathing they will be encouraged to stay home and contact a healthcare provider for guidance. | X | X |
3. Results to date
This trial’s NIDA U01 award was received in summer 2019 and planned for kick-off and initial recruitment in spring 2020, with active recruitment and follow-up during 2020–2024. COVID-19 quickly disrupted this timeline due to academic and correctional shutdowns of health service research, and recruitment began in February 2021.
4. Discussion
This multi-site RCT compares monthly XR buprenorphine and naltrexone formulations among justice-involved participants recruited in jails, prisons, and the community. The open-label pragmatic design relies on community treatment providers at each site for continued delivery of the study medication. An ETAU arm is expected to provide temporal, nonrandomized, quasi-experimental observational data for usual nonstudy OUD treatments, which will likely vary by site.
While designed as a pragmatic “real-world” open-label trial, several approaches are relatively unique to this protocol. One such approach is the re-introduction of buprenorphine and physical opioid dependence among persons with histories of OUD but now opioid-free during a prolonged incarceration. The strategy of initiating opioid nontolerant pre-release prisoners with a history of heroin addiction on methadone maintenance with low methadone doses and a slower than usual rate of increase was first reported over 50 years ago (Dole et al., 1969). More recently, a number of recent studies have carried out safe induction onto sublingual buprenorphine of this population (Garcia, Correa, Hernandez Viver, et al., 2007; S.A. Springer, Chen, & Altice, 2010; Vocci et al., 2015; Zaller et al., 2013). Using sublingual buprenorphine is now standard practice in CT, NJ, and NH DOCs and NYC jails, among other systems. As a health-services delivery study, the research team designed the approach to reflect MOUD treatment practices as they are currently being implemented in correctional settings. Given that overdose mortality rates of newly released adults are more than 10 times higher when leaving prison, any pre-release MOUD is arguably well-justified (Bins-wanger, Blatchford, Mueller, & Stern, 2013). We will also bypass Sublocade’s mandatory postmarketing Risk Evaluation and Mitigation Strategy requirements for commercial use by conducting the trial under an IND.
JCOIN and this study are intended to better inform adults with OUD, their providers, and the general public about effective strategies for risk reduction in justice-involved populations. A head:head comparison of the two monthly opioid agonist vs. antagonist injections as postrelease OUD treatments should further clarify the expected effects and benefits of these long-acting medications.
Acknowledgements and funding
NIDA U01DA047982 (Lee, Farabee, Marsch, Schwartz, Springer, Waddell); NIDA Independent Scientist Award K02 DA032322 (Springer); this protocol will receive in kind study drug from Indivior (Buprenoprhine-XR, Sublocade) and Alkermes (XR-Naltrexone, Vivitrol). We thank our collaborators in DE: Joanna Champney, MA and Kecia Winchester at DE DOC and Bill Northey, PhD, Vic Heresniak, DO, and Brenda Blackiston, NP at Connections Community Support Programs.
Disclosures
Drs. Lee, Springer, Farabee, and Waddell have received in-kind study drug from Indivior and Alkermes for multiple other federally funded trials. Dr. Springer has provided scientific consultation for Alkermes. Dr. Schwartz has provided consultation for Verily Life Sciences. Dr. Gryczynski is part owner of COG Analytics. Drs. Gryczynski, Lee, and Monico have received research funding from Indivior for conduct of a health survey study.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, & Stern MF (2013). Mortality after prison release: Opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Annals of Internal Medicine, 159(9), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi BE, Zheng X, Frank CA, Petrakis I, & Springer SA (2020). A literature review examining primary outcomes of medication treatment studies for opioid use disorder: What outcome should be used to measure opioid treatment success? The American Journal on Addictions, 29, 249–267. 10.1111/ajad.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, & Caine E (1969). Methadone treatment of randomly selected criminal addicts. New England Journal of Medicine, 280(25), 1372–1375. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, & Rutigliano P (2000). The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology, 68 (1), 134. [DOI] [PubMed] [Google Scholar]
- Farabee D, Condon T, Hallgren KA, & McCrady B (2020). A randomized comparison of extended-release naltrexone with or without patient navigation vs enhanced treatment-as-usual for incarcerated adults with opioid use disorder. Journal of Substance Abuse Treatment, 117, 108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Hoskinson R, Gordon M, Schwartz R, Kinlock T, Knight K, et al. (2012). Medication-assisted treatment in criminal justice agencies affiliated with the criminal justice-drug abuse treatment studies (CJ-DATS): Availability, barriers, and intentions. Substance Abuse, 33(1), 9–18. 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CA, Correa GC, Hernandez Viver AD, et al. (2007). Buprenorphine naloxone treatment for pre-release opioid-dependent inmate in Puerto Rico. Journal of Addiction Medicine, 1(3), 126–132. [DOI] [PubMed] [Google Scholar]
- Haight BR, Learned SM, Laffont CM, Fudala PJ, Zhao Y, Garofalo AS, … RB-US-13–0001 Study Investigators. (2019). Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England), 393 (10173), 778–790. 10.1016/S0140-6736(18)32259-1. [DOI] [PubMed] [Google Scholar]
- Hanmer J, Dewitt B, Yu L, Tsevat J, Roberts M, Revicki D, Pilkonis PA, Hess R, Hays RD, Fischhoff B and Feeny D (2018). Cross-sectional validation of the PROMIS-preference scoring system. PloS one, 13(7), p.e0201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indivior. (2017). Sublocade: Full prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209819s000lbl.pdf.
- Lee JD, Cheng A, Malone M, McDonald R, Matteo M, Katyal M, Mangat J, Giftos J, MacDonald M (2020, June 22–24). Buprenorphine extended-release in jail and at re-entry: Pilot proof-of-concept vs. daily sublingual buprenorphine-naloxone. Oral presentation at the meeting of college on problems of drug dependence, Virtual. [Google Scholar]
- Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, & Gourevitch MN (2015). Opioid treatment at release from jail using extended-release naltrexone: a pilot proof-of-concept randomized effectiveness trial. Addiction, 110(6), 1008–1014. [DOI] [PubMed] [Google Scholar]
- Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA Jr., et al. (2016). Extended-release naltrexone to prevent opioid relapse in criminal justice offenders. The New England Journal of Medicine, 374(13), 1232–1242. 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, et al. (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet (London, England), 391(10118), 309–318. 10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Nunes EV, Bailey GL, Sigmon SC, Kampman KM, … Kim S (2018). Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: A randomized clinical trial. JAMA Internal Medicine, 178(6), 764–773. 10.1001/jamainternmed.2018.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, Woody GE (1994). Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery (RAB): Enhancing the assessment of risk behaviors. AIDS Research and Human Retroviruses 1994;10(2):S281–3. [PubMed] [Google Scholar]
- Rich JD, Wakeman SE, & Dickman SL (2011). Medicine and the epidemic of incarceration in the United States. The New England Journal of Medicine, 364(22), 2081–2083. 10.1056/NEJMp1102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon AJ, Ling W, Hillhouse M, et al. (2013). Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug and Alcohol Dependence, 128(1–2), 71–76. 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Chen S, & Altice FL (2010). Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: The impact of buprenorphine treatment. Journal of Urban Health, 87(4), 592–602. 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Di Paola A, Azar MM, Barbour R, Biondi BE, Desabrais M, et al. (2018). Extended-release naltrexone improves viral suppression among incarcerated persons living with HIV with opioid use disorders transitioning to the community: Results of a double-blind, placebo-controlled randomized trial. Journal of Acquired Immune Deficiency Syndromes (1999), 78(1), 43–53. 10.1097/QAI.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Qiu J, Saber-Tehrani AS, & Altice FL (2012). Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One, 7(5), Article e38335. 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Latif ZE, Benth JŠ, Opheim A, Sharma-Haase K, … Kunøe N (2017). Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry, 74(12), 1197–1205. 10.1001/jamapsychiatry.2017.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Schwartz RP, Wilson ME, Gordon MS, Kinlock TW, Fitzgerald TT, … Jaffe JH (2015). Buprenorphine dose induction in non-opioid-tolerant pre-release prisoners. Drug and Alcohol Dependence, 156, 133–138. 10.1016/j.drugalcdep.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Azar MM, Cannon CM, Altice FL, & Springer SA (2015). Validation of a brief measure of opioid dependence: The rapid opioid dependence screen (RODS). Journal of Correctional Health Care, 21(1), 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaller N, McKenzie M, Friedmann PD, Green TC, McGowan S, & Rich JD (2013). Initiation of buprenorphine during incarceration and retention in treatment upon release. Journal of Substance Abuse Treatment, 45(2), 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


