Abstract
The diversity of ubiquitin modifications is immense. A protein can be monoubiquitylated, multi-monoubiquitylated, and polyubiquitylated with chains varying in size and shape. Ubiquitin itself can be adorned with other ubiquitin-like proteins and smaller functional groups. Considering different combinations of post-translational modifications can give rise to distinct biological outcomes, characterizing ubiquitylated proteoforms of a given protein is paramount. In this Opinion, we review recent advances in detecting and quantifying various ubiquitin proteoforms using mass spectrometry.
Introduction
Posttranslational modifications (PTMs) dramatically expand the functional capacity of proteins [1]. By modifying amino acid side chains of nascent or folded proteins, PTMs license new chemistry, create new recognition motifs, regulate enzymatic activity, and control protein stability and localization. In many instances, proteins exist in several modified forms referred to as proteoforms [2]. Individual proteoforms are capable of eliciting distinct biological responses. Thus, the combinatorial nature of PTMs enables exquisite regulation of biochemical events and equips cells with the ability to rapidly respond to developmental or physiological cues [3].
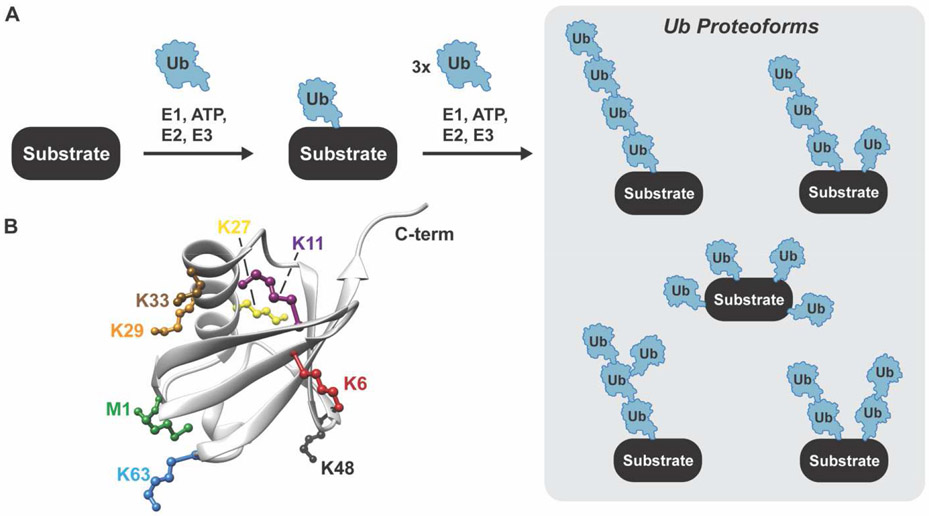
One of the more intricate PTMs is protein ubiquitylation. Ubiquitin is a highly conserved 76-residue protein with an exceptionally stable β-grasp fold [4]. The C-terminal glycine of ubiquitin (G76) is covalently attached to the ε-amino group of substrate lysines through the action of three enzymes: E1 (2 human enzymes), E2 (35 human enzymes), and E3 (>600 human enzymes). E1 enzymes activate the ubiquitin C-terminus and transfer the ubiquityl moiety to an active site cysteine of an E2 shuttling enzyme [5,6]. The ubiquitin-charged E2 then interacts with a substrate-bound E3 delivering ubiquitin to a single lysine (monoubiquitylation) or multiple lysines (multi-monoubiquitylation) (Figure 1A) [7-9]. The mechanisms by which ubiquitin is transferred to the substrate depend on the type of E3 [10]. Really Interesting New Gene (RING) E3s act as scaffolds, catalyzing transfer largely via a proximity-induced effect. Homologous to E6AP C-Terminus (HECT) and RING-Between-RING (RBR) E3s, on the other hand, utilize a covalent mechanism involving the intermediacy of a ubiquityl~E3 acyl-enzyme species (“~” refers to a reactive thioester).
Figure 1. Ubiquitin proteoforms.
A. Schematic showing the ubiquitylation cascade leading to the formation of different proteoforms. B. Structure of ubiquitin showing the seven lysines and amino terminus that are used to form ubiquitin chains.
The complexity of ubiquitylation stems from the ability to form ubiquitin oligomers (Figure 1A) [11,12]. Ubiquitin has seven lysines (K6, K11, K27, K29, K33, K48, and K63) and an amino-terminus (M1) that provide eight different attachment sites for the C-terminus of another ubiquitin molecule (Figure 1B). The resulting ubiquitin chains vary in length (number of ubiquitin molecules), linkage, and overall architecture (unbranched versus branched). The number of possibilities is astonishing. In addition, ubiquitin subunits can be adorned with smaller functional groups, e.g., phosphoryl [13,14], acetyl [15], and phosphoribosyl [16,17]. Such diversity, which we refer to as ubiquitin proteoforms, makes it difficult to assign biological function. Considering the flux through most signaling pathways is tightly regulated by ubiquitylation it is imperative to characterize the exact nature of the chain(s) attached to a substrate protein [18,19].
The presence of numerous proteoforms of the modified protein itself further complicates functional analysis. According to the Uniprot database there are many human proteins with annotated PTMs on more than one site and there is substantial evidence of crosstalk between PTMs [20,21]. Understanding the information embedded in a particular ubiquitin proteoform therefore requires each modification to be considered in the context of the other PTMs on the same protein, e.g., other ubiquitin modifications, phosphorylation, glycosylation, acetylation, etc. The problem, however, is that the pattern of PTMs also varies, resulting in a combinatorial explosion of proteoforms. Take for example a protein with four sites that can be monoubiquitylated. There are 16 (24) possible proteoforms of this multi-monoubiquitylated protein alone. So how do we characterize all proteoforms to develop a holistic view of the ubiquitin landscape? In this review we discuss different mass spectrometry-based strategies that are used to characterize ubiquitin proteoforms along with their limitations.
Bottom-Up Approach to Proteoform Analysis
The conventional approach to analyzing ubiquitin modifications involves bottom-up proteomics (BUP) [22,23]. As part of the BUP workflow, proteins are broken down into peptide fragments using a protease, typically trypsin or Lys-C [24,25]. Because the C-terminus of ubiquitin ends in Arg-Gly-Gly, trypsinolysis of ubiquitin conjugates leaves a diGly motif on the side chain of a substrate lysine residue (KGG). LC-MS/MS characterization of diGly-modified peptides then informs on the exact site of ubiquitylation. Due to the low abundance of diGly-modified peptides relative to their unmodified counterparts, an additional enrichment step using KGG-specific antibodies is required [26-29]. With the KGG enrichment approach, over 90,000 unique ubiquitylation sites have now been identified and the landscape of ubiquitin modifications has been analyzed in different cellular states [30-36]. In many of these studies, stimulus-dependent changes in the abundance ubiquitin chain linkages have bolstered the idea that distinct chains have distinct biological functions, which is the basis of the ‘ubiquitin code’ hypothesis. Whole cell KGG analyses, however, do not inform on the type of chain attached to a protein. This can be achieved using immunoprecipitation followed by MS, but a more common approach is to use linkage-specific antibodies [37-41], affimers [42,43], or binding domains [44-46] to enrich for a particular chain type and then use BUP to identify the modified proteins. We refer the reader to a series of excellent reviews discussing the utility of linkage-specific chain enrichment strategies [47,48].
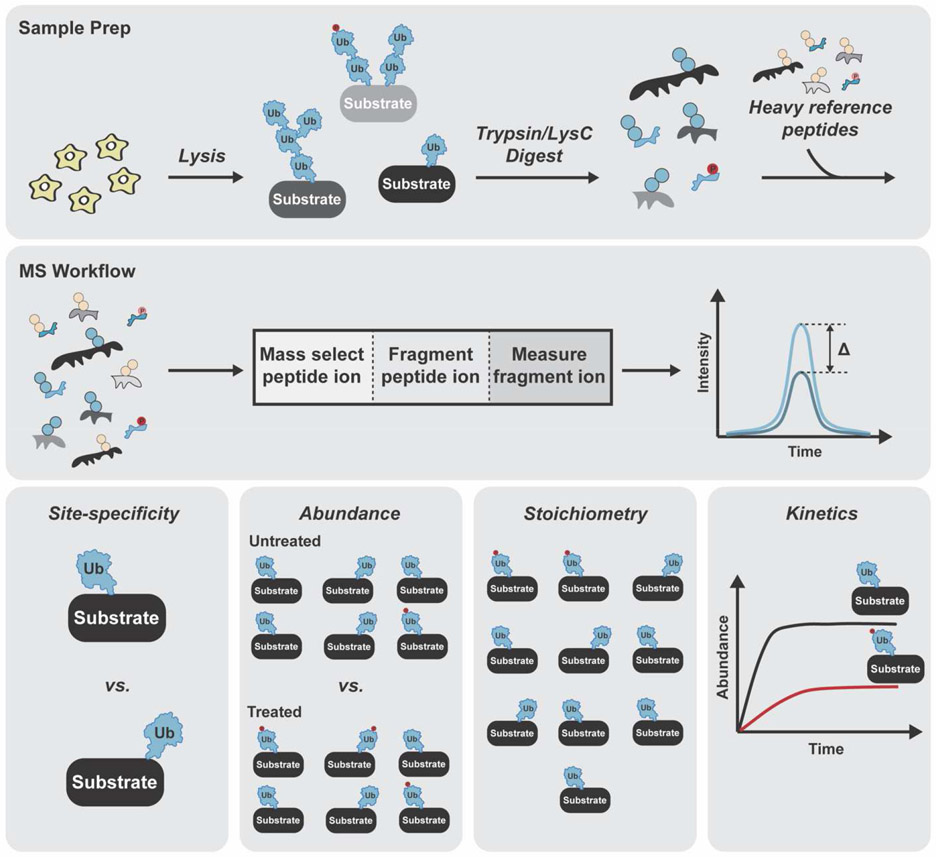
The challenge with BUP is that the precise nature of the chain cannot be discerned nor can the relationship between ubiquitylation and other PTMs. What BUP lacks in the ability to characterize proteoforms, is offset by the quantitative information that can be gleaned. Coupling BUP with targeted methods based on heavy reference peptides and selected ion monitoring techniques, e.g., parallel reaction monitoring (PRM) or selected reaction monitoring (SRM)-MS, can provide information on site-specificity, abundance, stoichiometry, and kinetics (Figure 2) [49]. Two recent studies nicely illustrate the power of targeted approaches in the analysis of proteoforms.
Figure 2. Ubiquitin proteoform analysis with BUP.
Scheme for selected ion monitoring using PRM or SRM-MS. Samples are digested with trypsin and Lys-C and mixed with heavy reference peptides corresponding to unmodified or modified forms of the protein prior to analysis by proteomics. MS1 and MS2 intensities are used to determine relative and absolute abundance of individual sites. Information on site-specificity, abundance, stoichiometry, and kinetics can be obtained using PRM/SRM-MS.
Damaged mitochondria are removed through a form of selective autophagy termed mitophagy [50]. The mitochondrially localized kinase PINK1 accumulates on the mitochondrial outer membrane (MOM) upon damage [51-53], where it phosphorylates ubiquitin to recruit and activate the PARKIN RING-Between-RING (RBR) ubiquitin ligase [54-63]. Ubiquitylation of numerous MOM-associated proteins along with the formation of ubiquitin chains then serves to mobilize autophagy receptors for proper disposal of damaged mitochondria [64-67]. To better understand how the coordination between phosphorylation and ubiquitylation control the rate of mitophagy, both label-free and PRM-MS-based quantitation were performed on MOM proteins [68]. Phosphorylated ubiquitin S65 (pS65-Ub) was found to comprise 20% of the ubiquitin pool purified with mitochondria. Increasing the fractional occupancy of pS65-Ub to ~60% reduces the association with mitophagy receptors, suggesting the stoichiometry of ubiquitin phosphorylation has been fine tuned to facilitate the recruitment of both PARKIN and mitophagy receptors. Establishing a temporal order of ubiquitylation by PRM-MS led to the identification of privileged PARKIN targets and the preferred sites of modification. Several lysines within the same target protein were modified with similar kinetics, suggesting multi-ubiquitylated proteoforms could be important for proper mitophagic flux. PRM-MS also offers a highly sensitive method for quantifying ubiquitin chain linkages [69], and in neurons K63-linked chains appear to be the preferred signal for PARKIN-dependent mitophagy [70]. Thus, the combination of heavy reference peptides and PRM-MS provides a powerful means to determine the absolute amounts of different proteoforms even though the precise nature can only be inferred.
Selected ion monitoring has also enabled the characterization of tau proteoforms. Tau is a microtubule-associated protein predominately expressed in neurons [71,72]. Misregulation leads to the formation of aggregates, which are the hallmark of a class of neurodegenerative diseases referred to as tauopathies [73]. Hyperphosphorylation of tau is considered an early event in the aggregation process [74]. A number of other PTMs, including ubiquitylation, have been implicated in disease progression. To define the tau PTMs relevant to tauopathies, specifically Alzheimer’s disease (AD), heavy full-length tau protein was spiked into samples containing postmortem brain tissue prior to proteolytic digestion [75]. The heavy reference peptides facilitate quantitation of the unmodified peptides as opposed to the modified variants [76]. In principle, the abundance of the unmodified peptide will decrease by an amount proportional to the modified form(s). MS-based detection of the unmodified peptide must therefore be highly reproducible, which is why a targeted ion monitoring approach must be employed. Coupling this strategy with statistical analyses uncovered common features in AD patients and identified combinations of PTMs that reflect disease progression. Phosphorylation of tau within the proline-rich region occurs at the earliest stages of disease. Ubiquitylation and acetylation of sites in the microtubule-binding domain occur later and are strongly associated with formation of high molecular weight, detergent-insoluble forms of tau that have prion-like behavior. These results together with cryo-EM data of tau suggest that distinct combinations of ubiquitylation and acetylation contribute to the stability of different filament structures [77]. Whether ubiquitin chains or other ubiquitin modifications play a role in filament formation and thus disease progression remains unclear.
Insights into Chain Architecture Using Middle-Down MS
Although the quantitative information afforded by BUP is unparalleled, the loss of connectivity between modified peptides means the structure of different proteoforms can only be inferred. The inference problem is particularly acute for ubiquitin chains, as insight into chain length and the extent of branching is completely lost.
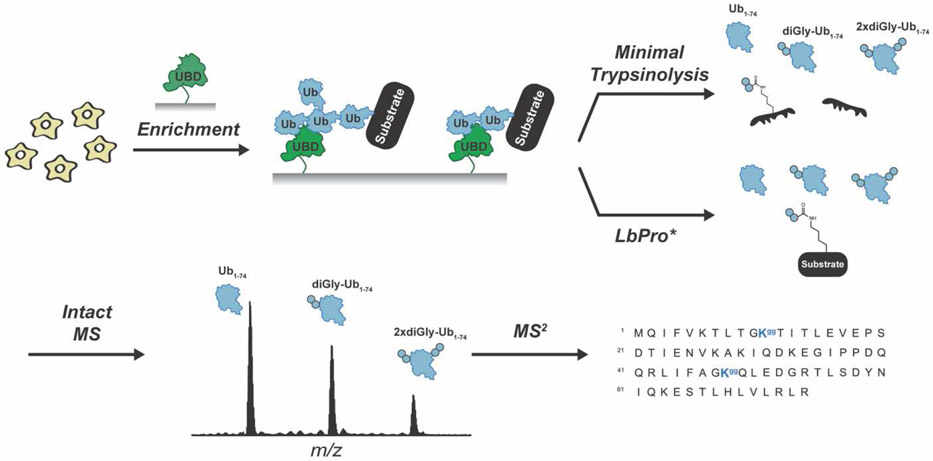
Branched chains have been implicated in a number of pathways ranging from the cell cycle to immune signaling [41,78-84]. Branched chains are composed of at least two or more linkages and have individual subunits that are modified with two or more ubiquitin molecules. When multiple modifications occur on non-adjacent lysines, BUP is unable to distinguish between branched and unbranched chains. Middle-down proteomics (MDP), however, facilitates the detection of branched chains by exploiting the recalcitrance of ubiquitin to tryptic cleavage. Under native conditions, the peptide bond between R74 and G75 of ubiquitin is the most susceptible to trypsinolysis [85]. With ubiquitin largely intact, distinct regions of a ubiquitin chain can be detected by MS: the caps (Ub1-74), the unbranched portion (diGly-Ub1-74), and the branchpoints (2xdiGly-Ub1-74) (Figure 3). Early studies showed that the ratio of Ub1-74 to diGly-Ub1-74 could be used to deduce the length of isolated chains [86]. By coupling MDP with electron-transfer dissociation (ETD) MS2 analysis, subsequent studies demonstrated that the extent to which different E3s assemble branched chains could be assessed and information on the linkages present in branchpoints could be obtained [87]. MDP can also be used to measure the abundance of branchpoints in cell extracts after enrichment of ubiquitin chains by immobilized ubiquitin-binding domains (UBDs) [88]. By availing a K11 linkage-specific antibody, for example, K11/K48 branched chains were found to represent ~4% of the total population of the enriched ubiquitin species in G2/M synchronized cells [89].
Figure 3. Middle-down MS analysis of ubiquitin chain architecture.
Ubiquitylated proteins are digested with trypsin under native conditions or treated with LbPro* to generate three distinct ubiquitin variants: Ub1-74, diGly-Ub1-74, and 2xdiGly-Ub1-74. The resulting variants are analyzed by MS1 and MS2 to determine the extent of chain branching and the modification sites.
MDP has also played an instrumental role in identifying UCHL5/UCH37 as a branched chain-selective deubiquitinase. UCH37 was first discovered as a deubiquitinase transiently associated with the 19S regulatory particle of the 26S proteasome [90]. For a long time, the premise was that the proteasomal subunit RPN13 stimulates the ability of UCH37 to trim the distal end of K48 chains, thereby rescuing proteins prematurely sent to the proteasome for degradation. However, it has been hard to reconcile this model with data showing that UCH37 cleaves homotypic K48 chains at very slow rates even in the presence of RPN13 and the proteasome [91-94]. A clue that UCH37 might be targeting K48 linkages in heterotypic chains was provided by quantitative analysis of chain linkages using PRM-MS [95]. It was MDP, however, that allowed for direct visualization of UCH37’s ability to remove K48 branchpoints and regulate proteasomal degradation through this activity.
A major limitation with trypsin-mediated MDP, however, is the need to carefully optimize the experimental conditions to avoid unwanted digestion. Another issue is that minimal trypsinolysis results in cleavage of the ubiquitin-modified protein. Thus, information is forfeited on other PTMs that may promote or be a consequence of ubiquitylation. In a groundbreaking study, the leader protease (LbPro*) of foot-and-mouth disease virus was engineered to specifically recognize and cleave ubiquitin after R74 [96]. With this protease, ubiquitin is ‘clipped’ at its C-terminus leaving behind the signature Kgg motif on the modified protein. The same ubiquitin variants—Ub1-74, phospho-Ub1-74, diGly-Ub1-74, phospho-diGly-Ub1-74, and 2xdiGly-Ub1-74—that can be detected by trypsin-mediated MDP are identified with ‘Ub-clipping’, but the protein attached to the chain or mono-ubiquitin is left intact (Figure 3). Ub-clipping has recently been used to investigate the phospho-ubiquitin proteoforms produced during PINK1/PARKIN-dependent mitophagic signaling [70]. The majority of ubiquitin phosphorylation was found to occur on monomeric ubiquitin or the caps of chains.
The Challenge of Chain Length
While advances in BUP and MDP enable the characterization and quantitation of ubiquitin chain linkages, ubiquitin PTMs, and the degree of branching in complex biological samples, the analysis of chain length has largely relied on gel mobility. As BUP experiments have shown, many substrates have multiple ubiquitylation sites making it difficult to discern whether the ‘ubiquitin smear’ on a gel or immunoblot is due to multi-monoubiquitylation or heterogeneity in chain length. Chains with the same number of ubiquitin molecules can also have different gel mobilities due to linkage type and branching. Thus, we have largely been left in the dark regarding the functional relevance of chain length.
The need for characterization methods is further underscored by mounting evidence suggesting that chain length controls the dynamics of ubiquitin-dependent pathways. A classic example is the in vitro study from the Pickart lab showing a K48 tetraubiquitin chain provides the minimal proteasome-targeting signal for the globular protein dihydrofolate reductase [97]. More recently, chain length has emerged as an important factor for substrate selection by the ubiquitin-dependent unfoldase Cdc48/p97/VCP [98-100], the deubiquitinase MINDY [101], and the ubiquitin-directed endoprotease DDI2/Ddi1 [102,103]. In each of these cases, longer chains bearing more than five subunits are preferentially recognized, suggesting there is certain threshold that must be achieved to trigger downstream events. Methods for characterizing chain length could help us understand how often this threshold is met, the degree to which it depends on the nature of the chain and substrate, and the cellular processes that rely on it.
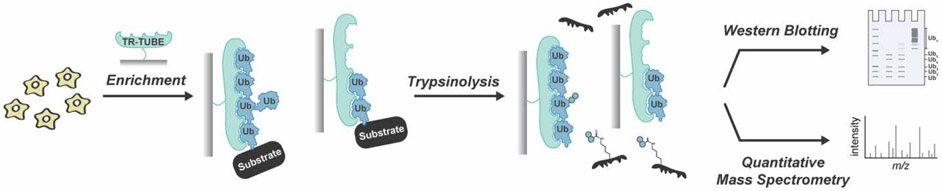
For the purposes of characterization, access to an enzyme that could remove chains en bloc from a substrate would be ideal, as chains would be left intact. If the same enzyme also possessed cleavage specificity for R74 of ubiquitin, then ubiquitylation sites along with other PTMs could be mapped on a substrate protein. In the absence of such a reagent, other approaches have been devised. In a method referred to as Ub-ProT (ubiquitin chain protection from trypsinization), trypsin is combined with a trypsin-resistant, multivalent display of ubiquitin-binding domains to release intact chains from substrates [104] (Figure 4). The lengths are then analyzed using a gel-based assay and the absolute abundance of different linkage type are assessed using BUP. In yeast, substrate-anchored ubiquitin chains were mainly found in the range of monomer to heptamer, with Cdc48 regulating chain length. The challenge is that not all ubiquitin chain linkages are fully protected from trypsinolysis and branched chains seem to be more prone to digestion than homotypic chains.
Figure 4. Ubiquitin chain Protection from Trypsinization (Ub-ProT).
Trypsin-Resistant Tandem Ubiquitin Binding Entities (TR-TUBEs) enable the isolation of polyubiquitinated proteins from cells. Following trypsinolysis, the modified substrate is digested but the chains are left intact. Western blotting and BUP can then be used for characterization.
Top-Down Proteomic Analyses
As the only MS-based platform that is not limited by the protein inference problem, top-down proteomics (TDP) should be well-suited for the analysis of proteoforms bearing ubiquitin chains of different lengths, linkages, and architectures. Proteoforms are left completely intact prior to mass analysis, and identification and localization of chemical modifications are determined by MS2 analysis of fragment ions [105]. The major challenge is that with increasing molecular weight the signal-to-noise (S/N) ratio decreases exponentially [106]. The low abundance of ubiquitylated proteins further compounds the problem. Thus, while TDP has been used to characterize well-defined ubiquitylated proteins [107,108], the success of TDP in the analysis of complex, heterogenous mixtures has not been realized. With advances in sample preparation, separation, and instrumentation [109], it will eventually be possible to capture the entire landscape of ubiquitylation events on a protein of interest and investigate the interplay with other PTMs. Indeed, by combining LbPro* with the enrichment of a protein of interest, the characterization of modification crosstalk using TDP is already within reach, and it will be exciting to see future applications of this strategy. The advent of new proteolytic tools that differentiate between ubiquitin-ubiquitin and ubiquitin-target protein linkages would also accelerate TDP analyses. In parallel with modification crosstalk, chain length distribution could be assessed while also avoiding complications associated with the low S/N ratios of polyubiquitylated proteins.
Conclusions and Future Directions
Complex cellular activities are made possible by the diversity of proteoforms that arise from splice variants and PTMs. Even within the same family of proteoforms, distinct combinations of PTMs influence both structure and function, enabling highly sophisticated cellular information processing. The complexity of PTMs like ubiquitylation, however, make it particularly challenging to identify and quantify proteoforms to understand function. Despite these obstacles, mass spectrometry has proven to be an invaluable tool in the study of ubiquitin-containing proteoforms. When combined with heavy isotope-labeled peptides and selective ion monitoring techniques such as PRM or SRM, BUP informs on the site-specificity, abundance, stoichiometry, and kinetics, but correlations between the target protein and the precise nature of the ubiquitin modification are completely lost. MDP offers insights into the architecture of chains, but chain length must be inferred. Since the ultimate goal is to characterize all ubiquitylated proteoforms within a given family and determine which proteoform(s) is/are responsible for a particular biological response, information on all sources of variability must be retained. Thus, TDP will play an increasingly important role in the characterization of ubiquitin proteoforms.
Acknowledgements
This research was supported by the National Institutes of Health Grant R01GM110543.
Footnotes
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Walsh C: Posttranslational Modification of Proteins: Expanding Nature’s Inventory. Roberts and Company Publishers, Englewood, CO; 2006. [Google Scholar]
- 2.Smith LL, Kelleher NL: Proteoforms as the next proteomics currency. Science (80-) 2018, doi: 10.1126/science.aat0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabakaran S, Lippens G, Steen H, Gunawardena J: Post-translational modification: Nature’s escape from genetic imprisonment and the basis for dynamic information encoding. Wiley Interdiscip Rev Syst Biol Med 2012, 4:565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burroughs AM, Balaji S, Iyer LM, Aravind L: Small but versatile: The extraordinary functional and structural diversity of the β-grasp fold. Biol Direct 2007, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman BA, Wade Harper J: Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat Rev Mol Cell Biol 2009, 10:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Y, Rape M: Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol 2009, 10:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshaies RJ, Joazeiro CAP: RING Domain E3 Ubiquitin Ligases. Annu Rev Biochem 2009, 78:399–434. [DOI] [PubMed] [Google Scholar]
- 8.Smit JJ, Sixma TK: RBR E3-ligases at work. EMBO Rep 2014, 15:142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buetow L, Huang DT: Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol 2016, 17:626–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deol KK, Lorenz S, Strieter ER: Enzymatic Logic of Ubiquitin Chain Assembly. Front Physiol 2019, 10: 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yau R, Rape M: The increasing complexity of the ubiquitin code. Nat Cell Biol 2016, 18:579–586. [DOI] [PubMed] [Google Scholar]
- 12.Swatek KN, Komander D: Ubiquitin modifications. Cell Res 2016, 26:399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaney DL, Beltrao P, Starita L, Guo A, Rush J, Fields S, Krogan NJ, Villén J: Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat Methods 2013, 10:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. : Global Survey of Phosphotyrosine Signaling Identifies Oncogenic Kinases in Lung Cancer. Cell 2007, 131:1190–1203. [DOI] [PubMed] [Google Scholar]
- 15.Ohtake F, Saeki Y, Sakamoto K, Ohtake K, Nishikawa H, Tsuchiya H, Ohta T, Tanaka K, Kanno J: Ubiquitin acetylation inhibits polyubiquitin chain elongation. EMBO Rep 2015, 16:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhogaraju S, Kalayil S, Liu Y, Bonn F, Colby T, Matic I, Dikic I: Phosphoribosylation of Ubiquitin Promotes Serine Ubiquitination and Impairs Conventional Ubiquitination. Cell 2016, 167:1636–1649.e13. [DOI] [PubMed] [Google Scholar]
- 17.Qiu J, Sheedlo MJ, Yu K, Tan Y, Nakayasu ES, Das C, Liu X, Luo ZQ: Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 2016, 533:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh E, Akopian D, Rape M: Principles of Ubiquitin-Dependent Signaling. Annu Rev Cell Dev Biol 2018, 34: 137–162. [DOI] [PubMed] [Google Scholar]
- 19.Harper JW, Bennett EJ: Proteome Complexity and the Forces that Drive Proteome Imbalance. Nature 2016, 537: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minguez P, Letunic I, Parca L, Bork P: PTMcode: A database of known and predicted functional associations between post-translational modifications in proteins. Nucleic Acids Res 2013, 41: D306–D311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan RM, Kupai A, Rothbart SB: Chromatin Regulation through Ubiquitin and Ubiquitin-like Histone Modifications. Trends Biochem Sci 2020, doi: 10.1016/j.tibs.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulzele A, Bennett EJ: Ubiquitin diGly Proteomics as an Approach to Identify and Quantify the Ubiquitin-Modified Proteome. Methods Mol Biol 2018, 1844: 363–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, Li H, Wang X, Ullah K, Xu G: Proteomic Approaches for the Profiling of Ubiquitylation Events and their Applications in Drug Discovery. J Proteomics 2021, 231: 103996. [DOI] [PubMed] [Google Scholar]
- 24. Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP: A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 2003, 21:921–926. •• Seminal study showing that the diGly remnant approach can be used to identify ubiquitylation sites.
- 25.Meierhofer D, Wang X, Huang L, Kaiser P: Quantitative analysis of global ubiquitination in HeLa cells by mass spectrometry. J Proteome Res 2008, 7:4566–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu G, Paige JS, Jaffrey SR: Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat Biotechnol 2010, 28:868–873. • First report describing the use of a Kgg-specific antibody to enrich peptides carrying the diGly signature motif.
- 27. Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. : Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 2011, 44:325–340. •• First comprehensive study using the Kgg-specific antibody to identify ubiquitylation sites on a proteome-wide scale.
- 28.Udeshi ND, Svinkina T, Mertins P, Kuhn E, Mani DR, Qiao JW, Carr SA: Refined preparation and use of anti-diglycine remnant (k-ε-gg) antibody enables routine quantification of 10,000s of ubiquitination sites in single proteomics experiments. Mol Cell Proteomics 2013, 12:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner SA, Beli P, Weinert BT, Schölz C, Kelstrup CD, Young C, Nielsen ML, Olsen JV., Brakebusch C, Choudhary C: Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol Cell Proteomics 2012, 11:1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akimov V, Barrio-Hernandez I, Hansen SVF, Hallenborg P, Pedersen A-K, Bekker-Jensen DB, Puglia M, Christensen SDK, Vanselow JT, Nielsen MM, et al. : UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat Struct Mol Biol 2018, 25: 631–640. • The authors describe the development of an antibody that recognizes the 13 amino acids at the C-terminus of ubiquitin, which allows for ubiquitylation to be distinguished from other modifications involving ubiquitin-like proteins, e.g., NEDD8 and ISG15.
- 31.Udeshi ND, Mani DC, Satpathy S, Fereshetian S, Gasser JA, Svinkina T, Olive ME, Ebert BL, Mertins P, Carr SA: Rapid and deep-scale ubiquitylation profiling for biology and translational research. Nat Commun 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen F, Tanzer M, Brüning F, Bludau I, Schulman B, Robles M, Karayel O, Mann M: Data-independent acquisition method for ubiquitinome analysis reveals regulation of circadian biology. Nat Commun 2021, 12: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steger M, Ihmor P, Backman M, Müller S, Daub H: Deep ubiquitination site profiling by single-shot data-independent acquisition mass spectrometry. bioRxiv 2020, doi: 10.1101/2020.07.23.218651. [DOI] [Google Scholar]
- 34.Povlsen LK, Beli P, Wagner SA, Poulsen SL, Sylvestersen KB, Poulsen JW, Nielsen ML, Bekker-Jensen S, Mailand N, Choudhary C: Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass. Nat Cell Biol 2012, 14:1089–1098. [DOI] [PubMed] [Google Scholar]
- 35.Elia AEH, Boardman AP, Wang DC, Huttlin EL, Everley RA, Dephoure N, Zhou C, Koren I, Gygi SP, Elledge SJ: Quantitative Proteomic Atlas of Ubiquitination and Acetylation in the DNA Damage Response. Mol Cell 2015, 59:867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner SA, Satpathy S, Beli P, Choudhary C: SPATA 2 links CYLD to the TNF-α receptor signaling complex and modulates the receptor signaling outcomes. EMBO J 2016, 35:1868–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, Komuves L, French DM, Ferrando RE, Lam C, Compaan D, Yu C, Bosanac I, Hymowitz SG, Kelley RF, Dixit VM: Ubiquitin Chain Editing Revealed by Polyubiquitin Linkage-Specific Antibodies. Cell 2008, 134: 668–678. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto ML, Wickliffe KE, Dong KC, Yu C, Bosanac I, Bustos D, Phu L, Kirkpatrick DS, Hymowitz SG, Rape M, Kelley RF, Dixit VM: K11-Linked Polyubiquitination in Cell Cycle Control Revealed by a K11 Linkage-Specific Antibody. Mol Cell 2010, 39: 477–484. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto ML, Dong KC, Yu C, Phu L, Gao X, Hannoush RN, Hymowitz SG, Kirkpatrick DS, Dixit VM, Kelley RF: Engineering and Structural Characterization of a Linear Polyubiquitin-Specific Antibody. J Mol Biol 2012, 418: 134–144. [DOI] [PubMed] [Google Scholar]
- 40.Emmerich CH, Cohen P: Optimising Methods for the Preservation, Capture and Identification of Ubiquitin Chains and Ubiquitylated Proteins by Immunoblotting. Biochem Biophys Res Commun 2015, 466, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yau RG, Doerner K, Castellanos ER, Haakonsen DL, Werner A, Wang N, Yang XW, Martinez-Martin N, Matsumoto ML, Dixit VM, et al. : Assembly and Function of Heterotypic Ubiquitin Chains in Cell-Cycle and Protein Quality Control. Cell 2017, 171:918–933. •• The authors report on the development and utility of a bispecific antibody targeting K11/K48 branched ubiquitin chains. Branched chains are not only produced during the cell cycle but also during proteotoxic stress, suggesting that these unique chains might have distinct functions relative to their linear counterparts.
- 42.Michel MA, Swatek KN, Hospenthal MK, Komander D: Ubiquitin Linkage-Specific Affimers Reveal Insights into K6-Linked Ubiquitin Signaling. Mol Cell 2017, 68: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gersch M, Gladkova C, Schubert AF, Michel MA, Maslen S, Komander D: Mechanism and Regulation of the Lys6-Selective Deubiquitinase USP30. Nat Struct Mol Biol 2017, 24: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva GM, Vogel C: Mass Spectrometry Analysis of K63-ubiquitinated Targets in Response to Oxidative Stress. Data Brief 2015, 4: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopitz-Otsoa F, Rodriguez-Suarez E, Aillet F, Casado-Vela J, Lang V, Matthiesen R, Elortza F, Rodriguez MS: Integrative Analysis of the Ubiquitin Proteome Isolated Using Tandem Ubiquitin Binding Entities (TUBEs). J Proteomics 2012, 75: 2998–3014. [DOI] [PubMed] [Google Scholar]
- 46.Silva GM, Finley D, Vogel C: K63 Polyubiquitination is a New Modulator of the Oxidative Stress Response. Nat Struct Mol Biol 2015, 22: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattern M, Sutherland J, Kadimisetty K, Barrio R, Rodriguez MS: Using Ubiquitin Binders to Decipher the Ubiquitin Code. Trends Biochem Sci 2019, 44: 599–615. [DOI] [PubMed] [Google Scholar]
- 48.Kliza K, Husnjak K: Resolving the Complexity of Ubiquitin Networks. Front Mol Biosci 2020, 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ordureau A, Münch C, Harper JW: Quantifying Ubiquitin Signaling. Mol Cell 2015, 58: 660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harper JW, Ordureau A, Heo J-M: Building and decoding ubiquitin chains for mitophagy. Nat Rev Mol Cell Biol 2018, 19:93–108. [DOI] [PubMed] [Google Scholar]
- 51.Lazarou M, Jin SM, Kane LA, Youle RJ: Role of PINK1 Binding to the TOM Complex and Alternate Intracellular Membranes in Recruitment and Activation of the E3 Ligase Parkin. Dev Cell 2012, 22:320–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ: PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 2010, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamano K, Youle RJ: PINK1 is degraded through the N-end rule pathway. Autophagy 2013, 9:1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, Youle RJ: PINK1 phosphorylates ubiquitin to activate parkin E3 ubiquitin ligase activity. J Cell Biol 2014, 205:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MMK: Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J 2014, 460:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazlauskaite A, Martínez-Torres RJ, Wilkie S, Kumar A, Peltier J, Gonzalez A, Johnson C, Zhang J, Hope AG, Peggie M, et al. : Binding to serine 65-phosphorylated ubiquitin primes Parkin for optimal PINK 1-dependent phosphorylation and activation . EMBO Rep 2015, 16:939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, et al. : Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 2014, 510:162–166. [DOI] [PubMed] [Google Scholar]
- 58.Okatsu K, Kimura M, Oka T, Tanaka K, Matsuda N: Unconventional PINK1 localization to the outer membrane of depolarized mitochondria drives Parkin recruitment. J Cell Sci 2015, 128:964–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, et al. : Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 2014, 56:360–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ordureau A, Heo J-M, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA, Harper JW: Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci 2015, 112:6637–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wauer T, Simicek M, Schubert A, Komander D: Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature 2015, 524: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gladkova C, Maslen SL, Skehel JM, Komander D: Mechanism of parkin activation by PINK1. Nature 2018, 559: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sauvé V, Sung G, Soya N, Kozlov G, Blaimschein N, Miotto LS, Trempe J-F, Lukacs GL, Gehring K: Mechanism of parkin activation by phosphorylation. Nat Struct Mol Biol 2018, 25:623–630. [DOI] [PubMed] [Google Scholar]
- 64.Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M: The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 2014, 510:370–375. [DOI] [PubMed] [Google Scholar]
- 65.Rose CM, Isasa M, Ordureau A, Prado MA, Beausoleil SA, Jedrychowski MP, Finley DJ, Harper JW, Gygi SP: Highly Multiplexed Quantitative Mass Spectrometry Analysis of Ubiquitylomes. Cell Syst 2016, 3:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, Harper JW: Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013, 496:372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW: The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol Cell 2015, 60:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ordureau A, Paulo JA, Zhang W, Ahfeldt T, Zhang J, Cohn EF, Hou Z, Heo J-M, Rubin LL, Sidhu SS, et al. : Dynamics of PARKIN-Dependent Mitochondrial Ubiquitylation in Induced Neurons and Model Systems Revealed by Digital Snapshot Proteomics. Mol Cell 2018, 70:211–227. •• In this tour de force study, the authors use targeted BUP to investigate the abundance, stoichiometry, site-specificity, and kinetics of PARKIN-dependent ubiquitylation during mitophagy. Important modulators are identified and the stoichiometry of ubiquitin phosphorylation is found to be crucial for controlling mitophagic flux.
- 69.Ohtake F, Tsuchiya H, Tanaka K, Saeki Y: Methods to measure ubiquitin chain length and linkage. In Methods in Enzymology. Academic Press Inc.; 2019:105–133. [DOI] [PubMed] [Google Scholar]
- 70.Ordureau A, Paulo JA, Wan Q, Komander D, Wade J, Correspondence H: Global Landscape and Dynamics of Parkin and USP30-Dependent Ubiquitylomes in iNeurons during Mitophagic Signaling. Mol Cell 2020, 77: 1124–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dixit R, Ross JL, Goldman YE, Holzbaur ELF: Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319:1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avila J, Lucas JJ, Perez M, Hernandez F: Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol Rev 2004, 84:361–384. [DOI] [PubMed] [Google Scholar]
- 73.Ballatore C, Lee VMY, Trojanowski JQ: Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 2007, 8:663–672. [DOI] [PubMed] [Google Scholar]
- 74.Mandelkow EM, Mandelkow E: Biochemistry and cell biology of Tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Biol 2011, 3:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, Fatou B, Guise AJ, Cheng L, Takeda S, et al. : Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183: 1699–1713. •• Using targeted BUP strategies and brain tissue samples, the authors provide a comprehensive analysis of tau proteoforms relevant to Alzheimer's disease. Phosphorylation, ubiquitylation, and acetylation occur with high occupancy and frequency in Alzheimer's disease.The data support a model in which significant crosstalk occurs between the different modifications. Hyperphosphorylation promotes the formation of toxic tau fibrils by neutralizing positive charges. Ubiquitylation and acetylation then provide additional mechanisms for charge neutralization to further stabilize tau filaments.
- 76.Mair W, Muntel J, Tepper K, Tang S, Biernat J, Seeley WW, Kosik KS, Mandelkow E, Steen H, Steen JA: FLEXITau: Quantifying Post-translational Modifications of Tau Protein in Vitro and in Human Disease. Anal Chem 2016, 88:3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arakhamia T, Lee CE, Carlomagno Y, Duong DM, Kundinger SR, Wang K, Williams D, DeTure M, Dickson DW, Cook CN, et al. : Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 2020, 180:633–644.e12. •• The authors provide a detailed account of the structures of tau filaments isolated from human brain tissue. In corroboration with the Wesseling et. al. study (ref. 60), the data demonstrate significant crosstalk between different PTMs. Cryo-EM structures show that ubiquitylation at distinct sites of tau results in the formation of different fibril structures.
- 78. Meyer HJ, Rape M: Enhanced protein degradation by branched ubiquitin chains. Cell 2014, 157:910–921. • The authors show that the anaphase promoting complex (APC) decorates substrates with K11/K48-linked branched ubiquitin chains to promote cell cycle progression. This is the first report demonstrating the formation of branched chains in a cellular pathway.
- 79.Samant RS, Livingston CM, Sontag EM, Frydman J: Distinct Proteostasis Circuits Cooperate in Nuclear and Cytoplasmic Protein Quality Control. Nature 2018, 563: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ohtake F, Tsuchiya H, Saeki Y, Tanaka K: K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains. Proc Natl Acad Sci 2018, 115:E1401–E1408. • The authors show that the introduction of K48 branchpoints alters the function of a preexisting K63 chain, transforming the modification into a proteasome-targeting signal.
- 81.Liu C, Liu W, Ye Y, Li W: Ufd2p synthesizes branched ubiquitin chains to promote the degradation of substrates modified with atypical chains. Nat Commun 2017, 8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohtake F, Saeki Y, Ishido S, Kanno J, Tanaka K: The K48-K63 Branched Ubiquitin Chain Regulates NF-kB Signaling. Mol Cell 2016, 64:251–266. [DOI] [PubMed] [Google Scholar]
- 83.Emmerich CH, Ordureau A, Strickson S, Arthur JSC, Pedrioli PGA, Komander D, Cohen P: Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A 2013, 110:15247–15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, Popovych N, Helgason E, Schoeffler A, Jeet S, et al. : Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature 2015, 528:370–375. [DOI] [PubMed] [Google Scholar]
- 85.Wilkinson KD, Cox MJ, Mayer AN, Frey T: Synthesis and Characterization of Ubiquitin Ethyl Ester, a New Substrate for Ubiquitin Carboxyl-Terminal Hydrolase. Biochemistry 1986, 25: 6644–6649. [DOI] [PubMed] [Google Scholar]
- 86.Xu P, Peng J: Characterization of polyubiquitin chain structure by middle-down mass spectrometry. Anal Chem 2008, 80:3438–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Valkevich EM, Sanchez NA, Ge Y, Strieter ER: Middle-Down mass spectrometry enables characterization of branched ubiquitin chains. Biochemistry 2014, 53: 4979–4989. • First study showing that middle-down MS can be used to characterize branched ubiquitin chains.
- 88.Crowe SO, Rana ASJB, Deol KK, Ge Y, Strieter ER: Ubiquitin Chain Enrichment Middle-Down Mass Spectrometry Enables Characterization of Branched Ubiquitin Chains in Cellulo. Anal Chem 2017, 89: 4428–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rana ASJB, Ge Y, Strieter ER: Ubiquitin Chain Enrichment Middle-Down Mass Spectrometry (UbiChEM-MS) Reveals Cell-Cycle Dependent Formation of Lys11/Lys48 Branched Ubiquitin Chains. J Proteome Res 2017, 16: 3363–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lam YA, Xu W, DeMartino GN, Cohen RE: Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 1997, 385:737–40. [DOI] [PubMed] [Google Scholar]
- 91.Hamazaki J, Iemura SI, Natsume T, Yashiroda H, Tanaka K, Murata S: A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J 2006, 25:4524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qiu XB, Ouyang SY, Li CJ, Miao S, Wang L, Goldberg AL: hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J 2006, 25:5742–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK, Washburn MP, Conaway RC, Conaway JW, Cohen RE: Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol 2006, 8:994–1002. [DOI] [PubMed] [Google Scholar]
- 94.Jørgensen JP, Lauridsen AM, Kristensen P, Dissing K, Johnsen AH, Hendil KB, Hartmann-Petersen R: Adrm1, a Putative Cell Adhesion Regulating Protein, is a Novel Proteasome-associated Factor. J Mol Biol 2006, 360:1043–1052. [DOI] [PubMed] [Google Scholar]
- 95. Deol KK, Crowe SO, Du J, Bisbee HA, Guenette RG, Strieter ER: Proteasome-Bound UCH37/UCHL5 Debranches Ubiquitin Chains to Promote Degradation. Mol Cell 2020, 80:796–809. •• The authors highlight the power of middle-down MS in identifying UCH37/UCHL5 as a DUB with specificity for K48 branchpoints. They show that the unique debranching activity of UCH37 is important for generating substrates suitable for proteosomal degradation.
- 96. Swatek KN, Usher JL, Kueck AF, Gladkova C, Mevissen TET, Pruneda JN, Skern T, Komander D: Insights into ubiquitin chain architecture using Ub-clipping. Nature 2019, 572: 533–537. •• In a major advance, the authors engineer the viral protease LbPro* to cleave between R74 and G75 of ubiquitin. Ubiquitin variants corresponding to different regions of a ubiquitin chain–the caps, the unbranched portion, and branchpoints–can all be identified by intact MS. Moreover, unlike trypsin, LbPro* does not cleave the ubiquitylated substrate, allowing for characterization of potential crosstalk between different PTMs.
- 97.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM: Recognition of the polyubiquitin proteolytic signal. EMBO J 2000, 19:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bodnar NO, Rapoport TA: Molecular Mechanism of Substrate Processing by the Cdc48 ATPase Complex. Cell 2017, 169: 722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blythe EE, Olson KC, Chau V, Deshaies RJ: Ubiquitin- and ATP-Dependent Unfoldase Activity of P97/VCP•NPLOC4•UFD1L is Enhanced by a Mutation that Causes Multisystem Proteinopathy. Proc Natl Acad Sci USA 2017, 114: E4380–E4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deegan TD, Mukherjee PP, Fujisawa R, Polo Rivera C, Labib K: CMG Helicase Disassembly is Controlled by Replication Fork DNA, Replisome Components and a Ubiquitin Threshold. eLife 2020, 9: e60371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdul Rehman SA, Kristariyanto YA, Choi SY, Nkosi PJ, Weidlich S, Labib K, Hofmann K, Kulathu Y: MINDY-1 is a Member of an Evolutionarily Conserved and Structurally Distinct New Family of Deubiquitinating Enzymes. Mol Cell 2016, 63: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dirac-Svejstrup AB, Walker J, Faull P, Encheva V, Akimov V, Puglia M, Perkins D, Kümper S, Hunjan SS, Blagoev B, Snijders AP, Powell DJ, Svejstrup JQ: DDI2 is a Ubiquitin-Directed Endoprotease Responsible for Cleavage of Transcription Factor NRF1. Mol Cell 2020, 79: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yip MCJ, Bodnar NO, Rapoport TA: Ddi1 is a Ubiquitin-Dependent Protease. Proc Natl Acad Sci USA 2020, 117: 7776–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tsuchiya H, Burana D, Ohtake F, Arai N, Kaiho A, Komada M, Tanaka K, Saeki Y: Ub-ProT reveals global length and composition of protein ubiquitylation in cells. Nat Commun 2018, 9: 524. •• The authors use a trypsin-resistant form of tandem ubiquitin-binding entities (TUBEs) to protect chains from cleavage during trypsinolysis. With chains left intact, their size can be assessed by gel electrophoresis. When combined with MS analysis, modification site on the protein substrate can also be identified.
- 105.Toby TK, Fornelli L, Kelleher NL: Progress in Top-Down Proteomics and the Analysis of Proteoforms. Annu Rev Anal Chem 2016, 9:499–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Compton PD, Zamdborg L, Thomas PM, Kelleher NL: On the Scalability and Requirements of Whole Protein Mass Spectrometry. Anal Chem 2011, 83: 6868–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Geis-Asteggiante L, Lee AE, Fenselau C: Analysis of the topology of ubiquitin chains. In Methods in Enzymology. Academic Press Inc.; 2019:323–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cannon JR, Martinez-Fonts K, Robotham SA, Matouschek A, Brodbelt JS: Top-Down 193-nm ultraviolet photodissociation mass spectrometry for simultaneous determination of polyubiquitin chain length and topology. Anal Chem 2015, 87:1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brown KA, Melby JA, Roberts DS, Ge Y: Top-Down Proteomics: Challenges, Innovations, and Applications in Basic and Clinical Research. Expert Rev Proteomics 2020, 17: 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]