Abstract
Background/Objectives:
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) have been suggested as obesogens but epidemiologic evidence is limited. We examined associations of serum PFAS concentrations with longitudinal trajectories of weight, waist circumference (WC), fat mass and proportion fat in midlife women.
Subjects/Methods:
This study included 1,381 midlife women, with a total of 15,000 repeated measures from the multi-racial/ethnic Study of Women’s Health Across the Nation between 1999 and 2018. The average follow-up was 14.9 (range: 0–18.6) years. Body size (objectively measured weight and WC) and body composition from dual-energy X-ray absorptiometry were assessed at near-annual visits. Linear mixed models with piecewise linear splines were utilized to model non-linear trajectories of body size and composition.
Results:
After multivariable adjustment, PFAS concentrations were positively associated with weight, WC, fat mass, and proportion fat at baseline and during follow-up. Comparing the highest to the lowest tertiles of PFAS concentrations, adjusted geometric mean weight was 73.9 kg vs. 69.6 kg for PFOS (P<0.0001), and 74.0 vs. 69.4 kg for linear PFOA (P<0.0001) at baseline. Women with the highest tertile of PFOS had an annual increase rate of 0.33% (95% CI: 0.27%, 0.40%) in weight, compared to the lowest tertile with 0.10% (95% CI: 0.04%, 0.17%) (P<0.0001). PFOS was also significantly related to higher increase rates in WC (difference=0.12% per year, P=0.002) and fat mass (difference=0.25% per year, P=0.0002). EtFOSAA and MeFOSAA showed similar effects to PFOS. Although PFHxS was not related to body size or fat at baseline, PFHxS was significantly associated with accelerated increases in weight (P<0.0001), WC (P=0.003), fat mass (P<0.0001), and proportion fat (P=0.0009). No significant results were found for PFNA.
Conclusions:
Certain PFAS were positively associated with greater body size and body fat, and higher rates of change over time. PFAS may be an underappreciated contributing factor to obesity risk.
INTRODUCTION
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are used to manufacture everyday products such as nonstick cookware, food packaging materials, waterproof clothing, and furniture and carpets.1 PFAS are widely present in ground water and drinking water; it has been estimated that more than 200 million United States residents consume drinking water contaminated by PFAS.2 Due to the ubiquity of PFAS exposure, as nearly all persons 12 years and older have detectable concentrations of at least one PFAS in their blood.3 To compound the problem of exposure is the acknowledgement that commonly used PFAS have relatively long elimination half-lives, of 2–9 years depending on the compound.4,5 Therefore, not only are most individuals exposed to PFAS, but some PFAS can remain in the body for many years.
PFAS exhibit both lipophobic and hydrophobic properties. Due to their structural similarities to fatty acids, PFAS - especially perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) - have been recognized as potential endocrine-disrupting chemicals (EDCs).6,7 PFAS exposure may alter endocrine regulation and modulate metabolic pathways, possibly through the activation of multiple nuclear receptors such as peroxisome proliferator-activated receptors (PPARs).8,9 PPARs promote adipogenesis and have a significant impact on inflammation and lipid metabolism.10
The associations between PFAS and body weight have been explored in adults. Although some studies observed significant relationships between PFAS and increased weight and risks of overweight and obesity,11–14 the remaining studies did not observe an association.15,16 Most prospective cohort studies were restricted to overweight and obese persons, or those with elevated glucose concentrations.13,14 Notably, studies that analyzed their findings by sex observed a more pronounced effect in women.12,14 Given that the hormonal changes across the menopausal transition (MT) substantially contribute to increased abdominal obesity17 and gains in fat mass18, and that PFAS can eliminate from the body through menstrual bleeding,4,19 women’s midlife years may represent a special period of vulnerability to PFAS exposure. However, no previous study exploring the relationship between PFAS and body size and composition focused on midlife women.
The overarching goal of this analysis was to investigate whether baseline serum PFAS concentrations were associated with body size and composition or their change in midlife women, using longitudinal data from the Study of Women’s Health Across the Nation (SWAN). We hypothesized that women with higher PFAS concentrations had larger weight, waist circumference (WC), body fat mass and proportion fat at baseline, and higher rates of changes in body size and composition over time.
MATERIALS/SUBJECTS AND METHODS
Study design and participants
The SWAN is a multi-site, multi-racial/ethnic, community-based longitudinal cohort of midlife women. It is designed to characterize physiological and psychosocial changes during the menopausal transition and subsequent health outcomes.20 From 1996 to 1997, 3,302 premenopausal or early perimenopausal women aged 42–52 years were recruited from seven study sites, including White women from all sites, Black women from Boston, Chicago, Southeast Michigan, and Pittsburgh, Hispanic women from Newark, Japanese women from Los Angeles, and Chinese women from Oakland, CA. Eligibility criteria included having an intact uterus, at least one menstrual period in the prior three months, and not having taken hormone medications within the prior three months. Data and biospecimens were collected annually or biannually. The institutional review board approved the study protocol, and all participants provided written informed consent at each participating site.
In 2016, the SWAN Multi-Pollutant Study (SWAN-MPS) was initiated to investigate the potential health effects of multiple chemicals in midlife women, using repository serum and urine samples from the SWAN visit 03 (1999–2000), which is the SWAN-MPS baseline for exposure assessments. The study designs and details of the SWAN-MPS were described elsewhere.4,19,21–23 PFAS concentrations were quantified in 1,400 participants at the SWAN-MPS baseline (identified as the baseline in the present study).The SWAN-MPS included White, Black, Chinese and Japanese women from five sites. Hispanic women (only available from the Newark, NJ site) were not included because MPS required women to have both blood and urine samples which were not collected at that site. Of the 1,400 participants enrolled, 19 did not have assessments of body size or body composition. Thus, we had a final analytic sample of 1,381 women with 15,000 repeated measures of body size or composition from 1999 to 2018. The average number of observations per participant was 10.9 observations.
Procedures
This analysis considered four outcomes, including weight (kg), waist circumference (WC, cm), fat mass (kg), and proportion of fat mass (%). All measurements were performed at each SWAN visit. A trained technician followed standardized protocols to measure weight and WC. Weight was measured to the nearest 0.1 kg without shoes and in light indoor clothing using a portable digital scale or balance beam scale. WC was recorded to the nearest 0.1 cm. Body composition, including fat mass and lean mass, was measured using dual-energy X-ray absorptiometry (DXA) using Hologic instruments (Hologic Inc.). The SWAN implemented a rigorous quality assurance and a calibration procedure that has been previously published.18 Body composition variables did not include the head from the calculation.
Serum samples were sent to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC) for environmental exposure assessments. The CDC laboratory’s involvement did not constitute engagement in human-subjects research. Perfluorohexane sulfonate (PFHxS), n-PFOS, sum of branched isomers of PFOS (Sm-PFOS), n-PFOA, sum of branched PFOA (Sb-PFOA), perfluorononanoate (PFNA), perfluorodecanoate (PFDA), perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoA), 2-(N-methyl-perfluorooctane sulfonamido) acetate (MeFOSAA), and 2-(N-ethyl-perfluorooctane sulfonamido) acetate (EtFOSAA) were quantified in 0.1 mL of serum using an online solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry.24 Total PFOS was computed as the sum of n-PFOS and Sm-PFOS. Because of low detection in Sb-PFOA (<20%), we did not include total PFOA in the analysis. The limit of detection (LOD) was 0.1 ng/mL for all the analytes. Measurements below the LODs were replaced by .25 Comprehensive QA/QC procedures were conducted. The coefficients of variation, which reflect both inter- and intra-day variations, were 5.9–12.1% for the low concentration QC pools; and 5.9–10.6% for the high QC concentration pools. Sb-PFOA, PFDA and PFUnDA with detection rates <50% were not included in the analysis, depending on the analyte.
Information on covariates was obtained from standardized interviews conducted at each visit. Sociodemographic variables included age at baseline (in years), self-defined race (Black, Chinese, Japanese, White), study site (Southeast Michigan, Boston, Pittsburgh, Oakland, Los Angeles), education (high school or less, some college, college degree or higher), and financial strain (very hard, somewhat hard, not hard at all). Time-varying covariates included self-reported lifestyle factors, namely smoking status (never smoked, past smoker, current smoker),26 environmental tobacco smoking (0, 1–4, ≥5 person-hours),27 total calorie intake (in kcal),28 and alcohol intake (<1 drink/month, >1 drink/month and ≤1/week, >1 drink/week).28 Physical activity was assessed in various domains, including sports/exercise, household/caregiving and daily routine, with a range of 3 to 15 (15 indicating the highest level of activity).29 Information on menopausal status was collected at each follow-up visit. Menopausal status was categorized into surgical postmenopausal, natural postmenopausal, late perimenopausal, early perimenopausal, premenopausal, or unknown due to HT using bleeding patterns and information about HT use.30 Dietary data were collected using a modified Block food frequency questionnaire. Based on previous studies,19 we selected fish consumption (including fried fish or fish sandwich, tuna, shellfish, and other fish), pizza, salty snacks (e.g. chips, popcorn and crackers), and French fries and fried potatoes.
Statistical analysis
Univariate statistics were calculated for baseline characteristics and serum PFAS concentrations at the SWAN-MPS baseline (1999–2000). Weight, WC and fat mass were log-transformed to ensure normality. Because proportion fat mass had approximate normal distributions, log-transformation was not applied. We utilized a 3-step approach to model the trajectories of body size and body fat:
Generalized additive mixed models with penalized splines to model the trajectories of weight, WC, body fat and proportion fat during follow-up.
Linear mixed models with piecewise linear splines to determine the best knot placement for the parametric outcome trajectories based on Bayesian Information Criterion (BIC) values. We evaluated knot selection by finding the lowest BIC value when each of the 2 knots were varied in 6-month intervals from 5 to 10 years after baseline for weight and WC, and 10 to 15 follow-up years for body fat mass and proportion fat, as suggested by smoothing curves (Figure S1). All outcome trajectories were composed of 2 linear segments with a knot placement. For weight and WC, the 2 segments were: 0 to 9.0 years since baseline, and 9.0 to 18.6 years during the follow-up. For body fat and proportion fat, the segments included: years 0 to 14.5 years and 14.5 to 18.6 years of follow-up.
- To examine each outcome’s rate of increase or decrease and how PFAS exposure influenced the changes, we utilized piecewise linear mixed-effect models adjusting for age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status. Linear splines with a single knot accounted for non-linear trends. Interaction terms between PFAS and time were included to estimate the change rates in each outcome by tertiles of PFAS concentrations.
where Outcomeij included log-transformed weight, log-transformed WC, log-transformed fat mass and proportion fat mass for the ith subject with the jth observation; and are dummy variables which represent the second and third tertiles of baseline PFAS concentrations, respectively, comparing with the first tertile; Timeij stands for follow-up time (in years); Covariatesij included age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status; C is the knot representing years after baseline; and b0j is the random intercept. For weight and WC, C equals to 9.0. For body fat mass and proportion fat mass, C equals to 14.5; (Timeij − C)+ is 0 if Timeij ≤ C, (Timeij − C)+ is (Timeij − C) if Timeij > C.
We created model-predicted trajectories of all biometrics. We also estimated rates of changes in each outcome’s two segments to determine whether PFAS concentrations influenced the rate of increase or decrease in the outcomes during each time segment. To obtain the average participant’s profile, age was centered at 45 years while all other continuous covariates were set to their means. All categorical variables fitted as dummies (i.e., coded as 0 or 1) were set to their means. In this case, variations by categorical variables such as race/ethnicity cannot be discerned in the predicted means or geometric means (GMs). Predicted GMs and 95% confidence intervals (95% CIs) of weight, WC, and fat mass by tertiles of PFAS concentrations were calculated at baseline, years 5, 10, and 15 during follow-up using effect estimates from the linear mixed models representing predicted GMs at age 45, 50, 55, and 60 years, respectively. Ratios of GMs were reported across the comparison groups in women with tertiles of PFAS concentrations. Because proportion fat mass was not log-transformed, predicted means and 95% CIs of proportion fat mass by tertiles of PFAS concentrations were computed. Differences in means were assessed by tertiles of PFAS concentrations.
In the sensitivity analyses, we explored linear relationships between PFAS and outcomes. Serum PFAS concentrations were log-transformed with base 2 to ensure normality. Furthermore, baseline body mass index was additionally controlled for in the models to evaluate its impact on change rates. Because consumption of fast food, salty snacks and fish are potential determinants of PFAS concentrations, the models were also adjusted for pizza, salty snacks, fries, and fish consumption. Moreover, PFAS have been associated with earlier natural menopause and thus it could be a mediator.31 We have conducted a sensitivity analysis with adjustment of time-varying menopausal status. Finally, we observed decreases in body weight and body fat at the later years of follow-up. We have conducted sensitivity analyses censoring women at the visit of developing diabetes or any type of cancer during the follow-up visits. The prevalence of other chronic conditions such as cardiovascular disease was very low and thus we decided not to consider that in the analyses.
RESULTS
In total, median age of the 1,381 women included in this study at baseline was 49.4 years with an interquartile range (IQR) of 47.3–51.4 years. Half (50.7%) of the women were White, 21.6% were Black, 12.8% were Chinese, and 14.9% were Japanese. The median (IQR) baseline serum concentration was 1.5 (1.0–2.4) ng/mL for PFHxS, 25.1 (17.7–35.8) ng/mL for total PFOS, 4.1 (2.9–5.8) ng/mL for n-PFOA, 0.6 (0.4–0.8) ng/mL for PFNA, 1.2 (0.7–2.2) ng/mL for EtFOSAA, and 1.5 (0.9–2.3) ng/mL for MeFOSAA. Participants had a median of 68.7 (IQR: 58.5–83.9) kg of body weight, 82.3 (IQR: 73.7–94.9) cm of waist circumference, 25.1 (IQR: 19.0–34.2) kg of body fat mass, and 40.4% (IQR: 35.6%−45.1%) of proportion fat mass. Descriptive statistics of other baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics, body size and composition, and serum concentrations of PFAS in midlife women from the Study of Women’s Health Across the Nation Multi-Pollutant Study (N=1,381).
| Characteristics | Median (IQR) or N (%) |
|---|---|
| Age, year | 49.4 (47.3–51.4) |
| Race | |
| White | 700 (50.7%) |
| Black | 298 (21.6%) |
| Chinese | 177 (12.8%) |
| Japanese | 206 (14.9%) |
| Study site | |
| SE Michigan | 250 (18.1%) |
| Boston | 228 (16.5%) |
| Oakland | 308 (22.3%) |
| Los Angeles | 363 (26.3%) |
| Pittsburgh | 232 (16.8%) |
| Education | |
| ≤High school | 248 (18.0%) |
| High school | 442 (21.2%) |
| College | 337 (24.5%) |
| Post-college | 347 (25.3%) |
| Difficulty paying for basics | |
| Very hard | 88 (6.4%) |
| Somewhat hard | 342 (24.9%) |
| Not hard at all | 945 (68.7%) |
| Smoking status | |
| Never smoked | 871 (63.1%) |
| Past smoker | 366 (26.5%) |
| Current smoker | 144 (10.4%) |
| Passive smoking | |
| 0 person-hrs | 824 (59.7%) |
| 1–4 person-hrs | 319 (23.1%) |
| ≥5 person-hrs | 238 (17.2%) |
| Alcohol intake | |
| None/Low use | 733 (53.0%) |
| Moderate use | 324 (23.5%) |
| High use | 324 (23.5%) |
| Physical activity score | 7.8 (6.6–9.0) |
| Menopausal status | |
| Surgical post | 47 (3.4%) |
| Natural post | 154 (11.1%) |
| Late perimenopausal | 111 (8.0%) |
| Early perimenopausal | 705 (51.1%) |
| Premenopausal | 161 (11.7%) |
| Unknown with hormone therapy | 203 (14.7%) |
| Total calorie intake | 1686 (1333–2167) |
| Body size and composition | |
| Weight, kg | 68.7 (58.5–83.9) |
| Waist circumference, cm | 82.3 (73.7–94.9) |
| Fat mass, kg | 25.1 (19.0–34.2) |
| Percent body fat, % | 40.4 (35.6–45.1) |
| PFAS | % >LOD | Median (IQR), ng/mL |
|---|---|---|
| PFHxS | 99.6 | 1.5 (1.0–2.4) |
| Total PFOS | NA | 25.1 (17.7–35.8) |
| n-PFOS | 100 | 17.6 (12.4–25.0) |
| sm-PFOS | 99.9 | 7.3 (4.7–11.2) |
| n-PFOA | 99.9 | 4.1 (2.9–5.8) |
| sb-PFOA | 18.3 | <LOD |
| PFNA | 97.2 | 0.6 (0.4–0.8) |
| PFDA | 41.0 | <LOD (<LOD-0.3) |
| PFUnDA | 31.9 | <LOD (<LOD-0.2) |
| EtFOSAA | 99.0 | 1.2 (0.7–2.2) |
| MeFOSAA | 99.6 | 1.5 (0.9–2.3) |
IQR = interquartile range; >LOD = above the limit of detection of 0.1 ng/mL
Figure 1 shows model-predicted trajectories of all biometrics by tertile of total PFOS concentrations. These are shown for each of the 2-time segments (0 to 9.0 years and 9.0 to 18.6 years since baseline for weight and WC; 0 to 14.5 years and 14.5 to 18.6 years since baseline for body fat mass and proportion fat). Trajectory parameters, i.e. annual rates of changes, are presented subsequently (Table S1). Overall, PFOS was significantly associated with trajectories of all biometrics characterized by greater weight, WC, body fat mass and proportion fat over time (Table 2). At baseline, predicted GM weight was 4.3 kg (ratio=1.06, 95% CI: 1.03, 1.09) higher in women with the highest tertile of total PFOS concentrations than those with the lowest tertile. Similarly, comparing the highest to the lowest tertile, predicted GM was 87.3 vs. 83.7 cm (ratio=1.04, 95% CI: 1.02, 1.07) for WC, 27.9 vs. 25.2 kg (ratio=1.10, 95% CI: 1.05, 1.16) for body fat mass, and 42.4% vs. 40.6% (difference=1.81%, 95% CI: 1.01%, 2.61%) for proportion fat. The significant associations with weight, WC and fat mass became larger during follow-up. As women reached year 15, predicted GM weight was 5.8 kg (ratio=1.08, 95% CI: 1.05, 1.11) in those with the higher tertile of total PFOS concentrations compared to the lowest tertile.
Figure 1.
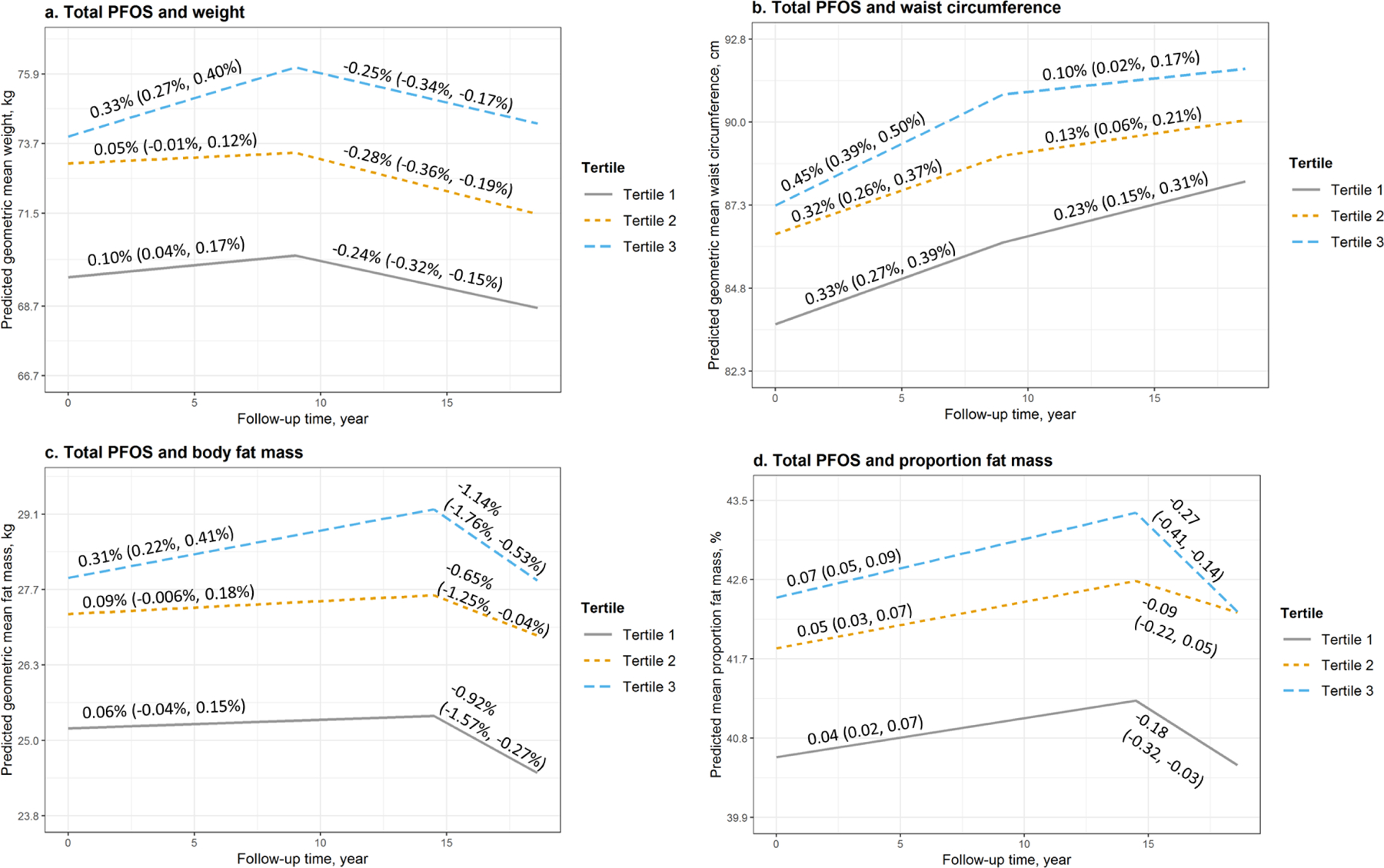
Predicted geometric mean (95% confidence interval, 95% CI) and annual rate of changes (95% CI) of a. weight, b. waist circumference, c. body fat mass, and d. proportion fat mass by tertiles of serum concentrations of total PFOS at baseline and during follow-up. Models were adjusted for age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status. Medians (ranges) of total PFOS concentrations by tertiles were 14.8 (2.0, 20.1) ng/mL for tertile 1, 25.1 (20.2, 30.9) ng/mL for tertile 2, and 42.9 (31.0, 376.0) ng/mL for tertile 3.
Table 2.
Predicted geometric mean (95% confidence interval, 95% CI) of weight, waist circumference and body fat mass, as well as predicted mean (95% CI) of proportion fat mass by tertile of total PFOSserum concentrations; and ratios of geometric mean or difference in mean across the comparison groups in women with the second the third tertiles vs. the lowest tertile as reference, from linear mixed-effect models.a
| Weight | |||||
|---|---|---|---|---|---|
| Geometric mean (95% CI), kg | Ratio of geometric mean (95% CI)b | ||||
| Tertile of total PFOS concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 69.6 (67.9, 71.3) | 73.1 (71.4, 74.8) | 73.9 (72.2, 75.7) | 1.05 (1.02, 1.08) | 1.06 (1.03, 1.09) |
| Year 5 | 69.9 (68.3, 71.6) | 73.3 (71.5, 75.0) | 75.2 (73.4, 77.0) | 1.05 (1.02, 1.08) | 1.07 (1.04, 1.11) |
| Year 10 | 70.1 (68.4, 71.8) | 73.2 (71.5, 75.0) | 76.0 (74.2, 77.8) | 1.04 (1.02, 1.07) | 1.08 (1.05, 1.12) |
| Year 15 | 69.2 (67.6, 70.9) | 72.2 (70.5, 74.0) | 75.0 (73.2, 76.8) | 1.04 (1.01, 1.07) | 1.08 (1.05, 1.11) |
| Waist circumference | |||||
| Geometric mean (95% CI), cm | Ratio of geometric mean (95% CI)b | ||||
| Tertile of total PFOS concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 83.7 (82.2, 85.1) | 86.4 (85.0, 87.9) | 87.3 (85.8, 88.9) | 1.03 (1.01, 1.05) | 1.04 (1.02, 1.07) |
| Year 5 | 85.1 (83.6, 86.5) | 87.8 (86.3, 89.3) | 89.3 (87.8, 90.9) | 1.03 (1.01, 1.05) | 1.05 (1.03, 1.07) |
| Year 10 | 86.4 (84.9, 87.9) | 89.1 (87.5, 90.6) | 91.0 (89.4, 92.6) | 1.03 (1.01, 1.05) | 1.05 (1.03, 1.08) |
| Year 15 | 87.4 (85.9, 88.9) | 89.6 (88.1, 91.2) | 91.5 (89.9, 93.1) | 1.03 (1.01, 1.05) | 1.05 (1.03, 1.07) |
| Body fat mass | |||||
| Geometric mean (95% CI), kg | Ratio of geometric mean (95% CI)b | ||||
| Tertile of total PFOS concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 25.2 (24.2, 26.3) | 27.2 (26.1, 28.3) | 27.9 (26.8, 29.0) | 1.08 (1.03, 1.13) | 1.10 (1.05, 1.16) |
| Year 5 | 25.3 (24.3, 26.3) | 27.3 (26.3, 28.4) | 28.3 (27.2, 29.5) | 1.08 (1.03, 1.13) | 1.12 (1.07, 1.17) |
| Year 10 | 25.4 (24.4, 26.4) | 27.5 (26.4, 28.6) | 28.8 (27.6, 29.9) | 1.08 (1.03, 1.13) | 1.13 (1.08, 1.19) |
| Year 15 | 25.3 (24.3, 26.4) | 27.5 (26.4, 28.6) | 29.0 (27.9, 30.2) | 1.08 (1.04, 1.14) | 1.15 (1.09, 1.20) |
| Proportion fat mass | |||||
| Mean (95% CI), % | Difference in mean (95% CI), % | ||||
| Tertile of total PFOS concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 40.6 (39.9, 41.3) | 41.8 (41.1, 42.5) | 42.4 (41.7, 43.1) | 1.24 (0.46, 2.01) | 1.81 (1.01, 2.61) |
| Year 5 | 40.8 (40.1, 41.5) | 42.1 (41.4, 42.8) | 42.7 (42.0, 43.4) | 1.28 (0.52, 2.04) | 1.92 (1.13, 2.71) |
| Year 10 | 41.0 (40.3, 41.7) | 42.3 (41.6, 43.1) | 43.1 (42.4, 43.8) | 1.92 (1.13, 2.71) | 2.03 (1.23, 2.83) |
| Year 15 | 41.1 (40.4, 41.9) | 42.5 (41.8, 43.3) | 43.2 (42.5, 43.9) | 1.40 (0.61, 2.19) | 2.08 (1.27, 2.90) |
Models were adjusted for age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status.
Weight, waist circumference, and body fat mass were log-transformed to ensure normality; thus ratios of geometric mean (95% CI) were calculated.
Medians (ranges) of total PFOS concentrations by tertiles were 14.8 (2.0, 20.1) ng/mL for tertile 1, 25.1 (20.2, 30.9) ng/mL for tertile 2, and 42.9 (31.0, 376.0) ng/mL for tertile 3.
In addition, total PFOS was also significantly associated with accelerated increases in weight during 0 to 9.0 years of follow-up (Table S1). Women with the lowest tertile of total PFOS concentrations had an annual rate of increase of 0.10% (95% CI: 0.04%, 0.17%) in year 0 to year 9; a significant increase with annual rates of 0.33% (95% CI: 0.27%, 0.40%) observed in women with the highest tertile of total PFOS concentrations (P for interaction<.0001). Similarly, compared to those with the lowest tertile, women with the highest tertile of serum concentrations had a 1.4-fold acceleration in annual rate of increases in WC (P for interaction=0.002). Total PFOS was also significantly associated with accelerated gains in body fat mass (P for interaction=0.0002), but not proportion fat mass (P for interaction=0.32) in year 0 to 14.5. Similar results were detected for n-PFOS (Figure S2 and Tables S2, S9) and Sm-PFOS (Figure S3 and Tables S3, S10). Weight, body fat mass and proportion fat had slight decreases in the 2nd segment of follow-up, while there was a continued increase in WC. PFOS was associated with higher weight, WC, body fat mass, and proportion fat over time. PFOS was also related to different rates of gains or loss in body size or body fat.
n-PFOA was also significantly associated with larger body size and body fat (Figure 2 and Table 3). At baseline, comparing the highest to the lowest tertile of n-PFOA concentrations, predicted GM was 74.0 vs. 69.4 kg (ratio=1.07, 95% CI: 1.03, 1.10) for weight, 87.6 vs. 83.9 cm (ratio=1.04, 95% CI: 1.02, 1.07) for WC, 87.6 vs. 83.9 cm (ratio=1.04, 95% CI: 1.02, 1.07) for fat mass, and 42.5% vs. 40.6% (Diff=1.91%, 95% CI: 1.07%, 2.75%) for proportion fat. At follow-up, the differences remained similar to the differences observed at baseline as n-PFOS was not associated with change rates in outcomes (Table S4).
Figure 2.
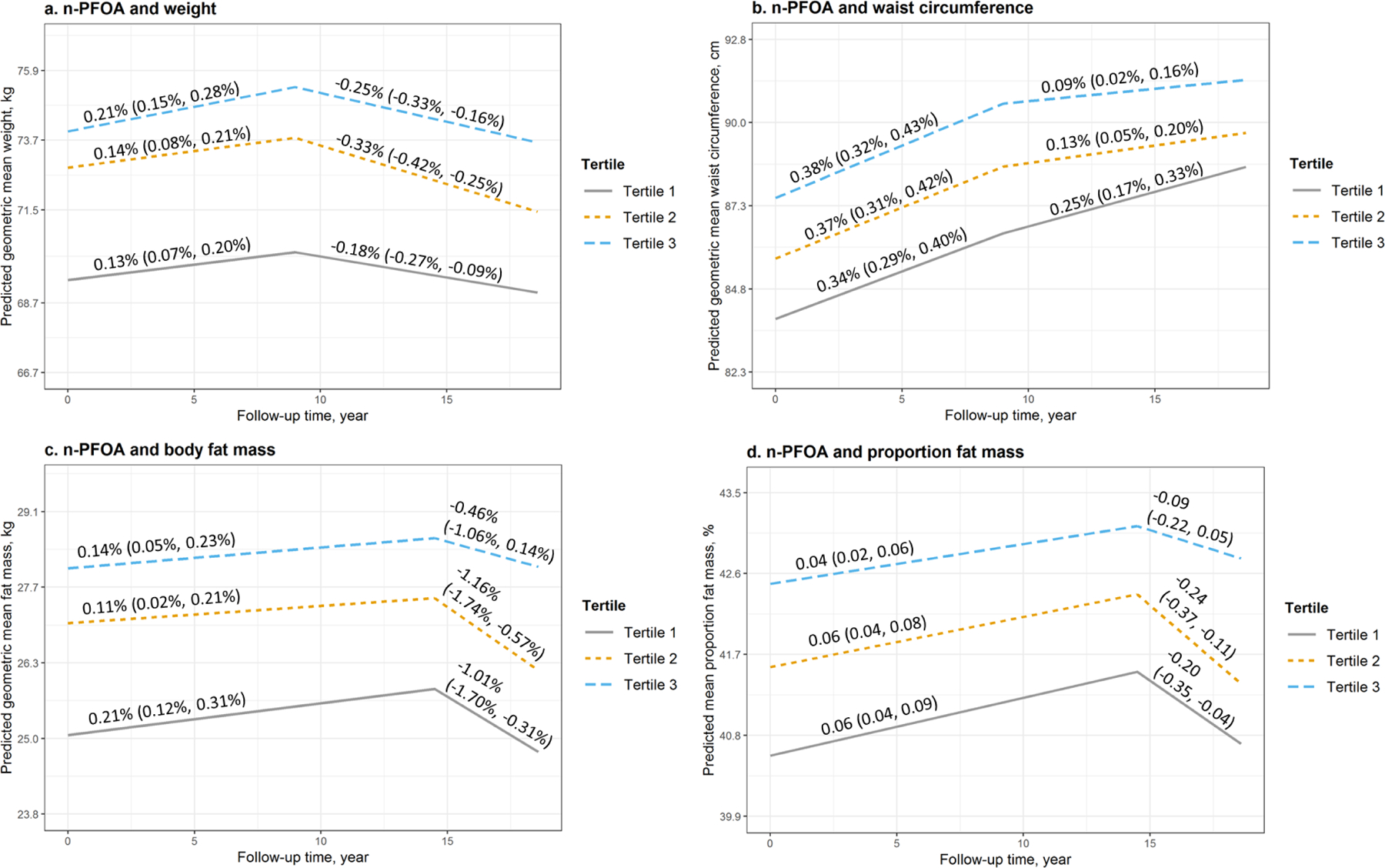
Predicted geometric mean (95% confidence interval, 95% CI) and annual rate of changes (95% CI) of a. weight, b. waist circumference, c. body fat mass, and d. proportion fat mass by tertiles of serum concentrations of n-PFOA at baseline and during the follow-up. Models were adjusted for age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status. Medians (ranges) of n-PFOA concentrations by tertiles were 2.4 (<LOD, 3.2) ng/mL for tertile 1, 4.1 (3.3, 5.1) ng/mL for tertile 2, and 6.8 (5.2, 56.5) ng/mL for tertile 3.
Table 3.
Predicted geometric mean (95% confidence interval, 95% CI) of weight, waist circumference and body fat mass, as well as predicted mean (95% CI) of proportion fat mass by tertile of n-PFOAserum concentrations; and ratios of geometric mean or difference in mean across the comparison groups in women with the second the third tertiles vs. the lowest tertile as reference, from linear mixed-effect models.
| Weight | |||||
|---|---|---|---|---|---|
| Geometric mean (95% CI), kg | Ratio of geometric mean (95% CI)b | ||||
| Tertile of n-PFOA concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 69.4 (67.6, 71.2) | 72.8 (71.1, 74.6) | 74.0 (72.3, 75.8) | 1.05 (1.02, 1.08) | 1.07 (1.03, 1.10) |
| Year 5 | 69.9 (68.1, 71.7) | 73.4 (71.6, 75.1) | 74.8 (73.0, 76.5) | 1.05 (1.02, 1.08) | 1.07 (1.04, 1.10) |
| Year 10 | 70.1 (68.3, 71.9) | 73.5 (71.8, 75.3) | 75.2 (73.5, 77.0) | 1.05 (1.02, 1.08) | 1.07 (1.04, 1.11) |
| Year 15 | 69.5 (67.7, 71.3) | 72.3 (70.6, 74.1) | 74.3 (72.6, 76.1) | 1.04 (1.01, 1.07) | 1.07 (1.04, 1.10) |
| Waist circumference | |||||
| Geometric mean (95% CI), cm | Ratio of geometric mean (95% CI)b | ||||
| Tertile of n-PFOA concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 83.9 (82.3, 85.4) | 85.7 (84.2, 87.2) | 87.6 (86.1, 89.1) | 1.02 (1.00, 1.04) | 1.04 (1.02, 1.07) |
| Year 5 | 85.3 (83.8, 86.9) | 87.3 (85.8, 88.8) | 89.3 (87.8, 90.8) | 1.02 (1.00, 1.04) | 1.05 (1.02, 1.07) |
| Year 10 | 86.7 (85.1, 88.3) | 88.7 (87.2, 90.3) | 90.7 (89.2, 92.3) | 1.02 (1.00, 1.04) | 1.04 (1.02, 1.07) |
| Year 15 | 87.8 (86.2, 89.4) | 89.3 (87.7, 90.8) | 91.1 (89.6, 92.7) | 1.02 (1.00, 1.04) | 1.04 (1.02, 1.06) |
| Body fat mass | |||||
| Geometric mean (95% CI), kg | Ratio of geometric mean (95% CI)b | ||||
| Tertile of n-PFOA concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 25.1 (24.0, 26.2) | 27.0 (25.9, 28.1) | 28.0 (26.9, 29.1) | 1.08 (1.03, 1.13) | 1.12 (1.06, 1.17) |
| Year 5 | 25.3 (24.3, 26.4) | 27.2 (26.1, 28.3) | 28.2 (27.1, 29.3) | 1.07 (1.02, 1.12) | 1.11 (1.06, 1.17) |
| Year 10 | 25.6 (24.5, 26.7) | 27.3 (26.2, 28.4) | 28.4 (27.3, 29.5) | 1.07 (1.02, 1.12) | 1.11 (1.06, 1.16) |
| Year 15 | 25.7 (24.6, 26.9) | 27.3 (26.2, 28.4) | 28.5 (27.4, 29.7) | 1.06 (1.01, 1.11) | 1.11 (1.05, 1.16) |
| Proportion fat mass | |||||
| Mean (95% CI), % | Difference in mean (95% CI), % | ||||
| Tertile of n-PFOA concentrationsc | |||||
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 2 vs. 1 | Tertile 3 vs. 1 | |
| Baseline | 40.6 (39.8, 41.3) | 41.6 (40.8, 42.3) | 42.5 (41.8, 43.2) | 0.99 (0.17, 1.80) | 1.91 (1.07, 2.75) |
| Year 5 | 40.9 (40.2, 41.6) | 41.8 (41.1, 42.5) | 42.7 (42.0, 43.4) | 0.94 (0.15, 1.74) | 1.81 (0.99, 2.64) |
| Year 10 | 41.2 (40.5, 42.0) | 42.1 (41.4, 42.8) | 42.9 (42.2, 43.6) | 0.90 (0.09, 1.71) | 1.71 (0.87, 2.55) |
| Year 15 | 41.4 (40.7, 42.2) | 42.2 (41.5, 43.0) | 43.1 (42.4, 43.8) | 0.84 (0.008, 1.67) | 1.67 (0.82, 2.53) |
Models were adjusted for age at baseline, race, study site, education, financial strain, smoking status, passive smoking, alcohol consumption, total calorie intake, physical activity, and menopausal status.
Weight, waist circumference, and body fat mass were log-transformed to ensure normality; thus ratios of geometric mean (95% CI) were calculated.
Medians (ranges) of n-PFOA concentrations by tertiles were 2.4 (<LOD, 3.2) ng/mL for tertile 1, 4.1 (3.3, 5.1) ng/mL for tertile 2, and 6.8 (5.2, 56.5) ng/mL for tertile 3.
Trajectories of all biometrics for other PFAS are shown in Figures S4–S7, rates of change in Tables S5–S8, and predicted values in Tables S11–S14. Two PFOS precursors, i.e. EtFOSAA and MeFOSAA, had similar effects to PFOS. PFHxS was not related to body size or body fat during follow-up (Table S11). Nonetheless, PFHxS was significantly associated with higher rates of increases in weight (P for interaction<0.0001) and WC (P for interaction=0.003) in years 0 to 9, as well as body fat mass (P for interaction<0.0001) and proportion fat (P for interaction=0.0009) in years 0 to 14.5 (Table S5). No significant findings were found for PFNA.
In the sensitivity analyses, the results of log-transformed PFAS were similar to our main findings using tertiles of PFAS concentrations (Tables S15–S16). Log-transformed PFOS, n-PFOA, EtFOSAA and MeFOSAA were positively associated with weight, waist circumference (WC), fat mass, and proportion fat at baseline. Higher PFOS concentrations were also associated with larger increase rates of weight (P for interaction <0.0001), WC (P for interaction=0.01), and fat mass (P for interaction <0.0001). EtFOSAA and MeFOSAA showed similar effects to PFOS (data not shown). Although PFHxS were not related to body size or body fat at baseline, PFHxS were associated with faster increases in weight (P for interaction <0.0001), fat mass (P for interaction=0.0005), and proportion fat (P for interaction=0.02) (data not shown). An additional adjustment for baseline body mass index did not impact the change rates (Tables S19–S20). The results remained almost the same after controlling for pizza, salty snacks, fries, and fish consumption (Table S21–S23). Further adjustment for time-varying menopausal status did not change the results (Tables S24–S26). The results also remained similar after censoring women at the visit when they developed diabetes or cancer (Tables S27–S29).
DISCUSSION
In this study, PFOS, PFOA, MeFOSAA and EtFOSAA was related to greater body weight, WC, fat mass and proportion fat mass at baseline; the associations persisted through the follow-up visits. Our study also quantified the effects of PFAS exposure on longitudinal trajectories of body size and composition and determined how PFAS was associated with the rates of changes during follow-up. Exposure to PFOS and PFHxS was significantly related to accelerated gains in weight, WC, and body fat.
Although an association between PFAS and larger body size has been reported in previous studies, our study provides a precise estimate of the timing of alterations in body size and body fat in midlife women. Previously, Cardenas and colleagues13 found a significant association of PFOS, PFNA and MeFOSAA with larger weight at baseline and during follow-up in a study of 802 adults with overweight or obesity; whereas no association was observed for other PFAS or for skinfold thickness and subcutaneous and visceral fat. Liu and colleagues14 found weight regain was significantly related to higher baseline plasma concentrations of PFOS, PFOA, PFNA, PFHxS and PFDA after a 6-month weight loss program followed by 18-month follow up in a group of 621 adults with overweight or obesity; whereas PFAS were not significantly related to weight or weight loss during the weight loss period. However, those prospective cohort studies did not include normal weight adults. A cross-sectional study of 1,612 Chinese adults found PFAS exposure associated with obesity and larger WC.12 In contrast, a retrospective cohort of 8,764 residents from communities with drinking water contaminated with PFOA aged 20–40 years from the C8 Health Project reported no relationship between early-life PFOA exposure and obesity risks.15 Two other studies11,16 also reported no significant association of body size with PFAS exposure. The main distinction between previous studies and ours is long-term follow-ups with the average number of repeated measurements of 10.9 in midlife women during and after the menopausal transition, a life-stage that confers susceptibility to metabolic changes in women. This rich data allows us to evaluate whether longitudinal trajectories of body size and composition over 15 years differ by different PFAS serum concentration groups. The present study supports the hypothesis that PFAS exposure may be associated with greater body weight and body fat, and contribute to higher rates of change in body weight, WC, and body fat.
The associations between PFAS and increased body size and body fat are biologically plausible. PFAS can activate PPARs. PPARα may be less responsive to PFAS exposure in humans than in mice; nonetheless, most perfluoroalkyl acids (e.g., PFOA and PFOS) can activate both PPARα and PPARγ.33,34 PPARs are known to involve in adipogenesis, inflammation, and lipid metabolism.10 Therefore, PFAS have the ability to promote metabolic disturbances and increase adipocyte differentiation.35 Further, epidemiologic studies have suggested that PFAS were associated with lower thyroid hormone levels; however, the findings are inconsistent.36,37 Moreover, PFAS exposure may also disrupt leptin signaling pathways, which could contribute to increased weight in mice when they reached midlife38; however, the underlying mechanisms of PFAS and leptin are not completely understood.
Growing evidence pinpoints estradiol (E2) and follicle stimulating hormone (FSH) as targets of PFAS toxicity.6,7 FSH and E2 affect energy homeostasis pathways. For example, mice with FSH blocked show increases in energy expenditure and oxygen consumption which are accompanied by increased physical activity as well as reductions in resting metabolic rate and body fat.39 Longitudinal data from women in SWAN displayed a positive association between FSH and body fat during menopausal transition.18,40 Thus, alterations in FSH are possible mechanisms of obesogenic toxicity of PFAS because PFAS were associated with increased FSH concentrations and accelerated ovarian aging in midlife women. PFAS had impact on steroid hormone synthesis.7 Besides interacting with specific receptors, PFAS can also exert metabolic effects by acting on non-specific molecular targets. For instance, PFOA and PFOS may cause decreases in global DNA cytosine methylation, while these chemicals may also contribute to increases in long interspersed nuclear element 1 (LINE-1) DNA methylation, and alterations in the expression of genes responsible for lipid metabolism in humans.41,42
Weight, WC, body fat and proportion fat increased in the 1st segment of follow-up. Interestingly, we observed slight declines in weight, body fat mass and proportion fat in the 2nd segment of follow-up, and yet a continued increase in WC (as a surrogate marker of visceral fat). Previous studies have shown that both men and women gain weight and fat mass until middle age and begin to lose weight and fat mass near age 60–65.43–45 Even in healthy, active older people, men and women may lose weight and body fat.44 It is similar to what we observed in SWAN (Figure S1). Weight and body fat decreased in midlife women over time, whereas waist circumference steadily increased with aging. The weight loss among older adults might be due to the loss of lean mass, while visceral fat (as represented by waist circumference) increased. A previous toxicologic study showed that exposure of human visceral preadipocytes in vitro to 5 and 50 μM PFOS promoted adipocyte differentiation and increased cellular lipid accumulation.46 It is possible that PFAS exposure may also determine fat distribution during the menopausal transition. Future studies are warranted to examine the associations between PFAS and fat distribution.
Study strengths are several. First, this analysis included a large cohort of midlife women followed for up to 18 years. Standard annual follow-up visits also provided reliable estimates of changes in body size and composition. Second, we analyzed DXA-quantified body composition and measured body weight and waist circumference, thus providing more convincing evidence for the potential obesogenic effects of PFAS. However, we cannot rule out residual confounding, although we have controlled for many known confounders. Also, some women lost to follow-up in the later years which may impact the precision of the study results. Finally, there is a lack of well-established statistical methods to assess the combined effects of chemical mixtures on longitudinal trajectories of outcomes.47,48 Given that people are exposed to a myriad of chemicals daily, it is critically important to examine the joint effects of PFAS mixtures in future research.
In a large, longitudinal cohort of multi-racial/ethnic midlife women with 18 years of follow up, higher serum concentrations of PFOS, PFOA, EtFOSAA, MeFOSAA were significantly associated with greater body weight, WC, body fat and proportion fat, accompanied by accelerated increased during follow-up. Our findings suggest that PFAS may be an underappreciated contributing factor to women’s obesity, especially during the critical time windows for the development of metabolic and cardiovascular risks. Following the discovery of polytetrafluoroethylene (Teflon) in 1938, thousands of different PFASs have been synthesized and used in industrial and consumer products. These chemicals, also known as “forever chemicals”, may predispose people to weight gain in later life and could play a significant role in the global obesity epidemic. Although PFOA and PFOS in the USA has been largely phased out of use,49,50 these compounds are still found in the blood of most people. Since PFAS is ubiquitous in the environment, especially in drinking water and ground water, it poses a significant and immediate threat to the general population in the United States and around the world. Consequently, there is a pressing need to raise awareness of PFAS toxicity and potential adverse human health effects.
Supplementary Material
ACKNOWLEDGMENT
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The study was supported by the SWAN Repository (U01AG017719).
This study was also supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
CDC Laboratory: Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA.
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013 - Present; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
COMPETING INTERESTS
The authors declared no competing interests.
REFERENCES
- 1.ATSDR. Toxicological Profile for Perfluoroalkyls, Draft for Public Comment 2018www.regulations.gov. (accessed 8 May2020).
- 2.Andrews DQ, Naidenko OV. Population-Wide Exposure to Per- And Polyfluoroalkyl Substances from Drinking Water in the United States. Environ Sci Technol Lett 2020; 7: 931–936. [Google Scholar]
- 3.CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Fourth Natl Rep Hum Expo to Environ Chem 2009; : 1–529. [Google Scholar]
- 4.Ding N, Harlow SD, Batterman S, Mukherjee B, Park SK. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women′s Health Across the Nation. Environ Int 2020; 135: 105381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 2007; 115: 1298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 2008; 31: 161–169. [DOI] [PubMed] [Google Scholar]
- 7.Ding N, Harlow SD, Randolph JF, Loch-Caruso R, Park SK. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update 2020; 26: 724–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 2011; 288: 8–17. [DOI] [PubMed] [Google Scholar]
- 9.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 2009; 304: 97–105. [DOI] [PubMed] [Google Scholar]
- 10.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta - Lipids Lipid Metab 1996; 1302: 93–109. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 2010; 118: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian YP, Zeng XW, Bloom MS, Lin S, Wang SQ, Yim SHL et al. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: Isomers of C8 Health Project in China. Environ Int 2019; 124: 130–138. [DOI] [PubMed] [Google Scholar]
- 13.Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, Fleisch AF et al. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Adiposity. JAMA Netw open 2018; 1: e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med 2018; 15. doi: 10.1371/journal.pmed.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry V, Darrow LA, Klein M, Winquist A, Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res 2014; 132: 62–69. [DOI] [PubMed] [Google Scholar]
- 16.Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ Pollut 2018; 242: 894–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D et al. Understanding weight gain at menopause. Climacteric 2012; 15: 419–429. [DOI] [PubMed] [Google Scholar]
- 18.Greendale GA, Sternfeld B, Huang MH, Han W, Karvonen-Gutierrez C, Ruppert K et al. Changes in body composition and weight during the menopause transition. JCI Insight 2019; 4. doi: 10.1172/jci.insight.124865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SK, Peng Q, Ding N, Mukherjee B, Harlow SD. Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ Res 2019; 175: 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santoro N, Taylor ES, Sutton-Tyrrell K. The SWAN Song: Study of Women’s Health Across the Nation’s Recurring Themes. Obstet Gynecol Clin North Am 2011; 38: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Mukherjee B, Batterman S, Harlow SD, Park SK. Urinary metals and metal mixtures in midlife women: The Study of Women’s Health Across the Nation (SWAN). Int J Hyg Environ Health 2019; 222: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Karvonen-Gutierrez CA, Herman WH, Mukherjee B, Harlow SD, Park SK. Urinary metals and incident diabetes in midlife women: Study of Women’s Health Across the Nation (SWAN). BMJ Open Diabetes Res Care 2020; 8: e001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Karvonen-Gutierrez CA, Mukherjee B, Herman WH, Park SK. Urinary metals and adipokines in midlife women: The Study of Women’s Health Across the nation (SWAN). Environ Res 2020. doi: 10.1016/j.envres.2020.110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 2011; 1218: 2133–2137. [DOI] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg 1990; 5: 46–51. [Google Scholar]
- 26.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978; 118: 1–120. [PubMed] [Google Scholar]
- 27.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol 1989; 130: 696–704. [DOI] [PubMed] [Google Scholar]
- 28.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986; 124: 453–469. [DOI] [PubMed] [Google Scholar]
- 29.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med (Baltim) 1999; 28: 313–323. [DOI] [PubMed] [Google Scholar]
- 30.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW et al. Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. In: Journal of Clinical Endocrinology and Metabolism The Endocrine Society, 2012, pp 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding N, Harlow SD, Randolph JF, Calafat AM, Mukherjee B, Batterman S et al. Associations of Perfluoroalkyl Substances with Incident Natural Menopause: The Study of Women’s Health across the Nation. J Clin Endocrinol Metab 2020; 105: E3169–E3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009; 20: 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf CJ, Takacs ML, Schmid JE, Lau C, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptor alpha by perfluoroalkyl acids of different functional groups and chain lengths. Toxicol Sci 2008; 106: 162–171. [DOI] [PubMed] [Google Scholar]
- 34.Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 2007; 95: 108–117. [DOI] [PubMed] [Google Scholar]
- 35.Watkins AM, Wood CR, Lin MT, Abbott BD. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 2015; 400: 90–101. [DOI] [PubMed] [Google Scholar]
- 36.Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int 2017; 99: 15–28. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Moon S, Oh BC, Jung D, Ji K, Choi K et al. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS One 2018; 13: e0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 2009; 304: 97–105. [DOI] [PubMed] [Google Scholar]
- 39.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, Demambro VE, Dhawan S et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017; 546: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sowers MF, Zheng H, Tomey K, Karvonen-Gutierrez C, Jannausch M, Li X et al. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J Clin Endocrinol Metab 2007; 92: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 2013; 57–58: 2–10. [DOI] [PubMed] [Google Scholar]
- 42.Watkins DJ, Wellenius GA, Butler RA, Bartell SM, Fletcher T, Kelsey KT. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ Int 2014; 63: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheehan TJ, DuBrava S, DeChello LM, Fang Z. Rates of weight change for black and white Americans over a twenty year period. Int J Obes 2003; 27: 498–504. [DOI] [PubMed] [Google Scholar]
- 44.Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional Age Differences in Body Composition in Persons 60 + Years of Age. Journals Gerontol Ser A Biol Sci Med Sci 1995; 50A: M307–M316. [DOI] [PubMed] [Google Scholar]
- 45.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr 2005; 82: 923–934. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol 2016; 290: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Mukherjee B, Batterman S, Harlow SD, Park SK. Urinary metals and metal mixtures in midlife women: The Study of Women’s Health Across the Nation (SWAN). Int J Hyg Environ Health 2019; 222: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Mukherjee B, Karvonen-Gutierrez CA, Herman WH, Batterman S, Harlow SD et al. Urinary metal mixtures and longitudinal changes in glucose homeostasis: The Study of Women’s Health Across the Nation (SWAN). Environ Int 2020; 145: 106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.USEPA. Health effects support document for perfluorooctane sulfonate (PFOS) 2016http://www.epa.gov/ (accessed 11 Mar2019).
- 50.USEPA. Health Effects Support Document for Perfluorooctanoic Acid (PFOA) 2016https://www.epa.gov/sites/production/files/2016-05/documents/pfoa_hesd_final-plain.pdf (accessed 11 Mar2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


