Abstract
Post-translational modifications (PTMs) of proteins extensively diversify the biological information flow from the genome to the proteome and thus have profound pathophysiological implications. Precise dissection of the regulatory networks of PTMs benefits from the ability to achieve conditional control through external optogenetic or chemogenetic triggers. Genetic code expansion provides a unique solution by allowing for site-specific installation of functionally masked unnatural amino acids (UAAs) into proteins, such as enzymes and enzyme substrates, rendering them inert until rapid activation through exposure to light or small molecules. Here, we summarize the most recent advances harnessing this methodology to study various forms of PTMs as well as generalizable approaches to externally control nodes-of-interest in PTM networks.
Keywords: post-translational modification, unnatural amino acid, genetic code expansion, protein phosphorylation, protein ubiquitination, protein methylation, protein SUMOylation
Introduction
Post-translational modifications (PTMs) of proteins greatly expand the biological information transferred from the genome to the proteome by chemically transforming peptides during and/or after protein translation. Addition of various groups to the amino acid residues is crucial for diversifying the functions of nascent proteins by regulating their enzymatic activity, substrate or cofactor specificity, localization, and stability [1–2]. Important PTMs include phosphorylation, ubiquitination, methylation, lipidation, glycosylation, etc. Studies of PTMs have profound pathophysiological implications as they are involved in almost all cellular processes, including proliferation, differentiation, cell death, and immune response, and thus dysregulation of PTMs is related to the pathogenesis of many human diseases [3]. Most PTMs display spatiotemporal dynamics as proteins can be transiently modified by writers and erasers at varied timepoints and subcellular locations during biological events. Further complexity is added through combinatorial modifications to one protein in an orchestrated manner to modulate the biological outcome, or PTM “crosstalk” [4–5]. Recent advances in the study of PTMs have employed chemical biology tools to address the aforementioned challenges [6]. Amongst these tools, unnatural amino acid mutagenesis through genetic code expansion has provided a powerful solution [7]. UAAs with a chemically masked functionality (for select examples important to the topic of this review, see Table 1; for more comprehensive lists of genetically encoded UAAs, see [8–9]) are site-specifically inserted during protein biosynthesis in response to an amber codon mutation in the mRNA. This is enabled by the expression of engineered tRNACUA/tRNA synthetase pairs that are orthogonal to the host organism. While stop codon suppression is widely adopted, other codons can also be reassigned, such as rare sense codons and quadruplet codons [10–11]. This strategy not only allows for site-specific and genetically encoded introduction of PTMs [12–16] or caged PTMs [17–20] but can also confer temporal and spatial control to PTM writers and erasers using light (optogenetic) or small molecule (chemogenetic) triggers of protein function [21–23]. In this review, we summarize recent progress in using optical and chemical triggers to control post-translational modifications via UAA mutagenesis.
Table 1.
Caged amino acids that have been genetically encoded in mammalian cells. For caging groups, only the general core structures are described, while derivatives are being included in the references.
| amino acid | caging group | trigger | references |
|---|---|---|---|
| lysine | nitrobenzyl | 365 nm light | [24–25] |
| coumarinyl | 405 and 760 nm light | [26] | |
| azidobenzyl | phosphine | [27–28] | |
| azidobenzyl | trans-cyclooctene | [29] | |
| trans-cyclooctenyl | tetrazine | [30] | |
| propargyl | Pd(II) complex | [31] | |
| tyrosine | nitrobenzyl | 365 nm light | [32,15] |
| allenyl | Pd(II) complex | [33] | |
| cysteine | nitrobenzyl | 365 nm light | [34–36] |
| homocysteine | nitrobenzyl | 365 nm light | [35] |
Caging the activity of PTM-writing enzymes
Protein phosphorylation is a key post-translational modification that is crucial for signal transduction networks composed of interconnected signaling pathways that cells use to make decisions in response to external and internal stimuli [37]. Classically, signal transduction from receptors at the cell surface to transcription factors in the cell nucleus is mediated by protein kinases that catalyze the transfer of γ-phosphate groups of ATP to the designated residue(s) on substrate proteins, which can be further passed onto downstream substrates in the form of a cascade of phosphorylation events. In contrast, protein phosphatases catalyze the reverse process by removing a phosphate modification from targeted residues. These enzymes work in collaboration to regulate cellular signaling pathways, notably the mitogen-activated protein kinase (MAPK) pathways including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 pathways in mammals [38–39]. In each of these cascades, three kinases are sequentially activated: a serine/threonine kinase classified as a MAPK kinase kinase (MAPKKK or M3K) phosphorylates and activates a dual-specificity MAPK kinase (MAPKK or M2K), which in turn phosphorylates and activates the MAPK. Of these, the ERK cascade (Raf/MEK/ERK) controls cell proliferation in response to growth factor stimulation, and the JNK (MKK4/MKK7/JNK) and p38 (MKK3/MKK6/p38) cascades are considered to respond with apoptosis to cellular stress and inflammatory signals [40–41].
In recent years, a universal strategy has been developed for the conditional control of enzymatic activity, including kinases of the MAPK family: a catalytically critical residue in the active site is substituted by a “caged” UAA which masks catalytic activity until lightor small molecule-induced restoration of the native residue and thus protein function is achieved (Figure 1A). This provides rapid, temporal control over PTM writing and erasing, thereby eliminating compensatory effects that are elicited by slow, genetic knock-down or knock-in approaches. Utilizing this approach, the Haugh and Deiters labs used a photocaged lysine (PCK) [24], which undergoes photolysis upon 365 nm light irradiation to restore a native lysine (Figure 1B), to achieve optical control of MKK6 activity and to interrogate the crosstalk between the MKK6 pathway and the ERK pathway [42]. Not only did the Haugh lab define MKK6 as a new pleiotropic signal transducer that promotes both pro-apoptotic and anti-proliferative signaling, but they also discovered that light-activated MKK6 downregulates the ERK pathway in the presence of p38 inhibitor (Figure 1C), which upended the conventional belief that the MKK6-ERK crosstalk is p38-dependent [43]. These results highlight the advantages of an optically triggered MKK6 for the elucidation of signaling network topologies, such as crosstalk between the p38 and ERK cascades, without network adaptation and premature triggering of cell apoptosis.
Figure 1.
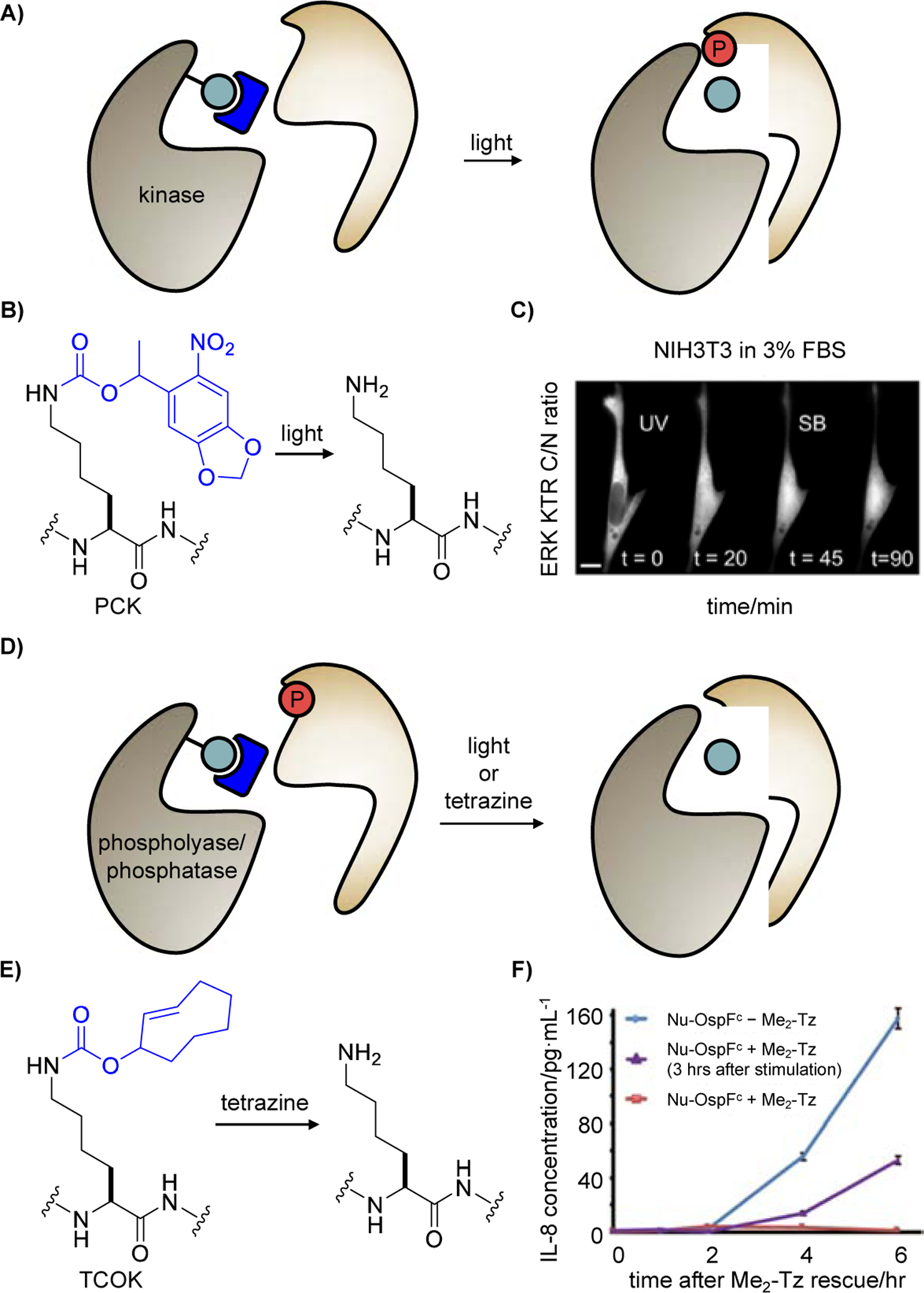
Control of enzymes adding protein post-translational modifications by caging catalytic residues. The catalytic residue is indicated in teal, the caging group is blue, and the tranferred phosphate PTM is red. A) Illustrating the general strategy for caging a conserved, catalytic lysine residue within kinases. B) Irradiation of the photocaged lysine PCK removes the caging group and restores a native lysine residue. C) NIH3T3 cell expressing an ERK KTR (kinase translocation reporter) and a caged MKK6 were preincubated with FBS (fetal bovine serum), then irradiated, and treated with a p38 inhibitor 45 min later. ERK activation is represented by the translocation of ERK KTR from the nucleus to the cytoplasm, despite p38 inhibition. Adapted from ref. [42] with permission. D) General strategy for caging the catalytic residue of a phospholyase or a phosphatase. E) Addition of a tetrazine (Me2-Tz, 3,6-dimethyl-1,2,4,5-tetrazine) removes the TCO caging group and restores a native lysine residue, thereby restoring enzymatic function. F) Signaling in Jurkat cells expressing OspF K134TCOK was activated by PMA (phorbol-12-myristate-13-acetate) and ionomycin, followed by Me2-Tz treatment after 10 min (red) or 3 h (purple) for nucleus-localized caged OspF (nu-OspFc) activation. Adapted from ref. [44] with permission.
Similar to kinases, the catalytic residue of phosphor-lyases or phosphatases can be caged to conditionally trigger dephosphorylation (Figure 1D). The Chen group achieved irreversible dephosphorylation of p38 (phosphor-pT180/Y182) and ERK (phospho-T202/Y204) MAPKs using a phosphor-lyase, OspF, from Shigella spp. [44]. A photocaged o-nitrobenzyl-oxycarbonyl-Nε-L-lysine (ONBK) [25] was incorporated at the catalytic lysine K134 and is decaged by 365 nm light to convert phosphoserine or phosphothreonine of p-p38 and p-ERK to β-methyldehydroalanine, rendering the sites incapable for re-phosphorylation [45]. Permanently suppressed p38/ERK activity led to an attenuated immune response and reduced expression of cytokine interleukin-8 (IL-8) showcasing light-induced modulation of MAPK activity in living cells (data not shown). Additionally, Chen introduced the trans-cyclooctenyl lysine TCOK into the same site for small molecule-triggered decaging through a tetrazine ligation followed by elimination (Figure 1E). Chemical rescue of nucleus-localized OspF function by Me2-Tz also conferred significantly reduced secretion of IL-8 during an immune response, providing precise tuning of the timing and strength of interleukin secretion in T cells (Figure 1F). Though light- and small molecule-activated phosphor-lyase can be used to conditionally regulate dephosphorylation of MAPK, it lacks substrate specificity.
A recent development by the Deiters group successfully addressed this issue by introducing the first light-activated mammalian protein phosphatase by photocaging MAPK phosphatase 3 (MKP3), which has high specificity for erasing ERK phosphorylation. Following the strategy described in Figure 1D, the catalytic cysteine was replaced with a caged cysteine [46], NVC, which would mask the nucleophilicity of nascent C293 until UV irradiation removes the caging group. Upon light activation of MKP3 C293NVC, ERK cascade activity was suppressed even in the presence of EGF (estrogen growth factor) stimulation (data not shown).
Apart from the most recent progress mentioned above, the Chen lab achieved chemogenetic control of Src (sarcoma kinase), FAK (focal adhesion kinase), and MEK1 via tetrazine-induced TCO cleavage in cells and animals [47]. The same decaging strategy was then used by Chen to isoform-specifically activate MEK1/2 mutants orthogonal to endogenous MEK1/2, which unveiled that MEK1 induces ERK phosphorylation more strongly than MEK2 and led to the discovery of four MEK1-specific inhibitors [48]. The Chin lab introduced an optically controlled LCK (lymphocyte-specific protein tyrosine kinase) to investigate its role in T-cell antigen receptor signaling, revealing that CD4/8 use a similar mechanism to enhance LCK recruitment for ZAP70 membrane translocation [49]. In the same year, the Deiters lab reported temporal control of MEK activity in zebrafish embryos using an optically active lysine, enabling studies of its role during embryonic development and RASopathy birth defects [50]. Photocaging of enzymatic activity, combined with constitutively active enzyme mutants, allows for direct activation and investigation of a particular node-of-interest or a particular sub-network by decoupling it from upstream regulators.
Caging the accessibility of protein substrates
Another approach to control phosphatase activity for control of target protein phosphorylation was also developed in the previously mentioned report by Deiters [51] (Figure 2A). Installation of a sterically hindered, charge-neutral photocaged hydroxycoumarin lysine (HCK) in the place of an arginine residue (R65) at the phosphatase-substrate interface breaks the electrostatic interaction between MKP3 R65 and ERK2 D319, and thus blocks ERK dephosphorylation. Optical triggering converts HCK to a positively charged lysine residue (Figure 2B), restores the electrostatic interaction, and initiates dephosphorylation of ERK by activated MKP3 (Figure 2C). This led to an optically triggered phosphatase with highly tunable activity, based on the duration of light exposure, and the approach is poised to be applicable to many of the 200 protein phosphatases. Compared to nitrobenzyl-caging groups in PCK, ONBK, and NVC, the hydroxycoumarin-caging group in HCK displays higher photosensitivity and can be photolyzed through irradiation with blue light [26].
Figure 2.
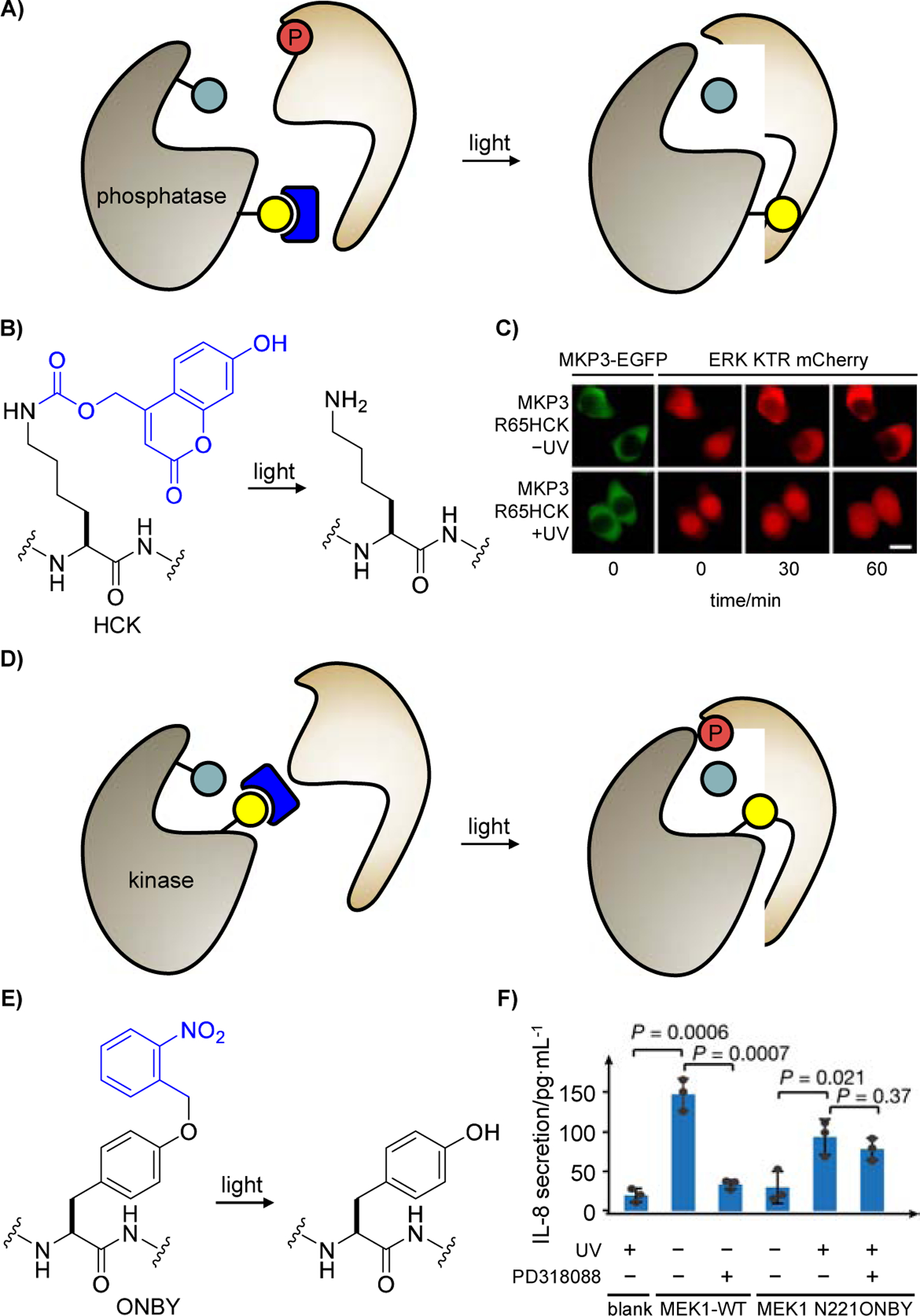
Control of protein post-translational modifications by regulating access of the substrate to the caged PTM-writing enzyme. A) Strategy utilized for regulating a phosphatase-substrate interface. The catalytic residue is shown in teal, the caging group is blue, the residue essential to the protein-protein interaction is marked in yellow, and the phosphate modification is red. B) Irradiation of the caged lysine HCK removes the caging group and restores a native lysine residue, thereby restoring the phosphatase-substrate interface. C) HEK293T cells expressing an ERK KTR mCherry and the caged MKP3 phosphatase were pretreated with epidermal growth factor and subsequently irradiated. ERK activation leads to translocation of the reporter from the nucleus to the cytoplasm. Adapted from ref. [51] with permission. D) The proximity caging approach introduces a caged residue, marked in yellow, close to the catalytic center of the enzyme. E) Irradiation of ONBY removes the caging group and restores a tyrosine residue that does not perturb enzyme function. F) Jurkat cells expressing MEK1 N221ONBY were incubated with or without PD318088. Secretion of IL-8 was only detected with MEK-WT (without PD318088) and light-activated MEK1 N221ONBY (with and without PD318088). Adapted from ref. [52] with permission.
The Chen lab reported a generalizable pipeline for creating caged enzymes aided by in silico screening using a set of predefined criteria [52]. As shown in Figure 2D, a photocaged tyrosine (ONBY) is placed in close proximity to the catalytic site of an enzyme, blocking accessibility to the cognate substrate until light irradiation relieves said hindrance and restores catalytic activity (Figure 2E), provided that neither ONBY nor the nascent tyrosine after decaging disturbs the native conformation of the enzyme. Chen and collaborators were able to use this universal strategy to photocage MEK1. In Jurkat cells, MEK1 N221ONBY blocked PMA-stimulated IL-8 secretion until light restored MEK1 kinase activity and triggered an immune response. Moreover, the proximal tyrosine mutation conferred resistance to a MEK1/2 inhibitor (PD318088) that targets endogenous MEK1 but not MEK1 N221Y, which could be leveraged to build tailor-made signaling cascades that are not interfered by their endogenous counterparts (Figure 2F). General applicability of this approach was demonstrated by caging the small GTPase KRAS through a Y23ONBY substitution.
Though the two aforementioned tactics both control writing and erasing of phosphate PTMs by regulating substrate accessibility, it should be noted that disruption of protein-protein interaction (PPI) interfaces with UAAs can be rationally designed based on structural information or through scanning of putative interfaces with photocaged amino acids, followed by activity readouts before and after optical stimulation.
Caging amino acid residues that are PTM targets
Apart from phosphorylation, control of other types of PTMs, including protein ubiquitination and SUMOylation (SUMO, small ubiquitin-like modifier), have also been achieved in the past few years through genetic code expansion. Ubiquitination and SUMOylation are two important post-translational modifications on lysine residues, regulating signal transduction, protein trafficking, protein stability, and transcription via protein fate determination [53]. Their dysregulation can lead to aberrant protein degradation and in turn contribute to disease [54]. Crosstalk between these two reversible modifications further underlines the demand for the optogenetic or chemogenetic triggering of ubiquitination and SUMOylation [55,52].
Towards this goal, the Deiters group [27] and the Lang group [56] used a Staudinger reduction to unveil a nucleophilic amino group for site-specific post-translational modification by ubiquitin (Ub) or SUMO (Figure 3A). Building onto an earlier report [28], the Deiters lab developed a second-generation protected lysine PABK which shows higher incorporation efficiency and superior decaging kinetics when exposed to the phosphine 2DPBM (Figure 3B). To control SUMOylation with small molecule triggers, a naturally SUMOylated lysine residue on Ran GTPase Activating Protein 1 (RanGAP1), K524, was replaced with PABK in NIH3T3 cells. Upon 2DPBM-triggered reduction, RanGAP1 was rapidly SUMOylated in the cytosol and translocated to the nuclear pore complex, similar to wild-type RanGAP1 and contrary to the SUMOylation-resistant K524R mutant (Figure 3C). This approach is expected to be broadly applicable to chemically control modification of specific lysines with SUMO and other PTMs.
Figure 3.
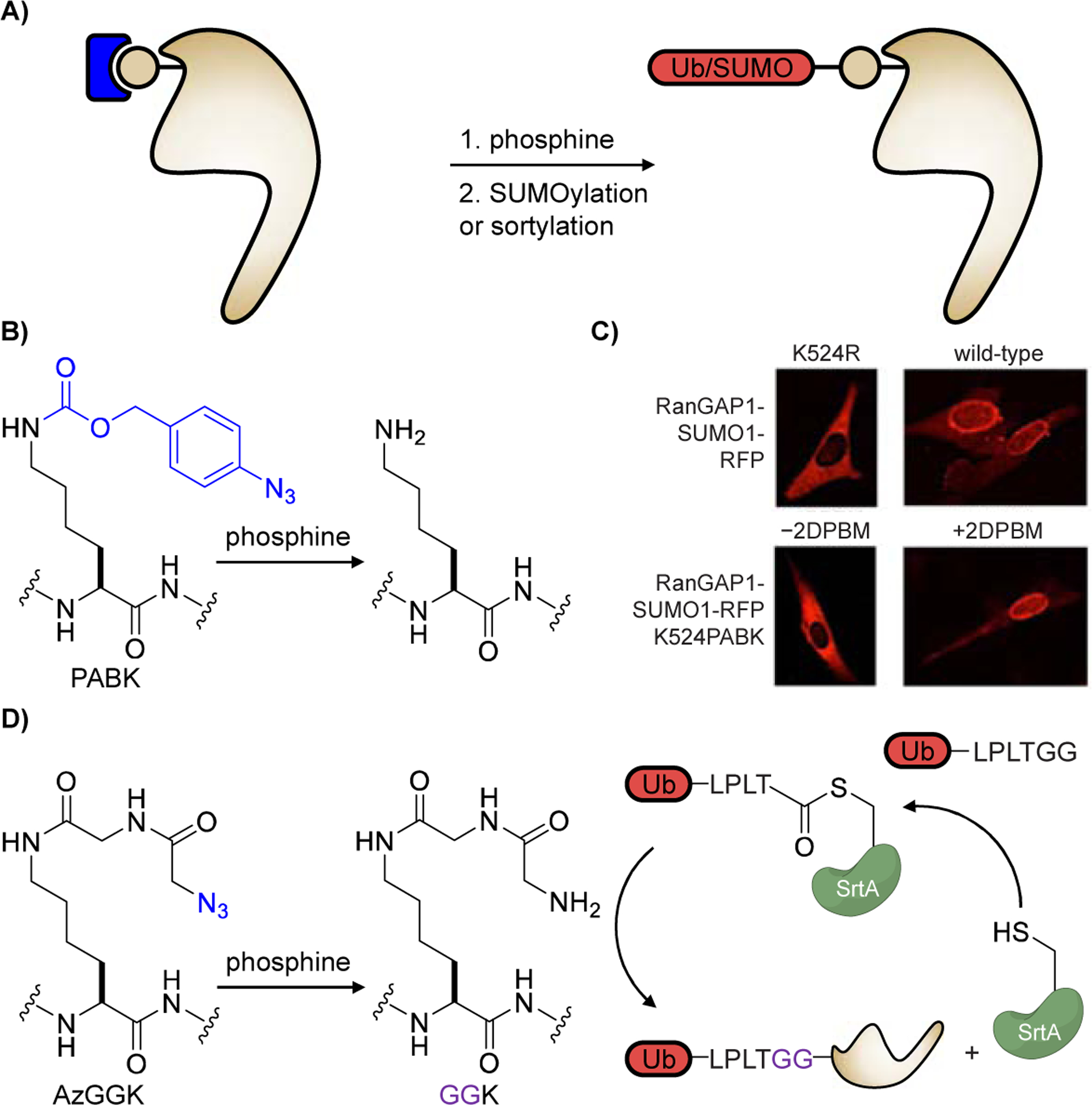
Control of post-translational modification by caging of amino acid residues receiving the PTM. A) General strategy for decaging residues before PTM. The caging group is shown in blue, the modification site/residue is grey, and the PTM is marked in red. B) Addition of 2DPBM (2-(diphenylphosphino)benzamide) removes the azido-caging group and restores a native lysine residue, which is then SUMOylated. C) NIH3T3 cells expressing RanGAP-K524-RFP mutants show that the K524PABK mutant remains cytosolic, but translocates to the nuclear membrane after treatment with 2DPBM and subsequent SUMOylation. Adapted from ref. [27] with permission. Copyright: 2019, Wiley‐VCH. D) Addition of 2DPBA (2-(diphenylphosphino)benzoic acid) converts an azide into an ɑ-amino group in the glycylglycine motif of GGK, allowing for sortase-mediated ubiquitination.
Aiming to site-specifically ubiquitylate and SUMOylate any target protein of interest in an inducible fashion, the Lang lab uniquely combined sortase-mediated transpeptidation and genetic code expansion. Here, sortase A (SrtA) forms a Ub-SrtA intermediate with Ub-LPLTGG, which is then site-specifically delivered to a lysine residue on the protein of interest (POI). The transfer is controlled through phosphine (2DPBA) reduction of an azido group on the genetically encoded AzGGK tripeptide UAA to the corresponding, nucleophilic amino group (Figure 3D). The LPLTGG linker, though different from the natural linker in ubiquitination, retains recognition by ubiquitin-binding domains. This method extends conditional control of ubiquitination and SUMOylation beyond naturally ubiquitinated/SUMOylated residues, which has been challenging with present chemical methods in native environments [57]. Sortylation is easily implementable and is poised to enable dissection of Ub/SUMO-regulated cellular signaling networks by obviating the requirement to activate upstream signaling components.
Summary and outlook
In the past few years, significant progress has been made in the field of optogenetic and chemogenetic control of protein post-translational modifications by applying unnatural amino acid mutagenesis in cells with an expanded genetic code. These efforts were aimed at conditional control of PTMs in a spatiotemporal and site-specific manner, paving the way for better understanding of transient, dynamic PTM events [58–59], dissecting their roles in spatially distinct cells and tissues during development [60–61], and unveiling previously obscured biological mechanisms of enzyme isoforms [62–63]. Optical and chemical triggers have been exploited in these studies, providing noninvasive, dose-dependent, and temporally precise control. While light uniquely enjoys spatial specificity and rapid activation, small molecules have advantages in ease of application without the need for special equipment and in better penetration into deep and non-transparent tissues. Several generally applicable methodologies have been established and validated, including caging enzymatic activity, controlling substrate accessibility, and masking the amino acid residue that receives the enzyme-assisted post-translational modification. Several labs have employed conditional control of phosphorylation to interrogate physiological processes, to unveil previously unknown crosstalk mechanisms [42], characterize time windows of MEK/ERK function in zebrafish development [50], and identify T cell receptor kinases interactions [49]. Overall, hypothesis-driven interrogation of PTM function will benefit from further technology development of spatiotemporal control, as well as an expanded panel of chemically functionalized amino acids beyond the most frequently adopted lysine and tyrosine derivatives.
Given the potential of controlling PTMs through unnatural amino acid mutagenesis, we envision that the combination of conditional trigger and multi-omics technologies will provide unique opportunities to interrogate PTM function at the systems level. Moreover, several PTMs have not been targeted with the approaches presented here, including lipidation, hydroxylation, glycosylation, and nitrosylation. UAA-enabled conditional control of PTM erasers, other than phosphatases, such as demethylases, deacylases, and deubiquitinating enzymes has also not been explored. The field of nucleic acid-modifying enzymes is also underdeveloped as it comes to UAA mutagenesis, with the exception of recent reports of light-triggered DNA 5’-methylcytosine oxidation [64] and RNA N6-methyladenosine demethylation [52]. In summary, UAA mutagenesis has a bright future in exploring PTM biology and other epigenetic mechanisms.
Acknowledgement
This work was supported by the NIH (R01GM132565) and the NSF (CHE-1904972).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Walsh C, Posttranslational modification of proteins: expanding nature’s inventory, Roberts and Co. Publishers, Englewood, Colo., 2006. [Google Scholar]
- [2].Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr., Protein posttranslational modifications: the chemistry of proteome diversifications, Angew Chem Int Ed Engl 44 (2005) 7342–7372. [DOI] [PubMed] [Google Scholar]
- [3].Uversky VN, Posttranslational Modification, in: Maloy S, Hughes K, Brenner’s Encyclopedia of Genetics (Second Edition), Academic Press, San Diego, 2013, pp. 425–430. [Google Scholar]
- [4].Rust HL, Thompson PR, Kinase consensus sequences: a breeding ground for crosstalk, ACS Chem Biol 6 (2011) 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hunter T, The age of crosstalk: phosphorylation, ubiquitination, and beyond, Mol Cell 28 (2007) 730–738. [DOI] [PubMed] [Google Scholar]
- [6].Conibear AC, Deciphering protein post-translational modifications using chemical biology tools, Nature Reviews Chemistry (2020). [DOI] [PubMed] [Google Scholar]
- [7].Lang K, Chin JW, Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins, Chem Rev 114 (2014) 4764–4806. [DOI] [PubMed] [Google Scholar]
- [8].Nodling AR, Spear LA, Williams TL, Luk LYP, Tsai YH, Using genetically incorporated unnatural amino acids to control protein functions in mammalian cells, Essays Biochem 63 (2019) 237–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dumas A, Lercher L, Spicer CD, Davis BG, Designing logical codon reassignment - Expanding the chemistry in biology, Chem Sci 6 (2015) 50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zeng Y, Wang W, Liu WR, Towards reassigning the rare AGG codon in Escherichia coli, Chembiochem 15 (2014) 1750–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neumann H, Wang K, Davis L, Garcia-Alai M, Chin JW, Encoding multiple unnatural amino acids via evolution of a quadruplet-decoding ribosome, Nature 464 (2010) 441–444. [DOI] [PubMed] [Google Scholar]
- [12].Porter JJ, Jang HS, Van Fossen EM, Nguyen DP, Willi TS, Cooley RB, Mehl RA, Genetically Encoded Protein Tyrosine Nitration in Mammalian Cells, ACS Chem Biol 14 (2019) 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang ZA, Kurra Y, Wang X, Zeng Y, Lee YJ, Sharma V, Lin H, Dai SY, Liu WR, A Versatile Approach for Site-Specific Lysine Acylation in Proteins, Angew Chem Int Ed Engl 56 (2017) 1643–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang MS, Brunner SF, Huguenin-Dezot N, Liang AD, Schmied WH, Rogerson DT, Chin JW, Biosynthesis and genetic encoding of phosphothreonine through parallel selection and deep sequencing, Nat Methods 14 (2017) 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arbely E, Torres-Kolbus J, Deiters A, Chin JW, Photocontrol of tyrosine phosphorylation in mammalian cells via genetic encoding of photocaged tyrosine, J Am Chem Soc 134 (2012) 11912–11915. [DOI] [PubMed] [Google Scholar]
- [16].Lemke EA, Summerer D, Geierstanger BH, Brittain SM, Schultz PG, Control of protein phosphorylation with a genetically encoded photocaged amino acid, Nat Chem Biol 3 (2007) 769–772. [DOI] [PubMed] [Google Scholar]
- [17].Ai HW, Lee JW, Schultz PG, A method to site-specifically introduce methyllysine into proteins in E. coli, Chem Commun (Camb) 46 (2010) 5506–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Groff D, Chen PR, Peters FB, Schultz PG, A genetically encoded epsilon-N-methyl lysine in mammalian cells, Chembiochem 11 (2010) 1066–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nguyen DP, Garcia Alai MM, Kapadnis PB, Neumann H, Chin JW, Genetically encoding N(epsilon)-methyl-L-lysine in recombinant histones, J Am Chem Soc 131 (2009) 14194–14195. [DOI] [PubMed] [Google Scholar]
- [20].Rothman DM, Petersson EJ, Vazquez ME, Brandt GS, Dougherty DA, Imperiali B, Caged phosphoproteins, J Am Chem Soc 127 (2005) 846–847. [DOI] [PubMed] [Google Scholar]
- [21].Chen H, Venkat S, McGuire P, Gan Q, Fan C, Recent Development of Genetic Code Expansion for Posttranslational Modification Studies, Molecules 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chin JW, Expanding and reprogramming the genetic code of cells and animals, Annu Rev Biochem 83 (2014) 379–408. [DOI] [PubMed] [Google Scholar]
- [23].Liu CC, Schultz PG, Adding new chemistries to the genetic code, Annu Rev Biochem 79 (2010) 413–444. [DOI] [PubMed] [Google Scholar]
- [24].Gautier A, Nguyen DP, Lusic H, An W, Deiters A, Chin JW, Genetically encoded photocontrol of protein localization in mammalian cells, J Am Chem Soc 132 (2010) 4086–4088. [DOI] [PubMed] [Google Scholar]
- [25].Chen PR, Groff D, Guo J, Ou W, Cellitti S, Geierstanger BH, Schultz PG, A facile system for encoding unnatural amino acids in mammalian cells, Angew Chem Int Ed Engl 48 (2009) 4052–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luo J, Uprety R, Naro Y, Chou C, Nguyen DP, Chin JW, Deiters A, Genetically encoded optochemical probes for simultaneous fluorescence reporting and light activation of protein function with two-photon excitation, J Am Chem Soc 136 (2014) 15551–15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wesalo JS, Luo J, Morihiro K, Liu J, Deiters A, Phosphine‐Activated Lysine Analogues for Fast Chemical Control of Protein Subcellular Localization and Protein SUMOylation, ChemBioChem 21 (2019) 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Fast small molecule activation of a caged lysine residue through a Staudinger reduction enabled temporal control of site-specific SUMOylation.
- [28].Luo J, Liu Q, Morihiro K, Deiters A, Small-molecule control of protein function through Staudinger reduction, Nat Chem 8 (2016) 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ge Y, Fan X, Chen PR, A genetically encoded multifunctional unnatural amino acid for versatile protein manipulations in living cells, Chem Sci 7 (2016) 7055–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Jia S, Chen PR, Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells, Nat Chem Biol 10 (2014) 1003–1005. [DOI] [PubMed] [Google Scholar]
- [31].Li J, Yu J, Zhao J, Wang J, Zheng S, Lin S, Chen L, Yang M, Jia S, Zhang X, Chen PR, Palladium-triggered deprotection chemistry for protein activation in living cells, Nat Chem 6 (2014) 352–361. [DOI] [PubMed] [Google Scholar]
- [32].Luo J, Torres-Kolbus J, Liu J, Deiters A, Genetic Encoding of Photocaged Tyrosines with Improved Light-Activation Properties for the Optical Control of Protease Function, Chembiochem 18 (2017) 1442–1447. [DOI] [PubMed] [Google Scholar]
- [33].Wang J, Zheng S, Liu Y, Zhang Z, Lin Z, Li J, Zhang G, Wang X, Li J, Chen PR, Palladium-Triggered Chemical Rescue of Intracellular Proteins via Genetically Encoded Allene-Caged Tyrosine, J Am Chem Soc 138 (2016) 15118–15121. [DOI] [PubMed] [Google Scholar]
- [34].Nguyen DP, Mahesh M, Elsasser SJ, Hancock SM, Uttamapinant C, Chin JW, Genetic encoding of photocaged cysteine allows photoactivation of TEV protease in live mammalian cells, J Am Chem Soc 136 (2014) 2240–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Uprety R, Luo J, Liu J, Naro Y, Samanta S, Deiters A, Genetic encoding of caged cysteine and caged homocysteine in bacterial and mammalian cells, Chembiochem 15 (2014) 1793–1799. [DOI] [PubMed] [Google Scholar]
- [36].Kang JY, Kawaguchi D, Coin I, Xiang Z, O’Leary DD, Slesinger PA, Wang L, In vivo expression of a light-activatable potassium channel using unnatural amino acids, Neuron 80 (2013) 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Day EK, Sosale NG, Lazzara MJ, Cell signaling regulation by protein phosphorylation: a multivariate, heterogeneous, and context-dependent process, Curr Opin Biotechnol 40 (2016) 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kyriakis JM, Avruch J, Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update, Physiol Rev 92 (2012) 689–737. [DOI] [PubMed] [Google Scholar]
- [39].Johnson GL, Lapadat R, Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases, Science 298 (2002) 1911–1912. [DOI] [PubMed] [Google Scholar]
- [40].Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H, p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents, Cancer Lett 344 (2014) 174–179. [DOI] [PubMed] [Google Scholar]
- [41].Zhang W, Liu HT, MAPK signal pathways in the regulation of cell proliferation in mammalian cells, Cell Res 12 (2002) 9–18. [DOI] [PubMed] [Google Scholar]
- [42].Rahman SMT, Zhou W, Deiters A, Haugh JM, Optical control of MAP kinase kinase 6 (MKK6) reveals that it has divergent roles in pro-apoptotic and anti-proliferative signaling, J Biol Chem 295 (2020) 8494–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** By substituting the catalytic lysine residue of MKK6 with a photocaged lysine, a light-activated MKK6 was developed and a previously unknown, p38-independent crosstalk with ERK signaling was discovered.
- [43].Westermarck J, Li SP, Kallunki T, Han J, Kahari VM, p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression, Mol Cell Biol 21 (2001) 2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao J, Liu Y, Lin F, Wang W, Yang S, Ge Y, Chen PR, Bioorthogonal Engineering of Bacterial Effectors for Spatial–Temporal Modulation of Cell Signaling, ACS Central Science 5 (2018) 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A tetrazine small molecule was used to trigger activation of a phospholyase, which dephosphorylates ERK and p38 in mammalian cells and provides temporal control over their function.
- [45].Zhu Y, Li H, Long C, Hu L, Xu H, Liu L, Chen S, Wang DC, Shao F, Structural insights into the enzymatic mechanism of the pathogenic MAPK phosphothreonine lyase, Mol Cell 28 (2007) 899–913. [DOI] [PubMed] [Google Scholar]
- [46].Ren W, Ji A, Ai HW, Light activation of protein splicing with a photocaged fast intein, J Am Chem Soc 137 (2015) 2155–2158. [DOI] [PubMed] [Google Scholar]
- [47].Zhang G, Li J, Xie R, Fan X, Liu Y, Zheng S, Ge Y, Chen PR, Bioorthogonal Chemical Activation of Kinases in Living Systems, ACS Cent Sci 2 (2016) 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zheng S, Fan X, Wang J, Zhao J, Chen PR, Dissection of Kinase Isoforms through Orthogonal and Chemical Inducible Signaling Cascades, Chembiochem 18 (2017) 1593–1598. [DOI] [PubMed] [Google Scholar]
- [49].Liaunardy-Jopeace A, Murton BL, Mahesh M, Chin JW, James JR, Encoding optical control in LCK kinase to quantitatively investigate its activity in live cells, Nat Struct Mol Biol 24 (2017) 1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu J, Hemphill J, Samanta S, Tsang M, Deiters A, Genetic Code Expansion in Zebrafish Embryos and Its Application to Optical Control of Cell Signaling, J Am Chem Soc 139 (2017) 9100–9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Courtney TM, Deiters A, Optical control of protein phosphatase function, Nat Commun 10 (2019) 4384. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Both, photocaged cysteine and lysine were used to engineer the first optically activated protein phosphatase, MKP3, by placing them into the active site and a protein-protein interface, respectively.
- [52].Wang J, Liu Y, Liu Y, Zheng S, Wang X, Zhao J, Yang F, Zhang G, Wang C, Chen PR, Time-resolved protein activation by proximal decaging in living systems, Nature 569 (2019) 509–513. [DOI] [PubMed] [Google Scholar]; ** A generalizable pipeline for creating caged enzymes, such as the kinases MEK1 and KRAS, aided by in silico screening was developed, based on the light-activation of a photocaged residue in close proximity to the active site catalytic amino acid.
- [53].Swatek KN, Komander D, Ubiquitin modifications, Cell Res 26 (2016) 399–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wei W, Lin HK, The key role of ubiquitination and sumoylation in signaling and cancer: a research topic, Front Oncol 2 (2012) 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bansal S, Tiwari S, Mechanisms for the temporal regulation of substrate ubiquitination by the anaphase-promoting complex/cyclosome, Cell Div 14 (2019) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fottner M, Brunner AD, Bittl V, Horn-Ghetko D, Jussupow A, Kaila VRI, Bremm A, Lang K, Site-specific ubiquitylation and SUMOylation using genetic-code expansion and sortase, Nat Chem Biol 15 (2019) 276–284. [DOI] [PubMed] [Google Scholar]; ** Through combination of phosphine-induced generation of a nucleophilic amino group and sortase-assisted ligation, site-specific protein ubiquitination and SUMOylation were achieved.
- [57].Stanley M, Virdee S, Chemical ubiquitination for decrypting a cellular code, Biochem J 473 (2016) 1297–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Black JC, Van Rechem C, Whetstine JR, Histone lysine methylation dynamics: establishment, regulation, and biological impact, Mol Cell 48 (2012) 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Perkins JR, Diboun I, Dessailly BH, Lees JG, Orengo C, Transient protein-protein interactions: structural, functional, and network properties, Structure 18 (2010) 1233–1243. [DOI] [PubMed] [Google Scholar]
- [60].Holder J, Poser E, Barr FA, Getting out of mitosis: spatial and temporal control of mitotic exit and cytokinesis by PP1 and PP2A, FEBS Lett 593 (2019) 2908–2924. [DOI] [PubMed] [Google Scholar]
- [61].Edwards AV, Edwards GJ, Schwammle V, Saxtorph H, Larsen MR, Spatial and temporal effects in protein post-translational modification distributions in the developing mouse brain, J Proteome Res 13 (2014) 260–267. [DOI] [PubMed] [Google Scholar]
- [62].Gujar H, Weisenberger DJ, Liang G, The Roles of Human DNA Methyltransferases and Their Isoforms in Shaping the Epigenome, Genes (Basel) 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hong JY, Oh IH, McCrea PD, Phosphorylation and isoform use in p120-catenin during development and tumorigenesis, Biochim Biophys Acta 1863 (2016) 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Palei S, Buchmuller B, Wolffgramm J, Munoz-Lopez A, Jung S, Czodrowski P, Summerer D, Light-Activatable TET-Dioxygenases Reveal Dynamics of 5-Methylcytosine Oxidation and Transcriptome Reorganization, J Am Chem Soc 142 (2020) 7289–7294. [DOI] [PubMed] [Google Scholar]


