Abstract
Prurigo nodularis is an understudied, chronic inflammatory skin disease that disproportionately affects African-Americans and presents with intensely pruritic nodules of unknown etiology. To better characterize immune dysregulation in prurigo nodularis, peripheral blood mononuclear cells and skin biopsies were obtained from majority African American patients with prurigo nodularis and healthy subjects matched by age, race, and sex. Flow cytometric analysis of functional T-cell response comparing prurigo nodularis to healthy subjects identified increased γδT-cells (CD3+CD4−CD8−γδTCR+) with Vδ2+ γδT-enrichment. Activated T-cells demonstrated uniquely increased IL-22 cytokine expression in prurigo nodularis patients when compared to healthy controls. CD4+ and CD8+ T-cells were identified as the source of increased circulating IL-22. Consistent with these findings, RNA-sequencing of lesional prurigo nodularis skin compared to non-lesional prurigo nodularis skin and biopsy site matched control skin demonstrated robust up-regulation of Th22-related genes and signaling networks implicated in impaired epidermal differentiation. Th22-related cytokine upregulation remained significant with stratifications by race and biopsy site. Importantly, the expression of the IL-22 receptors IL22RA1/A2 was significantly elevated in lesional prurigo nodularis skin. These results indicate both systemic and cutaneous immune responses in prurigo nodularis patients are skewed towards a Th22/IL-22 profile. Prurigo nodularis may benefit from immunomodulatory therapies directed at Th22-mediated inflammation.
Keywords: Prurigo nodularis, skin, dermatology, itch, pruritus, cytokines, Th1, Th2, Th17, Th22, γδ T-cells, African American, racial differences, Skin of color
INTRODUCTION
Prurigo nodularis (PN) is a chronic inflammatory skin disease characterized by intensely pruritic nodules on the extremities and trunk (Kwatra 2020). PN disproportionately affects African Americans and is associated with multiple systemic conditions such as cardiovascular disease and type II diabetes (Boozalis et al. 2018; Huang et al. 2020b; Huang et al. 2019; Whang et al. 2019b; Whang et al. 2019a). Among chronic pruritic dermatoses, PN has the greatest itch intensity and most significant impairment of quality of life (Steinke et al. 2018; Kwatra 2020; Huang et al. 2020; Williams et al. 2020). Given PN's unknown etiology and the lack of U.S. Food and Drug Administration (FDA) approved treatments, current therapeutic strategies for PN remain largely ineffective (Huang et al. 2020a; Kwatra 2020; Williams et al. 2020).
Prior investigations of PN have suggested roles for aberrant keratinocyte signaling, neuronal dysregulation, and cutaneous inflammation (Haas et al. 2010; Liang et al. 2000; Matsumura et al. 2015; Wong et al. 2020; Zhong et al. 2019). Targeted real-time polymerase chain (PCR) and immunohistochemical staining have demonstrated mixed evidence of dysregulation of neurotrophic factors and cytokines with studies suggesting PN to have variable amounts of Th2, Th17, or Th22 signaling (Bagci and Ruzicka 2018; Beck et al. 2014; Fukushi et al. 2011; Park et al. 2011; Wong et al. 2020). Recent studies of atopic dermatitis (AD), a chronic inflammatory pruritic skin disease that is comorbid in 11-19% of PN patients, have also revealed significant immune-based variations between racial groups (Boozalis et al. 2018; Iking et al. 2013; Noda et al. 2015; Sanyal et al. 2019). Notably, African American patients with atopic dermatitis display Th2/Th22 skewing (Sanyal et al. 2019). Such Th2/Th22 cytokine polarization may explain why African American patients with AD experience a more severe AD phenotype with extensor body area involvement and increased papular clinical manifestations (McColl et al. 2020).
IL-22, a member of the IL-20 cytokine subfamily and larger IL-10 cytokine family, is a major pro-inflammatory cytokine produced by circulating lymphocytes, including Th17 cells, Th22 cells, NK cells, and γδ T-cells (Mashiko et al. 2015; Rutz et al. 2014; Zheng and Li 2018). Elevated serum IL-22 is implicated in numerous chronic inflammatory conditions such as coronary artery disease (CAD) and type II diabetes (T2DM), both of which are highly associated comorbidities in PN patients (Gong et al. 2016; Rutz et al. 2014; Zheng and Li 2018). A prior study demonstrated a correlation of IL-22 plasma levels with disease severity in psoriasis patients, a condition also with known CAD and T2DM associations, suggesting that systemic IL-22 expression may be a key contributor to both PN disease pathogenesis and these associated comorbidities. (Daudén et al. 2013; Lønnberg et al. 2016; Wolk et al. 2006). In the epidermis, elevated levels of IL-22 promote keratinocyte hyperplasia and acanthosis as well as function synergistically with IL-17 to upregulate antimicrobial peptides (Boniface et al. 2005; Sonnenberg et al. 2011; Zheng et al. 2007). Since PN is characterized both clinically and histopathologically by keratinocyte hyperplasia and impaired epidermal differentiation, we hypothesized that PN would display Th22 polarization.
Improved molecular immune characterization has led to major breakthroughs in the management of chronic inflammatory diseases, such as atopic dermatitis (AD), psoriasis, and rheumatoid arthritis (Florian et al. 2020; Hawkes et al. 2017; O’Dell 2004; Thijs et al. 2017). When applied to PN, a similar strategy may better identify targeted therapies and guide the use of existing or emerging treatments. However, no study to date has performed circulating blood immunophenotyping and global cutaneous gene transcriptome analysis in PN patients. Therefore, to better characterize the immune response in patients with PN, we performed flow cytometry of peripheral blood mononuclear cells (PBMCs) along with RNA-sequencing and targeted immunohistochemistry of lesional PN, non-pruritic non-lesional PN, and healthy matched skin samples from majority African American patients (Table S1/S2). We observed that the systemic and cutaneous immune responses in patients with PN are skewed towards a Th22/IL-22 profile, and the degree of cutaneous upregulation correlates with itch severity.
RESULTS
Lesional PN skin is characterized histologically by marked inflammatory cell infiltrate and significant architectural changes
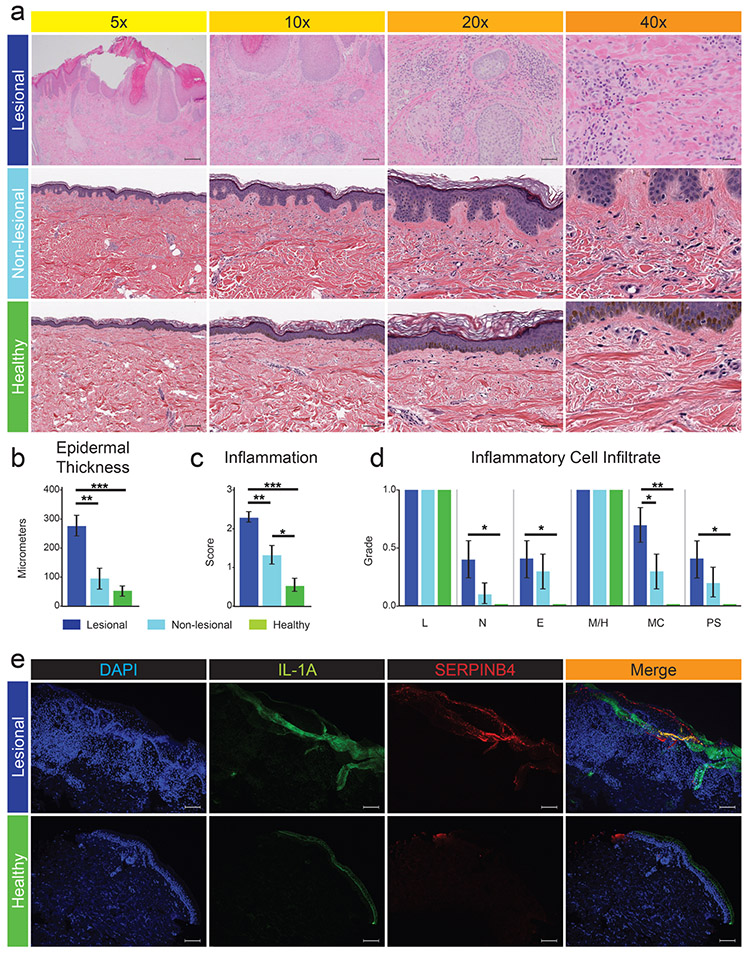
Histologic evaluation of lesional PN skin demonstrated significantly increased epidermal thickness as well as hyperkeratosis, hypergranulosis, acanthosis, spongiosis, increased vascularity and mild dermal fibrosis (Figure 1a-c) compared to non-lesional PN and healthy skin samples. Lesional PN skin displayed significantly greater inflammation with increased neutrophil, eosinophil, mast cell, and plasma cell infiltrates (Figure S1) compared to matched healthy skin (Figure 1d). Only mast cell infiltration was appreciably greater in lesional PN compared to non-lesional PN skin (Figure 1d).
Figure 1. PN lesional skin is characterized histologically by marked inflammatory cell infiltrate, robust architectural changes, and greater immunofluorescence signal intensity of IL1A and SERPINB4.
a, H&E staining of lesional PN, non-lesional PN, and matched healthy control skin tissue biopsies from African American females. b, Average epidermal thickness as measured at the thickest suprapapillary epidermal plate. c, Blinded dermatopathologist assessed degree of inflammation (0 = no, 3 = severe). d, Blinded dermatopathologist assessed presence (1) or absence (0) of specific inflammatory cell infiltrate across different skin samples with the average value shown. e, Immunofluorescence staining (IF) of DAPI, IL1A, and SERPINB4 in lesional PN and matched healthy skin from African American females with overlapping areas highlighted in gold. *p-value < .05, ** p-value < .01, and *** p-value < .001. PML, polymorphonuclear leukocyte; L, lymphocytes; N, neutrophils; E, eosinophils; M/H, macrophages / histiocytes; MC, mast cells; PC, plasma cells. 5x scale bar = 200um; 10x scale bar = 100um; 20x scale bar = 50um; 40x scale bar = 20um; IF scale bar = 200um.
Lesional PN skin demonstrates a distinct pattern of mRNA expression from non-lesional PN and healthy skin
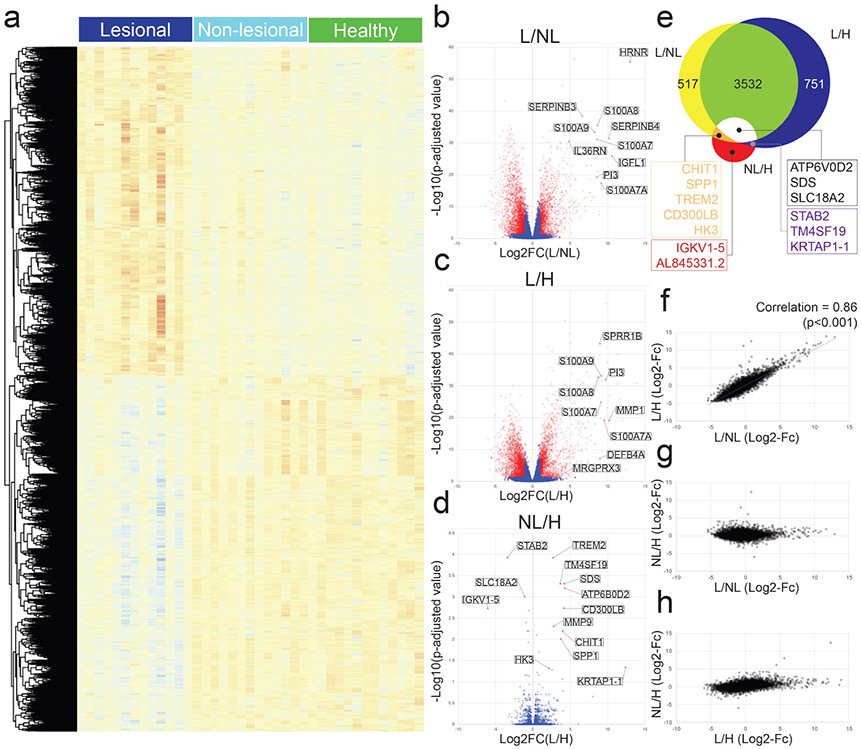
The skin transcriptome profile was performed in pruritic lesional PN skin, non-pruritic non-lesional PN skin, and matched healthy control skin (Figure 4a). We identified all differentially expressed genes (DEGs), defined as coding genes with a log2 fold change greater than 1 or less than −1 and a false discovery rate adjusted p-value less than 0.05 (Figure 4b-d).
Figure 4. Analyses of differentially expressed genes (DEGs) in lesional PN (n=13), non-lesional PN (n=13), and matched healthy skin (n=13).
DEGs defined as coding genes with a log base 2 fold change value less than −1 or greater than 1 and FDR adjusted p value less than 0.05 (−1 > logFc > 1, p<0.05). a, Heatmap of all DEGs by RNA-seq in lesional PN, non-lesional PN, and matched healthy control skin. Red, greater expression; blue, lower expression. b-d, Volcano plot compared gene expression in lesional to non-lesional PN skin (b) lesional to healthy skin (c) and non-lesional to healthy skin (d). e, Venn diagram comparison of DEGs. f-h, Plot comparison of DEG fold changes. L/H / L/NL (f). NL/H / L/NL (g). NL/H / L/H (h). L, lesional PN samples; NL, non-lesional PN samples; H, matched healthy control samples.
Lesional PN skin demonstrated a distinct pattern of mRNA expression compared to non-lesional PN and healthy skin (Figure 4e). A significant correlation in mRNA expression differences was observed between lesional PN versus non-lesional PN skin and lesional PN versus healthy skin (Figure 4f). Few significant differences in mRNA expression were observed between non-lesional PN and matched healthy skin (Figure 4e-h). Full lists of DEGs for all comparisons are provided in Tables S4-S8.
Lesional PN skin shows Th17/22 skewing
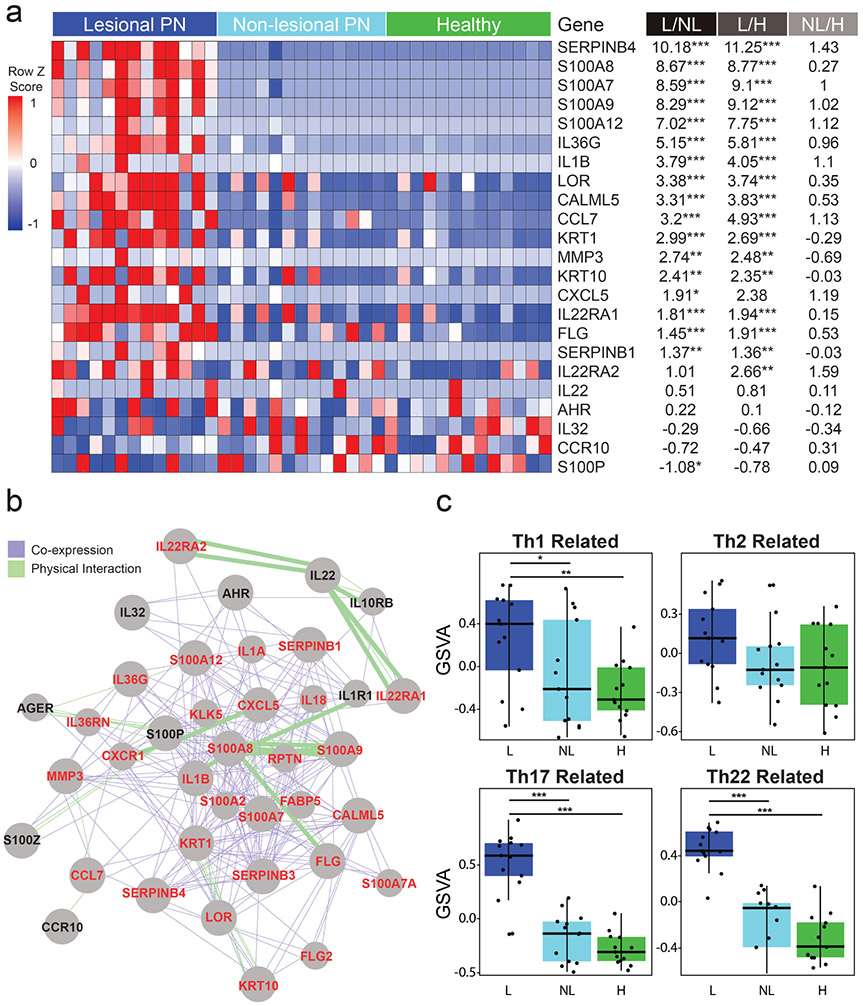
mRNA-sequencing revealed that lesional PN samples expressed significantly increased levels of Th17/Th22 associated S100 genes (S100A7/A8/A9/A12), LOR, and IL36G compared to non-lesional PN and healthy control skin samples. We also observed significantly increased expression of Th22/IL-22 associated genes, including SERPINB4, CALML5, MMP3, CCL7, CXCL5, IL-1β, as well as IL-22 receptors IL22RA1/A2 (Figure 5a,b, and Figure S4). We subsequently observed significantly increased expression of affected downstream genes such as IL-1β induced KRT6/17 and SERPINB4 induced IL1A. Increased SERPINB4 and IL-1α in lesional PN compared to matched healthy skin were also observed with immunofluorescence (Figure 1e). Increased expression of Th17/IL-17 induced genes was observed. Th2 associated genes STAT4 and CCL13 expressions were significantly decreased while no significant differences in expression were observed for several Th2-related genes, including IL-4/13, STAT6, CCL17/22, and CCR5 (P>0.05) (Figure S4).
Figure 5. Cutaneous mRNA analyses indicate Th22 immune polarization.
a, A heat map of cutaneous mRNA expression of select Th22/IL-22 related genes. Red, greater expression; blue, lower expression. Fold change shown is log base 2 fold-change. p-value shown is a false discovery rate adjusted p value. b, GeneMANIA functional association gene network for Th22-associated genes. The co-expression and physical interaction between genes are expressed as purple and green lines, respectively. Stronger associations are shown with thicker lines. Gene names shown in red are upregulated, while names in black show no significant difference in expression in PN lesional skin compared to healthy controls. c, Gene Set Variation Analyses (GSVA) comparison of immune mediators in lesional PN (n=13) to non-lesional (n=13) and healthy skin (n=13) found lesional PN skin is characterized by significantly increased expression of Th17/Th22-markers, mildly elevated Th1, and no significant difference in expression of Th2 related genes. *p-value < .05, ** p-value < .01, and *** p-value < .001. L, lesional PN samples; NL, non-lesional PN samples; H, matched healthy control samples.
Gene set variation analyses (GSVAs) were performed to compare the expression of Th1, Th2, Th17, and Th22 markers. Lesional PN skin demonstrated robust increased Th22-related gene expression. Additionally, we observed a significantly increased Th17-marker expression, mildly yet statistically significant increase in Th1-marker expression and no significant differences in Th2 related genes (Figure 5c). To correlate clinical findings with the degree of immune dysregulation, we compared itch severity with GSVA for Th1, Th2, Th17, and Th22 related genes in pruritic lesional PN skin and biopsy matched non-pruritic healthy control skin (Figure S5). Among these gene families, itch severity most strongly correlated with the degree of Th22 related gene dysregulation (R 0.89 p<0.001). GSVA by race subgroups revealed significant Th22/Th17 skewing to be present in both European and African Americans (Figure S7). We only observed a trend towards greater Th2 skewing in Europeans, with decreased expression of Th2 cytokines IL-4, IL-5, IL-13, and IL-31 in African Americans compared to Europeans (Table S7). GSVA by biopsy site subgroups (arms, legs, back) revealed only Th22 skewing to be consistent across all sites (Figure S8). Interestingly, no single anatomic site was clearly the most pruritic across patients.
Vδ2+ γδ T-cells and iNKT-cells are enriched in PN
To immunophenotype T-cells and their cytokine expression in PN, peripheral blood mononuclear cells (PBMCs) from PN and healthy controls were stimulated with a pan-T-cell cocktail containing phorbol 12-myristate 13-acetate (PMA) and Ionomycin for 4 hours at 37°C. The stimulated cells were evaluated for T-cell responses and their cytokine profile by flow cytometry. Cells were stained with surface T-cell markers, including CD3, CD4, CD8, γδTCR, and CD56, along with intracellular cytokine markers, including IL-22, TNF, IL-4, TGF-β, IFN-γ, IL-17, IL-10 and IL-13 respectively. T-cell populations were assayed (flow cytometry gating strategy is shown in the online supplemental data, Figure S2a-d).
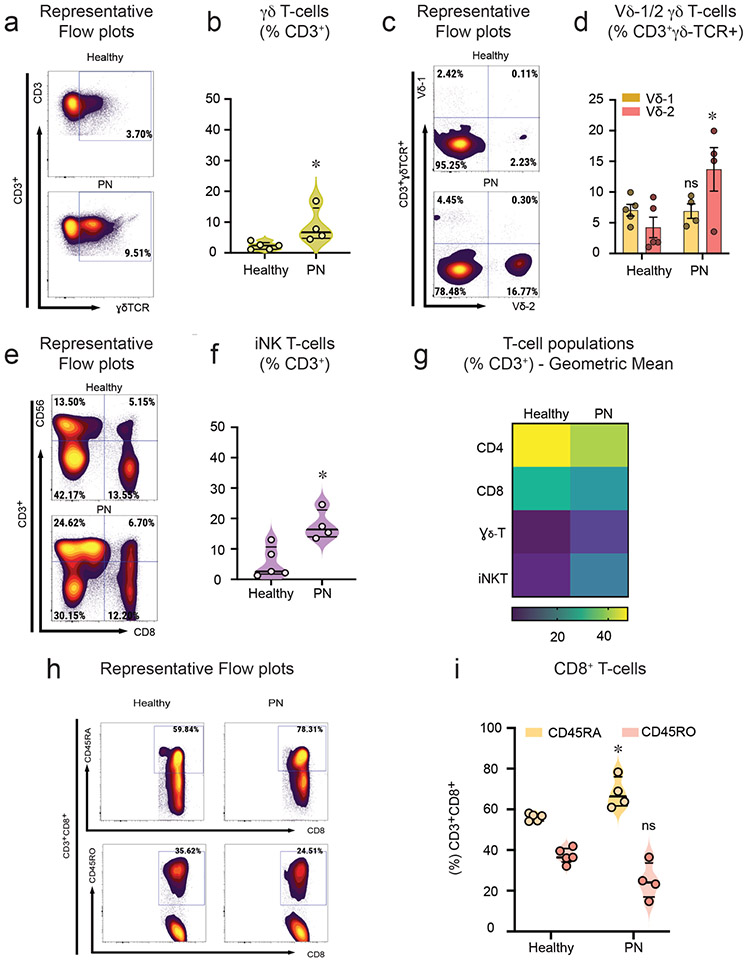
γδ T-cells (CD3+CD4−CD8−gdTCR+) were increased (Figure 2a,b), and within the γδ T-cells Vd2+ γδ T-cells were enriched upon stimulation in PN subjects when compared to healthy controls (Figure 2c,d). Interestingly, iNK T-cells (CD3+CD4−CD8−CD56+) were also significantly increased in PN (Figure 2e,f). The absolute numbers for cell populations γδ T-cells and iNKT cells are shown in online supplemental data, Fig S9 a-c. There were reduced CD4+ T-cells in PN samples compared to controls (Figure 2g). The CD4 (CD3+CD4+) and CD8 (CD3+CD8+) ratio and its corresponding absolute numbers are presented in Figure 2d-g. Naïve CD8 T-cells (CD3+CD8+CD45RA+) were significantly increased in PN subjects (Figure 2h,i). There were no differences in CD8+ memory T-cells (CD3+CD8+CD45RO+) or the expression of CD8+ T-cells in PN when compared to healthy controls (Figure 2g-i).
Figure 2. Vδ2+γδ T and iNKT-cells are enriched in PN patients.
PBMCs from PN (n=4) and healthy subjects (n=5) (67% African American) were stimulated with PMA and Ionomycin in combination with protein transport inhibitor for 4hrs. The differentiation of T-cell subtypes was assessed by flow cytometry. a, Representative flow cytometry plots for γδ T-cells (CD3+CD4− CD8−γδTCR+ cells). b, Percentage of CD3+ γδ T-cells (median ± ICR). c, Representative flow cytometry plots for Vδ1+ and Vδ2+ subsets of γδ – T cells. d, Percentage of sub-population of γδ-T cells (CD3+γδ-TCR+Vδ1+ or Vδ2+) (median ± ICR). e, Representative flow cytometry plots for iNKT-cells (CD3+CD4−CD8−CD56+). f, Percentage of CD3+ iNKT – cells (median ± ICR). g, Heat map representing geometric mean expression of T-cell populations from healthy and PN subjects as represented by CD4+ T helper (CD3+CD4+CD8−) cells, CD8+ T cytotoxic cells (CD3+CD8+ CD4−), γδ T-cells (CD3+CD4−CD8− TCR+ cells), iNKT-cells (CD3+CD4−CD8− CD56+). h, Representative flow cytometry plots for CD8 Naïve (CD3+CD8+CD45RA+) cells and CD8 Memory (CD3+CD8+CD45RO+) cells. i, Percentage of CD8 Naïve and CD8 Memory cells (median ± ICR). *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U-test. ns = non-significant.
Activated T-cells show Th22 skewing in PN
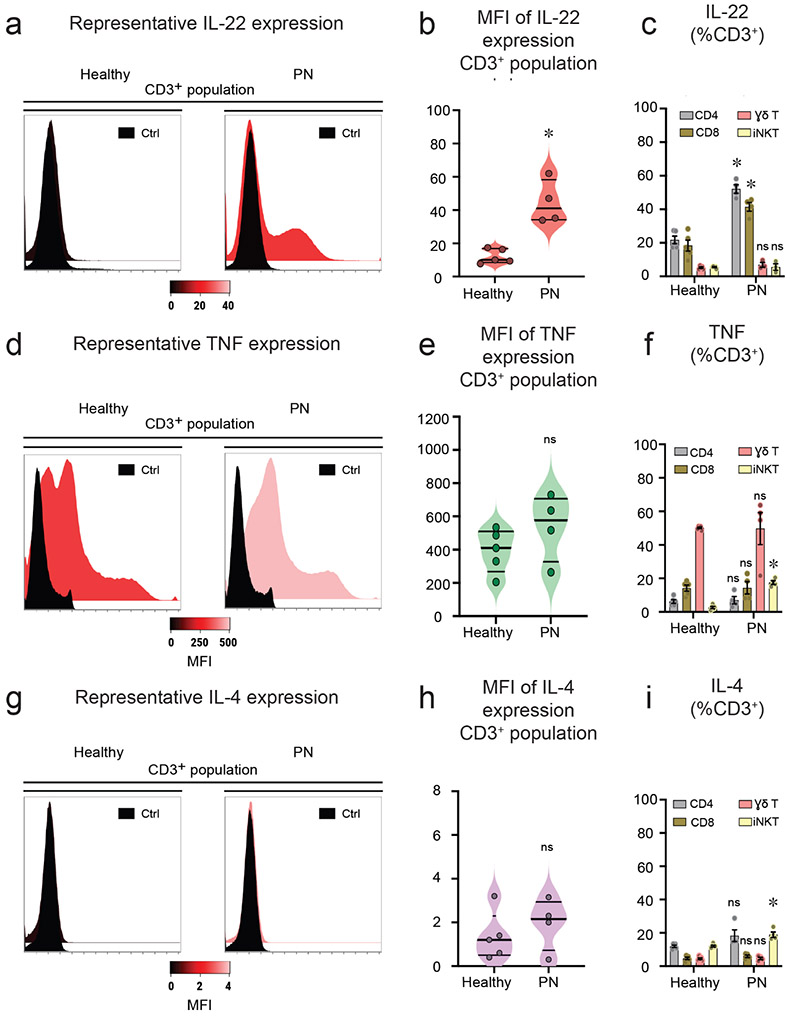
To determine the cytokine profile of T-cells in PN, stimulated PBMCs were stained for intracellular cytokine markers, including IL-22, TNF, IL-4, TGF-β, IFN-γ, IL-17, IL-10 and IL-13. T-cell populations and their cytokine profile were assayed. Activated T-cells showed increased IL-22 cytokine expression in PN compared to healthy controls (Figure 3a,b). CD4+ and CD8+ T-cell populations were identified as the source of increased circulating IL-22 (Figure 3c). The expression of TNF was marginally increased in PN subjects (Figure 3d,e). Among T-cell populations, iNK T-cells showed increased expression of TNF cytokine when compared to healthy controls (Figure 3f). There was no difference in the expression of IL-4 cytokine between PN and healthy controls (Figure 3g,h). However, there was a modest increase in IL-4 cytokine expression observed in iNK T-cells in PN subjects (Figure 3i). The absolute numbers for cell populations expressing IL-22, TNF and IL-4 are shown in Figure S10a-c. Interestingly, TGF-β expression in γδ T-cells was markedly reduced in PN subjects (Figure S3a). Furthermore, no differences in cytokine expression of IFN-γ, IL-17, IL-10, and IL-13 between PN and healthy controls were observed upon stimulation (Figure S3b-e). Interestingly, PBMCs from PN subjects did not express significant amounts of IL-17. To validate our flow expression of IL-17, PBMCs from patients with bacteremia showed increased IL-17 expression (Figure S11), suggesting that there were indeed less amounts of IL-17 expression from PN subjects.
Figure 3. Activated T-cells exhibit an IL-22 phenotype in PN.
PBMCs from PN (n=4) and healthy subjects (n=5) were stimulated with PMA and ionomycin combined with protein transport inhibitor for 4hrs. The cells were analyzed for intracellular cytokine expression by flow cytometry. a, Representative flow cytometry histograms of intracellular IL-22 cellular expression versus unstained control (Ctrl). b, Mean fluorescence intensity (MFI) of IL-22 expressing CD3+ cells (median ± ICR). c, Percentage of IL-22 expressing T-cell subsets including CD4, CD8, γδ T-cells, and iNKT – cells ± SEM. d, Representative flow cytometry histograms of intracellular TNF cellular expression versus unstained control (Ctrl). e, Mean fluorescence intensity (MFI) of TNF expressing CD3+ cells (median ± ICR). f, Percentage of TNF expressing T-cell subsets including CD4, CD8, γδ T-cells, and iNKT-cells ± SEM. g, Representative flow cytometry histograms of intracellular IL-4 cellular expression versus unstained control (Ctrl). h, Mean fluorescence intensity (MFI) of IL-4 expressing CD3+ cells (median ± ICR). i, Percentage of IL-4 expressing T-cell subsets including CD4, CD8, γδ T-cells, and iNKT-cells ± SEM. *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U- test. ns = non-significant.
DISCUSSION
Our results demonstrate that PN is characterized by distinct circulating and cutaneous Th22 immune dysregulation. We also identified circulating CD4+ and CD8+ T-cell populations as the source of increased IL-22 secretion. While AD and psoriasis have been grouped into dominant Th2 and Th17 immune phenotypes, respectively, similar analyses have not been explored in PN, a condition with equally severe comorbidities and greater pruritus intensity (Boozalis et al. 2018; Gudjonsson et al. 2010; Huang et al. 2019; Sanyal et al. 2019; Schedel et al. 2014; Suarez-Farinas et al. 2013; Whang et al. 2019a; Zhong et al. 2019).
The cytokine IL-22 is important in the modulation of tissue responses during skin inflammation and wound healing. Specifically, IL-22 is known to increase expression of epidermal differentiation complex related S100 genes (S100A7/A9/A12), matrix metalloproteinase (MMP3), chemokines (CCL7, CXCL5), serine proteinase inhibitors (SERPINB1/4), and proinflammatory cytokines like IL-1β (Boniface et al. 2005; Eyerich et al. 2009; Zheng and Li 2018). Activation of these genes drives numerous inflammatory processes such as IL-1β induced KRT6/17, known to be involved in cutaneous inflammation, wound repair, and keratinocyte hyperproliferation, as well as SERPINB4 induced IL-1α, known to increase expression of epidermal differentiation complex genes, proteases and antimicrobial transcripts (Komine et al. 2001; Mee et al. 2007; Titapiwatanakun et al. 2005; Zhang et al. 2019). Lesional skin among PN patients in our study demonstrated significantly increased mRNA levels of each of these genes. PN’s nodular clinical presentation, as well as our histological findings of epidermal hyperplasia, acanthosis, and significant inflammatory infiltrate in PN lesional skin, are highly reflective of Th22/IL-22 immune activation (Boniface et al. 2005; Eyerich et al. 2009; Zheng et al. 2007). Additionally, elevated IL-22 has been observed in patients with coronary artery disease and type II diabetes, suggesting a pathophysiologic link between PN and its associated increased risk of metabolic and coronary diseases (Boozalis et al. 2018; Gong et al. 2016; Huang et al. 2020b; Huang et al. 2019; Whang et al. 2019b; Whang et al. 2019a).
We observed significantly increased Vδ2+ γδ T-cells in PBMCs of PN patients and found that activated T-cells showed Th22 polarization. Previous studies have also observed γδ T-cells to be significant sources of IL-22; however, the role of circulating γδ T-cells in cutaneous disease pathogenesis remains poorly understood (Ness-Schwickerath and Morita 2011). Additionally, we observed elevated expression of IL-22 receptors IL22RA1/2 in lesional PN skin. As IL-22R expression is largely confined to epithelial cells, implying tissue specificity, our results suggest persistently elevated systemic IL-22 drives PN in combination with cutaneous IL-22R up-regulation with downstream cutaneous immunomodulation resulting in epidermal differentiation and accompanying severe pruritus (Lou et al. 2017; Wolk et al. 2004).
In addition to elevated Th22-markers in lesional PN skin, GSVAs showed Th17-marker elevation, relatively less up-regulation of Th1 associated genes, and no significant change in Th2 associated genes. Of note, elevated Th17-markers in PN skin were observed without corresponding elevations in circulating IL-17 or IFN-γ cytokine expression among PBMCs from patients with PN. The observed increase in localized Th17 associated genes in PN patients may, therefore, result from shared cutaneous Th17/22 gene signatures (Nograles et al. 2008). Alternatively, increased cutaneous Th17 related markers may reflect localized IL-17-mediated hyperkeratosis as observed in keloid pathogenesis or mast cell secreted IL-17 as seen in koebnerization among patients with psoriasis (Ji and Liu 2019; Lee et al. 2020). Previous studies have shown that mast cells are among the predominant cell types containing IL-17 in human skin, and consistent with previous studies, we observed notably increased mast cell infiltration in lesional PN compared to both non-lesional PN and healthy skin (Liang et al. 1998; Lin et al. 2011). Proteinase activated receptor (PAR)-2, which provoked mast cell accumulation in murine models, and IL-1β, known to induce human mast cell degranulation, were also significantly elevated in our lesional PN skin samples (Lin et al. 2011; Liu et al. 2016). Additionally, the effects of IL-22 are known to be context-dependent (Sonnenberg et al. 2011). IL-22, in combination with IL-17, promotes inflammation, inhibits keratinocyte differentiation, and increases keratinocyte proliferation (Deng et al. 2016; Sonnenberg et al. 2011). Therefore, the elevated cutaneous Th17-related markers may act as local catalysts to drive principally Th22 mediated inflammation.
Interestingly, our unstratified analyses did not observe significant IL-31 upregulation, a key mediator previously implicated in PN (Mikhak et al. 2019; Sonkoly et al. 2006). As IL-31 is primarily expressed on nerve tissue in the epidermis and spinal cord, IL-31 expression may be reduced in bulk RNA-Seq techniques such as used in our study (Cevikbas et al. 2014; Feld et al. 2016). However, when stratified by race, we observed increased IL-31 expression in Europeans compared to African Americans. Indeed, previous studies showing IL-31 upregulation consisted largely of European American PN patients (Mikhak et al. 2019; Sonkoly et al. 2006). This immune heterogeneity may be particularly important as a recent trial of the IL-31 inhibitor nemolizumab significantly reduced pruritus in PN patients, yet 97% of the patients were European Americans, highlighting the need for greater diversity in future clinical trials (Ständer et al. 2020).
Studies of patients with AD, widely accepted as Th2 centric, have revealed variation in cytokine predominance between racial groups, including the predominance of Th2/Th22 molecular phenotype in African American patients (Brunner and Guttman-Yassky 2019; Noda et al. 2015; Nomura et al. 2018; Sanyal et al. 2019). Our results reveal Th22 skewing in both African American and European patients. Given previously observed increased expression of IL-22 in two smaller studies of Asian patients with PN, our findings suggest Th22-related cytokine upregulation in PN may be independent of race (Park et al. 2011; Wong et al. 2020). In contrast, we observed significantly increased expression of Th2 cytokines including IL-4, IL-5 and IL-13 as well as greater Th2 activation by GSVA among European but not African American patients. Fukushi et al. previously showed Th2 as a principal driver of PN in a Japanese cohort (Fukushi et al. 2011). Similarly, case reports of PN responding to dupilumab, a monoclonal antibody targeting IL-4Rα, involved predominantly European or Hispanic patients (Calugareanu et al. 2019; Rambhia and Levitt 2019; Tanis et al. 2019). We observed cutaneous upregulation of IL-4Rα, without significant concomitant upregulation in cutaneous Th2-associated genes and minimal circulating IL-4 upregulation. It is therefore possible that dupilumab may be most effective in certain endotypes of PN patients with associated atopy, that IL-4Rα may also be involved in the signaling of other pruritogens in PN, or that our observed nonsignificant changes in Th2 expression in our African American cohort may represent a characteristic molecular phenotype of this specific population. Additionally, although prior studies have shown cutaneous inflammatory activation varies anatomically, we observed lesional Th22 skewing across all body sites (Del Duca et al. 2019).
In summary, our results indicate both the cutaneous and systemic immune responses in PN subjects exhibit a predominant Th22/IL-22 profile. These results suggest patients with PN may benefit from Th22/IL-22 inflammation modulating therapies. Limitations include sample size and a predominantly African American patient study population without atopy. Consequently, our results may be limited to this specific population of patients with PN. Future studies should assess the levels of IL-22 and Th22-associated genes at varying time points of PN progression. Furthermore, studies should determine immune polarization in PN patients of different races and genders.
MATERIALS & METHODS
A prospective Johns Hopkins IRB approved study was performed comparing PBMCs and skin biopsies from patients with PN and race-, sex- and age-matched healthy patients (Tables S1/S2). Written, informed consent was obtained from each study participant. Body site-matched skin biopsies were collected from healthy control skin (n=13) and pruritic lesional (n=13) and non-pruritic, non-lesional (n=13) skin from patients with PN. Lesional PN skin biopsy was obtained from the most pruritic nodule as self-reported by the patient. Non-lesional PN skin biopsy was obtained at least 10 cm from the PN lesional site on normal appearing skin; healthy control skin biopsy was body site-matched with PN biopsies. Informed consent was obtained from each study participant. A schematic of the study design is diagramed in Figure S6. Inclusion criteria for PN patients consisted of clinically diagnosed PN by an experienced dermatologist and pruritus greater than 7 on the Itch Numeric Rating Scale (Phan et al. 2012). Patients with a known underlying or history of atopic or cutaneous diseases such as psoriasis or AD were excluded from the study. Adult patients with clinically determined healthy skin were included as controls and were matched to participating PN patients by sex, race, and age.
Flow cytometry was performed using PBMCs from patients with PN (n=5) and matched healthy control subjects (n=5) were obtained (Table S2). Thirteen adults with histologically and clinically diagnosed PN and thirteen matched control subjects (13 lesional samples, 13 non-lesional samples and 13 healthy control samples) were included in the analysis (Table S1). Details of H&E staining, Immunofluorescence staining, Flow cytometry and mRNA-sequencing materials and methods are provided in the Supplemental Materials and Methods File.
Analyses were performed using Excel (Microsoft, Redding WA). Standard two-tailed t-tests were used to assess significant differences across continuous variables. In all analyses, a p-value threshold of <0.05 was considered statistically significant. FACS data are presented as mean ± standard error of the mean (SEM), and violin plots expressed with median and interquartile range (IQR). Mann-Whitney U test was performed between the healthy controls and PN samples. Statistics were analyzed in Prism 9.0. Normalization and differential expression of mRNA data were carried out using the DESeq2 Bioconductor package with the R statistical programming environment (Huber et al. 2015; Love et al. 2014). Paired or matched sample modeling included pairID as a cofactor. The false discovery rate was calculated to control for multiple hypothesis testing. Immune dysregulation of gene sets was assessed using GSVAs conducted with the GSVA R Bioconductor package using R version 3.6.3 (Hänzelmann et al. 2013). P values from paired t-tests were adjusted for multiple hypotheses using the Benjamini-Hochberg procedure (Benjamini and Hochberg 1995).
Supplementary Material
Figure S1. 80x magnification of H&E staining of PN lesional skin, which demonstrates a heterogeneous infiltrate of inflammatory cells, including: a. eosinophils (orange arrow), lymphocytes (blue arrow), and plasma cells (green arrow), b. mast cells (yellow arrow) and plasma cells (green arrow), c. eosinophils (orange arrow), lymphocytes (blue arrow), and neutrophils (black arrow), d. eosinophils (orange arrow) and lymphocytes (blue arrow). Scale bar = 40um.
Figure S2. Gating strategy for cell populations with representative flow plots for the CD3+ T cell population. Representative flow plots showing the gating strategy for CD8-T cells (CD3+CD8+CD4−), CD4-T cells (CD3+CD4+CD8−), iNKT – cells (CD3+CD4−CD8−CD56+), and γδ T-cells (CD3+CD4−CD8−γδTCR+), respectively.
Figure S3. Intracellular cytokine profile in PN patients. PBMCs from PN (n=4) and healthy subjects (n=5) were stimulated with PMA and Ionomycin combined with protein transport inhibitor for 4hrs. The cells were analyzed for intracellular cytokine expression by flow cytometry. a, Percentage of TGF-β expressing CD3+ T-cell populations ± SEM. b, Percentage of IFN-γ expressing CD3+ T-cell populations ± SEM. c, Percentage of IL-17 expressing CD3+ T-cell populations ± SEM. d, Percentage of IL-10 expressing CD3+ T-cell populations ± SEM. e, Percentage of IL-13 expressing CD3+ T-cell populations ± SEM. *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U- test. ns = nonsignificant.
Figure S4. Heat map of cutaneous gene expression with mRNA-sequencing highlights differences in immune signature in lesional PN skin. Red, greater expression; blue, lower expression. Fold change shown is log base 2 fold-change. p-value shown is a false discovery rate adjusted p value. * p-value < .05, ** p-value < .01, and *** p-value < .001. L, lesional PN samples; NL, non-lesional PN samples; H, matched healthy control samples.
Figure S5. Correlation of itch numeric rating scale values with GSVA scores of Th1, Th2, Th17, and Th22 related genes from PN patients and controls. Itch severity most strongly correlated with the degree of Th22 related gene expression.
Figure S6. Outline of experimental design. Under IRB approval, peripheral blood mononuclear cells (PBMCs) and skin samples were obtained from PN patients and healthy subjects matched by age, race, and gender. Flow cytometric analysis compared numbers and phenotypic characteristics of γδ+ T-cells in the peripheral blood of patients with PN and matched healthy controls. RNA-sequencing with immunohistochemistry validation compared gene expression in pruritic lesional PN, non-pruritic non-lesional PN, and biopsy site matched control skin.
Figure S7. Gene Set Variation Analysis of immune mediators in lesional PN (n=13 samples), non-lesional (n=13 samples), and matched healthy skin (n=13 samples), by race: African Americans (10 matched patients) and European Americans (3 matched patients). Th22 upregulation is observed in both European and African Americans. . * p-value < .05, ** p-value < .01, and *** p-value < .001. AA, African American; EA, European American.
Figure S8. Gene Set Variation Analysis of immune mediators in lesional PN (n=13), non-lesional (n=13), and matched healthy skin (n=13), by biopsy site: arms (n=4), legs (n=6), and back (n=3). Th22 upregulation is observed across all biopsy sites. . * p-value < .05, ** p-value < .01, and *** p-value < .001.
Figure S9. Absolute cell number for T cell population. Absolute cell numbers from PBMCs from PN (n=4) and healthy subjects (n=5) a, Absolute number of CD3+ γδ T-cells (median ± ICR). b, Absolute number of sub-populations of γδ-T cells ± SEM. c, Absolute number of iNKT – cells (median ± ICR). d, Representative flow cytometry plots for CD4+ T helper (CD3+CD4+CD8−) cells, and CD8+ T cytotoxic cells (CD3+CD8+ CD4−). e. Percentage of the ratio of CD4+ and CD8+ cell population ± SEM. f. Absolute number of CD4+ T-cells (median ± ICR). g. Absolute number of CD8+ T-cells (median ± ICR). h. Absolute number of CD8 Naïve and CD8 Memory cells (median ± ICR). *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U-test. ns = non-significant.
Figure S10. Absolute cell number of T-cell populations expressing cytokines. Absolute cell numbers from PBMCs from PN (n=4) and healthy subjects (n=5) a, Absolute number of IL-17 expressing T-cell populations ± SEM. b, Absolute number of TNF expressing T-cell populations. ± SEM. c, Absolute number of IL-4 expressing T-cell populations. ± SEM. *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U-test. ns = nonsignificant.
Figure S11. Representative histogram of IL-17 expression by CD3+ T cells. PBMCs from PN (n=4) and bacteremia (n=5) were stimulated with PMA and Ionomycin combined with protein transport inhibitor for 4hrs. The cells were analyzed for intracellular cytokine expression by flow cytometry. Representative flow cytometry histograms of intracellular IL-17 cellular expression versus unstained control (Ctrl).
Table. S1. Study cohort demographic information for matched patients recruited to provide skin samples, mRNA, and immunofluorescence analyses. PN, Prurigo nodularis; HC, healthy control; F, female; M, male; AA, African American; Itch NRS, itch numeric rating scale.
Table. S2. Study cohort demographic information for matched patients recruited to provide PBMCs for flow cytometry analyses. *Too few cells were collected, therefore patients were excluded from analyses. PN, Prurigo nodularis; HC, healthy control; F, female; M, male; AA, African American; Itch NRS, itch numeric rating scale.
Table. S3. The panel of mAbs and fluorescent-tagged dyes used for the detection of immune cell populations
Table. S4. Full list of DEGs (adjusted P<0.05) in lesional (n=13) vs. non-lesional PN (n=13)
Table. S5. Full list of DEGs (adjusted P<0.05) in lesional PN (n=13) vs. matched healthy skin (n=13)
Table. S6. Full list of DEGs (adjusted P<0.05) in non-lesional PN (n=13) vs. matched healthy skin (n=13)
Table. S7. Full list of DEGs (adjusted P<0.05) in African American (n=10) compared to European American (n=3) PN lesional skin. Select immune genes (IL4, IL5, IL13, IL31) are also shown.
Table. S8. Full list of DEGs (adjusted P<0.05) in African American (n=10) compared to European American (n=3) non-lesional PN skin.
ACKNOWLEDGMENTS
Thank you to the patients who agreed to participate in this study. We wish to thank the Johns Hopkins Clinical and Translational Research team for coordinating and assisting in this project as well as the GRCF Cell Center and Biorepository for all the technical supports in processing and storing PBMC. We also wish to thank the Skin of Color Society and the Dermatology Foundation Medical Dermatology Career Development Awards to SGK for their support. This research is also supported by grants from the National Institute of Health (NIH) 5T32GM007309, F30HL142131, and Kiniksa Pharmaceuticals, Ltd.
ABBREVIATIONS
- PN
Prurigo nodularis
- IL
Interleukin
- PBMCs
peripheral blood mononuclear cells
- DEGs
differentially expressed genes
- GSVAs
Gene set variation analyses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. Kwatra is an advisory board member/consultant for Abbvie, Galderma, Incyte Corporation, Pfizer Inc., Regeneron Pharmaceuticals, and Kiniksa Pharmaceuticals and has received grant funding from Galderma, Pfizer Inc. and Kiniksa Pharmaceuticals. Kent Bondesgaard and John F Paolini are employed by Kiniksa Pharmaceuticals, Corp., Lexington, MA, USA. All other authors have no relevant commercial associations that might pose a conflict of interest. This research is in part supported by a grant from Kiniksa Pharmaceuticals, Ltd.
DATA AVAILABILITY STATEMENT
All data referenced in this study are included in the figures, tables and supplemental files.
REFERENCES
- Bagci IS, Ruzicka T. IL-31: A new key player in dermatology and beyond. The Journal of allergy and clinical immunology. United States; 2018;141(3):858–66 [DOI] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. The New England journal of medicine. United States; 2014;371(2):130–9 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate : A Practical and Powerful Approach to Multiple Testing Author. Journal of the Royal Statistical Society. 1995;57(1):289–300 Available from: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Boniface K, Bernard F-X, Garcia M, Gurney AL, Lecron J-C, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. Journal of immunology (Baltimore, Md : 1950). United States; 2005;174(6):3695–702 [DOI] [PubMed] [Google Scholar]
- Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. Journal of the American Academy of Dermatology. United States; 2018;79(4):714–719.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. United States; 2019;122(5):449–55 [DOI] [PubMed] [Google Scholar]
- Calugareanu A, Jachiet M, Lepelletier C, De Masson A, Rybojad M, Bagot M, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. Journal of the European Academy of Dermatology and Venereology. England; 2019;33(8):1–2 [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology. 2014;133(2):448–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudén E, Castañeda S, Suárez C, García-Campayo J, Blasco AJ, Aguilar MD, et al. Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis. Journal of the European Academy of Dermatology and Venereology : JEADV. England; 2013;27(11):1387–404 [DOI] [PubMed] [Google Scholar]
- Deng Y, Chang C, Lu Q. The Inflammatory Response in Psoriasis: a Comprehensive Review. Clinical Reviews in Allergy & Immunology. 2016;50(3):377–89 Available from: 10.1007/s12016-016-8535-x [DOI] [PubMed] [Google Scholar]
- Del Duca E, Pavel AB, Dubin C, Song T, Wallace EB, Peng X, et al. Major Differences in Expression of Inflammatory Pathways in Skin from Different Body Sites of Healthy Individuals. The Journal of investigative dermatology. United States; 2019. p. 2228–2232.e10 [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. The Journal of clinical investigation. 2009;119(12):3573–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. The Journal of allergy and clinical immunology. United States; 2016;138(2):500–508.e24 [DOI] [PubMed] [Google Scholar]
- Florian P, Flechsenhar KR, Bartnik E, Ding-Pfennigdorff D, Herrmann M, Bryce PJ, et al. Translational drug discovery and development with the use of tissue-relevant biomarkers: Towards more physiological relevance and better prediction of clinical efficacy. Experimental dermatology. 2019/June/11. Denmark; 2020;29(1):4–14 Available from: https://pubmed.ncbi.nlm.nih.gov/30991456 [DOI] [PubMed] [Google Scholar]
- Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. The British journal of dermatology. England; 2011;165(5):990–6 [DOI] [PubMed] [Google Scholar]
- Gong F, Wu J, Zhou P, Zhang M, Liu J, Liu Y, et al. Interleukin-22 Might Act as a Double-Edged Sword in Type 2 Diabetes and Coronary Artery Disease. Mediators of inflammation. 2016;2016:8254797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Johnston A, Tejasvi T, Guzman AM, Nair RP, et al. Assessment of the Psoriatic Transcriptome in a Large Sample: Additional Regulated Genes and Comparisons with In Vitro Models. Journal of Investigative Dermatology. 2010;130(7):1829–40 Available from: http://www.sciencedirect.com/science/article/pii/S0022202X15348983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S, Capellino S, Phan NQ, Bohm M, Luger TA, Straub RH, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. Journal of dermatological science. Netherlands; 2010;58(3):193–7 [DOI] [PubMed] [Google Scholar]
- Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC bioinformatics. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. The Journal of allergy and clinical immunology. United States; 2017;140(3):645–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Canner JK, Kang S, Kwatra SG. Analysis of real-world treatment patterns in patients with prurigo nodularis. Journal of the American Academy of Dermatology. United States; 2020a;82(1):34–6 [DOI] [PubMed] [Google Scholar]
- Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. The Journal of investigative dermatology. United States; 2019; [DOI] [PubMed] [Google Scholar]
- Huang AH, Williams KA, Kwatra SG. Prurigo Nodularis: Epidemiology and Clinical Features. Journal of the American Academy of Dermatology. United States; 2020b; [DOI] [PubMed] [Google Scholar]
- Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nature methods. United States; 2015;12(2):115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. Journal of the European Academy of Dermatology and Venereology : JEADV. England; 2013;27(5):550–7 [DOI] [PubMed] [Google Scholar]
- Ji Y-Z, Liu S-R. Koebner phenomenon leading to the formation of new psoriatic lesions: evidences and mechanisms. Bioscience reports. 2019;39(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine M, Rao LS, Freedberg IM, Simon M, Milisavljevic V, Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. The Journal of investigative dermatology. United States; 2001;116(2):330–8 [DOI] [PubMed] [Google Scholar]
- Kwatra SG. Breaking the Itch-Scratch Cycle in Prurigo Nodularis. The New England journal of medicine. United States; 2020. p. 757–8 [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Kim EK, Seo HB, Choi JW, Yoo JH, Jung KA, et al. IL-17 Induced Stromal Cell-Derived Factor-1 and Profibrotic Factor in Keloid-Derived Skin Fibroblasts via the STAT3 Pathway. Inflammation. 2020;43(2):664–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. CGRP-immunoreactive nerves in prurigo nodularis--an exploration of neurogenic inflammation. Journal of cutaneous pathology. United States; 2000;27(7):359–66 [DOI] [PubMed] [Google Scholar]
- Liang Y, Marcusson JA, Jacobi HH, Haak-Frendscho M, Johansson O. Histamine-containing mast cells and their relationship to NGFr-immunoreactive nerves in prurigo nodularis: a reappraisal. Journal of cutaneous pathology. United States; 1998;25(4):189–98 [DOI] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. Journal of immunology (Baltimore, Md : 1950). 2011;187(1):490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang J, Zhang H, Zhan M, Chen H, Fang Z, et al. Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediators of inflammation. 2016/June/09. Hindawi Publishing Corporation; 2016;2016:6431574 Available from: https://pubmed.ncbi.nlm.nih.gov/27378825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of Psoriasis With the Risk for Type 2 Diabetes Mellitus and Obesity. JAMA dermatology. United States; 2016;152(7):761–7 [DOI] [PubMed] [Google Scholar]
- Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. Journal of immunology (Baltimore, Md : 1950). 2017;198(7):2543–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. England; 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. The Journal of allergy and clinical immunology. United States; 2015;136(2):351–9.e1 [DOI] [PubMed] [Google Scholar]
- Matsumura S, Terao M, Murota H, Katayama I. Th2 cytokines enhance TrkA expression, upregulate proliferation, and downregulate differentiation of keratinocytes. Journal of dermatological science. Netherlands; 2015;78(3):215–23 [DOI] [PubMed] [Google Scholar]
- McColl M, Boozalis E, Aguh C, Eseonu AC, Okoye GA, Kwatra SG. Pruritus in Black Skin: Unique Molecular Characteristics and Clinical Features. Journal of the National Medical Association. United States; 2020; [DOI] [PubMed] [Google Scholar]
- Mee JB, Johnson CM, Morar N, Burslem F, Groves RW. The Psoriatic Transcriptome Closely Resembles That Induced by Interleukin-1 in Cultured Keratinocytes: Dominance of Innate Immune Responses in Psoriasis. The American Journal of Pathology. 2007;171(1):32–42 Available from: http://www.sciencedirect.com/science/article/pii/S0002944010619402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhak Z, Bissonnette R, Siri D, Tyring SK, Tessari E, Gandhi R, et al. 560 KPL-716, anti-Oncostatin M receptor beta antibody, reduced pruritus in atopic dermatitis. Journal of Investigative Dermatology. 2019;139(5, Supplement):S96 Available from: http://www.sciencedirect.com/science/article/pii/S0022202X19308279 [Google Scholar]
- Ness-Schwickerath KJ, Morita CT. Regulation and function of IL-17A- and IL-22-producing γδ T cells. Cellular and molecular life sciences : CMLS. 2011;68(14):2371–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. Journal of Allergy and Clinical Immunology. 2015;136(5):1254–64 [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. The British journal of dermatology. 2008/August/05. 2008; 159(5):1092–102 Available from: https://www.ncbi.nlm.nih.gov/pubmed/18684158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Honda T, Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. International Immunology. 2018;30(9):419–28 Available from: 10.1093/intimm/dxy015 [DOI] [PubMed] [Google Scholar]
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. The New England journal of medicine. United States; 2004;350(25):2591–602 [DOI] [PubMed] [Google Scholar]
- Park K, Mori T, Nakamura M, Tokura Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. European journal of dermatology : EJD. France; 2011. p. 135–6 [DOI] [PubMed] [Google Scholar]
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta dermato-venereologica. Sweden; 2012;92(5):502–7 [DOI] [PubMed] [Google Scholar]
- Rambhia PH, Levitt JO. Recalcitrant prurigo nodularis treated successfully with dupilumab. JAAD case reports. United States; 2019. p. 471–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nature reviews Immunology. England; 2014;14(12):783–95 [DOI] [PubMed] [Google Scholar]
- Sanyal RD, Pavel AB, Glickman J, Chan TC, Zheng X, Zhang N, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. United States; 2019;122(1):99–110.e6 [DOI] [PubMed] [Google Scholar]
- Schedel F, Schurmann C, Metze D, Ständer S. [Prurigo. Clinical definition and classification]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. Germany; 2014;65(8):684–90 [DOI] [PubMed] [Google Scholar]
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. The Journal of allergy and clinical immunology. United States; 2006;117(2):411–7 [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature Immunology. 2011;12(5):383–90 Available from: 10.1038/ni.2025 [DOI] [PubMed] [Google Scholar]
- Stander S, Yosipovitch G, Legat FJ, Lacour J-P, Paul C, Narbutt J, et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. The New England journal of medicine. United States; 2020;382(8):706–16 [DOI] [PubMed] [Google Scholar]
- Steinke S, Zeidler C, Riepe C, Bruland P, Soto-Rey I, Storck M, et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: Results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. Journal of the American Academy of Dermatology. Elsevier; 2018;79(3):457–463.e5 Available from: 10.1016/j.jaad.2018.04.044 [DOI] [PubMed] [Google Scholar]
- Suarez-Farinas M, Dhingra N, Gittler J, Shemer A, Cardinale I, de Guzman Strong C, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. The Journal of allergy and clinical immunology. United States; 2013;132(2):361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis R, Ferenczi K, Payette M. Dupilumab Treatment for Prurigo Nodularis and Pruritis. Journal of drugs in dermatology : JDD. United States; 2019;18(9):940–2 [PubMed] [Google Scholar]
- Thijs JL, Strickland I, Bruijnzeel-Koomen CAFM, Nierkens S, Giovannone B, Csomor E, et al. Moving toward endotypes in atopic dermatitis: Identification of patient clusters based on serum biomarker analysis. The Journal of allergy and clinical immunology. United States; 2017;140(3):730–7 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B, Miyahira Y, Mayuzumi N, Ogawa H, Ikeda S. SCCA2-transfected human keratinocytes show increased secretion of IL-1alpha and IL-6, but not of TNF-alpha. Archives of dermatological research. Germany; 2005;297(6):274–7 [DOI] [PubMed] [Google Scholar]
- Whang KA, Kang S, Kwatra SG. Inpatient Burden of Prurigo Nodularis in the United States. Medicines (Basel, Switzerland). MDPI; 2019a;6(3):88 Available from: https://www.ncbi.nlm.nih.gov/pubmed/31405223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang KA, Khanna R, Thomas J, Aguh C, Kwatra SG. Racial and Gender Differences in the Presentation of Pruritus. Medicines (Basel, Switzerland). Switzerland; 2019b;6(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo Nodularis: Pathogenesis and Management. Journal of the American Academy of Dermatology. United States; 2020; [DOI] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. United States; 2004;21(2):241–54 [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Döcke W-D, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. European journal of immunology. Germany; 2006;36(5):1309–23 [DOI] [PubMed] [Google Scholar]
- Wong L-S, Yen Y-T, Lin S-H, Lee C-H. IL-17A Induces Endothelin-1 Expression through p38 Pathway in Prurigo Nodularis. The Journal of investigative dermatology. United States; 2020. p. 702–706.e2 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yin M, Zhang L-J. Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells. Switzerland; 2019;8(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. England; 2007;445(7128):648–51 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Li T. Interleukin-22, a potent target for treatment of non-autoimmune diseases. Human vaccines & immunotherapeutics. 2018;14(12):2811–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Wu X, Zhang W, Zhang J, Chen X, Chen S, et al. Aberrant Expression of Histamine-independent Pruritogenic Mediators in Keratinocytes may be Involved in the Pathogenesis of Prurigo Nodularis. Acta dermato-venereologica. Sweden; 2019;99(6):579–86 [DOI] [PubMed] [Google Scholar]
- R: The R Project for Statistical Computing [Internet]. Available from: https://www.r-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 80x magnification of H&E staining of PN lesional skin, which demonstrates a heterogeneous infiltrate of inflammatory cells, including: a. eosinophils (orange arrow), lymphocytes (blue arrow), and plasma cells (green arrow), b. mast cells (yellow arrow) and plasma cells (green arrow), c. eosinophils (orange arrow), lymphocytes (blue arrow), and neutrophils (black arrow), d. eosinophils (orange arrow) and lymphocytes (blue arrow). Scale bar = 40um.
Figure S2. Gating strategy for cell populations with representative flow plots for the CD3+ T cell population. Representative flow plots showing the gating strategy for CD8-T cells (CD3+CD8+CD4−), CD4-T cells (CD3+CD4+CD8−), iNKT – cells (CD3+CD4−CD8−CD56+), and γδ T-cells (CD3+CD4−CD8−γδTCR+), respectively.
Figure S3. Intracellular cytokine profile in PN patients. PBMCs from PN (n=4) and healthy subjects (n=5) were stimulated with PMA and Ionomycin combined with protein transport inhibitor for 4hrs. The cells were analyzed for intracellular cytokine expression by flow cytometry. a, Percentage of TGF-β expressing CD3+ T-cell populations ± SEM. b, Percentage of IFN-γ expressing CD3+ T-cell populations ± SEM. c, Percentage of IL-17 expressing CD3+ T-cell populations ± SEM. d, Percentage of IL-10 expressing CD3+ T-cell populations ± SEM. e, Percentage of IL-13 expressing CD3+ T-cell populations ± SEM. *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U- test. ns = nonsignificant.
Figure S4. Heat map of cutaneous gene expression with mRNA-sequencing highlights differences in immune signature in lesional PN skin. Red, greater expression; blue, lower expression. Fold change shown is log base 2 fold-change. p-value shown is a false discovery rate adjusted p value. * p-value < .05, ** p-value < .01, and *** p-value < .001. L, lesional PN samples; NL, non-lesional PN samples; H, matched healthy control samples.
Figure S5. Correlation of itch numeric rating scale values with GSVA scores of Th1, Th2, Th17, and Th22 related genes from PN patients and controls. Itch severity most strongly correlated with the degree of Th22 related gene expression.
Figure S6. Outline of experimental design. Under IRB approval, peripheral blood mononuclear cells (PBMCs) and skin samples were obtained from PN patients and healthy subjects matched by age, race, and gender. Flow cytometric analysis compared numbers and phenotypic characteristics of γδ+ T-cells in the peripheral blood of patients with PN and matched healthy controls. RNA-sequencing with immunohistochemistry validation compared gene expression in pruritic lesional PN, non-pruritic non-lesional PN, and biopsy site matched control skin.
Figure S7. Gene Set Variation Analysis of immune mediators in lesional PN (n=13 samples), non-lesional (n=13 samples), and matched healthy skin (n=13 samples), by race: African Americans (10 matched patients) and European Americans (3 matched patients). Th22 upregulation is observed in both European and African Americans. . * p-value < .05, ** p-value < .01, and *** p-value < .001. AA, African American; EA, European American.
Figure S8. Gene Set Variation Analysis of immune mediators in lesional PN (n=13), non-lesional (n=13), and matched healthy skin (n=13), by biopsy site: arms (n=4), legs (n=6), and back (n=3). Th22 upregulation is observed across all biopsy sites. . * p-value < .05, ** p-value < .01, and *** p-value < .001.
Figure S9. Absolute cell number for T cell population. Absolute cell numbers from PBMCs from PN (n=4) and healthy subjects (n=5) a, Absolute number of CD3+ γδ T-cells (median ± ICR). b, Absolute number of sub-populations of γδ-T cells ± SEM. c, Absolute number of iNKT – cells (median ± ICR). d, Representative flow cytometry plots for CD4+ T helper (CD3+CD4+CD8−) cells, and CD8+ T cytotoxic cells (CD3+CD8+ CD4−). e. Percentage of the ratio of CD4+ and CD8+ cell population ± SEM. f. Absolute number of CD4+ T-cells (median ± ICR). g. Absolute number of CD8+ T-cells (median ± ICR). h. Absolute number of CD8 Naïve and CD8 Memory cells (median ± ICR). *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U-test. ns = non-significant.
Figure S10. Absolute cell number of T-cell populations expressing cytokines. Absolute cell numbers from PBMCs from PN (n=4) and healthy subjects (n=5) a, Absolute number of IL-17 expressing T-cell populations ± SEM. b, Absolute number of TNF expressing T-cell populations. ± SEM. c, Absolute number of IL-4 expressing T-cell populations. ± SEM. *P<0.05, healthy controls versus PN subjects, as calculated by a non-parametric Mann Whitney U-test. ns = nonsignificant.
Figure S11. Representative histogram of IL-17 expression by CD3+ T cells. PBMCs from PN (n=4) and bacteremia (n=5) were stimulated with PMA and Ionomycin combined with protein transport inhibitor for 4hrs. The cells were analyzed for intracellular cytokine expression by flow cytometry. Representative flow cytometry histograms of intracellular IL-17 cellular expression versus unstained control (Ctrl).
Table. S1. Study cohort demographic information for matched patients recruited to provide skin samples, mRNA, and immunofluorescence analyses. PN, Prurigo nodularis; HC, healthy control; F, female; M, male; AA, African American; Itch NRS, itch numeric rating scale.
Table. S2. Study cohort demographic information for matched patients recruited to provide PBMCs for flow cytometry analyses. *Too few cells were collected, therefore patients were excluded from analyses. PN, Prurigo nodularis; HC, healthy control; F, female; M, male; AA, African American; Itch NRS, itch numeric rating scale.
Table. S3. The panel of mAbs and fluorescent-tagged dyes used for the detection of immune cell populations
Table. S4. Full list of DEGs (adjusted P<0.05) in lesional (n=13) vs. non-lesional PN (n=13)
Table. S5. Full list of DEGs (adjusted P<0.05) in lesional PN (n=13) vs. matched healthy skin (n=13)
Table. S6. Full list of DEGs (adjusted P<0.05) in non-lesional PN (n=13) vs. matched healthy skin (n=13)
Table. S7. Full list of DEGs (adjusted P<0.05) in African American (n=10) compared to European American (n=3) PN lesional skin. Select immune genes (IL4, IL5, IL13, IL31) are also shown.
Table. S8. Full list of DEGs (adjusted P<0.05) in African American (n=10) compared to European American (n=3) non-lesional PN skin.
Data Availability Statement
All data referenced in this study are included in the figures, tables and supplemental files.