Abstract
Updated Clostridioides difficile infection (CDI) guidelines published in 2018 recommend vancomycin as first-line treatment. Of 833 community-onset CDI cases in metropolitan Atlanta, Georgia in 2018, over half did not receive first-line treatment, although guideline adherence increased over the year. Second-line treatment was more common in patients treated in ambulatory settings.
Keywords: Clostridioides difficile infection, metronidazole, guideline-adherent treatment, vancomycin
Graphical Abstract
Clostridioides difficile infection (CDI) affects nearly 500,000 patients in the United States annually [1]. Although CDI is commonly associated with healthcare exposure [2], community-associated CDI represents nearly half of incident cases, and incidence of community-associated CDI is increasing [1].
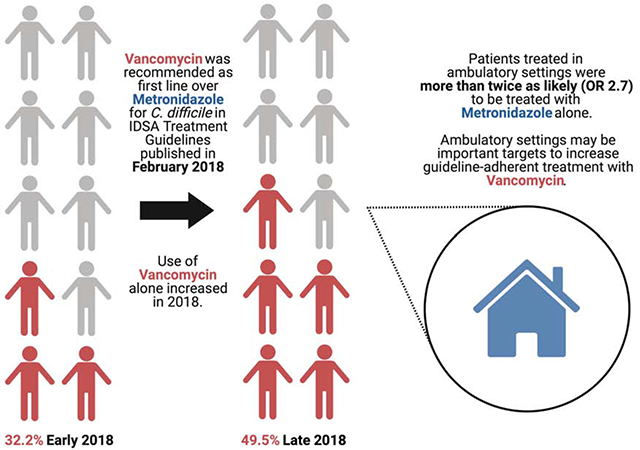
Although some patients only develop diarrhea, CDI complications can be life-threatening, and appropriate antibiotic therapy is important for preventing complications and achieving clinical cure [3, 4]. Until 2018, oral metronidazole was the recommended first line treatment for most initial cases of CDI [5] based on small randomized controlled trials that showed no difference between metronidazole and vancomycin coupled with metronidazole’s relative affordability [6]. In February 2018, the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) published revised guidelines recommending oral vancomycin as initial treatment for CDI [7] after studies demonstrated improved clinical cure rates with vancomycin as first line treatment, even among mild-moderate cases [4].
Knowledge of whether patients receive guideline-adherent treatment, and factors that contribute to receipt of second-line treatment, are crucial to inform efforts by professional organizations and healthcare institutions to ensure that all patients receive optimal medical care. We analyzed surveillance data of treatment for CDI during 2018 in metropolitan Atlanta, Georgia, USA to determine the impact of the updated IDSA/SHEA guidelines on antibiotic prescribing patterns for CDI treatment.
We used data collected by the Georgia Emerging Infections Program (EIP), which conducts Centers for Disease Control and Prevention (CDC)-funded active population-based surveillance of all incident CDI cases in the eight counties of metropolitan Atlanta, GA (population 4.1 million). An incident case was defined as a resident of the catchment area with a positive C. difficile molecular or toxin test without an additional positive test in the preceding eight weeks.
Epidemiologic classification was determined according to CDC criteria [8]. We excluded healthcare facility-onset cases, i.e., cases where stool was collected in an acute care facility either > 3 days after admission or at any time after admission from a long-term care facility (LTCF), or if stool was collected in a LTCF. A 1:3 random sample of non-healthcare facility-onset incident adult (> 17 years old) cases were selected for complete chart review and abstraction.
For sampled cases, trained surveillance staff abstracted relevant data from medical records including baseline demographics, laboratory variables, treatment location, antibiotics administered, prior CDI cases, and outcomes. Cases in 2017 were counted as prior incident cases for purposes of this analysis. Treatment categories were defined as monotherapy with either vancomycin or metronidazole, combination (either serial or concurrent), or other. We compared differences between cases receiving either vancomycin-only or metronidazole-only antibiotic regimens with the X2, Fisher’s exact, or Kruskal-Wallis test as appropriate. We determined odds ratios for use of metronidazole-only treatment according to quarter or infection and treatment location. We constructed a multivariable logistic regression model to test the associations of quarter of infection and treatment location (i.e., ambulatory vs. hospital-based) with receipt of metronidazole-only treatment. In this model, severe CDI (i.e., WBC ≥15,000) was included a priori as a covariate, and the remainder of potential covariates (quarter of infection, treatment location, age, race, chronic kidney disease [CKD], inflammatory bowel disease [IBD], and incident case number [i.e., 1 vs. ≥2]) were subjected to backwards elimination. Analyses were performed with SAS (version 9.4, SAS Institute Inc., Cary, NC, USA), and p-value < 0.05 was considered statistically significant. The data collection was exempted through the Georgia Department of Public Health and approved by the Emory University Institutional Review Board and the Atlanta VA Research and Development Office.
Of 4055 incident CDI cases in metropolitan Atlanta, GA in 2018, 833 adult community-onset cases (among 719 unique patients) were included (Figure 1). Overall, 355 cases (42.6%) were treated with vancomycin only, 207 (24.8%) with metronidazole only and 199 (23.9%) with a combination of vancomycin and metronidazole. The remaining cases (72, 8.6%) were either treated with other regimens or did not receive CDI treatment.
Figure 1. Study flow diagram.
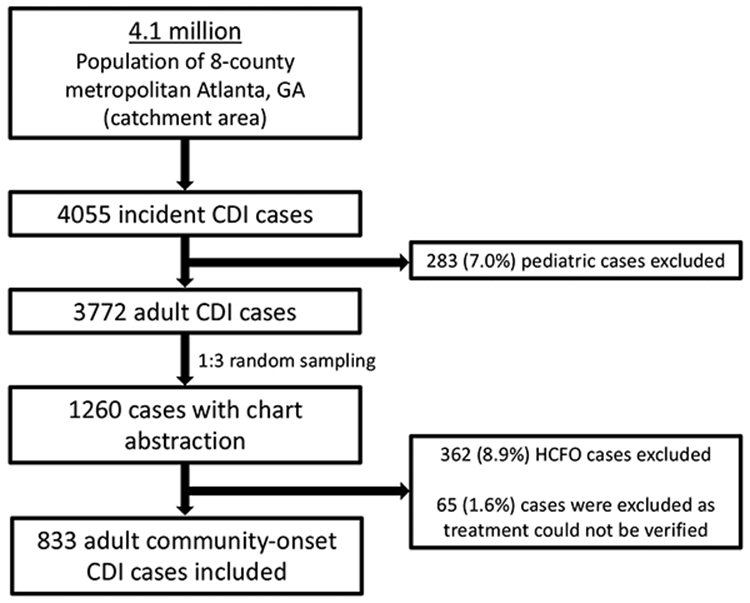
Abbreviations: CDI, Clostridioides difficile infection; HCFO, healthcare facility-onset. HCFO was defined as cases where stool was collected in an acute care facility either >3 days after admission or at any time after admission from a long-term care facility, or if stool was collected in a long-term care facility.
Cases receiving metronidazole-only treatment were younger than those receiving vancomycin-only treatment (median age 58 vs. 62 years, p<0.01) and were less likely to have diabetes mellitus (21.3% vs. 29.6%, p=0.03), CKD (12.1% vs. 24.5%, p<0.001), and IBD (3.9% vs. 9.0%, p=0.02) (Table 1). Metronidazole-only cases were more likely to be the first incident case (96.1% vs. 85.6%, p<0.001) and less likely to require ICU admission (2.4% vs. 7.0%, p=0.02), although proportion of severe cases (WBC ≥15000) did not differ between groups (9.2% vs. 14.1%, p=0.09). Overall mortality was 2.3% and did not differ between metronidazole-only and vancomycin-only treatment groups (p=1.00).
Table 1.
Characteristics of 833 incident cases of community-onset Clostridioides difficile infection in metropolitan Atlanta, Georgia, in 2018, according to treatment.
| Characteristic | Metronidazole -only (n=207) |
Vancomycin- only (n=355) |
P-valuea | Combination (n=199)b |
Other (n=72)c |
|---|---|---|---|---|---|
| Age, median (IQR) | 58 (37,69) | 62 (48,72) | <0.01 | 66 (54,77) | 61 (51,70) |
| Female, N (%) | 144 (69.6) | 228 (64.2) | 0.20 | 118 (59.3) | 47 (65.3) |
| Race, N (%) | <0.01 | ||||
| White | 92 (44.4) | 173 (48.7) | 115 (57.8) | 38 (52.8) | |
| Black | 72 (34.8) | 147 (41.1) | 73 (36.7) | 23 (31.9) | |
| Other/unknown | 43 (21.1) | 35 (9.9) | |||
| Hispanic, N (%) | 6 (2.9) | 12 (3.4) | 0.75 | 4 (2.0) | 2 (2.8) |
| Comorbidities, N (%) | |||||
| Diabetes mellitus | 44 (21.3) | 105 (29.6) | 0.03 | 61 (30.7) | 12 (16.7) |
| CKD | 25 (12.1) | 87 (24.5) | <0.001 | 51 (25.6) | 12 (16.7) |
| IBD | 8 (3.9) | 32 (9.0) | 0.02 | 13 (6.5) | 7 (9.7) |
| PUD | 2 (1.0) | 6 (1.7) | 0.49 | 6 (3.0) | 1 (1.4) |
| Case #, N (%) | <0.001 | ||||
| First | 199 (96.1) | 304 (85.6) | 166 (83.4) | 50 (69.4) | |
| Recurrent (#2-6) | 8 (3.9) | 51 (14.4) | 33 (16.6) | 22 (30.6) | |
| Epi classd, N (%) | <0.001 | ||||
| CA | 176 (85.0) | 234 (65.9) | 109 (54.8) | 51 (70.8) | |
| HACO | 31 (15.0) | 121 (34.1) | 90 (45.2) | 21 (29.2) | |
| Treatment location, N (%) | <0.001 | ||||
| Ambulatory | 116 (56.0) | 144 (40.6) | 15 (7.5) | 42 (58.3) | |
| Hospital-based | 91 (44.0) | 211 (59.4) | 184 (92.5) | 30 (41.7) | |
| Quarter of infection, N (%) | <0.001 | ||||
| Q1 (Jan – March) | 80 (38.6) | 67 (18.9) | 47 (23.6) | 14 (19.4) | |
| Q2 (April – June) | 61 (29.5) | 78 (22.0) | 45 (22.6) | 15 (20.8) | |
| Q3 (July – Sep) | 36 (17.4) | 103 (29.0) | 51 (25.6) | 21 (29.2) | |
| Q4 (Oct – Dec) | 30 (14.5) | 107 (30.1) | 56 (28.1) | 22 (30.6) | |
| Labs, N (%) | |||||
| WBC ≥ 15000/μ L | 19 (9.2) | 50 (14.1) | 0.09 | 76 (38.2) | 11 (15.3) |
| WBC ≤ 1000/μ L | 1 (0.5) | 4 (1.1) | 0.66 | 7 (3.5) | 0 (0.0) |
| Albumin ≤ 2.5 g/dL | 7 (3.4) | 39 (11.0) | <0.01 | 57 (28.6) | 5 (6.9) |
| Outcomes, N (%) | |||||
| ICU admission | 5 (2.4) | 25 (7.0) | 0.02 | 41 (20.6) | 5 (6.9) |
| Death | 2 (1.0) | 4 (1.1) | 1.00 | 9 (4.5) | 4 (5.6) |
P-value compares values between metronidazole-only and vancomycin-only groups using X2, Fisher’s exact, or Kruskal-Wallis test as appropriate.
Both vancomycin and metronidazole, either serial or concurrent.
Other antibiotics, fecal microbiota transplant, or no treatment.
Determined according to CDC criteria [8].
Abbreviations: CA, community-associated; HACO, healthcare-associated community-onset; CKD, chronic kidney disease; IBD, inflammatory bowel disease; ICU, intensive care unit, IQR, interquartile range; PUD, peptic ulcer disease; WBC, white blood cells.
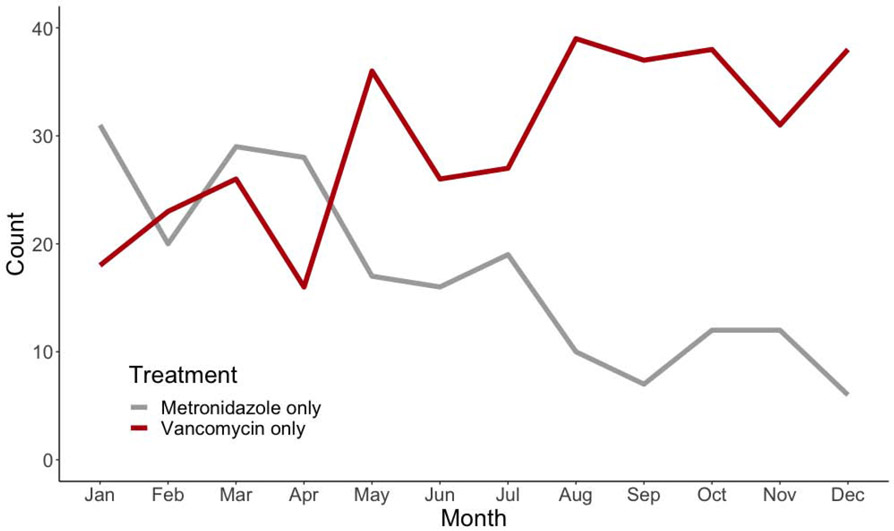
The proportion of cases treated with metronidazole only declined significantly over the year (Figure 2). In quarter four, 14.0% of cases were treated with metronidazole only compared to 38.5% in quarter one (odds ratio [OR] 0.26, 95% confidence interval [CI] 0.16-0.42). In addition, patients treated in an ambulatory setting were significantly more likely to receive metronidazole only compared to patients treated in a hospital-based setting (OR 2.7, 95% CI 2.0-3.7).
Figure 2.
Trends in antibiotic treatment of community-onset incident Clostridioides difficile infection cases in Atlanta, Georgia, during 2018.
These associations remained when controlling for multiple potential confounders. In a multivariable logistic regression model controlling for age, race, CKD, IBD, recurrent CDI, and severe CDI, both quarter of infection and treatment location were significantly associated with receipt of metronidazole-only treatment. Compared to quarter 1, odds of metronidazole-only treatment decreased over the year 2018 (ORs [95% CIs] for quarter 2, 3, and 4 = 0.61 [0.37-1.00], 0.25 [0.15-0.44], and 0.20 [0.12, 0.36], respectively). Similarly, compared to patients treated in a hospital-based setting, those treated in an ambulatory setting were significantly more likely to receive metronidazole-only treatment (OR 1.58, 95% CI 1.03-2.42).
In this analysis of over 800 cases of community-onset CDI in metropolitan Atlanta, GA in 2018, nearly 1 in 4 received second-line treatment with metronidazole, despite publication of updated IDSA/SHEA guidelines recommending vancomycin as first line treatment in February 2018. However, guideline-adherent treatment increased steadily over the course of the year; patients in the last quarter of the year were 70% less likely to receive second-line treatment than patients in the first quarter. Importantly, patients treated in an ambulatory setting were more than twice as likely to receive second-line treatment as patients treated in a hospital. These associations remained when controlling for several important confounders.
Incidence of community-associated CDI increased by nearly 30% from 2011 to 2017, and community-associated cases now make up nearly half of all CDI cases [1]. Given the increasing role of ambulatory settings in treating patients with CDI, it is important to understand why outpatient providers may inconsistently prescribe guideline-adherent treatment. Several factors may influence whether patients receive guideline-adherent treatment including treatment location (e.g. academic affiliation) [9], perceived cost of treatment, and explicit or implicit provider biases regarding patient age and race [10]. Our analysis suggests that outpatient providers are an important group to target for education efforts to increase guideline-adherent treatment for CDI to improve treatment and outcomes associated with CDI occurring in the community. Although vancomycin’s cost is a recognized barrier to access, the role of socioeconomic status or insurance coverage has not been well evaluated. Further study of treatment choice for initial episodes of CDI beyond 2018 will help to determine if poor adherence to guideline-based prescribing persists and whether economic factors are associated with non-guideline-adherent CDI treatment.
Measuring guideline-adherent treatment is an important step to evaluate success of dissemination of recommended improvements in healthcare quality. Two other studies have evaluated changes in prescribing after the IDSA/SHEA CDI guidelines were published; metronidazole prescriptions declined between 3-50% after guideline publication in these analyses [11, 12]. However, in the largest cohort capturing nearly 20 million antibiotic prescription courses, metronidazole was prescribed over 10 times more commonly than vancomycin even after guideline publication [12]. It is unclear what accounts for the discrepancy in use of metronidazole compared to vancomycin in these studies, as neither study analyzed prescribing differences by provider, treatment location, or CDI characteristics. Given our finding that ambulatory-based providers are more likely to prescribe metronidazole, the difference may be explained by prescribing location; further elucidating risk factors for receipt of second-line treatment will be an important step towards ensuring that all patients receive guideline-adherent care.
Our study has several limitations. First, factors that influence CDI treatment in the metropolitan Atlanta area, including treatment setting and provider characteristics, may be specific to this cohort. However, the large size of the catchment area (population of over 4 million across 8 counties) and the Georgia EIP’s systematic approach to data collection including community surveillance are important strengths. Second, patient income and insurance status were not included in this analysis, which may confound the association between treatment location and treatment type.
Overall, this population-based cohort of patients with community-onset CDI in metropolitan Atlanta, Georgia, in 2018 demonstrates a steady increase in adoption of guideline-adherent treatment in the 10 months after publication of new treatment guidelines. Treatment in an ambulatory setting was associated with receipt of second-line treatment. Our findings suggest important provider groups to target to increase uptake of CDI guideline-recommended treatment.
2018 Clostridioides difficile guidelines recommend treatment with vancomycin
Use of vancomycin increased throughout the year after guideline publication
Patients treated in an ambulatory setting were less likely to receive vancomycin
These findings can help target education to improve guideline-adherent prescribing
Acknowledgements:
We would like to thank the dedicated EIP staff who work incredibly hard to collect data on infections of public health importance, including CDI, and collected data used in this report. We would also like to thank Monica Farley, MD, for her support of this project.
Funding:
Georgia Emerging Infections Program surveillance was funded through the Centers for Disease Control and Prevention under U50CK000485. This research was also supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI144036 to MHW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: All authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med 2020; 382(14): 1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, O'Leary E, Janelle SJ, et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med 2018; 379(18): 1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative Effectiveness of Vancomycin and Metronidazole for the Prevention of Recurrence and Death in Patients With Clostridium difficile Infection. JAMA Intern Med 2017; 177(4): 546–53. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59(3): 345–54. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31(5): 431–55. [DOI] [PubMed] [Google Scholar]
- 6.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet 1983; 2(8358): 1043–6. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66(7): e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Multidrug-Resistant Organism & Clostridioides difficile Infection (MDRO/CDI) Module. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/12pscmdro_cdadcurrent.pdf.Accessed November 16, 2020.
- 9.Tam LM, Fonarow GC, Bhatt DL, et al. Achievement of guideline-concordant care and in-hospital outcomes in patients with coronary artery disease in teaching and nonteaching hospitals: results from the Get With The Guidelines-Coronary Artery Disease program. Circ Cardiovasc Qual Outcomes 2013; 6(1): 58–65. [DOI] [PubMed] [Google Scholar]
- 10.Blom EF, Ten Haaf K, Arenberg DA, de Koning HJ. Disparities in Receiving Guideline-Concordant Treatment for Lung Cancer in the United States. Ann Am Thorac Soc 2020; 17(2): 186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentry CA, Campbell DL, Williams RJ 2nd. Outcomes associated with recent guideline recommendations removing metronidazole for treatment of non-severe Clostridioides difficile infection: a retrospective, observational, nationwide cohort study. Int J Antimicrob Agents 2021: 106282. [DOI] [PubMed] [Google Scholar]
- 12.Clancy CJ, Buehrle D, Vu M, Wagener MM, Nguyen MH. Impact of revised Infectious Diseases Society of America and Society for Healthcare Epidemiology of America clinical practice guidelines on the treatment of Clostridium difficile infections in the United States. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]