Abstract
Rationale & Objective :
An early change in proteinuria is considered a reasonably likely surrogate endpoint in IgA nephropathy (IgAN), and can be used as basis for accelerated approval of therapies with verification in a post-marketing confirmatory trial. GFR slope is a recently validated surrogate endpoint for CKD progression and may be considered as the endpoint used for verification. We undertook a meta-analysis of clinical trials in IgAN to compare treatment effects on change in proteinuria to change in eGFR slope.
Study design:
Individual patient-level meta-analysis.
Setting and Study Populations:
Individual data of 1037 patients from 12 randomized trials.
Selection Criteria for Studies:
Randomized trials of IgAN with proteinuria measurements at baseline and 6-months (range 2.5–14) and at least one further year of follow-up for the clinical outcome.
Analytical Approach:
For each trial we estimated the treatment effects on proteinuria and on the eGFR slope, computed as either the total slope starting at baseline or the chronic slope starting 3-months after randomization. We used a Bayesian mixed effects analysis to relate the treatment effects on proteinuria to effects on GFR slope across these studies and developed a prediction model for the treatment effect on the GFR slope based on the effect on proteinuria.
Results:
Across all studies, treatment effects on proteinuria accurately predicted treatment effects on the total slope at 3 years (median R2=0.88 [95% Bayesian credible Interval (BCI) 0.06–1) and on the chronic slope (R2 0.98 [95% BCI 0.29–1]). For future trials, an observed treatment effect of approximately 30% reduction in proteinuria would confer probabilities of at least 90% for nonzero treatment benefits on the total and chronic slope of eGFR. We obtained similar results for proteinuria at 9 and 12 months and total slope at 2 years.
Limitations:
Study population restricted to 12 trials of small sample size, leading to wide BCIs. There was heterogeneity among trials with respect to study design and interventions.
Conclusions:
These results provide new evidence supporting that early reduction in proteinuria can be used as a surrogate endpoint for studies of CKD progression in IgAN.
Keywords: GFR slope, Urine protein, Surrogate endpoint, IgA Nephropathy, End-stage renal disease (ESRD)
Plain language summary
Drug regulatory agencies allow change in urine protein to be used as endpoints for trials in IgA nephropathy (IgAN), as part of accelerated approval of treatments, as long as there is confirmation of the treatment benefit following approval in post-marketing trials. We performed an individual patient meta-analysis including data from 1037 patients across 12 trials to assess how treatment effects on the change in proteinuria predicts the treatment effects on the change in eGFR, a surrogate outcome that has recently been validated. We found that treatment effects on urine protein accurately predicted treatment effects on the total GFR slope at 3 years and on chronic GFR slope. These results provide new evidence supporting that a change in proteinuria can be used as a surrogate endpoint in treatment trials of progression of IgAN.
Introduction
IgA nephropathy (IgAN) is rare, but the most common cause of glomerulonephritis, with few proven therapies. Trials early in the disease course are challenging to undertake because of low event rate for clinical endpoints, generally defined as kidney failure (treated end stage kidney disease (ESKD) or glomerular filtration rate (GFR) < 15 mL/min/1.73 m2) or doubling of serum creatinine (Scr). In many chronic kidney diseases (CKD), a large decline in GFR, assessed as a doubling of Scr from baseline, and more recently 30 or 40% decline in GFR, has often been used as a surrogate endpoint for kidney failure in randomized clinical trials (RCTs) of patients with low levels of GFR or rapidly progressive disease RCTs.1–3 In a rare disease such as IgAN, these endpoints may not be feasible because of the long duration of the disease, leading to large expense and complexity of trials that would be required to detect treatment effects on a large decline in GFR. In addition, the goal of most therapeutic strategies is to treat the disease early, prior to development of irreversible changes. These issues have likely contributed to the paucity of therapies. Recent evidence supports early changes in urine protein as a reasonably likely surrogate in IgAN.4,5
In the US, reasonably likely surrogate endpoints can be used as a basis for accelerated approval of therapies intended to treat serious or life-threating conditions, such as IgAN.6,7 The clinical benefit of products approved under this program would need to be verified in a post-marketing confirmatory trial.8 Recent empirical data demonstrated the validity of GFR slope as a surrogate endpoint for clinical benefit in general CKD progression studies.9–13 For IgAN, the slope of GFR decline would be a more viable endpoint for verification in post-marketing confirmatory trials given the low likelihood of sufficient clinical events. Here we report an individual patient-level meta-analysis of a pooled dataset of 1037 individuals from twelve RCTs in IgAN to evaluate the association of treatment effects on early change in urine protein compared to treatment effects on GFR slope. These data would be valuable for design of confirmatory trials.
Methods
Study selection and study populations
We identified studies through systematic searches of the medical literature on Ovid MedLine published from January 1, 1979 to December 15, 2016, as previously described10,14 (Item S3, Tables S1–S2, Figures S1–S2). Twelve studies were included that investigated four intervention types [renin angiotensin system blockade (RASB), fish oil, steroids, or other immunosuppression agents] (Table S3).15–28 Participants provided informed consent at inclusion in each study. This analysis was considered exempt from review by the Tufts Medical Center Institutional Review Board.
Early change in urine protein
We defined change in urine protein from baseline to 6 (range 2.5 to 14), 9 (2.5 to 14) and 12 (2.5 to 19) months, taking the value closest to the target month. For the primary analysis, we used change at 6 months to be consistent with the recent publication evaluating associations between treatment effects on changes in urine protein to those on the clinical endpoint.14 Urine protein was in units of grams/day (g/day) and was log transformed due to skewness of the data.
GFR slope
GFR was estimated using the CKD-EPI 2009 creatinine equation.29 Creatinine was standardized to isotope dilution mass spectroscopy traceable reference methods using direct comparison or was reduced by 5%.10 As we have previously described, we used a simplified linear mixed effects model based on a single slope starting at 3 months post randomization adjusted for baseline GFR with the model accounting for various sources of variation in GFR slopes between and within subjects and treatment arms.10,30 The slope was estimated for all participants in each study. For studies with greater than 15 dialysis or death events, we used a shared parameter model to account for informative censoring due to dialysis or death.16–18,22 Under this model, the differences between the randomized groups in the mean intercepts at 3 months follow-up, the mean slopes after 3 months, and the estimated mean changes from baseline to either 1, 2, or 3 years follow-up factored by the follow-up duration represented the treatment effects on the acute, chronic, and total slopes, respectively. To recreate a more realistic trial scenario, in sensitivity analysis, we also present the results for the chronic slope computed over a two-year period which we defined as last visit by 27 months (referred to as 2 year chronic slope).
Analyses
Objective
Our first goal was to evaluate the association of the treatment effects on the change in urine protein with treatment effects on GFR slope. Our second goal was to use these results to describe the probabilities of treatment benefit on GFR slope associated with varying treatment effects on urine protein for application to future studies. The supplement includes a more detailed description of the methods.
Trial level analysis
The trial level analysis requires two steps: intent-to-treat estimation of the treatment effects on both endpoints within each RCT, followed by a meta-regression to relate the treatment effects on the two endpoints of interest across RCTs.2,5,10,31 In the first step, treatment effects on change in urine protein were estimated by performing analyses of covariance within each study, with log urine protein change as the endpoint adjusting for treatment and log baseline urine protein. Treatment effects on urine protein were expressed as geometric mean ratios (GMR). Treatment effects on GFR slope were estimated using a shared parametric mixed effects model as described above and were expressed as mean differences in the GFR slopes between the treatment vs control groups, in units of ml/min/1.73m2/year.
In the second step, a Bayesian mixed effects meta-regression related the estimated treatment effects on one endpoint to the estimated treatment effects on the second endpoint with study as the unit of analysis (details in supplement). The model relates the treatment effects on the two endpoints after accounting for random errors in the estimated effects in each RCT. The meta-regression supports strong association between the two endpoints if 1) the slope of the meta-regression line is statistically significant as defined by Bayesian credible intervals (BCI) that do not cross 0, with a large magnitude, 2) the intercept is close to 0, implying absence of an average effect on the GFR slope when the treatment does not affect urine protein, 3) the R2 is high, so that treatment effects on urine protein account for most of the variation in treatment effects on the GFR slope, and 4) the root mean square error (RMSE) is low, assuring low variation in the GFR slope given a fixed treatment effect on urine protein. We also used separate random effects meta-analyses to summarize the distributions of the treatment effects on each endpoint across the 12 RCTs.
Predicting clinical benefit in future trials
From the trial level meta-regression, we computed 95% and 80% Bayesian credible prediction intervals and estimated the probabilities of treatment benefit on GFR slope (defined by a difference in slope > 0) for an infinite, modest or small sized RCT. Under the meta-regression model for an infinite sized RCT, a treatment effect has a 95% or 80% probability of falling within the prediction interval, a 2.5% and 10% probability of exceeding the upper limit, and a 2.5% and 10% probability or falling below the lower limit, respectively. A modest RCT was defined as having a sample size of 250 (SE 0.09) and a small RCT was defined as having a sample size of 100 (SE 0.15), assuming a standard deviation of 0.75 for change in log urine protein. We computed the threshold associated with the smallest observed treatment effect on a change in urine protein that provides a positive predictive value (PPV) of 97.5%, 95% and 90% for treatment benefit on the GFR slope.
Analyses were performed using SAS version 9.4M6 (SAS Institute, Cary, NC) and R 4.0.1 (R Project for Statistical Computing www.r-project.org.32
Results
Table 1 and Tables S4 and S5 summarize aggregate characteristics of the included studies. Of the 12 studies, two tested RAS blockade,15,16 3 tested steroids,23–27 2 tested fish oil,17,18 2 tested MMF,19,20 and 2 others tested azathioprine.21,22 STOP-IgAN contained two interventions, each at different levels of GFR. At higher GFR the intervention was steroids whereas at lower levels of GFR, the intervention was cyclophosphamide followed by azathioprine as well as steroids. Across the trials, the mean age of the study participants ranged from 32 to 46 years and the proportion of women ranged from 10% (2/20) to 72% (77/107). Average baseline eGFR and urine protein were 71 (standard deviation (SD) 30) ml/min/1.73 m2 and 1.8 (25th, 75th percentile: 1.2, 2.6) g/day, respectively, in the pooled dataset.
Table 1.
Patient characteristics, by study, for analysis of change in urine protein at 6 months
| Study | Intervention | N | Age (SD) | Female (%) | eGFR (SD) | UP (IQR) | FU (IQR) |
|---|---|---|---|---|---|---|---|
| Donadio 1999 | Fish oil | 91 | 38.8 (13.4) | 23 (25.3) | 65.8 (21.7) | 1.9 (1.2, 3.4) | 37.1 (26.4, 44.9) |
| Donadio 2001 | Fish oil | 66 | 46.4 (13.4) | 10 (15.2) | 41.8 (14.1) | 1.6 (0.7, 2.6) | 28.2 (25.1, 38.5) |
| Praga 2003 | RASB | 44 | 31.6 (11.5) | 17 (38.6) | 98.1 (26.5) | 1.7 (1.1, 2.4) | 76.0 (61.0, 129.5) |
| HKVIN | RASB | 107 | 40.1 (9.1) | 77 (72.0) | 75.6 (29.1) | 1.6 (1.1, 2.6) | 34.9 (34.8, 35.1) |
| Maes | IS | 34 | 44.8 (11.3) | 10 (29.4) | 62.2 (18.9) | 1.0 (0.6, 2.7) | 45.0 (33.0, 45.0) |
| Appel | IS | 20 | 37.6 (13.3) | 2 (10.0) | 47.4 (29.2) | 2.3 (1.6, 3.0) | 25.8 (15.1, 28.8) |
| Pozzi 2004 | Steroid | 83 | 38.6 (11.7) | 25 (30.1) | 87.2 (21.6) | 1.9 (1.4, 2.4) | 102.0 (66.0, 126.0) |
| Pozzi 2010 | IS | 190 | 39.3 (12.7) | 55 (28.9) | 74.0 (25.0) | 2.0 (1.5, 2.7) | 72.7 (52.6, 90.3) |
| Pozzi 2013 | IS | 44 | 42.1 (11.6) | 8 (18.2) | 27.9 (7.1) | 2.5 (1.5, 3.9) | 50.3 (35.2, 62.9) |
| Katafuchi | Steroid | 74 | 36.2 (11.4) | 44 (59.5) | 98.5 (21.8) | 1.3 (0.9, 2.6) | 78.0 (60.0, 90.0) |
| Schena | Steroid | 95 | 33.7 (11.1) | 29 (30.5) | 91.3 (23.7) | 1.6 (1.3, 2.5) | 66.0 (42.0, 78.0) |
| STOP-IgAN | IS | 142 | 44.5 (12.3) | 32 (22.5) | 59.5 (27.3) | 1.6 (1.1, 2.1) | 37.6 (37.2, 38.0) |
|
| |||||||
| Overall | 990 | 39.7 (12.5) | 332 (33.5) | 71.9 (29.8) | 1.8 (1.2, 2.6) | 42.6 (34.9, 78.0) | |
Values for categorical variables are given as number (percentage); values for continuous variables, as mean (standard deviation) or median (interquartile range represented as difference between 25th and 75th percentile). N, sample size; GFR, glomerular filtration rate; UP, urine protein in g/day; FU, follow-up in months; RASB, renin-angiotensin system blockade; IS, immunosuppression.
Over a 6 month (25th, 75th percentiles, 5.9, 6.9 months) period, the overall percent mean change in urine protein in the control and treatment arms was 35% reduction (25th, 75th percentiles 57% reduction, 18% increase) and 53% reduction (25th, 75th percentile, 68% reduction, 9% reduction), respectively, resulting in a treatment effect corresponding to a GMR of 0.75 [95th confidence intervals (CI) 0.61, 0.94)] which corresponds approximately to a 25% relative reduction in urine protein due to the treatment (95% CI: 39% reduction, 6% reduction); Figure 1, left panel; Table S6). Similar results were seen with the 9 or 12 month change in urine protein (Table S6; Figure S3).
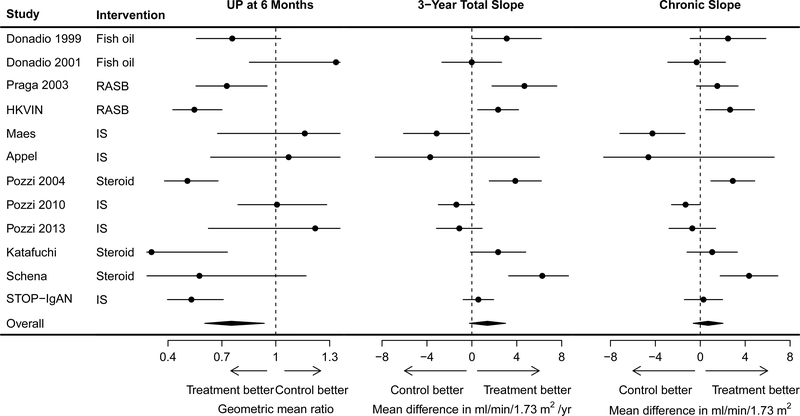
Figure 1: Treatment effect on change in urine protein at 6 months, on 3-year total GFR slope, and on chronic slope.
Treatment effects on urine protein are expressed as geometric mean ratios and were estimated by performing analyses of covariance within each study. Treatment effects on slope are difference in glomerular filtration rate between treatment and control arm and are expressed as ml/min/1.73m2/year and were estimated using a shared parameter mixed effects model. The circles represent the estimated treatment effects and the horizontal line the 95% confidence intervals. UP, urine protein measured in gram/day.
For most studies, the mean total GFR slope at 1, 2, and 3 years and chronic GFR slope was less steep (less negative) in the treatment arm compared to the control arm (Tables S7–S8; Figure S4–S5). The pooled mean total slope at 3 years was −3.51 (SE 0.83) ml/min/1.73 m2/year in the control arm and −1.91 (SE 0.54) ml/min/1.73m2/year in the treatment arm. The mean treatment effect on the total slope at 3 years (1.39 (95% CI −0.21, 2.99) ml/min/1.73 m2/year) was stronger than on the chronic slope (0.70 (95% CI −0.62, 2.02)) ml/min/1.73m2/year) with variation by study (Figure 1).
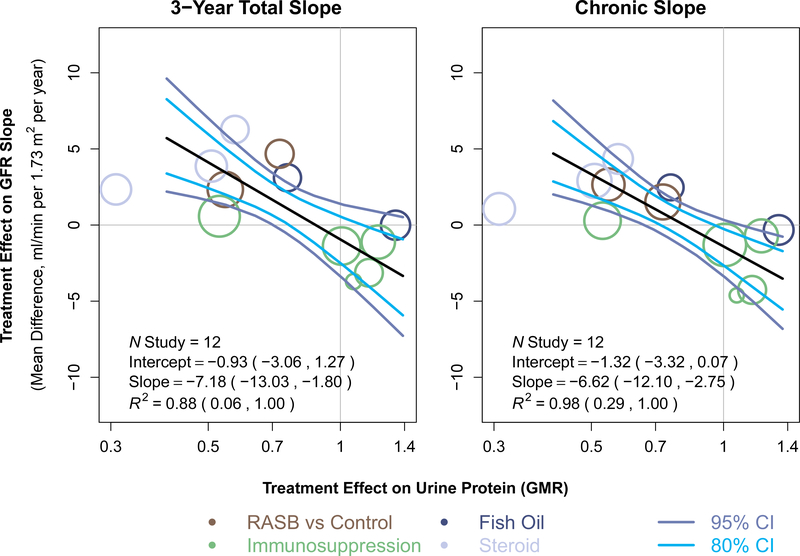
There appeared to be strong agreement between the treatment effects on the change in urine protein to treatment effects on total slope at 3 years (Figure 2; Table S9a; Figure S6). For change in urine protein at 6 months, the slope of the meta-regression line was −7.18 (95% BCI −13.03 to −1.80). A slope of −7.18 would imply that each additional 10% reduction in geometric mean urine protein by the treatment is associated with an additional 0.72 ml/min/1.73m2/year reduction in mean slope by the treatment. The intercept of the regression line was −0.93 (95% BCI −3.06 to 1.27) indicating that there is no evidence that treatments with no effect on the change in urine protein at 6 months have a nonzero average effect on the total slope at 3 years. The median posterior estimate for R2 was 0.88 (95% BCI 0.06 to 1.00; 80% BCI 0.30, 1.00). Results were similar for total slope at 2 years (R2 0.86, 95% BCI 0.03 to 1.0; 80% BCI 0.24 to 1.0). Higher R2 values were estimated for the chronic slope (R2 0.98, 95% BCI 0.29, 1.00; 80% BCI 0.68, 1). For total slope at 1 year, there was a nonsignificant association between the two treatment effects (Table S9a and Figure S6). Results were similar for chronic slope estimate over the entire study duration and over two years (Table S9a and Figure S7). Results were similar for change in urine protein at 9 and 12 months (Tables S9b and S9c, Table S10, and Figures S6 and S7).
Figure 2. Trial level associations between treatment effects on change in urine protein and treatment effects on total GFR slope at 3 years and chronic slope, for urine protein at 6 months.
Shown is the relationship between estimated treatment effects on the 3-year GFR slope on the vertical axis to estimated treatment effects on the change in urine protein on the horizontal axis. Treatment effects on GFR slope are expressed as mean difference in treatment and control and expressed in ml/min/1.73m2/year. Treatment effects on urine protein are expressed as geometric mean ratios. Each circle is a separate intervention with the size of the circle proportional to the number of events. The colors of the circles indicate intervention type. The black line is the line of regression through the studies. The dark blue lines represent the 95% confidence band and the light blue lines represent the 80% confidence band computed from the model. RASB, renin angiotensin system blockers; GMR, geometric mean ratio.
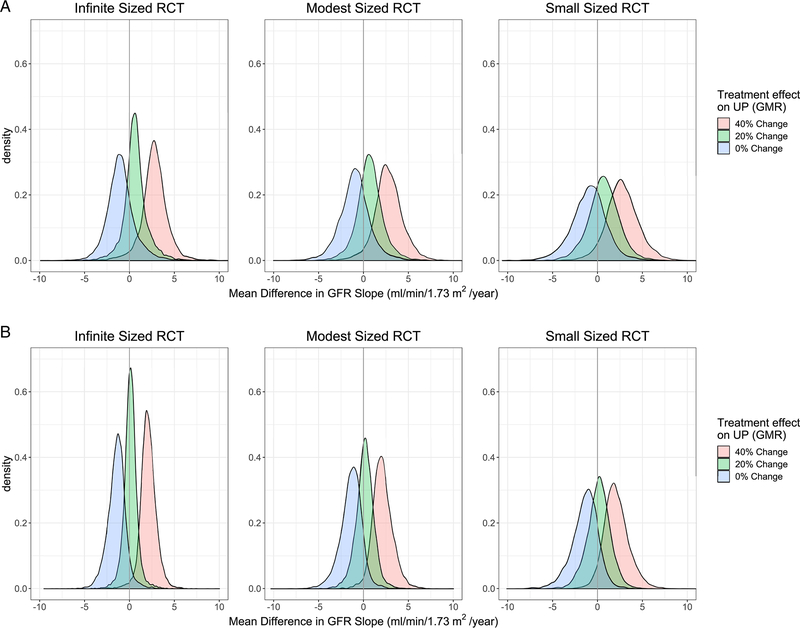
For future trials, an observed treatment effect of a 30% reduction in urine protein at 6 months confers probabilities of approximately 90% for nonzero treatment benefit on total GFR slope at 3 and 2 years and on the chronic GFR slope (Table 2 and Table S10). Predicted treatment effects on GFR slope are stronger at higher magnitude of treatment effect on urine protein (Figure 3, Table 2, Figure S8, Tables S10 and S11). For example, for future modest sized trials, the model predicts that a treatment effect of change in urine protein of 30% would predict a treatment effect on total GFR slope at 3 years of 1.62 (95% BCI −1.59, 4.91; 80% BCI −0.06, 3.48), whereas treatment effect of 40% would predict treatment effect of 2.73 (95% BCI −0.63, 6.15; 80% BCI 0.94, 4.79) (Table 2 and Table S10). Similar results were observed for changes in urine protein at 9 and 12 months (Tables S10 and S11; Figure S8).
Table 2:
Application of change in urine protein to predict GFR slope in new RCTs: predicted treatment effect on c inical endpoint and positive predictive values, for 6 months sample_
| GFR Slope | Observed Treatment effect on change in UP | Infinite sized RCT | Modest sized RCT (N=250) | Small sized RCT (N=100) | |||
|---|---|---|---|---|---|---|---|
| Median Treatment effect on GFR slope and 95% Prediction Interval | PPV | Median Treatment effect on GFR slope and 95% Prediction Interval | PPV | Median Treatment effect on GFR slope and 95% Prediction Interval | PPV | ||
| Total slope over 3 years | 0.5 | 4.10 (0.34, 7.46) | 0.98 | 4.07 (0.24, 8.01) | 0.98 | 4.01 (0.05, 8.53) | 0.98 |
| 0.6 | 2.77 (−0.49, 5.73) | 0.96 | 2.73 (−0.63, 6.15) | 0.96 | 2.70 (−0.86, 6.69) | 0.94 | |
| 0.7 | 1.61 (−1.26, 4.61) | 0.92 | 1.62 (−1.59, 4.91) | 0.89 | 1.62 (−1.96, 5.35) | 0.85 | |
| 0.8 | 0.61 (−2.13, 3.77) | 0.75 | 0.66 (−2.51, 4.00) | 0.70 | 0.68 (−2.98, 4.30) | 0.67 | |
| Threshold for treatment effect on UP to assure PPV ≥ 95% (90%) | 0.64 (0.72) | 0.62 (0.69) | 0.58 (0.66) | ||||
| Chronic slope | 0.5 | 3.27 (1.08, 5.83) | 0.99 | 3.25 (0.91, 6.36) | 0.99 | 3.19 (0.56, 6.96) | 0.99 |
| 0.6 | 2.06 (0.26, 3.92) | 0.98 | 2.04 (−0.01, 4.56) | 0.97 | 1.99 (−0.47, 5.18) | 0.95 | |
| 0.7 | 1.03 (−0.56, 2.60) | 0.94 | 1.03 (−1.03, 3.16) | 0.88 | 1.02 (−1.57, 3.82) | 0.81 | |
| 0.8 | 0.13 (−1.46, 1.74) | 0.58 | 0.16 (−2.05, 2.18) | 0.57 | 0.17 (−2.62, 2.73) | 0.56 | |
| Threshold for treatment effect on UP to assure PPV ≥ 95% (90%) | 0.69 (0.73) | 0.64 (0.68) | 0.60 (0.65) | ||||
UP, urine protein; PPV, positive predictive value.
Treatment effect on change in urine protein is expressed as geometric mean ratio. This can be converted to percent reduction in urine protein by 1-GMR *100. Treatment effect on GFR slope is expressed as mean difference between treatment arms. Prediction intervals are Bayesian credible intervals. PPV is defined as the probability that the treatment reduces the magnitude of the mean GFR slope by any amount which is greater than 0. The thresholds for the treatment effect on UP, is the change in UP required to assure PPV for a GFR slope > 0 of greater or equal to 95% (90%) modest RCT was defined as having a sample size of 250 (SE 0.09) and a small RCT was defined as having a sample size of 150 (SE 0.09), assuming a standard deviation of 0.75 for change in log urine protein. eTable10 includes the 80% Bayesian credible intervals for the predicted treatment effect on the GFR slope along with the 95% credible intervals shown here. eTable11 includes the threshold for treatment effect on UP to assure PPV ≥ 97.5%, along with the 95% and 90% as shown here.
Figure 3. Posterior predictive probabilities of true treatment effect on GFR Slope at 3 years and chronic slope given true treatment effect on change in urine protein.
Panel A shows the posterior predictive probabilities for total GFR Slope at 3 years and panel B shows posterior predictive probabilities for the chronic slope; UP, urine protein; GMR, geometric mean ratio, RCT, randomized controlled trial, GFR, glomerular filtration rate.
Discussion
Valid surrogate endpoints may improve the efficiency of clinical trials, particularly for clinical trials of chronic kidney disease in which progression to clinical endpoints can be slow. For studies of IgAN, use of surrogate endpoints also allows for evaluation of interventions early in the disease course prior to kidney scarring and irreversible changes when interventions might have additional value. There is sound biological and empirical basis for the hypothesis that an early change in urine protein is a valid surrogate endpoint for progression of IgAN. First, pathological data shows that the degree of urine protein correlates with greater evidence of disease.33–35 Second, baseline levels of urine protein are prognostic for long-term disease progression,36–43 and attenuation of urine protein after steroid therapy is associated with improved prognosis.44,45 Third, we have previously provided trial level analyses that demonstrate that early changes in urine protein are a moderately strong surrogate relative to the clinical endpoint across a broad collection of kidney diseases, including IgAN.5,14 A recent paper by the Kidney Health Initiative supports “the use of proteinuria reduction as a reasonably likely surrogate endpoint in future trials studying IgAN…. when accompanied by verification of the clinical benefits in a postmarketing confirmatory trial”.8 Drug development programs for treatments in IgAN using change in GFR are underway.46–48 The current report provides evidence useful for the design of such studies in IgAN.
Using the trial level approach, we found that across 12 studies of multiple interventions, there is a positive relationship between the treatment effects on urine protein and on GFR slope. The Bayesian credible intervals that did not cross 0. This suggests that observed treatment effects on the early changes in urine protein can inform investigators and sponsors on the longer-term treatment effects on GFR slope. The Bayesian credible intervals for the key parameters of our trial level meta-regression analysis are wide. This uncertainty leads to uncertainty in the Bayesian credible prediction intervals for the treatment effects on slope that can be expected for different observed treatment effects on urine protein in future trials. For example, our model would predict that even a treatment effect of 0.8 (20% reduction) on the change in urine protein the predicted treatment effect on GFR slope would have confidence intervals that cross 0. These limitations in precision lead to for the requirement for large thresholds for the treatment effects on the change in urine protein to demonstrate a high probability of benefit on slope. The imprecision is the result of the limited number of studies, all with small samples sizes, rather than an inherent limitation of urine protein as an endpoint in IgAN. Future analyses relating treatment effects on change in urine protein to treatment effects on GFR slope in the overall set of CKD studies, are expected to achieve higher level of precision.
Previously, we demonstrated that treatment effects on GFR slope have very strong associations with treatment effects on the clinical endpoint in 49 studies evaluating treatments for CKD progression, providing evidence for its validation.10,11 These associations are substantially stronger than that observed for treatment effects on change in albuminuria to the clinical endpoint in CKD,14 consistent with the biological nature of GFR decline as an intermediate endpoint along the path to kidney failure. The FDA has stated that they have “accepted GFR slope as an endpoint and basis for full approval of therapies for rare chronic kidney diseases”, and the EMA stated that GFR slope “offers promising potential for a surrogate endpoint in the confirmatory phase of a specific clinical program”13. This supplements the FDA’s prior acceptance of confirmed 40% and 30% decline in GFR as a basis for drug approval of therapies intended to treat common and rare chronic kidney diseases, respectively.12 Thus the results presented here together with our prior work evaluating treatment effects on urine protein and on GFR slope to clinical endpoints, suggest at least two potential uses for urine protein as a surrogate endpoint in IgAN studies.10 First, treatment effects on early change in urine protein can be used in early phase studies for proof-of-concept or dose finding, both of which may accommodate some degree of uncertainty. Second, change in urine protein can be used for initial regulatory approval followed by a confirmatory trial that uses mean difference in GFR slope as the endpoint. This can be performed as two separate trials or as part of an adaptive clinical trial design. In such a design, the treatment effect on early change in proteinuria at an interim analysis can be used to estimate conditional power for treatment effects on GFR slope, as an intermediate endpoint for the clinical endpoint. In this setting, treatment effects on both change in urine protein and on GFR slope could also be used in combination to jointly predict treatment effect on the clinical endpoint should the patients be followed sufficiently. The ultimate decision as to how urine protein is integrated into a trial design or drug development program rests with the sponsor and regulatory body and would be influenced by multiple factors, including but not limited to the intervention and population being studied. These data support these discussions.
Treatment effects on urine protein appeared to be more strongly associated with treatment effects on the chronic slope than on the total slope. In general, variation between effects on the total and chronic slope likely reflects the presence of acute or immediate effects that differ from the longer-term effects. It is possible that a stronger trial level association would also be observed between treatment effects on urine protein and on the total slope in trials investigating treatments without an acute effect. We have previously shown that use of chronic slope is one method to reduce the impact of the acute effect, and these results are consistent with that observation.10,11 For future trials, the decision about use of total vs chronic slope as the primary endpoint would rely on multiple considerations such as the drug’s mechanism of action, knowledge about acute effects and prior knowledge about the drug in similar or other populations. There has been reluctance to use the chronic slope as a sole primary endpoint because the chronic slope is computed from a post-baseline time point, after the GFR has already been modified by the treatment. In certain scenarios this incurs a risk of bias due to attenuation of the acute effect or early discontinuation of the study medication.49 Future work should guide us on how to minimize bias with endpoints which are designed to estimate and minimize the impact of the acute effect similarly to the chronic slope. Possibilities include employing off-treatment GFR measurements and application of different pre-randomization baseline measurements for the treatment and control arms following introduction of the treatment in a run-in phase, as seen in the recent studies evaluating tolvaptan in polycystic kidney disease.50 For some treatments, it may be appropriate to evaluate both slope endpoints as part of the totality of evidence in a drug development program.
Strengths of the current analysis include a systematic literature search to include all available published English language studies, uniform definitions of exposures and outcomes across studies, and a robust trial level analysis. There are also limitations. First, due to the rarity of the disease, the analysis is underpowered to estimate the associations given the small number of studies, all of which had small sample size. Second, our designation of the treatment arm in each trial as the group hypothesized to have the greater benefit was somewhat arbitrary. Since in the studies that compared azathioprine and steroids vs. steroids alone, the azathioprine + steroids was considered the active treatment group and steroids are considered effective, this could have biased the results.21,22 Third, we only included studies written in English that had sufficient data for our analyses and where the investigators were able to share data. Fourth, the evaluation of urine protein as a surrogate endpoint was limited to changes between approximately 6–12 months, and our findings may not extend changes in urine protein over longer (or shorter) time periods. However, our intention is to evaluate early changes in urine protein as if trials are longer, then GFR slope generally becomes more informative. Since the endpoint is defined by the change in urine protein, all participants must have survived to have the second measurement. Finally, IgAN is heterogeneous, and treated with heterogeneous treatments and these results apply to populations selected for inclusions and for treatments evaluated in the current analysis.
Overall, the evidence presented here, when considered in conjunction with prior studies, supports the use of urine protein as an initial surrogate endpoint followed by GFR slope for subsequent confirmatory studies and accumulation of safety data, or as parts of early phase studies. This presents a pathway for drug development that could facilitate studies for new treatments for IgAN.
Supplementary Material
Figure S1 Flowchart of study identification process
Figure S2 Assessment of bias in each study
Figure S3 Treatment effect on change in urine protein at 6, 9 and 12 months
Figure S4 Treatment effect on total slope at 1, 2 or 3 years
Figure S5 Treatment effect on chronic slope computed overall and at two years
Figure S6 Trial level association of UP with Total GFR slope at 1, 2 and 3 years
Figure S7 Trial level association of UP with overall chronic slope and 2 year chronic slope
Figure S8 Posterior predictive probabilities for True Treatment Effects on Total GFR slope at 1 and 2 years and 2 year chronic slope
Item S1 Abbreviations, Units, and Terms
Item S2 Study Funding Sources
Item S3 Protocol
Table S1 Search terms for systematic review
Table S2 Inclusion criteria for studies in systematic review
Table S3 Study characteristics and inclusion criteria
Table S4 Patient characteristics, by study, for analysis of change in urine protein
Table S5 Follow-up time, mean number of GFR measurements and average max eGFR visit time
Table S6 Change in urine protein by treatment arm and treatment effect, at 6, 9 and 12 months
Table S7 Total GFR slope by treatment arm and treatment effect at 1, 2 and 3 years
Table S8 Chronic GFR slope by treatment arm and treatment effect
Table S9 Trial level associations between treatment effect on change in urine protein and treatment effect on GFR slope
Table S10 Predicted GFR slope for future trials
Table S11 Threshold for treatment effect on urine protein change to assure PPV above range of target
Acknowledgements:
The authors thank the following CKD-EPI Investigators/Collaborators (study acronyms are listed in Item S1 in the supplement with other abbreviations): Bari: Francesco Paolo Schena, Manno Carlo, Pesce Francesco, Rossini Michele; Fukuoka: Ritsuko Katafuchi, MD; HKVIN: Philip K T Li, Kai Ming Chow, Cheuk Chun Szeto; Chi Bon Leung; Lecco: Francesco Locatelli, Lucia Del Vecchio, Claudio Pozzi, Simeone Andrulli; Leuven: Bart D Maes; Madrid: Manuel Praga, Eduardo Gutierrez, Fernando Caravaca-Fontan, Angel Sevillano; New York: Gerald B. Appel, Gershon Frisch; Rochester: James Donadio, Fernando C. Fervenza; STOP-IgAN: Jürgen Floege, Thomas Rauen, Christina Fitzner. Prior Presentation: Part of the results were presented at the American Society of Nephrology Kidney Week 2019.
Support:
Funding was provided by the National Kidney Foundation and Travere Therapeutics (formerly known as Retrophin) in agreement with Tufts Medical Center and University of Utah. A variety of sources have supported the RCTs included in the CKD-Epidemiology Collaboration. These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors listed in Item S2. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosure:
Lesley A Inker reports funding from National Institutes of Health (NIH), National Kidney Foundation (NKF), Travere Therapeutics, Omeros, Dialysis Clinics, Inc., and Reata Pharmaceuticals for research and contracts to Tufts Medical Center; and consulting agreements with Tricida. Andrew S Levey reports grants and contracts from NIH and NKF to Tufts Medical Center; and a clinical trial contract with Astra Zeneca. Hiddo Lambers-Heerspink is supported by a VIDI (917.15.306) grant from the Netherlands Organisation for Scientific Research and has served as a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Gilead, Janssen, NovoNordisk, Mundipharma, Mitsubishi Tanabe, and Travere Therapeutics; and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. Ulysses Diva is an employee of Travere Therapeutics, Inc. and may have an equity or other financial interest in Travere Therapeutics, Inc. Alex Mercer is a consultant to Travere Therapeutics, Inc. Jürgen Floege–consulting agreements with Calliditas, Travere Therapeutics and Omeros; clinical trial contract with Visterra. Gerald B Appel reports research grants and/or consulting fees from Achillion, Aurinia, BM Squibb, Callidiatas, ChemoCentryx, EMD Serono, Genentech – Roche, Travere Therapeutics, Mallinkrodt, NIH-NEPTUNE STUDY, Sanofi-Genzyme, Reata, Genentech, Sanofi-Genzyme, Alexion, Pfizer, Merck, Zyvers, and Omeros. Philip K T Li received speaker Honorarium from Fibrogen and Astra Zeneca. Bart D Maes hold the following positions: National Leader ASCEND ND and D trial – GSK, National Leader Nefigard trial – Calliditas, National Leader DUPLEX and PROTECT trial – Travere Therapeutics, Steering Committee Member LPN023 IgAN trial – Novartis. Manuel Praga has consulting agreements with Alexion and Travere Therapeutics and reports payment for lectures from Alexion Novartis and Otsuka. Tom Greene reports grants from NIH during the conduct of the study; personal fees from Janssen Pharmaceuticals, DURECT Corporation, Pfizer Inc., CSL, Boehringer-Ingelheim and Astrazeneca, outside the submitted work. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–835. [DOI] [PubMed] [Google Scholar]
- 2.Inker LA, Lambers Heerspink HJ, Mondal H, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64(6):848–859. [DOI] [PubMed] [Google Scholar]
- 3.Lv J, Zhang H, Wong MG, et al. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2017;318(5):432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachman P, Thompson A. Identifying Surrogate Endpoints for Clinical Trials in IgA Nephropathy. Am Soc Neph. Kidney Health Initiative (KHI) Web site. https://www.asn-online.org/khi/project.aspx?ID=58. Published 2017. Accessed 1/14/2019. [Google Scholar]
- 5.Inker LA, Mondal H, Greene T, et al. Early Change in Urine Protein as a Surrogate End Point in Studies of IgA Nephropathy: An Individual-Patient Meta-analysis. Am J Kidney Dis. 2016;68(3):392–401. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for Industry Expedited Programs for Serious Conditions – Drugs and Biologics. https://www.fda.gov/downloads/Drugs/Guidances/UCM358301.pdf. Published 2014. Accessed 1/16/2019.
- 7.United States Code. 21 CFR Part 314, Subpart H, Accelerated Approval of New Drugs for Serious or Life-Threatening Illnesses. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=2ec0bc1571c06727e63ffa2c72b2e08d&mc=true&n=pt21.5.314&r=PART&ty=HTML#sp21.5.314.h.Accessed 1/16/2019.
- 8.Thompson A, Carroll K, L AI, et al. Proteinuria Reduction as a Surrogate End Point in Trials of IgA Nephropathy. Clinical journal of the American Society of Nephrology : CJASN. 2019;14(3):469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grams ME, Sang Y, Ballew SH, et al. Evaluating Glomerular Filtration Rate Slope as a Surrogate End Point for ESKD in Clinical Trials: An Individual Participant Meta-Analysis of Observational Data. J Am Soc Nephrol. 2019;30(9):1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, Heerspink HJL, Tighiouart H, et al. GFR Slope as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Meta-Analysis of Treatment Effects of Randomized Controlled Trials. J Am Soc Nephrol. 2019;30(9):1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene T, Ying J, Vonesh EF, et al. Performance of GFR Slope as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Statistical Simulation. J Am Soc Nephrol. 2019;30(9):1756–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson A, Smith K, Lawrence J. Change in Estimated GFR and Albuminuria as End Points in Clinical Trials: A Viewpoint From the FDA. American Journal of Kidney Diseases. 2020;75(1):4–5. [DOI] [PubMed] [Google Scholar]
- 13.Holtkamp F, Gudmundsdottir H, Maciulaitis R, Benda N, Thomson A, Vetter T. Change in Albuminuria and Estimated GFR as End Points for Clinical Trials in Early Stages of CKD: A Perspective From European Regulators. American Journal of Kidney Diseases. 2020;75(1):6–8. [DOI] [PubMed] [Google Scholar]
- 14.Heerspink HJL, Greene T, Tighiouart H, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. The lancet Diabetes & endocrinology. 2019;7(2):128–139. [DOI] [PubMed] [Google Scholar]
- 15.Li PK, Leung CB, Chow KM, et al. Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis. 2006;47(5):751–760. [DOI] [PubMed] [Google Scholar]
- 16.Praga M, Gutierrez E, Gonzalez E, Morales E, Hernandez E. Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol. 2003;14(6):1578–1583. [DOI] [PubMed] [Google Scholar]
- 17.Donadio JV Jr., Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. Mayo Nephrology Collaborative Group. J Am Soc Nephrol. 1999;10(8):1772–1777. [DOI] [PubMed] [Google Scholar]
- 18.Donadio JV Jr., Larson TS, Bergstralh EJ, Grande JP. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol. 2001;12(4):791–799. [DOI] [PubMed] [Google Scholar]
- 19.Maes BD, Oyen R, Claes K, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney international. 2004;65(5):1842–1849. [DOI] [PubMed] [Google Scholar]
- 20.Frisch G, Lin J, Rosenstock J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20(10):2139–2145. [DOI] [PubMed] [Google Scholar]
- 21.Pozzi C, Andrulli S, Pani A, et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol. 2010;21(10):1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi C, Andrulli S, Pani A, et al. IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. Journal of nephrology. 2013;26(1):86–93. [DOI] [PubMed] [Google Scholar]
- 23.Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15(1):157–163. [DOI] [PubMed] [Google Scholar]
- 24.Pozzi C, Bolasco PG, Fogazzi GB, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353(9156):883–887. [DOI] [PubMed] [Google Scholar]
- 25.Locatelli F, Pozzi C, Del Vecchio L, et al. Role of proteinuria reduction in the progression of IgA nephropathy. Renal failure. 2001;23(3–4):495–505. [DOI] [PubMed] [Google Scholar]
- 26.Katafuchi R, Ikeda K, Mizumasa T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis. 2003;41(5):972–983. [DOI] [PubMed] [Google Scholar]
- 27.Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(12):3694–3701. [DOI] [PubMed] [Google Scholar]
- 28.Rauen T, Eitner F, Fitzner C, et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. New England Journal of Medicine. 2015;373(23):2225–2236. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonesh E, Tighiouart H, Ying J, et al. Mixed-effects models for slope-based endpoints in clinical trials of chronic kidney disease. Statistics in medicine. 2019;38(22):4218–4239. [DOI] [PubMed] [Google Scholar]
- 31.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311(24):2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 33.Shen P, Shen J, Li W, He L. Urinary podocyte can be an indicator for the pathogenetic condition of patients with IgA nephropathy. Clinical laboratory. 2014;60(10):1709–1715. [DOI] [PubMed] [Google Scholar]
- 34.Kamei K, Nakanishi K, Ito S, et al. Risk factors for persistent proteinuria after a 2-year combination therapy for severe childhood IgA nephropathy. Pediatr Nephrol. 2015;30(6):961–967. [DOI] [PubMed] [Google Scholar]
- 35.Coppo R, Troyanov S, Bellur S, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney international. 2014;86(4):828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Seminars in nephrology. 2004;24(3):179–196. [DOI] [PubMed] [Google Scholar]
- 37.Barbour SJ, Reich HN. Risk stratification of patients with IgA nephropathy. Am J Kidney Dis. 2012;59(6):865–873. [DOI] [PubMed] [Google Scholar]
- 38.Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney international. 2009;76(5):534–545. [DOI] [PubMed] [Google Scholar]
- 39.Frimat L, Briancon S, Hestin D, et al. IgA nephropathy: prognostic classification of end-stage renal failure. ĽAssociation des Nephrologues de l’Est. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1997;12(12):2569–2575. [DOI] [PubMed] [Google Scholar]
- 40.Reich HN, Troyanov S, Scholey JW, Cattran DC. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177–3183. [DOI] [PubMed] [Google Scholar]
- 41.Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association European Renal Association. 2002;17(7):1197–1203. [DOI] [PubMed] [Google Scholar]
- 42.Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. [DOI] [PubMed] [Google Scholar]
- 43.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T. Early Change in Proteinuria as a Surrogate End Point for Kidney Disease Progression: An Individual Patient Meta-analysis. Am J Kidney Dis. 2014;64(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatematsu M, Yasuda Y, Morita Y, et al. Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clinical and experimental nephrology. 2012;16(6):883–891. [DOI] [PubMed] [Google Scholar]
- 45.Hirano K, Kawamura T, Tsuboi N, et al. The predictive value of attenuated proteinuria at 1 year after steroid therapy for renal survival in patients with IgA nephropathy. Clinical and experimental nephrology. 2013;17(4):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barratt J, Rovin B, Diva U, Mercer A, Komers R. Implementing the Kidney Health Initiative Surrogate Efficacy Endpoint in Patients With IgA Nephropathy (the PROTECT Trial). Kidney international reports. 2019;4(11):1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).Identifier: NCT03643965, Efficacy and Safety of Nefecon in Patients With Primary IgA (Immunoglobulin A) Nephropathy (Nefigard). https://clinicaltrials.gov/ct2/show/NCT03643965.Accessed December 10, 2020. [Google Scholar]
- 48.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).Identifier: NCT03608033, Study of the Safety and Efficacy of OMS721 in Patients With Immunoglobulin A (IgA) Nephropathy. https://clinicaltrials.gov/ct2/show/NCT03608033.Accessed December 10, 2020. [Google Scholar]
- 49.Gassman JJ, Greene T, Wright JT Jr., et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol. 2003;14(7 Suppl 2):S154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. The New England journal of medicine. 2012;367(25):2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Flowchart of study identification process
Figure S2 Assessment of bias in each study
Figure S3 Treatment effect on change in urine protein at 6, 9 and 12 months
Figure S4 Treatment effect on total slope at 1, 2 or 3 years
Figure S5 Treatment effect on chronic slope computed overall and at two years
Figure S6 Trial level association of UP with Total GFR slope at 1, 2 and 3 years
Figure S7 Trial level association of UP with overall chronic slope and 2 year chronic slope
Figure S8 Posterior predictive probabilities for True Treatment Effects on Total GFR slope at 1 and 2 years and 2 year chronic slope
Item S1 Abbreviations, Units, and Terms
Item S2 Study Funding Sources
Item S3 Protocol
Table S1 Search terms for systematic review
Table S2 Inclusion criteria for studies in systematic review
Table S3 Study characteristics and inclusion criteria
Table S4 Patient characteristics, by study, for analysis of change in urine protein
Table S5 Follow-up time, mean number of GFR measurements and average max eGFR visit time
Table S6 Change in urine protein by treatment arm and treatment effect, at 6, 9 and 12 months
Table S7 Total GFR slope by treatment arm and treatment effect at 1, 2 and 3 years
Table S8 Chronic GFR slope by treatment arm and treatment effect
Table S9 Trial level associations between treatment effect on change in urine protein and treatment effect on GFR slope
Table S10 Predicted GFR slope for future trials
Table S11 Threshold for treatment effect on urine protein change to assure PPV above range of target