Abstract
Background and Purpose:
High blood pressure (BP) variability after endovascular stroke therapy (EVT) is associated with poor outcome. Conventional BP variability measures require long recordings, limiting their utility as a risk assessment tool to guide clinical decision making. Here, we performed rapid assessment of BP variability by spectral analysis and evaluated its association with early clinical improvement and long-term functional outcomes.
Methods:
We conducted a prospective study of 146 patients with anterior circulation ischemic stroke who underwent successful EVT. Spectral analysis of 5-minute recordings of beat-to-beat BP was used to quantify BP variability. Outcomes included initial clinical response and modified Rankin scale at 90 days.
Results:
Increased BP variability at high frequencies was independently associated with poor functional outcome at 90 days (adjusted odds ratio [aOR]=1.85, 95% CI 1.07 – 3.25, p=0.03; low/high frequency ratio aOR= 0.67, 0.46 – 0.92, p=0.02) and reduced likelihood of an early neurological recovery (aOR=0.62, 0.44 – 0.91, p=0.01 and aOR=1.37, 1.03 – 1.87, p=0.04, respectively).
Conclusions:
High-frequency BP oscillations after successful reperfusion may be harmful and associate with a decreased likelihood of neurological recovery and favorable functional outcomes. Rapid assessment of BP variability throughout the post-reperfusion period is feasible and may allow for a more personalized BP management.
Introduction
There is an increased risk of hemorrhagic transformation (HT) and worse outcome in patients with increased blood pressure (BP) during the first hours after EVT.1, 2 Because of impaired cerebral autoregulation with resulting susceptibility to cerebral hypo- or hyperperfusion,3 avoiding large BP swings after revascularization may be as important as treating high BP levels. Conventional measures of BP variability usually require 24 hours of BP monitoring, limiting their usefulness for clinical decisions making. This study aimed to assess BP variability from 5-minute beat-to-beat recordings using spectral analysis and evaluate its ability to predict early neurological recovery and long-term functional outcome.
Materials and Methods
Data availability
Data and statistical syntax is available for research purposes and can be obtained by a reasonable request to the corresponding author.
Study design and outcomes
We conducted a prospective study of adult patients with anterior circulation large-vessel occlusion (LVO) stroke who underwent successful EVT (modified Thrombolysis in Cerebral Infarction score [mTICI] 2b-3).4 Standard demographic/clinical variables were recorded. CT scans were centrally reviewed by an experienced neuroradiologist (SP) for lesions affecting the central autonomic network (Supplemental Figure I).5 The study received approval from the ethics committee at both institutions. Written informed consent was obtained from all participants or legally authorized representatives.
Functional outcome was assessed using the modified Rankin scale (mRS) score at 90 days. Additional outcomes at 24 hours included midline shift, HT and early neurological recovery, the reduction in NIHSS score of at least 4 points or NIHSS<2.
BP variability
Five-minute beat-to-beat BP recordings were obtained <24 hours from last-seen-well time using non-invasive finger photoplethysmography (Finometer MIDI, FMS, Netherlands). Details in Supplemental Data. Spectral analysis was performed using the Welch method.6 Power spectra were decomposed in very-low (VLF;<0.04 Hz), low (LF;0.04–0.15 Hz) and high (HF;0.15–0.40 Hz) frequency bands. Increased power implies more variability. Additionally, BP was assessed hourly (brachial cuff) to calculate mean and standard deviation (SD) over 24-hours.
Statistical Analysis
For details see Supplementary Data.
Results
Between September 2017 and January 2019, 146 patients were enrolled (Table I; Supplemental Figure II). Overall, unfavorable functional outcome was associated with higher systolic BP at admission and during the first 24 hours after EVT (Supplemental Table III). We found no association between BP variability measured as SD and any of the endpoints.
Normalized HF power was significantly lower in patients demonstrating faster neurological recovery and in those who were independent at 90 days (Table 1). Adjusting to baseline severity, higher normalized HF power (adjusted OR [aOR]=1.85, 95%CI 1.07 – 3.25, p=0.03) and LH/HF ratio (aOR=0.67, 0.46 – 0.92, p=0.02) were independently associated with poor functional outcome. Higher normalized HF power and lower LH/HF ratio also reduced the likelihood of neurological recovery (aOR=0.62, 0.44–0.91, p=0.01; aOR=1.37, 1.03 – 1.87, p=0.04); Supplemental Table III). Figure 1 shows representative cases.
Table 1.
BP parameters among subgroups of clinical outcome
| All (n=146) | Independent mRS 0–2 (n=63) | Dependent mRS 3–6 (n=58) | P value* | Early neurological recovery Yes (n=61) | Early neurological recovery No (n=60) | P* | |
|---|---|---|---|---|---|---|---|
| Admission | |||||||
| Systolic BP, mm Hg – mean (SD) | 141 (24) | 136 (26) | 146 (21) | 0.02 | 140 (24) | 143 (24) | 0.53 |
| Diastolic BP, mm Hg – mean (SD) | 77 (19) | 76 (24) | 79 (15) | 0.33 | 77 (21) | 77 (18) | 0.34 |
| 24-hour monitoring | |||||||
| Averaged Systolic BP, mm Hg – mean (SD) | 125 (16) | 121 (17) | 130 (15) | <0.01 | 124 (15) | 126 (17) | 0.41 |
| SD of Systolic BP, mm Hg – median (IQR) | 13 (10 – 16) | 13 (10 – 15) | 14 (11 – 16) | 0.12 | 13 (10 – 15) | 14 (10 – 17) | 0.10 |
| Averaged Diastolic BP, mm Hg – mean (SD) | 68 (11) | 68 (12) | 68 (10) | 0.75 | 69 (11) | 68 (11) | 0.61 |
| SD of Diastolic BP, mm Hg – median (IQR) | 9 (7 – 12) | 9 (7 – 12) | 10 (8 – 12) | 0.10 | 9 (7 – 11) | 10 (8 – 13) | 0.08 |
| Very Short-term systolic BP variability (5 min) | |||||||
| Total spectral power, mm Hg2 – median (IQR) | 45.3 (21.3 – 78.3) | 45.5 (20.3 – 74.1) | 43.2 (21.6 – 81.3) | 0.75 | 45.3 (21.0 – 76.7) | 43.2 (21.1 – 78.4) | 0.86 |
| VLF power, mm Hg2 – median (IQR) | 20.6 (10.2 – 41.5) | 21.2 (10.5 – 42.0) | 20.2 (9.28 – 39.4) | 0.69 | 19.1 (10.5 – 43.1) | 21.7 (9.2 – 39.8) | 0.92 |
| LF power, mm Hg2 – median (IQR) | 7.1 (3.5 – 17.4) | 7.8 (3.6 – 20.0) | 7.1 (3.3 – 14.9) | 0.41 | 7.0 (3.6 – 19.2) | 7.4 (3.2 – 14.3) | 0.59 |
| HF power, mm Hg2 – median (IQR) | 5.7 (2.5 – 17.8) | 4.5 (2.1 – 14.6) | 7.0 (2.7 – 25.3) | 0.07 | 4.5 (1.9 – 15.1) | 7.1 (2.8 – 20.8) | 0.12 |
| LF spectral power, nu – mean (SD) | 0.52 (0.25) | 0.59 (0.23) | 0.46 (0.25) | <0.01 | 0.57 (0.25) | 0.48 (0.24) | 0.02 |
| HF spectral power, nu – mean (SD) | 0.48 (0.25) | 0.41 (0.23) | 0.54 (0.25) | <0.01 | 0.43 (0.25) | 0.52 (0.24) | 0.02 |
| LF/HF ratio – median (IQR) | 1.09 (0.46 – 2.7) | 1.71 (0.64 – 3.94) | 0.71 (0.38 – 1.93) | <0.01 | 1.58 (0.58 – 3.78) | 0.83 (0.42 – 1.99) | 0.02 |
Abbreviations: Blood pressure (BP); High, Low and Very-Low Frequency (HF;LF;VLF); interquartile range (IQR); normalized units (nu); standard deviation (SD)
Differences between subgroups from chi-square/Fisher’s exact or Student’s t/Mann-Whitney tests.
Figure 1.
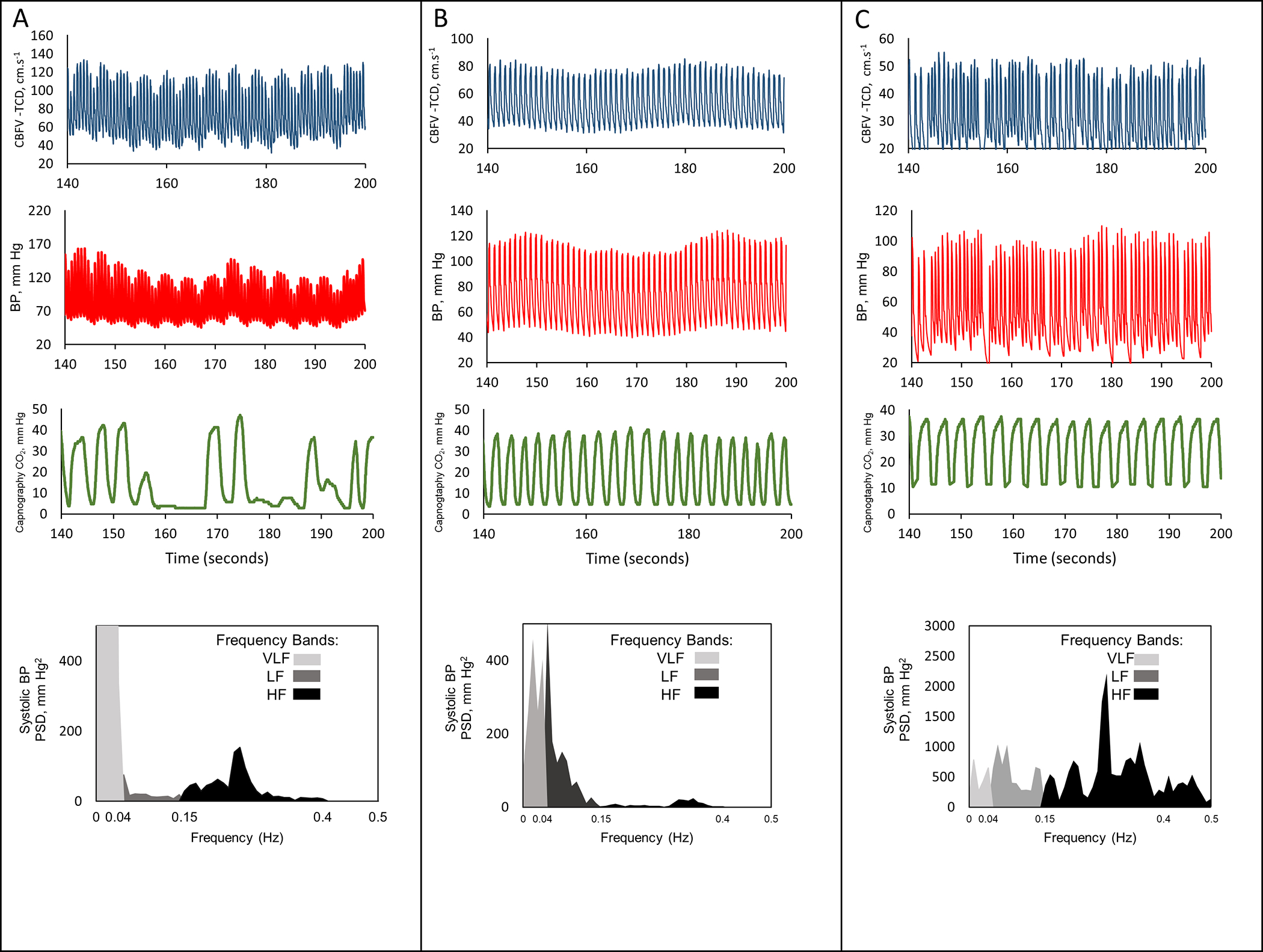
Representative cases (A, B and C) of very short-term variability. Red, blue and green lines represent continuous blood pressure (BP; Finometer), cerebral blood flow velocity (CBFV; transcranial Doppler) and expiratory carbon dioxide (CO2; capnography). At bottom, spectral density of systolic BP in very-low (VLF/light grey), low (LF/dark grey) and high (HF/ black) bands. A–poor prognosis; BP variability peak~0.3 Hz (HF range). B–good prognosis; increased LF over HF power. C–poor prognosis; atrial fibrillation, still shows a peak within HF band.
Considering the prognostic performance of BP parameters (Figure 2), we found that normalized HF power was the best predictor of early neurological recovery (AUC=0.63, 0.61 – 0.68, p=0.02) and functional outcome (AUC=0.65, 0.63 – 0.67, p<0.01) (Supplemental Figure III); optimal cuff-off at 0.45. HF>0.45 was significantly associated with a shift towards worse mRS classes (aOR=2.71, 1.44 – 5.14, p<0.01, Figure 2). Sensitivity analyses showed no bias due to atrial fibrillation, hypertension, or time of recanalization (Supplemental Figure IV).
Figure 2.
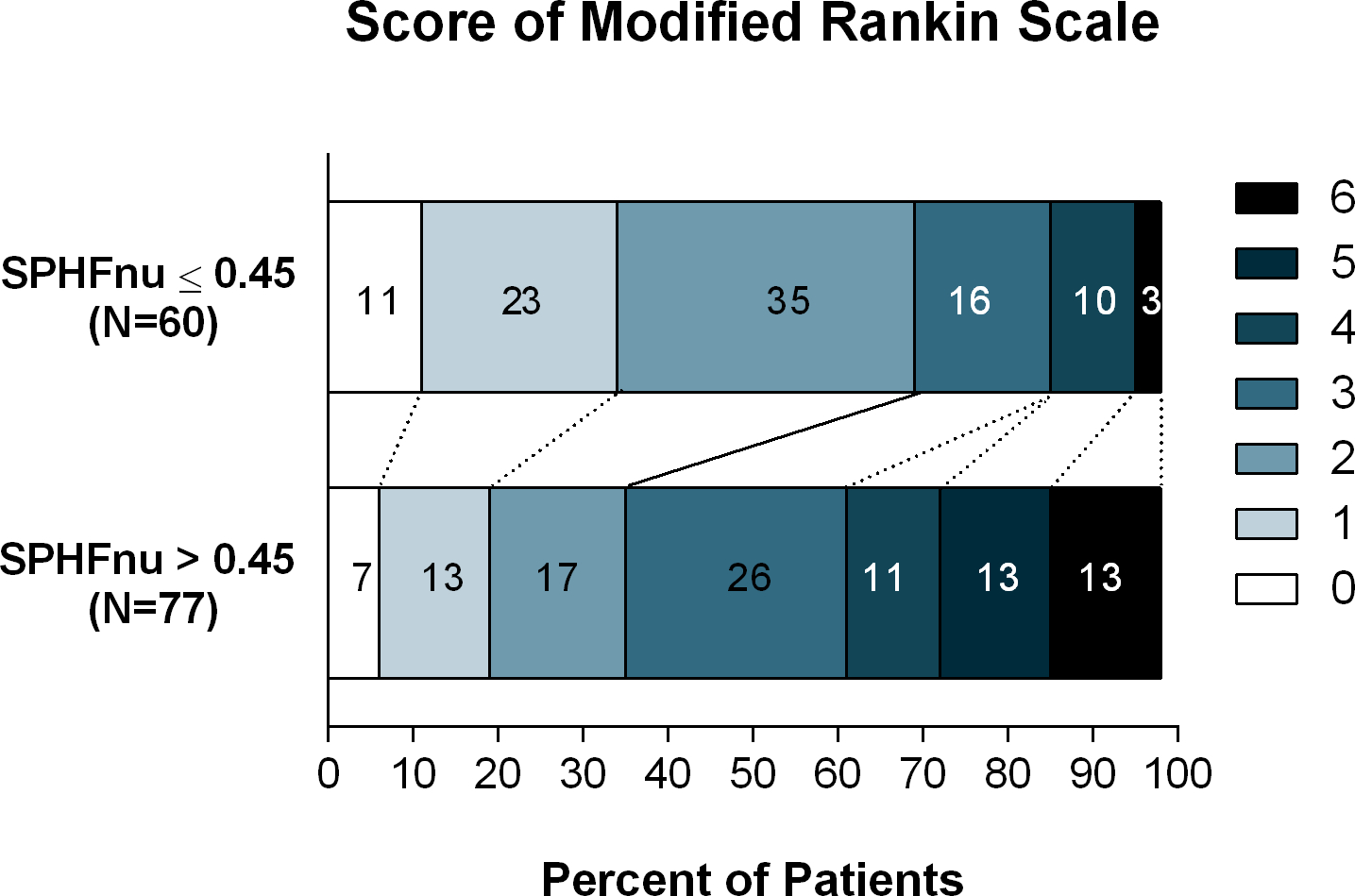
Distribution of scores on the modified Rankin scale at 90 days among patients with high versus low very-short-term BP variability (normalized units, SPHFnu)
Normalized HF power was associated with the development of significant cerebral edema (aOR=5.87, 1.01 – 44, p=0.04, Supplemental Table IV). Infarct volume or location did not relate to BP parameters (Supplemental Table V).
Discussion
In 146 consecutive patients with anterior circulation LVO stroke, increased high-frequency BP variability after successful EVT was associated with the development of significant cerebral edema, a lower probability of early neurological recovery and increased risk of unfavorable functional outcome
Our results suggest that the harmful effects of BP variability may, in part, be related to cerebral edema and possibly dysfunction of blood-barrier that follows successful EVT.7 Our results consistent with previous studies that showed increased variations of hourly BP measurements after stroke are associated with an increased risk of cerebral edema and HT.8–10 On the other hand, severe edema can cause BP changes by itself. However, unlike traditional methods, our approach can detect harmful patterns of BP variability within minutes, and thus can provide a real-time measure to guide BP management in individual patients.11–13
Autonomic dysfunction is common in stroke,14 despite strong conceptual reasons, we found no association between HF BP variability and injury to the structures of the central autonomic network or infarct volume.
Limitations concerns a modest sample size, restriction to successful EVT, and lack of MRI protocol to evaluate blood-barrier dysfunction.15 Spectral BPV should be studied also in unsuccessful recanalization and different collateral grades to broaden the extension of our results. Vasoactive drugs can be confounding factors for BP parameters, despite multivariate adjustment, as infection and other respiratory parameters. Finger plethysmography presents several challenges in the acute stroke settings (Supplementary Data).
Summary
Rapid bedside assessment of BP variability is feasible and carries prognostic significance. Further research is needed to test novel treatment strategies to minimize potentially harmful BP fluctuations.
Supplementary Material
Conflict(s)-of-Interest/Disclosure(s):
C.O.T. serves as data science consultant to Lokavant Inc. and receives consultancy fees.
N.P. Received grants from NIH/NINDS K23NS110980
S. P. received funding from the Doris Duke Charitable Foundation
F. S. received funding from National Institutes of Health (NIH)
Non-standard Abbreviations and Acronyms
- BP
Blood Pressure
- LVO
Large vessel occlusion
- EVT
Endovascular treatment
- VLF
Very-low frequency
- LF
Low frequency
- HF
High frequency
Footnotes
References
- 1.Anadani M, Orabi MY, Alawieh A, Goyal N, Alexandrov AV, Petersen N, Kodali S, Maier IL, Psychogios MN, Swisher CB, et al. Blood pressure and outcome after mechanical thrombectomy with successful revascularization. Stroke. 2019;50:2448–2454 [DOI] [PubMed] [Google Scholar]
- 2.Kim TJ, Park HK, Kim JM, Lee JS, Park SH, Jeong HB, Park KY, Rha JH, Yoon BW, Ko SB. Blood pressure variability and hemorrhagic transformation in patients with successful recanalization after endovascular recanalization therapy: A retrospective observational study. Ann Neurol. 2019;85:574–581 [DOI] [PubMed] [Google Scholar]
- 3.Castro P, Serrador JM, Rocha I, Sorond F, Azevedo E. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcome. Front Neurol. 2017;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–137 [DOI] [PubMed] [Google Scholar]
- 5.Sie J-H, Chen Y-H, Chang C-Y, Yen N-S, Chu W-C, Shiau Y-HJSr. Altered central autonomic network in baseball players: A resting-state fmri study. 2019;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimoto T, Eguchi K, Sakurai H, Ohmichi Y, Hashimoto T, Ohmichi M, Morimoto A, Yamaguchi Y, Ushida T, Iwase SJTJoPS. Frequency components of systolic blood pressure variability reflect vasomotor and cardiac sympathetic functions in conscious rats. 2011;61:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamner JW, Ishibashi K, Tan CO. Revisiting human cerebral blood flow responses to augmented blood pressure oscillations. J Physiol. 2019;597:1553–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Mederos R, Ribo M, Rovira A, Rubiera M, Munuera J, Santamarina E, Delgado P, Maisterra O, Alvarez-Sabin J, Molina CA. Prognostic significance of blood pressure variability after thrombolysis in acute stroke. Neurology. 2008;71:552–558 [DOI] [PubMed] [Google Scholar]
- 9.Cho BH, Kim JT, Lee JS, Park MS, Kang KW, Choi KH, Lee SH, Choi SM, Kim BC, Kim MKJEjon. Associations of various blood pressure parameters with functional outcomes after endovascular thrombectomy in acute ischaemic stroke. 2019;26:1019–1027 [DOI] [PubMed] [Google Scholar]
- 10.Mistry EA, Mehta T, Mistry A, Arora N, Starosciak AK, De Los Rios La Rosa F, Siegler JE 3rd, Chitale R, Anadani M, Yaghi S, et al. Blood pressure variability and neurologic outcome after endovascular thrombectomy: A secondary analysis of the best study. Stroke. 2020;51:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning LS, Rothwell PM, Potter JF, Robinson TGJS. Prognostic significance of short-term blood pressure variability in acute stroke: Systematic review. 2015;46:2482–2490 [DOI] [PubMed] [Google Scholar]
- 12.Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic significance of blood pressure variability on beat-to-beat monitoring after transient ischemic attack and stroke. Stroke. 2018;49:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb AJS, Lawson A, Wartolowska K, Mazzucco S, Rothwell PM. Progression of beat-to-beat blood pressure variability despite best medical management. Hypertension. 2021;77:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Raedt S, De Vos A, De Keyser JJJotns. Autonomic dysfunction in acute ischemic stroke: An underexplored therapeutic area? 2015;348:24–34 [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, Pile-Spellman JJNC. Reperfusion changes after stroke and practical approaches for neuroprotection. 2018;28:663–682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and statistical syntax is available for research purposes and can be obtained by a reasonable request to the corresponding author.


