Abstract
Background:
Middle meningeal artery embolization (MMAE) has been used as an effective minimally invasive treatment for chronic subdural hematomas (cSDH). The demographics and clinical outcomes after MMAE treatment for cSDH have not yet been studied using the large scale of a national database.
Methods:
We queried all MMAE cases up to October 7th, 2020, from the TriNetX Analytics Network. We identified patients >18 years old who underwent MMAE for the treatment of cSDH. Patient demographics, baseline characteristics, comorbidities, and clinical outcomes were evaluated within 180 days post-MMAE. 180-day mortality and recurrence analyses were performed after propensity score matching to control for baseline characteristics and comorbidities.
Results:
A total 191 patients were included (mean age: 71.2 ± 13.5, 73.3% male, 69.6% White, 13.6% Black/African American, and 16.8% other). Essential hypertension (71.3%), heart disease (62.8%), type 2 diabetes mellitus (27.2%), nicotine dependence (23.6%), chronic kidney disease (19.4%), and overweight/obesity (19.4%) were among the most prevalent comorbidities. At presentation, 20.4% and 40.3% were on antiplatelet and anticoagulation therapy, respectively. Outcomes within a 180-day follow-up were 6.3% (or 1.0–5.8% when propensity-matched) for mortality (12 patients), 7.3% for craniotomy/craniectomy after MMAE (14 patients), 0.52–5.2% for burr hole procedures (1–10 patients), and no patients with low vision/blindness.
Conclusion:
MMAE is a safe and effective minimally invasive procedure for the treatment of cSDH. This represents the first analysis of patients undergoing MMAE for cSDH using a national database.
Keywords: Chronic subdural hematoma, chronic subdural hemorrhage, refractory subdural hematoma, middle meningeal artery embolization
Introduction
Chronic Subdural Hematoma (cSDH) is one of the most prevalent pathologies encountered in neurosurgery. The incidence of cSDH is rising in the U.S., and it is estimated to be 60,000 cases per year by 2030 due to various factors such as the aging population and increased cardiovascular risk factors (e.g., metabolic syndrome, obesity, diabetes) requiring the use of anticoagulation.1 Treatment of cSDH with the traditional approach of surgical evacuation has a high recurrence rate of 2–37%,2–9 which makes it particularly challenging as the patients are commonly elderly with various cardiovascular comorbidities and coagulopathies. The high recurrence rate is in part due to not addressing the pathological cause of cSDH, which is hypothesized to result from elevated angiogenic factors due to chronic dural injury and inflammation.10–14 This results in thin and fragile capillaries along the subdural outer membrane of the hematoma, which captures its blood supply from the middle meningeal artery (MMA). Additionally, MMA enlargement has been observed in patients with cSDH, possibly due to an increase of blood supply to a cSDH from MMA, though the mechanism is unclear.15 Therefore, MMA appears to play an important role in the development of cSDH, and its embolization can prevent the extravasation of blood through these fragile and porous capillary networks and treat the underlying cause of cSDH.13, 16, 17
Middle meningeal artery embolization (MMAE) has been used as a primary or adjunctive treatment of cSDH with reported success.12, 18–22 Here, we present a cohort of 191 patients undergoing MMAE for cSDH from a national database to describe its effectiveness as a standalone or adjunctive procedure, safety in treating cSDH, and efficacy in preventing cSDH recurrence within a 180-day follow-up period.
Methods
Patient Selection and Data
The TriNetX Analytics Network, a global federated network that is comprised of de-identified electronic health records from 55 healthcare organizations (HCOs), was used for these analyses. The data within TriNetX is provided by HCOs, including academic medical centers and community hospitals in the U.S. Available data include demographics, diagnoses, procedures, medications, and laboratory measurement. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of HCOs and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. Thus, no protected health information or personal data is made available to the users of the platform. The data are updated daily, and the analyses are performed on the data queries submitted via the browser. The available data included information on baseline characteristics of patients for each diagnosis using International Classification of Diseases, 10th revision (ICD-10) codes.
Figure 1 demonstrates our inclusion and exclusion criteria. Our inclusion criteria consisted of a confirmed diagnosis of nontraumatic chronic subdural hemorrhage (ICD I62.03) and nontraumatic subdural hemorrhage (ICD I62.00) who had one of the following Current Procedural Terminology (CPT) codes 61624 or 61626 for transcatheter permanent occlusion or embolization within one month of the cSDH diagnosis. Our exclusion criteria were patients younger than 18 years old, patients who had a diagnosis of cerebral AVM (ICD Q28.2), malignant neoplasm of the brain (ICD C71), malignant neoplasm of the head, face, and neck (ICD C76.0), epistaxis (ICD R04.0), and cerebral aneurysm/acquired cerebral arteriovenous fistula (ICD I67.1) because the embolization could be for a separate indication. A cohort of 191 patients from 26 HCOs met the inclusion and exclusion criteria for outcome analysis.
Figure 1.

Patient Selection Flowchart.
For variables of interest, we identified a set of established comorbidities and risk factors for MMAE, as follows: age, sex, race (White, Black/African American, Asian), ethnicity (Hispanic/Latino, Not Hispanic/Latino, Unknown), essential/primary hypertension, type 2 diabetes mellitus, nicotine dependence, overweight/obesity, chronic kidney disease (CKD), atrial fibrillation/flutter, asthma, ischemic heart diseases within ten years (ICDs I20-I25), other heart diseases within ten years (ICDs I30-I52), antiplatelet medications (Aspirin, Clopidogrel, anticoagulation medications (Apixaban, Heparin, Rivaroxaban, and Warfarin), coagulation lab parameters (prothrombin time (P.T.) in plasma/blood, activated Partial thromboplastin time (aPTT), international normalized ratio (INR)), and complete blood count (hemoglobin, hematocrit, and platelets) in blood.23–26
For comparison of outcomes to the baseline risk of patients without a cSDH and undergoing embolization, we created a control cohort of patients who had an encounter for general adult examination without abnormal findings (ICD-10: Z00.00). The patients in the control cohort were propensity-matched to the cSDH with MMAE group based on age, sex, ethnicity, race, and comorbidities (type 2 diabetes, essential hypertension, CKD, history of nicotine use, obesity, ischemic heart disease, and other forms of heart diseases). Supplemental Table 1 provides the patient baseline characteristics before and after matching.
Outcome Analysis
Primary clinical outcomes were defined as the proportion of patients who had the outcome of interest. Outcomes studied included mortality, repeated MMAE, craniectomy or craniotomy procedures, twist drill/burr hole, and blindness/low vision.
Statistical analysis
Cohort statistics were collected for each outcome of interest. The cohort statistics include patients in the cohort, patients with the outcome, and risk of the outcome within a 180-day time window. Measures of association analysis were performed for each of the outcomes of interest; in particular, we evaluated the risk difference using a Z-test, risk ratio, and odds ratio with confidence intervals. The comparison to the control group allowed to elucidate the risk of the MMAE procedure when controlled for patient demographics and comorbidities. Kaplan Meier survival curve analysis was performed on the mortality outcome of patients over a 180-day period. A log-rank test was used to identify if the survival curves differ between the two cohorts.
Additionally, the Charlson Comorbidity Index (CCI), a measure of 1- and 10-year mortality risk based on patient’s comorbidities, was used to investigate the degree to which the difference in 6 months mortality and patients who are still alive can be attributed to their overall state of health prior to MMAE. CCI was calculated based on the individual patient’s medical history up to the day of the MMAE procedure. CCI calculations used a modified comorbidity v0.5.3.9000 package in R27, 28. The patient age was not factored into the calculation in the original package but was added in our calculations29. Kruskal Wallis test and Dunn’s multiple comparison tests were used for evaluating the statistical significance of the differences in the distribution of CCI between patients who had a mortality outcome in <6 months, 6 months to 5 years, and those still alive. The comparative and descriptive statistics were performed using GraphPad Prism 9.1.0.
Data for this study are not publicly available because of a data-use agreement. For requests to access the study data, please contact the corresponding author.
Results
Baseline Patient Characteristics
A total of 191 patients undergoing MMAE procedures were identified. Table 1 and Table 2 delineate the baseline characteristics and comorbidities of the MMAE cohort. Overall, essential hypertension, other heart diseases within ten years, ischemic heart diseases within ten years, smoking, and type 2 diabetes were the most common comorbidities. The mean age at MMAE was 71.2 ± 13.5, and 73.3% of patients were male. Based on self-reporting, 69.6% were white, 13.6% were Black/African American, and 16.8% were others/unknown. A total of 12.0% had a craniectomy/craniotomy prior to admission, and 15.7% had a previous twist drill/burr hole/trephine procedure prior to admission. A total of 20.4% were on antiplatelet medication within three months of admission. Of these, 17.8% were on Aspirin, and 0.52–5.2% were on clopidogrel. A total of 40.3% were on anticoagulation medication prior to admission. Of these, 25.1% were on Heparin, 8.4% were on Warfarin, 5.8% were on Apixaban, and 0.52–5.2% were on Rivaroxaban. Figure 2 shows the number of patients who were on antiplatelet and anticoagulant. Mean for P.T., aPTT, INR, hemoglobin, hematocrit, and platelet counts were within three months of admission, 13.3 ± 3.9, 33.6 ± 14, 1.2 ± 0.3, 11.8 ± 2.1, 36.3± 6.1, and 220 ± 92.9 respectively.
Table 1.
Patient Baseline Characteristics.
| Disease | N (%) |
|---|---|
| cSDH patients with MMA Embolization | 191 |
| Current Age (years) | 72.6 ± 13.4 |
| Age at Embolization (years) | 71.2 ± 13.5 |
| Sex | |
| Female | 51 (26.70%) |
| Male | 140 (73.30%) |
| Race | |
| White | 133 (69.63%) |
| Black or African American | 26 (13.61%) |
| Other | 32 (16.75%) |
| Ethnicity | |
| Hispanic or Latino | 18 (9.42%) |
| Not Hispanic or Latino | 134 (70.16%) |
| Unknown | 39 (20.42%) |
Table 2.
Patient Baseline Comorbidities.
| Disease | N (%) | |
|---|---|---|
| cSDH patients with MMA Embolization | 191 | |
| Comorbidity | ||
| Essential (primary) hypertension | 137 (71.73%) | |
| Type 2 diabetes mellitus | 52 (27.23%) | |
| Nicotine dependence | 45 (23.56%) | |
| Overweight and obesity | 37 (19.37%) | |
| Chronic kidney disease (CKD) | 37 (19.37%) | |
| Asthma | 12 (6.28%) | |
| Ischemic Heart Diseases within 10 Years (I20-I25) | 70 (36.65%) | |
| Other Heart Diseases within 10 Years (I30-I52) | 120 (62.83%) | |
| Previous Craniectomy or Craniotomy Procedures | 23 (12.04%) | |
| Previous Twist Drill, Burr Hole(s), or Trephine Procedures on the Skull, Meninges, and Brain | 30 (15.71%) | |
| Medication (within 3 month) | ||
| Antiplatelet medication type | 39 (20.42%) | |
| Aspirin | 34 (17.80%) | |
| Clopidogrel | 10 (5.24%) | |
| Anticoagulation type | 77 (40.31%) | |
| Heparin | 48 (25.13%) | |
| Warfarin | 16 (8.38%) | |
| Apixaban | 11 (5.76%) | |
| Rivaroxaban | 10 (5.24%) | |
| Labs (within 3 months) | N | Value |
|---|---|---|
| Coagulation | ||
| Prothrombin time (PT) in Plasma or Blood | 157 (53.77%) | 13.3 ± 3.9 |
| Activated partial thromboplastin time (aPTT) in Plasma or Blood | 147 (50.34%) | 33.6 ± 14 |
| INR in Plasma or Blood | 152 (52.05%) | 1.17 ± 0.288 |
| Complete Blood Count | ||
| Hemoglobin [Mass/volume] in Blood | 160 (54.79%) | 11.8 ± 2.13 |
| Hematocrit [Volume Fraction] of Blood | 160 (54.79%) | 36.3 ± 6.07 |
| Platelets [#/volume] in Blood | 160 (54.79%) | 220 ± 92.9 |
Figure 2.

Venn Diagram for patients on antiplatelet and anticoagulant.
Outcomes
Clinical outcomes are presented in Table 3. Outcomes within a 180-day follow-up were 6.3% (or 1.0–5.8% when subtracting propensity-matched control for 1–10 patients) for mortality (12 patients), 0.52–5.2% for repeat MMAE (1–10 patients) (for patients’ anonymization, the TriNetX Analytics Network reports 10 for any patient cohort identification that is between 1–10), 7.3% for craniotomy/craniectomy after MMAE (14 patients), 0.52–5.2% for twist drill, burr hole(s), or trephine procedures (1–10 patients), and no patients with low vision/blindness.
Table 3.
Clinical Patient Outcome in MMAE vs. Propensity-Matched Control Cohort.
| Outcome | MMAE | Control | ||||
|---|---|---|---|---|---|---|
| Patients in Cohort | Patients with Outcome | Risk | Patients in Cohort | Patients with Outcome | Risk | |
| Mortality | 191 | 12 | 6.28% | 191 | 10 | 5.24% |
| Repeat Procedure | 191 | 10 | 5.24% | 191 | 0 | 0.00% |
| Craniectomy or Craniotomy Procedures | 191 | 14 | 7.33% | 191 | 0 | 0.00% |
| Twist Drill, Burr Hole(s), or Trephine Procedures on the Skull, Meninges, and Brain | 191 | 10 | 5.24% | 191 | 0 | 0.00% |
| Blindness/Low vision* | 188 | 0 | 0 | 185 | 0 | 0.00% |
3 patients in MMAE and 6 patients in Control were excluded from results because they had the outcome prior to the time window. Any patient count of 1–10 for outcome is rounded up to 10 for deidentification purposes.
Figure 3 shows the Kaplan Meier survival curve between the MMAE and control cohorts. The survival probability at the end of the time window (i.e., 180 days) was 92.3% and 96.7% for the MMAE and control cohorts, respectively (p-value=0.063). Additionally, Figure 4 demonstrates CCI measured for patients in the MMAE cohort. The mean CCI was 7.6 (95% CI: 4.8–10.3), 8.5 (95% CI: 2.1–14.9), and 3.6 (95%: CI 3.2–4.1) for patients that died within 6 months, 6 months-5 years, and those still alive post-MMAE, respectively. The CCI was found to be significantly different by Dunn’s multiple comparisons test between patients with a mortality outcome in <6 months and those who are still alive (p-value = 0.003).
Figure 3.
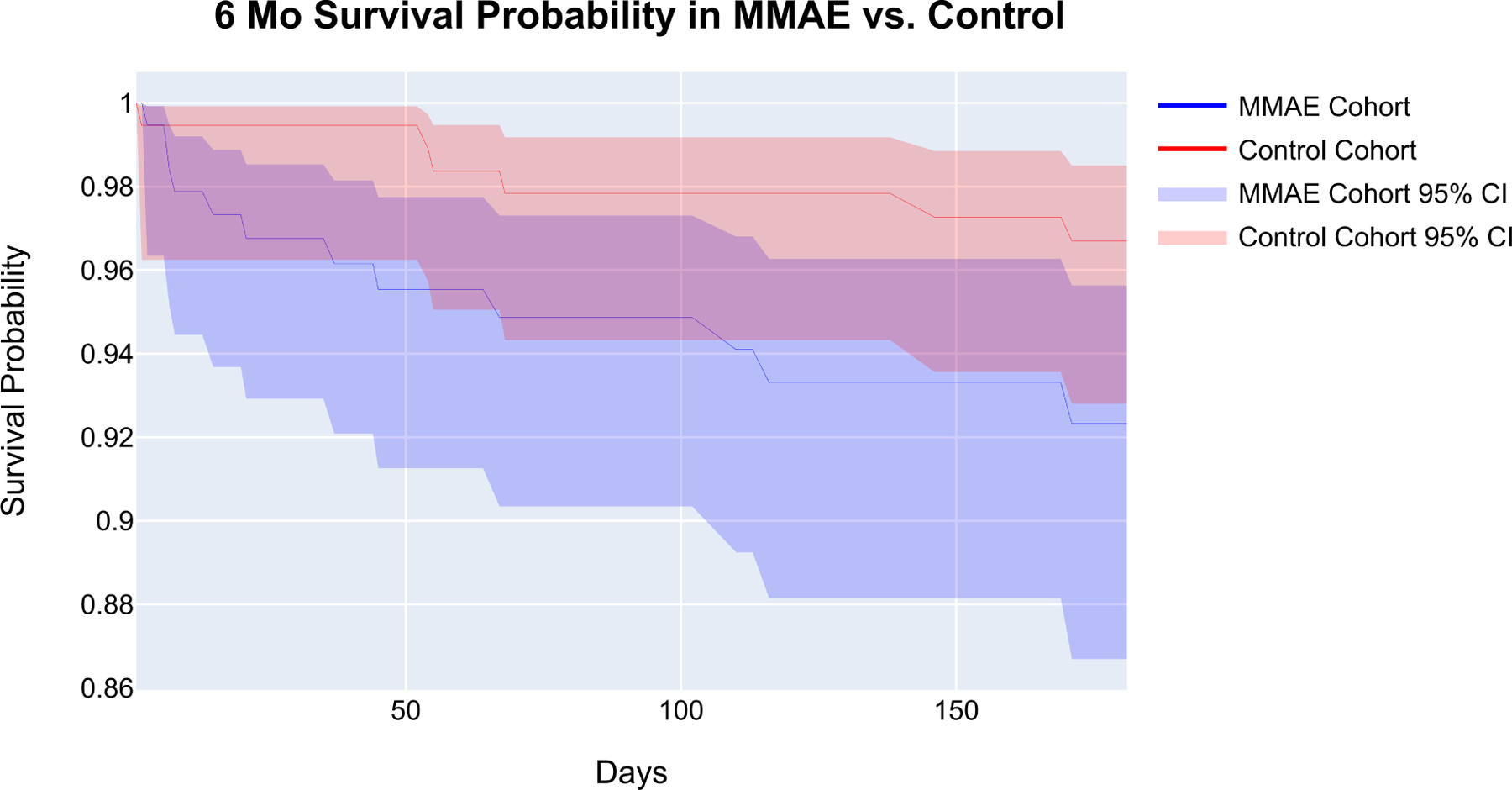
Kaplan-Meier Curves of MMAE and Propensity Matched Control Cohort Survivals. The shaded areas indicate the 95% confidence interval. The survival probability at the end of the time window is at 92.3% and 96.7% for the MMAE and control cohorts, respectively (p-value=0.063).
Figure 4.

Charlson Comorbidity Index. The bar plot represents the 2.5–97.5 percentiles of the CCI scores within each mortality cohort. A Kruskal Wallis test showed that that the CCI scores do not have the same distribution between each cohort (p-value=0.0006); more specifically, a Dunn’s multiple comparison test showed that there is a difference in CCI scores between those who had a mortality outcome within 6 months vs. those who are still alive (p-value = 0.003), denoted by **.
Discussion
Our study presents a large cohort derived from a national database within the United States, including 191 patients undergoing MMAE procedures for cSDH treatment within one month of diagnosis and with a 180-day follow-up. Currently, there is one multi-center study that investigated the clinical outcomes of MMAE for cSDH.30 Additional single-center studies have compared MMAE with surgical evacuation within a three-month follow-up.12, 21, 31, 32 To the best of our knowledge, this is the first study that utilized an independent data collection party to control for baseline characteristics and comorbidities for outcome analysis. Additionally, it provides the largest patient cohort from different institutions across the United States with a 180-day follow-up that corroborates the existing results.
The 180-day mortality for the study was 6.3% in the MMAE cohort; however, the mortality of the control group when propensity-matched for baseline characteristics and comorbidities was 0.52–5.2% (1–10 patients), signifying that the true mortality rate for our cohort is between 1.0–5.8%% rather than 6.3%. The difference in the survival probability of MMAE compared to the control cohort at the end of the time window from the Kaplan Meier analysis was 4.37%; however, the log-rank test showed that the difference is not significant (p-value=0.063). This is in line with Kan et al., who recently reported the 90-day mortality of 4.4% in a multi-center cohort of 154 patients.30 Furthermore, CCIs were calculated to characterize the 1- and 10- year comorbidity differences prior to embolization between 6-month mortality and those who are still alive within the MMAE cohort. Our data showed that patients who were deceased within 6-month post-MMAE had significantly higher CCI than those who are still alive. Overall, the statistically insignificant difference (p-value=0.063) in survival probability between the MMAE and control cohort combined with the statistically significant difference (p-value=0.003) in CCI between 6-month mortality and alive patients provide evidence that mortality is more likely to be attributed to the patients’ baseline health status within the cohort as opposed to the embolization procedure.
With the cohort, 7.3% of patients required craniectomy/craniotomy, and 0.52–5.2% required twist drill/burr hole within 180-days post-MMAE. The need for cSDH re-treatment has varied across prior reports: 0%, 1.4%, 3.6%, 5%, and 6.5%.12, 21, 30, 31, 33, 34 Tiwari et al. utilized the Wilson Score Interval to report an estimate of hematoma recurrence rate post embolization. The 95% confidence interval of the recurrence rate was reported as 0–12.5%.34 The maximal failure rate of MMAE is 12.5% in this study, as craniotomy/craniectomy and twist drill/burr hole for a SDH recurrence cannot be differentiated from a contralateral SDH due to the use of the same CPT and ICD codes in the electronic medical records and billing. Also, there is no information on whether cSDH was unilateral or bilateral at presentation, and if bilateral, whether the procedure was performed on a single side or on both sides. A contralateral hematoma is likely to progress after surgery if a procedure was performed on a single side. The incidence of bilateral cSDH has been observed to be 16%–24% in populations of cSDH.35–37 Thus, our reported 7.3% rate for craniectomy/craniotomy procedures post embolization is probably inflated due to a contralateral SDH rather than ipsilateral recurrence/treatment failure. Additionally, the need for surgical rescue, defined by post-MMAE burr holes or craniotomy in the studies mentioned, was previously reported within a 90-day follow-up as opposed to our 180-day follow-up. Moreover, the individual surgeon/institution preferences for patient selection and techniques result in variability between reported cSDH retreatment across different surgeons/institutions.
Our study provides additional evidence reinforcing the existing results in the literature that MMAE can be safe and effective in treating cSDH. MMAE has been previously reported as an effective standalone therapy even in the setting of midline shifts slightly greater than 5 mm and thickness above 10 mm in specific patient populations (i.e., asymptomatic or mildly symptomatic patients).38 As mentioned earlier, the mean age of our cohort was 71.2 ± 13.5, which makes surgical intervention particularly challenging in this elderly group, both due to operative risks and perioperative medical complications. In particular, cardiovascular complications and hospitalization periods are significantly higher in patients >60 years old with cSDH who undergo general anesthesia as compared to local anesthesia.39 Thus, MMAE can be an attractive minimally invasive treatment option in this population by mitigating cardiovascular and pulmonary complications associated with traditional surgical evacuation.
This study has several limitations. First, the radiographic data for evaluating baseline hematoma characteristics and SDH thickness reduction post-MMAE are not available in the TriNetX database. Several subpopulations of patients were included in the analysis due to the number of patients who had the procedure. In follow-up studies, when the procedure has been more widely adopted, it may be pertinent to investigate these subpopulations seperately based on whether MMAE was used as a standalone therapy, therapy post-surgical failure, or as an adjunctive post-surgical treatment. Additionally, functional scores such as NIH Stroke Scale/Score (NIHSS) and Modified Rankin Scale (mRS) were not consistently reported across the cohort to be utilized for estimating the gross functional state of the patients before and after the procedure. Nevertheless, our multi-institutional cohort study utilizing an up-to-date database of patient medical records corroborates the results of previous single-center studies and a multi-center study, with an advantage of independent data collection party, as well as utilizing a larger sample size and propensity-match scoring to control for a certain degree of variability to improve the generalizability of findings.
Conclusion
The present study is the first large multi-institutional series of MMAE for cSDH that utilized an independent data collection system. Our results are in agreement with the available literature that MMAE can be a safe and effective minimally invasive option for cSDH when propensity-matched for baseline characteristics and comorbidities.
Supplementary Material
Funding
The data access was supported by NIH grant TR001439, Clinical and Translational Science Award (UL1).
Abbreviations list
- aPTT
activated Partial thromboplastin time
- CCI
Charlson Comorbidity Index
- CKD
chronic kidney disease
- cSDH
chronic subdural hematomas
- HCO
healthcare organization
- ICD-10
International Classification of Diseases, 10th revision
- INR
international normalized ratio
- MMA
middle meningeal artery
- MMAE
middle meningeal artery embolization
- mRS
Modified Rankin Scale
- NIHSS
NIH Stroke Score
- P.T.
prothrombin time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- 1.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. Journal of neurosurgery. 2015;123(5): 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. Journal of neurosurgery. 2011;114(1): 72–76. [DOI] [PubMed] [Google Scholar]
- 3.Ducruet AF, Grobelny BT, Zacharia BE, et al. The surgical management of chronic subdural hematoma. Neurosurgical review. 2012;35(2): 155–169. [DOI] [PubMed] [Google Scholar]
- 4.Brodbelt A, Warnke P. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(8): 1209–1210. [PMC free article] [PubMed] [Google Scholar]
- 5.Almenawer SA, Farrokhyar F, Hong C, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34829 patients. Annals of surgery. 2014;259(3): 449–457. [DOI] [PubMed] [Google Scholar]
- 6.Ivamoto HS, Lemos HP Jr, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World neurosurgery. 2016;86: 399–418. [DOI] [PubMed] [Google Scholar]
- 7.Santarius T, Kirkpatrick PJ, Ganesan D, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. The Lancet. 2009;374(9695): 1067–1073. [DOI] [PubMed] [Google Scholar]
- 8.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6): 1125–1129. [DOI] [PubMed] [Google Scholar]
- 9.Tugcu B, Tanriverdi O, Baydin S, et al. Can recurrence of chronic subdural hematoma be predicted? A retrospective analysis of 292 cases. Journal of Neurological Surgery Part A: Central European Neurosurgery. 2014;75(01): 037–041. [DOI] [PubMed] [Google Scholar]
- 10.Jang K-M, Kwon J-T, Hwang S-N, Park Y-S, Nam T-K. Comparison of the outcomes and recurrence with three surgical techniques for chronic subdural hematoma: single, double burr hole, and double burr hole drainage with irrigation. Korean journal of neurotrauma. 2015;11(2): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mino M, Nishimura S, Hori E, et al. Efficacy of middle meningeal artery embolization in the treatment of refractory chronic subdural hematoma. Surg Neurol Int. 2010;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ban SP, Hwang G, Byoun HS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018;286(3): 992–999. [DOI] [PubMed] [Google Scholar]
- 13.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KL, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. Journal of neuroinflammation. 2017;14(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma: Case report. Journal of neurosurgery. 2000;93(4): 686–688. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa K, Sorimachi T, Ishizaka H, et al. Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma. Journal of neurosurgery. 2016;124(6): 1679–1683. [DOI] [PubMed] [Google Scholar]
- 16.Moshayedi P, Liebeskind DS. Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Frontiers in Neurology. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanisic M, Aasen AO, Pripp AH, et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflammation Research. 2012;61(8): 845–852. [DOI] [PubMed] [Google Scholar]
- 18.Yajima H, Kanaya H, Ogino M, Ueki K, Kim P. Middle meningeal artery embolization for chronic subdural hematoma with high risk of recurrence: A single institution experience. Clinical Neurology and Neurosurgery. 2020;197: 106097. [DOI] [PubMed] [Google Scholar]
- 19.Shotar E, Meyblum L, Premat K, et al. Middle meningeal artery embolization reduces the post-operative recurrence rate of at-risk chronic subdural hematoma. Journal of NeuroInterventional Surgery. 2020; 12(12): 1209–1213. [DOI] [PubMed] [Google Scholar]
- 20.Waqas M, Vakhari K, Weimer PV, Hashmi E, Davies JM, Siddiqui AH. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World neurosurgery. 2019;126: 228–236. [DOI] [PubMed] [Google Scholar]
- 21.Kim E Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World neurosurgery. 2017;101: 520–527. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T, Ohashi T, Watanabe D, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013;4: 104–104. 10.4103/2152-7806.116679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. The Lancet Infectious Diseases. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J-j, Dong X, Cao Y-y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223): 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821): 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasparini A comorbidity: An R package for computing comorbidity scores. Journal of Open Source Software. 2018;3(23): 648. [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 30.Kan P, Maragkos GA, Srivatsan A, et al. Middle meningeal artery embolization for chronic subdural hematoma: a multi-center experience of 154 consecutive embolizations. Neurosurgery. 2021;88(2): 268–277. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto H, Hanayama H, Okada T, et al. Which surgical procedure is effective for refractory chronic subdural hematoma? Analysis of our surgical procedures and literature review. Journal of Clinical Neuroscience. 2018;49: 40–47. [DOI] [PubMed] [Google Scholar]
- 32.Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. 2019;85(6): 801–807. [DOI] [PubMed] [Google Scholar]
- 33.Srivatsan A, Mohanty A, Nascimento FA, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World neurosurgery. 2019;122: 613–619. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari A, Dmytriw AA, Bo R, et al. Recurrence and Coniglobus Volumetric Resolution of Subacute and Chronic Subdural Hematoma Post-Middle Meningeal Artery Embolization. Diagnostics. 2021;11(2): 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM. Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012;70(4): 873–880. [DOI] [PubMed] [Google Scholar]
- 36.Chon K-H, Lee J-M, Koh E-J, Choi H-Y. Independent predictors for recurrence of chronic subdural hematoma. Acta neurochirurgica. 2012;154(9): 1541–1548. [DOI] [PubMed] [Google Scholar]
- 37.MacFarlane M, Weerakkody Y, Kathiravel Y. Chronic subdural haematomas are more common on the left than on the right. Journal of Clinical Neuroscience. 2009;16(5): 642–644. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Paz S, Akamatsu Y, Salem MM, et al. Upfront middle meningeal artery embolization for treatment of chronic subdural hematomas in patients with or without midline shift. Interv Neuroradiol. 2020: 1591019920982816. 10.1177/1591019920982816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SO, Jung SI, Won YS, Choi CS, Yang JY. A comparative study of local versus general anesthesia for chronic subdural hematoma in elderly patients over 60 years. Korean journal of neurotrauma. 2013;9(2): 47–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


