Abstract
Despite years of basic research and pioneering clinical work, ischemic stroke remains a major public health concern. Prior STAIR conferences identified both failures of clinical trial design and failures in preclinical assessment in developing putative ischemic stroke treatments. At STAIR XI, participants in Workshop #1 “Top Priorities for Neuroprotection” sought to redefine the “neuroprotection paradigm” and given the paucity of evidence underlying preclinical assessment, offer consensus-based recommendations. STAIR proposes the term “brain cytoprotection” or “cerebroprotection” to replace the term ‘neuroprotection’ when the intention of an investigation is to demonstrate that a new, candidate treatment benefits the entire brain. Although “time is still brain,” tissue imaging techniques have been developed to identify patients with both predicted core injury and penumbral, salvageable brain tissue, regardless of time after stroke symptom onset. STAIR XI workshop participants called this imaging approach a ‘tissue window’ to select patients for recanalization. Elements of the neurovascular unit show differential vulnerability evolving over differing time scales in different brain regions. STAIR proposes the term “target window”, to suggest therapies that target the different elements of the NVU at different times. Based on contemporary principles of rigor and transparency, the workshop updated, revised and enhanced the STAIR preclinical recommendations for developing new treatments in two phases: an exploratory ‘qualification phase’ and a definitive ‘validation phase’. For new, putative treatments, investigators should carefully characterize the mechanism of action, the pharmacokinetics/pharmacodynamics, demonstrate target engagement, and confirm penetration through the blood brain barrier. Prior to clinical trials, testing of candidate molecules in stroke models could proceed in a comprehensive manner using animals of both sexes and to include significant variables such as age and comorbid conditions. Comprehensive preclinical assessment might include multi-center, collaborative testing, e.g., network trials. In the absence of a proven cerebroprotective agent to use as a ‘gold standard’ however, it remains speculative whether such comprehensive preclinical assessment can effectively predict clinical outcome.
Introduction
Despite years of basic research and pioneering clinical work, ischemic stroke remains a major public health concern. Basic and clinical research has produced rational approaches for neuronal protection1, acute reperfusion therapies2, 3, various devices for mechanical revascularization3, and strategies for regeneration of brain tissue damaged by ischemia4, 5. All these innovations were based on widely accepted scientific principles and data from preclinical studies, yet no previous candidate neuroprotective therapy has successfully entered clinical practice. The reasons for this clinical-translational failure remain uncertain, but prior STAIR conferences identified failures of clinical trial design, as well as failures in the preclinical assessment approaches. At STAIR XI, participants in Workshop #1 “Top Priorities for Neuroprotection” sought to redefine the “neuroprotection paradigm”. Participants aimed to revise the STAIR recommendations for preclinical assessment in light of new information about differential vulnerability in the neurovascular unit and its dynamic time course, the emergence of new, pleiotropic agents to protect brain, and renewed commitment to scientific rigor.
In most prior efforts to develop stroke therapy, an emphasis was placed on testing single-action, single-target agents. This approach ignored the fact that ischemia produces a plethora of pathologic pathways proceeding both in series and in parallel.6 Workshop participants propose that a multiple-action, multiple-target approach for ischemic stroke could have a higher likelihood of success in stroke patients. Future ischemic stroke treatment might target the neurovascular unit using pleiotropic agents acting at multiple points in the ischemic cascade and at different times. For example, the novel drug, 3K3A-APC, acts on the protease activated receptor 1 (PAR–1) found on endothelial cells, astrocytes, pericytes, and neurons, allowing a treatment strategy that targets the entire neurovascular unit7. Alternatively, an agent might be developed to act on a key single target that serves as a hub for multiple cytodestructive downstream signaling pathways. NA-1, by acting specifically on the PSD-95 protein, disrupts glutamate receptor interactions with multiple effector molecules potentially reducing ischemic cell injury via several downstream mechanisms8, 9. Both of these drugs are in clinical development to determine if significant benefit accrues with their use10, 11.
Despite advances in revascularization therapies, there remains a large unmet need in ischemic stroke treatment: not all patients treated with thrombolysis or mechanical thrombectomy (MT) recover full or substantial function. Also, the risk of hemorrhage after recanalization therapies—although uncommon—dissuades some practitioners from using them12. Thus, adjuvant treatments are needed to complement recanalization therapies. In past stroke treatment trials, conducted prior to the advent of MT as a treatment option, it is likely that many patients failed to reperfuse; the candidate adjuvant treatments were tested in the more challenging setting of permanent rather than transient brain ischemia, and thus failed to show benefit in clinical trials. In modern clinical stroke trial design, candidate adjuvant therapy can be studied in concert with recanalization. In patients with large vessel occlusion, more than 80% treated with MT do substantially recanalize, although downstream reperfusion may be less than complete13. Thus, modern clinical trials using thrombolysis for all indicated patients and MT for large vessel occlusion patients ought to have a much greater probability that the candidate drug will succeed. Bridging agents—given to slow ischemic injury progression and increase volume of salvageable tissue at the time of definitive recanalization—might now demonstrate benefit. Studies of agents intended to prevent post-reperfusion injury will now have many more eligible patients for testing.
Once an adjuvant treatment shows benefit in combination with recanalization, then, subsequent additional trials could assess for benefit in patients who are ineligible for recanalization therapies. Although there is a tremendous need for treatment targeting patients ineligible for recanalization therapies, workshop participants concluded that for the time being clinical trials should target patients undergoing recanalization to optimize the chance of demonstrating a beneficial effect.
After presentations on the above topics, workshop participants developed the following recommendations.
Changing the paradigm: nomenclature.
For decades, stroke investigators used the term neuroprotection in two ways. Either, we meant protection of the entire brain during injury—usually ischemic stroke but also brain trauma and cerebral ischemia due to cardiac arrest. Or, we meant the salvage of neurons during injury in cell culture with glutamate application, other chemical injury, or simulated ischemia with oxygen-glucose deprivation (OGD). Using these in vitro neuronal protection models, we assumed that treatments that protected neurons in cell culture would translate into agents that would protect whole brain in stroke models, and subsequently translate into effective treatments for human stroke victims. This paradigm has failed to date, perhaps because the neurovascular unit encompasses more than just neurons.
Recent data confirms that the several elements of the neurovascular unit (NVU) behave differently during ischemia14. The NVU consists of several cell elements: neurons, astrocytes, endothelial cells, pericytes, oligodendroglia, and microglia are the most commonly studied; these cells all interact with blood and peripheral immune cells. The phenomenon called selective vulnerability was described many years ago15–17. Neurons were found to be most vulnerable, followed by astrocytes, followed by endothelial cells; this hierarchy of vulnerability was speculated to derive from the relative distances to each cell type from the nearby microcirculation18. Regional differences in the excitotoxic ratio—relative densities of excitotoxic glutamate versus inhibitory GABAergic synapses—also relate to selective vulnerability15. Recently this fundamental concept has been updated into the context of the NVU where elements of the NVU show differential vulnerability (Fig. 1)14. Unlike selective-vulnerability, which relates to regional differences in cell death during stroke, differential vulnerability refers to the innate susceptibility of NVU elements to ischemia in monocellular cultures. Here, neurons are still most vulnerable, but astrocytes exhibit the greatest resistance to ischemia (Fig. 1); the mechanisms underlying differential vulnerability remain obscure.
Figure 1. Differential Vulnerability.
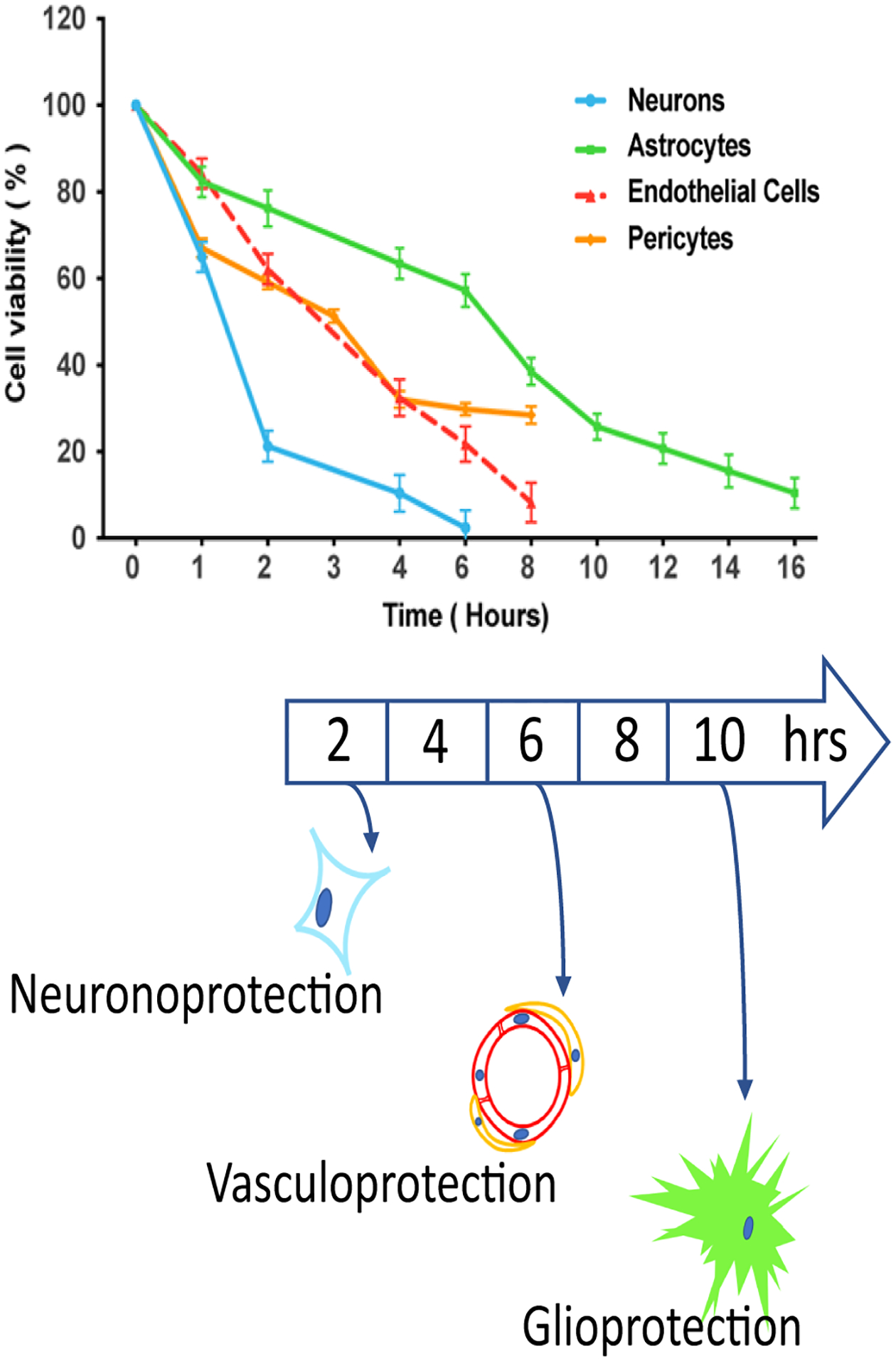
The several elements of the neurovascular unit each exhibit different susceptibility to oxygen-glucose deprivation14. Monocellular cultures underwent varying durations of OGD, and viability was measured 24 hours later. Neurons showed greatest vulnerability, astrocytes least vulnerability, and pericytes/endothelial cells were intermediate. The term ‘brain cerebroprotection’ includes protection of each element of the neurovascular unit: neuronoprotection, vasculoprotection, and glioprotection. Upper panel reprinted by permission of SAGE Publications, Ltd. Journal of Cerebral Blood Flow & Metabolism, 39(9):1693–1709. Copyright 2019 (International Society for Cerebral Blood Flow and Metabolism). Lower panel © Patrick Lyden MD.
In addition to differential vulnerability, several laboratories have shown that neurons signal astrocytes for help during injury but the identity of the neuronal ‘help-me’ signal remains to be determined with certainty19; candidates include thrombin20 and β2-estradiol21. Further, after ‘help-me’ signaling from neurons, astrocytes activate to protect adjacent neurons—a response now well-documented by several groups22–24. Studies of the mechanism of astrocyte-neuron paracrine protection could lead to the rational design of new therapeutic agents25–29.
The clinical importance of differential vulnerability/response remains to be proven but could be quite significant. In translational stroke research, investigators have applied cerebroprotective agents as if all elements of the NVU respond similarly. This presumed uniform response across all NVU elements may be wrong, and in fact, may partly explain some prior failures of human stroke clinical trials. To illustrate, therapeutic hypothermia (TH) was tested with respect to differential vulnerability. Brief, deep TH protected neurons during OGD and the brain during rodent middle cerebral artery occlusion (MCAo), but long durations of TH inhibited astrocytes, and abrogated the protective paracrine astrocyte response, thereby increasing neuronal death14. In other words, a cytoprotective strategy intended to protect one cell element of the NVU may cause unintended and significant harm in another element: agents that impact one element of the NVU beneficially may impact another element quite differently, causing unexpected and worse outcomes in clinical trials.
In light of the above considerations, at the prior STAIR X the term “brain cytoprotection” was proposed to replace the term ‘neuroprotection’30. At STAIR XI, this term was endorsed and further refinements were made to the proposed terminology.
Recommendation 1
Cerebroprotection should be clearly defined and stated, when the intention of an investigation is to demonstrate that a new, candidate treatment benefits the entire brain as measured by either tissue volume, neurologic function, or preferably both. Brain cytoprotection connotes the same intent.
The term ‘neuroprotection’ should be avoided when the investigator seeks to demonstrate pan-cellular brain protection.
- Brain Cytoprotection (Fig. 1).
- Neuronoprotection refers to the preservation/protection of neurons, either in cell culture or using cell identification techniques in vivo, as in selective neuronal vulnerability.
- Glioprotection refers to the preservation/protection of glia, mainly astrocytes but also oligodendroglia, in cell culture or in vivo.
- Vasculoprotection refers to preserving blood brain barrier (BBB) and reducing vascular leakage in vivo or the preservation of endothelial cells and pericytes in cell culture.
Timing: as shown in Figure 1, each version of brain cytoprotection will proceed on different time scales. Investigators must anticipate differential response in the NVU, depending on timing, and alter administration schedules accordingly.
Opportunity Windows.
The workshop participants next focused on approaches to offering therapy to patients when it can be most beneficial, i.e., avoiding futile or even hazardous interventions. For the few decades after publication of the NINDS rt-PA for Acute Stroke Trial, great attention focused on time windows because intravenous thrombolysis functioned best if used soon after stroke symptom onset (Fig. 2). The mantra “time is brain” reflected the importance of urgent recanalization, but clinicians and investigators recognized that in many patients some injured brain tissue might remain salvageable after the 3 hour time window used in the original NINDS study, or subsequently even after the 4.5 hour time window used in the ECASS 3 trial31, 32. As a result, tissue imaging techniques were developed to identify patients with both predicted core injury and penumbral, salvageable brain tissue, regardless of time after stroke symptom onset. Tracer perfusion techniques allow imaging of brain areas with slow blood flow (potentially salvageable) or zero flow (presumably completed infarction), and this ‘perfusion mismatch’ correlates generally with the ischemic penumbra33. Even closer to direct tissue imaging, magnetic resonance imaging allows demonstration of brain areas showing diffusion restriction, correlating with ischemic injury, and brain areas showing hyperintensity on Fluid Attenuated Inversion Recovery which roughly correlates with irreversibly injured brain34. STAIR XI workshop participants called this imaging approach a ‘tissue window’ to select patients for recanalization35 (Fig.2). Recent studies suggest benefit for rt-PA beyond 4.5 hours of symptom duration, or due to unwitnessed onset using these imaging-based patient selection techniques. Additional studies demonstrated benefit for endovascular thrombectomy at 6 to 24 hours, also using imaging-based patient selection34–37.
Figure 2. Opportunity Windows.
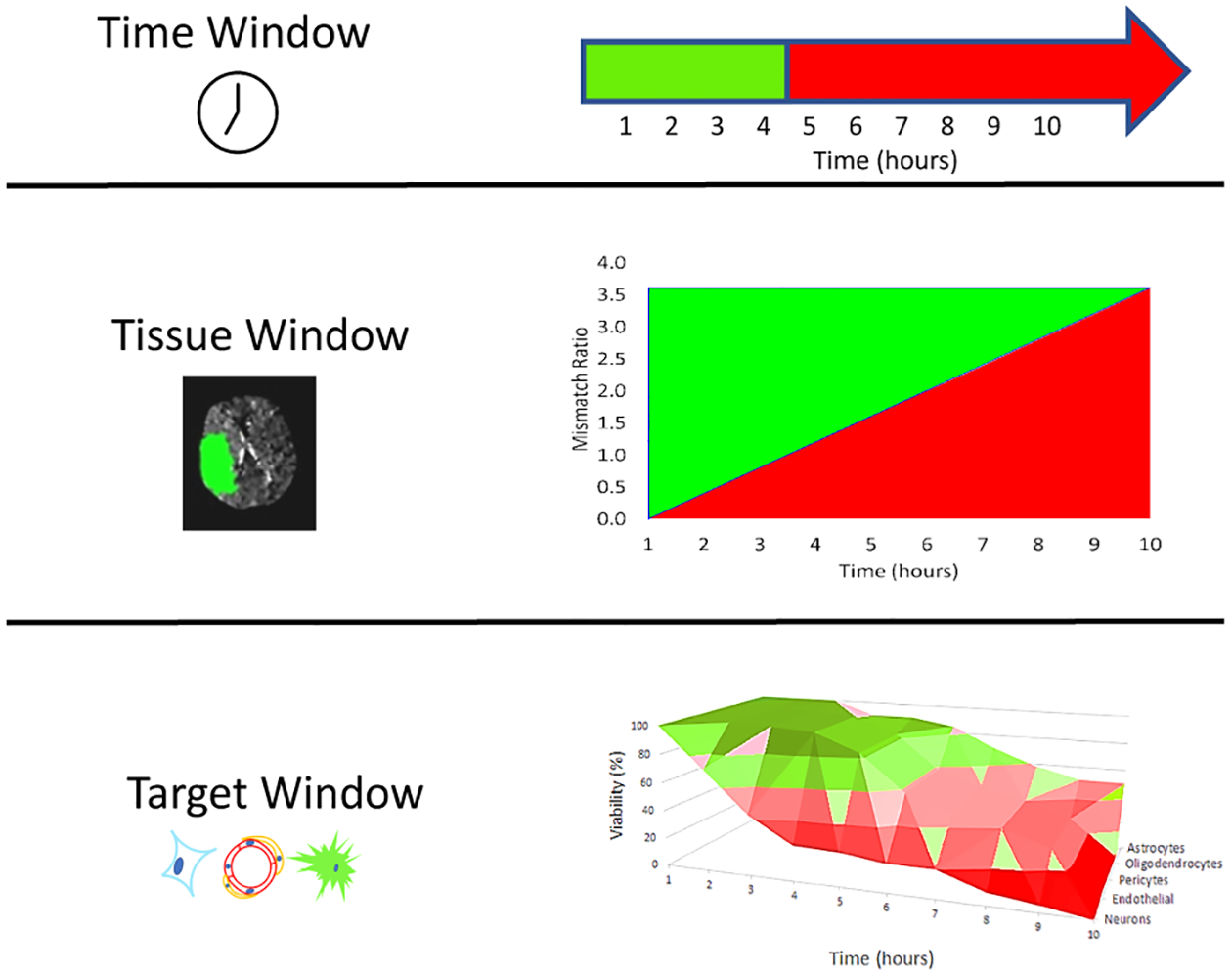
Scheme for understanding the historical evolution of successful treatment for acute ischemic stroke. Green indicates beneficial outcome, red indicates death or disability, and pink indicates a transition epoch where outcome may not be best, but not worst. These graphs are provided for illustration only, are not data-derived, and are not intended to suggest specific recommendations. A. Time Window. Initially thrombolysis and thrombectomy (together known as recanalization therapy) could only be targeted using clock time, defined as the time since the patient was last known well and free of new stroke deficits. B. Tissue Window. The development of perfusion imaging allowed for the estimation of perfusion mismatch and insight into the volume of salvageable tissue. Magnetic resonance-based techniques allowed even more direct imaging of tissue injury. Both perfusion and tissue injury methods allow recanalization therapy to target salvageable tissue, rather than depending on clock time. NB: despite the appearance of the figure, the relationship between time and mismatch is likely nonlinear. C. Target Window. In the future, both recanalization and brain cerebroprotection therapy might target different elements of the neurovascular unit at different times, knowing the differential progression of pathology in differentially vulnerable regions, or different brain regions using focal, targeted delivery (not illustrated). © Patrick Lyden, MD
Recommendation 2
Elements of the NVU show differential vulnerability evolving over differing time scales in different brain regions, suggesting the term ‘target window’. In the future, investigators could develop therapies that target the different elements of the NVU at different times.
Optimal designs for preclinical evaluation
In the past 5 years, two significant developments raise new hope for investigators developing brain cerebroprotectants: the appearance of new compounds with multiple mechanisms of action and the promulgation of new standards for rigorous preclinical development38–41. Workshop participants agreed to modify the existing STAIR preclinical recommendations to incorporate new developments. In Table 1 the original STAIR guidelines42 (1999) are compared to the modified version30, 43 (2009) along with ‘extra’ recommendations that were added at subsequent STAIRS. In Table 2 the workshop participants updated, revised and enhanced the STAIR preclinical recommendations. It should be noted however, that in the absence of any proven cerebroprotective stroke therapy, these recommendations cannot be ranked with respect to any supportive evidence base.
Table 1. Past STAIR Preclinical Recommendations.
Original recommendations from STAIR 1 (published in 1999) were updated in 2009. Extra recommendations were added at subsequent STAIRs, including STAIR XI.
| A. Initial STAIR Preclinical Recommendations (1999) | |
| Dose Response Curve | The drug should exhibit different effects over a range of doses |
| Time Window | Determine the maximum delay to treatment after which the treatment fails. |
| Permanent then transient occlusion | Treatment should be studied in both permanent and transient occlusion (reperfusion) models |
| Blinded, physiologically controlled, reproducible studies | Physiological variables should be monitored and maintained. Laser Doppler flow drop of at least 60% should be required. |
| Histological and behavioral outcomes | Outcomes should include both estimates of stroke lesion volume and behavioral outcomes |
| Sex* | Consider studying treatments in both males and females. |
| Multiple Mechanisms* | Consider combinatorial approaches |
| Rodent then gyrencephalic species | Demonstrate efficacy in at least 2 species |
| B. Additional STAIR Recommendations (2009) | |
| Sample Size Calculation | Report standard deviation and predicted estimated effect size |
| Inclusion/Exclusion criteria | E.g., required drop in laser Doppler flowmetry or symptom severity |
| Randomization | Group allocation should be randomized |
| Allocation concealment | Surgeon performing stroke remains unaware of treatment assignment |
| Reporting on excluded animals | Account for all drop out animals |
| Blinded assessment of outcome | Behavioral raters, image analysts unaware of treatment assignment |
| Reporting of investigator or institutional conflicts of interest | Any relationship that could be perceived to introduce conflict of interest should be disclosed |
| C. Extra Recommendations | |
| Age | Assess treatment effects in aging animals |
| Sex | Test in both sexes |
| Co-morbidities | Test in the presence of hypertension or diabetes |
| Multiple laboratories | Results should be confirmed in more than one laboratory |
| D. STAIR XI Extra Recommendations | |
| Mechanism | Studies of basic mechanisms should be clearly identified and differentiated from preclinical assessments for efficacy. Endpoints should reflect the mechanism under study |
| Sample size | Mechanistic studies must be adequately powered for the chosen endpoint and predicted effect size |
| Preclinical assessments | Studies seeking to qualify a candidate treatment for clinical trials should be clearly identified and designed accordingly |
Note: Although sex and multiple mechanisms were addressed in the 1999 version, these are not traditionally considered part of the original STAIR Preclinical Recommendations.
Table 2. Revised STAIR Recommendations.
After STAIR XI all prior recommendations were revised, consolidated and updated. Revised STAIR recommendations are separated for two experimental purposes. A. Candidate Treatment Qualification, which means early research and development of a novel, putative treatment. B. Preclinical Assessment and Validation, which means demonstrating efficacy in stroke models that have a likelihood of predicting success in subsequent patient clinical trials.
| A. Candidate Treatment Qualification | |
| Dose Response | Treatment effect varies with changes in dose |
| Time Window | Treatment remains effective when administered after clinically relevant delay times |
| Histological and behavioral outcomes | Beneficial effects can be demonstrated using measures of behavior and tissue damage |
| Target Engagement | Candidate treatment reaches presumed target and causes expected physiological effects |
| Barrier Penetration | Candidate treatment enters brain |
| B. Preclinical Assessment and validation | |
| Sample Size | Sample size should be pre-specified based on known or assumed standard deviation and predicted effect size |
| Inclusion/Exclusion criteria | Effective MCA occlusion is confirmed using laser Doppler or other flowmetry or symptom severity |
| Randomization | Animals are randomized prior to initiation of any study procedures |
| Allocation concealment | Surgeon performing stroke remains unaware of treatment assignment |
| Reporting on excluded animals | Subjects lost at each experimental step after randomization are summarized |
| Blinded assessment of outcome | Investigators remain unaware of treatment assignment during all assessments |
| Age | Consider effects of age on outcome |
| Sex | Males and females should be assessed. Dose response differences between sexes should be determined |
| Co-morbidities | Ideal models of stroke co-morbid conditions (e.g., diabetes or hypertension) need to be refined |
| Multiple laboratories | Concordant effects should be demonstrated across multiple laboratories using similar methods. |
| Gyrencephalic species | Demonstration of efficacy in gyrencephalic species, particularly non-human primates may contribute to predicting clinical efficacy |
| Circadian Effects | Preclinical testing of therapies during the awake phase of rodent models should be considered. |
| Reporting of investigator or institutional conflicts of interest | Investigator and institution conflicts are reported and managed |
NB: STAIR recommendations are not guidelines or protocols, but rather consensus suggestions from an expert panel for investigators to consider.
Qualification Phase
Stroke models can be used for two very different purposes, as codified in Table 2. For understanding a new candidate stroke therapy, i.e., exploratory research, we recommend that investigators should seek to understand the mechanism of action, the pharmacokinetics/pharmacodynamics, demonstrate target engagement, and confirm penetration through the BBB. These activities comprise “Candidate Treatment Qualification” as summarized in Table 2. In the qualification phase, simple animal models may be helpful in demonstrating target engagement, BBB transit, and other useful mechanistic studies. For reasons of expense and time, early screening studies of candidate treatments may be done in simpler stroke models, e.g., younger animals, but later should proceed to more costly studies of aged animals. In these screening studies, rodents are commonly used, typically without comorbidities. Both permanent and temporary occlusion models should be investigated with histological, behavioral and biomarker endpoints.
Validation Phase
Prior to embarking on an expensive clinical development program, investigators and regulators often seek assurance that the candidate treatment shows signals of efficacy and safety. One way to demonstrate promising signals is to use a clinically relevant animal stroke model. Prior STAIR conferences have recommended that preclinical assessment be approached with rigor and a commitment to scientific best practice, summarized in Table 2. In this phase, testing of candidate molecules in animal models could proceed in a comprehensive manner using animals of both sexes and to include significant variables such as age and comorbid conditions. Comprehensive preclinical assessment might include multi-center, collaborative testing, e.g., network trials. In the absence of a proven cerebroprotective agent to use as a ‘gold standard’ however, it remains speculative whether such comprehensive preclinical assessment can effectively predict clinical outcome.
Intense analysis of previous preclinical development programs in stroke and neurodegeneration have identified key problems that should be addressed, starting with a variety of biases that have limited the generalizability and validity of animal research in general, and stroke modeling specifically44, 45. The workshop participants recommend that investigators commit to significant improvement and advancement of preclinical development by implementing the following technical innovations in a preclinical stroke testing network: central randomization, masking treatment assignment, power analysis and rational sample sizing, replication in multiple laboratories, study with key factors that impact outcome e.g., diabetes, hypertension, age, sex. Again it must be noted that no support is available to rank these recommendations with respect to a level of evidence.
In addition to the above innovations, simulations have suggested the superiority of multi-site trials over larger single-laboratory studies46. The multi-site approach improves the external validity and may improve the likelihood of clinical success. Such collaborative science will require bringing together multiple sites to collaborate, agree on, and implement difficult protocols. Molecules that demonstrate promise in early screening studies could be investigated more completely in well-designed and performed multi-site studies before proceeding to clinical trial evaluation. Further, multi-site trials necessarily increase the heterogeneity of the study population, a key feature of human clinical trials usually missing in preclinical animal models46. That such heterogeneity improves clinical predictability remains a hypothesis to be tested.
The Stroke Preclinical Assessment Network (SPAN) was created by NINDS to develop an approach to studying putative stroke therapies in a manner that addresses the above issues. SPAN uses a novel system of distributed, masked evaluation. The SPAN platform allows each investigative lab to upload outcome data (images or behavior video recordings) in a masked fashion. Then, recordings are assigned for review to other site(s) for masked evaluation. A centralized database allows for data monitoring and quality control. The resultant data is summarized, analyzed, and when all data is locked, the code will be broken for analysis. This novel approach allows for a secure, masked, highly cost efficient, tightly managed system with built in central quality-control.
Randomization is central to eliminating bias and establishing rigor. Prior studies suggest that simple, benchside strategies (e.g., coin flipping, alternating odd/even days) retain some susceptibility to bias depending on the implementation. Successful parallel testing of multiple compounds with an adaptive strategy requires sophisticated approaches. Several authorities recommend that randomization occur PRIOR to the stroke surgery (regardless of ischemia method)39, 47. SPAN uses centralized randomization of subjects at enrollment, prior to stroke surgery.
Recommendation 3
Preliminary studies of treatments for stroke should include dose/response effect, time window characterization, behavioral and histological outcomes, target engagement and BBB penetration (Table 2A). We recommend that candidate treatments showing promise in such qualifying studies might then be tested more intensively (Table 2B), including consideration of these following factors:
Sample size: Investigators should know in advance the standard deviation and predicted effect size of the treatment they plan to study. Sample size should be estimated to detect the predicted effect size with reasonable power.
Inclusion/exclusion criteria: Subjects must be excluded from the final analysis only for pre-specified, objective criteria, again to reduce conscious or unconscious manipulation of the results. For example, appropriate drop of the LDF-measured cerebral blood flow is an essential inclusion criterion for MCAo models.
Randomization: animals should be randomized to treatment groups prior to any study procedures, including behavioral apparatus habituation or stroke-induction surgery. This helps prevent conscious or unconscious allocation bias.
Allocation concealment: Ideally the investigator performing the stroke does not know the treatment assignment of the subject, to avoid unconscious differences in surgical technique.
Reporting on excluded animals: Subjects may drop out at any phase of the protocol. Reporting these dropouts helps minimize any manipulation of the final results.
Blinded assessment of outcome: Investigators must remain unaware of group assignments when measuring any outcome. This is especially true of subjective assessments such as behavior scoring but applies also to semi-automated morphometry.
Age: Very few stroke patients in trials are younger than 20 years old. Almost all preclinical stroke modeling is performed in rodents 2–3 months old, which corresponds to a human age of 15–20 years48. At some point prior to launching human clinical trials, preclinical testing of therapies should include studies in rodents at least 10 months old (corresponding to middle-aged humans).
Sex: Molecules showing promise in male rodents should also be evaluated in aged female or oophorectimized female rodents to demonstrate efficacy in both sexes.
Comorbidities: Almost two thirds of stroke patients in trials are hypertensive. Almost all preclinical testing of therapies is performed in normotensive rodents. Investigators should consider the added value of preclinical testing in hypertensive rodents. Hyperglycemia due to diabetes mellitus is another co-morbidity known to influence stroke outcome.
Multiple laboratories: Prior to initiating clinical trials, it may be reasonable to test candidate cerebroprotectants for efficacy in multiple laboratories, and in multiple species39, 43.
Gyrencephalic species: Candidate stroke treatments have rarely been tested or shown to be effective in gyrencephalic species such as non-human primate stroke models8, 49. It remains to be proven whether such demonstration of efficacy in primates will prove superior or complementary to rodent models in predicting success for the candidate therapy in clinical trials.
Circadian context: Circadian biology affects all aspects of mammalian physiology50. Almost 80% of all currently approved drugs hit targets that show circadian rhythm51. It is now well-accepted that circadian biology profoundly affects cardiovascular medicine and immunology52, 53. Hence, it may be reasonable to consider the effects of circadian rhythms on stroke therapeutics. Over 90% of stroke patients in trials are enrolled in the daytime. For diurnal humans, this is the active (awake) phase. Almost all preclinical testing of therapies is also performed in rodents during the daytime, but for nocturnal rodents, this is their inactive (sleep) phase. A recent study found that stroke evolution and neuroprotectant effects may be different during active versus inactive phases in rodent stroke models54.
In conclusion, Workshop #1 of STAIR XI considered and presented a new paradigm for the evaluation of putative therapies that may work together with recanalization to improve outcome after stroke. This paradigm is presented for the consideration of the larger stroke research community, and for further testing and validation.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Lyden is supported by the National Institute of Neurological Disorders and Stroke grants U24 NS113452and R01NS075930
Non-standard Abbreviations and Acronyms
- APC
activated protein C
- BBB
blood brain barrier
- ECASS
European Cooperative Acute Stroke Study
- GABA
gamma aminobutyric acid
- LDF
laser Doppler flowmetry
- MCAo
middle cerebral artery occlusion
- MT
mechanical thrombectomy
- NINDS
National Institute of Neurological Disorders and Stroke
- NVU
neurovascular unit
- OGD
oxygen-glucose deprivation
- PAR
protease activated receptor
- PSD
post-synaptic density
- SPAN
Stroke Preclinical Assessment Network
- STAIR
Stroke Treatment Academic Industry Roundtable
- TH
therapeutic hypothermia
Footnotes
Disclosures Disclosures
Dr. Lyden is the Principal Investigator of the NIH sponsored Stroke Preclinical Assessment Network; a DSMB member for the Basilar Artery International Cooperation Study (unpaid) and the “PORTICO™ Re-sheathable Transcatheter Aortic Valve System US IDE Trial (PORTICO)” (Baim Institute); received royalties for “Thrombolytic Therapy for Acute Stroke”, 3rd Ed., Springer Press; and serves as a consultant to various plaintiff and defense legal firms and Apex Innovations.
Dr Buchan reports other from Brainomix during the conduct of the study.
Dr Fisher reports personal fees from AstraZeneca, personal fees from Simcere USA, and personal fees from ALLM outside the submitted work
References
- 1.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullen MT, Pisapia JM, Tilwa S, Messe SR, Stein SC. Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+intra-arterial thrombolysis. Stroke. 2012;43:2350–2355 [DOI] [PubMed] [Google Scholar]
- 4.Endres M, Engelhardt B, Koistinaho J, Lindvall O, Meairs S, Mohr JP, Planas A, Rothwell N, Schwaninger M, Schwab ME, et al. Improving outcome after stroke: Overcoming the translational roadblock. Cerebrovasc Dis. 2008;25:268–278 [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308 [PMC free article] [PubMed] [Google Scholar]
- 6.Lapchak PA. Emerging therapies: Pleiotropic multi-target drugs to treat stroke victims. Transl Stroke Res. 2011;2:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin JH, Mosnier LO, Fernandez JA, Zlokovic BV. 2016 scientific sessions sol sherry distinguished lecturer in thrombosis: Thrombotic stroke: Neuroprotective therapy by recombinant-activated protein c. Arterioscler Thromb Vasc Biol. 2016;36:2143–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DJ, Teves L, Tymianski M. Treatment of stroke with a psd-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483:213–217 [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Hayashi A, Sun H-S, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, et al. Pdz protein interactions underlying nmda receptor-mediated excitotoxicity and neuroprotection by psd-95 inhibitors. The Journal of Neuroscience. 2007;27:9901–9915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, Sawyer RN Jr., Claassen J, Adeoye O, Song S, et al. Final results of the rhapsody trial: A multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3k3a-apc, a recombinant variant of human activated protein c, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (escape-na1): A multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–887 [DOI] [PubMed] [Google Scholar]
- 12.Lyden PD, Pryor KE, Minigh J, Davis TP, Griffin JH, Levy H, Zlokovic BV. Stroke treatment with par-1 agents to decrease hemorrhagic transformation. Frontiers in Neurology. 2021;12:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ames A, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia 2. No-reflow phenomenon. Am J Path. 1968;52:437. [PMC free article] [PubMed] [Google Scholar]
- 14.Lyden PD, Lamb J, Kothari S, Toossi S, Boitano P, Rajput PS. Differential effects of hypothermia on neurovascular unit determine protective or toxic results: Toward optimized therapeutic hypothermia. J Cereb Blood Flow Metab. 2018:271678X18814614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mordecai Y, Globus T, Busto R, Martinez E, Valdes I, Ginsberg MD. Excitotoxic index - a biochemical marker of selective vulnerability. Stroke. 1991;22(1):128. [DOI] [PubMed] [Google Scholar]
- 16.Collins RC, Dobkin BH, Choi DW. Selective vulnerability of the brain: New insights into the pathophysiology of stroke. Annals of Internal Medicine. 1989;110:992. [DOI] [PubMed] [Google Scholar]
- 17.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neurolpathol. 1984;62:201. [DOI] [PubMed] [Google Scholar]
- 18.Mabuchi T, Lucero J, Feng A, Koziol JA, del Zoppo GJ. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266 [DOI] [PubMed] [Google Scholar]
- 19.Xing C, Lo EH. Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. Progress in Neurobiology. 2017;152:181–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajput PS, Lamb J, Kothari S, Pereira B, Soetkamp D, Wang Y, Tang J, Van Eyk JE, Mullins ES, Lyden PD. Neuron-generated thrombin induces a protective astrocyte response via protease activated receptors. Glia. 2020;68:246–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Sareddy GR, Wang J, Zhang Q, Tang F-L, Pratap UP, Tekmal RR, Vadlamudi RK, Brann DW. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. The Journal of Neuroscience. 2020;40:7355–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Sareddy GR, Lu Y, Pratap UP, Tang F, Greene KM, Meyre PL, Tekmal RR, Vadlamudi RK, Brann DW. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. The Journal of Neuroscience. 2020:JN-RM-0888–0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitt J, Wilcox KC, Tortelli V, Diniz LP, Oliveira MS, Dobbins C, Yu XW, Nandamuri S, Gomes FCA, DiNunno N, et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound abeta oligomers. Mol Biol Cell. 2017;28:2623–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha MK, Seo M, Kim JH, Kim BG, Cho JY, Suk K. The secretome signature of reactive glial cells and its pathological implications. Biochim Biophys Acta. 2013;1834:2418–2428 [DOI] [PubMed] [Google Scholar]
- 26.Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: Targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Luo W, Reiser G. Activation of protease-activated receptors in astrocytes evokes a novel neuroprotective pathway through release of chemokines of the growth-regulated oncogene/cytokine-induced neutrophil chemoattractant family. Eur J Neurosci. 2007;26:3159–3168 [DOI] [PubMed] [Google Scholar]
- 28.Lin CH, Cheng FC, Lu YZ, Chu LF, Wang CH, Hsueh CM. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Exp Neurol. 2006;201:225–233 [DOI] [PubMed] [Google Scholar]
- 29.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: Focus on ischemic preconditioning. Glia. 2005;50:307–320 [DOI] [PubMed] [Google Scholar]
- 30.Savitz SI, Baron JC, Fisher M, Consortium SX. Stroke treatment academic industry roundtable x: Brain cytoprotection therapies in the reperfusion era. Stroke. 2019;50:1026–1031 [DOI] [PubMed] [Google Scholar]
- 31.The national institute of neurological disorders and stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 32.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251 [DOI] [PubMed] [Google Scholar]
- 33.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot JM, Desmond P, et al. Rapid automated patient selection for reperfusion therapy: A pooled analysis of the echoplanar imaging thrombolytic evaluation trial (epithet) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Stroke. 2011;42:1608–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, et al. Mri-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622 [DOI] [PubMed] [Google Scholar]
- 35.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New England Journal of Medicine. 2018;378:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. New England Journal of Medicine. 2019;380:1795–1803 [DOI] [PubMed] [Google Scholar]
- 37.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
- 38.Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433–439 [DOI] [PubMed] [Google Scholar]
- 39.Macleod MR, Fisher M, O’Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman R, Minematsu K, et al. Good laboratory practice: Preventing introduction of bias at the bench. Stroke. 2009;40:e50–52 [DOI] [PubMed] [Google Scholar]
- 40.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. Rigor guidelines: Escalating stair and steps for effective translational research. Transl Stroke Res. 2013;4:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, Carmichael ST, Cho S, Cipolla MJ, Corbett D, et al. Translational stroke research: Vision and opportunities. Stroke. 2017;48:2632–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voelkl B, Vogt L, Sena ES, Wurbel H. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 2018;16:e2003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta P The laboratory rat: Relating its age with human’s. Int J Prev Med. 2013;4:624–630 [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall Jonathan WB, Cummings Rosalyn M, Bowes Laura J, Ridley Rosalind M, Green AR. Functional and histological evidence for the protective effect of nxy-059 in a primate model of stroke when given 4 hours after occlusion. Stroke. 2003;34:2228. [DOI] [PubMed] [Google Scholar]
- 50.Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Forster C, Hogenesch JB, et al. Medicine in the fourth dimension. Cell Metab. 2019;30:238–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esposito E, Li W, E TM, Park JH, Sencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K, et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582:395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


