Abstract
Introduction:
Veterans with opioid use disorder have an increased risk of suicide and overdose compared with the general population. Buprenorphine, a U.S. Food and Drug Administration–approved medication to treat opioid use disorder, has shown benefits, including decreased risk of illicit drug use and overdose. This study assesses the mortality outcomes with buprenorphine pharmacotherapy among Veterans up to 5 years from treatment initiation.
Methods:
This was a retrospective cohort study of Veterans receiving buprenorphine (2008–2017) across any Veterans Health Administration facility. Buprenorphine pharmacotherapy was evaluated as a time-varying covariate. The primary outcome was death up to 5 years from treatment initiation by suicide and overdose combined; secondary outcomes included suicide, overdose, opioid-specific overdose, and all-cause death. Secondary analyses included evaluating the risk of mortality in recent discontinuation and effect modification by select characteristics. All analyses were conducted in 2020.
Results:
Veterans who were not receiving buprenorphine were 4.33 (adjusted hazard ratio; 95% CI=3.60, 5.21) times more likely to die by suicide/overdose than those receiving buprenorphine pharmacotherapy on any given day, with similar protective associations with treatment across secondary outcomes. The risk of suicide/overdose was highest 8–14 days from treatment discontinuation (adjusted hazard ratio=6.54, 95% CI=4.32, 9.91) than in currently receiving buprenorphine pharmacotherapy. There was no evidence of effect modification by the selected covariates.
Conclusions:
Mortality risk was greater among Veterans who were not receiving buprenorphine pharmacotherapy than among those who were. Providers should consider whether buprenorphine pharmacotherapy, either intermittent or continuous, may provide health benefits for their patients and prevent mortality.
INTRODUCTION
Opioid use disorder (OUD) is a significant public health burden in the U.S., with nearly 500,000 people dying from an overdose involving opioids between 1999 and 2018.1 Veterans, in particular, have an increased risk of OUD,2–4 with previous work showing that the diagnosis of OUD within the Veterans Health Administration (VHA) was nearly 7 times greater than that within commercial health plans.5
Contributing factors that increase the risk of OUD within the Veteran population include higher rates of chronic pain, sleep issues, concurrent mental health issues such as depression or post-traumatic stress disorder, and the use of other substances such as alcohol or sedatives.6–8 Rurality is a risk factor pertaining to harmful outcomes of OUD9,10: nearly 25% of Veterans live in rural areas where a shortage of mental health providers and access to timely care are barriers.11,12 Veterans also have an increased risk of opioid-related adverse events such as overdose and death2,13 and suicide compared with non-Veteran civilians.14,15 Several of these risk factors for addiction or dependence are also factors that qualify someone for using VHA services.16
When considering the long-term health outcomes of Veterans with OUD, it is essential to weigh the role of clinical management in the VHA with pharmacotherapies that may mitigate the risk. Medication for OUD (MOUD) is an effective treatment for those with OUD and may be combined with counseling or therapy for those who need it.17 Three current pharmacotherapies approved by the U.S. Food and Drug Administration include methadone (an agonist), buprenorphine (a partial agonist), and naltrexone (an antagonist).18 Retention on MOUD, a marker of successful treatment, has psychosocial and health benefits.19,20 The extent to which long-term treatment (whether intermittent or continuous), recent discontinuation trends, and treatment impact the risk across specific demographics within the Veteran population needs further exploration. Because OUD is a risk factor for suicide and overdose,14 it is critical to examine the relationship between the status of buprenorphine pharmacotherapies and long-term health outcomes, including mortality.
The objective of this study is to evaluate the association between buprenorphine pharmacotherapy and suicide, overdose, and all-cause mortality among Veterans initiating buprenorphine pharmacotherapy within the VHA. Because recent discontinuation of buprenorphine pharmacotherapy may be associated with adverse outcomes,21 the secondary objective is to assess whether the risk of mortality is greater with a recent discontinuation or gap in buprenorphine pharmacotherapy.
METHODS
Study Sample
This was a retrospective cohort study of adult Veterans (aged ≥18 years) diagnosed with OUD and treated with buprenorphine or buprenorphine/naloxone within the VHA system between January 1, 2008 and December 31, 2017. The study sample was restricted to those who received a prescription for a sublingual, short-acting buprenorphine product with a diagnosis code for OUD in the previous 6 months using the International Classification of Diseases, Ninth Revision, Clinical Modification and ICD-10-CM. Incident prescription of buprenorphine was determined as the first outpatient pharmacy prescription dispensed within the VHA. Veterans who received these medications between 2006 and 2008 were excluded. Exclusion criteria included those receiving buprenorphine patches because these suggest pain management22 and those with metastatic tumor diagnosis (because this may influence subsequent medications, the continuation of buprenorphine pharmacotherapy, and mortality) within 2 years before buprenorphine initiation. This study was approved by the local IRB and Veterans Administration Research and Development Committee, and the manuscript is reported in accordance with the STROBE statement.23
Measures
The incident buprenorphine date for each Veteran was determined from the first oral outpatient buprenorphine prescription filled between January 1, 2008 and December 31, 2017. Buprenorphine pharmacotherapy continuity was evaluated by the longitudinal pattern of dispensed buprenorphine prescriptions; for each prescription dispensed, an episode of buprenorphine pharmacotherapy was generated by assessing the supply days from the day the prescription was filled through the number of supply days. Potential oversupply was accounted for when the prescription was filled before the expected supply exhaustion. Days receiving buprenorphine pharmacotherapy (exposed) were measured as days that buprenorphine was available to the Veteran, whereas days not receiving buprenorphine pharmacotherapy (unexposed) were evaluated as days where there was no buprenorphine supply available. The peak dose or highest dose of buprenorphine within the first 14 days of pharmacotherapy initiation was assessed as a marker of severity and converted to milligram morphine equivalents.24
Other MOUDs (naltrexone, methadone) utilized during follow-up were assessed to distinguish between (1) Veterans without any current MOUD and (2) Veterans without buprenorphine MOUD pharmacotherapy. Days receiving pharmacotherapy were assessed similarly from the prescription fill day through the exhaustion of the supply. For extended-release naltrexone, the days’ supply within the VHA pharmacy indicated 30 days per dose. Buprenorphine pharmacotherapy status on any given day was characterized while accounting for periods on naltrexone or methadone (1) up to death, (2) up to the end of the study period (December 31, 2017), or (3) up to 5 years from buprenorphine pharmacotherapy initiation.
To account for temporal trends in buprenorphine availability and access, pharmacotherapy initiation year was categorized in 2-year intervals. Sociodemographic factors assessed at baseline included age, sex, and potential homelessness (identified using previous methodologies).25,26 Geographic measures included driving distance and time to nearest primary/secondary care centers, and urban/rural designation status was determined by ZIP codes and 2010 Rural–Urban Commuting Area codes.27 A combination of primary and secondary codes was used to assign a rural designation as isolated rural, small rural, and large rural areas. Urban designation included urban tracts and those for which ≥30% commuted to a nearby metropolitan, micropolitan, or small-town core.27
Comorbidities diagnosed up to 2 years before buprenorphine initiation were identified from the International Classification of Diseases, Ninth Revision and ICD-10 inpatient and outpatient diagnosis codes. These conditions were classified using Quan’s algorithms and the Clinical Classifications Software Level 2 coding schema for mental health diagnoses from the Healthcare Cost and Utilization Project.28 Past-year psychiatric medications were identified from pharmacy files. Healthcare utilization was measured from inpatient (classified as any, mental health, suicide/self-harm, substance use), outpatient, urgent-care, and emergency department utilization from VHA stop codes up to 2 years before buprenorphine initiation.29
The primary outcome in this study was a combined outcome of death by suicide and overdose because the determination of suicide requires that the death be established as both self-inflicted and intentional, and this may be unclear in overdose.30 Secondary outcomes included suicide death, any overdose death, opioid-specific overdose death, and all-cause mortality. Data were obtained from the Suicide Data Repository, developed as a collaborative effort to include demographic and personnel data from the Joint Department of Veterans Affairs–Department of Defense and the National Death Index from the Centers for Disease Control and Prevention.31 Cause-specific death was determined by ICD-10 codes identified on death certificate data from the National Death Index (Appendix Methods 1, available online).
Statistical Analysis
Buprenorphine pharmacotherapy status was evaluated until death, with censoring occurring at the end of the study period (December 31, 2017) or at the end of maximum follow-up period of 5 years. Pharmacotherapy status was measured as a time-varying covariate to account for days Veterans received buprenorphine pharmacotherapy and days they did not receive it over time. In the first 30 days of the last known treatment, days were further classified as discontinuation/gaps within ≤7 days (first week), 8–14 days (second week), 15–30 days (third and fourth week), and >30 days.
The association between each demographic and clinical covariate and mortality was modeled through Cox proportional hazards regression. Variables identified as associated with the outcome (with a threshold of p<0.20) were considered in developing the final model. Final multivariable models were built through the inclusion of pertinent variables and evaluation of Akaike information criterion values. Analyses were conducted in 2020 using SAS, version 9.4.
Potential multiplicative effect modification of buprenorphine pharmacotherapy and outcome associations by key demographic variables (sex, race, rural–urban categorization, and maximum buprenorphine dose in the first 2 weeks) was evaluated by fitting the models with an interaction term of buprenorphine pharmacotherapy status across each variable stratum.
A total of 4 sensitivity analyses of the main outcome (suicide/overdose up to 5 years) were performed. First, the impact of duration of follow-up time on mortality was assessed to account for the potential change in the susceptible population over time.32 Second, because new users of VHA care would have incomplete comorbidity data, those at or below the 10th percentile of outpatient visits in the 2-year baseline period were excluded. Third, those who had <30 days of cumulative buprenorphine pharmacotherapy were excluded as a proxy for those who might receive care outside the VHA system. Finally, the potential for unmeasured confounding and bias was evaluated using E-values, the minimum strength of association that an unmeasured confounder would need to fully explain away the observed association, conditioned on covariates that are measured.33,34
RESULTS
There were 29,054 Veterans in the final study sample (Figure 1), among whom, 92.9% were male, 81.2% were white, and 90.6% were aged 25–64 years at the time of their initial buprenorphine prescription within the VHA (Table 1). Psychiatric comorbidities were prevalent in this population, with 73.2% of Veterans diagnosed with depression, 60.7% diagnosed with anxiety disorders, and >50% diagnosed with documentation of ≥1 other substance dependence.
Figure 1.
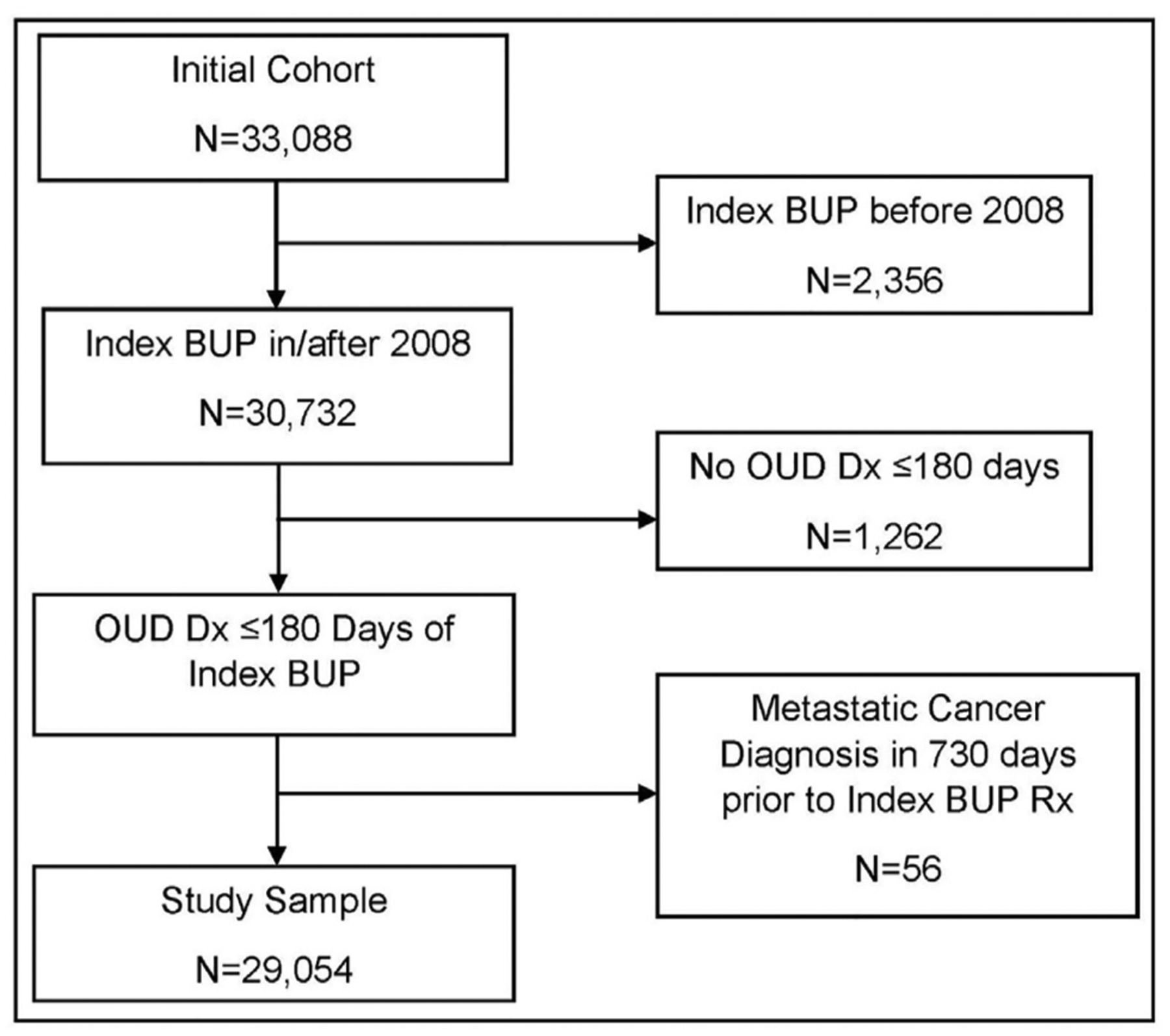
Flow chart of the study sample: Veterans treated for opioid use disorder with buprenorphine pharmacotherapy in the Veterans Health Administration, 2082–17.
BUP, buprenorphine; Dx, diagnosis; OUD, opioid use disorder; Rx, prescription.
Table 1.
Baseline Characteristics of Veterans Treated for OUD With Buprenorphine Pharmacotherapy and Associations With Suicide/Overdose Deaths in the VHA, 2008–2017
| Demographic, geographic, and clinical measures | Total (N=29,054) | % | Suicide/overdose, UHR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 2,060 | 7.1 | ref |
| Male | 26,994 | 92.9 | 1.39 (1.04, 1.87) |
| Age, years | |||
| 18–24 | 1,125 | 3.9 | 1.70 (1.09, 2.66) |
| 25–44 | 14,134 | 48.6 | 1.37 (0.94, 2.01) |
| 45–64 | 12,209 | 42.0 | 1.12 (0.76, 1.65) |
| ≥65 | 1,586 | 5.5 | ref |
| Race | |||
| White | 23,603 | 81.2 | ref |
| Black | 3,621 | 12.5 | 0.60 (0.47, 0.75) |
| Other | 670 | 2.3 | 0.74 (0.46, 1.20) |
| Unknown/missing | 1,160 | 4.0 | 1.08 (0.79, 1.47) |
| Ethnicity | |||
| Hispanic | 1,600 | 5.5 | ref |
| Non-Hispanic | 26,670 | 91.8 | 1.49 (1.04, 2.08) |
| Unknown/missing | 784 | 2.7 | 1.78 (1.10, 2.89) |
| Indication for homelessness/unstable housing | |||
| No | 20,666 | 71.1 | ref |
| Yes | 8,388 | 28.9 | 1.76 (1.55, 2.00) |
| Residential designation | |||
| Urban | 25,516 | 87.8 | ref |
| Rural | 3,275 | 11.3 | 0.76 (0.61, 0.95) |
| Unknown/missing | 263 | 0.9 | 2.61 (1.71, 3.99) |
| Distance to the closest primary care facility | |||
| 0–19 miles | 22,565 | 77.7 | ref |
| ≥20 miles | 6,419 | 22.1 | 0.86 (0.73, 1.00) |
| Unknown/missing | 70 | 0.2 | 19.63 (12.29, 31.37) |
| Distance to the closest secondary care facility | |||
| 0–19 miles | 12,529 | 43.1 | ref |
| ≥20 miles | 16,423 | 56.5 | 0.85 (0.75, 0.96) |
| Unknown/missing | 102 | 0.4 | 9.25 (5.85, 14.65) |
| Drive time to the closest primary care facility | |||
| 0–29 minutes | 23,889 | 82.2 | ref |
| ≥30 minutes | 5,095 | 17.5 | 0.79 (0.66, 0.95) |
| Unknown/missing | 70 | 0.2 | 19.54 (12.23, 31.21) |
| Drive time to the closest secondary care facility | |||
| 0–29 minutes | 14,032 | 48.3 | ref |
| ≥30 minutes | 14,920 | 51.4 | 0.85 (0.75, 0.97) |
| Unknown/missing | 102 | 0.4 | 9.36 (5.92, 14.80) |
| Treatment initiation era | |||
| 2008–2009 | 4,008 | 13.8 | ref |
| 2010–2011 | 5,347 | 18.4 | 1.11 (0.90, 1.36) |
| 2012–2013 | 5,952 | 20.5 | 1.34 (1.10, 1.64) |
| 2014–2015 | 6,433 | 22.1 | 1.75 (1.41, 2.16) |
| 2016–2017 | 7,314 | 25.2 | 1.91 (1.44, 2.54) |
| Clinical characteristics (ref=No) | |||
| Comorbidities | |||
| AIDS/HIV | 277 | 1.0 | 1.30 (0.74, 2.30) |
| Cardiac arrhythmia | 2,110 | 7.3 | 0.98 (0.76, 1.27) |
| Chronic Obstructive Pulmonary Disease | 3,955 | 13.6 | 1.18 (0.99, 1.41) |
| Congestive heart failure | 646 | 2.2 | 1.29 (0.85, 1.95) |
| Diabetes (with complications) | 933 | 3.2 | 1.36 (0.95, 1.94) |
| Diabetes (without complications) | 2,608 | 9.0 | 1.08 (0.86, 1.35) |
| Hypertension (without complications) | 9,419 | 32.4 | 1.08 (0.94, 1.23) |
| Hypertension (with complications) | 480 | 1.7 | 1.62 (0.97, 2.70) |
| Liver disease | 4,492 | 15.5 | 1.35 (1.14, 1.59) |
| Malignancy | 804 | 2.8 | 0.86 (0.56, 1.33) |
| Myocardial infarction | 308 | 1.1 | 0.77 (0.37, 1.63) |
| Nonmetastatic cancer | 707 | 2.4 | 0.84 (0.53, 1.35) |
| Obesity | 3,374 | 11.6 | 1.14 (0.94, 1.37) |
| Peripheral vascular disease | 710 | 2.4 | 1.47 (1.02, 2.13) |
| Renal disease | 564 | 1.9 | 1.20 (0.75, 1.91) |
| Weight loss | 1,042 | 3.6 | 0.82 (0.56, 1.21) |
| Psychiatric comorbidities | |||
| Attention deficit hyperactivity disorder | 1,659 | 5.7 | 1.41 (1.11, 1.81) |
| Adjustment | 4,427 | 15.2 | 1.26 (1.07, 1.48) |
| Anxiety disorder | 17,636 | 60.7 | 1.47 (1.28, 1.68) |
| Bipolar disorder | 5,352 | 18.4 | 1.51 (1.31, 1.75) |
| Depression | 21,258 | 73.2 | 1.55 (1.33, 1.82) |
| Personality disorder | 2,319 | 8.0 | 1.49 (1.22, 1.81) |
| Post-traumatic stress disorder | 11,516 | 39.6 | 1.32 (1.16, 1.50) |
| Schizophrenia | 1,735 | 6.0 | 1.34 (1.06, 1.70) |
| Other substance use dependence | |||
| Alcohol | 10,574 | 36.4 | 1.50 (1.32, 1.71) |
| Stimulants | 6,159 | 21.2 | 1.27 (1.09, 1.47) |
| Cannabis | 3,746 | 12.9 | 1.14 (0.94, 1.37) |
| Cocaine | 5,564 | 19.2 | 1.50 (1.30, 1.73) |
| Hallucinogen | 123 | 0.4 | 1.71 (0.81, 3.60) |
| Nicotine | 15,046 | 51.8 | 1.28 (1.13, 1.45) |
| Sedative | 2,619 | 9.0 | 1.70 (1.42, 2.05) |
| Recent medication use | |||
| Antidepressants | 18,874 | 65.0 | 1.41 (1.23, 1.62) |
| Antipsychotics | 6,632 | 22.8 | 1.62 (1.41, 1.85) |
| Anxiolytics | 9,820 | 33.8 | 1.29 (1.14, 1.47) |
| Mood stabilizers | 10,135 | 34.9 | 1.57 (1.38, 1.78) |
| Opioids | 11,050 | 38.0 | 1.05 (0.93, 1.20) |
| Stimulants | 1,014 | 3.5 | 1.45 (1.08, 1.96) |
| Healthcare utilization | |||
| Inpatient admission | |||
| Any reason | 13,331 | 45.9 | 1.71 (1.50, 1.94) |
| Mental health | 12,587 | 43.3 | 1.71 (1.50, 1.94) |
| Suicide or self harm | 2,938 | 10.1 | 1.59 (1.33, 1.90) |
| Substance use | 11,599 | 39.9 | 1.70 (1.50, 1.93) |
| Urgent care use | 4,400 | 15.1 | 1.45 (1.25, 1.70) |
| Emergency department use | |||
| Suicide or self harm | 2,266 | 7.8 | 1.58 (1.29, 1.94) |
| Substance use | 8,359 | 28.8 | 1.44 (1.26, 1.64) |
OUD, opioid use disorder; UHR, unadjusted hazard ratio; VHA, Veterans Health Administration.
There were 15.1 million person-days of buprenorphine treatment from initiation up to 5 years. Approximately 3.1% (n=892) of Veterans had ≥1 methadone prescription (258,355 person-days, 0.7% of follow-up time) (Appendix Table 1, available online). Similarly, 7.4% (n=2,137) of Veterans had ≥1 naltrexone prescription (177,339 person-days, 0.5% of follow-up time).
Over the 5 years of follow-up from the initial buprenorphine prescription, 3.3% of the cohort died of suicide/overdose, and 7.8% died of any cause. Among the suicide/overdose deaths, the majority (89.9%) were due to overdose, and 71.0% of the overdose deaths involved a prescription or illicit opiate. Male sex and younger age were associated with an increased risk of suicide/overdose (Table 1), although younger age was associated with a lower risk of all-cause mortality (Appendix Table 2, available online).
In the final adjusted model, the risk of suicide/overdose death was 4.33 (95% CI=3.60, 5.21) times greater among those who were not receiving buprenorphine pharmacotherapy than among those who were receiving it on any given day, even when accounting for any periods where they received methadone or naltrexone (Table 2). There was some suggestion of elevated risk in earlier weeks from last known buprenorphine pharmacotherapy; the risk of suicide/overdose was highest 8–14 days from treatment discontinuation (adjusted hazard ratio [AHR]=6.54, 95% CI=4.32, 9.91) than for currently receiving buprenorphine.
Table 2.
Association Between Buprenorphine Pharmacotherapy Receipt and Suicide/Overdose With up to 5 Years of Follow-Up From Treatment Initiation Among Veterans in the VHA, 2008–2017 (N=29,054)
| Buprenorphine pharmacotherapy status | Person-days at risk | n, deaths | IR | UHR (95% CI) | AHR (95% CI)a |
|---|---|---|---|---|---|
| Treated | 15,094,978 | 142 | 0.94 | ref | ref |
| Not treated, overall | 20,191,645 | 822 | 4.07 | 4.61 (3.84, 5.54) | 4.33 (3.60, 5.21) |
| Treated | 15,094,978 | 142 | 0.94 | ref | ref |
| Not treated, stratified | |||||
| ≤7 days since the last treatment | 543,673 | 28 | 5.15 | 5.13 (3.40, 7.75) | 4.56 (3.01, 6.90) |
| 8–14 days since the last treatment | 388,091 | 28 | 7.21 | 7.47 (4.95, 11.28) | 6.54 (4.32, 9.91) |
| 15–30 days since the last treatment | 708,080 | 26 | 3.67 | 3.95 (2.59, 6.04) | 3.45 (2.25, 5.29) |
| >30 days since the last treatment | 18,551,801 | 740 | 3.99 | 4.51 (3.74, 5.43) | 4.29 (3.55, 5.17) |
Note: Boldface indicates statistical significance (p<0.05). Suicide/overdose indicates suicide/overdose deaths (n=964). IR indicates deaths per 100,000 person-days.
Adjusted for demographics (age, sex, race, rural, homelessness, year initiating buprenorphine), clinical comorbidities (depression, peripheral vascular disease, liver disease), concurrent substance dependence (alcohol, cannabis), medications (antipsychotics, mood stabilizers), other MOUD (naltrexone, methadone), and healthcare utilization (overdose in ED, inpatient admission for substance use or dependence).
AHR, adjusted hazard ratio; ED, emergency department; IR, incidence rate MOUD, medications for opioid use disorder; UHR, unadjusted hazard ratio; VHA, Veterans Health Administration.
Consistent with the primary outcome, mortality risk was greater for those not receiving buprenorphine than for those receiving this medication, including suicide death (AHR=3.57, 95% CI=2.25, 5.66), overdose death (AHR=4.58, 95% CI=3.75, 5.59), opioid-related death (AHR=4.59, 95% CI=3.62, 5.80), and all-cause mortality (AHR=3.82, 95% CI=3.41, 4.28) (Appendix Table 3, available online).
There was no evidence of multiplicative effect modification by sex (p=0.75), age (p=0.98), race (p=0.08), rurality (p=0.84), or peak dose (p=0.29) (Appendix Figure 1, available online).
Mortality risk was highest with follow-up to 1 year, with slight attenuation as follow-up time increased to 5 years (Appendix Tables 4–7, available online, and Appendix Figure 2, available online). There was a slight attenuation of hazard ratios when excluding those with limited VHA contact before buprenorphine initiation and those with ≤30 days of cumulative buprenorphine pharmacotherapy (Table 3 and Appendix Table 8, available online). The evaluation of E-values and CIs showed that a significant amount of unmeasured confounding would have been needed to explain away the observed associations.
Table 3.
Comparison of Results Between Main and Sensitivity Analyses for the Association Between Buprenorphine Pharmacotherapy Receipt and Suicide/Overdose, With up to 5 Years of Follow-Up From Buprenorphine Pharmacotherapy Initiation Among Veterans Treated in the VHA, 2008–2017
| Outcome | Main analysis,a N=29,054 | SA 1: excluding low utilizers,b n=26,404 | SA 2: excluding infrequent buprenorphine utilizers,c n=24,405 | SA 3: E-valuesd |
|---|---|---|---|---|
| AHR (95% CI) | AHR (95% CI) | AHR (95% CI) | E-value/CI | |
| Suicide/overdose deaths | n=964 | n=899 | n=731 | |
| Buprenorphine pharmacotherapy status | ||||
| Treated | ref | ref | ref | ref |
| Not treated overall | 4.33 (3.60, 5.21) | 4.15 (3.44, 5.02) | 3.92 (3.23, 4.76) | 8.15/6.66 |
| Treated | ref | ref | ref | ref |
| Not treated stratified | ||||
| ≤7 days since last treatment | 4.56 (3.01, 6.90) | 4.23 (2.75, 6.50) | 4.16 (2.53, 6.83) | 8.59/5.47 |
| 8–14 days since the last treatment | 6.54 (4.32, 9.91) | 6.30 (4.12, 9.63) | 6.67 (4.18, 10.62) | 12.56/8.11 |
| 15–30 days since the last treatment | 3.45 (2.25, 5.29) | 3.19 (2.05, 4.98) | 3.27 (2.01, 5.32) | 6.36/3.93 |
| >30 days since last treatment | 4.29 (3.55, 5.17) | 4.12 (3.40, 5.00) | 3.87 (3.18, 4.71) | 8.05/6.56 |
Note: Boldface indicates statistical significance (p<0.05).
Main analysis: represents the results from the final models for each suicide/overdose outcome over 5 years of follow-up, as seen in Table 2.
SA 1: represents the results from excluding Veterans who had ≤14 outpatient encounters within the VHA system in the 2 years preceding the initial buprenorphine prescription. The purpose of this analysis was to assess whether there was potential unmeasured confounding from those who might not have had the opportunity to have comorbidities documented to the same extent as routine VHA users.
SA 2: represents the results from excluding Veterans who had a total of ≤30 days on buprenorphine treatment over 5 years of follow-up from the initial buprenorphine prescription. The purpose of this analysis was to account for potential misclassification of exposure, specifically among those who might have received treatment outside of the VHA but have been documented as not being on treatment in the main analysis.
SA 3: represents an assessment of unmeasured confounding and bias by evaluating the E-values, the minimum strength of association that an unmeasured confounder would need to fully explain away the observed association, conditioned on covariates that are measured. For example, for the primary suicide/overdose composite outcome over 5 years of follow-up, the observed HR of 4.33 could be explained away by an unmeasured confounder that was associated with both the treatment and the outcome by an HR of 8.15-fold each, above and beyond the measured confounders, but weaker confounding could not do so. The CI could be moved to include the null by an unmeasured confounder that was associated with both the treatment and the outcome by an HR of 6.66 folds each, above and beyond the measured confounders, but weaker confounding could not do so. AHR, adjusted hazard ratio; HR, hazard ratio; SA, sensitivity analysis; VHA, Veterans Health Administration.
DISCUSSION
A national cohort of nearly 30,000 Veterans who initiated buprenorphine pharmacotherapy for OUD was examined for the association between their medication course up to 5 years after buprenorphine initiation and mortality. One key finding in this study is further evidence that buprenorphine pharmacotherapy is lifesaving: Veterans who were not receiving buprenorphine pharmacotherapy on any given day had a >4-fold increase in suicide/overdose death compared with those who were receiving buprenorphine pharmacotherapy, even when accounting for time periods on other MOUDs. These findings are consistent with those of previous studies in smaller or regionalized populations with reduced all-cause, overdose, or opioid-related deaths.35–38 However, these estimates of overdose and all-cause mortality were elevated compared with the estimates of a 2017 meta-analysis that found a pooled incidence rate 3.3 times greater for those not receiving buprenorphine pharmacotherapy than for those who were receiving this pharmacotherapy.35 Differences in findings may be due to variable population characteristics, eligibility for benefits within the VHA, or study era.
Although not significantly different, mortality risk appeared highest in earlier periods after the last known buprenorphine pharmacotherapy. The estimates for suicide/overdose mortality showed a slight elevation during the second week (Days 8–14) of discontinuation or exhaustion of supply compared with those in the first week. One study examining the receipt of buprenorphine and overdose in a large-scale commercially insured population found no difference in the rate of overdose within the first 4 weeks of buprenorphine discontinuation,39 whereas others reported a higher risk for overdose mortality out of treatment.38,40
Despite the elevated estimates in the risk of mortality in the earlier periods after lapses in pharmacotherapy, there may be several reasons why these were not significantly different from those in later time periods. It is important to clarify that it is not known whether the absence of pharmacotherapy was due to a true discontinuation or an exhaustion of supply for any reason. A subset of Veterans may have also been hospitalized, incarcerated, or in another similar situation where they did not have the opportunity to refill their prescriptions. However, within the first week of a discontinuation/gap, approximately 35% of Veterans reinitiated buprenorphine pharmacotherapy, suggesting that they had been less than fully adherent or had exhausted their supply but intended to remain on buprenorphine therapy. Another explanation for the absence of significant findings in this earlier timeframe may be the persistent impact of buprenorphine after recent discontinuation; the relatively long half-life of buprenorphine (24–37 hours) after sublingual administration may sustain its protective effect through part of the first week after discontinuation.41 Finally, because this study relied on administrative data for prescriptions, an oversupply of medication may have lasted beyond the calculated end of buprenorphine pharmacotherapy such that the actual discontinuation was later than the apparent discontinuation date.
Previous findings from the Centers for Disease Control and Prevention and from other researchers have shown variations in pharmacotherapy initiation and retention with buprenorphine or overdose mortality by socioeconomic, demographic, and geographic characteristics.15,26,42 This study evaluated the potential effect modification, and 2 important themes found warrant further research. First, although there may be variability in access to providers or medications, pharmacotherapy initiation, or overall differences in sample characteristics for suicide or overdose-related mortality, the findings in this study suggest that the association between the receipt of buprenorphine pharmacotherapy and mortality does not vary across these specific variables. These findings should be approached with some caution; despite the large sample size overall, there were smaller subgroups of female, non-White, and rural Veterans, leading to less precision. The second theme was that although rurality independently sustained a protective effect against mortality, it did not modify the relationship between buprenorphine pharmacotherapy receipt and mortality. Although previous research has shown rurality to be a risk factor for mortality, this study now raises the following question: are there improved health outcomes for rural Veterans provided they initiated buprenorphine pharmacotherapy for OUD? In recent years, the VHA has expanded measures to advance health outcomes for rural Veterans to overcome barriers such as distance and access to specialized care.43–47 Perhaps, increased services and capabilities such as the adoption of telehealth networks and the flexibility to incorporate non-VHA providers into coordinated care to mitigate such barriers have improved the long-term health outcomes for Veterans. Future studies should examine the mechanisms by which these efforts influence OUD management for rural Veterans treated within the system and also should examine the potential gaps in programs through regions or Veteran cohorts who do not seek treatment within the VHA.
Limitations
There are several limitations to this study. First, Veterans may have received pharmacotherapies outside of the VHA, although there was only a slight attenuation of mortality risk when a subset of Veterans who had <30 days of buprenorphine pharmacotherapy was excluded. Administrative data were used for buprenorphine pharmacotherapy status, which could have led to potential errors in characterizing the availability of treatment on any given day. The use of these data may have also resulted in unmeasured confounding or bias beyond what was captured within this study. The evaluation of E-values showed that there would have needed to be a high degree (>6-fold) of unmeasured confounding to explain away the observed association. Finally, suicide and overdose classification were determined from death certificates, which may under-report true estimates; nonetheless, this standard is used as a valid source for epidemiologic studies for mortality.48,49
There were several strengths to this study, including identifying Veterans who used buprenorphine within the VHA over a 10-year window and the construction of pharmacotherapy episodes to account for medication status on any given day for each Veteran during follow-up. Finally, data were obtained from the National Death Index, a part of the Joint Department of Veteran Affairs and Department of Defense Suicide Data Repository, leading to higher accuracy of all-cause mortality.
CONCLUSIONS
The risk of overdose/suicide was substantially greater among Veterans who were not receiving buprenorphine than among those who were receiving buprenorphine pharmacotherapy on any given day within a cohort of incident buprenorphine users within the VHA, even after accounting for periods where other MOUDs were prescribed. Clinical efforts need to include long-term retention in treatment as a key outcome, integrate adequate mental health treatment to help prevent suicide and overdose death, and account for key demographics that are considered at risk for both treatment discontinuation and mortality. Meanwhile, future research efforts should expand access to treatment, identify barriers to pharmacotherapies, implement qualitative studies to identify the decision to discontinue treatment, and develop innovative methods for improving retention to prevent opioid relapse.
Supplementary Material
ACKNOWLEDGMENTS
The funding sources to this study had no role in the study design; collection, analysis, and interpretation of data; writing of this report; or the decision to submit the report for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
This work was supported by the National Institute on Drug Abuse’s R36 Drug Abuse Dissertation Grant [1R36DA050878-01] and a predoctoral training grant and a pilot grant from the Centers for Disease Control and Prevention/National Institute of Occupational Health and Safety/Heartland Center for Occupational Health and Safety (T420H008491). This material is the result of work supported with resources and the use of facilities at the (local) Department of Veterans Affairs Health Care System.
No other financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.02.026.
REFERENCES
- 1.Understanding the epidemic. Centers for Disease Control and Prevention. https://www.cdc.gov/drugoverdose/epidemic/index.html.Updated March 21, 2021. AccessedMay 3, 2021.
- 2.Teeters JB, Lancaster CL, Brown DG, Back SE. Substance use disorders in military veterans: prevalence and treatment challenges. Subst Abuse Rehabil. 2017;8:69–77. 10.2147/SAR.S116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393–396. 10.1097/MLR.0b013e318202aa27. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JF, Valenstein M, Kim HM, Ilgen M, Zivin K, Blow FC. Suicide mortality among patients receiving care in the Veterans Health Administration health system. Am J Epidemiol. 2009;169(8):1033–1038. 10.1093/aje/kwp010. [DOI] [PubMed] [Google Scholar]
- 5.Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans Health Administration. Pain Pract. 2014;14(5):437–445. 10.1111/papr.12097. [DOI] [PubMed] [Google Scholar]
- 6.Bachhuber MA, Roberts CB, Metraux S, Montgomery AE. Screening for homelessness among individuals initiating medication-assisted treatment for opioid use disorder in the Veterans Health Administration. J Opioid Manag. 2015;11(6):459–462. 10.5055/jom.2015.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meshberg-Cohen S, Black AC, DeViva JC, Petrakis IL, Rosen MI. Trauma treatment for veterans in buprenorphine maintenance treatment for opioid use disorder. Addict Behav. 2019;89:29–34. 10.1016/j.addbeh.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Kelley ML, Bravo AJ, Votaw VR, Stein E, Redman JC, Witkiewitz K. Opioid and sedative misuse among veterans wounded in combat. Addict Behav. 2019;92:168–172. 10.1016/j.addbeh.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26–S35. 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). Unintentional poisoning deaths–United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56(5):93–96. https://www.cdc.gov/mmwr//preview/mmwrhtml/mm5605a1.htm.AccessedMay 17, 2021. [PubMed] [Google Scholar]
- 11.Rural access to health care services request for Information. Health Resources and Services Administration. https://www.hrsa.gov/rural-health/rfi-rural-health-care-access.Updated January 2021. AccessedAugust 31, 2020. [Google Scholar]
- 12.UNC The Cecil G. Sheps Center for Health Services Research. NC Rural Health Research Program. Rural Health Snapshot (2017). Chapel Hill, NC: UNC The Cecil G. Sheps Center for Health Services Research; 2017. https://www.shepscenter.unc.edu/wp-content/uploads/dlm_uploads/2017/05/Snapshot2017.pdf. Published 2017. Accessed August 31, 2020.
- 13.Wilder CM, Miller SC, Tiffany E, Winhusen T, Winstanley EL, Stein MD. Risk factors for opioid overdose and awareness of overdose risk among veterans prescribed chronic opioids for addiction or pain. J Addict Dis. 2016;35(1):42–51. 10.1080/10550887.2016.1107264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon J, Volkow N. Suicide deaths are a major component of the opioid crisis that must be addressed. Bethesda, MD: National Institute of Mental Health; 2019. https://www.nimh.nih.gov/about/director/messages/2019/suicide-deaths-are-a-major-component-of-the-opioid-crisis-that-must-be-addressed.shtml.Published 2019. AccessedJuly 23, 2020. [Google Scholar]
- 15.Hedegaard H, Curtin SC, Warner M. Suicide mortality in the United States, 1999–2017. Hyattsville, MD: National Center for Health Statistics; 2018. https://www.cdc.gov/nchs/data/databriefs/db330-h.pdf.Published 2018. AccessedMarch 19, 2021. [Google Scholar]
- 16.Office of Public and Intergovernmental Affairs: chapter 1 health care benefits. U.S. Department of Veterans Affairs; 2018. https://www.va.gov/opa/publications/benefits_book/Chapter_1_Health_Care_Benefits.asp.AccessedJanuary 18, 2021. [Google Scholar]
- 17.Substance Abuse and Mental Health Services Administration. Medications for opioid use disorder for healthcare and addiction professionals, policymakers, patients, and families: treatment improvement protocol TIP 63. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document/PEP20-02-01-006.Published 2018. AccessedMarch 31, 2021. [Google Scholar]
- 18.MAT Medication, counseling, and related conditions. Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/medication-assisted-treatment/medications-counseling-related-conditions.AccessedFebruary 19, 2019.
- 19.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35(1):22–35. 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Bao YP, Wang RJ, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1868–1883. 10.1038/s41380-018-0094-5. [DOI] [PubMed] [Google Scholar]
- 21.Williams AR, Samples H, Crystal S, Olfson M. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2020;177(2):117–124. 10.1176/appi.ajp.2019.19060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MP, Pasternak G, Behm B. Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs. 2018;78(12):1211–1228. 10.1007/s40265-018-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare & Medicaid Services. Opioid morphine equivalent conversion factors. Baltimore, MD: Centers for Medicare & Medicaid Services; 2015. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-March-2015.pdf.Published 2015. AccessedAugust 9, 2020. [Google Scholar]
- 25.Peterson R, Gundlapalli AV, Metraux S, et al. Identifying homelessness among veterans using VA administrative data: opportunities to expand detection criteria. PLoS One. 2015;10(7):e0132664. 10.1371/journal.pone.0132664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manhapra A, Petrakis I, Rosenheck R. Three-year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. Am J Addict. 2017;26(6):572–580. 10.1111/ajad.12553. [DOI] [PubMed] [Google Scholar]
- 27.2010 Rural–urban commuting area (RUCA) codes. Economic Research Service, U.S. Department of Agriculture; 2020. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/.AccessedSeptember 11, 2020. [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 29.Harris AH, Reeder RN, Ellerbe L, Bowe T. Are VHA administrative location codes valid indicators of specialty substance use disorder treatment? J Rehabil Res Dev. 2010;47(8):699–708. 10.1682/jrrd.2009.07.0106. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control (CDC). Operational criteria for determining suicide. MMWR Morb Mortal Wkly Rep. 1988;37(50):773–780. https://www.cdc.gov/mmwr/preview/mmwrhtml/00001318.htm.AccessedMay 17, 2021. [PubMed] [Google Scholar]
- 31.Defense Suicide Prevention Office (DSPO). The Suicide Data Repository (SDR) fact sheet. Defense Suicide Prevention Office (DSPO); 2020. Alexandria, VA. https://www.dspo.mil/Portals/113/Documents/SDR%20Fact%20Sheet.pdf.Published 2020. AccessedAugust 9, 2020. [Google Scholar]
- 32.Hernán MA. The hazards of hazard ratios [published correction appears in Epidemiology. 2011;22(1):134] Epidemiology. 2010;21(1):13–15. 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45–e47. 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268–274. 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 35.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137–145. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ. 2010;341:c5475. 10.1136/bmj.c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry. 2015;2(10):901–908. 10.1016/S2215-0366(15)00366-1. [DOI] [PubMed] [Google Scholar]
- 39.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34–39. 10.1016/j.drugalcdep.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006–10. Addiction. 2015;110(6):996–1005. 10.1111/add.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cone EJ, Gorodetzky CW, Yousefnejad D, Buchwald WF, Johnson RE. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577–581. AccessedMarch 5, 2021. [PubMed] [Google Scholar]
- 42.Pierce M, Bird SM, Hickman M, et al. Impact of treatment for opioid dependence on fatal drug-related poisoning: a national cohort study in England. Addiction. 2016;111(2):298–308. 10.1111/add.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Health Resources and Services Administration. Funding cycle view: Rural Veterans Health Access Program. Rockville, MD: Health Resources and Services Administration; 2018. https://grants.hrsa.gov/2010/Web2External/Interface/FundingCycle/ExternalView.aspx?fCycleID=53babc47-a718-4552-b9bd-d683b947ebd7.Published 2018. AccessedMarch 24, 2021. [Google Scholar]
- 44.Jacobs JC, Blonigen DM, Kimerling R, et al. Increasing mental health care access, continuity, and efficiency for veterans through telehealth with video tablets. Psychiatr Serv. 2019;70(11):976–982. 10.1176/appi.ps.201900104. [DOI] [PubMed] [Google Scholar]
- 45.Pearson K, Burgess A, Gale J, Coburn A, Hansen A. Health information exchange: a strategy for improving access for rural veterans in the Maine Flex Rural Veterans Health Access. Portland, ME: University of Southern Maine, Muskie School of Public Service, Maine Rural Health Research Center; 2016. https://digitalcommons.usm.maine.edu/insurance/38/.Published 2016. AccessedMarch 19, 2021. [Google Scholar]
- 46.Weintraub E, Greenblatt AD, Chang J, Himelhoch S, Welsh C. Expanding access to buprenorphine treatment in rural areas with the use of telemedicine. Am J Addict. 2018;27(8):612–617. 10.1111/ajad.12805. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Department of Veterans Affairs. Health Services Research & Development. VA Telehealth: overview Washington, DC: U.S. Department of Veterans Affairs; 2018. https://www.hsrd.research.va.gov/publications/inprogress/dec18/default.cfm?InProgressMenu=dec18-1.Published 2018. AccessedMarch 24, 2021. [Google Scholar]
- 48.Miller M, Barber C, Young M, Azrael D, Mukamal K, Lawler E. Veterans and suicide: a reexamination of the National Death Index-linked National Health Interview Survey. Am J Public Health. 2012;102(suppl1):S154–S159. AccessedMarch 5, 2021. 10.2105/AJPH.2011.300409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speechley M, Stavraky KM. The adequacy of suicide statistics for use in epidemiology and public health. Can J Public Health. 1991;82(1):38–42. https://www.jstor.org/stable/41989983.AccessedMay 17, 2021. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


