Abstract
Pancreatic cancer (PC) is often associated with a poor prognosis. Long-standing diabetes mellitus is considered as an important risk factor for its development. This risk can be modified by the use of certain antidiabetic medications. On the other hand, new-onset diabetes can signal towards an underlying PC in the elderly population. Recently, several attempts have been made to develop an effective clinical tool for PC screening using a combination of history of new-onset diabetes and several other clinical and biochemical markers. On the contrary, diabetes affects the survival after treatment for PC. We describe this intimate and complex two-way relationship of diabetes and PC in this review by exploring the underlying pathogenesis.
Keywords: Chronic pancreatitis, Diabetes, New onset diabetes, Pancreatic adenocarcinoma, Pancreatic cancer, Type 3c diabetes
Core Tip: Type 2 diabetes mellitus can increase the risk of pancreatic cancer (PC) and certain antidiabetic medications can modify this risk. New onset diabetes in combination with other clinical and biochemical markers can serve as an effective screening tool for PC. On the contrary, the glycaemic status affects the treatment outcome of PC. More awareness among clinicians is required about the two-way relationship between diabetes mellitus and PC.
INTRODUCTION
Pancreatic cancer (PC) is one of the few malignancies associated with a dismal prognosis. Its incidence is on the rise and is one of the leading causes of cancer-related death worldwide[1]. Similarly, type 2 diabetes mellitus (T2DM) accounts for a significant morbidity and mortality owing to a global increase in its incidence. Its prevalence is predicted to reach up to 700 million by 2045[2]. Longstanding diabetes has been regarded as a modest risk factor for PC. On the other hand, new-onset diabetes mellitus (NOD), especially after the 5th decade of life is often observed as a harbinger of an underlying PC. There is also a simultaneous increase in obesity worldwide, which plays a key role in development of both T2DM and PC[3]. Thus, this surge in diabetes and obesity prevalence may eventually increase the risk of PC in a significant number of population in near future[4].
Diabetes and PC have a multifaceted relationship. There are different types of diabetes as per the American Diabetes Association but two types of diabetes, namely T2DM and type 3c diabetes, merit attention in relation to PC[5]. T2DM is a chronic non-communicable disease characterised by hyperglycaemia resulting from the defective insulin secretion due to progressive beta cell dysfunction in the face of ongoing insulin resistance (IR)[5]. The diabetes associated with different exocrine pancreatic disorders is known as type 3c diabetes. The duration of DM has an important relationship with development of PC. However, the time duration cut-offs to define different types of diabetes are arbitrary and are varied. The time duration taken to define NOD in the context of PC is between 2-3 years[6]. On the contrary, when the diabetes is present for more than 2-3 years before the diagnosis of PC, it is considered as a long-standing T2DM. However, differentiating between this two entities is very difficult in a given subject of PC, since many patients of T2DM have a long asymptomatic undiagnosed period[5].
T2DM can have an impact on the outcome of different treatment modalities of the PC. Moreover, different drugs used for treating T2DM can affect the risk of PC as well. Metformin has gained particular attention in this context. In the appropriate clinical context, a recent worsening glycaemic profile requiring insulin might point towards the development of PC in the elderly diabetes subjects. The main obstacle in the diagnosis of PC in DM is to identify the candidates to be screened as routine evaluation for PC is not recommended in them. Ongoing research in identifying the screening population based on clinical characteristics and biomarkers and developing different models based on the combination of such parameters continues. In this background, we aim to review the current literature for unfolding the complex but intricate relationship between diabetes and PC.
SEARCH STRATEGY
The PubMed search was carried out for relevant articles by three authors (AR, JS, PM). The references of the pertinent articles were also searched for additional appropriate studies. The keywords and combinations included in the search were: ‘diabetes’; ‘new onset diabetes’; ‘pancreatic cancer-related diabetes’; ‘pancreatic cancer’ and ‘diabetes’; ‘new onset diabetes’ and ‘pancreatic cancer’; ‘long term diabetes’ and ‘pancreatic cancer’; ‘pancreatic ductal adenocarcinoma’ and ‘diabetes’; ‘metformin’ and ‘pancreatic cancer’ and ‘diabetes’; ‘Type 3c diabetes’ and ‘pancreatic cancer’. The search was restricted to only English literature and predominantly focused on the recent evidence. The appropriate articles to be included in this review were selected by SK, DN and RK.
RISK OF PC IN LONG-STANDING DIABETES MELLITUS
The evidence of association between NOD and the PC is consistent (see below); however, the evidence for risk of development of pancreatic ductal adenocarcinoma (PDAC) in long-standing diabetes is mixed. PDAC is the most common form of PC. Moreover, the risk is cumulative and is in continuum with the fasting blood glucose levels and the risk consistently increases from normal glucose tolerance to prediabetes to diabetes[7].
The increased risk of PC in long-standing T2DM has been suggested across different population of the world, including Asians[8-10]. A recent report involving a large population (n = 112818 females and 46207 males respectively) over 30 years of cumulative exposure showed an increased risk of PDAC with long-standing diabetes over time (age-adjusted hazard ratio [HR] 2.16 [95%CI: 1.78-2.60])[11]. Another recently published meta-analysis also suggested an increased PC related mortality with T2DM (relative risk [RR] 1.67; [95%CI: 1.30-2.14])[12].
The summary of the evidence suggests that the reported RR for developing PDAC in long-term diabetes is modest and varies between 1.4-2.1[8,13]. The risk may persist even after adjustment for obesity and smoking, two important and independent risk factors for PDAC[14]. Additionally, PDAC risk is significantly more in NOD and although the risk reduces subsequently, it may remain significant as the duration of the diabetes gets longer as per few meta-analysis[13,15]. However, a 2015 summary review of the available meta-analysis questioned the robustness of diabetes and PDAC association[16]. Importantly, other population based studies did not find any association between long-standing diabetes and the development of PDAC[17,18]. Thus, the elevated risk of PC in long-standing T2DM is confounded by the factor that may originate from a common soil of obesity and IR. Further, the role of different anti-diabetic medications as a risk modifier cannot be ignored while assessing the risk.
The Mendelian randomization (MR) studies looking into causal association between long-standing diabetes and PC have yielded conflicting results. While some studies showed causal association, others did not[19,20]. A pooled analysis performed on MR studies including 8374 PC patients by Yuan et al[21], found an odds ratio (OR) of 1.08 (95%CI: 1.02-1.14; P = 0.009) for this association. Although this evidence suggests a modest increase in the risk of PDAC in long-standing T2DM, more studies are required to confirm this association in future.
RISK OF PC IN TYPE 3C DIABETES
Chronic pancreatitis is defined as the chronic progressive inflammation and fibrosis of the pancreas caused by various aetiology and finally results in both endocrine and exocrine pancreatic dysfunction[22]. Diabetes is found in 35%-50% of subjects with CP in the observational studies[23-25] and the prevalence of DM increases with the increasing duration of CP and may reach up to 90%[25]. This type of diabetes is known as type 3c diabetes. Diabetes is more common in patients with pancreatic calcifications, pancreatic exocrine insufficiency and those who underwent surgery[23,24]. In a meta-analysis including fifteen studies (8970 patients), the incidence of DM was 30% and the prevalence increased after 5 years of CP diagnosis[26]. Diabetes in CP is often difficult to manage as a significant proportion of subjects require insulin therapy[23]. Importantly, CP itself is a risk factor for the development of PC. Kirkegård et al[27] had shown the risk of PC in CP varies with the duration of the disease and the effect estimates were 16.16, 7.90 and 3.53 at 2, 5 and 9 years after the diagnosis of CP, respectively. Another important entity is fibro-calculous pancreatic diabetes (FCPD), also known as tropical calcific pancreatitis, a relatively common cause of type 3c diabetes in certain tropical countries. FCPD also carries a very high risk for the development of PDAC[28].
Thus, it is important to look for CP in a given patient of diabetes and a closer follow-up with appropriate imaging is needed for diagnosis of PC in suspected cases. Since CP patients are often malnourished, progressive weight loss or anorexia despite adequate glycaemic control should alert the clinician for the possibility of PDAC.
RISK OF PC IN NEW-ONSET DIABETES
NOD has been considered as an important metabolic marker for the development of PDAC within the first 2-3 years of its diagnosis. NOD serves as a harbinger of PDAC in patients more than 45-50 years of age and hence calls for a careful follow up[29-31]. An earlier study demonstrated a 0.85% chance of development of PC within 3 years of diagnosis of diabetes in persons aged 50 years or more[32]. This study also showed that the risk was almost 8 times higher in patients with NOD. In a large cohort of 2.3 million Israeli population, a very high risk for developing PC was observed both in women and men (HR of 15.24 and 13.88 respectively) during the first year after the diagnosis of diabetes[33]. Two meta-analyses[13,14] also showed a 5-7 times elevated risk of PDAC in NOD, particularly within first year of diagnosis. Such an association was confirmed in different ethnicities like African Americans, Latinos[34] and Asians[35].
Agarwal et al[36] reported a very high prevalence of DM (68 %) in patients with PC compared to age matched other cancers subjects or non-cancer controls. Similarly, the number of NOD within the preceding 36 mo was markedly higher in PC than the other two groups (40% vs 3.3% vs 5.7%). About 50%-74% of the PC related diabetes is of recent onset (< 2-3 years duration)[6,37]. The prevalence of dysglycaemia in PDAC was more when standard oral glucose tolerance test (OGTT) was used instead of fasting glucose levels for diagnosis (78% vs 45%)[36,38]. The abnormalities in glucose metabolism are frequently missed in PDAC. The importance of making a preoperative diagnosis of glucose abnormality needs to be emphasized in this setting as it is shown to influence the surgical policy in up to 15% of patients[39].
It was also observed that a significant proportion of NOD in patients with PDAC resolved after pancreatic resection[37]. This indicates that PDAC by itself is causally related to the development of NOD, which is an early and specific biomarker for PDAC rather than a mere consequence. Besides the NOD, a deterioration of the existing glycaemic control in the form of elevated glycated hemoglobin (HbA1c) has also been associated with the development of PDAC[38].
MECHANISM OF DEVELOPMENT OF PC IN LONG-STANDING T2DM
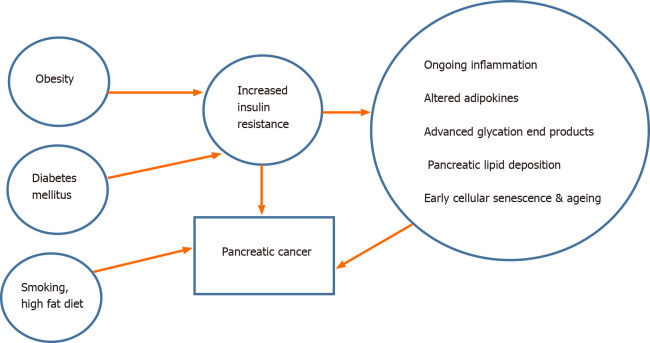
The potential mechanism responsible for the development of PDAC in long-standing diabetes is poorly understood (Figure 1). The proposed theories are: (1) IR and the resulting direct effect of hyperinsulinemia[40]. A very recent study performed in a large prospective cohort (> 0.5 million subjects with a median follow-up of 8.4 years) has shown that higher IR as assessed by homeostatic model assessment- IR (HOMA-IR) is an important and independent risk factor for PC related mortality even in patients without diabetes[41]; (2) Cancer promoting role of the IGFs[42]; (3) The potential role of hyperglycaemia itself to alter several biochemical pathways involved in the carcinogenesis; (4) The synergistic effect of obesity and inflammation (‘the common soil hypothesis’); and finally (5) Genetic predisposition to both these conditions. Experimental evidence is emerging to explain the molecular mechanism linking T2DM and PDAC. They include the roles of cellular senescence promoted by both T2DM and obesity[43], advanced glycation end products and its receptor[44], metabolic reprogramming by hyperglycaemia[45] and the interplay between non-alcoholic fatty pancreas development in the milieu of obesity and diabetes[46].
Figure 1.
Schematic diagram depicting the interplay of various proposed factors leading to development of pancreatic cancer in long-standing diabetes mellitus.
MECHANISM OF DEVELOPMENT OF NOD IN PC
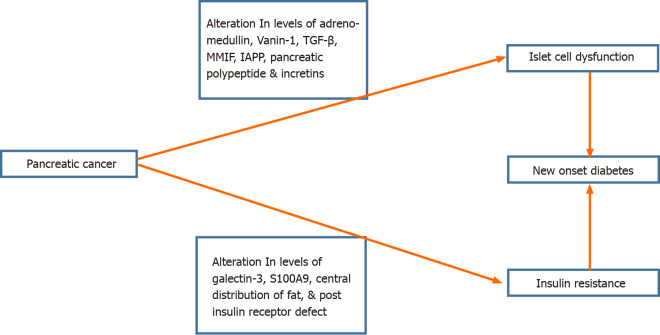
PDAC by itself induces a potential ‘diabetogenic’ state. PC-associated NOD is grouped under Type 3c diabetes, which also includes diabetes caused by CP[47]. The mechanisms linking PC and NOD are shown in Figure 2. The hypothesis that NOD is the result of destruction of the endocrine pancreas by PDAC is not a plausible explanation because NOD can be present even before PC becomes radiologically detectable[48] and has also been shown to improve after surgery[37]. Hence, it is essential to search for systemic mediators of NOD in PC; until now, only a few of them have been substantiated.
Figure 2.
Schematic diagram for the possible mechanism for the pathogenesis of new-onset diabetes mellitus in pancreatic cancer. IAPP: Islet amyloid polypeptide; MMIF: Macrophage migratory inhibiting factor; TGF-β: Transforming growth factor-beta.
Role of insulin resistance
Initial pioneering studies have shown that PC-associated NOD causes marked impairment in insulin action[49-51], more profound in patients who had diabetes[50]. Insulin mediated glucose entry at the level of skeletal muscle was particularly found to be impaired significantly[52]. Interestingly, Permert et al[49] also demonstrated an improvement in whole body insulin sensitivity following surgery by using a hyperglycaemic clamp, which is considered to be the gold standard in evaluation of insulin sensitivity.
Thus, IR is an important determinant of the PDAC-related NOD, but the underlying mechanisms remain to be further studied. Currently available studies have suggested that IR may be related to the post-insulin receptor defect, particularly involving glycogen synthesis and storage pathways[53]. Recent experimental studies have found that PDAC-associated exosomes can inhibit the insulin receptor signalling pathway downstream of the receptor causing IR in skeletal muscle[54]. Another proteomic study revealed that galectin-3 and S100A9, which are overexpressed in PDAC-related NOD, can induce IR and can also serve as markers in distinguishing this entity from T2DM[55].
The role of islet amyloid polypeptide (IAPP) in the development of IR was initially suspected but later its clinical utility was not proven[56]. It was also suggested that PDAC-related NOD is due to the differential effect of ectopic fat as PDAC is characterized by subcutaneous fat loss and preservation of visceral fat[57]. However, a recent study found that 30-18 mo before the diagnosis of PDAC, a significant proportion of patients had developed hyperglycaemia without any discernible change in the muscle or fat compartments[58,59].
Role of islet cell dysfunction
Pancreatic islet cell dysfunction is likely to be a crucial factor in the development of PDAC-related NOD. The morphology of the pancreatic islet in PC was recently characterized by Nagpal et al[60]. They demonstrated a significant reduction in islet density, beta and alpha cell area in PC compared to T2DM/control subjects. PC-related DM had lower IAPP deposition than T2DM. The lower IAPP deposit in PC related DM was also noted in an earlier study[61]. Future studies should explore the functional impact of such morphological changes.
Earlier studies have demonstrated a lower C-peptide response to glucagon stimulation suggestive of a beta cell secretory dysfunction in PDAC[62]. Beta cell function as assessed by HOMA-B was also found to be lower in PDAC patients with higher fasting glucose and diabetes[63]. In experimental studies, it was shown that beta cell in PDAC secrete increased amount of amylin preferentially while insulin secretion is diminished[64-66].
There is an experimental evidence for inhibition of insulin secretory function of the beta cell by adrenomedullin, which is released from PC-associated exosomes[67]. The role of adrenomedullin inhibiting beta cell insulin secretion in response to glucose was previously shown[68]. Moreover, adrenomedullin upregulation was noted in PC and its role in IR was also demonstrated[68]. Adrenomedullin was found to be a mediator for the increase in the exosome-induced lipolysis of the subcutaneous fat in PDAC[69]. The role of adrenomedullin as a screening biomarker is currently investigated in a prospective cohort study to identify patients with NOD and underlying PDAC[70].
Vanin-1 helps in hydrolysis of pantetheine and synthesis of vitamin B5 and cysteamine, which are required for lipid, energy and coenzyme A metabolism[71]. The role of vanin-1 is implicated in PDAC-induced paraneoplastic islet cell dysfunction, predominantly mediated by decreasing glutathione and elevating oxidative stress[72]. The same group earlier identified vanin-1 as a distinct marker of PDAC-related DM based on gene expression profile of the peripheral blood[73]. The role of transforming growth factor-beta (TGF-β) in the destruction of pancreatic beta cell had also been shown in animal studies[74,75] The macrophage migratory inhibiting factor was overexpressed in PDAC and was shown to decrease the beta cell secretory function[76].
A recent study has demonstrated that the markers of beta cell de-differentiations are consistently higher in non-diabetic PDAC patients suggesting the possible role of beta cell reprogramming in the early beta cell dysfunction even before the appearance of hyperglycaemia[77]. This dedifferentiation might be potentiated by the inflammatory milieu triggered by the PDAC.
Studies relating to alpha-cell function with PDAC-related DM are lacking. One study showed a higher glucagon/insulin ratio as a marker of NOD in PDAC[78]. Another small study revealed hyperglucagonemia in PDAC-related DM patients[79]. However, further studies are required to delineate the role of alpha cell dysfunction in PDAC-related diabetes.
Pancreatic polypeptide (PP) is released from the PP cells predominantly located in the head of the pancreas. PP cells have an important paracrine action including suppressive effect on glucagon secretion from alpha cell. Interestingly, one study had reported diminished PP response at 30 min following a mixed meal challenge in PC-related DM patients as compared to T2DM[80]. This was seen in tumours located in the ventral part of the pancreas. However, another study did not find any difference in fasting PP levels between PDAC-related DM, CP and T2DM[81]. Further studies should explore the role of PP in NOD and its use as an effective screening tool for PDAC.
Very few studies have evaluated the role of incretin hormones in the pathogenesis of PDAC-related DM. Interestingly, one study reported a lower gastric inhibitory polypeptide (GIP) and PP secretion in PDAC patients with diabetes as compared with T2DM patients, without any difference in glucagon-like peptide 1 (GLP-1) response[82]. Importantly, those with NOD or prediabetes with weight loss (> 2 kg) had significantly lower GIP. However, further studies are required to confirm this association. In-vitro studies had demonstrated that a lower GIP and GLP-1 response might be related to the inhibitory effect of the PDAC-exosomes on the proprotein convertase subtilisin/kexin type 1/3 enzyme which is responsible for cleaving the pro-glucagon molecule to generate the incretin peptides[83]. This study suggested the possibility of pancreatic exosome mediated dysfunction of the incretin hormones in the gut.
EARLY DETECTION AND/OR SCREENING MODELS FOR PC IN DM
Clinical indicators
Till now, the recommendations regarding systemic screening of a person with diabetes to identify PDAC are not standardized. But, whom to screen and how to screen is not defined clearly by any guidelines till now to the best of our knowledge. So, it is necessary to develop a screening tool based on NOD and other risk factors. Since the yield of screening in such population is low, whether systematic screening is cost-effective and practically feasible remains an area of active debate.
The screening of patients with diabetes for PC is based on filtering of diabetes patients based on presence of associated clinical factors or level of biomarkers or a combination of such factors. NOD within 3 years of diagnosis increases the risk of PDAC 6-8 times more than the general population, but the prevalence of PDAC in such circumstances is low (0.8%-1%)[32].
It is a challenge to differentiate PC induced NOD from the more commonly encountered T2DM based on clinical and biochemical factors in clinical practice (Table 1). There are many overlaps between these two entities[84-86]. Munigala et al[87], identified age ≥ 65 years, heavy smoking, non-obese status at diagnosis, history of CP or gallstones as different risk factors of PC in a prospective cohort of NOD. One study reported a 40% higher risk of PC in patients with dyslipidaemia, although the association with specific lipid parameter was not mentioned[88].
Table 1.
Factors that can help in differentiating pancreatic cancer associated new onset diabetes from type 2 diabetes mellitus
|
Clinical indicators
|
Biochemical markers
|
| Age > 65 yr | Carbohydrate antigen 19-9 |
| Heavy smoker | Galectin 3 |
| Low body mass index | S100A9 |
| History of chronic pancreatitis or gall stone disease | Insulin like growth factor-1 |
| Recent worsening of hyperglycemia in an elderly patient | Osteoprotegerin |
| Weight loss associated with diabetes onset | Pancreatic polypeptide |
| Loss of subcutaneous fat and muscle mass in imaging studies like dual energy X-ray absorptiometry or magnetic resonance imaging | Thrombospondins- 1 |
| Vanin 1 | |
| Matrix metalloproteinase-9 | |
| MicroRNAs |
Two other important factors that may provide a clinical clue for PC associated NOD are weight loss and worsening of hyperglycaemia. A continued weight loss in the presence of NOD was observed in a greater number of PC patients (59% vs 30%) than T2DM[89,90]. The amount of weight loss was also more in PC patients (8.3 ± 8.3 kg vs 0.8 ± 4.8 kg). Mueller et al[91] showed that weight loss of more than 10% had an adjusted OR of 3.58 (95%CI: 2.31-5.54) for development of PC. The presence of weight loss of more than 15% was not only associated with an increased odds of PC in NOD[91] but also in patients with long-standing diabetes[92]. Olson and colleagues showed that NOD and severe weight loss often occurred together before the diagnosis of PC[90]. Chen et al[11] observed that in a subject with NOD, when weight loss was unintentional or occurred in an individual with body mass index (BMI) less than 25 kg/m2, then it substantially increased the risk of PC. Hence, weight change should be actively sought in elderly diabetes and warrant further investigation for PC.
Sah et al[58] reported worsening of hyperglycaemia, in the 18 to 6 mo before the diagnosis of PDAC. Similarly, rapid elevation of both blood glucose and HbA1c was observed by Huang et al[93] in the months preceding the detection of PC. The worsening of hyperglycaemia more often required the use of insulin treatment[90]. Thus, rapid deterioration of glycaemic control should alert a physician to screen for PDAC.
Another important feature is the loss of muscle mass, which is also known as sarcopenia. Sah et al[58] observed loss of subcutaneous adipose tissue even 6 mo before PDAC diagnosis. It was suggested that the preferential loss of subcutaneous adipose depot with relative preservation of the visceral adipose tissue might explain the IR and the worsening of glycaemic status[57]. However, a recent study did not find any difference in the prevalence of cachexia, skeletal muscle loss or weight loss between PC patients with or without DM[59]. Overall, sarcopenia suggests advanced disease and often portend poor survival, but its relationship with diabetes development need to be assessed in future studies[94].
Screening models
Interestingly, another upcoming approach is the development of a predictive model based on easily available clinical features in NOD. This model can identify NOD patients to be screened for PC and thus improve the detection rate while significantly decreasing the cost[30]. Sharma et al[95] came up with a model known as the Enriching New-Onset Diabetes for Pancreatic Cancer (END-PAC). This is a risk prediction model based on three different factors: change in weight, change in blood glucose, and age at the onset of DM. A score of 3 or more identified 78% of patients (n = 7/9) with 85% specificity. In the initial model, a score of more than 3 predicted a significantly increased risk of PDAC (4.4-fold) and a low END-PAC score of less than 0 had a very low risk of development of PDAC. This model was further assessed in a retrospective cohort of NOD patients (n = 13947) and 2% of high risk population (62 out of 3038) were diagnosed with PDAC within 3 years yielding a sensitivity and specificity of 63% and 78% respectively at the score level of 3 or higher[96]. The positive predictive value (PPV) and negative predictive value were 2.0% and 99.7%, respectively. Another model was proposed by Boursi et al[97] based on The Health Improvement Network, which is a large primary care electronic research database from the United Kingdom. This prediction model included several easily available clinical parameters like age, BMI, change in BMI, presence or absence of smoking, use of proton pump inhibitors and other anti-diabetic medication including metformin. The laboratory parameters include levels of hemoglobin, creatinine, and alkaline phosphatase, HbA1c and cholesterol. The area under the curve (AUC) for the final model was 0.82 (95%CI: 0.75–0.89) and at a risk threshold of 1% for screening for PDAC, around 6% of patient with NOD would have to undergo systemic screening. The sensitivity, specificity and PPV at this level were 44.7%, 94.0% and 2.6%, respectively. Thus, though these model systems are encouraging and can narrow down on the screening population, they are limited by poor sensitivity and lower PPV[30] and further improvement is required before routine clinical use. Recently, a protocol of a multicentric, prospective observational study (NODES Trial) has been published, which intends to follow up new-onset (≤ 6 mo) diabetes patients over 60 years of age with both clinical and valid biomarkers[98]. The study also aims to evaluate for biomarkers that can distinguish patients with PDAC more precisely. Such studies will be invaluable in understanding and defining a screening protocol in NOD patients to identify PDAC as early as possible.
BIOMARKERS IN THE SCREENING OF PC IN DM
The role of different biomarkers in assisting the early diagnosis of PC among DM patients is crucial. A plethora of studies on different biomarkers have been published in the literature, though till now, none of them have reached the routine clinical use. The search for an easy-to-use clinically useful and cost-effective marker is still ongoing. Finding of suitable biomarkers is a difficult task in a relatively uncommon disease like PC and moreover, presence of diabetes can confound the measurements of different biomarkers in such setting[30]. A detailed discussion on this topic is out of the purview of this article, but a brief description on the latest biomarkers are discussed here. The proposed biomarkers are either measured in the blood or tissue fluids and they are the result of the ‘multi-omics’ studies involving proteomics, genomics and metabolomics.
Hormones involved in glucose homeostasis
The biomarkers, which draws our attention first is the biomarkers related to the glucose metabolism. Sharma et al[99] have shown that rising fasting plasma glucose itself predates the development of PDAC (36-60 mo before PDAC diagnosis) and is often related to the size of the tumour. Though fasting blood glucose levels increased concordantly with the volume of the tumour, no such relationship with the tumour gradation was reported. Another study reported a higher serum glucagon/insulin ratio with a cut-off of 7.4 ng/mIU could differentiate PC induced NOD from T2DM with 77% sensitivity and 69% specificity[78]. A study demonstrated a higher glucose stimulated glucagon in PC patients with DM, suggesting glucagon as a potential biomarker[79].
Incretins involved in glucose homeostasis
The role of different gut polypeptides involved in the glucose homeostasis was also studied in PDAC patients. It was found that a significantly lower plasma concentrations of GIP and PP in patients with PC irrespective of the degree of glucose intolerance as compared to the T2DM and normal healthy controls[82]. A diminished PP response to a mixed meal was also observed earlier in PDAC associated diabetes in a small study[80]. However, another study could not find a difference in fasting PP levels between PDAC patients with or without diabetes and T2DM[81]. Thus the blunted PP response in PDAC can serve as an important tool for screening for PDAC, but the time-line is not well established and studies with a larger sample size may further consolidate PP as an important biomarker.
Carbohydrate antigen 19-9
A study by Choe et al[100] has shown that in asymptomatic NOD patients, a higher carbohydrate antigen 19-9 (CA19-9) levels above the upper normal limit had a 5.5 times risk of developing PC within 2 years of diagnosis. Another retrospective analysis showed similar results and found that the odds for development of PC in NOD patients with elevated CA19-9 was consistently higher, particularly in patients with elevated bilirubin levels[101]. Murakami et al[102] proposed that a cut-off of serum CA19-9 level of 75 U/mL can discriminate between patients with diabetes with or without PC. At this cut off, the sensitivity and specificity of CA19-9 for PC was 69.5% and 98.2%, respectively, while the AUC was 0.875 (95%CI: 0.826-0.924). A combination of elevated CA19-9 and carcinoembryonic antigen was also shown to detect PC among DM patients[103]. However, it is important to note that the utility of CA19-9 may be limited by the fact that it is affected by the levels of glycemia. CA19-9 levels must be interpreted in the context of ongoing glycaemic control and patients with diabetes per se may have elevated CA19-9[104].Thus there is a need to optimize the CA19-9 cut off level in DM patients for PC detection.
Thrombospondin -1
Another promising biomarker is thrombospondin-1 (TSP-1), a multimeric protein with anti-angiogenic properties. TSP-1 levels were found to be lower in PDAC patients, particularly those with diabetes as compared to non-diabetes and this lower levels were detected even 24 mo before the diagnosis of PDAC[105]. According to this study, TSP-1 levels in combination with CA19-9 yielded an AUC 0.86 in the detection of PDAC. Importantly, a lower TSP-1 levels were also noted in PDAC associated diabetes but not in the long-standing T2DM.
Vanin-1 and matrix metalloproteinase 9
Vanin-1, a protein involved in the oxidative stress pathway was found to be associated with paraneoplastic islet cell dysfunction (see earlier) and can serve as a potential biomarker in detecting PC among DM patients. Huang et al[73] have shown that the levels of Vanin-1 genes were significantly upregulated in PDAC and an elevated levels of both vanin-1 and matrix metalloproteinase 9 (MMP9) in serum using quantitative real-time polymerase chain reaction could differentiate PDAC associated diabetes from T2DM. The AUC for the combination of both Vanin-1 and MMP9 was 0.950 with a sensitivity of 95% but the specificity of 76%. A combination of CA19-9 and MMP9 was also found to be helpful in discriminating PDAC-related diabetes from T2DM with an AUC of 0.886[106].
Galectin-3 and S100A9
Galectin-3 is a β-galactoside–binding lectin involved in the proliferation, migration and invasion of PC cells[107] whereas S100A9 protein is involved in the inflammation through toll-like receptor-4[108]. Liao et al[55] have shown that levels of both galectin-3 and S100A9 were higher in PDAC related DM than T2DM. They also found that the serum levels of both galectin-3 and S100A9 proteins can differentiate between PDAC related DM and T2DM with the AUC of 0.83 (95%CI: 0.74–0.92) and 0.77 (95%CI: 0.67–0.87) respectively.
MicroRNAs
Alteration in the profile of serum microRNAs (miRNAs) have been postulated as important biomarkers in recent times. One study reported that a panel of six miRNAs (miR-483-5p, miR-19a, miR-29a, miR-20a, miR-24, miR-25) could differentiate between PDAC related DM and T2DM[109]. The AUC for the differentiation of these two entities was 0.885 (95%CI: 0.784-0.986). However, the micro RNA profiles (miR-192, miR-196, miR-200, miR-21, miR-30 and miR-423) were found to be similar in PC patients with or without DM in a study by Skrha et al[110]. This list of miRNAs will be increasing in the future but the potential ones should come out from the prospective follow-up studies of the NOD patients.
Metabolomics
Studies have used extensive metabolomics approach either through liquid or gas chromatography and mass spectrometry or nuclear magnetic resonance imaging technique to identify various metabolites in order to identify specific biomarkers that can differentiate between PDAC-related DM and T2DM. One study revealed a distinct signature of 62 different serum metabolomes in PC related DM as compared to T2DM[111]. Out of them, two metabolites namely N-Succinyl-L-diaminopimelic-acid and PE (18: 2) had shown good sensitivity (93.3%) and specificity (93.1%) for PC in the logistic regression analysis. A recent nuclear magnetic resonance based study also identified a panel of eight metabolites with good accuracy (more than 80%) in the discrimination of PC and long-standing T2DM patients[112]. In future, possibly a panel of metabolites will help us to improve the precision medicine in identifying the cases requiring close follow-up for detection of PC among diabetes patients.
Other new biomarkers
There have been several other biomarkers proposed for the differentiation of the PDAC associated diabetes from T2DM. A very recent analysis of several immune related proteins including cytokines, chemokines and adhesion molecules revealed that a panel of different molecules (GM-CSF, IL-31, RANTES, RESISTIN, FASL, & ICAM1) were different between PDAC related DM and T2DM with an AUC of 0.96 (0.93–1.00)[113]. This study paved a new way in the screening of PC in diabetes patients. The other tools that can be used to screen PC are plasma free amino acid index[114], combination of either neutrophil-to-lymphocyte ratio or platelet-to-lymphocyte ratio and CA19-9[115], angiopoietin-like protein 2[116] among others with reported variable AUC.
In summary, although a great numbers of promising biomarkers have been studied to detect PC early in diabetes patients, a very few have reached routine clinical use as of now. As more translational research is emerging, the main requirement of a panel of clinically useful biomarkers for early detection of PC in DM will be fulfilled in near future.
DIABETES AND THE TREATMENT OUTCOMES OF PC
Diabetes has an important role to play as a prognostic marker in PC patients. Pancreatectomy is the initial management strategy in PDAC. Currently, the evidence that diabetes may portend an unfavourable impact on the overall outcome of PC, particularly after surgery is not concrete[117]. Whether the treatment of the diabetes modifies this risk is also not clear at present. Hank et al[118] showed that diabetes subjects had a poor median overall survival (18 vs 34 mo; P < 0.001). Moreover, diabetes was associated with higher 30-d mortality (3.2% vs 0.8%; P = 0.019). Importantly, a larger tumour size, a greater number of lymph node involvement and more peri-neural invasion were seen in diabetes patients with PC. A negative association of diabetes with overall survival was also noted in a meta-analysis[119]. However there are studies which do not agree on such association[120,121], and rather showed paradoxical reduction in the risk of death[122]. A 2013 review showed that diabetes patients had a higher risk of post-operative complications (45% vs 35%)[123]. Baseline HbA1c more that 6.5%-7.0% was also found to be associated with a shorter survival[124,125].
Long-standing DM vs new onset DM
Although studies have shown a poor outcome in all diabetes patients, the relative role of duration of diabetes on PC outcome needs further clarification. Very few studies have shown the stratified analysis based on diabetes duration. Long-standing diabetes was found to have an association with diminished survival in prospective studies[126]. This was also confirmed in a meta-analysis[127] involving 18 studies (16181 patients). Several other studies[117,128,129] did not find any significant effect of long-standing diabetes on the survival in PC. Jeon et al[117] reported impact of long-term diabetes on decreased survival in those with resectable PDAC (HR, 1.42; 95%CI: 1.13–1.78) but not in advanced disease suggesting a role of staging in the outcome. It is important to note that the association of diabetes with prognosis became non-significant in most of the studies, after adjusting confounders like age, gender, BMI, smoking status and staging of the disease[128]. The evidence that diabetes patients can have a relatively larger pancreatic tumour size is well established[118,121,130,131].
On the other hand, the evidence is more consistent for a poorer outcome associated with NOD. A 2017 meta-analysis showed that only NOD was associated with shorter survival but not long-standing DM[119]. Similarly, other studies found that only NOD was a significantly independent predictor of decreased survival[129,132]. Importantly, Lee et al[133] have shown that NOD carries a higher risk of recurrence after pancreatic resection and may be a factor responsible for the poorer outcome. In contrast Jeon et al[117] did not find any impact of NOD on survival. Another point to consider is that whether post-surgery improvement of NOD has any impact on outcome. Though a study reported increased survival in patients where diabetes was resolved following surgery[134], future studies should substantiate this finding.
Impact of DM on the outcome after chemotherapy
Studies assessing the impact of diabetes in PC patients receiving chemotherapy have shown that, a prior diabetes status might be associated with a higher risk of death. Kleef et al[130] demonstrated a higher mortality rate in diabetes patients receiving adjuvant chemotherapy [HR 1.19 (95%CI: 1.01-1.40)]. Similarly, Hank et al[118] showed that median overall survival was lower in diabetes patients who received neo-adjuvant chemotherapy as compared to non-DM patients (18 mo vs 54 mo; P < 0.001). Another study showed diabetes to further add to the poorer outcome in metastatic disease treated with gemcitabine[135]. A meta-analysis looking at the impact of diabetes on the outcome following chemotherapy in PC (1034 with DM and 3207 without DM) demonstrated a lower survival and higher risk of death after chemotherapy in DM patients[136]. A high preoperative HbA1c was also found to be associated with non-completion of adjuvant chemotherapy and a higher risk of metastasis[137]. Diabetes also affects the survival in very advanced PC patients receiving palliative chemotherapy[138].
The mechanism behind the poorer outcome in PC with diabetes is not certain. Diabetes is associated with larger tumour size and hence a higher tumour stage. Hyperglycaemia has been shown to hasten the tumour development via sterol regulatory element binding protein 1 pathway[139]. There is also suggestion for an alteration in the tumour microenvironment in the presence of an elevated blood glucose level. Indeed, experimental studies have shown that hyperglycaemia increases the metastatic ability of the PC through aggravated hypoxia[140] or by increasing the perineural invasion[141]. The role of glycaemic variability is also suggested as a risk factor for promoting local invasion and metastasis via the retinoic acid receptor beta-runt related transcription factor 3-type VI collagen alpha 1 chain pathway[142].
EFFECT OF PC TREATMENT ON DIABETES
There is a complex relationship existing between patients undergoing surgery for PC and their glycaemic status. Glycaemic control is expected to worsen following pancreatectomy considering a significant loss of beta cells. However this is not often observed in clinical practice, particularly in patients with NOD after surgery. Studies[143-146] have either shown a significant improvement in their glycaemic control (75%) or resolution of NOD (20%-65%) after pancreatic surgery. It has also been reported that resolution of preoperative NOD after pancreatectomy may be a sign of a favourable outcome[134]. NOD has also been described in 15%-20% of patients[143,145,146] after surgery. One study reported deterioration of the glycaemic control in up to 40% post-operatively[147],when formal tests like OGTT and i.v. glucagon stimulation test were used. In the meta-analysis by Beger et al[148], cumulative incidence of NOD was found to be 15.5% after pancreatico-duodenectomy for malignant pancreatic tumours. Hence, it is necessary to assess the glycaemic status after pancreatico-duodenectomy even in those with pre-operative normoglycemia to achieve a better metabolic control after the surgery.
ANTI-DIABETIC MEDICATIONS AND PC
Since diabetic patients will be receiving several medications for controlling hyperglycaemia, it is important to consider their effects in the context of PC. There are many excellent reviews[149] already available in this regard and we highlight salient points based on the recent available evidence.
Metformin
Metformin has garnered a lot of interest in recent times due to its anti-cancer effect and PC is not an exception. Metformin is the first line drug of choice for treating T2DM. A plethora of studies have looked into the three key aspects of metformin and their role in PC. They are: (1) Metformin as a risk modifier of PC development in T2DM[150-152]; (2) Effect of metformin on the overall survival following therapy[153]; and (3) Metformin as an adjuvant therapy in diagnosed PC[154].
Studies regarding metformin treatment as a risk modifier of PC have yielded mixed results. While some studies have shown risk reduction of PC in metformin users[155], other studies did not find such a beneficial impact of metformin in PC risk reduction[156,157] and even reported an increased association risk in metformin users[151]. Though earlier pooled analysis[150] had shown a decreased risk of PC, the studies included in those analysis were mostly retrospective and met with the significant lead time bias. Another complicating issue regarding the risk estimation is that the long duration of diabetes already present is itself a potential risk for PC and the NOD heralding the onset of PC often complicates the scenario further. Therefore, recent studies with better statistical designs are warranted to establish the role of metformin in PC prevention in a concrete manner.
Animal studies have shown that metformin decreases the PC cell proliferation[158,159], its invasiveness[160] and thereby reduces the metastatic potential of PDAC[161]. Studies have also shown that metformin has a sensitization effect on chemotherapy, particularly gemcitabine[161,162]. The inhibition of TGF-β pathway is one of the several underlying mechanisms proposed to explain these effects[75,160]. Considering this finding, metformin might be expected to have beneficial result in PC. However, the clinical studies performed to assess the benefits of metformin have shown conflicting results. While few studies have suggested the survival benefit of metformin in PC patients[163-165], two phase 2 randomized controlled trials (RCTs)[166,167], observational studies[168,169] and other meta-analysis[170] refuted such finding.
The benefits of metformin observed in some earlier studies may be attributed to the immortal time bias that is inherent to meta-analysis studies[171]. Indeed, a meta-analysis taking into the account of immortal time bias did not show any additional survival benefit of metformin in DM patients with PC[170]. According to this meta-analysis, the effect size of reduction in the risk of survival was exaggerated by 18%. Again, the null effect of metformin on survival shown by the two RCTs was also flawed by the fact that metformin treatment was started late in the disease course and metastasis has already happened, leaving a small room to assess for the effect of metformin on survival outcome[166,167]. For this reason, it is important to focus on this area with well-designed RCTs in an earlier stage, including both DM and non-DM population. Further evidence is required to recommend treatment with metformin in PDAC patients with concurrent DM. Mild hyperglycaemia with obesity in early stage PC patients may be an ideal indication to start metformin. In summary, the risk reduction of PC and the overall survival following metformin therapy are not observed in recent well designed studies with improved statistical analysis taking into consideration of immortal time bias. Future phase 3 RCTs will be helpful in this context, mainly in selected PC candidates[172].
Insulin and insulin secretagogue
Insulin has a definite role in the pathogenesis of PDAC as hyperinsulinemia and IR are important risk factors for the development of PDAC (see above). Whether clinical use of insulin has any impact on PDAC development is a contentious issue. Long-term insulin use was not found to be a risk factor of PDAC development[173]. On the other hand, short-term insulin user (< 3-5 years) was found to have an elevated risk of PDAC (OR 5.60, 95%CI: 3.75-8.35)[173]. Perhaps this data reflects the other way around. It is likely that the worsening of hyperglycaemia or the severe hyperglycaemia requiring insulin injection might reflect the onset of PDAC or effect of PDAC on the glycaemic control. Whereas few meta-analysis have suggested an elevated risk of PC in insulin users[174] the same evidence was not found in other studies[175] and also with newer insulin like glargine insulin[176]. In terms of survival benefit, insulin use had no impact on survival as shown in recent studies[120,177].
Insulin secretagogues like the sulfonylureas (SU) are also implicated as a risk modifier of PC. There are only few studies that have specifically looked into the link between SU and PC. However, studies including nation-wide cohorts[178], and meta-analyses[175] had pointed that SU use is associated an elevated risk of PC (OR varies between 1.5-1.7). However, with newer generation SU data is sparse and this association is further complicated by the effect of concurrent obesity and IR on the development of PC. Moreover, earlier analyses are met with significant methodological flaws and heterogeneity among studies[179].
Incretin based therapies: DPP-4 inhibitors and GLP-1 receptor agonist
Incretins are hormones secreted from the intestine and have a significant impact on the glycaemic control. GLP-1 analogues and inhibitors of dipeptidyl peptidase-4 (DPP-4) enzyme are established therapies for T2DM in clinical practice. Although there was a concern of acute pancreatitis and PC associated with their use from the initial preclinical[180] and adverse database review[181] studies, data regarding the risk for PC was inconsistent. Hence, both United States Food and Drug Administration and European Medicines Agency advised on continuous follow-up of patients started on these therapies for these two adverse events[182].
Earlier meta-analysis also did not find an increased risk of PC with DPP-4i treatment group[183]. Moreover, the recent meta-analysis involving 157 trials reporting PC (66897 patients in DPP-4 inhibitors and 61597 patients in control group) showed no associated risk with DPP-4 inhibitors use (OR: 0.84 [95%CI: 0.69-1.03], I2 [for heterogeneity] = 0%). This association was found across different types of DPP-4i molecules and thus possibly reflects a class effect. Data from large population based studies also showed similar reassuring findings[184]. Due to several limitations of the trials like a shorter follow-up, reporting bias, small number of PC cases, it is important to keep a watch over this association in future. Moreover, one meta-analysis of the large cardiovascular outcome trials on DPP-4i showed an 75% increased risk of pancreatitis[185]. Such findings warrant longer duration follow-up studies and continued vigilance. A study[186] has shown that DPP-4i may be associated with increased risk of pancreatitis and PC in short-term without any relationship with exposure duration, thus implying that it might be the result of reverse causality rather than the DPP-4i exposure itself.
Similarly, more data are now available for different GLP-1 analogues. The larger cardiovascular outcome trials did not find any elevated risk of PC in GLP-1 analogue users[187,188]. Consequently, an updated pooled analyses from the cardiovascular outcome trials also did not show an excess risk of PC or pancreatitis with use of GLP-1 analogues[185]. However, it is noteworthy that such trials are not primarily meant to detect any increased malignancy risk. Thus, although the data is reassuring, a continued vigilance is warranted.
Other drugs (thiazolidinediones and sodium-glucose co-transporter type 2 inhibitors)
The other antidiabetic drugs are thiazolidinediones (TZD) and sodium-glucose co-transporter type 2 (SGLT-2) inhibitors. TZDs like pioglitazone and rosiglitazone primarily act through activation of the peroxisome proliferator-activated receptor-gamma pathway. This activation has direct and indirect implications in the PC biology. TZDs have shown to have inhibiting effect on several aspects of PC including cell proliferation and metastasis[189-191]. It also has the potential to modify the risk of PC through insulin sensitization, modification of the obesity and the inflammation[192]. However, these promising experimental findings of benefits of TZD have been replicated in clinical studies with mixed results.
While two meta-analyses did not find any association between TZD use and the risk of PC[175,193], one population based study had shown a protective role of TZDs against PC[178]. On the other hand, Lewis et al[194] demonstrated that TZD use might be associated with an increased risk of PC. As far as the prognostic role is considered, TZDs did not have any effect on survival[195,196].
SGLT2-inhibitors are the newest class of oral antidiabetic medication and have already made its place in the therapeutic algorithm of diabetes, owing to its cardiovascular benefits. Functional SGLT-2 are detectable in PC cells and hence, it was hypothesized that SGLT-2 inhibitors can inhibit tumour growth by blocking the entry of the glucose within the cell[197]. An experimental study has shown canagliflozin, a SGLT-2 inhibitor to inhibit PC growth[198]. However, clinical studies are yet to confirm its effect on PDAC survival.
CONCLUSION
In this review, we have summarized the intricate relationship between DM and PC. Long-standing diabetes is considered as a risk factor for development of PC. On the other side, NOD in an elderly patient can be a manifestation of underlying PC. Though the exact mechanism remains to be eluded in future studies, the mechanism of the development of NOD in PC involves both IR and islet cell dysfunction. Diabetes has also been suggested to have an unfavourable effect on the overall survival of patients with PC.
Early detection of PC in a patient with DM is of utmost important and is a clinically challenging task. PC has a low prevalence in both general population and diabetes subjects. Thus, devising a strategy to screen diabetes population for PC is the need of the hour. There is an urgent need for a clinically useful and cost-effective screening tool to detect PC among patients with long-standing diabetes. The epiphenomenon of NOD can subserve as a potential clue along with recent onset worsening of glycaemic control and a continued weight loss. Apart from clinical pointers, many biomarkers have also been found to differentiate PC related DM from the commoner T2DM. Moreover, different clinical and biochemical parameters have been combined to develop different screening tools. Proper screening and early recognition of PC can improve the outcome of this devastating neoplasm.
Can we delay the occurrence or halt the progression of PC in a patient of DM? The strategies to improve IR like regular physical exercises, intermittent fasting, or low-fat diet can be explored in future. Moreover, other healthy behaviours like smoking cessation should be implemented in patients with long-standing DM. The role of glucose lowering medications like metformin in delaying the occurrence of PC needs to be explored further in longitudinal studies.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to report.
Manuscript source: Invited manuscript
Peer-review started: January 28, 2021
First decision: May 2, 2021
Article in press: July 7, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen R, Li J, Yang Y S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Ayan Roy, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India; Department of Endocrinology and Metabolism, All India Institute of Medical Sciences, Jodhpur 342005, India.
Jayaprakash Sahoo, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India. jppgi@yahoo.com.
Sadishkumar Kamalanathan, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Dukhabandhu Naik, Department of Endocrinology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Pazhanivel Mohan, Department of Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
Raja Kalayarasan, Department of Surgical Gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605006, India.
References
- 1.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2. IDF Diabetes Atlas 9th edition 2019. Sep 14, 2020. Available from: https://diabetesatlas.org/en/
- 3.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 4.Rawla P, Thandra KC, Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. 2019;12:285–291. doi: 10.1007/s12328-019-00953-3. [DOI] [PubMed] [Google Scholar]
- 5.and Classification of Diabetes Mellitus. Diabetes Care. 2014;37:S81. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 6.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo DH, Han KD, Park CY. The Incremental Risk of Pancreatic Cancer According to Fasting Glucose Levels: Nationwide Population-Based Cohort Study. J Clin Endocrinol Metab. 2019;104:4594–4599. doi: 10.1210/jc.2019-00033. [DOI] [PubMed] [Google Scholar]
- 8.Pang Y, Kartsonaki C, Guo Y, Bragg F, Yang L, Bian Z, Chen Y, Iona A, Millwood IY, Lv J, Yu C, Chen J, Li L, Holmes MV, Chen Z. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140:1781–1788. doi: 10.1002/ijc.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magliano DJ, Davis WA, Shaw JE, Bruce DG, Davis TM. Incidence and predictors of all-cause and site-specific cancer in type 2 diabetes: the Fremantle Diabetes Study. Eur J Endocrinol. 2012;167:589–599. doi: 10.1530/EJE-12-0053. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wu F, Saito E, Lin Y, Song M, Luu HN, Gupta PC, Sawada N, Tamakoshi A, Shu XO, Koh WP, Xiang YB, Tomata Y, Sugiyama K, Park SK, Matsuo K, Nagata C, Sugawara Y, Qiao YL, You SL, Wang R, Shin MH, Pan WH, Pednekar MS, Tsugane S, Cai H, Yuan JM, Gao YT, Tsuji I, Kanemura S, Ito H, Wada K, Ahn YO, Yoo KY, Ahsan H, Chia KS, Boffetta P, Zheng W, Inoue M, Kang D, Potter JD. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60:1022–1032. doi: 10.1007/s00125-017-4229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan C, Babic A, Khalaf N, Nowak JA, Brais LK, Rubinson DA, Ng K, Aguirre AJ, Pandharipande PV, Fuchs CS, Giovannucci EL, Stampfer MJ, Rosenthal MH, Sander C, Kraft P, Wolpin BM. Diabetes, Weight Change, and Pancreatic Cancer Risk. JAMA Oncol. 2020;6:e202948. doi: 10.1001/jamaoncol.2020.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, Yates T, Khunti K, Davies MJ, Zaccardi F. Association of Type 2 Diabetes With Cancer: A Meta-analysis With Bias Analysis for Unmeasured Confounding in 151 Cohorts Comprising 32 Million People. Diabetes Care. 2020;43:2313–2322. doi: 10.2337/dc20-0204. [DOI] [PubMed] [Google Scholar]
- 13.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol. 2014;21:2453–2462. doi: 10.1245/s10434-014-3625-6. [DOI] [PubMed] [Google Scholar]
- 14.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Tang H, Hassan MM, Holly EA, Bracci PM, Silverman DT. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22:189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 17.Gullo L, Pezzilli R, Morselli-Labate AM Italian Pancreatic Cancer Study Group. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 18.Liao KF, Lai SW, Li CI, Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012;27:709–713. doi: 10.1111/j.1440-1746.2011.06938.x. [DOI] [PubMed] [Google Scholar]
- 19.Carreras-Torres R, Johansson M, Gaborieau V, Haycock PC, Wade KH, Relton CL, Martin RM, Davey Smith G, Brennan P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina-Montes E, Coscia C, Gómez-Rubio P, Fernández A, Boenink R, Rava M, Márquez M, Molero X, Löhr M, Sharp L, Michalski CW, Farré A, Perea J, O'Rorke M, Greenhalf W, Iglesias M, Tardón A, Gress TM, Barberá VM, Crnogorac-Jurcevic T, Muñoz-Bellvís L, Dominguez-Muñoz JE, Renz H, Balcells J, Costello E, Ilzarbe L, Kleeff J, Kong B, Mora J, O'Driscoll D, Poves I, Scarpa A, Yu J, Hidalgo M, Lawlor RT, Ye W, Carrato A, Real FX, Malats N PanGenEU Study Investigators. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut. 2021;70:319–329. doi: 10.1136/gutjnl-2019-319990. [DOI] [PubMed] [Google Scholar]
- 21.Yuan S, Kar S, Carter P, Vithayathil M, Mason AM, Burgess S, Larsson SC. Is Type 2 Diabetes Causally Associated With Cancer Risk? Diabetes. 2020;69:1588–1596. doi: 10.2337/db20-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. doi: 10.1038/nrdp.2017.60. [DOI] [PubMed] [Google Scholar]
- 23.Bellin MD, Whitcomb DC, Abberbock J, Sherman S, Sandhu BS, Gardner TB, Anderson MA, Lewis MD, Alkaade S, Singh VK, Baillie J, Banks PA, Conwell D, Cote GA, Guda NM, Muniraj T, Tang G, Brand RE, Gelrud A, Amann ST, Forsmark CE, Wilcox CM, Slivka A, Yadav D. Patient and Disease Characteristics Associated With the Presence of Diabetes Mellitus in Adults With Chronic Pancreatitis in the United States. Am J Gastroenterol. 2017;112:1457–1465. doi: 10.1038/ajg.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen SS, Poulsen JL, Novovic S, Nøjgaard C, Kalaitzakis E, Jensen NM, Engjom T, Tjora E, Waage A, Hauge T, Haas SL, Vujasinovic M, Barauskas G, Pukitis A, Ozola-Zālīte I, Okhlobystin A, Parhiala M, Laukkarinen J, Drewes AM. Multiple risk factors for diabetes mellitus in patients with chronic pancreatitis: A multicentre study of 1117 cases. United European Gastroenterol J. 2020;8:453–461. doi: 10.1177/2050640620901973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan J, Xin L, Wang D, Liao Z, Lin JH, Li BR, Du TT, Ye B, Zou WB, Chen H, Ji JT, Zheng ZH, Hu LH, Li ZS. Risk Factors for Diabetes Mellitus in Chronic Pancreatitis: A Cohort of 2,011 Patients. Medicine (Baltimore) 2016;95:e3251. doi: 10.1097/MD.0000000000003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Liu D, Wei Q, Lin H, Zhi M, Chen Y, Qi L, Waldron RT, Lugea A, Pandol SJ, Li L. New-Onset Diabetes Mellitus After Chronic Pancreatitis Diagnosis: A Systematic Review and Meta-analysis. Pancreas. 2019;48:868–875. doi: 10.1097/MPA.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 27.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 28.Chari ST, Mohan V, Pitchumoni CS, Viswanathan M, Madanagopalan N, Lowenfels AB. Risk of pancreatic carcinoma in tropical calcifying pancreatitis: an epidemiologic study. Pancreas. 1994;9:62–66. doi: 10.1097/00006676-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698–710. doi: 10.1016/S2468-1253(19)30416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology. 2019;156:2024–2040. doi: 10.1053/j.gastro.2019.01.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dankner R, Boffetta P, Balicer RD, Boker LK, Sadeh M, Berlin A, Olmer L, Goldfracht M, Freedman LS. Time-Dependent Risk of Cancer After a Diabetes Diagnosis in a Cohort of 2.3 Million Adults. Am J Epidemiol. 2016;183:1098–1106. doi: 10.1093/aje/kwv290. [DOI] [PubMed] [Google Scholar]
- 34.Setiawan VW, Stram DO, Porcel J, Chari ST, Maskarinec G, Le Marchand L, Wilkens LR, Haiman CA, Pandol SJ, Monroe KR. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J Natl Cancer Inst. 2019;111:27–33. doi: 10.1093/jnci/djy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben Q, Cai Q, Li Z, Yuan Y, Ning X, Deng S, Wang K. The relationship between new-onset diabetes mellitus and pancreatic cancer risk: a case-control study. Eur J Cancer. 2011;47:248–254. doi: 10.1016/j.ejca.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42:198–201. doi: 10.1097/MPA.0b013e3182592c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, García Rodríguez LA, Malgerud L, González-Pérez A, Martín-Pérez M, Lagergren J, Bexelius TS. New-onset type 2 diabetes, elevated HbA1c, anti-diabetic medications, and risk of pancreatic cancer. Br J Cancer. 2015;113:1607–1614. doi: 10.1038/bjc.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roeyen G, Jansen M, Chapelle T, Bracke B, Hartman V, Ysebaert D, De Block C. Diabetes mellitus and pre-diabetes are frequently undiagnosed and underreported in patients referred for pancreatic surgery. A prospective observational study. Pancreatology. 2016;16:671–676. doi: 10.1016/j.pan.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Zhang AMY, Magrill J, de Winter TJJ, Hu X, Skovsø S, Schaeffer DF, Kopp JL, Johnson JD. Endogenous Hyperinsulinemia Contributes to Pancreatic Cancer Development. Cell Metab. 2019;30:403–404. doi: 10.1016/j.cmet.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Kim NH, Chang Y, Lee SR, Ryu S, Kim HJ. Glycaemic Status, Insulin Resistance, and Risk of Pancreatic Cancer Mortality in Individuals With and Without Diabetes. Am J Gastroenterol. 2020;115:1840–1848. doi: 10.14309/ajg.0000000000000956. [DOI] [PubMed] [Google Scholar]
- 42.Trajkovic-Arsic M, Kalideris E, Siveke JT. The role of insulin and IGF system in pancreatic cancer. J Mol Endocrinol. 2013;50:R67–R74. doi: 10.1530/JME-12-0259. [DOI] [PubMed] [Google Scholar]
- 43.Burton DGA, Faragher RGA. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19:447–459. doi: 10.1007/s10522-018-9763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menini S, Iacobini C, de Latouliere L, Manni I, Ionta V, Blasetti Fantauzzi C, Pesce C, Cappello P, Novelli F, Piaggio G, Pugliese G. The advanced glycation end-product Nϵ -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention. J Pathol. 2018;245:197–208. doi: 10.1002/path.5072. [DOI] [PubMed] [Google Scholar]
- 45.Velazquez-Torres G, Fuentes-Mattei E, Choi HH, Yeung SJ, Meng X, Lee MH. Diabetes mellitus type 2 drives metabolic reprogramming to promote pancreatic cancer growth. Gastroenterol Rep (Oxf) 2020;8:261–276. doi: 10.1093/gastro/goaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi M, Hori M, Ishigamori R, Mutoh M, Imai T, Nakagama H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018;109:3013–3023. doi: 10.1111/cas.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer(CPDPC) Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226–237. doi: 10.1016/S2468-1253(16)30106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 49.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 50.Permert J, Adrian TE, Jacobsson P, Jorfelt L, Fruin AB, Larsson J. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumour-associated factor? Am J Surg. 1993;165:61–66; discussion 66. doi: 10.1016/s0002-9610(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 51.Cersosimo E, Pisters PW, Pesola G, McDermott K, Bajorunas D, Brennan MF. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Agustsson T, D'souza MA, Nowak G, Isaksson B. Mechanisms for skeletal muscle insulin resistance in patients with pancreatic ductal adenocarcinoma. Nutrition. 2011;27:796–801. doi: 10.1016/j.nut.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Knezetic JA, Strömmer L, Permert J, Larsson J, Adrian TE. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J Clin Endocrinol Metab. 2000;85:1232–1238. doi: 10.1210/jcem.85.3.6400. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Zhang B, Zheng W, Kang M, Chen Q, Qin W, Li C, Zhang Y, Shao Y, Wu Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7:5384. doi: 10.1038/s41598-017-05541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao WC, Huang BS, Yu YH, Yang HH, Chen PR, Huang CC, Huang HY, Wu MS, Chow LP. Galectin-3 and S100A9: Novel Diabetogenic Factors Mediating Pancreatic Cancer-Associated Diabetes. Diabetes Care. 2019;42:1752–1759. doi: 10.2337/dc19-0217. [DOI] [PubMed] [Google Scholar]
- 56.Chari ST, Klee GG, Miller LJ, Raimondo M, DiMagno EP. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–645. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 57.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sah RP, Sharma A, Nagpal S, Patlolla SH, Kandlakunta H, Anani V, Angom RS, Kamboj AK, Ahmed N, Mohapatra S, Vivekanandhan S, Philbrick KA, Weston A, Takahashi N, Kirkland J, Javeed N, Matveyenko A, Levy MJ, Mukhopadhyay D, Chari ST. Phases of Metabolic and Soft Tissue Changes in Months Preceding a Diagnosis of Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2019;156:1742–1752. doi: 10.1053/j.gastro.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao WC, Chen PR, Huang CC, Chang YT, Huang BS, Chang CC, Wu MS, Chow LP. Relationship between pancreatic cancer-associated diabetes and cachexia. J Cachexia Sarcopenia Muscle. 2020;11:899–908. doi: 10.1002/jcsm.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagpal SJS, Kandlakunta H, Her T, Sharma A, Sannapaneni S, Smyrk TC, Velamala P, Garg SK, Rakshit K, Majumder S, Chari S, Matveyenko A. Pancreatic ductal adenocarcinoma is associated with a unique endocrinopathy distinct from type 2 diabetes mellitus. Pancreatology. 2020;20:929–935. doi: 10.1016/j.pan.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saruc M, Iki K, Pour PM. Morphometric studies in human pancreatic cancer argues against the etiological role of type 2 diabetes in pancreatic cancer. Histol Histopathol. 2010;25:423–432. doi: 10.14670/HH-25.423. [DOI] [PubMed] [Google Scholar]
- 62.Basso D, Plebani M, Fogar P, Del Favero G, Briani G, Meggiato T, Panozzo MP, Ferrara C, D'Angeli F, Burlina A. Beta-cell function in pancreatic adenocarcinoma. Pancreas. 1994;9:332–335. doi: 10.1097/00006676-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Chari ST, Zapiach M, Yadav D, Rizza RA. Beta-cell function and insulin resistance evaluated by HOMA in pancreatic cancer subjects with varying degrees of glucose intolerance. Pancreatology. 2005;5:229–233. doi: 10.1159/000085276. [DOI] [PubMed] [Google Scholar]
- 64.Mäkimattila S, Hietaniemi K, Kiviluoto T, Timonen T, Yki-Järvinen H. In vivo glucose-stimulated amylin secretion is increased in nondiabetic patients with pancreatic cancer. Metabolism. 2001;50:1036–1042. doi: 10.1053/meta.2001.25801. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Larsson J, Abdiu A, Gasslander T, Westermark P, Adrian TE, Permert J. Dissociated secretion of islet amyloid polypeptide and insulin in serum-free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol. 1997;21:157–164. doi: 10.1007/BF02822387. [DOI] [PubMed] [Google Scholar]
- 66.Ding X, Flatt PR, Permert J, Adrian TE. Pancreatic cancer cells selectively stimulate islet beta cells to secrete amylin. Gastroenterology. 1998;114:130–138. doi: 10.1016/s0016-5085(98)70641-9. [DOI] [PubMed] [Google Scholar]
- 67.Javeed N, Sagar G, Dutta SK, Smyrk TC, Lau JS, Bhattacharya S, Truty M, Petersen GM, Kaufman RJ, Chari ST, Mukhopadhyay D. Pancreatic Cancer-Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin Cancer Res. 2015;21:1722–1733. doi: 10.1158/1078-0432.CCR-14-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ, Rumie Vittar NB, de Andrade M, Mukhopadhyay D, Petersen GM, Fernandez-Zapico ME, Logsdon CD, Chari ST. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in β cells and mice. Gastroenterology 2012; 143: 1510-1517. :e1. doi: 10.1053/j.gastro.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST, Mukhopadhyay D. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 2016;65:1165–1174. doi: 10.1136/gutjnl-2014-308350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antolino L, Rocca M, Todde F, Catarinozzi E, Aurello P, Bollanti L, Ramacciato G, D'Angelo F. Can pancreatic cancer be detected by adrenomedullin in patients with new-onset diabetes? Tumori. 2018;104:312–314. doi: 10.5301/tj.5000693. [DOI] [PubMed] [Google Scholar]
- 71.Bartucci R, Salvati A, Olinga P, Boersma YL. Vanin 1: Its Physiological Function and Role in Diseases. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20163891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang M, Qin W, Buya M, Dong X, Zheng W, Lu W, Chen J, Guo Q, Wu Y. VNN1, a potential biomarker for pancreatic cancer-associated new-onset diabetes, aggravates paraneoplastic islet dysfunction by increasing oxidative stress. Cancer Lett. 2016;373:241–250. doi: 10.1016/j.canlet.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 73.Huang H, Dong X, Kang MX, Xu B, Chen Y, Zhang B, Chen J, Xie QP, Wu YL. Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol. 2010;105:1661–1669. doi: 10.1038/ajg.2010.32. [DOI] [PubMed] [Google Scholar]
- 74.Parajuli P, Nguyen TL, Prunier C, Razzaque MS, Xu K, Atfi A. Pancreatic cancer triggers diabetes through TGF-β-mediated selective depletion of islet β-cells. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.201900573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma X, Cui Z, Du Z, Lin H. Transforming growth factor-β signalling, a potential mechanism associated with diabetes mellitus and pancreatic cancer? J Cell Physiol. 2020;235:5882–5892. doi: 10.1002/jcp.29605. [DOI] [PubMed] [Google Scholar]
- 76.Tan L, Ye X, Zhou Y, Yu M, Fu Z, Chen R, Zhuang B, Zeng B, Ye H, Gao W, Lin Q, Li Z, Zhou Q. Macrophage migration inhibitory factor is overexpressed in pancreatic cancer tissues and impairs insulin secretion function of β-cell. J Transl Med. 2014;12:92. doi: 10.1186/1479-5876-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Ni Q, Sun J, Xu M, Xie J, Zhang J, Fang Y, Ning G, Wang Q. Paraneoplastic β Cell Dedifferentiation in Nondiabetic Patients with Pancreatic Cancer. J Clin Endocrinol Metab. 2020;105 doi: 10.1210/clinem/dgz224. [DOI] [PubMed] [Google Scholar]
- 78.Kolb A, Rieder S, Born D, Giese NA, Giese T, Rudofsky G, Werner J, Büchler MW, Friess H, Esposito I, Kleeff J. Glucagon/insulin ratio as a potential biomarker for pancreatic cancer in patients with new-onset diabetes mellitus. Cancer Biol Ther. 2009;8:1527–1533. doi: 10.4161/cbt.8.16.9006. [DOI] [PubMed] [Google Scholar]
- 79.Stern JH, Arriaga Y, Gupta A, Verma U, Karri S, Syed S, Khosama L, Mansour J, Meyer J, Scherer PE, Beg MS. Fasting and Glucose-Stimulated Changes in Plasma Glucagon in Pancreatic Cancer: Potential Biomarkers for Detection? Pancreas. 2019;48:e1–e3. doi: 10.1097/MPA.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 80.Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology. 2015;15:162–166. doi: 10.1016/j.pan.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Nagpal SJS, Bamlet WR, Kudva YC, Chari ST. Comparison of Fasting Human Pancreatic Polypeptide Levels Among Patients With Pancreatic Ductal Adenocarcinoma, Chronic Pancreatitis, and Type 2 Diabetes Mellitus. Pancreas. 2018;47:738–741. doi: 10.1097/MPA.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Škrha J, Bušek P, Uhrová J, Hrabal P, Kmochová K, Laclav M, Bunganič B, Frič P. Lower plasma levels of glucose-dependent insulinotropic peptide (GIP) and pancreatic polypeptide (PP) in patients with ductal adenocarcinoma of the pancreas and their relation to the presence of impaired glucoregulation and weight loss. Pancreatology. 2017;17:89–94. doi: 10.1016/j.pan.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Huang S, Li P, Chen Q, Li Y, Zhou Y, Wang L, Kang M, Zhang B, Yang B, Dong X, Wu Y. Pancreatic cancer-derived exosomes suppress the production of GIP and GLP-1 from STC-1 cells in vitro by down-regulating the PCSK1/3. Cancer Lett. 2018;431:190–200. doi: 10.1016/j.canlet.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 84.Yoon BH, Ang SM, Alabd A, Furlong K, Yeo CJ, Lavu H, Winter JM. Pancreatic Cancer-Associated Diabetes is Clinically Distinguishable From Conventional Diabetes. J Surg Res. 2021;261:215–225. doi: 10.1016/j.jss.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 85.Dugnani E, Gandolfi A, Balzano G, Scavini M, Pasquale V, Aleotti F, Liberati D, Di Terlizzi G, Petrella G, Reni M, Doglioni C, Bosi E, Falconi M, Piemonti L. Diabetes associated with pancreatic ductal adenocarcinoma is just diabetes: Results of a prospective observational study in surgical patients. Pancreatology. 2016;16:844–852. doi: 10.1016/j.pan.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 86.McWilliams RR, Maisonneuve P, Bamlet WR, Petersen GM, Li D, Risch HA, Yu H, Fontham ET, Luckett B, Bosetti C, Negri E, La Vecchia C, Talamini R, Bueno de Mesquita HB, Bracci P, Gallinger S, Neale RE, Lowenfels AB. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas. 2016;45:311–316. doi: 10.1097/MPA.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munigala S, Singh A, Gelrud A, Agarwal B. Predictors for Pancreatic Cancer Diagnosis Following New-Onset Diabetes Mellitus. Clin Transl Gastroenterol. 2015;6:e118. doi: 10.1038/ctg.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tseng CH. New-onset diabetes with a history of dyslipidaemia predicts pancreatic cancer. Pancreas. 2013;42:42–48. doi: 10.1097/MPA.0b013e3182571ba9. [DOI] [PubMed] [Google Scholar]
- 89.Hart PA, Kamada P, Rabe KG, Srinivasan S, Basu A, Aggarwal G, Chari ST. Weight loss precedes cancer-specific symptoms in pancreatic cancer-associated diabetes mellitus. Pancreas. 2011;40:768–772. doi: 10.1097/MPA.0b013e318220816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olson SH, Xu Y, Herzog K, Saldia A, DeFilippis EM, Li P, Allen PJ, O'Reilly EM, Kurtz RC. Weight Loss, Diabetes, Fatigue, and Depression Preceding Pancreatic Cancer. Pancreas. 2016;45:986–991. doi: 10.1097/MPA.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mueller AM, Meier CR, Jick SS, Schneider C. Weight change and blood glucose concentration as markers for pancreatic cancer in subjects with new-onset diabetes mellitus: A matched case-control study. Pancreatology. 2019;19:578–586. doi: 10.1016/j.pan.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 92.Mueller AM, Meier CR, Jick SS, Schneider C. The Potential of Glycaemic Control and Body Weight Change as Early Markers for Pancreatic Cancer in Patients With Long-standing Diabetes Mellitus: A Case-Control Study. Pancreas. 2018;47:807–815. doi: 10.1097/MPA.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 93.Huang BZ, Pandol SJ, Jeon CY, Chari ST, Sugar CA, Chao CR, Zhang ZF, Wu BU, Setiawan VW. New-Onset Diabetes, Longitudinal Trends in Metabolic Markers, and Risk of Pancreatic Cancer in a Heterogeneous Population. Clin Gastroenterol Hepatol. 2020;18:1812–1821.e7. doi: 10.1016/j.cgh.2019.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng YC, Wu CH, Tien YW, Lu TP, Wang YH, Chen BB. Preoperative sarcopenia is associated with poor overall survival in pancreatic cancer patients following pancreaticoduodenectomy. Eur Radiol. 2021;31:2472–2481. doi: 10.1007/s00330-020-07294-7. [DOI] [PubMed] [Google Scholar]
- 95.Sharma A, Kandlakunta H, Nagpal SJS, Feng Z, Hoos W, Petersen GM, Chari ST. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology. 2018;155:730–739.e3. doi: 10.1053/j.gastro.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen W, Butler RK, Lustigova E, Chari ST, Wu BU. Validation of the Enriching New-Onset Diabetes for Pancreatic Cancer Model in a Diverse and Integrated Healthcare Setting. Dig Dis Sci. 2021;66:78–87. doi: 10.1007/s10620-020-06139-z. [DOI] [PubMed] [Google Scholar]
- 97.Boursi B, Finkelman B, Giantonio BJ, Haynes K, Rustgi AK, Rhim AD, Mamtani R, Yang YX. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer Among Patients With New-Onset Diabetes. Gastroenterology. 2017;152:840–850.e3. doi: 10.1053/j.gastro.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Illés D, Ivány E, Holzinger G, Kosár K, Adam MG, Kamlage B, Zsóri G, Tajti M, Svébis MM, Horváth V, Oláh I, Márta K, Váncsa S, Zádori N, Szentesi A, Czakó B, Hegyi P, Czakó L. New Onset of DiabetEs in aSsociation with pancreatic ductal adenocarcinoma (NODES Trial): protocol of a prospective, multicentre observational trial. BMJ Open. 2020;10:e037267. doi: 10.1136/bmjopen-2020-037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology. 2018;155:490–500.e2. doi: 10.1053/j.gastro.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choe JW, Kim JS, Kim HJ, Hwang SY, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT. Value of Early Check-Up of Carbohydrate Antigen 19-9 Levels for Pancreatic Cancer Screening in Asymptomatic New-Onset Diabetic Patients. Pancreas. 2016;45:730–734. doi: 10.1097/MPA.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 101.Choe JW, Kim HJ, Kim JS, Cha J, Joo MK, Lee BJ, Park JJ, Bak YT. Usefulness of CA 19-9 for pancreatic cancer screening in patients with new-onset diabetes. Hepatobiliary Pancreat Dis Int. 2018;17:263–268. doi: 10.1016/j.hbpd.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Murakami M, Nagai Y, Tenjin A, Tanaka Y. Proposed cut-off value of CA19-9 for detecting pancreatic cancer in patients with diabetes: a case-control study. Endocr J. 2018;65:639–643. doi: 10.1507/endocrj.EJ17-0380. [DOI] [PubMed] [Google Scholar]
- 103.Guo Q, Kang M, Zhang B, Chen Y, Dong X, Wu Y. Elevated levels of CA 19-9 and CEA in pancreatic cancer-associated diabetes. J Cancer Res Clin Oncol. 2010;136:1627–1631. doi: 10.1007/s00432-010-0820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Esteghamati A, Hafezi-Nejad N, Zandieh A, Sheikhbahaei S, Emamzadeh-Fard S, Nakhjavani M. CA 19-9 is associated with poor glycaemic control in diabetic patients: role of insulin resistance. Clin Lab. 2014;60:441–447. doi: 10.7754/clin.lab.2013.121243. [DOI] [PubMed] [Google Scholar]
- 105.Jenkinson C, Elliott VL, Evans A, Oldfield L, Jenkins RE, O'Brien DP, Apostolidou S, Gentry-Maharaj A, Fourkala EO, Jacobs IJ, Menon U, Cox T, Campbell F, Pereira SP, Tuveson DA, Park BK, Greenhalf W, Sutton R, Timms JF, Neoptolemos JP, Costello E. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin Cancer Res. 2016;22:1734–1743. doi: 10.1158/1078-0432.CCR-15-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moz S, Basso D, Padoan A, Bozzato D, Fogar P, Zambon CF, Pelloso M, Sperti C, Vigili de Kreutzenberg S, Pasquali C, Pedrazzoli S, Avogaro A, Plebani M. Blood expression of matrix metalloproteinases 8 and 9 and of their inducers S100A8 and S100A9 supports diagnosis and prognosis of PDAC-associated diabetes mellitus. Clin Chim Acta. 2016;456:24–30. doi: 10.1016/j.cca.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 107.Song S, Ji B, Ramachandran V, Wang H, Hafley M, Logsdon C, Bresalier RS. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signalling. PLoS One. 2012;7:e42699. doi: 10.1371/journal.pone.0042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 109.Dai X, Pang W, Zhou Y, Yao W, Xia L, Wang C, Chen X, Zen K, Zhang CY, Yuan Y. Altered profile of serum microRNAs in pancreatic cancer-associated new-onset diabetes mellitus. J Diabetes. 2016;8:422–433. doi: 10.1111/1753-0407.12313. [DOI] [PubMed] [Google Scholar]
- 110.Škrha P, Hořínek A, Pazourková E, Hajer J, Frič P, Škrha J, Anděl M. Serum microRNA-196 and microRNA-200 in pancreatic ductal adenocarcinoma of patients with diabetes mellitus. Pancreatology. 2016;16:839–843. doi: 10.1016/j.pan.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 111.He X, Zhong J, Wang S, Zhou Y, Wang L, Zhang Y, Yuan Y. Serum metabolomics differentiating pancreatic cancer from new-onset diabetes. Oncotarget. 2017;8:29116–29124. doi: 10.18632/oncotarget.16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michálková L, Horník Š, Sýkora J, Habartová L, Setnička V, Bunganič B. Early Detection of Pancreatic Cancer in Type 2 Diabetes Mellitus Patients Based on 1H NMR Metabolomics. J Proteome Res. 2021;20:1744–1753. doi: 10.1021/acs.jproteome.0c00990. [DOI] [PubMed] [Google Scholar]
- 113.Park WG, Li L, Appana S, Wei W, Stello K, Andersen DK, Hughes SJ, Whitcomb DC, Brand RE, Yadav D, Habtezion A Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer. Unique circulating immune signatures for recurrent acute pancreatitis, chronic pancreatitis and pancreatic cancer: A pilot study of these conditions with and without diabetes. Pancreatology. 2020;20:51–59. doi: 10.1016/j.pan.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mizuno S, Isayama H, Nakai Y, Ishigaki K, Saito K, Sato T, Takeda T, Hakuta R, Saito T, Takahara N, Kogure H, Ijichi H, Tateishi K, Tada M, Shikata N, Tagami T, Kikuchi S, Yamamoto H, Yamakado M, Koike K. Diagnostic yield of the plasma free amino acid index for pancreatic cancer in patients with diabetes mellitus. Pancreatology. 2019;19:695–698. doi: 10.1016/j.pan.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Qin S, Lu Y, Chen S, Hu Z, Chen H, Zhong J, Li S, Chen Z. The Relationship of Neutrophil-to-Lymphocyte Ratio or Platelet-to-Lymphocyte Ratio and Pancreatic Cancer in Patients with Type 2 Diabetes. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2019.181226. [DOI] [PubMed] [Google Scholar]
- 116.Yoshinaga T, Niou T, Niihara T, Kajiya Y, Hori E, Tomiyoshi A, Tokudome E, Nishimata H, Takei T, Yoshida M. Angiopoietin-like Protein 2 is a Useful Biomarker for Pancreatic Cancer that is Associated with Type 2 Diabetes Mellitus and Inflammation. J Cancer. 2018;9:4736–4741. doi: 10.7150/jca.25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jeon CY, Li D, Cleary S, Stolzenberg-Solomon R, Bosetti C, La Vecchia C, Porta M, Toriola AT, Hung RJ, Kurtz RC, Olson SH. The Association of Recently Diagnosed Diabetes and Long-term Diabetes With Survival in Pancreatic Cancer Patients: A Pooled Analysis. Pancreas. 2018;47:314–320. doi: 10.1097/MPA.0000000000000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hank T, Sandini M, Qadan M, Weniger M, Ciprani D, Li A, Ferrone CR, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Diabetes mellitus is associated with unfavourable pathologic features, increased postoperative mortality, and worse long-term survival in resected pancreatic cancer. Pancreatology. 2020;20:125–131. doi: 10.1016/j.pan.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Lv X, Qiao W, Leng Y, Wu L, Zhou Y. Impact of diabetes mellitus on clinical outcomes of pancreatic cancer after surgical resection: A systematic review and meta-analysis. PLoS One. 2017;12:e0171370. doi: 10.1371/journal.pone.0171370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bitterman DS, Winter KA, Hong TS, Fuchs CS, Regine WF, Abrams RA, Safran H, Hoffman JP, Benson AB 3rd, Kasunic T, Mulcahy M, Strauss JF, DiPetrillo T, Stella PJ, Chen Y, Plastaras JP, Crane CH. Impact of Diabetes and Insulin Use on Prognosis in Patients With Resected Pancreatic Cancer: An Ancillary Analysis of NRG Oncology RTOG 9704. Int J Radiat Oncol Biol Phys. 2021;109:201–211. doi: 10.1016/j.ijrobp.2020.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hart PA, Law RJ, Frank RD, Bamlet WR, Burch PA, Petersen GM, Rabe KG, Chari ST. Impact of diabetes mellitus on clinical outcomes in patients undergoing surgical resection for pancreatic cancer: a retrospective, cohort study. Am J Gastroenterol. 2014;109:1484–1492. doi: 10.1038/ajg.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beg MS, Dwivedi AK, Ahmad SA, Ali S, Olowokure O. Impact of diabetes mellitus on the outcome of pancreatic cancer. PLoS One. 2014;9:e98511. doi: 10.1371/journal.pone.0098511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raghavan SR, Ballehaninna UK, Chamberlain RS. The impact of perioperative blood glucose levels on pancreatic cancer prognosis and surgical outcomes: an evidence-based review. Pancreas. 2013;42:1210–1217. doi: 10.1097/MPA.0b013e3182a6db8e. [DOI] [PubMed] [Google Scholar]
- 124.Fan KY, Dholakia AS, Wild AT, Su Z, Hacker-Prietz A, Kumar R, Hodgin M, Hsu CC, Le DT, De Jesus-Acosta A, Diaz LA Jr, Laheru DA, Hruban RH, Fishman EK, Brown TD, Pawlik TM, Wolfgang CL, Tran PT, Herman JM. Baseline hemoglobin-A1c impacts clinical outcomes in patients with pancreatic cancer. J Natl Compr Canc Netw. 2014;12:50–57. doi: 10.6004/jnccn.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheon YK, Koo JK, Lee YS, Lee TY, Shim CS. Elevated hemoglobin A1c levels are associated with worse survival in advanced pancreatic cancer patients with diabetes. Gut Liver. 2014;8:205–214. doi: 10.5009/gnl.2014.8.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toriola AT, Stolzenberg-Solomon R, Dalidowitz L, Linehan D, Colditz G. Diabetes and pancreatic cancer survival: a prospective cohort-based study. Br J Cancer. 2014;111:181–185. doi: 10.1038/bjc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen H, Zhan M, Wang W, Yang D, Wang J. Impact of diabetes mellitus on the survival of pancreatic cancer: a meta-analysis. Onco Targets Ther. 2016;9:1679–1688. doi: 10.2147/OTT.S95744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tseng CM, Wang HH, Wang WL, Lee CT, Tai CM, Tseng CH, Chen CC, Tsai YN, Sun MS, Hsu YC. Prognostic impact of diabetes mellitus on overall survival in a nationwide population-based cohort of patients with pancreatic cancer. Endocr Pract. 2020 doi: 10.4158/EP-2019-0565. [DOI] [PubMed] [Google Scholar]
- 129.Balzano G, Dugnani E, Gandolfi A, Scavini M, Pasquale V, Aleotti F, Liberati D, Di Terlizzi G, Petrella G, Reni M, Doglioni C, Bosi E, Falconi M, Piemonti L. Effect of Diabetes on Survival after Resection of Pancreatic Adenocarcinoma. A Prospective, Observational Study. PLoS One. 2016;11:e0166008. doi: 10.1371/journal.pone.0166008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kleeff J, Costello E, Jackson R, Halloran C, Greenhalf W, Ghaneh P, Lamb RF, Lerch MM, Mayerle J, Palmer D, Cox T, Rawcliffe CL, Strobel O, Büchler MW, Neoptolemos JP. The impact of diabetes mellitus on survival following resection and adjuvant chemotherapy for pancreatic cancer. Br J Cancer. 2016;115:887–894. doi: 10.1038/bjc.2016.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chu CK, Mazo AE, Goodman M, Egnatashvili V, Sarmiento JM, Staley CA, Galloway JR, Adsay NV, Jacobs S, Kooby DA. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17:502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 132.Li D, Mao Y, Chang P, Liu C, Hassan MM, Yeung SJ, Abbruzzese JL. Impacts of new-onset and long-term diabetes on clinical outcome of pancreatic cancer. Am J Cancer Res. 2015;5:3260–3269. [PMC free article] [PubMed] [Google Scholar]
- 133.Lee S, Hwang HK, Kang CM, Lee WJ. Adverse Oncologic Impact of New-Onset Diabetes Mellitus on Recurrence in Resected Pancreatic Ductal Adenocarcinoma: A Comparison With Long-standing and Non-Diabetes Mellitus Patients. Pancreas. 2018;47:816–822. doi: 10.1097/MPA.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 134.He XY, Li JF, Yao WY, Yuan YZ. Resolution of new-onset diabetes after radical pancreatic resection predicts long-term survival in patients with pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20:3809–3816. doi: 10.1245/s10434-013-3095-2. [DOI] [PubMed] [Google Scholar]
- 135.Iizumi S, Kuchiba A, Okusaka T, Ikeda M, Sakamoto Y, Kondo S, Morizane C, Ueno H, Osame K, Mitsunaga S, Ohno I, Imaoka H, Hashimoto Y, Takahashi H, Sasaki M, Ohashi K. Impact of the Duration of Diabetes Mellitus on the Outcome of Metastatic Pancreatic Cancer Treated with Gemcitabine: A Retrospective Study. Intern Med. 2019;58:2435–2441. doi: 10.2169/internalmedicine.2539-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma J, Wang J, Ge L, Long B, Zhang J. The impact of diabetes mellitus on clinical outcomes following chemotherapy for the patients with pancreatic cancer: a meta-analysis. Acta Diabetol. 2019;56:1103–1111. doi: 10.1007/s00592-019-01337-2. [DOI] [PubMed] [Google Scholar]
- 137.Rajamanickam ES, Christians KK, Aldakkak M, Krepline AN, Ritch PS, George B, Erickson BA, Foley WD, Aburajab M, Evans DB, Tsai S. Poor Glycaemic Control Is Associated with Failure to Complete Neoadjuvant Therapy and Surgery in Patients with Localized Pancreatic Cancer. J Gastrointest Surg. 2017;21:496–505. doi: 10.1007/s11605-016-3319-4. [DOI] [PubMed] [Google Scholar]
- 138.Zeiss K, Parhofer KG, Heinemann V, Haas M, Laubender RP, Holdenrieder S, Schulz C, Boeck S. Glucose and lipid metabolism in patients with advanced pancreatic cancer receiving palliative chemotherapy. Anticancer Res. 2013;33:287–292. [PubMed] [Google Scholar]
- 139.Zhou C, Qian W, Li J, Ma J, Chen X, Jiang Z, Cheng L, Duan W, Wang Z, Wu Z, Ma Q, Li X. High glucose microenvironment accelerates tumour growth via SREBP1-autophagy axis in pancreatic cancer. J Exp Clin Cancer Res. 2019;38:302. doi: 10.1186/s13046-019-1288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li W, Liu H, Qian W, Cheng L, Yan B, Han L, Xu Q, Ma Q, Ma J. Hyperglycaemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput Struct Biotechnol J. 2018;16:479–487. doi: 10.1016/j.csbj.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J, Ma J, Han L, Xu Q, Lei J, Duan W, Li W, Wang F, Wu E, Ma Q, Huo X. Hyperglycemic tumour microenvironment induces perineural invasion in pancreatic cancer. Cancer Biol Ther. 2015;16:912–921. doi: 10.1080/15384047.2015.1040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jian Z, Cheng T, Zhang Z, Raulefs S, Shi K, Steiger K, Maeritz N, Kleigrewe K, Hofmann T, Benitz S, Bruns P, Lamp D, Jastroch M, Akkan J, Jäger C, Huang P, Nie S, Shen S, Zou X, Ceyhan GO, Michalski CW, Friess H, Kleeff J, Kong B. Glycaemic Variability Promotes Both Local Invasion and Metastatic Colonization by Pancreatic Ductal Adenocarcinoma. Cell Mol Gastroenterol Hepatol. 2018;6:429–449. doi: 10.1016/j.jcmgh.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shingyoji A, Mikata R, Ogasawara S, Kusakabe Y, Yasui S, Sugiyama H, Ohno I, Kato J, Takano S, Yoshitomi H, Ohtsuka M, Kato N. Diverse transitions in diabetes status during the clinical course of patients with resectable pancreatic cancer. Jpn J Clin Oncol. 2020;50:1403–1411. doi: 10.1093/jjco/hyaa136. [DOI] [PubMed] [Google Scholar]
- 144.Wu JM, Kuo TC, Yang CY, Chiang PY, Jeng YM, Huang PH, Tien YW. Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20:242–249. doi: 10.1245/s10434-012-2577-y. [DOI] [PubMed] [Google Scholar]
- 145.Canto MI, Kerdsirichairat T, Yeo CJ, Hruban RH, Shin EJ, Almario JA, Blackford A, Ford M, Klein AP, Javed AA, Lennon AM, Zaheer A, Kamel IR, Fishman EK, Burkhart R, He J, Makary M, Weiss MJ, Schulick RD, Goggins MG, Wolfgang CL. Surgical Outcomes After Pancreatic Resection of Screening-Detected Lesions in Individuals at High Risk for Developing Pancreatic Cancer. J Gastrointest Surg. 2020;24:1101–1110. doi: 10.1007/s11605-019-04230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Singh AN, Pal S, Kilambi R, Madhusudhan KS, Dash NR, Tandon N, Sahni P. Diabetes after pancreaticoduodenectomy: can we predict it? J Surg Res. 2018;227:211–219. doi: 10.1016/j.jss.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 147.Roeyen G, Jansen M, Hartman V, Chapelle T, Bracke B, Ysebaert D, De Block C. The impact of pancreaticoduodenectomy on endocrine and exocrine pancreatic function: A prospective cohort study based on pre- and postoperative function tests. Pancreatology. 2017;17:974–982. doi: 10.1016/j.pan.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 148.Beger HG, Poch B, Mayer B, Siech M. New Onset of Diabetes and Pancreatic Exocrine Insufficiency After Pancreaticoduodenectomy for Benign and Malignant Tumors: A Systematic Review and Meta-analysis of Long-term Results. Ann Surg. 2018;267:259–270. doi: 10.1097/SLA.0000000000002422. [DOI] [PubMed] [Google Scholar]
- 149.Andersen DK, Korc M, Petersen GM, Eibl G, Li D, Rickels MR, Chari ST, Abbruzzese JL. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes. 2017;66:1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, Ren ZG, Jiang GL. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:19–26. doi: 10.1016/j.diabres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 151.Farmer RE, Ford D, Mathur R, Chaturvedi N, Kaplan R, Smeeth L, Bhaskaran K. Metformin use and risk of cancer in patients with type 2 diabetes: a cohort study of primary care records using inverse probability weighting of marginal structural models. Int J Epidemiol . 2019;48:527–537. doi: 10.1093/ije/dyz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol. 2012;107:620–626. doi: 10.1038/ajg.2011.483. [DOI] [PubMed] [Google Scholar]
- 153.Shi YQ, Zhou XC, Du P, Yin MY, Xu L, Chen WJ, Xu CF. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21687. doi: 10.1097/MD.0000000000021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Broadhurst PJ, Hart AR. Metformin as an Adjunctive Therapy for Pancreatic Cancer: A Review of the Literature on Its Potential Therapeutic Use. Dig Dis Sci. 2018;63:2840–2852. doi: 10.1007/s10620-018-5233-y. [DOI] [PubMed] [Google Scholar]
- 155.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walker EJ, Ko AH, Holly EA, Bracci PM. Metformin use among type 2 diabetics and risk of pancreatic cancer in a clinic-based case-control study. Int J Cancer. 2015;136:E646–E653. doi: 10.1002/ijc.29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.You JH, Song SO, Kang MJ, Cho YY, Kim SW, Suh SH, Lee S, Lee YH, Lee BW. Metformin and Gastrointestinal Cancer Development in Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea. Clin Transl Gastroenterol. 2020;11:e00254. doi: 10.14309/ctg.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kato K, Iwama H, Yamashita T, Kobayashi K, Fujihara S, Fujimori T, Kamada H, Kobara H, Masaki T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: Study of the microRNAs associated with the antitumor effect of metformin. Oncol Rep. 2016;35:1582–1592. doi: 10.3892/or.2015.4496. [DOI] [PubMed] [Google Scholar]
- 159.Chen K, Qian W, Jiang Z, Cheng L, Li J, Sun L, Zhou C, Gao L, Lei M, Yan B, Cao J, Duan W, Ma Q. Metformin suppresses cancer initiation and progression in genetic mouse models of pancreatic cancer. Mol Cancer. 2017;16:131. doi: 10.1186/s12943-017-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duan W, Qian W, Zhou C, Cao J, Qin T, Xiao Y, Cheng L, Li J, Chen K, Li X, Ma J, Ma Q. Metformin suppresses the invasive ability of pancreatic cancer cells by blocking autocrine TGFβ1 signalling. Oncol Rep. 2018;40:1495–1502. doi: 10.3892/or.2018.6518. [DOI] [PubMed] [Google Scholar]
- 161.Wang C, Zhang T, Liao Q, Dai M, Guo J, Yang X, Tan W, Lin D, Wu C, Zhao Y. Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell. 2021;12:128–144. doi: 10.1007/s13238-020-00760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gu Y, Zhang B, Gu G, Yang X, Qian Z. Metformin Increases the Chemosensitivity of Pancreatic Cancer Cells to Gemcitabine by Reversing EMT Through Regulation DNA Methylation of miR-663. Onco Targets Ther. 2020;13:10417–10429. doi: 10.2147/OTT.S261570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cerullo M, Gani F, Chen SY, Canner J, Pawlik TM. Metformin Use Is Associated with Improved Survival in Patients Undergoing Resection for Pancreatic Cancer. J Gastrointest Surg. 2016;20:1572–1580. doi: 10.1007/s11605-016-3173-4. [DOI] [PubMed] [Google Scholar]
- 164.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Amin S, Mhango G, Lin J, Aronson A, Wisnivesky J, Boffetta P, Lucas AL. Metformin Improves Survival in Patients with Pancreatic Ductal Adenocarcinoma and Pre-Existing Diabetes: A Propensity Score Analysis. Am J Gastroenterol. 2016;111:1350–1357. doi: 10.1038/ajg.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 167.Reni M, Dugnani E, Cereda S, Belli C, Balzano G, Nicoletti R, Liberati D, Pasquale V, Scavini M, Maggiora P, Sordi V, Lampasona V, Ceraulo D, Di Terlizzi G, Doglioni C, Falconi M, Piemonti L. (Ir)relevance of Metformin Treatment in Patients with Metastatic Pancreatic Cancer: An Open-Label, Randomized Phase II Trial. Clin Cancer Res. 2016;22:1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 168.Frouws MA, Sibinga Mulder BG, Bastiaannet E, Zanders MMJ, van Herk-Sukel MPP, de Leede EM, Bonsing BA, Mieog JSD, Van de Velde CJH, Liefers GJ. No association between metformin use and survival in patients with pancreatic cancer: An observational cohort study. Medicine (Baltimore) 2017;96:e6229. doi: 10.1097/MD.0000000000006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chaiteerakij R, Petersen GM, Bamlet WR, Chaffee KG, Zhen DB, Burch PA, Leof ER, Roberts LR, Oberg AL. Metformin Use and Survival of Patients With Pancreatic Cancer: A Cautionary Lesson. J Clin Oncol. 2016;34:1898–1904. doi: 10.1200/JCO.2015.63.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei M, Liu Y, Bi Y, Zhang ZJ. Metformin and pancreatic cancer survival: Real effect or immortal time bias? Int J Cancer. 2019;145:1822–1828. doi: 10.1002/ijc.32254. [DOI] [PubMed] [Google Scholar]
- 171.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 172.Broadhurst PJ, Hart AR. An observational study to justify and plan a future phase III randomized controlled trial of metformin in improving overall survival in patients with inoperable pancreatic cancer without liver metastases. J Cancer Res Clin Oncol. 2020;146:1369–1375. doi: 10.1007/s00432-020-03177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bosetti C, Rosato V, Li D, Silverman D, Petersen GM, Bracci PM, Neale RE, Muscat J, Anderson K, Gallinger S, Olson SH, Miller AB, Bas Bueno-de-Mesquita H, Scelo G, Janout V, Holcatova I, Lagiou P, Serraino D, Lucenteforte E, Fabianova E, Baghurst PA, Zatonski W, Foretova L, Fontham E, Bamlet WR, Holly EA, Negri E, Hassan M, Prizment A, Cotterchio M, Cleary S, Kurtz RC, Maisonneuve P, Trichopoulos D, Polesel J, Duell EJ, Boffetta P, La Vecchia C, Ghadirian P. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol. 2014;25:2065–2072. doi: 10.1093/annonc/mdu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Janghorbani M, Dehghani M, Salehi-Marzijarani M. Systematic review and meta-analysis of insulin therapy and risk of cancer. Horm Cancer. 2012;3:137–146. doi: 10.1007/s12672-012-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR, Chari ST. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:510–519; quiz 520. doi: 10.1038/ajg.2013.7. [DOI] [PubMed] [Google Scholar]
- 176.Tang X, Yang L, He Z, Liu J. Insulin glargine and cancer risk in patients with diabetes: a meta-analysis. PLoS One. 2012;7:e51814. doi: 10.1371/journal.pone.0051814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cho J, Scragg R, Pandol SJ, Goodarzi MO, Petrov MS. Antidiabetic Medications and Mortality Risk in Individuals With Pancreatic Cancer-Related Diabetes and Postpancreatitis Diabetes: A Nationwide Cohort Study. Diabetes Care. 2019;42:1675–1683. doi: 10.2337/dc19-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee DY, Yu JH, Park S, Han K, Kim NH, Yoo HJ, Choi KM, Baik SH, Seo JA. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: a nationwide population-based study in Korea. Sci Rep. 2018;8:9719. doi: 10.1038/s41598-018-27965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dankner R, Roth J. More recent, better designed studies have weakened links between antidiabetes medications and cancer risk. Diabet Med. 2020;37:194–202. doi: 10.1111/dme.14179. [DOI] [PubMed] [Google Scholar]
- 180.Lamont BJ, Andrikopoulos S. Hope and fear for new classes of type 2 diabetes drugs: is there preclinical evidence that incretin-based therapies alter pancreatic morphology? J Endocrinol. 2014;221:T43–T61. doi: 10.1530/JOE-13-0577. [DOI] [PubMed] [Google Scholar]
- 181.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med. 2014;370:794–797. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- 183.Overbeek JA, Bakker M, van der Heijden AAWA, van Herk-Sukel MPP, Herings RMC, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2018;34:e3004. doi: 10.1002/dmrr.3004. [DOI] [PubMed] [Google Scholar]
- 184.Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, Durand M, Juurlink DN, Targownik LE, Turin TC, Paterson JM, Ernst P Canadian Network for Observational Drug Effect Studies Investigators. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ. 2016;352:i581. doi: 10.1136/bmj.i581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: a meta-analysis based on cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22:699–704. doi: 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- 186.Lee M, Sun J, Han M, Cho Y, Lee JY, Nam CM, Kang ES. Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors. Diabetes Care. 2019;42:2057–2064. doi: 10.2337/dc18-2195. [DOI] [PubMed] [Google Scholar]
- 187.Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 188.Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB LEADER Publication Committee on behalf of the LEADER Trial Investigators. Neoplasms Reported With Liraglutide or Placebo in People With Type 2 Diabetes: Results From the LEADER Randomized Trial. Diabetes Care. 2018;41:1663–1671. doi: 10.2337/dc17-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Galli A, Ceni E, Crabb DW, Mello T, Salzano R, Grappone C, Milani S, Surrenti E, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARgamma independent mechanisms. Gut. 2004;53:1688–1697. doi: 10.1136/gut.2003.031997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li Y, Zhang DW, Lin DQ, Cao LQ. Peroxisome proliferator-activated receptor-γ inhibits pancreatic cancer cell invasion and metastasis via regulating MMP-2 expression through PTEN. Mol Med Rep. 2015;12:6255–6260. doi: 10.3892/mmr.2015.4224. [DOI] [PubMed] [Google Scholar]
- 191.Koga H, Selvendiran K, Sivakumar R, Yoshida T, Torimura T, Ueno T, Sata M. PPARγ potentiates anticancer effects of gemcitabine on human pancreatic cancer cells. Int J Oncol. 2012;40:679–685. doi: 10.3892/ijo.2011.1237. [DOI] [PubMed] [Google Scholar]
- 192.Polvani S, Tarocchi M, Tempesti S, Bencini L, Galli A. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J Gastroenterol. 2016;22:2441–2459. doi: 10.3748/wjg.v22.i8.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18:148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, Mamtani R, Bilker W, Vaughn DJ, Nessel L, Van Den Eeden SK, Ferrara A. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314:265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 195.Beg MS, Gupta A, Sher D, Ali S, Khan S, Gao A, Stewart T, Ahn C, Berry J, Mortensen EM. Impact of Concurrent Medication Use on Pancreatic Cancer Survival-SEER-Medicare Analysis. Am J Clin Oncol. 2018;41:766–771. doi: 10.1097/COC.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou DC, Gong H, Tan CQ, Luo JQ. Prognostic significance of anti-diabetic medications in pancreatic cancer: A meta-analysis. Oncotarget. 2017;8:62349–62357. doi: 10.18632/oncotarget.17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA. 2015;112:E4111–E4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu D, Zhou Y, Xie X, He L, Ding J, Pang S, Shen B, Zhou C. Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter1 and lactate dehydrogenase A. Int J Oncol. 2020;57:1223–1233. doi: 10.3892/ijo.2020.5120. [DOI] [PubMed] [Google Scholar]