Abstract
BACKGROUND
Antibiotic resistance to Helicobacter pylori (H. pylori) infection, which ultimately results in eradication failure, has been an emerging issue in the clinical field. Recently, to overcome this problem, an antibiotic sensitivity-based tailored therapy (TT) for H. pylori infection has received attention.
AIM
To investigate the efficacy and safety profiles of TT for H. pylori infection treatment compared to a non-bismuth quadruple therapy, concomitant therapy (CT) regimen.
METHODS
We included patients (> 18 years) with an H. pylori infection and without a history of Helicobacter eradication who visited the Gil Medical Center between March 2016 and October 2020. After being randomly assigned to either the TT or CT treatment group in 1 to 1 manner, patient compliance, eradication success rate (ESR), and patient-reported side effects profiles were assessed and compared between the two groups. H. pylori infection was diagnosed using a rapid urease test, Giemsa stain, or dual priming oligonucleotide polymerase chain reaction (DPO-PCR). Tailored eradication strategy based through the presence of a 23S ribosomal RNA point mutation. For the TT group, a DPO-PCR test, which detected A2142G and/or A2143G point mutations, and a clarithromycin resistance test were performed. Patients in the clarithromycin-resistant group were treated with a bismuth-containing quadruple combination therapy, while those with sensitive results were treated with the standard triple regimen.
RESULTS
Of the 217 patients with a treatment naive H. pylori infection, 110 patients [mean age: 58.66 ± 13.03, men, n = 55 (50%)] were treated with TT, and 107 patients [mean age: 56.67 ± 10.88, men, n = 52 (48.60%)] were treated with CT. The compliance (TT vs CT, 100% vs 98.13%, P = 0.30), and follow-up loss rates (8.18% vs 9.35%, P = 0.95) were not significantly different between the groups. The ESR after treatment was also not statistically different between the groups (TT vs CT, 82.73% vs 82.24%, P = 0.95). However, the treatment-related and patient-reported side effects were significantly lower in the TT group than in the CT group (22.77% vs 50.52%, P < 0.001).
CONCLUSION
The DPO-based TT regimen shows promising results in efficacy and safety profiles as a first-line Helicobacter eradication regimen in Korea, especially when physicians are confronted with increased antibiotic resistance rates.
Keywords: Helicobacter pylori, Eradication, Tailored therapy, Concomitant therapy regimen
Core Tip: We investigated the efficacy and safety profiles of a tailored therapy (TT) as a first line Helicobacter pylori (H. pylori) eradication treatment compared to a concomitant therapy (CT) regimen in Korea, where clarithromycin resistance rates are high. Of 217 treatment-naïve H. pylori infection patients, 107 patients were treated with CT and 101 patients with TT. Although the eradication success rate was not statistically different between the groups, the treatment-related side effect rate was significantly lower in the TT group. Therefore, the TT regimen might be a promising solution to overcoming the problem of increased antibiotic resistance rates for Helicobacter eradication.
INTRODUCTION
Helicobacter pylori (H. pylori) eradication is associated with a reduced risk of stomach cancer, including adenocarcinoma and mucosa-associated lymphoid tissue lymphoma, thus the rising global rates of antibiotic resistant H. pylori infections is concerning[1-4]. Currently, the Maastricht V/Florence Consensus guidelines, and Korean H. pylori treatment guidelines recommend proton pump inhibitor-based standard triple therapy[5-7]. However, the treatment success rate of standard tailored therapy (TT) has been declining worldwide over the past few decades as H. pylori drug resistance has increased year-over-year[4,7-9]. In Korea, a recent studying assessing the use of the standard triple therapy regimen for H. pylori eradication showed a treatment success rate of less than 70%[8-11]. Given that the ideal eradication success rate (ESR) should be over 80% in intention-to-treatment (ITT) analyses, and 90% in per-protocol (PP) analyses according to the 1997 Asia-Pacific Agreement Report for H. pylori treatment, conventional standard triple therapy with an empirically chosen policy has lost its role in H. pylori eradication practice[12].
Recently, to overcome the aforementioned problems of empirically chosen eradication policies, the concept of ‘TT’ has been introduced in the eradication policy for H. pylori infection[12-18]. TT for H. pylori eradication is based on a pre-treatment antibiotic resistance test using stool or stomach biopsy samples[18,19]. However, tissue culture based antibiotic resistance testing for H. pylori is not ideal in that it is costly, time consuming, and not all of the antibiotics used in the regimen can be tested. Instead, dual priming oligonucleotide polymerase chain reaction (DPO-PCR) has been used[20-22]. DPO-PCR tests are cost effective and less time consuming than tissue culture based tests[23,24]. However, DPO-PCR test is currently only available for clarithromycin (CLR) resistance testing, as a method for rapid metronidazole (MTZ) resistance testing for H. pylori has been invented clinically. However, Korea is a region with high CLR resistance. In fact, the resistance rate has gradually increased, from 22.9% in 2003–2005 to 37.0% in 2007–2009, with the major barriers for H. pylori eradication success being CLR resistance, prompting clinical data to be accumulated using DPO-PCR tests in H. pylori eradication regimens[9,25].
Unfortunately, there is little data on the efficacy and safety profiles of the TT regimen compared to the concomitant therapy (CT) regimen in first-line H. pylori eradication.
Herein, we investigated the efficacy (ESR) and safety profiles (treatment-related side effect events) of the TT regimen as compared to those of CT in patients with treatment-naïve H. pylori infections in Korea, where the CLR resistance rate is high (> 15%).
MATERIALS AND METHODS
Institutional review board approval
The Institutional Review Board of the Gil Medical Center (GMC) reviewed the study protocol and ethics. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the GMC.
Enrolled study population
We enrolled patients (> 18 years) with evidence of an H. pylori infection who were treatment naïve for H. pylori eradication that visited the GMC (Incheon, Korea) between March 2016 and October 2020. Exclusion criteria for this study were as follows: (1) Patients under 18 years of age; (2) Patients with a history of previous eradication; (3) Patients with a history of an allergy to any medication used in this study; (4) Patients with any operation history regarding the stomach; (5) Seriously ill patients with critical medical history [heart failure (≥ New York Heart Association class II), severe respiratory illness, decompensated liver cirrhosis, terminal or supportive care stage of malignancy, etc.]; (6) Patients who could not afford to revisit the hospital for follow-up after medication; and (7) Patients who could not take medication orally.
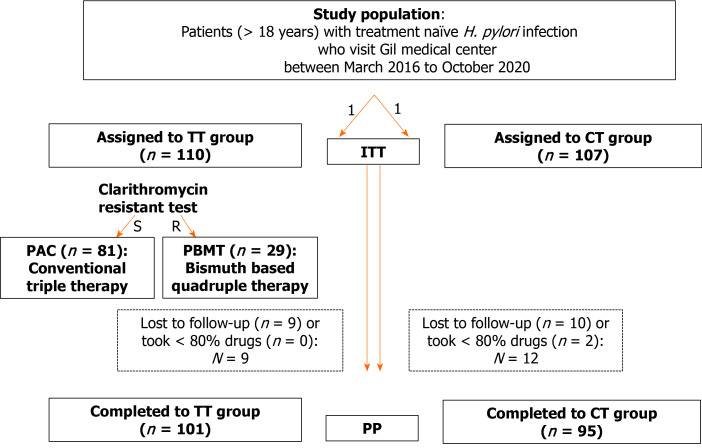
Enrolled patients were randomly assigned to either the TT group or the CT group in a 1 to 1 manner and their compliance rates, ESRs, and treatment-related side effect rates were assessed (Figure 1).
Figure 1.
Flow chart. TT: Tailored therapy; CT: Concomitant regimen; S: Sensitive; R: Resistance; PAC: Pancreatic adenocarcinoma; PBMT: Bismuth-containing quadruple combination; ITT: Intention-to-treat; PP: Per-protocol; H. pylori: Helicobacter pylori.
Diagnosis of H. pylori infection
All patients underwent an upper gastrointestinal endoscopy and showed positive results through a rapid urease test, Giemsa stain, or DPO-PCR.
PCR guided CLR resistance test for TT group
We used DPO-PCR to detect the presence of either a 2142G or 2143G point mutation on 23S rRNA. The point mutation in the V domain of 23S rRNA has been depicted as a major risk factor for CLR resistance against H. pylori, and 2142G and 2143G are the most frequent sites for 23S rRNA point mutations. The wild type for both 2142G and 2143 G mutations was defined as CLR sensitive.
Eradication regimen for TT group, and CT group
For the TT group, after the DPO-PCR test, patients who were deemed CLR resistant were administered a bismuth-containing quadruple combination (PBMT), and those who were CLR sensitive with a standard pancreatic adenocarcinoma (PAC) regimen (Figure 1). The bismuth-containing quadruple regimen consisted of 30 mg of lansoprazole twice daily + 500 mg MTZ twice daily + 300 mg bismuthate four times daily + 500 mg tetracycline four times daily for 10 d. The PAC regimen consisted of 30 mg lansoprazole + 500 mg CLR + 1000 mg amoxicillin (AMX), administered twice daily for 14 d.
The CT regimen consisted of 30 mg lansoprazole twice a day + 1 g AMX twice a day + 500 mg MTZ twice a day + 500 mg CLR twice a day for 10 d.
Follow up strategy, and outcome interpretation (efficacy and safety profiles)
Four weeks after finishing their eradication medication, patients were recommended to visit the GMC and undergo the 13C-ure breath test (UBT; UBiTkit; Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) with a cut-off value of delta 13CO2 < 2.5‰ to evaluate eradication success. During this visit, the patients reported treatment-related side effects and drug compliance rates were also recorded by the physician.
Definition for treatment-related adverse events
Physicians interviewed enrolled patients regarding treatment-related adverse events at follow up. Patients were asked for their treatment-related adverse effects type as both of open-ended, and closed-ended questions. Items for closed-ended questions regarding treatment-related side effect types were as follows: (1) Taste disturbance; (2) Nausea/vomiting; (3) Diarrhea/Loose stool/constipation; (4) Abdominal discomfort, dyspepsia; (5) General weakness, myalgia; (6) Dizziness, head ache; and (7) Skin rash.
The degree of treatment-related side effect were classified as ‘mild’, ‘moderate’, and ‘severe’ events according to the degree of tolerance of patients’ daily activities as follows: No adverse events; mild (without limitation in daily activities); moderate (partly limited daily activities); and severe (completely limited daily activities). Patients were instructed to visit hospital immediately when any severe adverse events occurred.
Definition for treatment compliance
Treatment compliance was defined according to the status of the consumption of the prescribed drugs through personal interviews at the follow-up visit. The compliance level was investigated through patients’ self-reported questionnaire, and consumed/ remained medication pill counts. Patients who consumed > 80% of the scheduled prescription were classified as having good compliance.
Statistics
Treatment outcomes (efficacy profiles and safety profiles) were analyzed using an ITT analysis and PP analysis. In the ITT analysis, after excluding patients meeting the exclusion criteria, all of the enrolled study population were included. In PP analyses, patients who were lost to follow-up or those with poor compliance (< 80%) were excluded. Categorical variables were analyzed as percentiles and compared between the TT and CT groups using the chi-square test. Continuous variables were represented as mean ± SD and were compared between groups using Student’s t-test. Statistical significance was set to P < 0.05. The Statistical Package for the Social Sciences (SPSS) software (version 22.0; IBM Corp., Armonk, NY, United States) was used for statistical analyses.
RESULTS
Clinical characteristics
Of the 217 patients with a treatment-naive H. pylori infection, 110 [men: n = 55 (50.00%); mean age: 58.66 ± 13.03] were treated with TT, and 107 with CT [men: n = 52 (48.60%); mean age: 56.67 ± 10.88] (Table 1). The rates of endoscopy, smoking, drinking, and comorbidity status were not significantly different between the groups (Table 1).
Table 1.
Baseline characteristics of the study population
|
|
TT group (n = 110)
|
CT group (n = 107)
|
P
value
|
| Age, mean ± SD (yr) | 58.66 ± 13.03 | 56.67 ± 10.88 | 0.22 |
| Men, n (%) | 55 (50.00) | 52 (48.60) | 0.89 |
| Body mass index (m2/kg) | 24.31 ± 3.23 | 23.87 ± 2.91 | 0.35 |
| Smoking, n (%) | 24 (22.82) | 22 (20.59) | 0.74 |
| Drinking, n (%) | 30 (30.00) | 28 (26.17) | 0.39 |
| Comorbidity | 0.81 | ||
| Hypertension, n (%) | 26 (23.64) | 23 (21.50) | |
| Diabetes mellitus, n (%) | 22 (20.00) | 25 (23.36) | |
| Cardiovascular disease, n (%) | 2 (1.82) | 2 (1.86) | |
| Reasons for eradication | 0.37 | ||
| Peptic ulcer disease | 49 (44.54) | 43 (40.19) | |
| Post ESD due to EGC | 4 (3.63) | 2 (1.87) | |
| MALToma | 0 (0.00) | 1 (0.93) | |
| Chronic atrophic gastritis with intestinal metaplasia | 57 (51.82) | 61 (57.01) | |
| Clarithromycin resistance diagnosed by DPO-PCR | |||
| No, n (%) | 81 (73.64) | ||
| A2142G positive, n (%) | 4 (3.64) | ||
| A2143G positive, n (%) | 25 (22.73) | ||
| Follow up loss, n (%) | 9 (8.18) | 10 (9.35) | 0.95 |
| Poor compliance, n (%) | 0 (0.00) | 2 (0.02) | 0.30 |
TT: Tailored therapy; CT: Concomitant therapy; DPO-PCR: Dual priming oligonucleotide polymerase chain reaction; ESD: Endoscopic submucosal dissection; EGC: Early gastric cancer; MALToma: Mucosa associated lymphoid tissue lymphoma.
After evaluating CLR resistance status using DPO-PCR test, of the 101 patients who were initially allocated to the TT group, 29 (26.36%) tested positive for CLR-resistant H. pylori, while 81 (73.64%) were classified as CLR-sensitive (Table 1). Among the 29 patients with CLR-resistant H. pylori, four patients showed a A2142G point mutation, while the other 25 patients showed a A2143G point mutation (Table 1).
Follow up loss rate and compliance status of TT vs CT group
The follow-up loss (TT vs CT; 8.18% vs 9.35%, P = 0.95) and poor compliance rates (0% vs 1.87%, P = 0.33) were not significantly different between the groups (Table 1).
Efficacy profiles (ESR) of TT vs CT group
In the ITT protocol, ESR after treatment was not significantly different between the groups (82.73% vs 82.24%, P = 0.95) (Table 2).
Table 2.
Helicobacter pylori eradication success rates
|
Eradication rate
|
TT group (n = 110)
|
CT group (n = 107)
|
P
value
|
| Intention-to-treat | 91/110 (82.73) | 88/107 (82.24) | 0.95 |
| Per-protocol | 91/101 (90.10) | 87/951 (91.58) | 0.72 |
Of 107 patients who were initially allocated into concomitant therapy group, 10 were lost to follow-up, and two patients showed poor compliance for taking medication (one patient showed eradication success, and the other eradication failure). Therefore, a total of 95 patients were included in our per-protocol analysis. TT: Tailored therapy; CT: Concomitant therapy.
Additionally, in the PP protocol, the TT group showed a lower ESR than the CT group (90.10% vs 91.58%, P = 0.72), but the difference was not statistically significant (Table 2).
ESR in TT group according to DPO-PCR results
When we determined ESR in the TT group according to the DPO-PCR results, a total of 72 patients showed CLR sensitive results (treated with PAC regimen), with the ESR for those patients being 80.25%, and 87.84% in the ITT and PP analyses, respectively (Table 3). Four patients showed an A2142G point mutation with ESR of (3/4) 75.00% and (3/3) 100% in the ITT and PP analyses, respectively (Table 3). Twenty-five patients with an A2143G point mutation showed an ESR of 92.00% and 95.83% in ITT and PP analyses, respectively (Table 3).
Table 3.
Eradication success rate in tailored therapy group according to dual priming oligonucleotide polymerase chain reaction results
|
Eradication rate
|
No mutation
|
A2142G positive
|
A2143G positive
|
| Number of patients who initially enrolled | 81 | 4 | 25 |
| Number of patients with eradication failure | 9 | 0 | 1 |
| Number of patients lost to follow up | 7 | 1 | 1 |
| Number of patients with poor compliance | 0 | 0 | 0 |
| Eradication success rate | |||
| Intention-to-treat | 65/81 (80.25%) | 3/4 (75.00%) | 23/25 (92.00%) |
| Per-protocol | 65/74 (87.84%) | 3/3 (100%) | 23/24 (95.83%) |
Patient reported treatment-related side effect profiles of TT vs CT group
Treatment-related and patient-reported side effect events were significantly lower in the TT group than in the CT group (22.77% vs 50.52%, P < 0.001) (Table 4).
Table 4.
Treatment related adverse events, n (%)
|
|
TT group (n = 1011)
|
CT group (n = 972)
|
P
value
|
| Eradication related Side effects | < 0.001 | ||
| No | 78 (77.23) | 48 (49.48) | |
| Yes | 23 (22.77) | 49 (50.52) | |
| Taste disturbance | 2 (1.98) | 18 (18.56) | |
| Nausea/vomiting | 10 (9.90) | 15 (15.46) | |
| Diarrhea/loose stool/constipation | 6 (5.94) | 9 (9.28) | |
| Abdominal discomfort, dyspepsia | 3 (2.97) | 3 (3.09) | |
| General weakness, myalgia | 1 (0.99) | 1 (1.03) | |
| Dizziness, headache | 1 (0.99) | 2 (2.06) | |
| Skin rash | 0 (0.00) | 1 (1.03) |
Of the 110 patients who were initially allocated into the tailored therapy (TT) group, 9 were not followed up. Therefore, a total of 101 patients were included in the TT group.
Of the 107 patients who were initially allocated into the concomitant therapy (CT) group, 10 were lost to follow up. Therefore, a total of 97 patients were included in the CT group, regardless of compliance status. TT: Tailored therapy; CT: Concomitant therapy.
The most common side effect was nausea/vomiting [n = 9 (10.23%)] in the TT group and taste disturbance [n = 18 (18.56%)] in the CT group (Table 4).
DISCUSSION
In this study, we investigated the efficacy (ESR) and safety profiles of TT as a first-line H. pylori eradication regimen compared to those of CT in Korea, where the CLR resistance rate is increasing (> 15%). According to our study results, the ESR between the TT and CT groups was not statistically different between the groups. However, the TT group showed a significantly lower treatment-related side effect rate as compared to that of the CT regimen. Considering that lower exposure to antibiotics and appropriate drug use are the best policies for reducing the spread of antibiotic-resistant bacteria[26,27], the TT regimen might be a promising H. pylori eradication strategy in an era where increased risk for antibiotic resistance has become a huge medical burden, as is the case in Korea.
Even though the CT regimen for H. pylori eradication has shown high ESR in Korea until recently, when compared to that of conventional TT, and even sequential treatment, several concerns arise with this regimen[14,28-34]. First, since the CT regimen includes multiple antibiotic medications for H. pylori eradication such as CLR, AMX, and MTZ, there is a possibility of antibiotic overuse[30,31,35]. Given that indiscriminate misuse of antibiotics is related to the emergence of multidrug resistance bacteria, not just limited to H. pylori, appropriate use of antibiotics based on drug sensitivity analysis has been emphasized in solving the current multidrug resistance problems of bacteria[27,35]. Even if the CT regimen is effective in H. pylori eradication, there is room for antibiotic overuse, which might result in violating antibiotic stewardship, thus physicians should only use a CT regimen with extreme caution[13,27,30,36,37]. Second, because the CT regimen contains multiple antibiotic options, higher incidences of treatment-related side effects, which are related to poor treatment compliance, and ultimately result in eradication failure, have been reported in previous studies[31,32,38,39]. According to our findings, the CT group showed a statistically higher incidence of treatment-related side effects than the TT group.
The paradigm shift from empirically chosen eradication policy to bacteria-specific targeted therapy in H. pylori eradication, TT, originated from the rise of precision medicine and ab accumulation of data on resistance mechanisms of H. pylori infection[11,15,16,25,37,40-42]. According to a meta-analysis by Venerito et al[43] that analyzed the effect of antibiotic sensitivity of H. pylori on ESR, a conventional triple regimen yielded an ESR of 80%–95% among CLR-sensitive strains, but dropped to 0%–48% among CLR resistant strains[43,44]. Therefore, CLR resistance significantly affected the ESR of the conventional triple regimen[43]. However, MTZ resistance was not significantly associated with the ESR of PBMT even in MTZ-resistant strains as compared to that in MTZ-sensitive strains[43]. Therefore, confirming CLR susceptibility before eradication in order to use a tailored eradication was closely associated with an improved ESR as compared to that of the empirically chosen conventional triple regimen, regardless of the MTZ resistance test results[19-21,41,45-47].
To date, several antibiotic resistance mechanisms of H. pylori infection have been proposed[48]. First, antimicrobial genes that are expressed as key targeted structures, such as cell membranes, nucleic acids, DNA gyrases, DNA-dependent RNA polymerases, and redox enzymes, mutate to evade antibiotics, such as quinolone series resistant H. pylori. Second, the efflux systems of H. pylori change to prohibit the intracellular accumulation of antibiotics. Third, H. pylori enzymes that inactivate antibiotic compounds are activated or produced. Among these mechanisms, the CLR resistance phenomenon for H. pylori infection mainly originates from the mutation of the cellular target genes, especially associated with protein translation in the V domain of 23S rRNA (A2143G, A2142C, A2142G, A2143C, etc.)[48-50]. In our study, we used the DPO-PCR test for CLR resistance detection, which specifically targeted the A2143G and A2142G point mutations. Among the 101 patients in the TT group, 72 of them showed CLR sensitivity, while 29 showed CLR resistance. Even among patients with a negative DPO-PCR test result (n = 72), who were classified with wild-type H. pylori strains against CLR, and prescribed the conventional triple regimen, the ESRs were 70.83% and 78.46% in the ITT and PP analyses, respectively. This phenomenon might have resulted from mutations in the V domain of 23S rRNA other than A2142G and A2143G point mutations, which were not detected in the DPO-PCR test we used. Considering cost effectiveness, we targeted H. pylori strains with the most common site of point mutations resulting in CLR resistance. It is possible that the H. pylori strains classified as wild type through the DPO-PCR test were not really wild type. However, among the patients who showed a 2142G or A 2143 G point mutation via the DPO-PCR test, the ESR of the PBMT regimen was 100% and 90.48%, respectively, in the PP analysis. Efforts to develop cost-effective and multiple point mutation detection kits for H. pylori should be continued in order to improve the ESR of TT[23,24,51].
Even though there is limited data on the efficacy of the TT regimen in H. pylori treatment policy, there have been several studies assessing TT efficacy profiles compared to that of the CT regimen[45,52,53]. For example, Ong et al[52] conducted a randomized controlled study comparing the treatment outcomes of TT regimen vs CT regimen, and reported that in ITT analyses, the TT regimen group showed a higher eradication rate compared the CT regimen group[52]. As for treatment-related side effect events, the TT group also showed fewer events during treatment than the CT group. While this study was conducted in Pusan, Korea, which is far from Incheon, Korea, where the GMC is located, similar treatment outcomes were induced even though the CLR resistance rates differed between the cities.
Furthermore, Lesprit et al[27] reported that a tailored H. pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes CAM resistance in patients with H. pylori infection is more cost effective than empirical treatment[27]. Kim et al[25] also conducted an economic modeling study comparing TR based on DPO-PCR and empirical treatment.
Even efficacy of TT regimen in treating H. pylori infection has been evaluated, cost-effectiveness of TT regimen should be evaluated to be widely used in clinical practice in Korea. Tailored regimen needs additional diagnostic procedure of antibiotic resistance test such as DPO-PCR. Even it varies depending on insurance coverages, DPO-PCR costs approximately $55.24 more than rapid urease test in Korea[23]. In this regards, several previous studies investigated medical costs of H. pylori tailored eradication strategy as compared to empirical first line eradication strategy (CLR based triple regimen) in Korea[23]. Cho et al[23] reported that it is acceptable level of predictive additional costs (only an extra $3.96 per eradicated patient) for tailored H. pylori eradication strategy with DPO-CPR as compared to CLR based conventional triple therapy[23]. In our study, we compared efficacy and safety level of TT vs CT regimen, and should discuss the medical cost effectiveness of TT vs CT regimen. However, there has been little data to investigate medical cost of TT strategy as compared to CT regimen. One would say medical cost of TT regimen might be much higher than that of CT regimen, since ESR of empirical CT regimen is generally much higher than empirical CLR based triple regimen. Not just considering each patient’s medical cost during Helicobacter eradication, but also given that worrisome issues on increased prevalence of drug resistance bacteria in worldwide, tailored approaches in treating H. pylori infection should be considered following the major principle of antibiotic use guidelines, antibiotic sensitivity result-based treatment.
Limits of the study
There are several limitations to this study. First, although we prospectively and randomly assigned patients to either the TT or CT group, we retrospectively reviewed the data. Second, since this study was conducted in a tertiary center located in Incheon, Korea, we applied our research results to other regions with caution. In a previous study, researchers investigated the CLR resistance rate for H. pylori infection in Incheon, and reported a CLR resistance rate of approximately 30%[54]. Although the aforementioned study was not conducted in our hospital, the GMC, the CLR resistance for H. pylori infection is similar to our study. Given that different regions might differ in H. pylori infection status and antibiotic resistance status, more multicenter and multinational studies are needed. Third, since we did not culture H. pylori for the antibiotic sensitivity test, but instead replaced it with a DPO-PCR test, and are therefore subject to the pit falls of a DPO-PCR. Nevertheless, in a previous study, the DPO-PCR test was validated alongside a culture-based antibiotic resistance test and showed approximately 98% accuracy[20,55].
CONCLUSION
Despite the aforementioned limitations, this study focused on the efficacy and safety profiles of the TT regimen compared to those of the CT regimen in a relatively large dataset in Korea. For the rapidly rising antibiotic resistance rate, the most active countermeasure is to perform an antibiotic resistance test for the strain and actively select the appropriate antibiotic and then decide on the treatment.
In conclusion, according to our study results, the TT regimen showed promising results in terms of efficacy and safety profiles for the first-line regimen of H. pylori eradication as compared to those of the CT regimen. Therefore the DPO-based TT regimen might be a successful option for H. pylori eradication, especially when physicians are confronted with increased antibiotic resistance rates for H. pylori eradication.
ARTICLE HIGHLIGHTS
Research background
Antibiotic resistance to Helicobacter pylori (H. pylori) infection has been an emerging issue in the clinical field. Recently, to overcome this problem, an antibiotic sensitivity-based tailored therapy (TT) for H. pylori infection has got attention.
Research motivation
However, there is limited data regarding efficacy of TT strategy in treatment of H. pylori infection in Korea as compared to that of concomitant therapy (CT) regimen.
Research objectives
To investigate the efficacy and safety profiles of TT for H. pylori infection treatment compared to a non-bismuth quadruple therapy, CT.
Research methods
We included treatment naive H. pylori infection patients (> 18 years) who visited the Gil Medical Center between March 2016 and October 2020. After randomly assigned to either the TT or CT treatment group in 1 to 1 manner, patient compliance, eradication success rate (ESR), and patient-reported side effects profiles were compared between the two groups. For the TT group, a dual priming oligonucleotide polymerase chain reaction (DPO-PCR) test, which detected A2142G and/or A2143G point mutations, and a clarithromycin (CLR) resistance test were performed. Patients in the CLR-resistant group were treated with a bismuth-containing quadruple combination therapy, while those with sensitive results were treated with the standard triple regimen.
Research results
Of the 217 patients with a treatment naive H. pylori infection, 110 patients [mean age: 58.66 ± 13.03, men, n = 55 (50%)] were treated with TT, and 107 patients [mean age: 56.67 ± 10.88, men, n = 52 (48.60%)] were treated with CT. The compliance (TT vs CT, 100% vs 98.13%, P = 0.30), and follow-up loss rates (8.18% vs 9.35%, P = 0.95) were not significantly different between the groups. The ESR after treatment was also not statistically different between the groups (TT vs CT, 82.73% vs 82.24%, P = 0.95). However, the treatment-related and patient-reported side effects were significantly lower in the TT group than in the CT group (22.77% vs 50.52%, P < 0.001).
Research conclusions
The DPO-based TT regimen shows promising results in efficacy and safety profiles as a first-line Helicobacter eradication regimen in Korea, especially when physicians are confronted with increased antibiotic resistance rates.
Research perspectives
The DPO-based TT regimen might role as a first-line Helicobacter eradication regimen with similar efficacy and safety profiles as compared to CT regimen.
Footnotes
Institutional review board statement: The Institutional Review Board of the Gil Medical Center (GMC) reviewed the study protocol and ethics. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the GMC.
Informed consent statement: Patients were not required to give the informed consent to the study because the analysis used the anonymous data that were collected after each patient agreed to treatment.
Conflict-of-interest statement: All the Authors have no conflict of interest related to the manuscript.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Invited manuscript
Peer-review started: April 29, 2021
First decision: June 3, 2021
Article in press: July 29, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Couto ME, Smith SM S-Editor: Fan JR L-Editor: A P-Editor: Xing YX
Contributor Information
Youn I Choi, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea.
Jun-Won Chung, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea. junwonchung@hanmail.net.
Kyoung Oh Kim, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea.
Kwang An Kwon, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea.
Yoon Jae Kim, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea.
Jung Ho Kim, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea.
Ja Young Seo, Department of Laboratory Medicine, Gil Medical Center, Gachon University, Inchoen 21565, South Korea.
Dong Kyun Park, Division of Internal Medicine, Department of Gastroenterology, Gachon University College of Medicine, Gil Medical Center, Inchoen 21565, South Korea; Health IT Research Center, Gachon University Gil Hospital, Incheon 21565, South Korea.
Data sharing statement
The data used to support the findings of this study are available from the corresponding author upon request at (junwonchung@gilhospital.com)
References
- 1.Park JM. Quality Indicator for Gastric Cancer Detection Based on Helicobacter pylori Status. Clin Endosc. 2020;53:629–630. doi: 10.5946/ce.2020.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishibashi F, Kobayashi K, Fukushima K, Tanaka R, Kawakami T, Kato J, Sugihara K. Quality Indicators for the Detection of Helicobacter pylori-Negative Early Gastric Cancer: A Retrospective Observational Study. Clin Endosc. 2020;53:698–704. doi: 10.5946/ce.2019.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddique O, Ovalle A, Siddique AS, Moss SF. Helicobacter pylori Infection: An Update for the Internist in the Age of Increasing Global Antibiotic Resistance. Am J Med. 2018;131:473–479. doi: 10.1016/j.amjmed.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, Maldonado-Garza HJ, Garza-González E. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Rev Gastroenterol Hepatol. 2018;12:819–827. doi: 10.1080/17474124.2018.1496017. [DOI] [PubMed] [Google Scholar]
- 5.Zagari RM, Rabitti S, Eusebi LH, Bazzoli F. Treatment of Helicobacter pylori infection: A clinical practice update. Eur J Clin Invest. 2018;48 doi: 10.1111/eci.12857. [DOI] [PubMed] [Google Scholar]
- 6.Alba C, Blanco A, Alarcón T. Antibiotic resistance in Helicobacter pylori. Curr Opin Infect Dis. 2017;30:489–497. doi: 10.1097/QCO.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 8.Boyanova L, Hadzhiyski P, Kandilarov N, Markovska R, Mitov I. Multidrug resistance in Helicobacter pylori: current state and future directions. Expert Rev Clin Pharmacol. 2019;12:909–915. doi: 10.1080/17512433.2019.1654858. [DOI] [PubMed] [Google Scholar]
- 9.Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, Kim SE, Lim HC, Kim JH, Nam SY, Shin WG, Park JM, Choi IJ, Kim JG, Choi M Korean College of Helicobacter and Upper Gastrointestinal Research. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver. 2021;15:168–195. doi: 10.5009/gnl20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JY, Kim N, Nam RH, In Choi S, Lee JW, Lee DH. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. doi: 10.1111/hel.12660. [DOI] [PubMed] [Google Scholar]
- 11.Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat Rev Gastroenterol Hepatol. 2017;14:383–384. doi: 10.1038/nrgastro.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology. 2019;157:44–53. doi: 10.1053/j.gastro.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg B, Srinivasan A, Chambers HF. New Societal Approaches to Empowering Antibiotic Stewardship. JAMA. 2016;315:1229–1230. doi: 10.1001/jama.2016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgopoulos SD, Papastergiou V, Karatapanis S. Helicobacter pylori Eradication Therapies in the Era of Increasing Antibiotic Resistance: A Paradigm Shift to Improved Efficacy. Gastroenterol Res Pract. 2012;2012:757926. doi: 10.1155/2012/757926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stollman N. Helicobacter pylori Infection in the Era of Antibiotic Resistance. Gastroenterol Hepatol (N Y) 2016;12:122–125. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JW, Kim N, Nam RH, Lee SM, Kwon YH, Sohn SD, Kim JM, Lee DH, Jung HC. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. 2019;24:e12561. doi: 10.1111/hel.12561. [DOI] [PubMed] [Google Scholar]
- 18.Ierardi E, Giorgio F, Iannone A, Losurdo G, Principi M, Barone M, Pisani A, Di Leo A. Noninvasive molecular analysis of Helicobacter pylori: Is it time for tailored first-line therapy? World J Gastroenterol. 2017;23:2453–2458. doi: 10.3748/wjg.v23.i14.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Dang Y, Zhou X, Liu B, Liu S, Zhang G. Tailored Therapy Versus Empiric Chosen Treatment for Helicobacter pylori Eradication: A Meta-Analysis. Medicine (Baltimore) 2016;95:e2750. doi: 10.1097/MD.0000000000002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon YH, Kim N, Lee JY, Choi YJ, Yoon K, Nam RH, Suh JH, Lee JW, Lee DH. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol. 2016;51:270–276. doi: 10.3109/00365521.2015.1095352. [DOI] [PubMed] [Google Scholar]
- 21.Delchier JC, Bastuji-Garin S, Raymond J, Megraud F, Amiot A, Cambau E, Burucoa C HELICOSTIC Study Group. Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med Mal Infect. 2020;50:492–499. doi: 10.1016/j.medmal.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto M, Uotani T, Sahara S, Ichikawa H, Yamade M, Sugimoto K, Furuta T. Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter. 2014;19:312–318. doi: 10.1111/hel.12128. [DOI] [PubMed] [Google Scholar]
- 23.Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol. 2019;34:700–706. doi: 10.1111/jgh.14383. [DOI] [PubMed] [Google Scholar]
- 24.Chang YW, Shin GY, Kim JW, Moon JC, Chang EJ, Oh CH, Jang JY. Cost-Effectiveness of Empirical Bismuth-Based Quadruple Therapy and Tailored Therapy After Clarithromycin Resistance Tests for Helicobacter pylori Eradication. Dig Dis Sci. 2021 doi: 10.1007/s10620-021-06938-y. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Choi DJ, Chung JW. Antibiotic treatment for Helicobacter pylori: Is the end coming? World J Gastrointest Pharmacol Ther. 2015;6:183–198. doi: 10.4292/wjgpt.v6.i4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Gómez P, Jordán-Castro JA, Abanades-Tercero M, Blanco-González JJ, Andrés Esteban EM, Valle-Muñoz J. Macrolide use in the previous years is associated with failure to eradicate Helicobacter pylori with clarithromycin-containing regimens. Helicobacter. 2018;23 doi: 10.1111/hel.12452. [DOI] [PubMed] [Google Scholar]
- 27.Lesprit P, Brun-Buisson C. Hospital antibiotic stewardship. Curr Opin Infect Dis. 2008;21:344–349. doi: 10.1097/QCO.0b013e3283013959. [DOI] [PubMed] [Google Scholar]
- 28.Choe JW, Jung SW, Kim SY, Hyun JJ, Jung YK, Koo JS, Yim HJ, Lee SW. Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter. 2018;23:e12466. doi: 10.1111/hel.12466. [DOI] [PubMed] [Google Scholar]
- 29.Jha SK, Mishra MK, Saharawat K, Jha P, Purkayastha S, Ranjan R. Comparison of concomitant therapy vs standard triple-drug therapy for eradication of Helicobacter pylori infection: A prospective open-label randomized controlled trial. Indian J Gastroenterol. 2019;38:325–331. doi: 10.1007/s12664-019-00949-4. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther. 2018;16:679–687. doi: 10.1080/14787210.2018.1511427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MJ, Chen CC, Chen YN, Fang YJ, Lin JT, Wu MS, Liou JM Taiwan Gastrointestinal Disease Helicobacter Consortium. Systematic Review with Meta-Analysis: Concomitant Therapy vs. Triple Therapy for the First-Line Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2018;113:1444–1457. doi: 10.1038/s41395-018-0217-2. [DOI] [PubMed] [Google Scholar]
- 32.Park SM, Kim JS, Kim BW, Ji JS, Choi H. Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 2017;32:589–594. doi: 10.1111/jgh.13510. [DOI] [PubMed] [Google Scholar]
- 33.Lee BE, Kim JS, Kim BW, Kim JH, Kim JI, Chung JW, Jeon SW, Lee JH, Kim N, Lee JY, Seo SY, Park SY, Kim SE, Joo MK, Song HJ, Kim KB, Bang CS, Kim HJ. Consistency of Helicobacter pylori eradication rates of first-line concomitant and sequential therapies in Korea: A nationwide multicenter retrospective study for the last 10 years. Helicobacter. 2021;26:e12780. doi: 10.1111/hel.12780. [DOI] [PubMed] [Google Scholar]
- 34.Bae HJ, Kim JS, Kim BW, Nam YJ. Concomitant or Sequential Therapy as the First-line Therapy for Eradication of Helicobacter pylori Infection in Korea: A Systematic Review and Meta-analysis. Korean J Gastroenterol. 2018;71:31–37. doi: 10.4166/kjg.2018.71.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiotani A, Lu H, Dore MP, Graham DY. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med. 2017;84:310–318. doi: 10.3949/ccjm.84a.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core Elements of Outpatient Antibiotic Stewardship. MMWR Recomm Rep. 2016;65:1–12. doi: 10.15585/mmwr.rr6506a1. [DOI] [PubMed] [Google Scholar]
- 37.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 38.Kim BJ, Lee H, Lee YC, Jeon SW, Kim GH, Kim HS, Sung JK, Lee DH, Kim HU, Park MI, Choi IJ, Yoon SM, Kim SW, Baik GH, Lee JY, Kim JI, Kim SG, Kim J, Lee J, Kim JG, Kim JJ Korean College of Helicobacter Upper Gastrointestinal Research. Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver. 2019;13:531–540. doi: 10.5009/gnl19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SJ, Chung JW, Woo HS, Kim SY, Kim JH, Kim YJ, Kim KO, Kwon KA, Park DK. Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial. World J Gastroenterol. 2019;25:6790–6798. doi: 10.3748/wjg.v25.i46.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto M, Furuta T. Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J Gastroenterol. 2014;20:6400–6411. doi: 10.3748/wjg.v20.i21.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawai T, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Sofuni A, Itoi T, Sakai Y, Moriyasu F, Osaka Y, Takagi Y, Aoki T, Rimbara E, Noguchi N, Sasatsu M. Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J Gastroenterol Hepatol. 2008;23 Suppl 2:S171–S174. doi: 10.1111/j.1440-1746.2008.05408.x. [DOI] [PubMed] [Google Scholar]
- 42.Gisbert JP. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? Therap Adv Gastroenterol. 2020;13:1756284820968736. doi: 10.1177/1756284820968736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy vs clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33–45. doi: 10.1159/000350719. [DOI] [PubMed] [Google Scholar]
- 44.Houben MH, van de Beek D, Hensen EF, de Craen AJ, Rauws EA, Tytgat GN. A systematic review of Helicobacter pylori eradication therapy--the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–1055. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Zhang J, Song Z, He L, Li Y, Qian J, Bai P, Xue Y, Wang Y, Lin S. Tailored vs Triple plus Bismuth or Concomitant Therapy as Initial Helicobacter pylori Treatment: A Randomized Trial. Helicobacter. 2016;21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 46.Choi YI, Chung JW, Park DK, Kim KO, Kwon KA, Kim YJ, Seo JY. Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J Gastroenterol. 2019;25:6743–6751. doi: 10.3748/wjg.v25.i46.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valle Muñoz J, Muñoz Gómez P, Sierra Bernal C, de Andrés E, Gómez Hernando C, Gómez Rodríguez R. Tailored Helicobacter pylori eradication based on prior intake of macrolide antibiotics allows the use of triple therapy with optimal results in an area with high clarithromycin resistance. Rev Esp Enferm Dig. 2019;111:655–661. doi: 10.17235/reed.2019.6198/2019. [DOI] [PubMed] [Google Scholar]
- 48.Gazi S, Karameris A, Christoforou M, Agnantis N, Rokkas T, Stefanou D. Real-Time PCR detection and quantitation of Helicobacter pylori clarithromycin-resistant strains in archival material and correlation with Sydney classification. Ann Gastroenterol. 2013;26:226–232. [PMC free article] [PubMed] [Google Scholar]
- 49.Gong Y, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Crit Rev Microbiol. 2018;44:371–392. doi: 10.1080/1040841X.2017.1418285. [DOI] [PubMed] [Google Scholar]
- 50.Gehlot V, Mahant S, Mukhopadhyay AK, Das K, Alam J, Ghosh P, Das R. Low prevalence of clarithromycin-resistant Helicobacter pylori isolates with A2143G point mutation in the 23S rRNA gene in North India. J Glob Antimicrob Resist. 2016;6:39–43. doi: 10.1016/j.jgar.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Lim K, Joo M, Park J, Lee B, Kim S, Chun H, Lee S, Kim W, Yoo A. Efficacy and Cost-Effectiveness of Helicobacter pylori Eradication: Comparison of Tailored Therapy Based on Clarithromycin Resistance and Concomitant Therapy. Gut Liver. 2019:13. [Google Scholar]
- 52.Ong S, Kim SE, Kim JH, Yi NH, Kim TY, Jung K, Park MI, Jung HY. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter. 2019;24:e12654. doi: 10.1111/hel.12654. [DOI] [PubMed] [Google Scholar]
- 53.Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy vs standard triple therapy for clarithromycin-susceptible Helicobacter pylori and vs sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269–276. doi: 10.1111/j.1523-5378.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, Kim BW, Park JC, Jung HK, Cho SJ, Shin CM, Choi YJ, Lee SH, Kim JH, Lee WS, Sung JK, Chung JW, Cheung DY, Lee H, Min YW, Kim JJ, Kim SY Korean College of Helicobacter; Upper Gastrointestinal Research. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 2019;24:e12592. doi: 10.1111/hel.12592. [DOI] [PubMed] [Google Scholar]
- 55.Kwon YH, Jeon SW, Nam SY, Lee HS, Park JH. Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: A single-center prospective pilot study. Helicobacter. 2019;24:e12585. doi: 10.1111/hel.12585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request at (junwonchung@gilhospital.com)