Abstract
Gastric cancer accounts for a significant proportion of worldwide cancer-related morbidity and mortality. The well documented precancerous cascade provides an opportunity for clinicians to detect and treat gastric cancers at an endoscopically curable stage. In high prevalence regions such as Japan and Korea, this has led to the implementation of population screening programs. However, guidelines remain ambiguous in lower prevalence regions. In recent years, there have been many advances in the endoscopic diagnosis and treatment of early gastric cancer and precancerous lesions. More advanced endoscopic imaging has led to improved detection and characterization of gastric lesions as well as superior accuracy for delineation of margins prior to resection. In addition, promising early data on artificial intelligence in gastroscopy suggests a future role for this technology in maximizing the yield of advanced endoscopic imaging. Data on endoscopic resection (ER) are particularly robust in Japan and Korea, with high rates of curative ER and markedly reduced procedural morbidity. However, there is a shortage of data in other regions to support the applicability of protocols from these high prevalence countries. Future advances in endoscopic therapeutics will likely lead to further expansion of the current indications for ER, as both technology and proceduralist expertise continue to grow.
Keywords: Gastric cancer, Endoscopy, Endoscopic imaging, Endoscopic mucosal resection, Endoscopic submucosal dissection
Core Tip: There have been numerous recent advances in the endoscopic detection, characterization and treatment of precancerous gastric lesions and early gastric cancers. Accumulating evidence suggests that endoscopic submucosal dissection results in at least equivalent disease-related outcomes with a marked reduction in morbidity compared to gastrectomy. Supportive data are robust in regions with high gastric cancer prevalence, however a paucity of western data results in inconsistencies in clinical practice in these regions. This article serves to review existing evidence regarding endoscopic imaging and therapeutics in gastric cancer, as well as identify future areas for research and development.
INTRODUCTION
Gastric cancer remains a leading cause of cancer-related morbidity and mortality, accounting for 780000 deaths and more than 1 million new diagnoses worldwide in 2020 alone[1,2]. Conventional surgical management with gastrectomy is associated with significant morbidity and mortality[3]. Countries with a high prevalence of gastric cancer have implemented systematic screening programs and demonstrated the benefit of early detection and endoscopic resection (ER) of precancerous gastric lesions and early gastric cancers (EGCs), offering curative treatment with considerably less morbidity[4,5]. Future challenges and opportunities remain, including the judicious surveillance of gastric metaplasia in lower prevalence communities, utilising improvements in endoscopic imaging and further refining existing ER techniques, while at all times accumulating high-quality collaborative data sets to inform and shape future management algorithms.
ENDOSCOPIC DIAGNOSIS OF GASTRIC NEOPLASIA
Endoscopic gastric cancer screening
Rates of gastric cancer in Eastern Asia (particularly Japan and Korea) are markedly higher than Western regions, which has resulted in implementation of population screening programs as an evidence based, cost effective measure for preventing gastric cancer-related mortality and morbidity[4-6]. Screening programs facilitate the detection of precancerous lesions and EGCs at an endoscopically resectable stage. Japanese guidelines suggest biennial or triennial endoscopic screening for those over the age of 50. This approach could prevent up to 63% of gastric cancer related mortality, resulting in 27.2 quality-adjusted life years gained per 1000 individuals[4]. In Korea, guidelines similarly suggest biennial screening but commencing at age 40. This is supported by data showing a 38% reduction in age-standardised mortality rate in the screening group at large, at a cost of less than the average gross expenditure product per capita[5].
In Western regions including North America and Australasia, the age-standardised incidence of gastric cancer is 6.5-8.8 per 100000 population, four-times lower than East Asia[6]. The lower incidence may be attributable to a combination of reduced Helicobacter pylori prevalence as well as environmental, lifestyle (diet, smoking) and genetic factors[6]. Given this comparatively low gastric cancer incidence, Western countries are yet to adopt population screening. Instead, guidelines in these regions are varied. The American Gastroenterology Association does not recommend population screening, only surveillance for patients with gastric intestinal metaplasia (GIM), while the British Society of Gastroenterology (BSG) recommends endoscopic screening in patients with multiple risk factors for gastric cancer (male, smoker, pernicious anaemia, family history in first degree relative), citing low-grade evidence[7,8]. Unsurprisingly, low disease prevalence combined with variable guidelines leads to inconsistencies in clinical practice, as demonstrated in a 2016 survey of American endoscopists[9]. An additional challenge in these low prevalence regions is the management of individuals who have migrated from areas where gastric cancer incidence is high, as data show persistently elevated gastric cancer risk for at least two generations, yet the infrastructure and guidelines in terms of screening are not established[10,11]. These factors would undoubtedly contribute to the discrepancy in gastric cancer survival between Western and East Asian regions, highlighting the need for additional research in these populations[12].
Progression of precancerous gastric lesions
Gastric cancer develops through a well-established precancerous cascade: From atrophic gastritis (AG) to GIM, low-grade dysplasia (LGD), high-grade dysplasia (HGD) and eventually carcinoma, with the likelihood of progression increasing as this cascade advances[13]. Although eradication of Helicobacter Pylori has been clearly demonstrated to reduce rates of development of this precancerous cascade, data have been more variable regarding prevention of progression from GIM, implying a ‘point of no return’ in the gastric cancer cascade[14-16]. These observations underpin the utility of screening and surveillance endoscopy, providing the opportunity to identify and treat curable lesions. In order to maximise yield, guidelines also suggest routine ‘mapping’ biopsies according to the updated Sydney protocol (including two biopsies taken from the antrum and one from each of the incisura, lesser curve and greater curve) in addition to biopsies of any suspicious areas during surveillance endoscopy, which results in improved AG and GIM detection compared to non-systematic biopsies[8,17].
Japanese data suggests an overall 5-year cumulative gastric cancer incidence of 1.9%-10% in AG and 5.3%-9.8% in GIM[18]. The Operative Link on Gastritis/Intestinal Metaplasia Assessment (OLGIM) scoring system further assists in differentiating between degrees of AG and GIM with regard to gastric cancer risk[19]. Those with high-risk lesions meeting OLGIM stage III/IV have an odds ratio for gastric cancer of 2.41 and 3.99 respectively[20]. Accordingly, guidelines suggest that patients with OLGIM stage III/IV AG or with GIM have 3-yearly surveillance endoscopy or 1- to 2-yearly endoscopy in the presence of a family history of gastric cancer[21]. Although these scoring systems are consistently reported in high-prevalence regions, the limited experience in lower prevalence regions again results in inconsistencies, as pathologists rarely report according to OLGIM staging and thus endoscopist adherence to GIM surveillance guidelines is variable[9].
In the context of biopsy-detected LGD and HGD, the risks of progression to gastric cancer are reportedly between 2.8%-11.5% and 10%-68.8% respectively; therefore guidelines suggest endoscopic removal of any defined lesion with dysplasia[22-26]. Importantly, lesions are also frequently upgraded histologically once endoscopically resected. In a 2015 study, 25% of lesions initially diagnosed as LGD were upgraded after ER; 16.7% to HGD and 6.9% to carcinoma[27]. Accordingly, gastric lesions with any degree of biopsy proven dysplasia should be regarded as potentially housing adenocarcinoma.
Endoscopic imaging techniques
The endoscopic detection and characterisation of EGCs and precancerous lesions is therefore critical in establishing cancer risk and directing appropriate treatment and surveillance. Consensus guidelines from the BSG and the Japanese Gastroenterological Endoscopy Society (JGES) define endoscopic standards to maximise detection of gastric mucosal lesions, including a minimum 7 min gastric examination time, as well as adequate mucosal visualisation using sufficient air insufflation, mucosal cleaning techniques (mucolytic and defoaming agents) and consideration of peristalsis inhibiting medications if views are obstructed by movement[28,29]. While a consensus has not yet been reached regarding the role of advanced endoscopic imaging techniques, a number of recent advances in endoscopic imaging have demonstrated higher detection rates and more accurate characterisation of mucosal lesions.
White light endoscopy: White light endoscopy (WLE) is the conventional endoscopic imaging modality. It is sufficient for detecting some features of carcinomatous transformation of gastric lesions, including red discolouration of the mucosal surface, depressed-type lesions and mucosal ulceration[30,31]. However, the sensitivity for WLE in detecting GIM, LGD/HGD and EGC has been reported to be as low as 29%-59.1%, 51%-74% and 48%-72% respectively[32-36]. As a result, more advanced endoscopic imaging techniques are now used for lesion detection and characterisation.
Chromoendoscopy: Traditional chromoendoscopy requires the topical application of stains or dyes (usually methylene blue, indigo carmine and/or acetic acid) in an effort to enhance tissue characterisation[37,38]. Studies have demonstrated the superiority of chromoendoscopy in the detection of EGCs and precancerous gastric lesions, with a 2016 meta-analysis demonstrating pooled sensitivity of 90% and specificity of 82%[38]. However, the requirement for local dye application has limited its use in the context of the wide field required for gastric lesion detection, particularly following the development of virtual chromoendoscopy.
Narrow-band imaging: Narrow-band imaging (NBI) is the most commonly used form of virtual chromoendoscopy; utilising a rotating optical interference filter to restrict incident light into two narrow bands of different wavelengths (Figure 1). This enhances the definition of the surface mucosa, while emphasising the contrast of the vascular network[39]. NBI is superior to WLE in detection of early precancerous gastric lesions, with pooled sensitivity of 69% and specificity of 91% for GIM[40]. Prospective trials have demonstrated a 40% increase in detection of all focal gastric lesions and more than twice the detection rates for GIM using NBI compared to WLE[32,41].
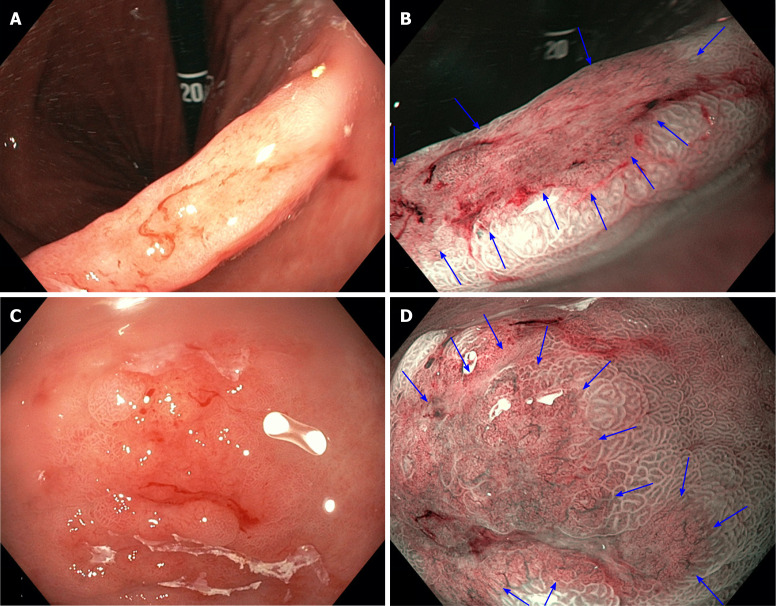
Figure 1.
White light endoscopy compared to narrow-band imaging in gastric lesions, demonstrating clear demarcation lines and irregular microvascular/microsurface patterns on narrow-band imaging. A: Early gastric cancer at the incisura seen on white light endoscopy (WLE); B: The same lesion seen on narrow-band imaging (NBI) (blue arrows); C: Early gastric cancer in the antrum seen on WLE; D: The same lesion seen on NBI (blue arrows).
High-magnification NBI: High-magnification endoscopy (Figure 2) uses a movable lens in the tip of the endoscope to allow up to 150 times optical zoom without any degradation of image quality[42]. This is usually combined with the use of a translucent cap to stabilize the focal length between the lens and the target tissue, as well as near-focus imaging that allows the endoscope to be positioned closer to the mucosal surface[42]. High-magnification endoscopy has been combined with NBI (ME-NBI) to facilitate detailed assessment of the mucosa and enhance lesion detection and characterisation.
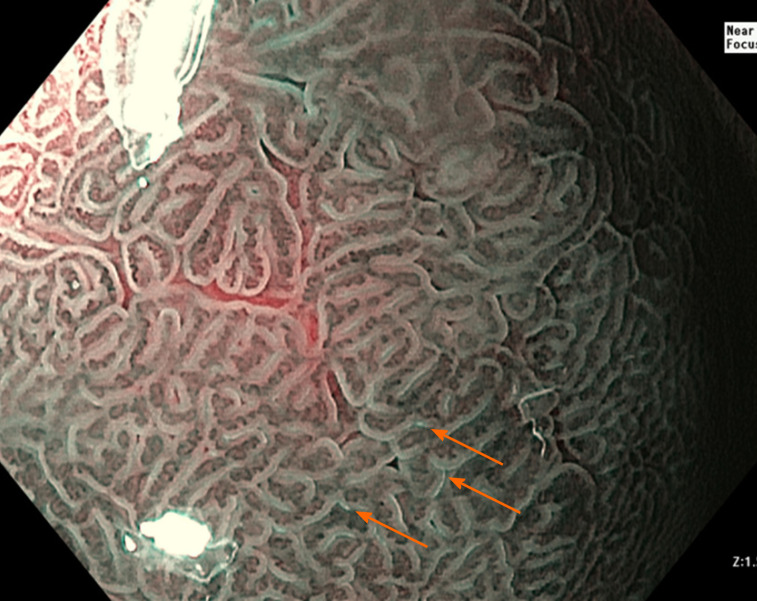
Figure 2.
Narrow-band imaging demonstrating the ‘light blue crest’ (orange arrows) consistent with intestinal metaplasia.
Uedo et al[42] first described the ‘light blue crest’ seen on ME-NBI (Figure 2) which has been demonstrated to detect GIM with a sensitivity of 80%-89% and a specificity of 93%-96%[36,43,44]. In regard to characterisation of dysplasia, a prospective multicentre study in 2016 showed an improvement in sensitivity from 74% to 92% with the use of ME-NBI over WLE[36]. Detailed assessment of surface microvascular patterns on polypoid lesions using ME-NBI results in sensitivity of up to 86.2% and specificity of up to 97% for the presence of dysplasia[45].
In the assessment of EGCs, there have been a number of classification systems using ME-NBI. Yao et al[45] first described the ‘VS’ system in 2008, which has a sensitivity of 86%-97% for EGCs[46-49]. This system requires detailed assessment of the microvascular and surface mucosal pattern to ascertain features of irregularity, which in the presence of a demarcation line is suggestive of carcinoma[46]. The VS classification was further simplified by Yamada et al[49] in 2014, who demonstrated that the presence of a demarcation line with an irregular microvascular pattern was 95% sensitive and 96% specific for EGC[49].
The accuracy of ME-NBE was confirmed in a 2015 meta-analysis of a combined 2171 patients, demonstrating 86% sensitivity and 96% specificity for the diagnosis of EGC[50,51]. Direct comparator studies of ME-NBI vs WLE have also shown up to twice the rate of EGC detection with ME-NBI[52-54].
In addition, ME-NBI is valuable in determining tumour margins at the time of ER, resulting in 97.4%-98.1% accuracy of endoscopic markings[55-57]. Comparator studies have demonstrated the superior precision of ME-NBI vs chromoendoscopy in this context, with 17%-20% higher rates of accurate endoscopic markings[55,57]. The use of ME-NBI is also beneficial when lesion margins are unclear on chromoendoscopy, as demonstrated by Nagahama et al[57] who found that 72.6% of lesions with an unclear margin on chromoendoscopy were able to be clearly delineated using ME-NBI[57].
Blue laser imaging: Blue laser imaging (BLI) uses laser to create a highly narrowed blue band, enhancing vascular structures without using a filter as is required for NBI, thereby resulting in a higher light intensity[58,59]. There is a paucity of evidence regarding BLI in comparison to other endoscopic imaging, however recent studies have demonstrated superior sensitivity (93%-94%) in detection of EGCs compared to WLE (46%-50%)[60,61]. While direct comparator studies are limited, Kaneko et al[61] compared BLI and NBI in 39 patients with gastrointestinal neoplasia and found that BLI maintained sufficient brightness and contrast up to 40 mm while NBI deteriorated beyond 20 mm. This could be of relevance in detection of EGCs where endoscopists may benefit from increased field of view, however further studies in this area are required.
Confocal laser endomicroscopy: Confocal laser endomicroscopy (CLE) uses a low-power laser to illuminate tissue, detecting reflected fluorescent light and allowing high-resolution endoscopic histological assessment[62,63]. CLE was first assessed in precancerous gastric lesions by Guo et al[63] who reported sensitivity and specificity of 98% and 95% for the detection of GIM[63]. A more recent meta-analysis demonstrated the accuracy of CLE for the diagnosis of all stages of precancerous gastric lesions and EGCs, with pooled sensitivity of 92% for GIM, 81% for LGD/HGD and 91% for EGC[64,65]. However, its use is limited by the equipment required (either a dedicated endoscopic system or a probe-based system inserted via the therapeutic channel), the time required for image acquisition, as well as the learning curve of image interpretation[63].
Future prospects
Artificial intelligence (AI) has demonstrated efficacy in detection and classification of multiple gastrointestinal lesions[66]. Real-time AI gastric lesion detection has not yet been studied, however convolutional neural networks have been generated from endoscopic images with a sensitivity of 92% using WLE and 97% using ME-NBI[67-69]. This could be of particular benefit for improving lesion detection and characterisation for endoscopists without expertise in advanced imaging interpretation.
Texture and colour enhancement imaging (TXI) is a recently developed technology aiming to improve lesion detection by enhancing texture, brightness and colour tone (Figure 3) using stacked images, while maintaining a similar colour spectrum to WLE[70]. By improving lesion detection within WLE, TXI may facilitate superior detection rates during endoscopic screening, although studies are required to investigate this.
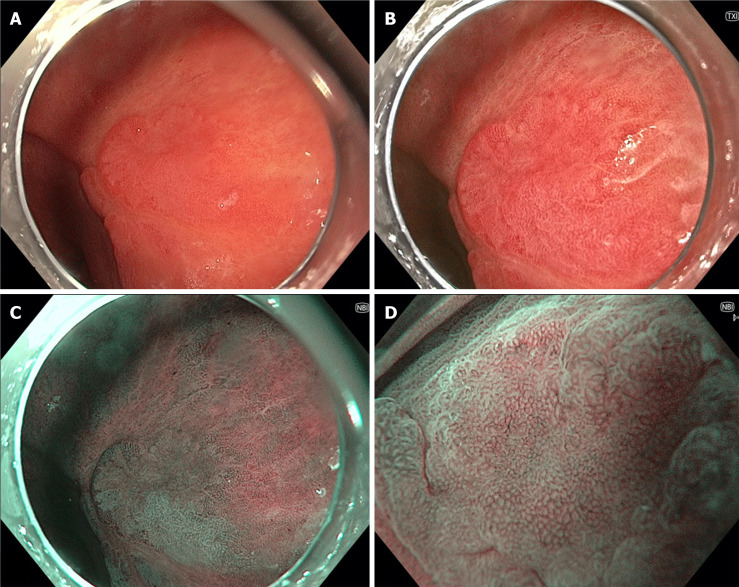
Figure 3.
Gastric body lesion with low-grade dysplasia seen on multiple forms of endoscopic imaging. A: White light endoscopy; B: Texture and colour enhancement imaging; C: Narrow-band imaging (NBI); D: high-magnification NBI.
Whilst WLE remains the mainstay for the majority of endoscopists, accumulating evidence as presented above points to a significant additive benefit in repeat interrogation of suspicious areas with chromoendoscopy. The trade-off between time, cost, availability and feasibility means that most proceduralists employ virtual chromoendoscopy with ME-NBI.
Assessment of invasion depth and nodal metastases
Endoscopic ultrasound: While computed tomography (CT) and positron emission tomography/CT are endorsed by guidelines for the assessment of distant metastases and locally advanced gastric cancers, endoscopic ultrasound (EUS) has been recommended for EGCs, in particular for distinction of T1 and T2 lesions[71]. However, data on the utility of EUS in assessment of EGC depth of invasion have been varied. Han et al[72] reported under-staging in 16.7% of T2 gastric cancers with EUS, and Choi et al[71] reported no advantage of EUS over conventional endoscopic assessment using surface nodularity and fold convergence[71]. In comparison, Mouri et al[73] reported that lesions classified by EUS as being intramucosal or submucosal border lesions were histologically either intramucosal or invading < 500 μm into the submucosal space (SM1) in 99% and 87% of cases[73]. A 2015 Cochrane review also demonstrated 87% sensitivity for submucosal invasion[74,75]. Regarding specific EUS characteristics, Kim et al[76] reported that an arch-shaped submucosal deformity on EUS was associated with a negative predictive value for SM2 (> 500 μm submucosal invasion) or greater of 94.4%, with a sensitivity and specificity of 84% and 83%[76]. More recently, EUS miniature probes have been demonstrated to have higher resolution but less penetration compared to conventional EUS[77]. Miniature probes have demonstrated superiority for assessment of submucosal invasion, with an overall accuracy of 84.5% vs 61.6% using conventional EUS when assessing lesions less than 2 cm in diameter without endoscopic features of submucosal invasion[78]. While the role of conventional EUS in the confirmation of suitability for ER remains unclear, miniature probe EUS may have a role in < 2 cm lesions which are indeterminate endoscopically.
Concurrent to these improvements in EUS, the addition of technologies such as CT with multiplanar reformations and virtual gastroscopy has led to overall accuracy as high as 94% for the diagnosis of EGC[79]. In addition, high-speed magnetic resonance imaging (MRI) and diffusion-weighted imaging have also addressed many of the limitations of MRI for the assessment of T-stage, though this remains infrequently used in the assessment of EGCs[80,81]. While a detailed comparison of radiological methods for staging gastric cancers is beyond the scope of this review, improvements in these technologies may facilitate accurate non-invasive assessment of EGCs in the future.
With respect to exclusion of nodal disease prior to ER for EGC, the use of EUS has again been associated with variable accuracy. In a 2008 meta-analysis, the pooled sensitivity of EUS for N1 disease was as low as 58.2%[82]. More recent data have shown an improved sensitivity and specificity of 74%-83% and 67%-70% in detecting nodal positivity[75,83,84]. However, this improved accuracy still remains inadequate to guide consideration of endoscopic management of disease with metastatic potential. Despite technological advances, CT also remains inaccurate for the detection of nodal metastases, with sensitivity between 62.5%-91.9%[85]. Accordingly, gastric cancers with a significant potential for lymph node metastasis based on lesion characteristics are generally managed surgically with lymphadenectomy to evaluate for lymphatic involvement, as reliable non-invasive exclusion of lymph node metastases (LNM) remains elusive.
ENDOSCOPIC TREATMENT OF GASTRIC NEOPLASIA
ER is indicated for all discrete gastric lesions with histological evidence of dysplasia given the risk of concurrent or future EGC. In addition, data supports ER for lesions histologically indefinite for dysplasia, as the rate of true dysplasia or EGC in resected specimens is as high as 90.8%, particularly in males; lesions > 5 mm in diameter; or lesions with erosions[86]. For gastric mucosal lesions < 1 cm in diameter with LGD, either endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) can be performed, however ESD is preferred in larger lesions and those demonstrating HGD/EGC[87].
Indications for ESD
In the context of histologically confirmed carcinoma, ESD was initially indicated only for the resection of macroscopically intramucosal (T1a) differentiated gastric carcinomas < 2 cm in diameter without ulcer or scar[87]. These have subsequently been labelled ‘absolute criteria’, as data now support the efficacy of ESD for the resection of a broader range of EGCs termed ‘expanded criteria’. In the 2015 JGES guidelines, expanded criteria lesions include all differentiated T1a EGCs > 2 cm without ulcer/scar, differentiated T1a lesions < 3 cm with ulcer/scar, and undifferentiated EGCs < 2 cm in diameter, while the 2015 European Society of Gastrointestinal Endoscopy (ESGE) guidelines also include differentiated lesions < 3 cm with superficial submucosal invasion (SM1 ≤ 500 μm)[87,88].
Another evolving potential indication for ESD is for the resection of submucosal gastric tumours. He et al[88] first demonstrated the efficacy and safety of ESD for gastrointestinal stromal tumours (GIST) in 2013, when they reported successful ESD in 25 large gastric GISTs with a mean diameter of 2.7 cm[88]. More recently, An et al[89] published data on 168 cases of ESD for gastric GISTs, with complete and en bloc resection in 100% of lesions[89]. Delayed bleeding occurred in only 1.2% and there were no other significant complications. While additional data are required before recommending ESD as a standard of care option for GIST management, early data support this as a potential future indication.
Efficacy of ESD
Data on the efficacy of ESD (Table 1) are particularly robust in East Asian countries where the incidence of gastric cancer is significantly higher than it is in Western countries. Studies in Japan and South Korea have demonstrated en bloc resection in 95.3%-99.2%, complete resection in 87.7%-95.5% and curative resection in 81.7%-84.1%[90-94]. In Western countries however, data are more limited and results are variable. En bloc resection rates are between 92%-97.8%, with complete resection in 75.6%-89% and curative resection in 72.2%-79.2%[95-100].
Table 1.
Studies reporting en bloc, complete and curative resection rates using endoscopic submucosal dissection
| Ref. | Location | Total lesions | En bloc resection | Complete resection | Curative resection |
| Suzuki et al[90], 2019 | Japan | 10821 | 99.2% | 91.6% | 81.7% |
| Ryu et al[91], 2018 | Korea | 1541 | 97.3% | 95.5% | N/A |
| Chung et al[92], 2009 | Korea | 1000 | 95.3% | 87.7% | N/A |
| Watanabe et al[93], 2017 | Japan | 511 | 97.4% | 92.9% | 84.1% |
| Ngamruengphong et al[94], 2020 | North America | 347 | 92% | 82% | N/A |
| Manta et al[95], 2020 | Italy | 299 | 97.6% | 89% | 72.5% |
| Abdelrahim et al[96], 2019 | Europe | 175 | 92.5% | 83.4% | N/A |
| Tate et al[99], 2019 | Australia | 135 | 94.8% | 86.7% | 79.2% |
| Pagano et al[98], 2019 | Italy | 41 | 97.8% | 75.6% | 72.2% |
N/A: No application.
One contributing factor to this discrepancy is the differing incidence of gastric cancer, resulting in comparatively limited experience in Western centres. This was supported by a 2020 Italian survey where only 41% of included interventionalists had performed more than 80 ESDs and 31% had performed < 40[98]. This study also demonstrated that rates of en bloc resection were higher and rates of perforation lower in more experienced proceduralists. However, there are multiple other contributing factors. Firstly, there are differences in lesion classification, as the Japanese classification of gastric carcinoma includes EGCs which would by European guidelines be classified as HGD[101]. The non-aggressive and non-invasive nature of HGD would result in bias in favour of Japanese outcomes. Further to this, there appear to be differences in disease behaviour in East Asian compared to Western populations. Studies in Korea reviewing rates of LNM in surgical specimens at gastrectomy have reported LNM in 0.3% of absolute criteria lesions and 0.4% in expanded criteria, while marginally higher at 3.2% in undifferentiated carcinomas[102-104]. In contrast, American studies report LNM in 7.5%-13.6% of expanded criteria lesions at gastrectomy[105,106]. Guidelines developed based on data from East Asian populations should therefore be used with caution in Western populations until further evidence is able to clarify this.
EMR vs ESD: ESD is associated with longer procedure times and an almost three times higher perforation rate compared to EMR, however multiple meta-analyses have established the superiority of ESD over EMR in regard to en bloc resection rates (OR 9-10 for ESD), complete resection rates (OR 5.7-8.4) and curative resection rates (OR 2.9[38,107,108]). Tao et al[107] also reported a reduction in local recurrence (OR 0.18), likely resulting from the aforementioned superior rates of en bloc, complete and curative resection[107].
ESD vs surgery: Increasing evidence suggests that ESD is associated with equivalent overall survival compared to gastrectomy, while reducing procedural complications. A 2015 retrospective Chinese study used propensity score matching to compare outcomes for surgery and ESD in 176 patients with a median follow up of 77 mo[109]. There was no significant difference in overall survival, and while local recurrence occurred in 1.7% of ESD, all but one of the recurrent lesions were treated endoscopically. There was no difference in early complication rates, but late complications were significantly more common in the surgical group (6.8% vs 0%). A similar 2017 Korean study found no difference in 5-year survival between groups, with a complication rate of 15% in gastrectomy vs 5.1% in ESD[110]. In regard to expanded criteria lesions, data have also reported equivalent survival with a reduction in complications using ESD, with a 2017 study actually demonstrating improved 5-year survival after propensity score matching in the ESD group (97.1% vs 85.8%)[109,111,112]. Unsurprisingly, a recent meta-analysis reported higher rates of local recurrence and metachronous cancer in the ESD group, resulting in lower disease-free survival (HR 4.58)[113]. However, the majority of recurrence is able to be treated with repeat ESD and thus lower disease-free survival is not reflected in any difference in disease-specific survival[109,113]. Further to this, the same meta-analysis reported a mean 128 min shorter operation time, 7 d shorter hospital stay, lower procedure-related death and fewer complications[113].
In regard to complications of surgical management, gastrectomy is associated with significant post-operative complication rates of 9.6%-18.1% for laparoscopic and 17.4%-29.3% for open gastrectomy, as well as a 30-d mortality rate of 2%-4.1%[3,114-116]. In addition, Yu et al[116] assessed quality of life scores after gastrectomy and found that fatigue, diarrhoea, reflux, dysphagia and eating restriction scores all remained persistently worse at 5-year follow-up after surgery[116].
A 2015 study demonstrated the lower overall medical costs of ESD compared to surgery for EGC, with ESD costing a median $2374 USD compared to $4954 USD for surgery (P < 0.001)[117,118]. This was confirmed by Shin et al[109] who reported approximate total hospital stay costs averaging $1871 USD for ESD vs $5925 USD for subtotal gastrectomy and $6476 for total gastrectomy[109].
Long-term outcomes: Long-term data on ESD for EGCs has been extensively reported in Japan and South Korea, where studies including up to 5-9 years of follow-up have documented local recurrence rates of 0%-1.8% in absolute criteria lesions and 0.6%-7.0% in expanded criteria lesions[119-123]. In these same cohorts there were no cases of LNM in absolute criteria lesions, and LNM rates were between 0%-0.48% in expanded criteria lesions. Min et al[123] reported long-term outcomes (48 mo follow-up) for only lesions meeting criteria for curative resection, in whom 0.3% of absolute and no expanded criteria lesions had local recurrence[123]. In a study by Suzuki et al[124] in 2015 (again reporting only on curative resections), despite overall 5-year overall survival being as low as 92.2% due to other comorbidities, the 5-year disease-specific survival was 99.9% in both absolute and expanded criteria lesions[124]. In Western populations, long-term data are limited, but studies have reported local recurrence rates of 4.8%-7% for expanded criteria lesions[125-127].
ESD in ‘outside of criteria’ lesions: ESD is often also employed for the treatment of lesions outside of even the expanded criteria when patients are unsuitable for major surgery. Abe et al[127] followed 14 patients who had ESD for undifferentiated non-curatively resected lesions who were not suitable for or had declined further surgery[127]. Over a median of 76.4 mo follow-up, there was only one case of local recurrence which was managed with repeat ESD. A 2013 Japanese study reviewed 104 patients who undergone ESD despite being outside of criteria in regard to lesion characteristics, who had declined or been deemed unsuitable for surgical management[128,129]. Patients were followed-up for a median of 47 mo after resection, with 5-year overall survival of 70% but disease-specific survival of 91.5%. In patients with comorbidities precluding them from major surgery, ESD may therefore be beneficial even in lesions outside of the expanded criteria.
Approach to incomplete and non-curative resection
Incomplete resection: The limited available evidence supports repeat ESD where possible for lesions with positive horizontal margins after initial resection. Jung et al[129] reported 28 patients with a positive horizontal margin after initial ESD, in whom the curative resection rate of repeat ESD was 89.3%[129]. In a 2018 study, Jeon et al[130] also reported improved disease-free survival following repeat ESD for non-curative resections due to positive lateral margins (89.2% vs 69.1%)[130].
Non-curative resection: The management of lesions determined to be beyond the expanded criteria histologically after ER (non-curative resection) remains controversial. A large 2019 meta-analysis demonstrated improved 5-year overall and disease-specific survival (OR 3.5 and 3.99 respectively) following additional surgery after non-curative resection[131,132]. However, these data are retrospective and are therefore susceptible to bias associated with the selection of less systemically unwell and comorbid patients in the additional surgery group. Other studies have reported rates of LNM or residual tumour as low as 5.1%, with differences in overall survival but no difference in disease-specific survival between those treated with additional gastrectomy and those managed with surveillance[133,134]. These studies have hypothesised that further data may delineate a group in whom surveillance after non-curative resection is associated with equivalent long-term outcomes.
Multiple studies have addressed factors to stratify the risk of LNM or residual tumour in patients who have had non-curative ESD. In regard to LNM, lymphovascular invasion (LVI) and depth of submucosal invasion histologically are reported to be the most consistent predictors[135,136]. Goto et al[134] reported that the presence of depth of invasion > SM1 or LVI was 100% sensitive and 86% specific for the presence of LNM[135]. For predicting the presence of residual tumour, a 2020 meta-analysis reported significant risk factors to be tumour size > 30 mm and the presence of positive horizontal margins[137]. Although additional data are required to guide decision making, patients who have non-curative resections may not warrant additional surgery provided the invasion depth is ≤ SM1 without evidence of LVI, while patients with tumour size > 30 mm or positive horizontal margin may be appropriate for repeat ESD.
This concept led to a 2017 study by Hatta et al[137], who developed the eCura scoring system for predicting the risk of LNM using data from 1101 patients who underwent radical surgery after non-curative ESD[137]. The presence of a positive vertical margin, submucosal invasion > SM1, tumour size > 30 mm and vascular invasion were each assigned 1 point, while lymphatic invasion was assigned 3 points. Patients scoring 0-1 points were classified as low-risk (2.5% risk of LNM), 2-4 points intermediate-risk (6.7% risk of LNM) and 5-7 points high-risk (22.7% risk of LNM). The scoring system was then validated on 905 patients with non-curative ESD who declined surgery. 5-year cancer-specific survival was 99.6% in the low-risk group (60.4% of patients), 96% in the intermediate risk group (27.6% of patients) and 90% in the high-risk group (11.9% of patients), suggesting that ESD without additional surgical management may be sufficient in low-risk patients according to the eCura system[138].
These risk stratification tools are of particular importance when considering patients with radiologically enlarged lymph nodes that may or may not signify the presence of LNM. Lee et al[138] reported 47 cases of ESD for expanded criteria lesions, where patients had at least one enlarged lymph node on CT[138]. 12 of 47 patients had surgical resection, with no evidence of metastatic disease, while the remaining 35 patients had serial CT monitoring over a median 56 mo. Of these 35 patients, 21 patients had reduction in size or complete resolution of lymphadenopathy, while 13 patients had no change. Only one case had progressive lymph node enlargement but declined biopsy due to age. Assessment of the risk of LNM may therefore help direct decision making regarding the need for additional surgery in the context of radiologically enlarged lymph nodes.
In patients at risk of LNM, future studies may explore the role of ER with additional laparoscopic lymph node resection. A 2011 study by Cho et al[139] described 9 patients who had endoscopic full thickness resection (FTR) with laparoscopic regional lymph node resection[139]. Despite 4 lesions having histological LVI, there were no cases of LNM. There may also be a future role for targeted lymph node resection using sentinel node detection strategies. While data have been inconsistent regarding the accuracy of gastric sentinel node detection, these strategies have been most successful in T1 EGCs and thus may be of use following higher-risk ESD[140,141]. Studies using dye have reported a sensitivity of only 75%, however in the context of dual tracer with radiolabelled tin colloid and blue dye, the sensitivity for LNM is up to 93%, with an overall accuracy of 99%[142]. Further studies are therefore required to assess the adequacy of sentinel node guided laparoscopic lymph node resection as a treatment strategy after non-curative ESD.
Complications of ESD
While ESD offers the promise of reduced morbidity, both acute (bleeding, perforation) and chronic (stenosis, recurrence, metachronous lesions) complications can occur[107,108,113].
Bleeding: The risk of delayed bleeding with ESD is equivalent to that in EMR, with most studies reporting rates between 4% and 6%[143-146]. Nam et al[145] retrospectively reviewed 1,864 cases of ESD, in which post-procedural bleeding occurred in 4.1% of patients, with the majority occurring within 24 h of ESD[145]. In their multivariate analysis, predictors of delayed bleeding were patient age ≤ 65 years, resection size > 30 mm, procedure time > 20 min, lesions located in the lower third of the stomach and the presence of erosions. In regard to treatment, delayed bleeding after ESD is generally amenable to routine endoscopic management[146].
Studies have addressed prevention strategies for post-ESD bleeding, with data supporting post-operative proton-pump inhibitor (PPI) use after ESD with an OR of 0.4-0.49 for delayed bleeding[147,148]. A 2016 meta-analysis on the use of PPI prior to ESD found a reduction in gastric pH at the time of procedure, but no pooled difference in delayed bleeding[149]. Previously, routine re-look endoscopies were performed in many centres after ESD in an attempt to prevent rebleeding; a method extrapolated from data supporting second endoscopy after treatment of bleeding peptic ulcers[150]. However, Goto et al[144] reported no difference in rates of bleeding before or after routine follow-up endoscopy within one week of initial ESD, suggesting limited benefit from this strategy[144].
Perforation: Perforation occurs more frequently in ESD than in EMR[107,108]. Rates of perforation are generally reported between 1.5% and 9.6%, with a large meta-analysis by Arezzo et al[150] demonstrating a pooled perforation rate of 4.9%[95,100,151-156]. Risk factors for perforation include lesions located in the upper third of the stomach, the presence of submucosal invasion or fibrosis and longer procedure times[152,153,157]. Longer procedure times may reflect either more complicated resections or less proceduralist experience, while perforations in upper third gastric lesions likely reflect thinner proximal gastric wall in comparison to the antrum[158].
When macroscopic perforations occur during ESD, more than 97% of cases are able to be treated endoscopically with clip closure[155,156]. Micro-perforations, or suspected perforations that are not endoscopically visible, are usually able to be conservatively managed with fasting and intravenous antibiotics[155].
Delayed perforation, when no intraprocedural perforation occurs but symptoms of peritonism and radiological evidence of perforation develop post-procedure, is a phenomenon that appears to be specific to ESD. It is most common in the thin upper third of the stomach and the majority of cases occur within 24 h[159,160]. Yamamoto et al[158] reported 5 cases of delayed perforation in a 2017 case series, of which 4 out of 5 were able to be managed endoscopically[158].
Stenosis: Post-ESD stenosis occurs most commonly following resection of gastric antral (occurring in up to 7% of cases) or cardiac lesions, presenting with either oesophageal or gastric outlet obstruction[161,162]. Sumiyoshi et al[160] analysed predisposing factors, with the only risk factor after multivariate analysis being a > 75% circumferential post-resection defect[160]. In regard to treatment, while data is limited, most patients have improvement in their symptoms following either balloon dilation if severe, or conservative management if mild-moderate symptoms[162,163].
Local recurrence and metachronous gastric lesions: Guidelines recommend close ongoing surveillance after ESD for EGC, with ESGE and JGES guidelines suggesting 3- to 6-monthly and 6- to 12-monthly surveillance endoscopy respectively[87,88]. The aim of surveillance after ESD is for early detection of not only local recurrence but also metachronous precancerous gastric lesions. These lesions occur in up to 6.9%-13% of patients after ESD, with a 2019 study reporting a cumulative incidence of metachronous gastric cancer of 33.2 cases per 1000 patient-years[95,164,165]. In regard to treatment of metachronous lesions, Kim et al[165] reported 117 cases of metachronous gastric neoplasms, of which 77% were able to be retreated with curative ESD[165].
FUTURE DIRECTIONS FOR THE ENDOSCOPIC TREATMENT OF GASTRIC NEOPLASIA
Methods for generating counter-traction
Generating counter-traction during ESD is of benefit in reducing procedural time and complications[166,167]. Depending on the position of the lesion being resected, gravity may not provide sufficient access to submucosal tissue planes. There have been a number of recent developments with regard to endoscopic equipment and techniques to generate counter-traction.
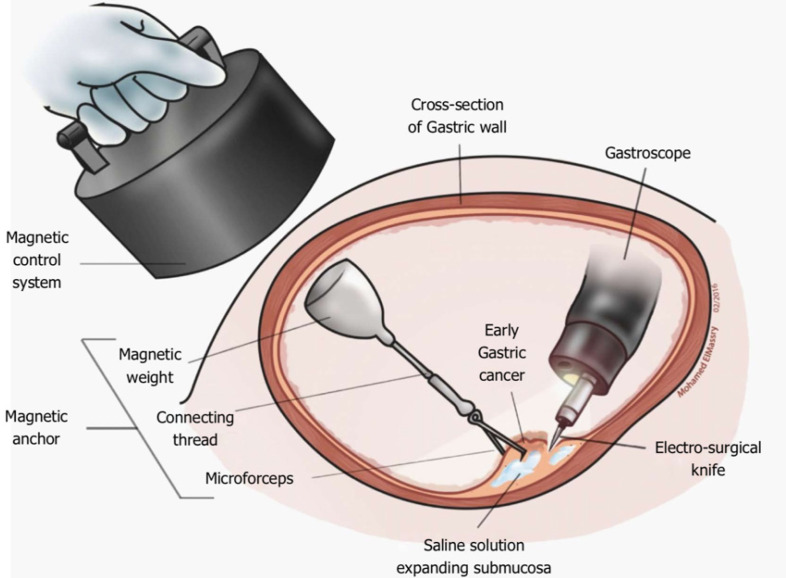
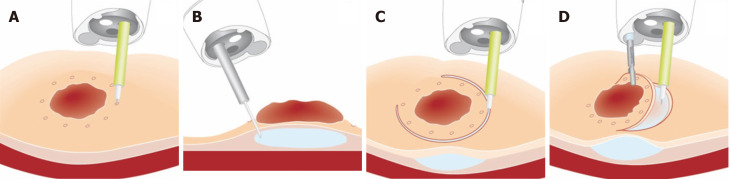
Magnetic anchor guided ESD: Magnetic anchor guided ESD uses a large external electromagnet combined with an internal magnet attached to the lesion via a clip (Figure 4). No comparative studies have assessed the advantages of this technique over conventional ESD, however studies have shown feasibility as well as subjective benefit from the point of view of endoscopists[168,169]. The main limitations of this technique are the equipment required and the coupling strength of the magnets relative to the abdominal wall thickness[170].
Figure 4.
Magnetic anchor-guided endoscopic submucosal dissection[169]. Citation: Mortagy M, Mehta N, Parsi MA, Abe S, Stevens T, Vargo JJ, Saito Y, Bhatt A. Magnetic anchor guidance for endoscopic submucosal dissection and other endoscopic procedures. World J Gastroenterol 2017; 23: 2883-2890. ©The Author(s) 2017. Published by Baishideng Publishing Group Inc.
Dual channel endoscope: This technique requires a dedicated dual working channel endoscope, often employed for the ‘grasp-and-snare’ technique in EMR[171]. Hua et al[171] compared conventional ESD (24 patients) with dual channel ESD (22 patients), using one channel for dissection while grasping the lesion via the second channel[171]. Mean procedure times were significantly shorter with dual channel ESD (20.5 min vs 49.1 min) with no other differences in outcomes or complications. There are two main limitations of dual channel endoscopy: The requirement for a dedicated endoscope and the limited distance between working channels inhibiting angulation of traction.
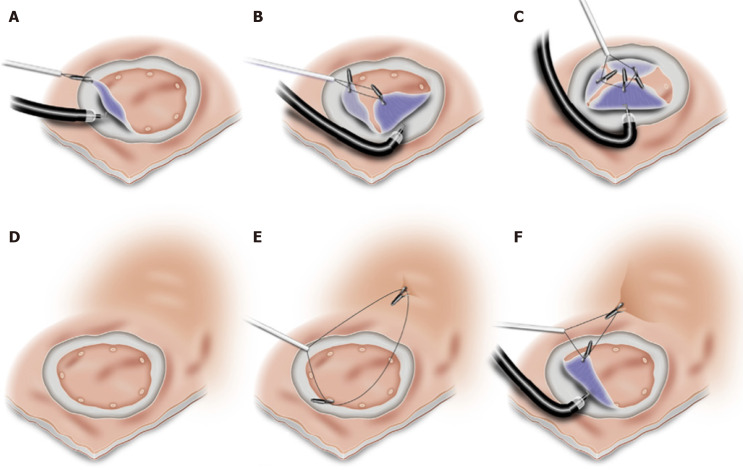
Additional working channel: The use of an attachable additional working channel (Figure 5) aims to overcome some of these limitations of dual channel endoscopy by providing greater distance between working channels as well as allowing the use of a conventional therapeutic endoscope. The additional working channel consists of a flexible attachment, a long shaft and an adaptor for fixation at the endoscope handle[172,173]. This technique has been compared with conventional ESD in porcine stomachs, where it resulted in a reduction in procedure time (24.5 min vs 32.5 min) and lower rates of muscularis damage (3.13% vs 18.75%)[173]. However, minimal data exist in humans apart from feasibility studies reporting successful resection of 8 lesions by ESD and 6 lesions by EMR[174,175]. The main limitation of this method is the inability to generate significant angulation on the grasping forceps, preventing the most effective direction of counter-traction.
Figure 5.
Endoscopic submucosal dissection using an additional working channel[172]. A: Lesion marked via usual working channel; B: Submucosal injection; C: Near-circumferential incision made; D: Lesion grasped for countertraction via additional working channel while dissection underway. Citation: Knoop RF, Wedi E, Petzold G, Bremer SCB, Amanzada A, Ellenrieder V, Neesse A, Kunsch S. Endoscopic submucosal dissection with an additional working channel (ESD+): a novel technique to improve procedure time and safety of ESD. Surg Endosc 2021; 35: 3506-3512. ©The Author(s) 2021. Published by Springer Open Access Article.
Double endoscopes: Using two separate endoscopes allows maximal angulation for the grasping forceps, optimising counter-traction. Generally, the first endoscope is inserted and a circumferential incision is made. Following this, a second smaller calibre endoscope is inserted to grasp and lift the edge of the lesion while the proceduralist proceeds with dissection. In 2017, Ogata et al[175] demonstrated the efficacy of this method in 122 patients, with a 97.5% en bloc resection rate and 86.9% curative resection rate, with perforation and delayed bleeding in 3.3% and 2.5% respectively[175]. While there are no direct comparator trials with conventional ESD, Çolak et al[176] employed this method in 6 patients where positioning made conventional ESD difficult[176]. They reported no difference in resection times compared to more straightforward ESDs. There are a number of obvious limitations to this method, including the requirement for two endoscopists, endoscopes and light sources, as well as space limitations in both the oral cavity and within the lumen where one endoscope can obstruct the view of the other[176]. Other groups have reported various methods to overcome some of these limitations. Higuchi et al[177] reported double endoscope ESD using a single light source, where the light source from the first endoscope is removed and attached to the second endoscope for insertion until the lesion is grasped[177]. Following this, the light source is reattached to the initial main endoscope. This technique resulted in improved accuracy of dissection compared to historical controls, without any serious intraprocedural complications. Alternatively, Ahn et al[178] employed a trans-nasal endoscope for applying traction, maximising space within the oral cavity and within the gastrointestinal tract due to the smaller calibre endoscope[178].
Endo-lifter: The ‘endo-lifter’ (Figure 6) consists of a transparent hood with grasping forceps, mounted on the tip of an endoscope, with the forceps running externally to the endoscope to allow dissection via the working channel[179]. The attachment of the forceps to the hood produces an arc that aims to provide superior angulation for countertraction. Minimal data exist using the endo-lifter in animal models, with variable results. Schölvinck et al[180] reported shortened procedure times when used by ESD-experienced but not inexperienced endoscopists[180]. Teo et al[179] reported increased visualisation of the submucosa and a reduction in subjective procedural difficulty rating from endoscopists[179].
Figure 6.
‘Endo-lifter’ (Olympus-Tokyo, Japan)[192]. Citation: Harlow C, Sivananthan A, Ayaru L, Patel K, Darzi A, Patel N. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc 2020; 13: 2631774520957220. ©The Author(s) 2020. Published by Open Access Article.
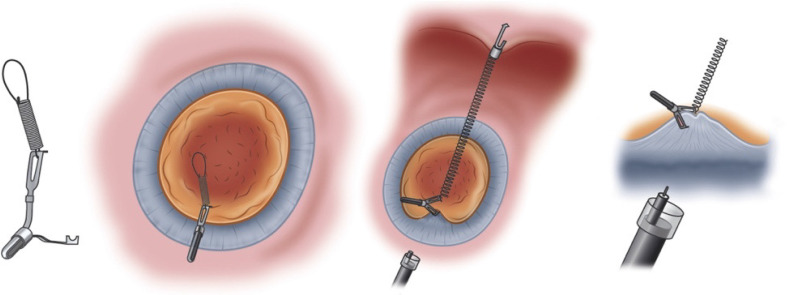
Spring and loop clip traction (using an S-O clip): This method uses the S-O clip (Figure 7) which is attached to a spring and then a loop of nylon[181,182]. One clip is attached to the edge of the lesion, while the other clip is attached to an area of opposing gastric wall to provide traction. Nagata[181] reported data from 140 patients of which 51 had spring and loop clip traction, with shorter procedure times and no difference in complication rates compared to conventional ESD[181]. The main limitations of this technique are the requirement for specific equipment (i.e., the S-O clip) as well as only allowing one direction of traction once the clips are deployed.
Figure 7.
Spring and loop clip traction[193]. Citation: Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc 2021; 93: 1097-1109. ©The Author(s) 2021. Published by Open Access Article.
Other clip methods: Various methods have been described employing readily available endoscopic equipment with clips to minimise cost. Yoshida et al[182] reported the efficacy of the ‘dental floss clip’ (DFC) traction device using dental floss tied to the proximal end of a clip before attaching the clip to the edge of the lesion, with the dental floss running externally to the endoscope[182]. There was no difference in procedure time overall, however in upper and middle greater curvature lesions DFC traction reduced procedure time by approximately 50%. In addition, the use of DFC traction reduced perforation rates compared to conventional ESD (0.3% vs 2.2%)[183]. Noda et al[183] added a polypectomy snare sheath external to the endoscope through which the thread (dental floss) was passed, which was then inserted along with the endoscope. The use of the sheath allows the thread to be moved without interference from the endoscope, as well as allowing both ‘pull’ and ‘push’ motions by advancing the entire sheath. In an 88 patient study comparing polypectomy snare sheath ESD with conventional ESD, this technique resulted in a reduction in procedure time and lower rates of significant bleeding[183]. Yoshida et al[184] also reported a reduction in procedure time by simplifying this technique to their ‘clip and snare’ technique: A snare with its sheath external to the endoscope is attached to the proximal end of a clip, then inserted and attached to the lesion[184]. Zhang et al[185] then modified the clip and snare technique (Figure 8), by using additional clips to attach the snare to the circumference of the lesion, allowing multi-focal lifting of the lesion, as well as placing clips attached to the snare on opposing gastric wall mucosa to alter the direction of traction[185].
Figure 8.
Modified endo-clip and snare[185]. A-C: Multiple clips used to provide multifocal traction; D-F: Clip applied to opposing gastric wall for countertraction. Citation: Zhang Q, Yao X, Wang Z. A modified method of endoclip-and-snare to assist in endoscopic submucosal dissection with mucosal traction in the upper GI tract. VideoGIE 2018; 3: 137-141. ©The Author(s) 2018. Published by Open Access Article.
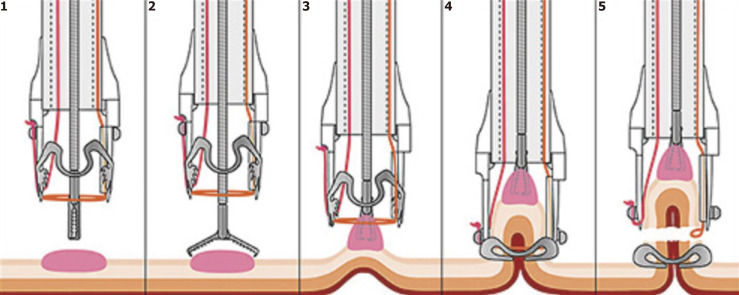
Endoscopic full-thickness resection
FTR can either be performed free-hand, attempting to maintain the serosa although often resulting in macroscopic perforation, or device assisted (Figure 9) using an ‘over-the-scope’ clip[186,187]. While some data exist regarding the use of FTR for gastric GIST where successful resection rates are as high as 96.8%, there is no evidence regarding the use of FTR in EGCs[188]. Theoretical roles for gastric FTR could include treatment of recurrent lesions within EMR/ESD scar limiting repeat resection, or using FTR in combination with laparoscopic nodal resection in non-candidates for gastrectomy with lesions outside of expanded criteria for ESD. Chae et al[188] reported a successful case of FTR for an EGC with a positive horizontal margin after initial ESD which had resulted in severe fibrosis[188]. Cho et al[138] reported a series of 9 patients treated with FTR and laparoscopic lymph node resection who had lesions outside of expanded criteria, in whom lymph nodes were positive in only 1 case[138].
Figure 9.
‘Over-the-scope clip’ (Ovesco, Germany) full-thickness resection[194]. Citation: Mão de-Ferro S, Castela J, Pereira D, Chaves P, Dias Pereira A. Endoscopic Full-Thickness Resection of Colorectal Lesions with the New FTRD System: Single-Center Experience. GE Port J Gastroenterol 2019; 26: 235-241. ©The Author(s) 2019. Published by Open Access Article.
Robot-assisted ESD
The ‘Master And Slave Transluminal Endoscopic Robot’ (MASTER) was developed in an attempt to mitigate the technical difficulties commonly encountered in interventional endoscopy[189-191]. Initial feasibility studies were performed in 2010 in ex-vivo and in-vivo porcine stomachs, where 20 gastric lesions were successfully resected using MASTER assisted ESD with no difference in procedure time compared to conventional ESD[190]. Subsequently, a small case series was reported in 2012, using MASTER assisted ESD in 5 patients, demonstrating clear resection margins in all cases with no major complications[192]. More studies are required to further explore the role of robot-assisted ESD, however this technique will clearly be limited by the cost and availability of equipment, while proceduralists already successfully perform more conventional ESD techniques at a fraction of the cost.
CONCLUSION
Advances in endoscopic imaging and therapeutics have enhanced the detection, characterisation and treatment of precancerous gastric lesions and EGC, allowing for pre-emptive minimally invasive intervention at an early stage. Endoscopic treatment of precancerous lesions and EGCs not only reduces rates of advanced carcinoma, but also avoids the requirement for high-risk surgical interventions with associated short- and long-term complications. The significant disparity in gastric cancer outcomes between East Asian and Western regions reflects extensive endoscopist experience, exhaustive research and clear guidelines in East Asia. Accordingly, there is a requirement for high-quality collaborative data from Western populations to aid in the development of clear management algorithms. Globally there are a multitude of opportunities for further research in both endoscopic imaging and resection techniques to optimise lesion detection and outcomes of ER, as well as potentially establish new indications for ER into the future.
Footnotes
Conflict-of-interest statement: No authors have any conflicts of interest to report.
Manuscript source: Invited manuscript
Peer-review started: March 31, 2021
First decision: June 23, 2021
Article in press: August 5, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Parry AH S-Editor: Fan JR L-Editor: A P-Editor: Li JH
Contributor Information
Edward Young, Department of Gastroenterology, Lyell McEwin Hospital, Elizabeth Vale 5112, SA, Australia; Faculty of Health and Medical Sciences, University of Adelaide, Adelaide 5000, SA, Australia. edward.young@sa.gov.au.
Hamish Philpott, Department of Gastroenterology, Lyell McEwin Hospital, Elizabeth Vale 5112, SA, Australia.
Rajvinder Singh, Department of Gastroenterology, Lyell McEwin Hospital, Elizabeth Vale 5112, SA, Australia; Faculty of Health and Medical Sciences, University of Adelaide, Adelaide 5000, SA, Australia.
References
- 1.International Agency for Research on Cancer. Globocan 2020 - Stomach Cancer. [cited 9 February 2021]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf .
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, Wilkinson NW. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21:3008–3014. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 4.Huang HL, Leung CY, Saito E, Katanoda K, Hur C, Kong CY, Nomura S, Shibuya K. Effect and cost-effectiveness of national gastric cancer screening in Japan: a microsimulation modeling study. BMC Med. 2020;18:257. doi: 10.1186/s12916-020-01729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh YS, Lee J, Woo H, Shin D, Kong SH, Lee HJ, Shin A, Yang HK. National cancer screening program for gastric cancer in Korea: Nationwide treatment benefit and cost. Cancer. 2020;126:1929–1939. doi: 10.1002/cncr.32753. [DOI] [PubMed] [Google Scholar]
- 6.GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. doi: 10.1016/S2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta S, Li D, El Serag HB, Davitkov P, Altayar O, Sultan S, Falck-Ytter Y, Mustafa RA. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology. 2020;158:693–702. doi: 10.1053/j.gastro.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vance RB Jr, Kubiliun N, Dunbar KB. How Do We Manage Gastric Intestinal Metaplasia? Dig Dis Sci. 2016;61:1870–1878. doi: 10.1007/s10620-016-4107-4. [DOI] [PubMed] [Google Scholar]
- 10.Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis. 2004;14:431–439. [PubMed] [Google Scholar]
- 11.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84:18–28. doi: 10.1016/j.gie.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol. 2013;20:2328–2338. doi: 10.1245/s10434-012-2862-9. [DOI] [PubMed] [Google Scholar]
- 13.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu KS, Wong IO, Leung WK. Helicobacter pylori associated gastric intestinal metaplasia: Treatment and surveillance. World J Gastroenterol. 2016;22:1311–1320. doi: 10.3748/wjg.v22.i3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166–175. doi: 10.1007/s10120-015-0462-7. [DOI] [PubMed] [Google Scholar]
- 16.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS; China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Lash JG, Genta RM. Adherence to the Sydney System guidelines increases the detection of Helicobacter gastritis and intestinal metaplasia in 400738 sets of gastric biopsies. Aliment Pharmacol Ther. 2013;38:424–431. doi: 10.1111/apt.12383. [DOI] [PubMed] [Google Scholar]
- 18.Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618–624. doi: 10.1016/j.gie.2016.03.791. [DOI] [PubMed] [Google Scholar]
- 19.Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579–587. doi: 10.1007/s10120-018-0812-3. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 22.Rugge M, Cassaro M, Di Mario F, Leo G, Leandro G, Russo VM, Pennelli G, Farinati F Interdisciplinary Group on Gastric Epithelial Dysplasia (IGGED) The long term outcome of gastric non-invasive neoplasia. Gut. 2003;52:1111–1116. doi: 10.1136/gut.52.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 24.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 25.You WC, Li JY, Blot WJ, Chang YS, Jin ML, Gail MH, Zhang L, Liu WD, Ma JL, Hu YR, Mark SD, Correa P, Fraumeni JF Jr, Xu GW. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer. 1999;83:615–619. doi: 10.1002/(sici)1097-0215(19991126)83:5<615::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Jeon SW, Jung MK, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–970. doi: 10.1097/MEG.0b013e3283013d58. [DOI] [PubMed] [Google Scholar]
- 27.Zhao G, Xue M, Hu Y, Lai S, Chen S, Wang L. How Commonly Is the Diagnosis of Gastric Low Grade Dysplasia Upgraded following Endoscopic Resection? PLoS One. 2015;10:e0132699. doi: 10.1371/journal.pone.0132699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao K, Uedo N, Kamada T, Hirasawa T, Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, Yoshida M, Miyashiro I, Fujimoto K, Tajiri H. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32:663–698. doi: 10.1111/den.13684. [DOI] [PubMed] [Google Scholar]
- 29.Jung MK, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim YJ, An J, Lee JJ, Cho JH, Kim KO, Chung JW, Kwon KA, Park DK, Kim JH. Endoscopic features suggesting gastric cancer in biopsy-proven gastric adenoma with high-grade neoplasia. World J Gastroenterol. 2014;20:12233–12240. doi: 10.3748/wjg.v20.i34.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017;86:857–865. doi: 10.1016/j.gie.2017.03.1528. [DOI] [PubMed] [Google Scholar]
- 32.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917–927. doi: 10.1016/j.gie.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Wang F, Chen ZY, Wang Z, Zhi FC, Liu SD, Bai Y. Comparison of the diagnostic efficacy of white light endoscopy and magnifying endoscopy with narrow band imaging for early gastric cancer: a meta-analysis. Gastric Cancer. 2016;19:543–552. doi: 10.1007/s10120-015-0500-5. [DOI] [PubMed] [Google Scholar]
- 34.Capelle LG, Haringsma J, de Vries AC, Steyerberg EW, Biermann K, van Dekken H, Kuipers EJ. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010;55:3442–3448. doi: 10.1007/s10620-010-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pimentel-Nunes P, Libânio D, Lage J, Abrantes D, Coimbra M, Esposito G, Hormozdi D, Pepper M, Drasovean S, White JR, Dobru D, Buxbaum J, Ragunath K, Annibale B, Dinis-Ribeiro M. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy. 2016;48:723–730. doi: 10.1055/s-0042-108435. [DOI] [PubMed] [Google Scholar]
- 36.ASGE Technology Committee. Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, Mishkin DS, Shah RJ, Somogyi L, Tierney WM, Petersen BT. Chromoendoscopy. Gastrointest Endosc. 2007;66:639–649. doi: 10.1016/j.gie.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016;31:1539–1545. doi: 10.1111/jgh.13313. [DOI] [PubMed] [Google Scholar]
- 38.Larghi A, Lecca PG, Costamagna G. High-resolution narrow band imaging endoscopy. Gut. 2008;57:976–986. doi: 10.1136/gut.2007.127845. [DOI] [PubMed] [Google Scholar]
- 39.Song J, Zhang J, Wang J, Guo X, Liu Y, Dong W. Meta-analysis: narrow band imaging for diagnosis of gastric intestinal metaplasia. PLoS One. 2014;9:e94869. doi: 10.1371/journal.pone.0094869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ang TL, Pittayanon R, Lau JY, Rerknimitr R, Ho SH, Singh R, Kwek AB, Ang DS, Chiu PW, Luk S, Goh KL, Ong JP, Tan JY, Teo EK, Fock KM. A multicenter randomized comparison between high-definition white light endoscopy and narrow band imaging for detection of gastric lesions. Eur J Gastroenterol Hepatol. 2015;27:1473–1478. doi: 10.1097/MEG.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 41.ASGE Technology Committee. High-definition and high-magnification endoscopes. Gastrointest Endosc. 2014;80:919–927. doi: 10.1016/j.gie.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Uedo N, Ishihara R, Iishi H, Yamamoto S, Yamada T, Imanaka K, Takeuchi Y, Higashino K, Ishiguro S, Tatsuta M. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–824. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 43.Savarino E, Corbo M, Dulbecco P, Gemignani L, Giambruno E, Mastracci L, Grillo F, Savarino V. Narrow-band imaging with magnifying endoscopy is accurate for detecting gastric intestinal metaplasia. World J Gastroenterol. 2013;19:2668–2675. doi: 10.3748/wjg.v19.i17.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omori T, Kamiya Y, Tahara T, Shibata T, Nakamura M, Yonemura J, Okubo M, Yoshioka D, Ishizuka T, Maruyama N, Kamano T, Fujita H, Nakagawa Y, Nagasaka M, Iwata M, Arisawa T, Hirata I. Correlation between magnifying narrow band imaging and histopathology in gastric protruding/or polypoid lesions: a pilot feasibility trial. BMC Gastroenterol. 2012;12:17. doi: 10.1186/1471-230X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao K, Takaki Y, Matsui T, Iwashita A, Anagnostopoulos GK, Kaye P, Ragunath K. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415–433, vii. doi: 10.1016/j.giec.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Muto M, Yao K, Kaise M, Kato M, Uedo N, Yagi K, Tajiri H. Magnifying endoscopy simple diagnostic algorithm for early gastric cancer (MESDA-G) Dig Endosc. 2016;28:379–393. doi: 10.1111/den.12638. [DOI] [PubMed] [Google Scholar]
- 47.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–467. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 48.Yao K, Doyama H, Gotoda T, Ishikawa H, Nagahama T, Yokoi C, Oda I, Machida H, Uchita K, Tabuchi M. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer. 2014;17:669–679. doi: 10.1007/s10120-013-0332-0. [DOI] [PubMed] [Google Scholar]
- 49.Yamada S, Doyama H, Yao K, Uedo N, Ezoe Y, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Saito Y, Muto M. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc. 2014;79:55–63. doi: 10.1016/j.gie.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Hu YY, Lian QW, Lin ZH, Zhong J, Xue M, Wang LJ. Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: A meta-analysis. World J Gastroenterol. 2015;21:7884–7894. doi: 10.3748/wjg.v21.i25.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010;72:523–529. doi: 10.1016/j.gie.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 52.Maki S, Yao K, Nagahama T, Beppu T, Hisabe T, Takaki Y, Hirai F, Matsui T, Tanabe H, Iwashita A. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013;16:140–146. doi: 10.1007/s10120-012-0160-7. [DOI] [PubMed] [Google Scholar]
- 53.Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, Ishikawa H, Takeuchi Y, Kaneko Y, Saito Y. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017–2025.e3. doi: 10.1053/j.gastro.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Kiyotoki S, Nishikawa J, Satake M, Fukagawa Y, Shirai Y, Hamabe K, Saito M, Okamoto T, Sakaida I. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010;25:1636–1641. doi: 10.1111/j.1440-1746.2010.06379.x. [DOI] [PubMed] [Google Scholar]
- 55.Horii Y, Dohi O, Naito Y, Takayama S, Ogita K, Terasaki K, Nakano T, Majima A, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Yagi N, Yanagisawa A, Itoh Y. Efficacy of Magnifying Narrow Band Imaging for Delineating Horizontal Margins of Early Gastric Cancer. Digestion. 2019;100:93–99. doi: 10.1159/000494053. [DOI] [PubMed] [Google Scholar]
- 56.Uchita K, Yao K, Uedo N, Shimokawa T, Iwasaki T, Kojima K, Kawada A, Nakayama M, Okazaki M, Iwamura S. Highest power magnification with narrow-band imaging is useful for improving diagnostic performance for endoscopic delineation of early gastric cancers. BMC Gastroenterol. 2015;15:155. doi: 10.1186/s12876-015-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video) Gastrointest Endosc. 2011;74:1259–1267. doi: 10.1016/j.gie.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Weigt J, Malfertheiner P, Canbay A, Haybaeck J, Bird-Lieberman E, Link A. Blue Light Imaging and Linked Color Imaging for the Characterization of Mucosal Changes in Chronic Gastritis: A Clinicians View and Brief Technical Report. Dig Dis. 2020;38:9–14. doi: 10.1159/000501265. [DOI] [PubMed] [Google Scholar]
- 59.Dohi O, Yagi N, Naito Y, Fukui A, Gen Y, Iwai N, Ueda T, Yoshida N, Kamada K, Uchiyama K, Takagi T, Konishi H, Yanagisawa A, Itoh Y. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: a randomized controlled study. Gastrointest Endosc. 2019;89:47–57. doi: 10.1016/j.gie.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 60.Dohi O, Yagi N, Majima A, Horii Y, Kitaichi T, Onozawa Y, Suzuki K, Tomie A, Kimura-Tsuchiya R, Tsuji T, Yamada N, Bito N, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Yanagisawa A, Itoh Y. Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: a prospective study. Gastric Cancer. 2017;20:297–303. doi: 10.1007/s10120-016-0620-6. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko K, Oono Y, Yano T, Ikematsu H, Odagaki T, Yoda Y, Yagishita A, Sato A, Nomura S. Effect of novel bright image enhanced endoscopy using blue laser imaging (BLI) Endosc Int Open. 2014;2:E212–E219. doi: 10.1055/s-0034-1390707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ASGE Technology Committee. Confocal laser endomicroscopy. Gastrointest Endosc. 2014;80:928–938. doi: 10.1016/j.gie.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547–553. doi: 10.1055/s-2007-995633. [DOI] [PubMed] [Google Scholar]
- 64.Zhang HP, Yang S, Chen WH, Hu TT, Lin J. The diagnostic value of confocal laser endomicroscopy for gastric cancer and precancerous lesions among Asian population: a system review and meta-analysis. Scand J Gastroenterol. 2017;52:382–388. doi: 10.1080/00365521.2016.1275770. [DOI] [PubMed] [Google Scholar]
- 65.Abadir AP, Ali MF, Karnes W, Samarasena JB. Artificial Intelligence in Gastrointestinal Endoscopy. Clin Endosc. 2020;53:132–141. doi: 10.5946/ce.2020.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanesaka T, Lee TC, Uedo N, Lin KP, Chen HZ, Lee JY, Wang HP, Chang HT. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018;87:1339–1344. doi: 10.1016/j.gie.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 67.Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki J, Tada T. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer. 2018;21:653–660. doi: 10.1007/s10120-018-0793-2. [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Singh R, Shin SH, Ho KY. Artificial intelligence in upper GI endoscopy - current status, challenges and future promise. J Gastroenterol Hepatol. 2021;36:20–24. doi: 10.1111/jgh.15354. [DOI] [PubMed] [Google Scholar]
- 69.Olympus News Release: Olympus launches EVIS X1, its most advanced endoscopy system to date [Internet]. Olympus Global 2020; 1-4. [cited 9 February 2021]. Available from: https://www.olympus-global.com/news/2020/contents/nr01635/nr01635_00002.pdf .
- 70.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 71.Choi J, Kim SG, Im JP, Kim JS, Jung HC, Song IS. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–713. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]
- 72.Han C, Nie C, Shen X, Xu T, Liu J, Ding Z, Hou X. Exploration of an effective training system for the diagnosis of pancreatobiliary diseases with EUS: A prospective study. Endosc Ultrasound. 2020;9:308–318. doi: 10.4103/eus.eus_47_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318–322. doi: 10.1097/MCG.0b013e3181775966. [DOI] [PubMed] [Google Scholar]
- 74.Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015:CD009944. doi: 10.1002/14651858.CD009944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takamaru H, Yoshinaga S, Takisawa H, Oda I, Katai H, Sekine S, Taniguchi K, Saito Y. Endoscopic Ultrasonography Miniature Probe Performance for Depth Diagnosis of Early Gastric Cancer with Suspected Submucosal Invasion. Gut Liver. 2020;14:581–588. doi: 10.5009/gnl19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim SJ, Choi CW, Kang DH. Endoscopic features of submucosal invasion in undifferentiated type early gastric cancer sized less than 2 cm without ulceration. JCO . 2019;47:76. [Google Scholar]
- 77.Kim J, Kim SG, Chung H, Lim JH, Choi JM, Park JY, Yang HJ, Han SJ, Oh S, Kim MS, Kim HJ, Hong H, Lee HJ, Kim JL, Lee E, Jung HC. Clinical efficacy of endoscopic ultrasonography for decision of treatment strategy of gastric cancer. Surg Endosc. 2018;32:3789–3797. doi: 10.1007/s00464-018-6104-5. [DOI] [PubMed] [Google Scholar]
- 78.Wani AH, Parry AH, Feroz I, Choh NA. Preoperative Staging of Gastric Cancer Using Computed Tomography and Its Correlation with Histopathology with Emphasis on Multi-planar Reformations and Virtual Gastroscopy. J Gastrointest Cancer. 2021;52:606–615. doi: 10.1007/s12029-020-00436-6. [DOI] [PubMed] [Google Scholar]
- 79.De Vuysere S, Vandecaveye V, De Bruecker Y, Carton S, Vermeiren K, Tollens T, De Keyzer F, Dresen RC. Accuracy of whole-body diffusion-weighted MRI (WB-DWI/MRI) in diagnosis, staging and follow-up of gastric cancer, in comparison to CT: a pilot study. BMC Med Imaging. 2021;21:18. doi: 10.1186/s12880-021-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi JI, Joo I, Lee JM. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J Gastroenterol. 2014;20:4546–4557. doi: 10.3748/wjg.v20.i16.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? World J Gastroenterol. 2008;14:4011–4019. doi: 10.3748/wjg.14.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J, Zhao G, Wang Y. Analysis of lymph node metastasis in early gastric cancer: a single institutional experience from China. World J Surg Oncol. 2020;18:57. doi: 10.1186/s12957-020-01834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19–S26. doi: 10.1007/s10120-011-0115-4. [DOI] [PubMed] [Google Scholar]
- 84.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 85.Nam HS, Choi CW, Kim SJ, Kang DH, Kim HW, Park SB, Ryu DG. Endoscopic submucosal dissection for gastric indefinite for neoplasia: which lesions should be resected? Surg Endosc. 2019;33:3976–3983. doi: 10.1007/s00464-019-06686-1. [DOI] [PubMed] [Google Scholar]
- 86.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 87.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 88.He Z, Sun C, Zheng Z, Yu Q, Wang T, Chen X, Cao H, Liu W, Wang B. Endoscopic submucosal dissection of large gastrointestinal stromal tumors in the esophagus and stomach. J Gastroenterol Hepatol. 2013;28:262–267. doi: 10.1111/jgh.12056. [DOI] [PubMed] [Google Scholar]
- 89.An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, Wang D, Li ZS, Shi XG. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31:4522–4531. doi: 10.1007/s00464-017-5511-3. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, Yano T, Tanaka S, Endo M, Nakagawa M, Toyonaga T, Doyama H, Hirasawa K, Matsuda M, Yamamoto H, Fujishiro M, Hashimoto S, Maeda Y, Oyama T, Takenaka R, Yamamoto Y, Naito Y, Michida T, Kobayashi N, Kawahara Y, Hirano M, Jin M, Hori S, Niwa Y, Hikichi T, Shimazu T, Ono H, Tanabe S, Kondo H, Iishi H, Ninomiya M Ichiro Oda for J-WEB/EGC group. Short-term outcomes of multicenter prospective cohort study of gastric endoscopic resection: 'Real-world evidence' in Japan. Dig Endosc. 2019;31:30–39. doi: 10.1111/den.13246. [DOI] [PubMed] [Google Scholar]
- 91.Ryu DG, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Nam HS. Pathologic outcomes of endoscopic submucosal dissection for gastric epithelial neoplasia. Medicine (Baltimore) 2018;97:e11802. doi: 10.1097/MD.0000000000011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe K, Hikichi T, Nakamura J, Takagi T, Suzuki R, Sugimoto M Md, Waragai Y, Kikuchi H, Konno N, Asama H, Takasumi M, Obara K, Ohira H. Endoscopic submucosal dissection for early gastric cancer in very elderly patients age 85 or older. Endosc Int Open. 2017;5:E17–E24. doi: 10.1055/s-0042-122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ngamruengphong S, Ferri L, Aihara H, Draganov PV, Yang DJ, Perbtani YB, Jue TL, Munroe CA, Boparai ES, Mehta NA, Bhatt A, Kumta NA, Othman MO, Mercado M, Javaid H, Aadam AA, Siegel A, James TW, Grimm IS, DeWitt JM, Novikov A, Schlachterman A, Kowalski T, Samarasena J, Hashimoto R, Chehade NEH, Lee J, Chang K, Su B, Ujiki MB, Mehta A, Sharaiha RZ, Carr-Locke DL, Chen A, Chen M, Chen YI, Pourmousavi Khoshknab M, Wang R, Kerdsirichairat T, Tomizawa Y, von Renteln D, Kumbhari V, Khashab MA, Bechara R, Karasik M, Patel NJ, Fukami N, Nishimura M, Hanada Y, Wong Kee Song LM, Laszkowska M, Wang AY, Hwang JH, Friedland S, Sethi A, Kalloo AN. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin Gastroenterol Hepatol. 2021;19:1611–1619.e1. doi: 10.1016/j.cgh.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 95.Manta R, Galloro G, Pugliese F, Angeletti S, Caruso A, Zito FP, Mangiafico S, Marmo R, Zullo A, Esposito G, Annibale B, Mutignani M, Conigliaro R. Endoscopic Submucosal Dissection of Gastric Neoplastic Lesions: An Italian, Multicenter Study. J Clin Med. 2020;9 doi: 10.3390/jcm9030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abdelrahim M, Kandiah K, Masseli R, Invernizzi M, Varytimiadis L, Al-Kandari A, Hossain E, Seewald S, Repici A, Bhandari P, Arndtz S. Endoscopic submucosal dissection of early gastric neoplasia: experience from three european tertiary centres. Gut. 2019:A146–A147. [Google Scholar]
- 97.Maselli R, Iacopini F, Azzolini F, Petruzziello L, Manno M, De Luca L, Cecinato P, Fiori G, Staiano T, Rosa Rizzotto E, Angeletti S, Caruso A, Coppola F, Andrisani G, Viale E, Missale G, Panarese A, Mazzocchi A, Cesaro P, Campanale M, Occhipinti P, Tarantino O, Crosta C, Brosolo P, Sferrazza S, Rondonotti E, Amato A, Fuccio L, Costamagna G, Repici A. Endoscopic submucosal dissection: Italian national survey on current practices, training and outcomes. Dig Liver Dis. 2020;52:64–71. doi: 10.1016/j.dld.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Pagano N, Frazzoni L, La Porta M, Fuccio L, Bazzoli F, Zagari RM. Endoscopic submucosal dissection for superficial premalignant and malignant epithelial neoplasms of the digestive tract: a real-life experience in Italy. Eur Rev Med Pharmacol Sci. 2019;23:8354–8359. doi: 10.26355/eurrev_201910_19146. [DOI] [PubMed] [Google Scholar]
- 99.Tate DJ, Klein A, Sidhu M, Desomer L, Awadie H, Lee EYT, Mahajan H, McLeod D, Bourke MJ. Endoscopic submucosal dissection for suspected early gastric cancer: absolute versus expanded criteria in a large Western cohort (with video) Gastrointest Endosc. 2019;90:467–479.e4. doi: 10.1016/j.gie.2019.04.242. [DOI] [PubMed] [Google Scholar]
- 100.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 101.Oh SY, Lee KG, Suh YS, Kim MA, Kong SH, Lee HJ, Kim WH, Yang HK. Lymph Node Metastasis in Mucosal Gastric Cancer: Reappraisal of Expanded Indication of Endoscopic Submucosal Dissection. Ann Surg. 2017;265:137–142. doi: 10.1097/SLA.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 102.Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, Cho CM, Tak WY, Kweon YO. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10. doi: 10.1055/s-2007-966750. [DOI] [PubMed] [Google Scholar]
- 103.Choi KK, Bae JM, Kim SM, Sohn TS, Noh JH, Lee JH, Choi MG, Kim S. The risk of lymph node metastases in 3951 surgically resected mucosal gastric cancers: implications for endoscopic resection. Gastrointest Endosc. 2016;83:896–901. doi: 10.1016/j.gie.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 104.Hanada Y, Choi AY, Hwang JH, Draganov PV, Khanna L, Sethi A, Bartel MJ, Goel N, Abe S, De Latour RA, Park K, Melis M, Newman E, Hatzaras I, Reddy SS, Farma JM, Liu X, Schlachterman A, Kresak J, Trapp G, Ansari N, Schrope B, Lee JY, Dhall D, Lo S, Jamil LH, Burch M, Gaddam S, Gong Y, Del Portillo A, Tomizawa Y, Truong CD, Brewer Gutierrez OI, Montgomery E, Johnston FM, Duncan M, Canto M, Ahuja N, Lennon AM, Ngamruengphong S. Low Frequency of Lymph Node Metastases in Patients in the United States With Early-stage Gastric Cancers That Fulfill Japanese Endoscopic Resection Criteria. Clin Gastroenterol Hepatol. 2019;17:1763–1769. doi: 10.1016/j.cgh.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 105.Pereira MA, Ramos MFKP, Dias AR, Faraj SF, Yagi OK, Safatle-Ribeiro AV, Maluf-Filho F, Zilberstein B, Cecconello I, de Mello ES, Ribeiro U Jr. Risk Factors for Lymph Node Metastasis in Western Early Gastric Cancer After Optimal Surgical Treatment. J Gastrointest Surg. 2018;22:23–31. doi: 10.1007/s11605-017-3517-8. [DOI] [PubMed] [Google Scholar]
- 106.Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555–563. doi: 10.4253/wjge.v6.i11.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tao M, Zhou X, Hu M, Pan J. Endoscopic submucosal dissection versus endoscopic mucosal resection for patients with early gastric cancer: a meta-analysis. BMJ Open. 2019;9:e025803. doi: 10.1136/bmjopen-2018-025803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho JH, Cha SW, Kim HG, Lee TH, Cho JY, Ko WJ, Jin SY, Park S. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc. 2016;30:3762–3773. doi: 10.1007/s00464-015-4672-1. [DOI] [PubMed] [Google Scholar]
- 109.Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc. 2017;50:170–178. doi: 10.5946/ce.2016.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga K, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143–152. doi: 10.1016/j.gie.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 111.Ryu SJ, Kim BW, Kim BG, Kim JH, Kim JS, Kim JI, Park JM, Oh JH, Kim TH, Kim JJ, Park SM, Park CH, Song KY, Lee JH, Kim SG, Kim DJ, Kim W. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: a retrospective multicenter study on immediate and long-term outcome over 5 years. Surg Endosc. 2016;30:5283–5289. doi: 10.1007/s00464-016-4877-y. [DOI] [PubMed] [Google Scholar]
- 112.Liu Q, Ding L, Qiu X, Meng F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int J Surg. 2020;73:28–41. doi: 10.1016/j.ijsu.2019.11.027. [DOI] [PubMed] [Google Scholar]
- 113.Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509–1520. doi: 10.1007/s00464-012-2661-1. [DOI] [PubMed] [Google Scholar]
- 114.Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590–1597. doi: 10.1001/jamaoncol.2020.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li SS, Costantino CL, Mullen JT. Morbidity and Mortality of Total Gastrectomy: a Comprehensive Analysis of 90-Day Outcomes. J Gastrointest Surg. 2019;23:1340–1348. doi: 10.1007/s11605-019-04228-7. [DOI] [PubMed] [Google Scholar]
- 116.Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological Changes of Quality of Life in Long-Term Survivors after Gastrectomy for Gastric Cancer. Cancer Res Treat. 2016;48:1030–1036. doi: 10.4143/crt.2015.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soh JS, Kim JK, Lim H, Kang HS, Park JW, Kim SE, Moon SH, Kim JH, Park CK, Cho JW, Lim MS, Kim KO. Comparison of endoscopic submucosal dissection and surgical resection for treating gastric subepithelial tumours. Scand J Gastroenterol. 2016;51:633–638. doi: 10.3109/00365521.2015.1124451. [DOI] [PubMed] [Google Scholar]
- 118.Tanabe S, Ishido K, Matsumoto T, Kosaka T, Oda I, Suzuki H, Fujisaki J, Ono H, Kawata N, Oyama T, Takahashi A, Doyama H, Kobayashi M, Uedo N, Hamada K, Toyonaga T, Kawara F, Tanaka S, Yoshifuku Y. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer. 2017;20:45–52. doi: 10.1007/s10120-016-0664-7. [DOI] [PubMed] [Google Scholar]
- 119.Choi MK, Kim GH, Park DY, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250–4258. doi: 10.1007/s00464-013-3030-4. [DOI] [PubMed] [Google Scholar]
- 120.Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 121.Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136. doi: 10.1007/s10120-013-0241-2. [DOI] [PubMed] [Google Scholar]
- 122.Nakamura K, Honda K, Akahoshi K, Ihara E, Matsuzaka H, Sumida Y, Yoshimura D, Akiho H, Motomura Y, Iwasa T, Komori K, Chijiiwa Y, Harada N, Ochiai T, Oya M, Oda Y, Takayanagi R. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol. 2015;50:413–422. doi: 10.3109/00365521.2014.940377. [DOI] [PubMed] [Google Scholar]
- 123.Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015;18:618–626. doi: 10.1007/s10120-014-0378-7. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205. doi: 10.1007/s10120-015-0469-0. [DOI] [PubMed] [Google Scholar]
- 125.Pimentel-Nunes P, Mourão F, Veloso N, Afonso LP, Jácome M, Moreira-Dias L, Dinis-Ribeiro M. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy. 2014;46:933–940. doi: 10.1055/s-0034-1377348. [DOI] [PubMed] [Google Scholar]
- 126.Probst A, Schneider A, Schaller T, Anthuber M, Ebigbo A, Messmann H. Endoscopic submucosal dissection for early gastric cancer: are expanded resection criteria safe for Western patients? Endoscopy. 2017;49:855–865. doi: 10.1055/s-0043-110672. [DOI] [PubMed] [Google Scholar]
- 127.Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703–707. doi: 10.1055/s-0033-1344396. [DOI] [PubMed] [Google Scholar]
- 128.Kakushima N, Hagiwara T, Tanaka M, Sawai H, Kawata N, Takizawa K, Imai K, Takao T, Hotta K, Yamaguchi Y, Matsubayashi H, Ono H. Endoscopic submucosal dissection for early gastric cancer in cases preoperatively contraindicated for endoscopic treatment. United European Gastroenterol J. 2013;1:453–460. doi: 10.1177/2050640613508550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jung DH, Youn YH, Kim JH, Park JJ, Park H. Secondary endoscopic submucosal dissection for locally recurrent or incompletely resected gastric neoplasms. World J Gastroenterol. 2018;24:3776–3785. doi: 10.3748/wjg.v24.i33.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jeon MY, Park JC, Hahn KY, Shin SK, Lee SK, Lee YC. Long-term outcomes after noncurative endoscopic resection of early gastric cancer: the optimal time for additional endoscopic treatment. Gastrointest Endosc. 2018;87:1003–1013.e2. doi: 10.1016/j.gie.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Li D, Luan H, Wang S, Zhou Y. Survival benefits of additional surgery after non-curative endoscopic resection in patients with early gastric cancer: a meta-analysis. Surg Endosc. 2019;33:711–716. doi: 10.1007/s00464-018-6570-9. [DOI] [PubMed] [Google Scholar]
- 132.Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679–689. doi: 10.1007/s10120-016-0651-z. [DOI] [PubMed] [Google Scholar]
- 133.Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? J Gastroenterol. 2017;52:175–184. doi: 10.1007/s00535-016-1210-4. [DOI] [PubMed] [Google Scholar]
- 134.Goto A, Nishikawa J, Hideura E, Ogawa R, Nagao M, Sasaki S, Kawasato R, Hashimoto S, Okamoto T, Ogihara H, Hamamoto Y, Sakaida I. Lymph node metastasis can be determined by just tumor depth and lymphovascular invasion in early gastric cancer patients after endoscopic submucosal dissection. Eur J Gastroenterol Hepatol. 2017;29:1346–1350. doi: 10.1097/MEG.0000000000000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sunagawa H, Kinoshita T, Kaito A, Shibasaki H, Kaneko K, Ochiai A, Ohtsu A, Nishida T. Additional surgery for non-curative resection after endoscopic submucosal dissection for gastric cancer: a retrospective analysis of 200 cases. Surg Today. 2017;47:202–209. doi: 10.1007/s00595-016-1353-1. [DOI] [PubMed] [Google Scholar]
- 136.Jiang B, Zhou L, Lu J, Wang Y, Guo J. Predictors of lymph node metastasis and residual tumor in early gastric cancer patients after noncurative endoscopic resection: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2020;13:1756284820935033. doi: 10.1177/1756284820935033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874–881. doi: 10.1038/ajg.2017.95. [DOI] [PubMed] [Google Scholar]
- 138.Lee DS, Park JK, Lee SJ, Cheon GJ. Clinical significance of regional lymph node enlargement in patients with EGC within the expanded criteria for ESD. BMC Gastroenterol. 2020;20:51. doi: 10.1186/s12876-020-01197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134–139. doi: 10.1055/s-0030-1255955. [DOI] [PubMed] [Google Scholar]
- 140.Wang Z, Dong ZY, Chen JQ, Liu JL. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol. 2012;19:1541–1550. doi: 10.1245/s10434-011-2124-2. [DOI] [PubMed] [Google Scholar]
- 141.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, Takagane A, Mohri Y, Nabeshima K, Uenosono Y, Kinami S, Sakamoto J, Morita S, Aikou T, Miwa K, Kitajima M. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 142.Furuhata T, Kaise M, Hoteya S, Iizuka T, Yamada A, Nomura K, Kuribayashi Y, Kikuchi D, Matsui A, Ogawa O, Yamashta S, Mitani T. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer. 2017;20:207–214. doi: 10.1007/s10120-015-0588-7. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goto O, Fujishiro M, Kodashima S, Ono S, Niimi K, Hirano K, Yamamichi N, Koike K. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241–248. doi: 10.1016/j.gie.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 145.Nam HS, Choi CW, Kim SJ, Kim HW, Kang DH, Park SB, Ryu DG. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for gastric neoplasm. Sci Rep. 2019;9:2674. doi: 10.1038/s41598-019-39381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uedo N, Takeuchi Y, Yamada T, Ishihara R, Ogiyama H, Yamamoto S, Kato M, Tatsumi K, Masuda E, Tamai C, Higashino K, Iishi H, Tatsuta M. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 147.Martin J, Kim M, Bainbridge D, Cheng D. Do proton pump inhibitors prevent bleeding from ulcers after endoscopic submucosal dissection or endoscopic mucosal resection? Gastrointest Endosc. 2009;69:AB103. [Google Scholar]
- 148.Nishizawa T, Suzuki H, Akimoto T, Maehata T, Morizane T, Kanai T, Yahagi N. Effects of preoperative proton pump inhibitor administration on bleeding after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. United European Gastroenterol J. 2016;4:5–10. doi: 10.1177/2050640615588023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiu PW, Lam CY, Lee SW, Kwong KH, Lam SH, Lee DT, Kwok SP. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403–1407. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4:18–29. doi: 10.1177/2050640615585470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM, Lee KJ, Kim JH, Cho SW. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456–2464. doi: 10.1007/s00464-012-2211-x. [DOI] [PubMed] [Google Scholar]
- 152.Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 153.Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:71–78. doi: 10.1111/j.1443-1661.2012.01376.x. [DOI] [PubMed] [Google Scholar]
- 154.Kim HJ, Chung H, Jung DH, Park JC, Shin SK, Lee SK, Lee YC. Clinical outcomes of and management strategy for perforations associated with endoscopic submucosal dissection of an upper gastrointestinal epithelial neoplasm. Surg Endosc. 2016;30:5059–5067. doi: 10.1007/s00464-016-4854-5. [DOI] [PubMed] [Google Scholar]
- 155.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 156.Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679–683. doi: 10.1055/s-0029-1214979. [DOI] [PubMed] [Google Scholar]
- 157.Pickhardt PJ, Asher DB. Wall thickening of the gastric antrum as a normal finding: multidetector CT with cadaveric comparison. AJR Am J Roentgenol. 2003;181:973–979. doi: 10.2214/ajr.181.4.1810973. [DOI] [PubMed] [Google Scholar]
- 158.Yamamoto Y, Nishisaki H, Sakai H, Tokuyama N, Sawai H, Sakai A, Mimura T, Kushida S, Tsumura H, Sakamoto T, Miki I, Tsuda M, Inokuchi H. Clinical Factors of Delayed Perforation after Endoscopic Submucosal Dissection for Gastric Neoplasms. Gastroenterol Res Pract. 2017;2017:7404613. doi: 10.1155/2017/7404613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hanaoka N, Uedo N, Ishihara R, Higashino K, Takeuchi Y, Inoue T, Chatani R, Hanafusa M, Tsujii Y, Kanzaki H, Kawada N, Iishi H, Tatsuta M, Tomita Y, Miyashiro I, Yano M. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2010;42:1112–1115. doi: 10.1055/s-0030-1255932. [DOI] [PubMed] [Google Scholar]
- 160.Sumiyoshi T, Kondo H, Minagawa T, Fujii R, Sakata K, Inaba K, Kimura T, Ihara H, Yoshizaki N, Hirayama M, Oyamada Y, Okushiba S. Risk factors and management for gastric stenosis after endoscopic submucosal dissection for gastric epithelial neoplasm. Gastric Cancer. 2017;20:690–698. doi: 10.1007/s10120-016-0673-6. [DOI] [PubMed] [Google Scholar]
- 161.Lee JU, Park MS, Yun SH, Yang MA, Han SH, Lee YJ, Jung GM, Kim JW, Cho YK, Cho JW. Risk factors and management for pyloric stenosis occurred after endoscopic submucosal dissection adjacent to pylorus. Medicine (Baltimore) 2016;95:e5633. doi: 10.1097/MD.0000000000005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tsunada S, Ogata S, Mannen K, Arima S, Sakata Y, Shiraishi R, Shimoda R, Ootani H, Yamaguchi K, Fujise T, Sakata H, Iwakiri R, Fujimoto K. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc. 2008;67:979–983. doi: 10.1016/j.gie.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 163.Kim YI, Park JY, Kim BJ, Hwang HW, Hong SA, Kim JG. Risk of metachronous gastric neoplasm occurrence during intermediate-term follow-up period after endoscopic submucosal dissection for gastric dysplasia. Sci Rep. 2020;10:6747. doi: 10.1038/s41598-020-63722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim JG, Park JY, Kim Y-I. Incidence of metachronous gastric neoplasia after endoscopic submucosal dissection for gastric dysplasia. J Clin Oncol. 2019;37:20. [Google Scholar]
- 165.Kim JL, Kim SG, Kim J, Park JY, Yang HJ, Kim HJ, Chung H. Clinical Outcomes of Metachronous Gastric Cancer after Endoscopic Resection for Early Gastric Cancer. Gut Liver. 2020;14:190–198. doi: 10.5009/gnl18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tziatzios G, Ebigbo A, Gölder SK, Probst A, Messmann H. Methods that Assist Traction during Endoscopic Submucosal Dissection of Superficial Gastrointestinal Cancers: A Systematic Literature Review. Clin Endosc. 2020;53:286–301. doi: 10.5946/ce.2019.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos) Gastrointest Endosc. 2009;69:10–15. doi: 10.1016/j.gie.2008.03.1127. [DOI] [PubMed] [Google Scholar]
- 168.Matsuzaki I, Hattori M, Hirose K, Esaki M, Yoshikawa M, Yokoi T, Kobayashi M, Miyahara R, Hirooka Y, Goto H. Magnetic anchor-guided endoscopic submucosal dissection for gastric lesions (with video) Gastrointest Endosc. 2018;87:1576–1580. doi: 10.1016/j.gie.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 169.Mortagy M, Mehta N, Parsi MA, Abe S, Stevens T, Vargo JJ, Saito Y, Bhatt A. Magnetic anchor guidance for endoscopic submucosal dissection and other endoscopic procedures. World J Gastroenterol. 2017;23:2883–2890. doi: 10.3748/wjg.v23.i16.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shetty A, Suarez AL, Dufault DL, Mcvey MC, Elmunzer BJ. Endoscopic mucosal resection with grasp-and-snare technique for challenging lesions. Gastrointest Endosc. 2016;84:738–739. doi: 10.1016/j.gie.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 171.Hua XL, Jun LL, Wen ZC, Lin JY, Ye T, Liang LX. Using a double-channel gastroscope reduces procedural time in performing gastric endoscopic submucosal dissection. Pak J Med Sci. 2016;32:617–621. doi: 10.12669/pjms.323.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Knoop RF, Wedi E, Petzold G, Bremer SCB, Amanzada A, Ellenrieder V, Neesse A, Kunsch S. Endoscopic submucosal dissection with an additional working channel (ESD+): a novel technique to improve procedure time and safety of ESD. Surg Endosc. 2021;35:3506–3512. doi: 10.1007/s00464-020-07808-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sportes A, Cfm J, Gromski MA, Koehler P, Seif Amir Hosseini A, Kauffmann P, Ellenrieder V, Wedi E. Novel modified endoscopic mucosal resection of large GI lesions (> 20 mm) using an external additional working channel (AWC) may improve R0 resection rate: initial clinical experience. BMC Gastroenterol. 2020;20:195. doi: 10.1186/s12876-020-01344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walter B, Schmidbaur S, Krieger Y, Meining A. Improved endoscopic resection of large flat lesions and early cancers using an external additional working channel (AWC): a case series. Endosc Int Open. 2019;7:E298–E301. doi: 10.1055/a-0824-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ogata K, Yanai M, Kuriyama K, Suzuki M, Yanoma T, Kimura A, Kogure N, Toyomasu Y, Ohno T, Mochiki E, Kuwano H. Double Endoscopic Intraluminal Operation (DEILO) for Early Gastric Cancer: Outcome of Novel Procedure for Endoscopic Submucosal Dissection. Anticancer Res. 2017;37:343–347. doi: 10.21873/anticanres.11327. [DOI] [PubMed] [Google Scholar]
- 176.Çolak Ş, Gürbulak B, Çakar E, Bektaş H. Resection of Mucosal and Submucosal Gastrointestinal Lesions and a Double Endoscope Experience. JSLS. 2019;23 doi: 10.4293/JSLS.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Higuchi K, Tanabe S, Azuma M, Sasaki T, Katada C, Ishido K, Naruke A, Mikami T, Koizumi W. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video) Gastrointest Endosc. 2013;78:266–273. doi: 10.1016/j.gie.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 178.Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, Kim JH. Transnasal endoscope-assisted endoscopic submucosal dissection for gastric adenoma and early gastric cancer in the pyloric area: a case series. Endoscopy. 2011;43:233–235. doi: 10.1055/s-0030-1256037. [DOI] [PubMed] [Google Scholar]
- 179.Teoh AY, Chiu PW, Hon SF, Mak TW, Ng EK, Lau JY. Ex vivo comparative study using the Endolifter® as a traction device for enhancing submucosal visualization during endoscopic submucosal dissection. Surg Endosc. 2013;27:1422–1427. doi: 10.1007/s00464-012-2583-y. [DOI] [PubMed] [Google Scholar]
- 180.Schölvinck DW, Goto O, Bergman JJ, Yahagi N, Weusten BL. The Efficacy of an Endoscopic Grasp-and-Traction Device for Gastric Endoscopic Submucosal Dissection: An Ex Vivo Comparative Study (with Video) Clin Endosc. 2015;48:221–227. doi: 10.5946/ce.2015.48.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nagata M. Internal traction method using a spring-and-loop with clip (S-O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg Endosc. 2020;34:3722–3733. doi: 10.1007/s00464-020-07590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoshida M, Takizawa K, Suzuki S, Koike Y, Nonaka S, Yamasaki Y, Minagawa T, Sato C, Takeuchi C, Watanabe K, Kanzaki H, Morimoto H, Yano T, Sudo K, Mori K, Gotoda T, Ono H CONNECT-G Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video) Gastrointest Endosc. 2018;87:1231–1240. doi: 10.1016/j.gie.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 183.Noda H, Ogasawara N, Koshino A, Fukuta S, Nagoya T, Hoshino H, Nagao K, Sugiyama T, Kondo Y, Ito Y, Izawa S, Ebi M, Funaki Y, Sasaki M, Kasugai K. Thread-Traction with a Sheath of Polypectomy Snare Facilitates Endoscopic Submucosal Dissection of Early Gastric Cancers. Gastroenterol Res Pract. 2016;2016:9415497. doi: 10.1155/2016/9415497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yoshida N, Doyama H, Ota R, Takeda Y, Nakanishi H, Tominaga K, Tsuji S, Takemura K. Effectiveness of clip-and-snare method using pre-looping technique for gastric endoscopic submucosal dissection. World J Gastrointest Endosc. 2016;8:451–457. doi: 10.4253/wjge.v8.i12.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Q, Yao X, Wang Z. A modified method of endoclip-and-snare to assist in endoscopic submucosal dissection with mucosal traction in the upper GI tract. VideoGIE. 2018;3:137–141. doi: 10.1016/j.vgie.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17–24. doi: 10.1111/den.13003. [DOI] [PubMed] [Google Scholar]
- 187.Jain D, Mahmood E, Desai A, Singhal S. Endoscopic full thickness resection for gastric tumors originating from muscularis propria. World J Gastrointest Endosc. 2016;8:489–495. doi: 10.4253/wjge.v8.i14.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chae JM, Jang JY, Hong S, Kim JW, Chang YW. A case of endoscopic full-thickness resection in a patient with gastric high-grade dysplasia unsuitable for endoscopic submucosal dissection. Clin Endosc. 2014;47:353–357. doi: 10.5946/ce.2014.47.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Phee SJ, Low SC, Huynh VA, Kencana AP, Sun ZL, Yang K. Master and slave transluminal endoscopic robot (MASTER) for natural orifice transluminal endoscopic surgery (NOTES) Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:1192–1195. doi: 10.1109/IEMBS.2009.5333413. [DOI] [PubMed] [Google Scholar]
- 190.Ho KY, Phee SJ, Shabbir A, Low SC, Huynh VA, Kencana AP, Yang K, Lomanto D, So BY, Wong YY, Chung SC. Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER) Gastrointest Endosc. 2010;72:593–599. doi: 10.1016/j.gie.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 191.Phee SJ, Reddy N, Chiu PW, Rebala P, Rao GV, Wang Z, Sun Z, Wong JY, Ho KY. Robot-assisted endoscopic submucosal dissection is effective in treating patients with early-stage gastric neoplasia. Clin Gastroenterol Hepatol. 2012;10:1117–1121. doi: 10.1016/j.cgh.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 192.Harlow C, Sivananthan A, Ayaru L, Patel K, Darzi A, Patel N. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc. 2020;13:2631774520957220. doi: 10.1177/2631774520957220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos) Gastrointest Endosc. 2021;93:1097–1109. doi: 10.1016/j.gie.2020.09.049. [DOI] [PubMed] [Google Scholar]
- 194.Mão de-Ferro S, Castela J, Pereira D, Chaves P, Dias Pereira A. Endoscopic Full-Thickness Resection of Colorectal Lesions with the New FTRD System: Single-Center Experience. GE Port J Gastroenterol. 2019;26:235–241. doi: 10.1159/000493808. [DOI] [PMC free article] [PubMed] [Google Scholar]