Abstract
Systemic sclerosis is a connective tissue disease that presents with significant gastrointestinal involvement, commonly in the esophagus. Dysphagia is a common clinical manifestation of systemic sclerosis and is strongly related to esophageal dysmotility. However, there are multiple other contributing factors in each step in the physiology of swallowing that may contribute to development of severe dysphagia. The oral phase of swallowing may be disrupted by poor mastication due to microstomia and poor dentition, as well as by xerostomia. In the pharyngeal phase of swallowing, pharyngeal muscle weakness due to concurrent myositis or cricopharyngeal muscle tightening due to acid reflux can cause disturbance. The esophageal phase of swallowing is most commonly disturbed by decreased peristalsis and esophageal dysmotility. However, it can also be affected by obstruction from chronic reflux changes, pill-induced esophagitis, or Candida esophagitis. Other contributing factors to dysphagia include difficulties in food preparation and gastroparesis. Understanding the anatomy and physiology of swallowing and evaluating systemic sclerosis patients presenting with dysphagia for disturbances in each step can allow for development of better treatment plans to improve dysphagia and overall quality of life.
Keywords: Systemic sclerosis, Esophageal motility disorders, Deglutition, Deglutition disorders, Gastroesophageal reflux, Esophagitis
Core Tip: Systemic sclerosis presents with significant gastrointestinal involvement, with dysphagia being a common clinical symptom. Normal swallowing physiology is broken down into the oral phase, pharyngeal phase, and esophageal phase of swallowing; systemic sclerosis can cause disease processes that affect and disrupt each stage of swallowing. We describe the disruptions to swallowing that occur in each phase and potential therapeutic options to alleviate symptoms.
INTRODUCTION
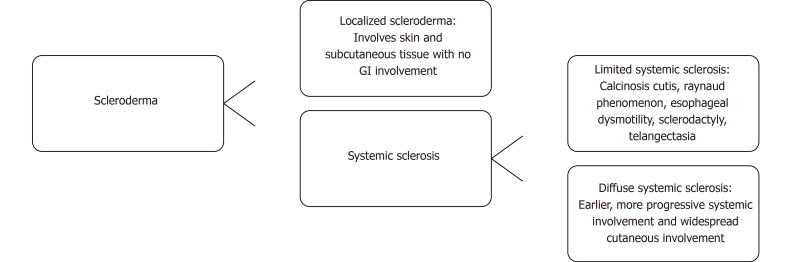
Scleroderma is a connective tissue disease that may affect any organ system, creating variegated patient-to-patient manifestations. Scleroderma can occur as localized scleroderma or systemic sclerosis (SSc). Localized Scleroderma involves the skin and subcutaneous tissue, but does not manifest with Raynaud phenomenon, digital ischemia, or visceral organ involvement[1]. SSc can be further divided into the limited form, formerly known as CREST syndrome, or the diffuse form. Limited SSc symptomology includes calcinosis cutis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia[1], while diffuse SSc involves widespread cutaneous involvement and earlier, more progressive systemic involvement[1]. The breakdown of types of scleroderma and features of each are shown in Figure 1.
Figure 1.
The types of scleroderma and features of each disease. Scleroderma can be broken down into localized scleroderma or systemic sclerosis. Systemic sclerosis can be further broken down into limited and diffuse types. GI: Gastrointestinal.
In SSc, gastrointestinal (GI) involvement, specifically esophageal disease, is very common in both limited and diffuse forms, and may be the first manifestation of disease[2]. A previous study evaluating quality of life has shown significant correlation between GI symptoms and lower quality of life scores[3]. Dysphagia, specifically, is a common presentation of GI involvement in SSc[4]. A recent study using screening questionnaires to evaluate physical, functional, and emotional outcomes due to dysphagia shows mild disability in 74% of patients and moderate to severe disability in the remaining 26%[5]. While many studies report that dysphagia is a result of esophageal dysfunction, oral and pharyngeal involvement can also lead to oropharyngeal causes of dysphagia in SSc[5]. Given the significant impact of dysphagia on quality of life in SSc patients, swallowing dysfunction should be thoroughly worked up so it may be treated appropriately[5].
The aim of this study is to review pathophysiology of SSc as well as the physiology of swallowing and reflux to understand the various disruptions that result in dysphagia in SSc. Understanding this will allow for development of more effective therapeutic plans for SSc patients suffering from dysphagia.
PATHOPHYSIOLOGY OF SSC
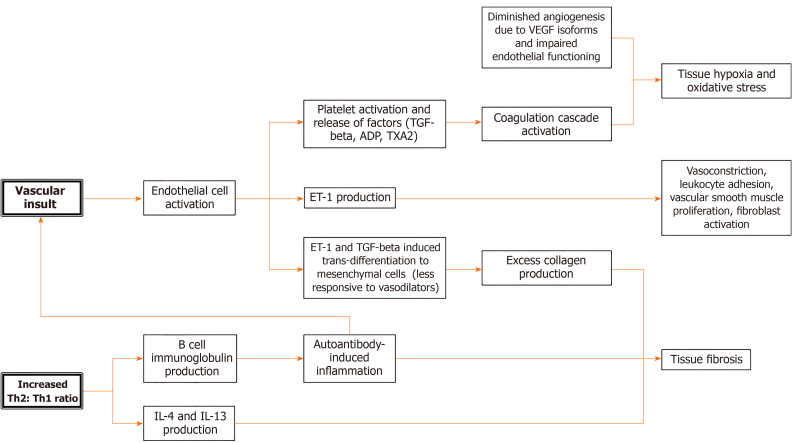
The pathophysiology of SSc is complex and has not been fully elucidated. SSc is defined by vascularity changes, autoimmunity, and tissue fibrosis[1]. Vascular insult, which can be due to autoantibody effects, infectious or inflammatory causes, environmental exposure, or any number of catalysts, results in activation of endothelial cells. This leads to activation of platelets, which release various factors, such as thromboxane A2 (TXA2), ADP, and transforming growth factor beta (TGF-beta), that promote platelet aggregation and activation of the coagulation cascade. Activated endothelial cells release endothelin 1 (ET-1), a potent vasoconstrictor that also causes leukocyte adhesion, proliferation of vascular smooth muscle cells, and activation of fibroblasts[1]. Under the influence of ET-1 and TGF-beta, endothelial cells undergo trans-differentiation into mesenchymal cells that have a blunted response to vasodilators such as nitrous oxide[1,6]. This milieu contributes to tissue hypoxia and oxidative stress, exacerbated by diminished angiogenesis secondary to the impaired functioning of endothelial progenitor cells in SSc[1]. Furthermore, SSc may be associated with vascular endothelial growth factor isoforms that are antiangiogenic[6].
Immune system involvement also plays a role in SSc pathogenesis, with dysregulation of both innate and humoral immunity. SSc presents an imbalance of T helper cells defined by a predominance of type 2 T helper (Th2) cells[1]. The cytokines produced by these overexpressed Th2 cells, including IL-4 and IL-13, are pro-fibrotic[7]. Th2 cells also induce immunoglobulin production by B cells; these upregulated autoantibodies contribute to inflammation. Limited SSc is associated with anti-centromere antibodies while the diffuse form is associated with anti-Scl-70 antibodies or anti-DNA topoisomerase I antibodies, as well as anti-RNA polymerase III antibodies. There is an association between anti-RNPC3 antibodies and severity of SSc GI disease, with the presence of these antibodies predictive of moderate or severe GI disease. The subunit of RNA polymerase III that is targeted by autoantibodies can further stratify GI severity in SSc patients[8]. Activation of the immune system in this way leads to fibrosis. The excess collagen produced by myofibroblasts contributes to this process of structural damage[7]. The basic pathophysiology of SSc is outlined in Figure 2.
Figure 2.
Simplified pathophysiology of systemic sclerosis. SSc: Systemic sclerosis; Th2: Type 2 T helper; Th1: Type 1 T helper; TGF-beta: Transforming growth factor beta; TXA2: Thromboxane A2; ET-1: Endothelin 1; VEGF: Vascular endothelial growth factor; IL: Interleukin.
Autonomic dysfunction may also contribute to SSc symptomology and severity. Vascular damage to the vasa nervorum is important in the development of this dysfunction[9]. Autoantibodies against nicotinic acetylcholine receptor at autonomic ganglia (gAChR) may also play a role in GI autonomic dysfunction, as anti-gAChRα3 autoantibodies were significantly higher in patients with SSc GI disease than in SSc patients without GI manifestations[10]. Anti-muscarinic-3 receptor autoantibodies may also contribute by inhibiting contraction of smooth muscle cells[11].
SSc esophageal involvement
GI involvement, specifically esophageal involvement, is the most common internal organ complication of SSc, affecting over 90% of patients[8]. While patients with the diffuse form have greater skin and muscular involvement, there is no difference in GI involvement between the diffuse and limited forms of SSc[12]. Since smooth muscle is targeted in the pathophysiology of SSc, the lower two-thirds of the esophagus and lower esophageal sphincter (LES) are preferentially affected, as the upper third of the esophagus is primarily composed of skeletal muscle. Esophageal symptomatology typically includes dysphagia, pseudo-obstruction, regurgitation, heartburn, nausea, or vomiting, all of which can lead to poor eating and severe weight loss[7]. Esophageal dysfunction permits gastric reflux, which can lead to erosive esophagitis, bleeding, and ulceration, as well as eventual stricture and fistulae formation and an achalasia-like syndrome. Barrett’s esophagus and adenocarcinoma may result from long-term disease. Micro-aspiration due to esophageal dysmotility is also associated with interstitial lung disease (ILD)[7].
Diagnostic delay may occur in SSc GI disease since 30% of patients with esophageal involvement may be asymptomatic, especially early in the disease course[7]. Various diagnostic studies can be used in detection of esophageal disease including manometry, pH monitoring with or without impedance, and esophagogastroduodenoscopy (EGD)[4]. Radiographic imaging may also be used: Videofluorography swallow study of the esophagus can also show altered peristalsis and a standard chest computed tomography (CT) may reveal a dilated esophagus[13]. The diagnostic studies are outlined in Table 1. When patients are symptomatic, associated GI symptoms can be scored in various ways according to symptomology. UCLA Scleroderma Clinical Trial Consortium GIT 2.0 (UCLA SCTC GIT 2.0) is a scoring system used to assess GI tract symptom severity as well as health-related quality of life[14]. The scoring system has 34 questions assessing 7 different scales, which include reflux, distention/bloating, diarrhea, fecal soilage, constipation, emotional well-being, and social functioning, as outlined in Table 2[14]. The score is based on a questionnaire that asks about severity and frequency of symptoms, ranging from “no gut symptoms” to “very severe symptoms”[14]. This scoring system is found to be reliable and feasibly applicable as it takes only 6-8 min to complete[14]. The UCLA STCT GIT 2.0 is very useful in SSc patients not only for evaluating severity of GI disease but also for following improvement in symptoms with various treatments[14].
Table 1.
Features of various diagnostic studies in systemic sclerosis diagnosis
| Diagnostic study |
Role in SSc |
| Esophagogastroduodenoscopy | Evaluates for esophageal causes of dysphagia[4] |
| Shows reflux-related complications: erosive esophagitis, strictures, Barrett’s esophagus, esophageal adenocarcinoma[4] | |
| Reveals esophageal findings in asymptomatic patients[4] | |
| Esophageal manometry | Detects esophageal dysmotility, even in early stages of SSc[4] |
| Shows decreased lower esophageal sphincter pressure and absent peristalsis in distal two-thirds of esophagus[4] | |
| Pharyngeal manometry | Evaluates for oropharyngeal dysphagia by assessing upper esophageal sphincter relaxation and pharyngeal propulsion[38] |
| Esophageal pH monitoring (with or without impedance) | Gold standard for gastroesophageal reflux detection[4] |
| Used for patients with resistant reflux[4] | |
| Videofluorography swallow study of esophagus | Shows esophageal dysmotility with decreased peristalsis in distal 2/3 of esophagus[13] |
| Shows decrease of lower esophageal sphincter pressure[13] | |
| Shows dilated lumen of esophagus[13] | |
| CT chest | Shows esophageal dilation[13] |
SSc: Systemic sclerosis; CT: Computed tomography.
Table 2.
Scoring system of gastrointestinal symptoms based on UCLA Scleroderma Clinical Trial Consortium GIT 2.0[14]
| Scales |
None-to-mild symptoms |
Moderate symptoms |
Severe-to-very severe symptoms |
| Reflux | |||
| Distension/bloating | |||
| Diarrhea | |||
| Constipation | |||
| Fecal soilage | |||
| Emotional well being | |||
| Social functioning | |||
| Total GIT score |
SSc patients, especially those with diffuse SSc in which complications typically occur more rapidly, report adverse effects of the disease on their quality of life. Patients describe fatigue, manual activity limitations, distress related to manifestations and unpredictability of their disease, sleep difficulties, and low self-esteem; patients do not feel fully equipped to handle their disease mentally or physically[15]. Anxiety related to the disease is prominent in the SSc population, further dampening quality of life[15]. The prevalence of GI disease in SSc, as well as the harsh symptomology that can result from GI forms of the disease, likely makes GI involvement a contributor to poor quality of life in these patients.
PHYSIOLOGY OF SWALLOWING
Dysphagia is common in SSc and may be related to disease affecting the smooth muscle of the distal two-thirds of the esophagus and the LES as well as other disease processes in SSc[1,16]. It is therefore necessary to first review the normal swallowing physiology to understand how various manifestations of SSc can cause disruption to each step of this process. Physicians can then apply this knowledge clinically by recommending treatment plans targeting these factors through lifestyle changes, medications, and procedures to improve dysphagia symptoms and quality of life.
Swallowing is a coordinated, physiological process that is important for consumption of food and fluid. It is essential for sustenance and any disruption in it affects perception of quality of life[17]. Difficult or disordered swallowing can cause further medical complications such as malnutrition, dehydration, and aspiration pneumonia[17,18].
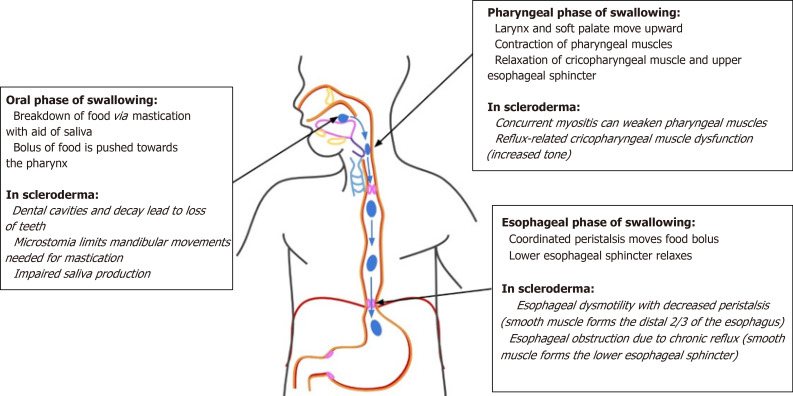
The physiological process of swallowing is broken down into three stages: the oral stage, the pharyngeal stage, and the esophageal stage[17]. The oral stage is the voluntary first step, and involves the lips, teeth, muscles of mastication, and tongue[17]. In the oral preparatory phase of the oral stage, mastication allows the breakdown of food as it is moved around the oral cavity and lubricated with saliva[17,19]. During the propulsive stage of the oral phase, the food bolus is first positioned on the superior surface of the tongue[17]. Tongue flexion beginning anteriorly and moving posteriorly pushes the bolus towards the pharynx, to begin the second stage of swallowing[17]. In the pharyngeal phase of swallowing, the larynx and soft palate move upwards to block off the airway and nasopharynx[19]. Several muscles of the anterior portion of the pharynx contract to cause forward displacement of the larynx and pharynx[19]. This is followed by relaxation of the cricopharyngeal muscle and relaxation and opening of the upper esophageal sphincter (UES)[19]. As the UES opens, the bolus will pass through to the esophagus, beginning the esophageal phase of swallowing[20]. Once the bolus enters the esophageal lumen, it is transported by coordinated, sequential muscular contractions and relaxations known as peristalsis[19]. This peristalsis is triggered by distension of esophagus and allows for the bolus to travel towards the stomach[19]. This primary peristalsis may be followed by a secondary wave. Secondary peristalsis is limited to the smooth muscle portion in the bottom 2/3 of the esophagus, and functions to clear remnants of the bolus leftover from the primary wave and to remove refluxed gastric contents[19]. The final step in the esophageal phase of swallowing is relaxation of the LES and movement of the bolus into the stomach[19]. Relaxation occurs in a coordinated manner with the preceding peristalsis[19]. The LES remains tonically contracted at rest and is supported by the crural diaphragm[19].
Dysphagia, defined as difficulty in swallowing that results in delay in passage of food or liquid bolus[21], is a common symptom in SSc patients due to various disturbances in the normal swallowing process[1]. In these patients, each stage of swallowing can be disrupted in various ways, each leading to a different classification of dysphagia[21]. Good history-taking followed by necessary diagnostic testing in SSc patients can define the type of dysphagia so an appropriate therapeutic plan may be recommended. The oral phase of swallowing in SSc may be disrupted by loss of teeth and microstomia limiting mastication. Impaired saliva production may further complicate the oral phase. Pharyngeal dysphagia commonly occurs due to uncoordinated contractions, muscular weakness causing decreased contraction, or anatomic anomalies causing obstruction[21]. In SSc, there may be weakening of pharyngeal muscles due to concurrent myositis or cricopharyngeal muscle tightening secondary to reflux. Esophageal dysphagia is commonly due to mechanical obstructions or motility disorders[21]. Dysmotility characterized by decreased peristalsis or obstruction secondary to chronic reflux changes may result in esophageal dysphagia in SSc. The various disease processes affecting each stage of swallowing in SSc are outlined in Figure 3. The potential treatment options for these processes are outlined in Table 3.
Figure 3.
Disruptions of normal swallowing physiology due to disease processes of scleroderma.
Table 3.
Treatment options for the various disease processes that contribute to the development of dysphagia in systemic sclerosis
| Disease process |
Therapeutic plan |
| Xerostomia | Drinking water more frequently[2] |
| Using artificial saliva as needed[2] | |
| Using special toothpastes and mouthwashes[2] | |
| Avoiding medications that exacerbate dry mouth[2] | |
| Microstomia | Performing exercises and massages to stretch the mouth[2] |
| Dental decay | Planning regular follow-up with experienced dentist[2] |
| Concurrent myositis | Frequent screening for myositis in patients with SSc and suggestive symptoms[37] |
| Treating concurrent myositis with immunomodulatory therapy and interventional procedures[37] | |
| Esophageal Dysmotility | Lifestyle management (taking smaller bites, chewing food thoroughly, drinking adequate water with food)[16] |
| GERD | Medications (PPIs, H2RAs)[2,52] |
| Dietary modifications (avoiding acidic foods)[52] | |
| Lifestyle modifications (avoiding meals before bedtime, elevating the head of the bed while sleeping)[2,57] | |
| Candida esophagitis | Screening for fungal esophagitis in patients with SSc and suggestive symptoms[73] |
| Prompt antifungal treatment[73] | |
| Pill esophagitis | Avoiding medications at high risk of causing esophagitis[79] |
| Screening for esophagitis in SSc patients taking culprit medications with suggestive symptoms[79] | |
| Gastroparesis | Medications (prokinetic agents)[2] |
| Dietary modifications of small frequent meals with fiber[2] |
SSc: Systemic sclerosis; PPIs: Proton pump inhibitors; H2RAs: H2-receptor antagonists; GERD: Gastroesophageal reflux disease.
CAUSES OF ORAL DYSPHAGIA IN SSC PATIENTS
Pre-oral phase food preparation
Hand function impairment in SSc can commonly lead to difficulty in food preparation and presentation. Hand dysfunction in SSc is widely recognized and is typically due to skin thickening and contracture of the hands. Tests such as the Hand Mobility in Scleroderma (HAMIS) test are frequently used to assess finger and wrist mobility[22]. In a study of 30 patients with SSc, it was found that hand dexterity was reduced to 68%-80%, and grip force was reduced to 46%-65% compared to a healthy person. Finger flexion and extension were the most impaired in hand mobility[23]. Ischemic digital ulceration in SSc also contributes to impairment of hand function as well as increased pain[24]. These impairments lead to difficulty in preparing or ingesting food in patients with SSc. These quality-of-life changes affecting eating are addressed in the Scleroderma Health Assessment Questionnaire, which asks questions such as “Are you able to cut your meat?” and “Are you able to lift a full glass to your mouth?”[25]. Those who do endorse difficulty cutting their food may have further swallowing symptoms due to ingestion of larger pieces of food. Inability to lift a glass can also limit water intake, that would have facilitated swallowing.
Disruption in mastication and food bolus preparation
The oral voluntary phase of swallowing may be also severely limited in SSc due to various factors that can subsequently impact the following swallowing phases. This adds to the burden of diminished esophageal motility function and dysphagia in SSc patients. Studies show that SSc patients may suffer from microstomia due to sclerofibrosis of perioral tissue and malfunctioning of the temporomandibular joint[26]. This limits SSc patients’ ability to open their mouths beyond 40 mm, and results in limited mandibular movements[27]. This causes disturbances in mastication and food bolus preparation, affecting the oral preparatory stage of the oral phase of swallowing and contributing to dysphagia in SSc patients.
Other disturbances in SSc lead to poor dental hygiene in this patient population. Teeth enamel can be eroded due to gastric acid reflux[2] and xerostomia[28]. In addition, SSc patients may have sclerodactyly and hand dysfunction, which can limit their ability to brush and care for their teeth[26]. This results in dental cavities, decay, and ultimately loss of teeth, and causes disruption in chewing of the food bolus for mastication in the oral phase of swallowing[26]. Other findings in SSc patients include widening of the periodontal ligament around the premolar and molar teeth[29], mandibular bone resorption[26], and gingival recession[26].
There are very few treatments to ameliorate the issue of poor dentition in SSc. Typical treatments for poor dentition include dentures and implants, which are not feasible in SSc. Dentures are difficult to apply due to impaired hand function as well as oral cavity narrowing and tongue rigidity in SSc patients[30]. Once applied, salivary hypofunction may also reduce denture retention[30]. Implants are also difficult due to low bone mineral density in SSc patients[31]. Mandibular bone resorption, commonly seen in SSc patients, is thought to be secondary to pressure ischemia from sclerosed skin and muscle atrophy[32]. Proper dental care and hygiene is essential in preventing and treating dysphagia in SSc patients. Patients may require assistance in brushing their teeth and should be encouraged through routine checks with experienced dentists.
Impaired saliva production and concomitant Sjogren’s syndrome
Patients with SSc have a higher prevalence of concomitant Sjogren’s Syndrome, an autoimmune disease characterized by inflammation of exocrine glands resulting in dryness of mucosal surfaces. Specifically, there is a loss of salivary glands that leads to xerostomia[33]. Sjogren’s disease is seen in 17%-29% patients with SSc[33]. Saliva is necessary for mastication, digestion, oral cleansing, and speech articulation[34]. It contains water, minerals, electrolytes, buffers, and proteins that maintain neutral pH and aid in enzymatic digestion. Loss of the bicarbonate buffer contained within saliva disrupts one defense mechanism against acid reflux, and may result in more severe gastroesophageal reflux disease (GERD) symptoms[19]. Salivary dysfunction also impairs the oral preparatory stage of the oral phase of swallowing, as saliva is needed for lubrication of food and mastication[17,35]. This disturbance may contribute to the overall prevalence of dysphagia in SSc patients.
CAUSES OF PHARYNGEAL DYSPHAGIA IN SSC PATIENTS
Concomitant myositis
Patients with SSc are more prone to suffering from concurrent myopathies that may contribute to the pathophysiology of dysphagia. Prior studies note anywhere between 5%-81% of SSc patients suffer from myopathies, depending on the definition of muscle involvement. Myositis in patients with SSc is termed as Scleroderma Polymyositis Overlap. Scleroderma Polymyositis Overlap is characterized by a more active fibrotic disease and is associated with an increased rate of systemic complications as well as a higher mortality rate[36]. Polymyositis is a myopathy characterized by muscle impairment and systemic symptoms secondary to muscle inflammation of striated skeletal muscles[37]. Because coordinated contraction of the striated skeletal muscles of the oropharynx and upper esophagus is essential to the swallowing process, inflammation and dysfunction of these muscles, as seen in polymyositis, can cause pharyngeal dysphagia[37]. Dysphagia is reported in up to 36% of patients with myositis[37]. On pharyngeal high-resolution impedance manometry, there may be globally weak pressure generation, with absent hypo-pharyngeal constrictor activity as well as abnormal UES contractility[38]. These findings correlate with increased post-swallow residue and aspiration risk[38]. Given the risk of concomitant myositis in SSc patients, pharyngeal dysphagia due to striated muscle weakness should be considered a potential cause of dysphagia in SSc patients. The possibility of additional disruption in the pharyngeal phase of swallowing may result in a more severe dysphagia in SSc patients.
Cricopharyngeal muscle disorder
Cricopharyngeal muscle disorders can commonly develop in SSc patients due to changes from chronic reflux. A previous study found that 51% of patients with gastroesophageal reflux have pharyngoesophageal dysphagia due to cricopharyngeal muscle dysfunction[39]. Another study in which HCl was infused proximal to the gastro-esophageal sphincter revealed an increase in UES tone 1 min after infusion, revealing the reactivity of the cricopharyngeal muscle to acid reflux[40]. Cricopharyngeal muscle dysfunction resulting in increased tone may prevent passage of food bolus from pharynx through UES into the esophagus. Given the high prevalence of reflux in SSc patients, cricopharyngeal muscle dysfunction may be an important complication to consider. Several case reports have also shown SSc patients specifically presenting with UES stenosis[41,42]. This upper esophageal involvement in SSc is an important contributor to dysphagia during the pharyngeal phase of swallowing.
CAUSES OF ESOPHAGEAL DYSPHAGIA IN SSC PATIENTS
Esophageal dysmotility
SSc commonly presents with GI involvement, manifested primarily in the esophagus as dysmotility[16]. The distal two-thirds of the esophagus is affected due to its smooth muscle makeup[2]; this area of smooth muscle is likely commonly cited as the first affected location of the GI tract because esophageal changes are readily sensed by patients due to the daily frequency of swallowing. While loss of peristalsis in other areas of the GI tract may become noticeable to patients with time, the fact that swallowing is under partial voluntary control and performed multiple times per day presumably highlights any associated discomfort. Further, perhaps the two-layered muscularis of the esophagus is more quickly affected than the thicker three-layered muscularis of the stomach, for example[43].
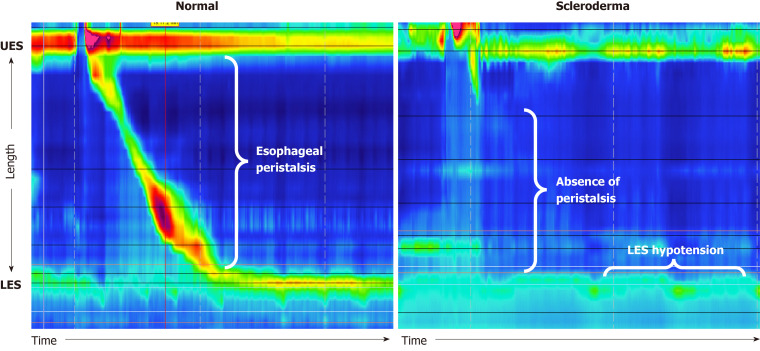
Prospective studies show esophageal dysmotility confirmed via manometry in about 70%-75% of SSc patients[16]. Esophageal involvement results in decreased amplitude of esophageal peristalsis as well as decreased tone of the LES. Esophageal manometry can confirm scleroderma as the cause of esophageal dysmotility by showing absent peristalsis in the distal two-thirds of the esophagus and a hypotensive LES[44], as seen in Figure 4. Clinically, patients most commonly present with dysphagia to both solids and liquids[16]. The lack of peristalsis in scleroderma results in esophageal dysphagia due to a motility issue, as lack of coordinated contractions/relaxations in the esophagus prevent food from being efficiently transported to the stomach[21]. Other clinical symptoms related to esophageal dysmotility in scleroderma include globus sensation, reflux, heartburn, or chest discomfort with swallowing[16]. Complications related to esophageal dysmotility include gastroesophageal reflux, stasis of foods in the esophagus, and aspiration into the lungs[16]. While there are no medications that effectively treat esophageal dysmotility, symptoms can be alleviated by lifestyle management. This includes taking smaller bites, chewing food thoroughly, and hydrating with water when having any solid, dry foods[16]. While esophageal dysmotility is commonly thought to contribute to dysphagia in SSC[1], the development and progression of further complications can cause more severe disruption to the normal swallowing physiology and may worsen dysphagia symptoms.
Figure 4.
Normal manometry findings showing peristalsis during swallowing and lower esophageal sphincter tone compared to manometry findings in scleroderma showing an absence of peristalsis and lower esophageal sphincter hypotension. LES: Lower esophageal sphincter; UES: Upper esophageal sphincter.
Gastroesophageal reflux
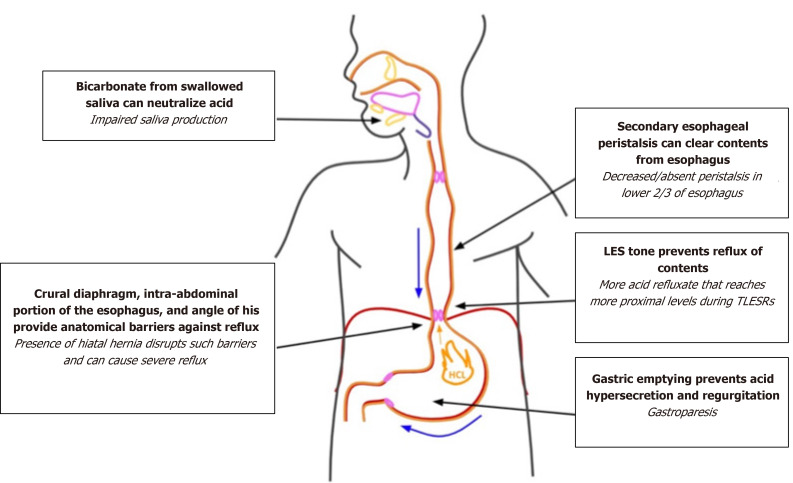
In individuals with a normal esophagus, throughout the day, only small amounts of gastric contents reflux into the esophagus due to presence of multiple defensive barriers[19]. These include inherent LES tone with surrounding muscles, like the crural diaphragm, augmenting tone, secondary peristalsis to rapidly clear refluxed materials, bicarbonate in swallowed saliva to neutralize acid, local bicarbonate and mucus secretion, and resistance of the epithelium to degradation[19]. In patients with GERD, gastric contents with acid or pepsin reflux into the esophagus and cause inflammation or esophageal injury, possibly due to defective defensive mechanisms[19]. If the LES is impaired by disease or inflammation, it may allow contents to reflux. Defective peristalsis may limit clearance of contents, causing further injury and inflammation with longer contact times[19]. In addition, reduced salivation will decrease neutralization of refluxed contents[19]. Certain anatomic irregularities can also worsen reflux frequency and severity. For example, hiatal hernias decrease the overlap between the crural diaphragm and LES, thereby decreasing LES tone as it is no longer supported by the diaphragm[19,45]. In addition, transient LES relaxation (TLESR) has a major role in GERD pathology[19,45]. Physiologically, TLESRs occur after meals as they are triggered by gastric distension to allow the release of gas from the stomach as belching[45]. In patients with GERD, TLESR can allow acid in addition to gas to be refluxed into the esophagus[45]. Studies have shown that most episodes of acid reflux occur during TLESRs[46]. In patients with GERD, TLESRs occur more frequently, acid refluxes into the esophagus more often, and refluxed contents reach more proximal levels in the esophagus than in patients without GERD[46].
GERD is a very common diagnosis in SSc patients due to various disruptions of reflux defense mechanisms, as outlined in Figure 5. Based on large retrospective studies, the prevalence of esophageal reflux in SSc patients is 34.8%[2,47]. Clinically, patients report classic heartburn and reflux symptoms as well as dysphagia, odynophagia, laryngitis, chronic cough, hoarseness, or asthma[16]. In SSc, esophageal dysmotility prevents secondary peristalsis from efficiently clearing acidic refluxed materials[16], highly predisposing SSc patients to developing severe GERD[2]. The loss of tone in the LES also contributes to the development of GERD and complications from reflux[2]. GERD-related complications such as reflux esophagitis, strictures, Barrett’s esophagus, and adenocarcinoma may cause further dysphagia symptoms[2]. Gastroparesis (Gp) and small bowel dysmotility are also common symptoms in scleroderma and may worsen GERD symptoms[48]. SSc is also associated with impaired saliva production, which impedes the ability to neutralize the refluxed contents[48].
Figure 5.
Disturbances of defense mechanisms against reflux in scleroderma. LES: Lower esophageal sphincter.
The presence of hiatal hernia in SSc patients may exacerbate GERD symptoms as well. One study comparing 25 SSc patients to 25 control patients found presence of hiatal hernia in 16 out of 25 SSc patients and only 3 out of 25 control patients[49]. Another cross-sectional study of SSc patients found that those with gastroesophageal symptoms had a significantly higher frequency of hiatal hernia than asymptomatic patients[50]. Concurrent hiatal hernia likely contributes to the occurrence and severity of GERD symptoms in SSc patients. Loss of multiple defense mechanisms against reflux through the various disease processes of SSc may explain why SSc-related GERD is associated with more severe symptoms and more complications[51].
In SSc patients that present with GERD, treatment is imperative to prevent development of acid-induced complications. Medical management via proton pump inhibitors (PPIs) is effective in relieving GI symptoms[16]. H2-receptor antagonists (H2RAs) can also be used, though less effective than PPIs[16,52]. This medication therapy should be coupled with lifestyle measures. This includes avoiding acidic foods, abstaining from drinking alcohol and smoking, losing weight, eating smaller meals, and elevating the head of the bed[52].
TLESR
TLESRs are important in development of GERD[45], as studies indicate that TLESRs may be more important than intervals of persistently low LES tone for reflux episodes[45,46]. Given that TLESRs are triggered by gastric distension[45], they may occur more frequently in SSc patients who suffer from Gp-related distension. In patients with GERD, reflux during TLESRs was found to be more acidic and reach more proximal levels of the esophagus than in normal patients[46]. This was thought to be related to decreased esophageal contraction in response to acid reflux[46]. Loss of esophageal motility in scleroderma patients may predispose patients to more acid reflux than can reach more proximal levels of the esophagus during TLESRs. The increase in frequency of TLESRs with refluxate that can reach more proximal levels of the esophagus likely increases severity of GERD symptoms in SSc patients. There has been some evidence suggesting that baclofen may decrease incidence of TLESRs, thereby reducing the number and length of reflux episodes in patients with GERD[16,53].
Delayed esophageal clearance
Despite the significant overlap between the clinical presentations of SSc esophagus and GERD, there are additional mechanisms in SSc pathophysiology to consider. While GERD is primarily due to reflux of acidic gastric contents up into the esophagus, SSc esophagus has reflux coupled with the stasis of acidic contents due to delayed esophageal clearance, exacerbating symptoms[19,54]. Consequences of delayed esophageal clearance secondary to dysmotility include both severe GERD symptoms from esophageal stasis-related acidity and a dilated distal esophagus[54].
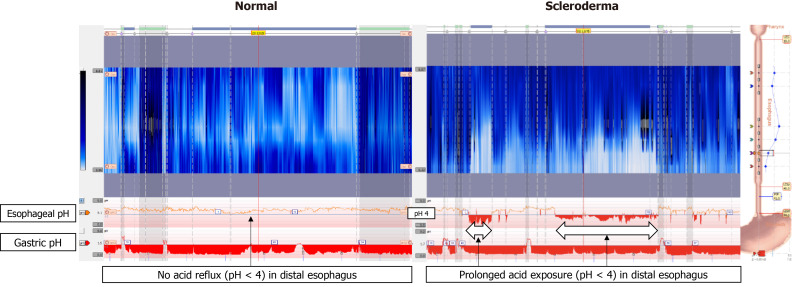
It is important to recognize patients who present with refractory GERD symptoms despite adequate treatments and suspect SSc as a possible diagnosis. In these patients, an EGD will show a dilated esophagus with retained secretions and saliva suggestive of esophageal stasis, and CT may confirm dilated esophagus[54]. Twenty-four-hour ambulatory pH monitor with impedance monitoring and manometry may demonstrate ineffective esophageal motility with subsequent stasis of acidic contents in the esophagus, by documenting prolonged esophageal acidity compared to acid exposure in the stomach[54]. Findings indicative of esophageal stasis on 24-h impedance pH monitoring may be seen in Figure 6. If delayed esophageal clearance is noted through diagnostic testing, it should be addressed promptly, as it is likely a major exacerbator of esophageal acidity. Esophageal stasis of acidic contents may contribute to the severity of GERD symptoms and high rate of complications in SSc patients[51], and should therefore be addressed in therapeutic plans. It is also important to note that esophageal involvement of SSc is linked to development of ILD, a major cause of morbidity and mortality in SSc[2]. ILD severity has been shown to be associated with more active reflux disease, but development may be possible even in SSc patients with esophageal dilation but no clinical esophageal symptoms[2,55]. A retrospective cross-sectional study has shown that the diameter of the esophagus in SSc patients correlates with progression of ILD, as measured by diffusion capacity of the lung for carbon monoxide[2,56]. Given that delayed esophageal clearance can result in both increased acid damage to the esophagus and development of ILD, developing an appropriate treatment plan is essential. Possible lifestyle modifications to consider include eating smaller meals, remaining in an upright position after eating, elevating the head of the bed while sleeping, and avoiding eating in the three hours before sleeping[57,54]. These changes utilize the effect of gravity to promote esophageal clearance of acidic contents[54]. Lifestyle changes can be supplemented with medical therapy targeting esophageal acidity[57].
Figure 6.
Twenty-four hour impedance pH monitoring normal findings compared to findings in scleroderma. In normal esophagus, there are no episodes of acid reflux, as indicated by no drops of pH below 4 in the esophagus. In scleroderma esophagus, there is prolonged acid exposure in the distal esophagus.
Erosive esophagitis
SSc patients are more prone to developing erosive esophagitis as a complication of severe GERD, which can further contribute to their dysphagia. A collection of studies report that 30%-60% of SSc patients develop erosive esophagitis[58-60]. One study found that 24.2% of SSc patients had mild to moderate erosive esophagitis while 3.6% of patients had severe erosive esophagitis[61]. Mechanisms for development of this pathology all pertain to impaired acid clearance in the esophagus and increased acid exposure, such as impaired peristalsis, impaired LES tone, and pathologic nighttime gastroesophageal reflux[58,59]. Symptoms of dysphagia were also found to correlate with erosive esophagitis[58]. This is likely related to both the development of the erosions in esophagitis as well as their sequela, such as strictures.
Strictures and mechanical obstruction
Strictures are a large contributor to dysphagia in SSc and are found in 17%-29% of SSc patients[62]. A diagnosis of SSc increases the odds ratio of developing strictures to 12[62]. Strictures result from excess deposition of collagen during healing of erosive esophagitis and are often due to chronic gastroesophageal reflux[57]. The presence of these strictures leads to an obstructive dysphagia as they narrow the esophageal lumen and reduce esophageal distensibility[63]. This leads to further impairment of acid clearance from the esophagus, resulting in more esophageal insult and potential for dysphagia. Schatzki rings have also been found in SSc patients, with one study finding 12.5% of SSc patients with the lower esophageal rings. Though they may be more amenable to treatment via dilation, these rings also contribute to dysphagia[64]. However, obstructive dysphagia is not always due to strictures and Schatzki rings in SSc — though rare, adenocarcinoma may also cause obstructive esophageal dysphagia.
Barrett’s esophagus and esophageal adenocarcinoma
Reflux-related disorders such as SSc are naturally conducive to the development of Barrett’s esophagus. Pathophysiology of Barrett’s esophagus is tied to increased acidic insult of esophageal epithelium. Clinically, patients with Barrett’s esophagus tend to have longer durations of dysphagia and relatively impaired LES tone[65]. It is unclear whether this association is causation or correlation, as risk factors for Barrett’s esophagus may predispose patients to other causes of dysphagia as well[65]. Barrett’s esophagus has been found in up to 37% of patients with SSc[62], and is more likely to develop in patients with limited SSc[65]. Patients with SSc that have Barrett’s esophagus have an increased risk of developing further complications, such as strictures and adenocarcinoma[66]. Esophageal adenocarcinoma specifically may develop as a result of metaplasia and dysplasia found in Barrett’s esophagus. Development is more common in SSc patients, as esophageal adenocarcinoma has an incidence of 0.7% per year in patients with SSc and Barrett’s esophagus, while incidence is only 0.45% per year in patients with Barrett’s esophagus without SSc[67]. Though other complications of SSc may have higher incidence, esophageal adenocarcinoma should still be considered and ruled out in patients presenting with dysphagia. Esophageal cancer most commonly presents with mechanical obstruction leading to esophageal dysphagia, so it must be considered as a potential contributor to dysphagia symptoms in SSc patients[66].
Sequelae of SSc treatment
Candida, an opportunistic fungus, is normally a symbiont of the esophagus that rarely gives rise to severe disease indications. Yet Candida infection remains the most common cause of infectious esophagitis, with an incidence of up to 88%[68,69]. In the setting of an impaired host-defense system, uncontrolled proliferation of Candida in the esophageal mucosa can occur, leading to the formation of characteristic adhesive plaques, which are visualized by endoscopy. Studies have reported that Candida esophagitis (CE) can be a result of poor emptying of the esophagus, acid suppression, and immunosuppressive therapy[62]. Connective tissue diseases, such as SSc, can promote esophageal stasis and can, therefore, lead to fungal colonization of the esophagus. In addition, acid suppression via PPIs and H2RAs are commonly used in SSc patients with complaints of GERD[2]. For other various manifestations of SSc such as ILD, immunosuppression therapy is commonly prescribed[70]. As a result, CE and secondary stricture formation is likely to occur with SSc, further complicating dysphagia and esophageal dysfunction in these patients[71]. Patients with CE respond well to anti-fungal treatment, limiting the progression of disease to stricture formation and necrotizing CE[72,73]. Therefore, although CE is rare, early detection and treatment of this condition in SSc esophagus is very important to avoid development of esophagus-related complications that have high mortality rates. Pill-induced esophagitis, which is characterized as direct injury to the esophageal mucosa due to use of certain culprit medications[74], is another important consideration in SSc patients. Interestingly, patient-related risk factors for pill-induced esophagitis are commonly associated with extended transit time of culprit medications in the esophagus. Studies suggest that altered anatomy, as found in SSc, can lead to delayed esophageal transit time and stasis, therefore increasing the risk for pill-induced esophagitis[74]. Other patient-related risk factors leading to increased transit time of medications include position of patient while taking pill and size of pill. Reduced water intake while ingesting pill, potentially due to impaired hand function in SSc also increases risk. Decreased saliva production secondary to Sjogren’s syndrome, a condition known to have a higher prevalence in SSc patients, may also lead to dysphagia and impaired swallowing of the pill[74].
Notable culprit medications leading to pill-induced esophagitis include non-steroidal anti-inflammatory drugs, aspirin, bisphosphonates, potassium chloride, antibiotics (namely tetracycline and clindamycin), and iron. Prolonged contact of the pill with the esophageal mucosa can lead to direct irritation of the mucosa[75]. Several pathophysiologic mechanisms have been implicated in this condition including disruption of the cytoprotective prostaglandin barrier of the mucosa, caustic injury, and vascular injury resulting from hyperosmotic properties of the medication[74,76-78]. Pill-induced esophagitis often occurs at the site of esophageal narrowing which, in SSc patients, can result from stricture formation, decreased LES tone, or decreased esophageal peristalsis. This condition is typically self-limiting, but if left untreated, can lead to strictures, ulcerations, and even perforations, which can complicate dysphagia in SSc esophagus[79]. Ultimately, pill-induced esophagitis should be considered in SSc patients presenting with heartburn, dysphagia, or odynophagia and culprit medications should be avoided in this patient subset whenever possible. Special attention should be given to SSc patients that develop iron deficiency anemia, which often occurs secondary to microhemorrhages from telangiectasias in the GI mucosa and severe malabsorption along the GI tract in advanced disease[80]. Treatment with iron supplementation may be indicated, but usage of oral iron pills should be closely followed, as studies have shown that this can cause direct mucosal injury to the esophagus, leading to pill-induced esophagitis and dysphagia[81].
OTHER CONTRIBUTING FACTORS TO DYSPHAGIA
Gp
Gp is the delay of gastric emptying without mechanical obstruction, resulting in symptoms of bloating, nausea, and upper abdominal pain[82]. In SSc, Gp may develop due to fibrotic infiltration, resulting in subsequent dysfunction of autonomic nerves, smooth muscle, and enteric neurons[82]. Previous studies in SSc patients exhibiting GI symptoms have shown the prevalence of Gp to be 50%-67%[83]. GERD is commonly associated with Gp, with about 27% of patients with Gp suffering from concomitant moderate to severe GERD[84]. The pathophysiology of this may be related to increased volume of gastric contents resulting in stomach distension and decreased LES pressure with more frequent TLESRs, causing regurgitation of contents into the esophagus[84]. This likely explains why GERD symptoms are found to be most severe in patients with Gp and other pre-existing conditions, such as SSc[3]. The complex interplay between Gp and severe GERD may be the reason why patients with SSc are found to have significantly greater reflux symptoms and complications than those with idiopathic reflux[51]. Correspondingly, treating GERD in patients with SSc may therefore involve treating the underlying Gp.
CONCLUSION
While it may be intuitive to blame esophageal dysmotility and reflux as the culprit for dysphagia in SSc patients, understanding the physiology of swallowing and anti-reflux defense mechanisms can provide better insight on causative and contributive factors in patient symptomatology. Understanding the range of potential sources for dysphagia, from oral to esophageal, can help develop more effective therapeutic plans that can improve symptoms and result in a better quality of life. Xerostomia may be managed through lifestyle changes, including drinking water frequently, using artificial saliva as needed, utilizing special toothpastes and mouthwashes, and avoiding medications that may exacerbate symptoms[2,85]. SSc patients should regularly perform exercises and massages to stretch their mouth and prevent debilitation from microstomia[2]. Regular follow-up with an experienced dentist can improve dental hygiene and oral health[2]. SSc patients presenting with dysphagia should also be screened for concurrent myositis, and treated accordingly with appropriate immunomodulatory therapy and other interventional procedures[37].While studies show limited efficacy of treatment options targeting esophageal dysmotility, appropriate GERD management is essential in limiting the development of dysphagia[2]. GERD management includes dietary and lifestyle modifications as well as medication therapy to limit progression of reflux and reflux-related complications[2,57]. Delayed esophageal clearance may also be best managed through lifestyle changes such as avoiding meals before bedtime and elevating the head of the bed while sleeping[54]. Given that Candida infection and certain culprit medications can also cause esophagitis, providers should carefully screen for these in SSc patients. Signs of fungal infection should be treated promptly to prevent complications and culprit medications should be generally avoided[73,79].Underlying Gp may also contribute to symptoms and should be managed with prokinetic agents and dietary modification[2,57]. Being cognizant of the various contributing factors that result in dysphagia can allow physicians to develop better, more well-rounded therapeutic plans. This may allow better control of the various disease processes in SSc and improved symptoms and quality of life for SSc patients.
ACKNOWLEDGEMENTS
The authors thank Tawfik A for help in drawing the figures.
Footnotes
Conflict-of-interest statement: The authors disclose no conflicts of interest or external funding for this publication.
Manuscript source: Invited manuscript
Peer-review started: March 23, 2021
First decision: April 29, 2021
Article in press: July 30, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aksionchyk M, Chiba T, Morozov S, Tabuchi M S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
Contributor Information
Anusri Kadakuntla, Albany Medical College, Albany, NY 12208, United States.
Ankit Juneja, Albany Medical College, Albany, NY 12208, United States.
Samantha Sattler, Albany Medical College, Albany, NY 12208, United States.
Anusha Agarwal, Albany Medical College, Albany, NY 12208, United States.
Drishti Panse, Albany Medical College, Albany, NY 12208, United States.
Nardin Zakhary, Department of Dentistry, Ministry of Health, Alexandria 21500, Egypt.
Anusha Pasumarthi, Albany Medical College, Albany, NY 12208, United States.
Lee Shapiro, Division of Rheumatology, Albany Medical Center, Albany, NY 12208, United States.
Micheal Tadros, Division of Gastroenterology, Albany Medical Center, Albany, NY 12208, United States. tadrosm1@amc.edu.
References
- 1.Adigun R, Goyal A, Bansal P, Hariz A. Systemic Sclerosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- 2.McFarlane IM, Bhamra MS, Kreps A, Iqbal S, Al-Ani F, Saladini-Aponte C, Grant C, Singh S, Awwal K, Koci K, Saperstein Y, Arroyo-Mercado FM, Laskar DB, Atluri P. Gastrointestinal Manifestations of Systemic Sclerosis. Rheumatology (Sunnyvale) 2018;8 doi: 10.4172/2161-1149.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandqvist G, Hesselstrand R. Validity of the Swedish version of the systemic sclerosis quality of life questionnaire (SSCQoL): A novel measure of quality of life for patients with systemic sclerosis. Ann Rheum Dis. 2019;78:855–857. doi: 10.1136/annrheumdis-2018-214260. [DOI] [PubMed] [Google Scholar]
- 4.Denaxas K, Ladas SD, Karamanolis GP. Evaluation and management of esophageal manifestations in systemic sclerosis. Ann Gastroenterol. 2018;31:165–170. doi: 10.20524/aog.2018.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli J, Marchese MR, De Canio C, Mandiello M, Mangone GM, Padula AA, Abignano G, Santandrea L, Paludetti G. Upper dysphagia in patients affected by systemic sclerosis: prevalence and features. Acta Otorhinolaryngol Ital. 2020;40:204–210. doi: 10.14639/0392-100X-N0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutolo M, Soldano S, Smith V. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol. 2019;15:753–764. doi: 10.1080/1744666X.2019.1614915. [DOI] [PubMed] [Google Scholar]
- 7.Frech TM, Mar D. Gastrointestinal and Hepatic Disease in Systemic Sclerosis. Rheum Dis Clin North Am. 2018;44:15–28. doi: 10.1016/j.rdc.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahan ZH. Gastrointestinal involvement in systemic sclerosis: an update. Curr Opin Rheumatol. 2019;31:561–568. doi: 10.1097/BOR.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun-Moscovici Y, Brun R, Braun M. Systemic Sclerosis and the Gastrointestinal Tract-Clinical Approach. Rambam Maimonides Med J. 2016;7 doi: 10.5041/RMMJ.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakane S, Umeda M, Kawashiri SY, Mukaino A, Ichinose K, Higuchi O, Maeda Y, Nakamura H, Matsuo H, Kawakami A. Detecting gastrointestinal manifestations in patients with systemic sclerosis using anti-gAChR antibodies. Arthritis Res Ther. 2020;22:32. doi: 10.1186/s13075-020-2128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Singh J, Kedika R, Mendoza F, Jimenez SA, Blomain ES, DiMarino AJ, Cohen S, Rattan S. Role of muscarinic-3 receptor antibody in systemic sclerosis: correlation with disease duration and effects of IVIG. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1052–G1060. doi: 10.1152/ajpgi.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furst DE, Clements PJ, Saab M, Sterz MG, Paulus HE. Clinical and serological comparison of 17 chronic progressive systemic sclerosis (PSS) and 17 CREST syndrome patients matched for sex, age, and disease duration. Ann Rheum Dis. 1984;43:794–801. doi: 10.1136/ard.43.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutka K, Garkowski A, Karaszewska K, Łebkowska U. Imaging in Diagnosis of Systemic Sclerosis. J Clin Med. 2021;10 doi: 10.3390/jcm10020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna D, Nagaraja V, Gladue H, Chey W, Pimentel M, Frech T. Measuring response in the gastrointestinal tract in systemic sclerosis. Curr Opin Rheumatol. 2013;25:700–706. doi: 10.1097/01.bor.0000434668.32150.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierakowska M, Doroszkiewicz H, Sierakowska J, Olesińska M, Grabowska-Jodkowska A, Brzosko M, Leszczyński P, Pawlak-Buś K, Batko B, Wiland P, Majdan M, Bykowska-Sochacka M, Romanowski W, Zon-Giebel A, Jeka S, Ndosi M. Factors associated with quality of life in systemic sclerosis: a cross-sectional study. Qual Life Res. 2019;28:3347–3354. doi: 10.1007/s11136-019-02284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shreiner AB, Murray C, Denton C, Khanna D. Gastrointestinal Manifestations of Systemic Sclerosis. J Scleroderma Relat Disord. 2016;1:247–256. doi: 10.5301/jsrd.5000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasegbon A, Hamdy S. The anatomy and physiology of normal and abnormal swallowing in oropharyngeal dysphagia. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.13100. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos C, Brito-de la Fuente E, Clavé P, Costa A, Assegehegn G. Nutritional Aspects of Dysphagia Management. Adv Food Nutr Res. 2017;81:271–318. doi: 10.1016/bs.afnr.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Barrett KE. Gastrointestinal physiology. McGraw Hill, 2014. [Google Scholar]
- 20.Duffy KL. Dysphagia in Children. Curr Probl Pediatr Adolesc Health Care. 2018;48:71–73. doi: 10.1016/j.cppeds.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Azer SA, Kshirsagar RK. Dysphagia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- 22.Young A, Namas R, Dodge C, Khanna D. Hand Impairment in Systemic Sclerosis: Various Manifestations and Currently Available Treatment. Curr Treatm Opt Rheumatol. 2016;2:252–269. doi: 10.1007/s40674-016-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandqvist G, Eklund M, Akesson A, Nordenskiöld U. Daily activities and hand function in women with scleroderma. Scand J Rheumatol. 2004;33:102–107. doi: 10.1080/03009740410006060. [DOI] [PubMed] [Google Scholar]
- 24.Mouthon L, Carpentier PH, Lok C, Clerson P, Gressin V, Hachulla E, Bérezné A, Diot E, Khau Van Kien A, Jego P, Agard C, Duval-Modeste AB, Sparsa A, Puzenat E, Richard MA ECLIPSE Study Investigators. Ischemic digital ulcers affect hand disability and pain in systemic sclerosis. J Rheumatol. 2014;41:1317–1323. doi: 10.3899/jrheum.130900. [DOI] [PubMed] [Google Scholar]
- 25.Pope J. Measures of systemic sclerosis (scleroderma): Health Assessment Questionnaire (HAQ) and Scleroderma HAQ (SHAQ), physician- and patient-rated global assessments, Symptom Burden Index (SBI), University of California, Los Angeles, Scleroderma Clinical Trials Consortium Gastrointestinal Scale (UCLA SCTC GIT) 2.0, Baseline Dyspnea Index (BDI) and Transition Dyspnea Index (TDI) (Mahler's Index), Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), and Raynaud's Condition Score (RCS) Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S98–111. doi: 10.1002/acr.20598. [DOI] [PubMed] [Google Scholar]
- 26.Jagadish R, Mehta DS, Jagadish P. Oral and periodontal manifestations associated with systemic sclerosis: A case series and review. J Indian Soc Periodontol. 2012;16:271–274. doi: 10.4103/0972-124X.99275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmary Y, Glaiss R, Pisanty S. Scleroderma: oral manifestations. Oral Surg Oral Med Oral Pathol. 1981;52:32–37. doi: 10.1016/0030-4220(81)90169-9. [DOI] [PubMed] [Google Scholar]
- 28.Hadj Said M, Foletti JM, Graillon N, Guyot L, Chossegros C. Orofacial manifestations of scleroderma. A literature review. Rev Stomatol Chir Maxillofac Chir Orale. 2016;117:322–326. doi: 10.1016/j.revsto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Dagenais M, MacDonald D, Baron M, Hudson M, Tatibouet S, Steele R, Gravel S, Mohit S, El Sayegh T, Pope J, Fontaine A, Masseto A, Matthews D, Sutton E, Thie N, Jones N, Copete M, Kolbinson D, Markland J, Nogueira-Filho G, Robinson D, Gornitsky M. The Canadian Systemic Sclerosis Oral Health Study IV: oral radiographic manifestations in systemic sclerosis compared with the general population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:104–111. doi: 10.1016/j.oooo.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panchbhai A, Pawar S, Barad A, Kazi Z. Review of orofacial considerations of systemic sclerosis or scleroderma with report of analysis of 3 cases. Indian J Dent. 2016;7:134–139. doi: 10.4103/0975-962X.186702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Lei L, Pan J, Zhao C. A meta-analysis of fracture risk and bone mineral density in patients with systemic sclerosis. Clin Rheumatol. 2020;39:1181–1189. doi: 10.1007/s10067-019-04847-0. [DOI] [PubMed] [Google Scholar]
- 32.Crincoli V, Fatone L, Fanelli M, Rotolo RP, Chialà A, Favia G, Lapadula G. Orofacial Manifestations and Temporomandibular Disorders of Systemic Scleroderma: An Observational Study. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucuk U, Sarioglu S, Cetin P, Sari I, Birlik M. Histopathological differences between primary Sjögren's syndrome and Sjögren's syndrome accompanied by scleroderma. Indian J Pathol Microbiol. 2018;61:319–322. doi: 10.4103/IJPM.IJPM_416_17. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen AML, Sørensen CE, Proctor GB, Carpenter GH, Ekström J. Salivary secretion in health and disease. J Oral Rehabil. 2018;45:730–746. doi: 10.1111/joor.12664. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen A, Sørensen CE, Proctor GB, Carpenter GH. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018;24:1399–1416. doi: 10.1111/odi.12867. [DOI] [PubMed] [Google Scholar]
- 36.Bhansing KJ, van Riel PL, van Engelen BG, Fransen J, Vonk MC. Patients with Systemic Sclerosis/polymyositis Overlap Have a Worse Survival Rate Than Patients Without It. J Rheumatol. 2016;43:1838–1843. doi: 10.3899/jrheum.151425. [DOI] [PubMed] [Google Scholar]
- 37.Labeit B, Pawlitzki M, Ruck T, Muhle P, Claus I, Suntrup-Krueger S, Warnecke T, Meuth SG, Wiendl H, Dziewas R. The Impact of Dysphagia in Myositis: A Systematic Review and Meta-Analysis. J Clin Med. 2020;9 doi: 10.3390/jcm9072150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cock C, Omari T. Diagnosis of Swallowing Disorders: How We Interpret Pharyngeal Manometry. Curr Gastroenterol Rep. 2017;19:11. doi: 10.1007/s11894-017-0552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson RD, Woolf C, Marryatt G. Pharyngoesophageal dysphagia and gastroesophageal reflux. Laryngoscope. 1976;86:1531–1539. doi: 10.1288/00005537-197610000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol. 1978;13:821–826. doi: 10.3109/00365527809182197. [DOI] [PubMed] [Google Scholar]
- 41.Rajapakse CN, Bancewicz J, Jones CJ, Jayson MI. Pharyngo-oesophageal dysphagia in systemic sclerosis. Ann Rheum Dis. 1981;40:612–614. doi: 10.1136/ard.40.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeffery CC, Jung M. Severe Dysphagia Due to Rapidly Progressive Pharyngoesophageal Segment Stenosis in Systemic Sclerosis. J Scleroderma Relat Disord . 2016;1 [Google Scholar]
- 43.Chaudhry SR, Liman MNP, Peterson DC. Anatomy, Abdomen and Pelvis, Stomach. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 44.D'Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 45.Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202–1213. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- 46.Sifrim D, Tack J, Lerut T, Janssens J. Transient lower esophageal sphincter relaxations and esophageal body muscular contractile response in reflux esophagitis. Dig Dis Sci. 2000;45:1293–1300. doi: 10.1023/a:1005539600303. [DOI] [PubMed] [Google Scholar]
- 47.Alastal Y, Hammad TA, Renno A, Khalil B, Pierre J, Kwaah B, Khuder SA, Nawras A. Gastrointestinal manifestations associated with systemic sclerosis: results from the nationwide inpatient sample. Ann Gastroenterol. 2017;30:498–503. doi: 10.20524/aog.2017.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bakhos CT, Petrov RV, Parkman HP, Malik Z, Abbas AE. Role and safety of fundoplication in esophageal disease and dysmotility syndromes. J Thorac Dis. 2019;11:S1610–S1617. doi: 10.21037/jtd.2019.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuber-Jerger I, Müller A, Kullmann F, Gelbmann CM, Endlicher E, Müller-Ladner U, Fleck M. Gastrointestinal manifestation of systemic sclerosis--thickening of the upper gastrointestinal wall detected by endoscopic ultrasound is a valid sign. Rheumatology (Oxford) 2010;49:368–372. doi: 10.1093/rheumatology/kep381. [DOI] [PubMed] [Google Scholar]
- 50.Petcu A, Ghib LJ, Grad SM, Popovici C, Rogojan L, Rednic NV, Rednic S. Upper gastrointestinal involvement in systemic sclerosis: Findings in a real-life setting. Exp Ther Med. 2019;18:5095–5100. doi: 10.3892/etm.2019.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuda R, Yamamichi N, Shimamoto T, Sumida H, Takahashi Y, Minatsuki C, Kodashima S, Ono S, Niimi K, Tsuji Y, Sakaguchi Y, Saito I, Kataoka Y, Asada-Hirayama I, Kakimoto H, Yakabi S, Takeuchi C, Matsumoto Y, Tamaki Z, Fujishiro M, Asano Y, Sato S, Koike K. Gastroesophageal Reflux Disease-Related Disorders of Systemic Sclerosis Based on the Analysis of 66 Patients. Digestion. 2018;98:201–208. doi: 10.1159/000489848. [DOI] [PubMed] [Google Scholar]
- 52.Hart AM. Evidence-based recommendations for GERD treatment. Nurse Pract. 2013;38:26–34; quiz 34. doi: 10.1097/01.NPR.0000431881.25363.84. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Shi S, Chen F, Lin J. The effects of baclofen for the treatment of gastroesophageal reflux disease: a meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2014;2014:307805. doi: 10.1155/2014/307805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasumarthi A, Mago S, Banerjee P, Tadros M. Differentiating Delayed Esophageal Clearance From Reflux in Scleroderma. Cureus. 2020;12:e11553. doi: 10.7759/cureus.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marie I, Dominique S, Levesque H, Ducrotté P, Denis P, Hellot MF, Courtois H. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum. 2001;45:346–354. doi: 10.1002/1529-0131(200108)45:4<346::AID-ART347>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 56.Richardson C, Agrawal R, Lee J, Almagor O, Nelson R, Varga J, Cuttica MJ, Dematte JD, Chang RW, Hinchcliff ME. Esophageal dilatation and interstitial lung disease in systemic sclerosis: A cross-sectional study. Semin Arthritis Rheum. 2016;46:109–114. doi: 10.1016/j.semarthrit.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagaraja V, McMahan ZH, Getzug T, Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015;1:82–105. doi: 10.1007/s40674-014-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aubert A, Lazareth I, Vayssairat M, Fiessinger JN, Petite JP. [Esophagitis in progressive systemic scleroderma. Prevalence and risk factors in forty-six patients] Gastroenterol Clin Biol. 1991;15:945–949. [PubMed] [Google Scholar]
- 59.De Castro Parga ML, Alonso P, García Porrua C, Prada JI. [Esophageal mucosal lesions and scleroderma: prevalence, symptoms and risk factors] Rev Esp Enferm Dig. 1996;88:93–98. [PubMed] [Google Scholar]
- 60.Zamost BJ, Hirschberg J, Ippoliti AF, Furst DE, Clements PJ, Weinstein WM. Esophagitis in scleroderma. Prevalence and risk factors. Gastroenterology. 1987;92:421–428. doi: 10.1016/0016-5085(87)90137-5. [DOI] [PubMed] [Google Scholar]
- 61.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal involvement in scleroderma: clinical, endoscopic, and manometric features. ISRN Rheumatol. 2011;2011:325826. doi: 10.5402/2011/325826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebert EC. Esophageal disease in scleroderma. J Clin Gastroenterol. 2006;40:769–775. doi: 10.1097/01.mcg.0000225549.19127.90. [DOI] [PubMed] [Google Scholar]
- 63.Desai JP, Moustarah F. Esophageal Stricture. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 64.Lovy MR, Levine JS, Steigerwald JC. Lower esophageal rings as a cause of dysphagia in progressive systemic sclerosis--coincidence or consequence? Dig Dis Sci. 1983;28:780–783. doi: 10.1007/BF01296899. [DOI] [PubMed] [Google Scholar]
- 65.Katzka DA, Reynolds JC, Saul SH, Plotkin A, Lang CA, Ouyang A, Jimenez S, Cohen S. Barrett's metaplasia and adenocarcinoma of the esophagus in scleroderma. Am J Med. 1987;82:46–52. doi: 10.1016/0002-9343(87)90376-7. [DOI] [PubMed] [Google Scholar]
- 66.Saint Luke's Health System EResources. Barrett Esophagus. Elsevier, 2020. [Google Scholar]
- 67.Wipff J, Coriat R, Masciocchi M, Caramaschi P, Derk CT, Hachulla E, Riccieri V, Mouthon L, Krasowska D, Ananyeva LP, Kahan A, Matucci-Cerinic M, Chaussade S, Allanore Y. Outcomes of Barrett's oesophagus related to systemic sclerosis: a 3-year EULAR Scleroderma Trials and Research prospective follow-up study. Rheumatology (Oxford) 2011;50:1440–1444. doi: 10.1093/rheumatology/ker110. [DOI] [PubMed] [Google Scholar]
- 68.Kliemann DA, Pasqualotto AC, Falavigna M, Giaretta T, Severo LC. Candida esophagitis: species distribution and risk factors for infection. Rev Inst Med Trop Sao Paulo. 2008;50:261–263. doi: 10.1590/s0036-46652008000500002. [DOI] [PubMed] [Google Scholar]
- 69.Walsh TJ, Hamilton SR, Belitsos N. Esophageal candidiasis. Managing an increasingly prevalent infection. Postgrad Med. 1988;84:193–196, 201. doi: 10.1080/00325481.1988.11700377. [DOI] [PubMed] [Google Scholar]
- 70.Manno R, Boin F. Immunotherapy of systemic sclerosis. Immunotherapy. 2010;2:863–878. doi: 10.2217/imt.10.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sam JW, Levine MS, Rubesin SE, Laufer I. The "foamy" esophagus: a radiographic sign of Candida esophagitis. AJR Am J Roentgenol. 2000;174:999–1002. doi: 10.2214/ajr.174.4.1740999. [DOI] [PubMed] [Google Scholar]
- 72.Adarsh MB, Sharma SK, Sinha SK, Bhattacharya A, Rana S, Dhir V, Singh S. Gastrointestinal Dysmotility and Infections in Systemic Sclerosis- An Indian Scenario. Curr Rheumatol Rev. 2018;14:172–176. doi: 10.2174/1573397113666170425145405. [DOI] [PubMed] [Google Scholar]
- 73.Natsui K, Tsuchiya A, Terai S. Refractory hemorrhagic esophageal ulcers by Candida esophagitis with advanced systemic sclerosis. JGH Open. 2020;4:1007–1008. doi: 10.1002/jgh3.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saleem F, Sharma A. Drug Induced Esophagitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 75.Ebert EC. Esophageal disease in progressive systemic sclerosis. Curr Treat Options Gastroenterol. 2008;11:64–69. doi: 10.1007/s11938-008-0008-8. [DOI] [PubMed] [Google Scholar]
- 76.Zografos GN, Georgiadou D, Thomas D, Kaltsas G, Digalakis M. Drug-induced esophagitis. Dis Esophagus. 2009;22:633–637. doi: 10.1111/j.1442-2050.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 77.de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–1021. doi: 10.1056/NEJM199610033351403. [DOI] [PubMed] [Google Scholar]
- 78.Carlborg B, Densert O, Lindqvist C. Tetracycline induced esophageal ulcers. a clinical and experimental study. Laryngoscope. 1983;93:184–187. doi: 10.1288/00005537-198302000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management. Drug Saf. 2000;22:237–249. doi: 10.2165/00002018-200022030-00007. [DOI] [PubMed] [Google Scholar]
- 80.Wielosz E, Majdan M. Haematological abnormalities in systemic sclerosis. Reumatologia. 2020;58:162–166. doi: 10.5114/reum.2020.96655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haig A, Driman DK. Iron-induced mucosal injury to the upper gastrointestinal tract. Histopathology. 2006;48:808–812. doi: 10.1111/j.1365-2559.2006.02448.x. [DOI] [PubMed] [Google Scholar]
- 82.Liu N, Abell T. Gastroparesis Updates on Pathogenesis and Management. Gut Liver. 2017;11:579–589. doi: 10.5009/gnl16336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marie I, Gourcerol G, Leroi AM, Ménard JF, Levesque H, Ducrotté P. Delayed gastric emptying determined using the 13C-octanoic acid breath test in patients with systemic sclerosis. Arthritis Rheum. 2012;64:2346–2355. doi: 10.1002/art.34374. [DOI] [PubMed] [Google Scholar]
- 84.Jehangir A, Parkman HP. Reflux Symptoms in Gastroparesis: Correlation With Gastroparesis Symptoms, Gastric Emptying, and Esophageal Function Testing. J Clin Gastroenterol. 2020;54:428–438. doi: 10.1097/MCG.0000000000001190. [DOI] [PubMed] [Google Scholar]
- 85.Jung S, Martin T, Schmittbuhl M, Huck O. The spectrum of orofacial manifestations in systemic sclerosis: a challenging management. Oral Dis. 2017;23:424–439. doi: 10.1111/odi.12507. [DOI] [PubMed] [Google Scholar]