Abstract
Background
To explore the development of central nervous system (CNS) symptoms and clinical application in predicting the clinical outcomes of SARS-COV-2 patients.
Methods
A retrospective cohort study was performed on the hospitalized patients with SARS-COV-2 recruited from four hospitals in Hubei Province, China from 18 January to 10 March 2020. The patients with CNS symptoms were determined. Data regarding clinical symptoms and laboratory tests were collected from medical records.
Results
Of 1268 patients studied, 162 (12.8%) had CNS symptoms, manifested as unconsciousness (71, 5.6%), coma (69, 5.4%), dysphoria (50, 3.9%), somnolence (34, 2.7%) and convulsion (3, 0.2%), which were observed at median of 14 (interquartile range 9–18) days after symptom onset and significantly associated with older age (OR = 5.71, 95% confidence interval [CI] 2.78–11.73), male (OR = 1.73, 95% CI 1.22–2.47) and preexisting hypertension (OR = 1.78, 95% CI 1.23–2.57). The presence of CNS symptoms could be predicted by abnormal laboratory tests across various clinical stages, including by lymphocyte counts of <0.93 × 109/L, LDH≥435 U/L and IL-6≥28.83 pg/L at 0–10 days post disease; by lymphocyte count<0.86 × 109/L, IL-2R ≥ 949 U/L, LDH≥382 U/L and WBC≥8.06 × 109/L at 11–20 days post disease. More patients with CNS symptoms developed fatal outcome compared with patients without CNS symptoms (HR = 33.96, 95% CI 20.87–55.16).
Conclusion
Neurological symptoms of COVID-19 were related to increased odds of developing poor prognosis and even fatal infection.
Keywords: Central nervous system, SARS-CoV-2, Fatal outcome, Prediction
Introduction
The recent outbreak of COVID-19 caused by SARS-CoV-2 coronavirus has turned the world into chaos with its ominously high rate of transmissions. More than 169 million cases of confirmed COVID-19 have been reported worldwide.1 Most patients manifest mild symptoms, with most of the deaths occurring in patients over 60 years of age or those having underlying diseases such as hypertension, diabetes and cardiovascular disease.2 , 3 Although the predominant clinical presentation is respiratory diseases, neurological manifestations that related to SARS-CoV-2 are increasingly recognized. At early epidemic, occasional development of neurological signs and symptoms in COVID-19 patients, together with the isolation of SARS-CoV from the brain of COVID-19 patient who had exhibited neurological features,4 , 5 led to the postulation of its neuropathy potential. Actually the expression and distribution of angiotensin converting enzyme two (ACE2), the functional receptor for SARS-CoV-2, has been recently identified in multiple human organs, including nervous system,6 , 7 indicative of the capacity of SARS-CoV-2 in gaining access to the central nervous system (CNS) and causing neurological symptoms through direct or indirect mechanisms. As the case incidence in the worldwide has increased, there was more evidence showing the neurological complications caused by COVID-19 infection from both retrospective case series or case reports, or even serve as the initial symptom of COVID-19.8 , 9 The diagnosis of encephalitis was made from some case series,4 , 10 , 11 and the increased risk of cerebrovascular disease and necrotizing encephalopathy in association with COVID-19 was replicated in other studies.12 , 13
In the current study, we performed a retrospective study on COVID-19 patients in multiple medical centers to explore the presence and outcome of CNS symptoms in COVID-19 patients, and to determine the role of commonly seen clinical features and laboratory findings involved in the clinical progression of CNS in COVID-19.
Methods
Study design and participants
A retrospective cohort study was performed at four designated hospitals for COVID-19 treatment in Hubei province, China (The People's Hospital Affiliated Wuhan University, Wuhan No.1 Hospital, Tongji Medical College in Huazhong University of Science and Technology, and Huangmei People's Hospital) to collect data on all patients who were hospitalized due to COVID-19 starting from 18 January to 10 March 2020. During the process of the study, standard diagnosis and therapy procedures were administered following the guideline of WHO interim guideline.14 Briefly, the patients were confirmed as SARS-COV-2 infection by testing throat swab by real-time RT-PCR assay using the SARS-CoV-2 nucleic acid detection kit according to the manufacturer's protocol (DAAN Gene, BioGerm, TIANLONG Technology and HUIRUI Co., Ltd).15 The study was performed in accordance to the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Data collection
The data regarding the demographic characteristics, presence of comorbidities, together with clinical symptoms and signs, laboratory findings, and chest computed tomographic (CT) scan findings within 60 days of admission, were reviewed and extracted from electronic medical records for each patient by a trained team of physicians using a standard data form. The laboratory parameters were delineated by using the median (IQR) value at three clinical stages separately, i.e., 1–10 days (stage 1), 11–20 days (stage 2) and 21–30 days after symptom onset. The date of COVID-19 disease onset was defined as the day when the initial symptom, mostly fever or dry cough or anorexia was noticed. According to the clinical diagnostic criteria of viral encephalitis, CNS symptoms were determined to include symptoms of dysphoria, convulsion, somnolence, unconsciousness and coma, for which onset date and resolving date were also recorded.16 The clinical phenotype of the COVID-19 patients was defined as mild, severe and critical disease, according to the guidelines for the diagnosis of COVID-19.15
The comorbidities that were used for the current analysis included hypertension (ICD-10 I10), diabetes mellitus (DM, both type I and type II, ICD-10 E10-E11), chronic heart diseases (CHD, cardiac heart failure and coronary heart disease, ICD-10 I50 and I25.101), cerebrovascular diseases (CVD, ICD-10 I67.800), chronic obstructive pulmonary diseases (COPD, ICD-10 J44.900) and chronic viral hepatitis (CVH, both HBV and HCV, ICD-10 B18.951). The reference values of laboratory findings were listed in Appendix Table 1.
Statistical analysis
Continuous variables were described as mean and standard deviation, or median and interquartile range (IQR), for which the comparison was made by using the unpaired Wilcox rank-sum test. Categorical variables were expressed as counts and percentages, for which the inter-group comparison was made using the χ2 test. Multivariate analyses were performed using logistic regression model to adjust the effect from age, sex and underlying conditions. The Kaplan–Meier method was used to generate survival curves and subject to log-rank test. The hazard ratio (HR) and 95% confidence interval (CI) were estimated by Cox regression model. All analyses were performed using STATA 17.0 (Stata Corp LLP, College Station, TX) and P < 0.05 was considered statistically significant.
Results
The recruit of patients with SARS-CoV-2 infection
A total of 1268 hospitalized patients with SARS-CoV-2 infection were included. Their median age was 64 (IQR 47–70) years, and 631 (49.8%) were females. Of these patients, 561 (44.2%) had at least one of the following underlying conditions: hypertension (406, 32.0%), diabetes mellitus (DM) (180, 14.2%), chronic heart diseases (CHD) (118, 9.3%), chronic obstructive pulmonary diseases (COPD) (56, 4.4%), cerebrovascular diseases (CVD) (41, 3.2%) and chronic viral hepatitis (CVH) (22, 1.7%). The median interval from symptom onset to hospital admission was nine (IQR 6–14) days.
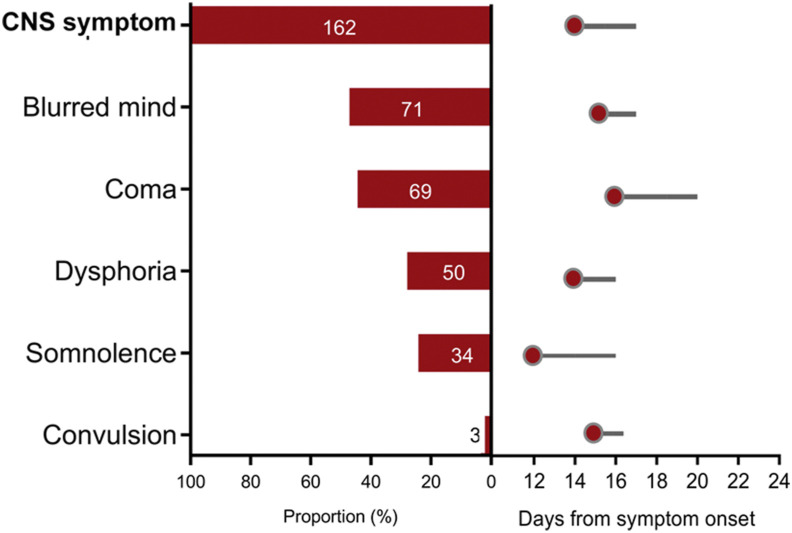
Among 1268 patients, 162 (12.8%) had CNS symptoms, comprised of unconsciousness (71, 5.6%), coma (69, 5.4%), dysphoria (50, 3.9%), somnolence (34, 2.7%) and convulsion (3, 0.2%) (Appendix Table 2). These symptoms appeared at median of 14 (IQR 9–18) days after symptom onset, corresponding to around five days after hospital admission and last for three (IQR 1–8) days (Fig. 1 ). When the symptoms were separately considered, somnolence was the earliest one, presenting at 12 (IQR 6–18) days, and coma was the latest one presenting at 16 (12–22) days after symptom onset.
Figure 1.
The proportions and duration of central nervous system (CNS) symptoms in the COVID-19 patients. The proportions of CNS symptom (any one of the listed symptoms), and each symptom were separately denoted. The red circle denotes the median time of CNS symptoms occurrence (in days post disease onset); the grey line was the median duration of CNS symptoms (in days post disease onset).
Baseline information between patients with vs. without CNS symptoms
Compared with patients without CNS symptoms, the patients with CNS symptoms had more male (61.7% vs. 48.5%, P = 0.002), and older age (median 72 vs. 58 years old, P < 0.001), with higher frequency of hypertension (54.9% vs. 28.7%, P < 0.001), DM (22.8% vs. 12.9%, P = 0.001), CHD (18.5% vs. 8.0%, P < 0.001), and CVD (7.4% vs. 2.6%, P = 0.001). Multivariate logistic regression model disclosed that the association with CNS development remained significant for older age (OR = 5.71, 95% CI 2.78–11.73), male (OR = 1.73, 95% CI 1.22–2.47) and presence of hypertension (OR = 1.78, 95% CI 1.23–2.57) (Appendix Table 3).
Clinical manifestations and laboratory abnormalities in patients with vs. without CNS symptoms
For all the recruited patients, the most common symptoms at onset of illness were fever (1015, 80.0%), cough (906, 71.5%) and asthenia (425, 33.5%) (Table 1 ). The comparison between two groups disclosed the significant over-presence of anorexia, anhelation, sputum production, chest tightness, dyspnea and cyanotic lips in patients with CNS symptoms vs. without CNS symptoms (48.2% vs. 29.4%, 52.5% vs. 23.2%, 32.1% vs. 18.8%, 27.2% vs. 18.4%, 38.3% vs. 6.1% and 11.1% vs. 2.3%, respectively, all P < 0.05) (Table 1). The other manifestations were evenly distributed between two groups.
Table 1.
Basic information and clinical manifestation of the COVID-19 patients with or without CNS symptoms.
| Characteristic | Total (N = 1268) | CNS symptoms (n = 162) | Non-CNS symptoms (n = 1106) | P |
|---|---|---|---|---|
| Age, year, median (IQR) | 64 (47–70) | 72 (63–81) | 58 (45–67) | <0.001 |
| ≤45 | 297 (23.4) | 9 (5.6) | 288 (26.0) | <0.001 |
| 45-60 | 335 (26.4) | 24 (14.8) | 311 (28.1) | |
| >60 | 636 (50.2) | 129 (79.6) | 507 (45.8) | |
| Sex, female, n (%) | 631 (49.8) | 62 (38.3) | 569 (51.5) | 0.002 |
| Interval from symptom onset to admission, days, median (IQR) | 9 (6–14) | 9 (6–14) | 9 (6–14) | 0.855 |
| ≤7 | 501 (39.5) | 62 (38.3) | 439 (39.7) | 0.730 |
| >7 | 767 (60.5) | 100 (61.7) | 667 (60.2) | |
| Comorbidities, n (%) | 561 (44.2) | 108 (66.7) | 453 (41.0) | <0.001 |
| Hypertension | 406 (32.0) | 89 (54.9) | 317 (28.7) | <0.001 |
| DM | 180 (14.2) | 37 (22.8) | 143 (12.9) | 0.001 |
| CHD | 118 (9.3) | 30 (18.5) | 88 (8.0) | <0.001 |
| COPD | 56 (4.4) | 11 (4.1) | 45 (6.8) | 0.115 |
| CVD | 41 (3.2) | 12 (7.4) | 29 (2.6) | 0.001 |
| CVH | 22 (1.7) | 4 (2.5) | 18 (1.6) | 0.444 |
| Severity of COVID-19 | ||||
| Mild | 921 (72.6) | 15 (9.3) | 906 (81.9) | <0.001 |
| Severe | 206 (16.3) | 61 (37.7) | 145 (13.1) | |
| Critical | 141 (11.1) | 86 (53.1) | 55 (5.0) | |
| Clinical manifestation and vital sign | ||||
| Rapid pulse >100 times/min | 131 (10.3) | 38 (23.5) | 93 (8.4) | <0.001 |
| Respiratory rate >24 times/min | 89 (7.0) | 32 (19.8) | 57 (5.2) | <0.001 |
| Fever | 1015 (80.0) | 127 (78.4) | 888 (80.3) | 0.573 |
| Cough | 906 (71.5) | 111 (68.5) | 795 (71.9) | 0.376 |
| Asthenia | 425 (33.5) | 57 (35.2) | 368 (33.3) | 0.630 |
| Anorexia | 403 (31.8) | 78 (48.2) | 325 (29.4) | <0.001 |
| Tachypnea | 341 (26.9) | 85 (52.5) | 256 (23.2) | <0.001 |
| Expectoration | 260 (20.5) | 52 (32.1) | 208 (18.8) | <0.001 |
| Chest tightness | 248 (19.6) | 44 (27.2) | 204 (18.4) | 0.009 |
| Diarrhea | 175 (13.8) | 16 (9.9) | 159 (14.4) | 0.121 |
| Dyspnea | 129 (10.2) | 62 (38.3) | 67 (6.1) | <0.001 |
| Nausea | 95 (7.5) | 9 (5.6) | 86 (7.8) | 0.316 |
| Chill | 62 (4.9) | 9 (5.6) | 53 (4.8) | 0.674 |
| Cyanotic lips | 43 (3.4) | 18 (11.1) | 25 (2.3) | <0.001 |
| Pharyngalgia | 30 (2.4) | 5 (3.1) | 25 (2.3) | 0.518 |
| Vomiting | 30 (2.4) | 5 (3.1) | 25 (2.3) | 0.518 |
| Headache | 22 (1.7) | 3 (1.9) | 19 (1.7) | 0.903 |
| Rhinorrhea | 18 (1.4) | 2 (1.2) | 16 (1.4) | 0.831 |
COVID-19, coronavirus disease 2019; CNS, central nervous system; DM, diabetes mellitus; CHD, chronic heart diseases; CVD, cerebrovascular diseases; CKD, chronic kidney diseases; COPD, chronic obstructive pulmonary diseases; CVH, chronic viral hepatitis; IQR, interquartile range.
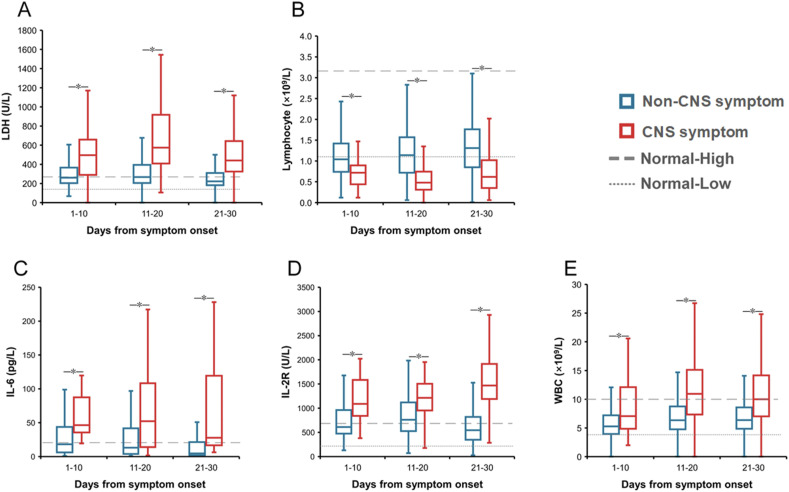
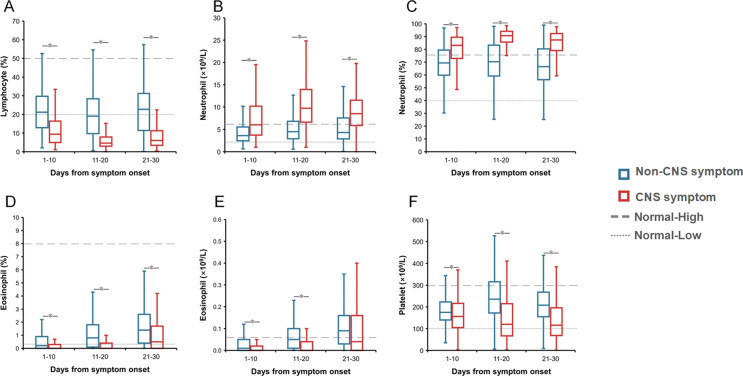
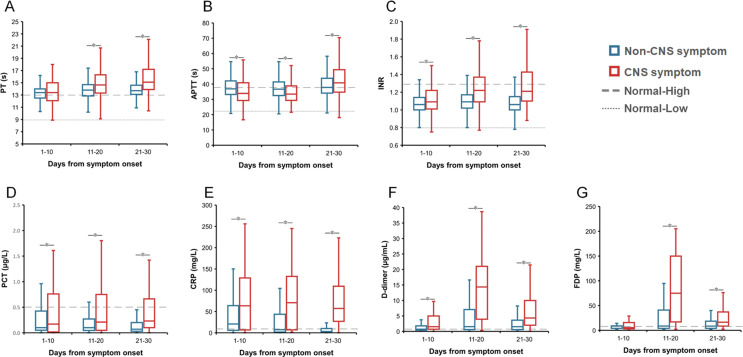
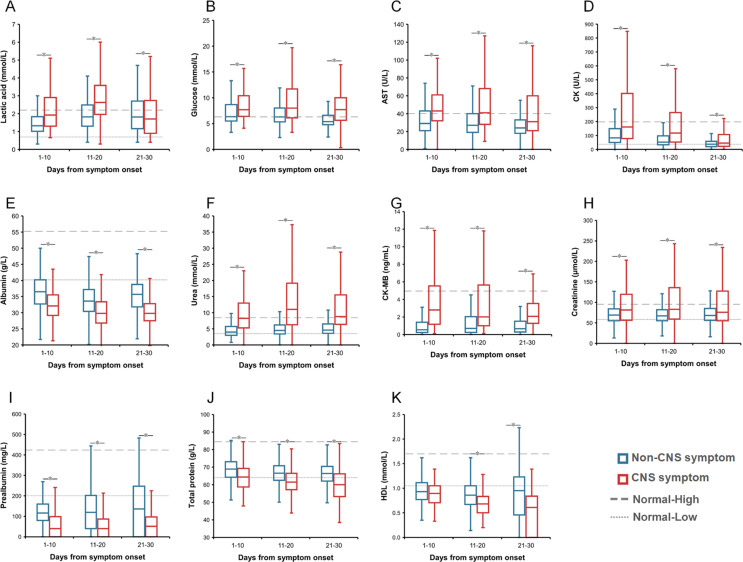
Inter-group comparison demonstrated that totally 28 abnormal findings were significantly different, including 19 with elevated levels in patients with CNS vs. non-CNS symptoms, i.e., lactic dehydrogenase (LDH), interleukin-6 (IL-6), fibrin degradation product (FDP), neutrophil count, neutrophil percent, white blood cell (WBC), prothrombin time (PT), international normalized ratio (INR), D-dimer, procalcitonin, C-reaction protein (CRP), IL-2R, lactic acid, glucose, aspartate aminotransferase (AST), creatine kinase (CK), urea, CK-MB, and creatinine; and 9 with decreased levels in patients with CNS symptoms, i.e., lymphocyte count, lymphocyte percent, eosinophil count, eosinophil percent, platelet count, albumin, prealbumin, total protein and high-density lipoprotein (HDL) at all three stages (Fig. 2 and Appendix Figs. 1–3).
Figure 2.
The dynamic profile of laboratory parameters related to the risk of CNS symptoms of the COVID-19 patients. The lines and error bars indicate the median and interquartile range, respectively. The median and interquartile range were compared between two groups for three clinical stages separately by Mann-Whiter U to hospital admission and comorbidities. ∗P < 0.05. A, lactic dehydrogenase (LDH); B, lymphocyte count; C, interleukin 6 (IL-6); D, IL-2R; E, white blood cell (WBC).
During the first stage of 1–10 days post disease, altogether 10 indicators showed significantly different measurement between two groups, attaining an AUC >0.7 in discriminating between two groups (Appendix Table 4). Patients with CNS symptoms had lower lymphocyte percent and counts, higher neutrophil percent and counts, abnormal liver function indicators of decreased prealbumin and albumin, increased LDH and urea levels, as well as increased inflammatory indicators of IL-6 and IL-2R.
During the second stage of 11–20 days post disease, altogether 18 indicators showed significantly different measurement, attaining an AUC >0.7 in discriminating between two groups. Patients with CNS symptoms had lower levels of platelet count, eosinophil percent, albumin, lymphocyte percent and count, and prealbumin and higher levels of lactic acid, FDP, INR, CRP, WBC, neutrophil percent and count, D-Dimer, LDH, urea, IL-6 and IL-2R (Appendix Table 4).
The discrimination of patients with CNS symptoms by applying multivariate analysis
Based on multivariate logistic regression model that were applied at the first stage, ten indictors with AUC greater than 0.7 were entered the model, together with age, sex and underlying comorbidities. The development of CNS symptoms was significantly associated with clinical presence of rapid pulse, dyspnea, anhelation, anorexia, as well as lymphocyte counts of <0.93 × 109/L, LDH≥435 U/L and IL-6≥28.83 pg/L (Table 2 and Appendix Table 5). For the second stage, the development of CNS symptoms was significantly associated with clinical presence of rapid pulse, cough, dyspnea, as well as lymphocyte count<0.86 × 109/L, IL-2R ≥ 949 U/L, LDH≥382 U/L and WBC≥8.06 × 109/L. For both stages, older age and preexisting hypertension conferred higher risk of CNS symptoms development.
Table 2.
The factors associated with the CNS symptoms of the COVID-19 patients by multivariate logistic regression model.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| For the first stage | |||
| Age | |||
| ≤45 | Reference | ||
| 45-60 | 1.658 | 0.706–3.895 | 0.246 |
| >60 | 4.287 | 1.949–9.427 | <0.001 |
| Sex | |||
| Male | 1.539 | 1.030–2.298 | 0.035 |
| Female | Reference | ||
| Hypertension | 1.611 | 1.066–2.432 | 0.023 |
| Rapid pulse >100 times/min | 3.533 | 1.946–6.411 | <0.001 |
| Dyspnea | 2.523 | 1.379–4.618 | 0.003 |
| Tachypnea | 1.665 | 1.097–2.527 | 0.017 |
| Anorexia | 1.673 | 1.098–2.550 | 0.017 |
| Lymphocyte ( × 109/L) | |||
| <0.93 | 2.329 | 1.036–5.236 | 0.041 |
| ≥0.93 | Reference | ||
| LDH (U/L) | |||
| ≥435 | 2.639 | 1.166–5.972 | 0.020 |
| <435 | Reference | ||
| IL-6 (pg/L) | |||
| ≥28.83 | 13.06 | 1.540–110.767 | 0.018 |
| <28.83 | Reference | ||
| For the second stage | |||
| Age | |||
| ≤45 | Reference | ||
| 45-60 | 1.607 | 0.641–4.028 | 0.311 |
| >60 | 3.891 | 1.683–8.996 | 0.001 |
| Hypertension | 1.572 | 1.012–2.442 | 0.044 |
| Rapid pulse >100 times/min | 2.096 | 1.115–3.943 | 0.022 |
| Cough | 0.535 | 0.333–0.860 | 0.010 |
| Dyspnea | 3.162 | 1.615–6.192 | 0.001 |
| Lymphocyte ( × 109/L) | |||
| <0.86 | 2.480 | 1.313–4.684 | 0.005 |
| ≥0.86 | Reference | ||
| IL-2R (U/L) | |||
| ≥949 | 3.240 | 1.231–8.528 | 0.017 |
| <949 | Reference | ||
| LDH (U/L) | |||
| ≥382 | 2.164 | 1.019–4.596 | 0.045 |
| <382 | Reference | ||
| WBC ( × 109/L) | |||
| ≥8.06 | 1.886 | 1.020–3.489 | 0.043 |
| <8.06 | Reference | ||
COVID-19, coronavirus disease 2019; CNS, central nervous system; OR, odds ratio; CI, confidence interval; LDH, lactic dehydrogenase; IL, interleukin; WBC, white blood cell.
Association between CNS symptoms and clinical outcome
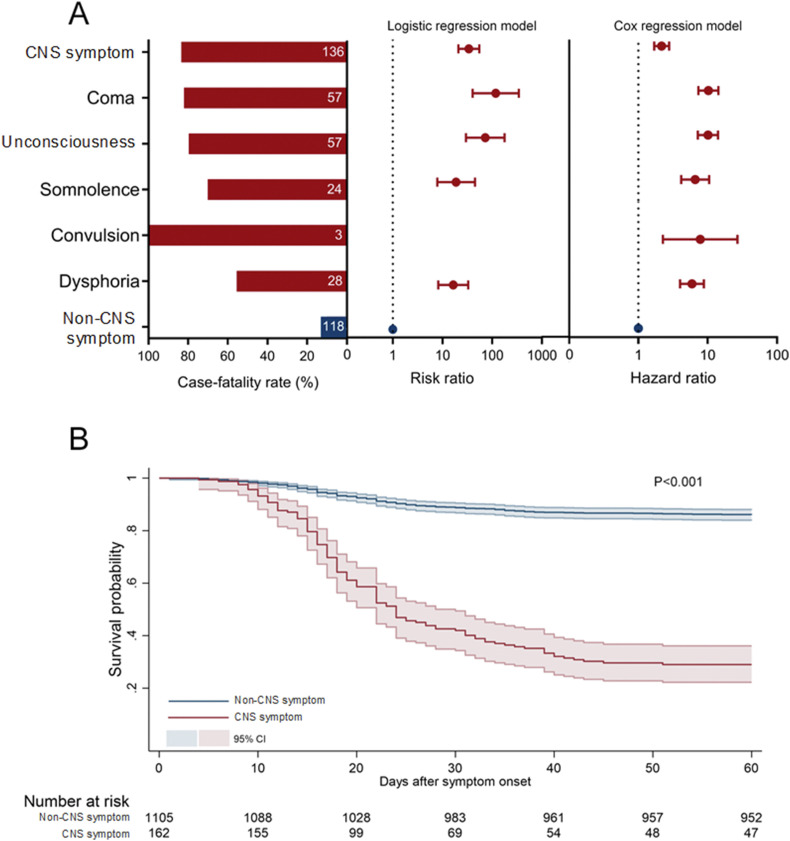
Totally 84.0% (136/162) of the patients with CNS symptoms developed fatal outcome, significantly higher than patients without CNS symptoms (10.7%, 118/1103, P < 0.001) (Fig. 3 and Appendix Table 6). The development of CNS symptoms remained significantly associated with fatal outcome (RR = 33.93, 95% CI 20.87–55.16) by performing logistic regression model to exclude the effects from age, sex, delay from symptom onset to hospital admission, and preexisting comorbidities. Each of the CNS features, when analyzed separately, was significantly related to higher case fatality. The highest risk on fatal outcome was conferred from coma (RR = 118.34, 95% CI 40.55–345.38), followed by unconsciousness (RR = 72.53, 95% CI 29.68–177.28), somnolence (RR = 18.87, 95% CI 7.91–45.02) and dysphoria (RR = 16.46, 95% CI 8.25–32.84) (Fig. 3 and Appendix Table 6). These associations were verified by survival analysis showing that the CNS symptoms as a whole, as well as each CNS symptoms, were significantly related to the increased risk of fatal outcome (Appendix Table 7).
Figure 3.
Association between CNS symptoms and fatal outcome for the COVID-19 patients. A, from left to right: the case fatality rate of the COVID-19 patients with CNS symptoms; the risk ratio of fatal outcome by logistic regression model; and the hazard ratio of fatal outcome by cox regression model. B, the survival analysis of fatal outcome based on the CNS symptoms. Log-rank test was used for comparison.
The discrimination of fatal patients with CNS symptoms
We made further analysis within the patients with CNS symptoms to identify the clinical and laboratory features that can be used to predict the development of fatal outcome (Table 3 and Appendix Table 8). For the first stage model, the fatal outcome could be predicted by thrombin time≥15.1 s (OR = 23.230, 95% CI 1.308–412.432), platelet count<150 × 109/L (OR = 21.968, 95% CI 1.565–308.430), clinical presence of cyanotic lips (OR = 0.033, 95% CI 0.001–0.816), and female gender (OR = 4.283, 95% CI 1.365–13.440). For the second stage model, two factors contributed significantly to fatal COVID-19, with male (OR = 3.435 95% CI 1.208–9.771) and LDH≥397 U/L (OR = 10.890, 95% CI 1.487–79.770), which leading to the AUC of 0.808.
Table 3.
The factors associated with death of the COVID-19 patients with CNS symptoms by multivariate logistic regression model.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| For the first stage | |||
| Sex | |||
| Male | 4.283 | 1.365–13.440 | 0.013 |
| Female | Reference | ||
| Cyanotic lips | 0.033 | 0.001–0.816 | 0.037 |
| Platelet ( × 109/L) | |||
| <151 | 21.968 | 1.565–308.430 | 0.022 |
| ≥151 | Reference | ||
| Thrombin time (s) | |||
| ≥15.1 | 23.230 | 1.308–412.432 | 0.032 |
| <15.1 | Reference | ||
| For the second stage | |||
| Sex | |||
| Male | 3.435 | 1.208–9.771 | 0.021 |
| Female | Reference | ||
| LDH (U/L) | |||
| ≥397 | 10.890 | 1.487–79.770 | 0.019 |
| <397 | Reference | ||
COVID-19, coronavirus disease 2019; CNS, central nerve system; OR, odds ratio; CI, confidence interval; LDH, lactic dehydrogenase.
Discussion
In the current study, we reported the occurrence of CNS symptoms in >10% of the hospitalized patients with SARS-COV-2 infections, among whom 84.0% progressed into fatal outcome, with over 30-fold increased risk of death than those without CNS symptoms. The clinical symptoms or syndromes, as well as the commonly tested laboratory indicators in clinical practice, were associated with the occurrence of this important adverse complications in COVID-19 disease. Among these significant factors, lower lymphocyte and LDH levels remained to be effective predictors at both early and middle stages of the diseases, which warrant close monitoring. The abnormalities that were predictive of fatal outcome following development of CNS symptoms included blood coagulation dysfunction (platelet level and thrombin time) and liver injury (increased LDH). All these abnormal evaluations indicated organ injury, which could be used as predictors referring to patients with severe cases. The exploring of significant predictors has allowed a differential diagnosis of cases at early stage before the neurological manifestation before the advanced stage, which could be reasonably lifesaving.
As has been increasingly studied, the neurological signs and symptoms observed in the COVID-19 cases could be a manifestation of complicated process with interactive mechanism involved.17 The interaction between the coronavirus and microvascular endothelial cells conduces to the access of CNS symptoms. Immune-mediated hyperplasia leads to extensive glial cell proliferation, inflammatory cell infiltration and neuronal necrosis.18 , 19 Moreover the cytokine storm syndrome and dysregulation of homeostasis due to SARS-CoV-2 infection can elicit CNS symptoms.20 , 21 Here we demonstrated that two cytokines, IL-6 and IL-2R, were elevated remarkably before clinical deterioration, further corroborating their potential association with the development of CNS symptoms. This was also in line with previous studies showing a panel of cytokines/chemokines that had significant effect on severe or fatal outcome after SARS-COV-2 infection.9 , 22 Despite of these findings, whether these cytokines were released in response to SARS-COV-2 infection and directly involved in the brain immunopathology process need to be elucidated by further study.
It's noteworthy that both thrombocytopenia and prolongation of thrombin time were associated with death in COVID-19 patients. This agreed with the current death predictors from the patients with CNS symptoms, that TT and decreased PT, both indicative of DIC, were determined as important prognostic factors of death. SARS-COV-2 infection causes cytokine storm syndrome, which was associated with changes in coagulation homeostasis, including D-dimer and platelet levels, all these injuries might trigger multiple organs failure, featured by hypoxia, respiratory, and metabolic acidosis at an advanced stage of the disease, further aggravating the condition of COVID-19 patients to a death end.20 , 21 , 23 , 24
In this study, male had a higher risk of CNS symptoms and death than women, consistent with findings from gender-disaggregated data showing higher mortality rate in men.25 Genetic susceptibility caused by sex may be due to the differences in ACE2 and TMPRSS2 regulation and mosaic advantage activated by X-heterozygous.26 The bisexual differences in lifestyle, comorbidities and treatment might also play roles.25 , 27 Notably, we observed presence of lip cyanosis as a protective factor for death in this study. It's hypothesized that these apparent adverse clinical symptoms might render more aggressive intervention measures, such as non-invasive ventilation, invasive ventilation etc., which reduced the risk of death.
The major limitation of the current study is that patients with CNS symptoms were determined based on clinical manifestations, without direct cerebrospinal fluid evidence or image study, such as brain computerized tomography, magnetic resonance imaging or cerebrospinal fluid (CSF) profile. The identification of SARS-COV-2 in CSF and neuroimaging, preferably by MRI in the patients with CNS symptoms might offer an in-depth understanding of the relationship between the CNS and the neuroinversion. Misclassification of patients with CNS symptoms is difficult to be avoided based on only clinical manifestations, since unconsciousness, dysphoria, convulsion, and drowsiness could also be caused by high fever instead. Also, the CNS manifestations collected in this study were limited due to the emergency during early outbreak and the absence of specific laboratory tests. For example, altered mental status which usually happens in patients with severe infection is influenced by age and underlying conditions, such as neurodegenerative diseases, sepsis, hypoxia, intensive care unit admission, and sedative medications, which were not included in the current analysis. Except for CNS symptoms or diseases, we made no attempts to investigate the peripheral nervous system (PNS) symptoms (hypogeusia, hyposmia, hyposmia, and neuralgia).
In conclusion, in agreement with the known capacity of SARS-CoV-2 in afflicting nervous system, and related to increased odds of developing poor prognosis and even fatal infection. The CNS symptoms mostly occurred at around two weeks after disease onset, and can predict fatal outcome. It's accordingly suggested that a low threshold for imaging and CSF analysis in COVID-19 patients displaying unexpected neurological symptoms should be applied. Taken this together, the current preliminary results need to be corroborated using a confirmed case cohort in the future investigation.
Acknowledgments
Authors' contributions
ZTS, ZHL, ZX, CX and JWL wrote the original manuscript; LHY, ZJ, YY, PXF, LJC, YT and LBC collected the data; ZTS, LQB and DJ analyzed data; LJH, ZXA, FLQ, LQB and LW contributed to the conception of the study; All authors revised the manuscript.
Availability of data
Data will be made available on reasonable request.
Funding
This work was supported by the China Mega-Project on Infectious Disease Prevention [No. 2017ZX10103004, No. 2018ZX10101003, No. 2018ZX10713002] and the National Natural Science Funds (No. 81825019, No. 82073617), and Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (No. BMU2021PY005).
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2021.07.010.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1The dynamic profile of the parameters in blood routine test in the COVID-19 patients with and without CNS symptoms. The lines and error bars indicate the median and interquartile range, respectively. The median and interquartile range were compared between two groups for three clinical stages separately by Mann-Whiter U. ∗P < 0.05. A, lymphocyte percent; B, neutrophil count; C, neutrophil percent; D, eosinophil percent; E, eosinophil count; G, platelet count.
figs2.
The dynamic profile of the parameters of inflammation and coagulation in the COVID-19 patients with and without CNS symptoms. The lines and error bars indicate the median and interquartile range, respectively. The median and interquartile range were compared between two groups for three clinical stages separately by Mann-Whiter U. ∗P < 0.05. A, prothrombin time (PT); B, activated partial thromboplastin time (APTT); C, international normalized ratio (INR); D, procalcitonin (PCT); E, C reaction protein (CRP); F, D-dimer; G, fibrinogen degradation products (FDP).
figs3.
The dynamic profile of the parameters of biochemistry in the COVID-19 patients with and without CNS symptoms. The lines and error bars indicate the median and interquartile range, respectively. The median and interquartile range were compared between two groups for three clinical stages separately by Mann-Whiter U. ∗P < 0.05. A, lactic acid; B, blood glucose; C, aspartate aminotransferase (AST); D, creatine kinase (CK); E, albumin; F, urea; G, CK-MB; H, creatinine; I, prealbumin; J, total protein; K, high-density lipoprotein.
References
- 1.WHO . 2021. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhama K., Khan S., Tiwari R., Sircar S., Bhat S., Malik Y.S., et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4) doi: 10.1128/CMR.00028-20. e00028-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: underestimated damage to nervous system. Trav Med Infect Dis. 2020;36:101642. doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z., Zixian Z., Yujia W., Yueqing Z., Yu M., Wei Z. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. Am J Respir Crit Care Med. 2020;202(5):756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Y., Lu Y., Xia L., Yuan X., Li G., Li X., et al. Analysis of 2019 novel coronavirus infection and clinical characteristics of outpatients: an epidemiological study from a fever clinic in Wuhan, China. J Med Virol. 2020;16(10):1002. doi: 10.1002/jmv.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong P., Craik S., Newman P., Makan A., Srinivasan K., Crawford E., et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med. 2020;20(3):293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohal S., Mossammat M. COVID-19 presenting with seizures. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–e120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . 2020. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- 15.National Health Commission of the People’s Republic of China . 2020. Diagnosis and treatment scheme of new coronavirus infected pneumonia (7th)http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml [Google Scholar]
- 16.Cui N., Liu R., Lu Q.B., Wang L.Y., Qin S.L., Yang Z.D., et al. Severe fever with thrombocytopenia syndrome bunyavirus-related human encephalitis. J Infect. 2015;70(1):52–59. doi: 10.1016/j.jinf.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Korteweg C., McNutt M.A., Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133(1):4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., et al. 2020. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) 2020.2002.2010.20021832. [Google Scholar]
- 23.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females Be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci. 2020;21(10):3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on reasonable request.