Abstract
Background
There is growing evidence that super-spreading events (SSEs) and multiple-spreading events (MSEs) are a characteristic feature of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, data regarding the possibility of SSEs or MSEs in healthcare settings are limited.
Methods
This study was performed at a tertiary-care hospital in Korea. We analysed the nosocomial COVID-19 cases that occurred in healthcare workers and inpatients and their caregivers between January and 20th December 2020. Cases with two to four secondary cases were defined as MSEs and those with five or more secondary cases as SSEs.
Findings
We identified 21 nosocomial events (single-case events, N = 12 (57%); MSE + SSE, N = 9 (43%)) involving 65 individuals with COVID-19. Of these 65 individuals, 21 (32%) were infectors. The infectors tended to have a longer duration between symptom onset and diagnostic confirmation than did the non-infectors (median two days vs zero days, P=0.08). Importantly, 12 (18%) individuals were responsible for MSEs and one (2%) for an SSE, which collectively generated 35 (54%) secondary cases.
Conclusion
In a hospital with thorough infection-control measures, approximately 70% of the nosocomial cases of COVID-19 did not generate secondary cases, and one-fifth of the infectors were responsible for SSEs and MSEs, which accounted for approximately half of the total cases. Early case identification, isolation, and extensive contact tracing are important for the prevention of transmission and SSEs.
Keywords: SARS-CoV-2, COVID-19, Transmission
Introduction
The World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) as a pandemic on 11th March 2020. As of 19th December 2020, more than 76.8 million cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection had occurred globally, and more than 1.7 million (2.2%) patients had died from it. Super-spreading events (SSEs) have been well documented for many infectious diseases [1]; in terms of seasonal influenza A virus infection, the top 20% of the most infectious adults were responsible for 78–82% of the total cases [1]. In addition, SSEs were a common feature of SARS-associated coronavirus and Middle East respiratory syndrome coronavirus [2,3].
As for SARS-CoV-2, an epidemiologic study based on a community setting in Hong Kong reported that 19% of SARS-CoV-2 infections were responsible for 80% of all transmissions, while 69% of cases did not transmit the virus to others [4]. However, there are limited data on the characteristics of SSEs or multiple-spreading events (MSEs) of SARS-CoV-2 infection in nosocomial settings where contact tracing and infection-control practice are more strictly performed. Herein, we describe our analysis of the clusters of nosocomial SARS-CoV-2 infections at a hospital in the Republic of Korea, where the community spread of SARS-CoV-2 infection has been relatively well controlled.
Methods
Setting
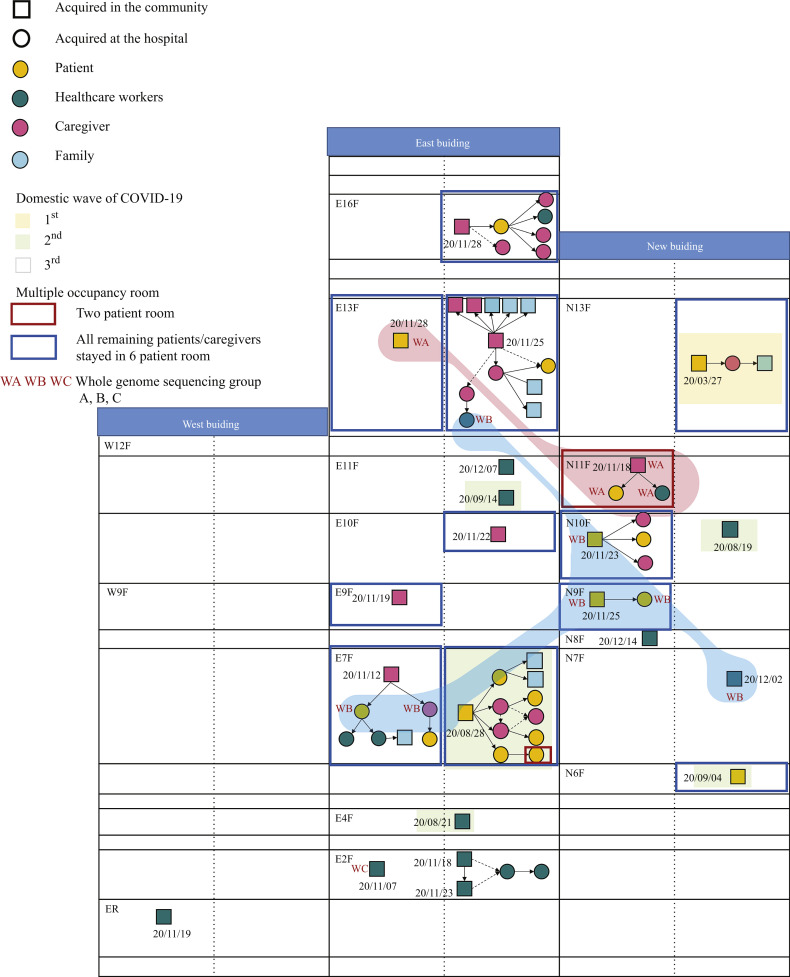
This retrospective observational study was performed at Asan Medical Center, a 2700-bed tertiary care centre in Seoul, Republic of Korea, with 8800 employees including healthcare workers (HCWs). Asan Medical Center has three buildings (West, East, and New) with a total of 55 wards, including 14 wards in the West building (6th floor–12th floor), 24 wards in the East building (7th floor–18th floor), and 17 wards in the New building (6th floor–15th floor; Figure 1 ). All wards have single-occupancy rooms, two-patient rooms, and six-patient rooms. In addition, one ward was designated as an isolation ward for cohorting patients with suspected COVID-19 or patients who had close contact with a patient with confirmed COVID-19 or epidemiologic risk factors for COVID-19.
Figure 1.
Floor plan of the hospital with transmission chains. The first date when the index cases in each cluster were determined to be infectious is shown. If the index cases were asymptomatic at diagnosis, they were regarded as having been infectious two days before diagnosis. If the index cases were symptomatic at diagnosis, they were regarded as having been infectious four days before symptom onset.
Since February 2020, we have performed symptom- and epidemiologic risk-based screenings in our hospital as a whole (i.e. outpatient clinic, emergency room, pre-admission, and inpatient); since 29th April 2020, we have implemented a universal pre-admission screening policy for SARS-CoV-2 using reverse transcription polymerase chain reaction (RT-PCR) in nasopharyngeal swab specimens, which was applied to individuals without symptoms associated with COVID-19, epidemiologic risk factors, or links with recent outbreaks in the community or hospitals. The caregivers of inpatients were subjected to daily screening with temperature monitoring for symptoms and epidemiologic links; if the caregivers showed any symptoms or epidemiologic links, they underwent SARS-CoV-2 PCR testing and left the hospital grounds immediately. A universal mask-wearing policy was mandated in the hospital; however, inpatients and caregivers were often unmasked in their rooms. Mandatory daily monitoring of symptoms by all HCWs and free PCR testing for all HCWs who had symptoms or epidemiologic links have been implemented. Patients with suspected COVID-19 were isolated in a negative-pressure airborne isolation room, and HCWs used N95 respirators (along with eye protection, gown or coverall, and gloves) when caring for them. We recommend alcohol hand rub to all HCWs unless visible contamination was present on their hands or they were caring for patients with suspected or confirmed Clostridioides difficile infection. Washing with water and medical soap is needed in the latter cases. Hand sanitizers for alcohol handrub were located at all beds and entrances of patients' rooms. Although the supply of N95 masks was limited, other personal protective equipment (PPE), including FFP2 equivalent masks, gowns, gloves, goggles, and face shields, were available. During the study period, the rates of observed adherence of the HCWs to hand hygiene and mask-wearing were 90% (6678/7458) and 96% (3327/3458), respectively. The institutional review board of Asan Medical Center evaluated and approved the medical, scientific, and ethical aspects of our study protocol (2021–0024).
Response to nosocomial cases and definitions of contact
Whenever a nosocomial case of COVID-19 was detected, we performed thorough contact tracing. We reviewed the closed-circuit television (CCTV) footage to identify the contacts, which included patients, guardians, visitors, and HCWs who stayed at or visited the ward. All contacts were interviewed especially regarding wearing a mask, face shield or goggles, and gloves and categorized according to the nature of the activity during exposure, duration of exposure, and PPE worn at the time of exposure. Close contacts were defined as (1) those who were in close proximity (<6 feet) to each other for a cumulative duration of at least 15 min from two days before symptom development in the symptomatic index or two days before positive specimen collection date in the asymptomatic index, (2) inpatients or guardians who shared the same (multi-patient) room with the case patient or anyone who had a meal with the index (equivalent exposure to household), or (3) contact with the index patient during aerosol-generating procedures without appropriate PPE (N95 or FFP2 equivalent respirator, face shield/goggle, gown, and gloves). HCWs and inpatients or guardians who came into contact with the case underwent SARS-CoV-2 PCR testing and were monitored for COVID-19-related symptoms on a daily basis until 14 days after the last exposure.
Whole-genome sequencing and phylogenetic analysis
When the diagnosis of non-close contacts who had possible temporal or spatial relationships with the index patient was confirmed by SARS-CoV-2 PCR testing, we performed whole-genome sequencing (WGS) for the specimens from the patients whose respiratory samples were available, caregivers, and HCWs to confirm or refute their association with the cluster. Patients, HCWs, and caregivers with epidemiologic associations but with WGS data of more than two single-nucleotide polymorphisms (SNPs) apart were deemed to not be cluster-related. To determine the viral genomic sequences from the original sample, we extracted viral RNA using the QIAamp viral RNA Mini Kit (Qiagen). To isolate pure SARS-CoV-2 RNA only, we depleted human ribosomal RNA using the NEBNext rRNA Depletion Kit (NEB). Library preparations were performed using the Truseq RNA sample prep kit v2 (Illumina) protocol. The enriched libraries were quantified using the Kapa Library Quantification Kit (Roche), and all sequencing described in this study was performed using the Miseq platform (Illumina) with Miseq reagent kit v2 (300 cycles) (Illumina). Sequencing analysis was performed using the CLC Genomics Workbench 10 (QIAGEN). Base-called reads in FASTQ were trimmed and mapped to a reference sequence (NCBI Reference: NC_045512).
In addition, full-length nucleotide sequences (N = 128) of SARS-CoV-2 used for phylogenetic analysis were downloaded from Global Initiative on Sharing All Influenza Data (GISAID) EpiCoV database on 21 May 2021. Phylogenetic tree was generated by maximum likelihood method on MEGA X software under the general time reversible (GTR) model with gamma distribution and invariant sites (G+I) with 1000 bootstrap replicates. Each lineage was classified according to the Phylogenetic Assignment of Named Global Outbreak Lineages (Pangolin) (https://pangolin.cog-uk.io/; last accessed May 2021).
Definition
We analysed the nosocomial COVID-19 cases between January and 20th December 2020. Nosocomial COVID-19 cases were defined as cases of SARS-CoV-2 acquisition in hospital without epidemiologic links outside the hospital, and cases transmitted in the community from discharged individuals with SARS-CoV-2 acquired at the hospital. We performed WGS to confirm or refute the epidemiologic association. We determined the index patient and the directionality of infection transmission based on the symptom onset and spatiotemporal relationship through intensive discussion with government epidemiologists. Infectors were defined as individuals who transmitted the virus to one or more contact persons. Non-infectors were defined as individuals who transmitted the virus to no-one. An SSE was defined as a patient transmitting SARS-CoV-2 infection to five or more patients, as described previously [5] and an MSE as a patient transmitting SARS-CoV-2 to two to four patients. Caregivers were defined as the individuals who provide care to inpatients, which included family members or hired professional caregivers. HCWs included physicians, nurses, emergency medical personnel, dental professionals and students, medical and nursing students, laboratory technicians, pharmacists, hospital volunteers and administrative staff.
Statistical analysis
Categorical variables were analysed using the chi-squared test or Fisher's exact test, as appropriate. Normally and non-normally distributed continuous variables were analysed using Student's t-test and Mann–Whitney U test, respectively. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA), and P-values of <0.05 were considered statistically significant.
Results
Cluster analysis
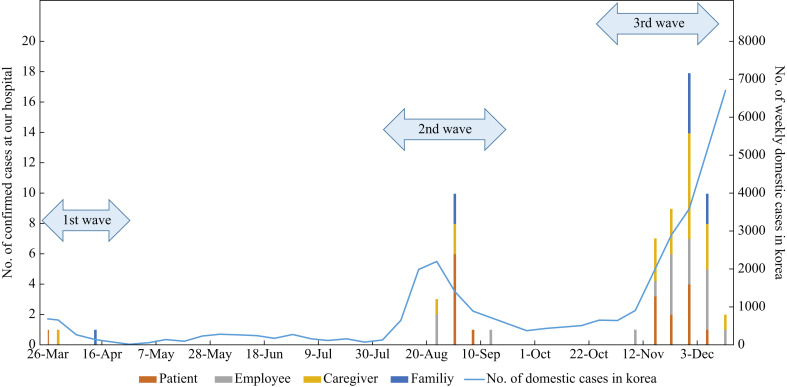
During the study period, there were 21 nosocomial events at our hospital, which involved 65 individuals with COVID-19 (caregivers, N = 21 (32%); patients, N = 18 (28%); HCWs, N = 17 (26%); family members infected in the community setting, N = 9 (14%)). The detailed spatial and temporal information of the nosocomial events is pictographically presented in Figure 1. Figure 2 shows the epidemic curve of the weekly number of laboratory-confirmed SARS-CoV-2 infection cases at our hospital by the symptom onset date and the weekly number of domestic cases in the Republic of Korea. During the first wave of COVID-19 cases in the Republic of Korea, there was one nosocomial cluster at our hospital; during the second and third waves, there were five and 15 nosocomial events, respectively.
Figure 2.
Epidemic curve of the weekly cases of laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection at the hospital according to the date of symptom onset (bar graph) and the weekly number of domestic cases in the Republic of Korea (line graph).
Of the total of 21 events, 12 (57%) were single-case events, and the remaining nine (43%) were clusters with one or more secondary cases. In these nine clusters, the median cluster size was four (interquartile range (IQR), 3–9) cases, with the largest one involving 12 cases. Of the nine clusters with secondary cases, the index case was a caregiver in four clusters, a patient in another four clusters, and an HCW in one cluster. Nine (43%) of the 21 events exclusively involved HCWs, of which all but one were single-case events without further transmission. The remaining 12 clusters involved inpatients or caregivers, of which 11 (92%) clusters occurred in six-patient rooms.
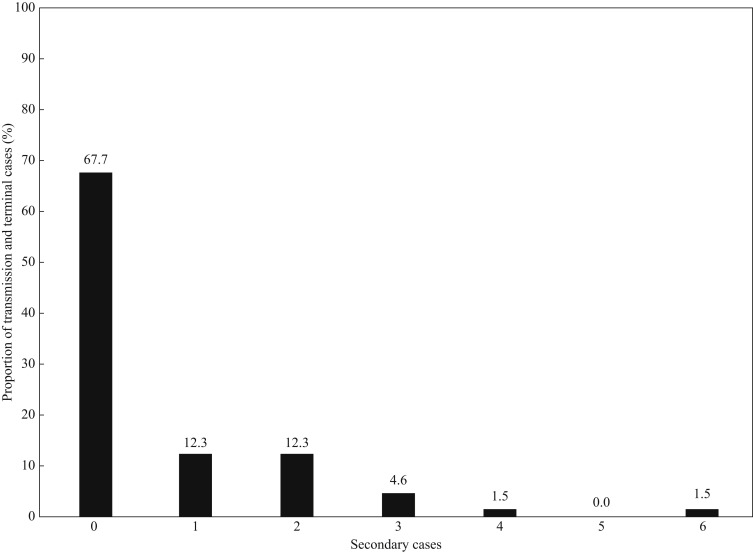
Of the 65 individuals with COVID-19, 44 (68%) were non-infectors, and the remaining 21 (32%) were infectors; their characteristics are shown in Table I . The infectors tended to have a longer duration between symptom onset and diagnostic confirmation than did the non-infectors (P=0.08; Table I). Importantly, 12 (18%) infectors were responsible for MSEs and one (2%) for an SSE, which collectively generated 35 (54%) cases. The median number of transmissions from an MSE or an SSE was 2 (IQR, 2–3). The observed offspring distribution of the SARS-CoV-2 cases is shown in Figure 3 . Notably, the HCWs were significantly less likely to be infectors than were the caregivers or patients (14% vs 41%, P=0.04) (Table I), and none of the HCWs were determined to have transmitted SARS-CoV-2 infection to the patients or caregivers.
Table I.
Comparison between infectors and non-infectors
| Infectors (N = 21) a | Non-infectors (N = 35) a | P | |
|---|---|---|---|
| Age, years, median (IQR)b | 55 (41–62) | 47 (33–56) | 0.20 |
| Male sex | 11 (52) | 16 (46) | 0.63 |
| Patient | 8 (38) | 10 (29) | 0.56 |
| Caregiver | 10 (48) | 11 (31) | 0.23 |
| Underlying diseases | |||
| Immunocompromisedc | 3 (14) | 3 (9) | 0.66 |
| Healthcare worker | 3 (14) | 14 (40) | 0.04 |
| Symptomatic at diagnosis | 18 (86) | 24 (69) | 0.33 |
| Duration from symptom onset to confirmation, days, median (IQR) | 2 (0–5) | 0 (0–2) | 0.08 |
| Disease severity at time of diagnosis | |||
| Mild | 21 (100) | 34 (97) | > 0.99 |
| Moderate | 0 | 0 | NA |
| Severe | 0 (0) | 1 (3) | > 0.99 |
| Stay in a six-patient room d | 17/18 (94) | 19/21 (90) | > 0.99 |
Data are presented as N (%) of patients unless otherwise indicated. IQR, interquartile range.
Family cases (N = 9) were excluded because they did not acquire severe acute respiratory syndrome coronavirus 2 infection at the hospital.
Data from one individual were unavailable.
All six immunocompromised patients had haematologic malignancy.
Analysed for the patients and caregivers.
Figure 3.
Offspring distribution of the nosocomial cases of COVID-19 (N = 65).
WGS and phylogenetic analysis
WGS was performed in a total of 12 specimens from eight clusters (six patients, two caregivers, and four HCWs) where each cluster had vague temporal or spatial links (Figure 1). In the WGS analysis, there were three groups: group A (background red colour in Figure 1), group B (background blue colour in Figure 1, seven-nucleotide differences from group A), and group C (13-nucleotide differences from group B). Groups A and B involved multiple clusters and wards, respectively.
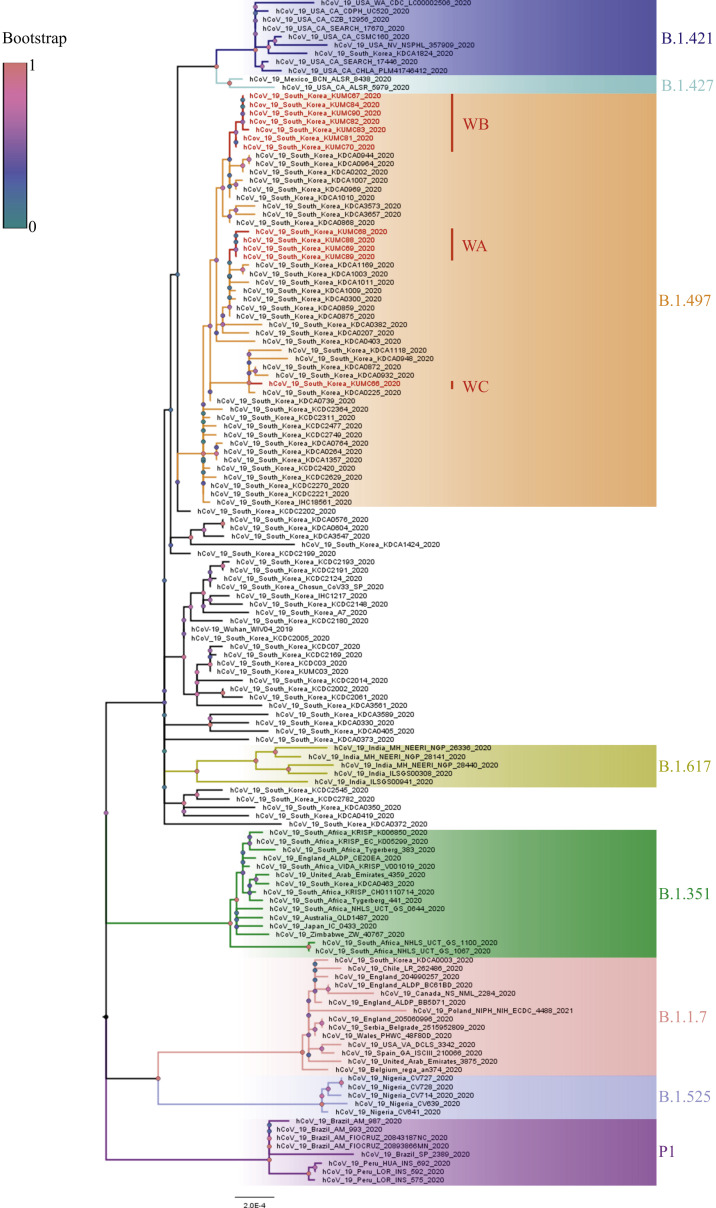
We conducted a phylogenetic analysis to determine the genetic distance of the SARS-CoV-2 sequences acquired from the SARS-CoV-2 circulating in Korea and worldwide. As shown in Figure 4 , all 12 specimens belonged to the B.1.497 lineage (GISAID Clade GH) reported circulating in Korea from May 2020 (https://cov-lineages.org/lineages/lineage_B.1.497.html). In particular, WGS groups A, B, and C (Figure 1) reported in this study were genetically distinguished in the phylogenetic tree. Moreover, the B.1.497 lineage that predominantly circulates in Korea was genetically distant from the currently reported SARS-CoV-2 variants B.1.1.7, B.1.351, B.1.525, P1, and B.1.617 (first detected in the UK, South Africa, the UK, Brazil, and India, respectively) and was genetically closer to the B.1.421 and B.1.427 lineages (first detected in the USA).
Figure 4.
Phylogenetic tree of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequences from Asan Medical Center. A phylogenetic tree was constructed via maximum likelihood estimation (1000 replicates) using MEGA X software. Bootstrap scores were represented at each node. Different branch colours represent different clades and subclades. The scale bar indicates nucleotide substitution per site. Red indicates 12 specimen sequences acquired from Asan Medical Center (whole-genome sequencing (WGS) group A: WA; WGS group B: WB; and WGS group C: WC).
The patient in E13F (ward 133) and the HCW in E13F (ward 134) had a partial spatial relationship; thus, we performed WGS analysis. Unexpectedly, the sequences were different between them. However, the patient in E13F (ward 133) had the same sequences as the N11F cluster (no epidemiologic relationship, group A in the WGS analysis), and the HCW in E13F had the same sequences as the E7F cluster (group B in the WGS analysis). In addition, we found that the E7F cluster had the same sequences as the N9F cluster (group B). Therefore, we re-investigated the epidemiologic association; however, there were no epidemiologic links or contacts found using the CCTV footage. There was one exception wherein the caregiver in ward 73 (7th floor in the East building) and a patient in ward 105 (10th floor in the New building) were in the waiting room in the outdoor screening clinic without face-to-face contact for 4 min on November 21. Both wore masks, and the distance between them was 2 m (Supplementary Figure S1). The caregiver developed fever on 21st November and revealed positive SARS-CoV-2 PCR testing results on the same day. The patient in ward 105 showed negative admission test results on 21st November but subsequently showed positive routine follow-up test results on 25th November. He developed symptoms later.
There was one transmission case that was clearly identified in the WGS analysis in a cluster with a vague epidemiologic link (no SNP difference, N11F cluster in Figure 1). The nurse met the caregiver (index) face-to-face for 3 min in a small room (11.53 m2) on 20th November. The index caregiver wore a mask, and the nurse wore a surgical mask without eye protection (Supplementary Figure S2). The index had a positive PCR testing result on 21st November, while the nurse revealed a negative PCR testing result on 22nd November during testing for the entire ward; however, she developed fever and had a positive PCR testing result on 23rd November. All the family members of this nurse had negative PCR testing results. We later found that the air inlet and outlet in the ceiling of the room were functioning well.
Discussion
In this study, we found that approximately 70% of the individuals with COVID-19 did not transmit SARS-CoV-2 infection in the hospital setting and that one-fifth were responsible for MSEs or SSEs, which accounted for approximately half of the nosocomial events that we observed during the study period. Compared with a previous analysis of transmission events of SARS-CoV-2 infection in a community setting [4], our study found that the proportion of MSEs or SSEs was similar in the hospital setting. However, the cluster size was smaller; the largest cluster was 106 in the Hong Kong study and 12 in our study. While there was no linear relationship between increasing delay in confirming infectors and more secondary cases in the Hong Kong study [4], we found that the infectors tended to have a longer duration from symptom onset to isolation than the non-infectors in our study (median 2 days vs 0 days; P=0.08). In addition, the median duration from symptom onset to isolation was less than two days in our study, which was shorter than that in previous studies [4,6]. Early detection and isolation of patients with COVID-19 with infection control measures, including universal mask-wearing, universal screening, and rigorous symptom-based screening for all inpatients and HCWs with PCR testing, will probably contribute to preventing large sizes of SSEs in hospital settings.
It has been reported that HCWs are less likely to cause transmissions within hospital settings owing to extensive mask-wearing [7]. Similarly, there was only one cluster with transmission among the HCWs who had close contact with other HCWs and ate lunch while being unmasked in our study. This shows that while HCWs are not likely to act as spreaders to inpatients, they may occasionally spread the virus to their co-workers. Adequate systematic support to maintain mask-wearing among HCWs is important, and a dedicated, well-ventilated space must be provided for them for breaks and meals [7].
We performed WGS when diagnosis of the non-close contacts who had possible temporal or spatial relationships with the index patients was confirmed by SARS-CoV-2 PCR testing. Notably, we found one transmission case that was consolidated in the WGS analysis. One HCW was infected by one caregiver while talking for 3 min in a small room; both were wearing masks appropriately. We initially thought of this epidemiologic link as vague in terms of mask-wearing and short contact time. However, both epidemiologic links (no known source for the HCW, including all family members) and WGS data (no SNP difference) support the direct transmission from the caregiver to the HCW. A previous study reported that a 10-min contact period caused transmission owing to short-range aerosol despite the use of surgical masks and face shields [8]. Thus, our data suggest that both mask-wearing and a relatively short contact time may not guarantee the absence of transmission, especially in a small room.
When there were no epidemiologic links, we found that WGS was not helpful in identifying the unexpected epidemiologic links even by re-investigating the possible links through CCTV footage tracing and interview. Although the same sequence was shown in the WGS analysis between the patient and caregiver who had contact in the outdoor waiting room while both were masked, it was difficult to determine whether the transmission actually occurred in the outdoor waiting room without face-to-face contact between these two appropriately masked individuals. In addition, we did not find any epidemiologic links in our thorough re-investigation between the different clusters and wards with the same sequences. Between November and December 2020, the number of cases suddenly increased in the community setting of Seoul. It is believed that the same strains circulating in the community were simultaneously imported to our hospital, as shown in the phylogenetic tree in Figure 4. Although we could not completely exclude missed epidemiologic links that might have occurred on multiple floors between the different buildings owing to airborne transmission, we assumed that the discriminative power of the WGS analysis during the sharp increase in the number of COVID-19 cases in the surrounding community might not be too high. Further studies are needed in this area.
Our study has several limitations. Firstly, we might not have uncovered all epidemiologic links between the nosocomial clusters; thus, the cluster sizes could have been underestimated. However, this is unlikely considering the extensive epidemiologic investigations performed by our infection control team with the help of the government epidemiologic investigators, the breadth of which included casual contacts as well as close contacts. Secondly, this study was a single-centre study wherein thorough infection control measures were implemented; the degree of community spread of SARS-CoV-2 infection was relatively well controlled; and extensive SARS-CoV-2 PCR testing was possible. Thus, our findings may not be extrapolated well in other settings. Finally, there may be a concern regarding the third wave in Korea being associated with the new UK or South African variant that is more contagious. However, there were only 10 COVID-19 cases with these variants (nine cases with lineage B.1.1.7 and one case with B.1.351) in Korea until 2nd January 2021 [9]. Although we did not perform WGS for all cases, it is less likely that the cases in our hospital were of different variants. The WGS data and phylogenetic tree from 12 patients further support this hypothesis. Despite these limitations, our study provides important knowledge on the nosocomial clusters of COVID-19 and policy-making decisions for hospital infection control practice.
In conclusion, approximately 70% of the nosocomial cases of COVID-19 did not generate secondary cases, and one-fifth of the infectors were responsible for SSEs and MSEs, which accounted for approximately half of the total cases in the healthcare setting. The HCWs were less likely to transmit the virus than were the patients and caregivers. Early case identification, isolation, and extensive contact tracing are important for the prevention of transmission and SSEs during the COVID-19 pandemic.
Acknowledgements
The authors greatly appreciate the healthcare workers and the staff members at Asan Medical Center who helped overcome the outbreak.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.06.012.
Author contributions
Conceptualization: J.J., S.-H. Kim. Methodology, data curation, and formal analysis: J.J., S.Y.L., J.L., J.-Y.B. Funding acquisition: S.-H. Kim., M.-S.P. Investigation: J.J., S.Y.L., J.L., J.-Y.B. Visualization: J.J., S.Y.L., J.L. Writing – original draft: J.J., S.Y.L., J.L., S.-H. Kim. Writing – review and editing: Y.-J.L., M.J.H., S.H. Kwak, E.O.K., H.S., M.-N.K., M.-S.P.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, which is funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HW20C2062), and from the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science and ICT, Republic of Korea (NRF-2018M3A9H4056537).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lau L.I., Ip D.K.M., Nishiura H., Fang V.J., Chan K.H., Peiris J.S. Heterogeneity in virus shedding among medically-attended influenza A virus infections. J Infect Dis. 2013;207:1281–1285. doi: 10.1093/infdis/jit034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Z., Ning F., Zhou W., He X., Lin C., Chin D.P. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh M.D., Park W.B., Park S.W., Choe P.G., Bang J.H., Song K.H. Middle East respiratory syndrome: what we learned from the 2015 outbreak in the Republic of Korea. Korean J Intern Med. 2018;33:233–246. doi: 10.3904/kjim.2018.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam D.C., Wu P., Wong J.Y., Lau E.H., Tsang T.K., Cauchemez S. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nature Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 5.Korea Centers for Disease Control and Prevention Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6:269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richterman A., Meyerowitz E.A., Cevik M. Hospital-acquired SARS-CoV-2 infection: lessons for public health. JAMA. 2020;324(21):2155–2156. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 8.Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D. A SARS-CoV-2 cluster in an acute care hospital. Ann Intern Med. 2021;174:794–802. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korea Centers for Disease Control and Prevention. The Update of COVID-19 in Korea as of January 2, 2021. Available at: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=362859&contSeq=362859&board_id=140&gubun=BDJ [last accessed January 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.