Graphical abstract

Keywords: Chlorpyrifos, Endocannabinoids, CB1 receptor, Microbiota, Obesogen, Δ9Tetrahydrocannabinol: Δ9THC
Highlights
-
•
Chlorpyrifos (CPF) + Δ9THC are co-exposed from pesticide use on cannabis.
-
•
CPF & Δ9THC have mechanisms through the endocannabinoid system (eCBS)
-
•
eCBS regulates energy balance and microbiota.
-
•
CPF causes dysbiosis and increases body weight in most age groups.
-
•
Δ9THC has actions that can protect against or reverse obesogenic effects.
Abstract
Marilyn Silva.
Retired from a career in toxicology and risk assessment.
Increased childhood and adult obesity are associated with chlorpyrifos (CPF), an organophosphate pesticide. Cannabis (Δ9Tetrahydrocannabinol: Δ9THC) use has increased globally with legalization. CPF applications on cannabis crops lacks federally regulated tolerances and may pose health risks through exposure during development and in adulthood. Both CPF and Δ9THC affect the endocannabinoid system (eCBS), a regulator of appetite, energy balance, and gut microbiota, which, if disrupted, increases risk for obesity and related diseases. CPF inhibits eCB metabolism and Δ9THC is a partial agonist/antagonist at the cannabinoid receptor (CB1R). Effects of each on obesogenic parameters were examined via literature search. Male rodents with CPF exposure showed increased body weights, dysbiosis, inflammation and oxidative stress, potentially associated with increased eCBs acting through the gut-microbiota-adipose-brain regulatory loop. Δ9THC generally decreased body weights via partial agonism at the CB1R, lowering levels of eCBs. Dysbiosis and/or oxidative stress associated inflammation occurred with CPF, but these parameters were not tested with Δ9THC. Database deficiencies included limited endpoints to compare between chemicals/age-groups, inter-study variables (dose ranges, dosing vehicle, rodent strain, treatment duration, etc.). CPF and Δ9THC were not tested together, but human co-chemical effects would depend on exposure ratio, subject age, exposure duration, and health status, among others. An overriding concern is that both chemicals are well-documented developmental neurotoxins in addition to their low dose effects on energy balance. A co-exposure risk assessment is warranted with increased use and lack of federal CPF regulation on cannabis.
Introduction1
Obesity in the United States has rapidly increased in adults to 42% (2017–2018) and in children (age 2–19) to 20% (2019; Overweight & Obesity | CDC). Childhood overweight and obesity present an increased risk for type-2 diabetes (T2D), hypertension and cardiovascular diseases in later life (Weihrauch-Blüher and Wiegand, 2018). Excessive lipid accumulation in adipose tissue has been attributed, in part, to environmental obesogenic chemicals, including pesticides (Blanco et al., 2020, Radi and Hasni, 2014). One such chemical is the organophosphate insecticide, chlorpyrifos (CPF, ethyl) which primarily acts through inhibition of the serine hydrolase: acetylcholinesterase (AChE), in the peripheral and central nervous systems (CNS) (Casida, 2017). It has been extensively used in agricultural settings throughout much of the United States and internationally (CDPR, 2018, EFSA, 2014, US EPA, 2020). Although it generally does not bioaccumulate, it is sufficiently lipophilic to be stored in fatty tissues and appear in colostrum or milk of lactating women (Weldon et al., 2011). Agriculture workers or bystanders living near fields where CPF is used are potentially at high risk for long term effects of exposure (CDPR, 2018, US EPA, 2020). Exposure in early development (Reygner et al., 2016), during pubescence or in adulthood (Acker and Nogueira, 2012, Elsharkawy et al., 2013) has resulted in conditions related to diabetes, obesity, and metabolic syndrome2 in rodent models and potentially also in humans (Velmurugan et al., 2017).
The use of medicinal and recreational cannabis (main component Δ9Tetrahydrocannabinol: Δ9THC) has increased globally by 60% from 2010 to 2019, with 8.7 billion dollars in sales between 2014 and 2021 (Marijuana Statistics 2020, Usage, Trends and Data - AmericanMarijuana). While at least half of the United States has legalized cannabis, it is not federally approved (https://www.dea.gov/controlled-substances-act). This means that pesticide use on marijuana plants can be unregulated, lacking tolerance limits normally set by the United States Environmental Protection Agency (USEPA; https://www.epa.gov/pesticide-tolerances). Moreover, CPF residues have been detected in cannabis plants and products and together they potentially pose health risks (Sandler et al., 2019, Voelker and Holmes, 2015). The risks depend on several variables, including use patterns (e.g., location, sex, age, race, frequency, etc.) and product (edibles, dermal/topical use, smoking dry flowers or vaping) (Schauer et al., 2016, Sexton et al., 2016, Shiplo et al., 2016). In some cases, as with waxes and concentrated extracts, concentrated CPF residues can occur (Raber et al., 2015). The combined effects of CPF and chemicals in cannabis are therefore of interest but have not been evaluated.
Background and theory
CPF and Δ9THC have in common their effects on the endocannabinoid system (eCBS) (Carr et al., 2020, Di Marzo et al., 2011, Medina-Cleghorn et al., 2014). It is generally composed of (1) a G-protein-coupled receptor, cannabinoid 1 (CB1R), which is highly concentrated in the CNS, but also abundant in the peripheral tissues; (2) two principal endogenous endocannabinoids (eCB) 2-arachidonoylglycerol (2-AG) and anandamide (AEA)); and (3) the serine hydrolases monoacylglycerol lipase (MAGL), and fatty acid amide hydrolase (FAAH) that metabolize 2-AG and AEA (di Marzo and Matias, 2005, Matias and di Marzo, 2007). eCBS helps to shape neuronal connectivity in the brain during development and into adulthood (Mato et al., 2003), however, it is also involved in numerous pathways controlling appetite, including the gut-microbiota-adipose-brain regulatory loop and other parameters directly-related to energy balance (Jo et al., 2005, Quarta et al., 2010, Silvestri and di Marzo, 2013, Forte et al., 2020).
Gut microbiota participate in gut-brain crosstalk to maintain host energy homeostasis, where increased circulating eCBs disrupt this balance (Forte et al., 2020). Most gut microbiota (~90%) are from 5 phyla consisting primarily of Bacteroidetes and Firmicutes at 90–99% but also Actinobacteria, Proteobacteria and Verrucomicrobia and within those phyla are a wide variety of species and strains in the intestine (Rinninella et al., 2019). Dysbiosis occurs when there is a decrease in bacterial diversity or in the ratio of healthy bacteria (Bacteroidetes/Firmicutes) compared to bacterial strains associated with pathogenic activity (e.g., Enterobacteriaceae family of Proteobacteria) (Castaner et al., 2018, Levy et al., 2017) caused by environmental toxins like CPF. When pathogenic bacteria predominate (Cani et al., 2014, Levy et al., 2017), the eCB system is activated in the gut, along with increases in CB1 receptors, while FAAH is downregulated (Marzo, 2008b, Dipatrizio, 2016, Matias and di Marzo, 2007). Circulating lipopolysaccharides (LPS) endotoxins from G negative bacteria cause metabolic endotoxemia-induced inflammation, a “leaky gut” that increases risk for insulin resistance and obesity (Cani et al., 2007, Cani et al., 2008, di Marzo and Silvestri, 2019). Forte et al. (2020) reviewed data showing that increased LPS and eCB are associated with increased fasting glucose and insulin, hepatic adipose, and whole-body weight gains. These risk factors are associated with obesity, T2D, gastrointestinal cancer and inflammatory bowel disease (Forte et al., 2020)
CPF and Δ9THC are metabolized by the following P450s: CYP1A2, 3A4, 2C9, 2C19, 2B6, 2D6 and eliminated by glucuronidation (Qian et al., 2019, Testai et al., 2010, Watanabe et al., 2007). With co-exposures, CYP induction could reach a tipping point for irreversible adverse effects even when the individual CPF and Δ9THC doses are within regulatory limits (Saili et al., 2020, Shah et al., 2016). The tipping point also depends on an individual’s ability to handle various exposure loads based on age, genetic makeup, health status, and diet, among other influences (Hewitt et al., 2007, Bernasconi et al., 2019). These risk factors are often difficult to characterize in humans since hepatic metabolism studies are, by necessity, generally performed in vitro (Bernasconi et al., 2019).
While both CPF and Δ9-THC affect the eCBS, their modes of action (MOA) are different. After absorption into tissues and/or interacting at the CB1R, CPF inhibits FAAH and MAGL centrally and peripherally, preventing the breakdown of eCBs and allowing their buildup (Buntyn et al., 2017, Carr et al., 2020). This action has been shown to occur at doses of less than or equal to 0.5 mg/kg/day (Buntyn et al., 2017, Carr et al., 2020, Carr et al., 2017, Leung et al., 2019). Increased eCB levels inhibit the release of excitatory or inhibitory neurotransmitters (e.g., ɣ-aminobutyric acid [GABA] or glutamate, norepinephrine and ACh) in the CNS and peripheral tissues (Leung et al., 2019) affecting various aspects of appetite and energy balance (Di Marzo and Matias, 2005). The effects would occur with the overabundance of and overstimulation by eCBs as shown in Fig. 1.
Fig. 1.
This diagram is an indication of where CPF or an obesogenic diet would lead to an over-active eCBS. Δ9-THC intake would downregulate CB1R levels and potentially attenuate these effects.
Over activation of the eCBS also affects the microbiome. Fig. 2 depicts the effects on an imbalance in normal microbiota (dysbiosis) resulting from an environmental stressor such as CPF. It is known that the eCBS is activated by eCB crosstalk with increased plasma levels of LPS from Gram negative gut microbiota (Muccioli et al., 2010).
Fig. 2.
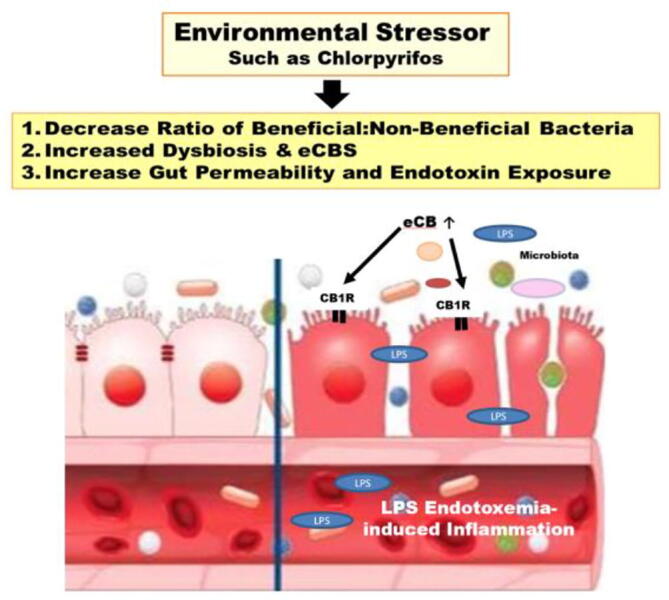
Dysbiosis occurring from an environmental stressor such as CPF. Δ9-THC would tend to promote a healthy balance in microbiota (discussed in the text).
Δ9-THC acts as a partial agonist/antagonist at the CB1R and the extent of agonist activity affecting food intake pathways generally depends on dose and treatment durations (Di Marzo, 2011). There is a bimodal dose effect in mice receiving acute intraperitoneal (i.p.) Δ9-THC treatment (Bellocchio et al., 2010). At 1 mg/kg Δ9-THC mice showed increased food intake, where at 2.5 mg/kg/day, food intake was decreased; however, at both doses there was increased glutamatergic and decreased GABAergic transmission in the CNS. Glutamatergic transmission through CB1R stimulates food intake, where CB1R action in GABAergic neurons inhibits food intake (Di Marzo, 2011). Thus, aspects of the Δ9-THC MOA involve selection of neuronal type in the brain, depending on exposure. Epidemiological studies showed that acute cannabis use was associated with appetite stimulation, while chronic use decreased risk of obesity, insulin resistance and diabetes mellitus (reviewed in: Farokhnia et al. (2020). These data support cross talk between cannabis, insulin, and the pancreas.
While it remains unknown how the combined exposures might disrupt the eCBS, in theory, exposure to both CPF and Δ9-THC could have opposing effects on energy balance. Δ9-THC intake would downregulate CB1R levels and potentially attenuate the effects of eCBS overactivation by CPF (di Marzo et al., 2004, Forte et al., 2020, Horváth et al., 2012). The current study presents a qualitative assessment of CPF and Δ9-THC effects on parameters influencing energy balance associated with the eCBS, along with discussion of potential consequences from co exposures.
Methods
Data sources and search strategy
A literature search was conducted by methods similar to the Patient, Intervention, Comparison, Outcome (PICO) of evidence-based practice (Eriksen and Frandsen, 2018, Santos et al., 2007). It was not a conventional Systematic Review requiring two or more persons to review an abstract (PICO vs. Systematic Review described in: Eriksen and Frandsen (2018)) but it was designed to obtain data involving search terms that most closely focused on the subject.
-
•
Problem Identification: A concern was the potential for exposure to CPF and Δ9-THC through use of cannabis products.
-
•
Specific Question: Is there a potential for disruption of the eCBS by either chemical and is there evidence that they affect energy balance or contribute to risk factors for obesity?
-
•
Search Strategy: The search strategy sought to curate through electronic sources peer-reviewed journal articles, books, and reports containing the toxicological data available to explore the subject. The open access Abstract Sifter tool was used to query PubMed, facilitate compilation of results and sort, and select pertinent articles (Baker et al., 2017). Searches were also performed with Google Scholar, Science Direct and Research Gate, as needed to obtain articles related to the primary subjects. PubMed Medical Subject Headings (MeSH: https://meshb.nlm.nih.gov/search) were queried, along with other index terms for CPF and Δ9-THC in relation to risk factors for obesity or imbalance in energy utilization (Supplemental Table 1). Δ9-THC was chosen as the cannabis component of concern, to focus on the ingredient with the likelihood of a dominant exposure. Among the search terms were: “adipose”, “body weight,” body weight gain OR body weight change,” “cholesterol,” “food intake OR food consumption,” “ghrelin,” “glucose,” “HDL OR high-density lipoprotein,” “high fat,” “insulin,” “insulin resistance,” “LDL OR low-density lipoprotein,” “leptin,” “gut microbiome OR microbiota (gut microorganisms),” “obese OR obesity,” “oxidative stress,” “triglycerides,” “VLDL OR very low-density lipoprotein”, “environmental obesogen,” and others (Supplemental Table 1). Literature was also obtained from citations listed in screened studies. Review articles were a source of additional articles.
Table 1.
Measured Parameters Associated with Risk Factors for Obesity from Treatment with CPF or Δ9-THC in Male Rodents.
 |
Abbreviations: F: fasted; G: body weight gain measured; i.p.: intraperitoneal; IR: insulin resistance measured; LOEL: lowest observed effect level; PND: post-natal day; s.c. subcutaneous; S: serum insulin levels measured; TC: total cholesterol; TG: triglyceride; W: body weight measured
References: 1.Joly Condette et al. (2014); 2. Joly et al. (2013); 3. Reygner et al. (2016); 4. Lassiter and Brimijoin (2008); 5. Slotkin et al. (2005); 6. Perez-Fernandez et al. (2020a); 7. Perez-Fernandez et al. (2020b); 8. Li et al. (2019); 9. Liang et al. (2019); 10. Łukaszewicz-Hussain (2011); 11. Akhtar et al. (2009); 12. Uchendu et al. (2017); 13. Uchendu et al. (2018); 14. Kopjar et al. (2018); 15. Fang et al. (2018); 16. Wang et al. (2009); 17. Alvarez et al. (2008); 18. Roshanravan et al. (2020); 19. Meggs and Brewer (2007); 20. Zhao et al. (2016); 21. Zhang et al. (2021); 22. Peris-Sampedro et al. (2015b); 23. Gillies et al. (2020); 24. Gupta and Elbracht (1983); 25. O'Shea and Mallet (2005); 26. Keeley et al. (2015); 27. Dow-Edwards and Zhao (2008); 28. Beydogan et al. (2019); 29. NTP (1998); 30. Weed et al. (2016); 31. Rahminiwati and Nishimura (1999); 32. Coskun and Bolkent (2014).
Symbols: “*” – Indicates only dose used in the study; #- Inflammation in liver; @-Serum levels of proinflammatory cytokines; $-Intestinal epithelium; X-Oxidative stress was based on lipid peroxidation associated with membrane fluidity; Green, yellow, and red indicate increase, equivocal or decreased compared to controls, respectively.
Data inclusion or exclusion
Study selection based on route of exposure
Oral, intravenous (i.v.), intraperitoneal (i.p.) or subcutaneous (s.c.) and inhalation are commonly used and well-characterized routes given that absorption is assumed to be 100% for CPF (Griffin et al., 1999, Testai et al., 2010, US Epa, 2011, CDPR, 2018) and Δ9-THC (Wiley et al., 2021, Nguyen et al., 2016, Nguyen et al., 2020a, Nguyen et al., 2020b, Taffe et al., 2020), where dermal studies are less sensitive due to the skin as a barrier to absorption. Dermal studies for CPF were excluded because absorption is low (~1–3%) and anticipated toxicological effects would not exceed those observed by other routes (US Epa, 2011, Nolan et al., 1984). The Health Effects Test Guideline 21-day dermal rat study showed no effects at the highest CPF dose (10 mg/kg/day) (US EPA, 2011). Another CPF dermal study in mice treated only the tail skin for 2 weeks and showed a slight decrease in plasma ChE at 101 mg/kg/day (only dose) (Krishnan et al., 2012). CPF inhalation studies were also excluded because four Health Effects Test Guideline 90-day inhalation studies showed no systemic or cholinesterase effects at the highest attainable vapor concentration (Corley et al., 1986; US Epa, 2011, US EPA, 2014) and other repeated dose inhalation studies performed with technical grade CPF were not found in the open literature. While both dermal and inhalation exposures to agricultural workers and inhalation exposure by spray drift to bystanders are a concern, the most sensitive point of departure for CPF risk assessment was based on developmental neurotoxicity after oral treatment in animal studies (~0.1 mg/kg/day) (CDPR, 2018). That value was used to calculate human risk by all routes.
There were 13 inhalation studies performed with technical grade Δ9-THC in the open literature, but they were excluded for the following reasons: 1) they were acute (single administration), 2) the doses used in the repeat intervals were above the LOEL (~5.0 mg/kg/day) and into the range of overt toxicity (10–20 mg/kg/day), 3) a graded dose regimen was used, or 4) only female animals were examined. Δ9-THC lacked dermal toxicity characterization (CompTox Chemicals Dashboard (epa.gov) in the open literature. While inhalation and dermal exposures are expected in users of cannabis products, the Δ9-THC risk assessment (EFSA, 2015) determined that neurotoxicity from oral administration to human subjects provided the most sensitive point of departure (~2.5 mg/kg/day) as a basis for calculating human risk by all routes.
Data Inclusion
-
•
Studies performed in rat and mouse models throughout all life stages were selected initially to determine whether age at exposure affected risk (Fig. 3; Supplemental Table 2).
-
•
Studies including healthy male and female rodents (rats and mice) on a standard diet.
-
•
Repeated dose studies were selected to capture effects from long-term, low-dose exposure scenarios which is a likely representation of cannabis use patterns, with potential CPF co-exposure.
-
•
Study acceptance was not limited by treatment vehicle (e.g., DMSO, corn oil, etc.).
-
•
Year of publication and/or geographical locations were not restricted, but the articles needed to be in English or English translations (Fig. 3).
Fig. 3.
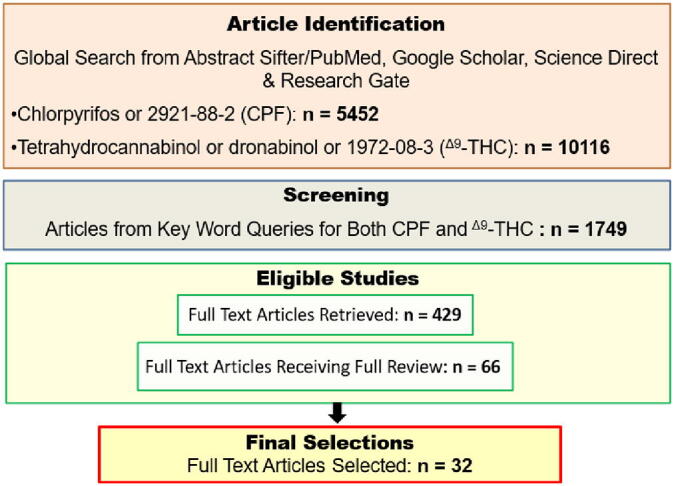
Sequence of steps involved in the review to filter for potential CPF and Δ9-THC effects on the eCB system associated with energy imbalance or obesity.
Data exclusion
-
•
Acute studies were excluded because the major concern is chronic exposure leading to irreversible effects, where there is greater potential for reversal and repair with acute exposure.
-
•
Use of diabetic, obese, or genetically modified rodent strains were excluded because the purpose was to investigate effects of CPF and Δ9-THC on healthy animals on a standard, non-obesogenic diet.
-
•
Studies were excluded where the animal strain and age at treatment were not described.
-
•
If exposure was to “cannabis” (cannabinoid mixtures, cannabinoids other than Δ9-THC or synthetic cannabinoids) or CPF as a formulated product or if the chemical characterization was not described, the study was excluded because cannabinoids other than Δ9-THC or inerts added to CPF formulations could stimulate effects unrelated to the active ingredients under investigation.
-
•
Studies using dose levels known to induce overt toxicity were obtained in the initial search but were excluded because the exposures would not be representative of what would generally occur in humans (e.g., CPF > 5.0 mg/kg/day or Δ9-THC 10–20 mg/kg/day) (Ambali et al., 2011, Elmazoudy et al., 2011, NTP, 1998).
-
•
If the full text article was unavailable after corresponding with authors through email or Research Gate, then it was excluded.
References were selected and their “pdf” copies were sent to Endnote20 (October 23, 2020, release). Each study was reviewed and the specifics (e.g., chemical exposure, vehicle, sex, animal age at treatment, strain, dosing schedule, doses used, testing dates post-treatment and endpoints) were compiled (Fig. 3; Supplemental Table 2).
Review of studies
The selected studies were reviewed for effects related to treatment by CPF or Δ9-THC associated with increases, decreases or no effect on body weight/body weight gain, food intake, adipose, free-fatty acids (FFA), triglycerides (TG), total cholesterol (TC), low or very low-density lipoproteins (LDL/VLDL), high density lipoproteins (HDL), glucose, insulin, insulin resistance, ghrelin, leptin, inflammation, oxidative stress, or microbiota. Initial reviews included both male and female rats and mice and these data are in Supplemental Table 2. Ultimately studies in male rodents were selected for analysis because most of the available results were in males.
Results
Search results
The data in Table 1 present an overall summary of studies. Not all parameters were measured at each life-stage or for each chemical, which limited direct comparisons of effects between chemicals. General age at treatment, treatment regimen, age at testing, dose(s) used, and effect in male rats and mice were reported. Female data were presented in Supplemental Table 2, along with complete descriptions of protocols for studies selected for this work. Some parameters were not added to Table 1 (FFA, leptin, ghrelin, HDL, LDL and VLDL) because they were measured in few studies and the results did not add to overall data interpretation (data for these parameters included in Supplemental Table 2).
Data summary
Overall conclusions were based on qualitative observations because most of the studies were not performed according to Health Effects Test Guidelines (US EPA, 1998) but were designed to examine a specific effect (i.e., body weight, etc.; Table 1). As such, they may have used only one dose which precluded determination of a LOEL or Benchmark Dose due to lack of a dose–response. Δ9-THC studies were mainly designed to test for neurotoxicity, but body weights and sometimes other parameters related to energy balance were available. Variables among studies included rat strains (6), mouse strains (5), doses administered, method of administration (i.e., gavage, diet, intravenous (i.v.), intraperitoneal (i.p.), subcutaneous (s.c.)), vehicle (i.e., olive oil, sesame oil, corn oil, soya oil, rapeseed oil, DMSO, methanol, Tween80, Tween20, propylene glycol, saline, cremaphor, ethanol), treatment durations (~4–175 days), age at treatment and testing, dietary composition, and test laboratory. Of the 10 measured parameters, or categories listed in Table 1, most were examined only with CPF. In most cases, fasting serum glucose was measured but it was not always stated in the methods section of the respective studies. In some cases, both serum insulin and insulin resistance were tested as designated in Table 1. Studies were initially organized by life-stage, but the overall results did not support or highlight differences. While the sequence of the studies in Table 1 is by treatment age, it is for convenience but does not indicate specific age-related effects.
Body weight effects
CPF (Table 1): Body weights were increased at doses as low as 0.3 mg/kg/day and up to 5.0 mg/kg/day in rats and mice treated during gestation- to weaning, at weaning and in adulthood (Table 1). There were no effects on body weights from neonatal/perinatal or adolescent treatment. In some cases, body weight increases were accompanied by increased adiposity but not necessarily by increased food intake.
In utero CPF exposure at 1.0 mg/kg/day would be from metabolism in treated dams for the entire gestation period and include placental transfer of the major metabolite CPF-oxon (Mattsson et al., 2000). Pup body weights could be affected from eCBS overactivity based the knowledge of placental CPF-oxon transfer and evidence of eCBS central and peripheral disruptions in juvenile rats at 0.5 mg/kg/day (Fig. 2) (Buntyn et al., 2017, Carr et al., 2020).
Body weight was not a sensitive endpoint in Sprague-Dawley and Wistar male pups treated neo/perinatally (PND 1–4 or PND 10–15) at 1.0 mg/kg/day CPF, possibly due to immature metabolic capacity (Perez-Fernandez et al., 2020a, Perez-Fernandez et al., 2020b, Slotkin et al., 2005) (Table 1). Neonatal/perinatal pediatric CPF-metabolizing nuclear receptor (e.g., pregnane-x-receptor: PXR) and CYP enzyme (CYP1A2, 3A4, 2C9, 2C19, 2B6) induction in Phase 1 metabolism may be 0–60% of adult values depending upon the CYP subfamily, inter- and intraindividual variability, assay test methods and available specimens (Allegaert and van den Anker, 2019, Sadler et al., 2016, Vyhlidal et al., 2006). Time of peak AChE inhibition for CPF administered by gavage (corn oil vehicle) at 3.0 mg/kg/day in brain of PND 11 pups vs. adult Sprague-Dawley females was 8 and 6 h, respectively, indicating that CPF metabolism was slower in younger animals (Marty et al., 2012). Hence, the eCBS may not have been disrupted to the extent that body weight effects occurred due to lack of metabolic activation of CPF to CPF-oxon. It is also possible that the longer treatment at other life stages had effects simply based on the greater exposure duration.
CPF at 0.3 mg/kg/day showed increased body weights in weanling rats (0.3 mg/kg/day; Tween20/saline vehicle; Table 1) but not in rats at 3.0 mg/kg/day during adolescence (corn oil vehicle; Table 1). The higher absorptive properties of Tween20/saline vs. corn oil vehicles when CPF was administered by gavage may explain the difference (Marty et al., 2007, Smith et al., 2009). It may also be that metabolic capability of rats during adolescence is increased, compared to weaning, such that the tipping point for toxicity was not reached. Activation to detoxification ratios for CPF depend on age, as well as many other interindividual and intraindividual ability to handle various exposure loads (Eaton et al., 2008, Ginsberg et al., 2004, Ginsberg et al., 2009, Ginsberg et al., 2017). Interpretation of these data was challenging because the Li et al. (2019) study used only 0.3 mg/kg/day CPF and measured absolute body weights, where Akhtar et al. (2009) treated at 3.0, 6.0 and 9.0 mg/kg/day and measured body weight gain.
Adult Wistar rats in two different studies showed either no effect or an increase in body weights at 0.3 mg/kg/day CPF with the same vehicle and chronic treatment of 63-, or 196-days (Fang et al., 2018, Li et al., 2019). While there is not a clear understanding of why the difference occurred, it is possible that 0.3 mg/kg/day CPF is a threshold for body weight effects in Wistar rats. The differences in the controls, study methods or times of assessment may have determined a result above or below the threshold.
Adult KM 3and C57BL/6 mice treated with CPF at 1.0 mg/kg/day by gavage (corn oil) for 30 or 85 days, respectively did not have effects on body weight (Zhang et al., 2021, Zhao et al., 2016). Adult C57BL/N6 mice treated at a higher dose had increased body weight and food intake at 2.0 mg/kg/day when treated 56 days with CPF in diet (Peris-Sampedro et al., 2015a). The vehicle used the above studies (corn oil vs. diet) may have affected CPF absorption. While 2.0 mg/kg/day CPF is above the dose initiating effects on the eCBS, it may be on the border of where body weight effects might occur in mice.
Δ9-THC (Table 1): One of the most consistent findings from Δ9-THC treatment was body weight decrease in rodents (Table 1). Since Δ9-THC acts through the eCBS, it is assumed that the body weight decrements occurred by agonism/antagonism at the CB1R. The threshold for Δ9-THC effect on body weight, regardless of age, vehicle, or treatment duration, appeared to be 3.0 mg/kg/day.
Body weights, pancreatic weights and glucose in plasma were decreased in Wistar male offspring of dams treated i.p. GD 6–22 at 3.0 mg/kg/day Δ9-THC (Gillies et al., 2020). PND 1 pup body weight decreases could have occurred through partial agonist activity of 9-THC at the placental CB1 receptor in the dams, while not affecting maternal food intake or body weights. Glucose, transported through the placenta to the fetus by glucose transporter proteins (e.g., GLUT1) (Acosta et al., 2015), is decreased by 35% in the placenta after 9-THC exposure during gestation, resulting in a decreased fetal body weight (Natale et al., 2020). In human subjects, developmental delays associated with low birth weight and cannabis use during pregnancy are known to occur (reviewed in: Fried and Smith (2001)). Hence, while protecting against potential risks for obesity, Δ9-THC negatively affects neuronal pathways during critical hormonal and neurodevelopmental changes (Berghuis et al., 2007, Berghuis, 2005, Fride, 2004, Fride, 2008). Moreover, low birth weight and body weight decreases potentially due to Δ9-THC partial agonist activity at the CB1R, can increase risk for diseases related to T2 diabetes, and hypertension in later life (Schieve et al., 2016, Alexander, 2014).
Body weight gains were decreased in male Ivanovas (Sprague-Dawley) and Wistar pups treated peri/postnatally by i.p. (PND 16–87) with Δ9-THC at 4.0 mg/kg/day (Gupta and Elbracht, 1983) or s.c. (PND 4–14) at 5.0 mg/kg/day (O'Shea and Mallet, 2005). Previous reports have shown that Δ9-THC>2.5 mg/kg/day in rats, results in decreased body weights (Bellocchio et al., 2010). However, as with low birth weights, a decrease in body weight during early development can be an adverse health effect. CB1R activation is essential in initiation of milk suckling in neonatal mice by facilitating innervation and activation of tongue muscles (Fride et al., 2005, Fride et al., 2009). Partial Δ9-THC agonism/antagonism (dose-dependent) at the CB1R will affect neurotransmission and suckling behavior in neonates, ultimately affecting body weight and inability to thrive. Δ9-THC exposure during the perinatal/preweaning period has been shown to have lasting negative effects on neurodevelopmental behaviors (Campolongo et al., 2011, Mohammed et al., 2018, Newsom and Kelly, 2008).
Long-Evans and Wistar males treated i.p. at weaning/peri-adolescence (PND 21–35)4 with 5.0 mg/kg/day Δ9-THC showed decreased body weight gains after 2 weeks of treatment (Keeley et al., 2015). Sprague-Dawley rats were treated PND 22–40 at 5.0 mg/kg/day but there were no effects on body weight (Dow-Edwards and Zhao, 2008). It is notable that body weight effects were different in two studies at the same dose with treatment for approximately the same duration. However, the rat strains differed, along with routes of administration and vehicle, among other variables. The i.p. injection exposure route and vehicle (cremaphor/saline) facilitated absorption, with greater tissue availability as compared to exposure by gavage (Iwaniec and Turner, 2013). For example, Dow-Edwards and Zhao (2008) attributed the lack of significant behavioral effects at 5.0 mg/kg/day to slow absorption of Δ9-THC administered orally compared to i.p. injection.
At 1.5 mg/kg/day Δ9-THC dose, Sprague-Dawley rats treated for 28 days by i.p. in adolescence showed slight but not significant decreases in body weight (Beydogan et al., 2019). Decreased body weights in adolescent male Fischer 344 and Long-Evans rats were seen at higher doses (5.0 mg/kg/day: gavage; 5.6 mg/kg/day: i.p.) (NTP, 1998, Weed et al., 2016). The decreased body weights in Long-Evans rats were not accompanied by effects on food intake (Weed et al., 2016), however, human subjects showed decreased Body Mass Index with chronic cannabis use along with increased caloric intake (Alshaarawy and Anthony, 2019, Smit and Crespo, 2001).
Effects on the developing brain with peri-adolescent/adolescent exposure may outweigh the protective effects on energy balance. Human exposure to Δ9-THC in childhood/peri-adolescence would more likely be second hand (e.g., second-hand smoke/vaping), rather than direct. Yet even these exposures could adversely affect brain development since areas of the cerebellum and amygdala continue to develop into early adulthood (Clancy et al., 2001, Rice and Barone, 2000). Cannabis use during adolescence and early adult ages results in cognitive and behavioral deficits (Camchong et al., 2017, Kasten et al., 2017, Nguyen et al., 2020b, Rubino et al., 2009). Further, current increases in cannabis use trends among pre-teens and adolescents (ages > 12 years) have shown that flavored vaping is perceived as beneficial compared to smoking (Knapp et al., 2019). Pilin et al. (2021) have stated this age is a time of experimentation with cannabis; often influenced by parental or peer behaviors.
Glucose and Insulin/Insulin resistance
CPF (Table 1): Overall, it appeared that insulin and insulin resistance were more sensitive targets than glucose, potentially related to dose and treatment duration. At higher CPF doses, insulin effects could have inhibited breakdown of eCBs, resulting in overstimulation in peripheral organs (Marzo, 2008a, Buntyn et al., 2017). eCBs modulate insulin-regulated glucose uptake in vitro, and the affected glucose and insulin levels may be indicators of insulin resistance (Bellocchio et al., 2008, Gasperi et al., 2007, Motaghedi and McGraw, 2008). The lack of effects on glucose or insulin levels in Sprague-Dawley pups treated at 1.0 mg/kg/day PND 1-4 (s.c. DMSO) may be due to the short exposure duration at a dose where the rats could metabolize and eliminate CPF (Slotkin et al., 2005).
Decreased glucose and (fasted) serum insulin occurred in adult Wistar rats treated 63 days with CPF at 0.3 mg/kg/day (Fang et al., 2018). At a low CPF dose (i.e., 0.3 mg/kg/day), the insulin decrease should coincide with a normal decrease in glucose associated with eCBs affecting insulin-regulated glucose uptake (Bellocchio et al., 2008, Gasperi et al., 2007, Motaghedi and McGraw, 2008).
Δ9-THC: It was concerning that after gestational treatment, pups showed decreases in glucose with Δ9-THC at 3.0 mg/kg/day, because an imbalance in glucose during brain development can have detrimental effects (Chugani, 1998). It was also notable because the regulatory No-observed-effect-level in rodents for Δ9-THC is 5.0 mg/kg/day (National Institute of Technology and Evaluation, Tokyo, Japan: Search Results - NITE-CHRIP (NITE Chemical Risk Information Platform based on NTP (1998)) and 2.5 mg/kg/day in humans (EFSA, 2015, EFSA, 2020). Therefore, fetuses exposed to supposedly “safe” levels of Δ9-THC, may end up with low birth weight and developmental deficits.
Δ9-THC had no effects on insulin at 1.5 or 3.0 mg/kg/day across life stages (Beydogan et al., 2019, Coskun and Bolkent, 2014, Gillies et al., 2020).
Triglycerides and total cholesterol
TG was a more sensitive endpoint than TC for CPF but there did not appear to be a dose, strain or treatment-related association (Table 1). TC was a sensitive endpoint for Δ9-THC, with an increase at lower doses (1.5 and 3.0 mg/kg/day) . The response for TG did not appear to be related to dose or dosing regimen when measured in adolescence and adulthood, however, TC was increased at both doses. The greater effects on TC at 1.5 mg/kg/day Δ9-THC may be due to the methanol vehicle administered i.p., since methanol, due to its greater lipophilicity, would facilitate Δ9-THC absorption to a greater extent than saline (PubChem (nih.gov); CompTox Chemicals Dashboard | Home (epa.gov)). Saline, with lower absorptive properties for lipophilic compounds may have resulted in fewer effects even though the higher dose (3.0 mg/kg/day Δ9-THC, also administered i.p., in the same strain of rat, showed no effects.
Inflammation, oxidative stress and microbiota
Across all life-stages, at CPF doses of 0.01 to 5.0 mg/kg/day, inflammation, oxidative stress, and microbiota changes occurred. Microbiota changes after CPF treatment at 0.3- to 5.0 mg/kg/day showed bacterial count patterns seen in obese humans or those with T2D (Karlsson et al., 2013, Qin et al., 2012). Dysbiosis leads to an increase in eCB activity, gut permeability and LPS release that would lead to an inflammatory response (Fig. 2) (Cani et al., 2016, Forte et al., 2020, Levy et al., 2017). The imbalance in beneficial to opportunistic (Gram negative) bacteria may account for the increases in LPS measured in mice (C57BL/6 and ICR) when treated PND 21–84 (Liang et al., 2019) and C57BL/6 mice treated as adults (Zhang et al., 2021) (Table 1). Both mouse strains in Liang et al. (2019) also had increased inflammation in liver, epididymal fat, ilium, and colon associated with microbiota changes (Table 1). Wistar rats treated PND 21–196 at the low dose of 0.3 mg/kg/day showed evidence of inflammation (proinflammatory cytokines in serum: MCP-1 and TNFα), however there were no effects on microbiota (Li et al., 2019). In the same study, adult rats treated PND 56–196 had increased inflammation and dysbiosis. Between the age of weaning and adulthood P450 enzymes that activate CPF and the eCBS are still developing (Berghuis et al., 2007, Fride, 2008, Tanaka, 1998, Upreti and Wahlstrom, 2016, Watanabe et al., 1993). CPF in weanlings, while absorbed through the gut, may primarily affect the brain eCBS because the P450s are not fully developed locally in the gut/liver. Inhibition of eCB breakdown in brain would affect the appetite center and potentially lead to the weight increase seen in adulthood after prolonged CPF treatment (Di Marzo, 2011) (Table 1). On the other hand, in adulthood, at low doses, CPF may be metabolized in the gut where eCBs are synthesized locally along with activation of CB1R (Di Marzo et al. 2004). The direct effects of CPF on the eCBS in the gut may lead to the inflammation and microbiota changes reported in Li et al. (2019). Further supporting the potential of a local eCBS disruption was the lack of body weight increases in treated adults indicating that CPF may not have reached the brain in sufficient levels to affect appetite.
KM mice treated with CPF at 1.0 mg/kg/day for 30 days had changes in the microbiota, accompanied by increased LPS in serum and liver inflammation (Zhao et al., 2016). The presence of proinflammatory cytokines in addition to dysbiosis, support the proposed pathway acting through disruptions in the eCBS (Cani et al., 2016, Forte et al., 2020, Levy et al., 2017).
The CB1R is involved with pro-oxidative stress, pro-inflammatory responses and cardiovascular disease (Rajesh et al., 2012). Oxidative stress likely contributed to inflammation, and both are linked to atherosclerotic diseases (Matthews and Ross, 2015). For example, oxidative stress (MDA increase; SOD and CAT decrease) was reported in conjunction with inflammation in liver after CPF treatment at 4.8 mg/kg/day (gavage, soya oil) in adolescent Wistar rats for 112–120 days (Uchendu et al., 2017, Uchendu et al., 2018). Notably, oxidative stress was observed in Wistar males even at 0.01 mg/kg/day CPF which is also below the point of departure determined by some regulatory agencies (Kopjar et al., 2018, CDPR, 2018). RBC SOD, plasma catalase and ferric reducing ability of plasma assay (FRAP: measures “antioxidant power”) were increased.
Wistar rats exposed to CPF at 5.0 mg/kg/day (corn oil vehicle) for 3 or 14 days showed increased MDA in aorta, liver, plasma, and kidney and after 3 days at 2.5 mg/kg/day, SOD was increased in plasma (Alvarez et al., 2008). Another study with Wistar males gavaged with CPF at 5.0 mg/kg/day (olive oil vehicle) showed increased oxidative stress on hematological parameters after 45 days of treatment (Roshanravan et al., 2020). However, Wistar males treated for 91 days at 1.3 mg/kg/day CPF (corn oil vehicle) showed no effects on oxidative stress possibly because this parameter was measured by lipid peroxidation in membranes and associated membrane fluidity, rather than on oxidative enzymes (e.g., SOD CAT), as was done is the other studies (Wang et al., 2009). On the other hand, the faster absorption of CPF at 1.3 mg/kg/day by the DMSO/Tween20/saline vehicle may have led to more rapid metabolism and elimination, since this dose was unlikely to overwhelm the system as might occur at higher doses (2.5 and 5.0 mg/kg/day) with corn oil or olive oil vehicles (Marty et al., 2007, Smith et al., 2009). The increased oxidative stress seen at 0.01 mg/kg/day CPF could also have been enhanced by the ethanol vehicle there is a reported increase in oxidative stress with ethanol alone compared to the negative control (Kopjar et al., 2018).
While there were no studies with Δ9-THC that measured effects on microbiota or oxidative stress, acting as a partial agonist/antagonist, Δ9-THC would decrease circulating eCBs through downregulation of CB1R to potentially counteract microbiota imbalance (Ceccarini et al., 2015, Villares, 2007). Mehrpouya-Bahrami et al. (2017) have shown that inhibition of the CB1 receptor, as would occur from Δ9-THC exposure, can attenuate inflammation and microbiota imbalance observed in obesity. Inflammation was measured in adult Sprague-Dawley rats at 3.0 mg/kg/day Δ9-THC and there were no effects (Coskun and Bolkent, 2014) but it would not be expected to occur at a dose below the NOEL/LOEL value of 5.0 mg/kg/day (NTP 1998).
Summary of observations
Predicted and observed effects on parameters related to dysbiosis and risk factors for T2D, obesity and related disorders were based on literature reviews and reports. CPF studies focused on obesogenic effects resulting from treatment and Δ9-THC studies focused on alleviating the effects of obesity or an obesogenic diet through Δ9-THC. These, among other factors limited data interpretation and the variability within and between age groups for CPF and Δ9-THC made direct comparisons of effects across studies challenging.
Examples of variability included:
-
•
11 different rodent species/strains
-
•
5 routes of administration
-
•
A variety of ages at treatment
-
•
Duration of treatment ranged from 4 to 175 days
-
•
Variable endpoints tested across studies using different technique
-
•
Use of a single dose rather than a range of doses and a limited dose selection with the presumed intention of getting an effect rather than determining a point of departure.
-
•
Treatment vehicles (~13)
-
•
Inter-test laboratory variability
-
•
Lack of parameters directly testing eCBS
-
•
No studies with both chemicals tested concurrently
Fig. 4 proposes a mode-of-action summary of the presumptive pathways for CPF and Δ9-THC leading to body weight effects, the most consistent parameter seen for both chemicals. The pathways are based on the known and/or related effects of each chemical on the eCBS and the observations in Table 1. Both chemical pathways include the neurodevelopmental effects associated with low birth weights and/or effects on the eCBS. Due to the variability listed above, and the fact that the two chemicals were not tested concurrently, conclusions were qualitative.
Fig. 4.
Proposed modes of action for CPF or Δ9-THC and the observations associated with those pathways in rodents (Abbreviations: eCB: endocannabinoid; LPS: lipopolysaccharide; TC: total cholesterol; TG: triglyceride) A) CPF interactions with eCBS and microbiota leading to CPF effects on body weight and accompanying neurodevelopmental effect; B) Δ9-THC effects on the eCBS leading to decreases in body weight while inducing adverse effects on neurodevelopment.
Discussion
The collective data indicated that increases in body weights/weight gains with CPF and decreases with Δ9-THC generally occurred across most age groups, independent of other variables. Organophosphates like CPF are implicated in the epidemic of obesity in the United States (Blanco et al., 2020, Meggs and Brewer, 2010, Radi and Hasni, 2014). CPF influence on many aspects of energy balance provided support for that association. Body weight effects could be attributed to CPF direct inhibition of FAAH and MAGL, leading to build up of AEA and 2-AG in the brain (Carr et al., 2020, Carr et al., 2017), over stimulation of the hypothalamus and nucleus accumbens resulting in increased hunger and motivation to eat (Di Marzo and Matias, 2005). Increased peripheral eCBs affecting a multitude of pathways, especially at low CPF doses, were likely associated with dysbiosis and inflammation, (Silvestri and Di Marzo, 2013) related to many diseases (e.g., T2D, metabolic syndrome, hypertension) (Borrelli and Izzo, 2009, Després et al., 2006).
Oxidative stress was a sensitive endpoint for CPF treatment in adolescence and adulthood at the lowest doses. It depleted antioxidants in tissues (i.e., SOD, CAT) and with depletion, increased inflammation occurred (Alvarez et al., 2008). Oxidative stress and/or dysbiosis, when tested together with CPF were accompanied by biomarkers for inflammation which can be precursors of obesity (Furukawa et al., 2017).
Chronic Δ9-THC treatment resulted in CB1R downregulation in male Wistar rat brain after treatment i.p. with 6.4 mg/kg/day (Tween80 vehicle) for 7 days (De Fonseca et al., 1994). It was also seen in human subjects by brain imaging (Hirvonen et al., 2012). Decreases in CB1R due to agonism/antagonism by Δ9-THC exposure was shown to increase the levels of beneficial microbiota that could alleviate inflammation associated with LPS, other endotoxins and oxidative stress observed in obesity and related diseases (Horváth et al., 2012, Cani et al., 2016, di Marzo and Silvestri, 2019, Dipatrizio, 2016). While studies showing Δ9-THC effects on microbiota in non-obese rodents on a standard diet were not available for the current study, Δ9-THC exposure demonstrated health-protective effects from a high fat diet, where subjects experienced T2D and/or obesity (Cluny et al., 2015, Sharkey and Wiley, 2016, Muccioli et al., 2010, Assa-Glazer et al., 2020). Dietary induced obese (DIO) male C57Bl/6N mice treated i.p. with Δ9-THC at 2.0 mg/kg/day for 6 weeks had decreased weight gain, adipose mass, and food intake, compared to DIO controls (Cluny et al., 2015). These effects were accompanied by increased beneficial gut bacteria (Akkermansia muciniphila), known to control fat storage and adipose metabolism associated with weight loss (Everard et al. 2013).
Conclusions
While there is generally control over exposure to Δ9-THC, except in the form of second-hand smoke or during development in utero, exposures to CPF are usually unknown because residues can concentrate in cannabis products (Raber et al., 2015). There are currently several data gaps of concern associated with organophosphates affecting the eCBS which need further study, including: 1) the obesogenic effects of organophosphates like CPF on critical systems like the gut-microbiota-adipose-brain regulatory loop and appetite; 2) effects of pesticides on the eCBS during all developmental stages; and 3) MOA for co-exposures of cannabis and CPF on energy balance, obesity, and associated diseases. A useful approach could provide a standardized battery of in vivo and in vitro tests to determine a tipping point for adverse health effects or a physiologically based pharmacokinetic model to characterize co-exposures to CPF and Δ9THC. Data suggest that the concern about CPF residues on cannabis might be alleviated because the MOAs for CPF and Δ9THC could counteract each other and many health protective advantages of cannabis have been reported. However, the answers to these concerns are complex and would depend on working through the numerous variables mentioned above.
Even though cannabis could counter act adverse effects from CPF related to energy balance, it cannot be overlooked that both chemicals are neurodevelopmental toxicants (Leung et al., 2019, Silva, 2020, Fride et al., 2009, Fried and Smith, 2001). This is a research area that deserves further study. It is hopeful that these concerns will be addressed, and regulations will be considered.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
I, Marilyn H. Silva, Ph.D., DABT am the sole author of this work. I initiated the concept, curated the data, analyzed the data, and was not funded for the work. I am currently retired and now I can work on projects that are of interest to me and hopefully to the scientific community as well. I performed the scientific investigations, used open access methodologies, and wrote the paper etc. I have no co-authors.
CRediT authorship contribution statement
Marilyn H. Silva: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
I would like to thank Ms. Ruthie H. Musker, M.S. for her help in designing the manuscript images and W. Kenneth Musker, Ph.D. for his help reviewing this article.
Footnotes
Abbreviations: AEA: anandamide; 2-AG: 2-arachidonoylglycerol CPF: chlorpyrifos; endocannabinoids (eCB); eCBS: eCB system; FAAH: fatty acid amide hydrolase; HDL: high density lipoproteins; i.p.: intraperitoneal; i.v.: intravenous; LDL/VDL: low or very low-density lipoproteins; LOEL: lowest observed effect level; MAGL: monoacylglycerol lipase; PND: post-natal day; s.c.: subcutaneous; Δ9THC: Δ9Tetrahydrocannabinol; TC: total cholesterol; TG: triglycerides
Metabolic syndrome is characterized by high blood pressure, insulin resistance, increased glucose, triglycerides and LDL, large waste circumference and potentially also obesity GRUNDY, S. M. 2016. Metabolic syndrome update. Trends in Cardiovascular Medicine, 26, 364–373.
Chinese Kun Ming (KM) mouse strain most closely genetically related to BALB/C and C57BL/6 strains ZHANG, X., ZHU, Z., HUANG, Z., TAN, P. & MA, R. Z. 2007. Microsatellite Genotyping for Four Expected Inbred Mouse Strains from KM Mice. Journal of Genetics and Genomics, 34, 214–222..
Rat adolescence is initiated approximately PND 28–42; SPEAR, L. P. 2000. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews, 24, 417–463, ibid., ibid..
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2021.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Acker C.I., Nogueira C.W. Chlorpyrifos acute exposure induces hyperglycemia and hyperlipidemia in rats. Chemosphere. 2012;89:602–608. doi: 10.1016/j.chemosphere.2012.05.059. [DOI] [PubMed] [Google Scholar]
- Acosta O., Ramirez V.I., Lager S., Gaccioli F., Dudley D.J., Powell T.L., Jansson T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am. J. Obstet. Gynecol. 2015;212:227.e1–227.e7. doi: 10.1016/j.ajog.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Akhtar N., Srivastava M.K., Raizada R.B. Assessment of chlorpyrifos toxicity on certain organs in rat, Rattus norvegicus. J Environ Biol. 2009;30:1047–1053. [PubMed] [Google Scholar]
- Alexander, B. T., Henry Dasinger, J. & Intapad, S. 2014. Effect of Low Birth Weight on Women’s Health. Clin. Therapeut., 36, 1913-1923. [DOI] [PMC free article] [PubMed]
- Allegaert K., van den Anker J. Ontogeny of Phase I Metabolism of Drugs. J. Clin. Pharmacol. 2019;59:S33–S41. doi: 10.1002/jcph.1483. [DOI] [PubMed] [Google Scholar]
- Alshaarawy O., Anthony J.C. Are cannabis users less likely to gain weight? Results from a national 3-year prospective study. Int. J. Epidemiol. 2019;48:1695–1700. doi: 10.1093/ije/dyz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A.A., Ramirez-Sanjuan E., Canizalez-Roman A. Chlorpyrifos induces oxidative stress in rats. Toxicol. Environ. Chem. 2008;90:1019–1025. [Google Scholar]
- Ambali S., Shittu M., Ayo J., Esievo K., Ojo S. Vitamin C alleviates chronic chlorpyrifos induced alterations in serum lipids and oxidative parameters in male wistar rats. Am. J. Pharmacol. Toxicol. 2011;6:109–118. [Google Scholar]
- Assa-Glazer T., Gorelick J., Sela N., Nyska A., Bernstein N., Madar Z. Cannabis Extracts Affected Metabolic Syndrome Parameters in Mice Fed High-Fat/Cholesterol Diet. Cannabis Cannabinoid Res. 2020;5:202–214. doi: 10.1089/can.2020.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, N. C., Knudsen, T. & Williams, A. L. 2017. Abstract Sifter: a comprehensive front-end system to PubMed. F1000Research, 6, Chem Inf Sci-2164. [DOI] [PMC free article] [PubMed]
- Bellocchio L., Cervino C., Pasquali R., Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008;20:850–857. doi: 10.1111/j.1365-2826.2008.01728.x. [DOI] [PubMed] [Google Scholar]
- Bellocchio L., Lafenêtre P., Cannich A., Cota D., Puente N., Grandes P., Chaouloff F., Piazza P.V., Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- BERGHUIS, P. 2005. Brain-derived neurotrophic factor and endocannabinoid functions in GABAergic interneuron development. Karolinska Instituet, Stockholm 2007.
- Berghuis P., Rajnicek A.M., Morozov Y.M., Ross R.A., Mulder J., Urbán G.M., Monory K., Marsicano G., Matteoli M., Canty A. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- Bernasconi Camilla, Pelkonen Olavi, Andersson Tommy B., Strickland Judy, Wilk-Zasadna Iwona, Asturiol David, Cole Thomas, Liska Roman, Worth Andrew, Müller-Vieira Ursula, Richert Lysiane, Chesne Christophe, Coecke Sandra. Validation of in vitro methods for human cytochrome P450 enzyme induction: Outcome of a multi-laboratory study. Toxicol. In Vitro. 2019;60:212–228. doi: 10.1016/j.tiv.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydogan A.B., Coskun Z.M., Bolkent S. The protective effects of Δ9-tetrahydrocannabinol against inflammation and oxidative stress in rat liver with fructose-induced hyperinsulinemia. J. Pharm. Pharmacol. 2019;71:408–416. doi: 10.1111/jphp.13042. [DOI] [PubMed] [Google Scholar]
- Blanco J., Guardia-Escote L., Mulero M., Basaure P., Biosca-Brull J., Cabré M., Colomina M.T., Domingo J.L., Sánchez D.J. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food Chem. Toxicol. 2020;137 doi: 10.1016/j.fct.2020.111171. [DOI] [PubMed] [Google Scholar]
- Borrelli F., Izzo A.A. Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance. Best Practice Res. Clin. Endocrinol. Metabol. 2009;23:33–49. doi: 10.1016/j.beem.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Buntyn R.W., Alugubelly N., Hybart R.L., Mohammed A.N., Nail C.A., Parker G.C., Ross M.K., Carr R.L. Inhibition of Endocannabinoid-Metabolizing Enzymes in Peripheral Tissues Following Developmental Chlorpyrifos Exposure in Rats. Int. J. Toxicol. 2017;36:395–402. doi: 10.1177/1091581817725272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J., Lim K.O., Kumra S. Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb. Cortex. 2017;27:1922–1930. doi: 10.1093/cercor/bhw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P., Trezza V., Ratano P., Palmery M., Cuomo V. Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology. 2011;214:5–15. doi: 10.1007/s00213-010-1892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., Neyrinck, A. M., Fava, F., Tuohy, K. M., Chabo, C., Waget, A., Delmée, E., Cousin, B., Sulpice, T., Chamontin, B., Ferrières, J., Tanti, J., Gibson, G. R., Casteilla, L., Delzenne, N. M., Alessi, M. C. & Burcelin, R. 2007. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes, 56, 1761. [DOI] [PubMed]
- Cani, P. D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A. M., Delzenne, N. M. & Burcelin, R. 2008. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes, 57, 1470-1481. [DOI] [PubMed]
- Cani P.D., Geurts L., Matamoros S., Plovier H., Duparc T. Glucose metabolism: Focus on gut microbiota, the endocannabinoid system and beyond. Diabet. Metabol. 2014;40:246–257. doi: 10.1016/j.diabet.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Plovier H., van Hul M., Geurts L., Delzenne N.M., Druart C., Everard A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016;12:133. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- Carr Russell L., Alugubelly Navatha, de Leon Kathryne, Loyant Louise, Mohammed Afzaal N., Patterson M. Elizabeth, Ross Matthew K., Rowbotham Nicole E. Inhibition of fatty acid amide hydrolase by chlorpyrifos in juvenile rats results in altered exploratory and social behavior as adolescents. NeuroToxicol. 2020;77:127–136. doi: 10.1016/j.neuro.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R.L., Armstrong N.H., Buchanan A.T., Eells J.B., Mohammed A.N., Ross M.K., Nail C.A. Decreased anxiety in juvenile rats following exposure to low levels of chlorpyrifos during development. NeuroTox. 2017;59:183–190. doi: 10.1016/j.neuro.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida, J. E. 2017. Organophosphorus Xenobiotic Toxicology. Annu. Rev. Pharmacol. Toxicol. 57, 309-327. [DOI] [PubMed]
- Castaner, O., Goday, A., Park, Y.-M., Lee, S.-H., Magkos, F., Shiow, S.-A., E., T. & Schröder, H. 2018. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol., 2018, 4095789. [DOI] [PMC free article] [PubMed]
- CDPR 2018. Evaluation of Chlorpyrifos as a Toxic Air Contaminant: Addendum to the Risk Characterization of Spray Drift, Dietary, and Aggregate Exposures to Residential Bystanders Human. Human Health Assessment Branch, Department of Pesticide Regulation, California Environmental Protection Agency, Sacramento, CA, http://www.cdpr.ca.gov/docs/whs/active_ingredient/chlorpyrifos.htm.
- Ceccarini J., Kuepper R., Kemels D., van Os J., Henquet C., van Laere K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict. Biol. 2015;20:357–367. doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- Chugani, H. T. 1998. A Critical Period of Brain Development: Studies of Cerebral Glucose Utilization with PET. Prevent. Med., 27, 184-188. [DOI] [PubMed]
- Clancy B., Darlington R.B., Finlay B.L. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Cluny N.L., Keenan C.M., Reimer R.A., le Foll B., Sharkey K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Delta9-Tetrahydrocannabinol. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORLEY, R. A., LANDRY, T. D., CALHOUN, L. L., DITTENBER, D. A. & LOMAX, L. G. 1986. Chlorpyrifos: 13-week nose-only vapor inhalation exposure study in Fischer 344 rats. Dow Chemical Company, Midland, MI, Study #HET K-044793-077, DPR Vol. 342-0343 #071389
- Coskun Z.M., Bolkent S. Biochemical and immunohistochemical changes in delta-9-tetrahydrocannabinol-treated type 2 diabetic rats. Acta Histochem. 2014;116:112–116. doi: 10.1016/j.acthis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- de Fonseca F.R., Gorriti M., Fernandez-Ruiz J., Palomo T., Ramos J. Downregulation of rat brain cannabinoid binding sites after chronic Δ9-tetrahydrocannabinol treatment. Pharmacol. Biochem. Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Després J.P., Lemieux I., Alméras N. Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int. J. Obesity. 2006;30:S44–S52. doi: 10.1038/sj.ijo.0803278. [DOI] [PubMed] [Google Scholar]
- Di Marzo, V. 2008a. CB(1) receptor antagonism: biological basis for metabolic effects. Drug Discov Today, 13, 1026-41. [DOI] [PubMed]
- Di Marzo, V. 2008b. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia, 51, 1356. [DOI] [PubMed]
- Di Marzo, V. 2011. Endocannabinoids: An appetite for fat. PNAS, 108, 12567-12568. [DOI] [PMC free article] [PubMed]
- di Marzo V., Bifulco M., de Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discovery. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- di Marzo V., Matias I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- DI MARZO, V., PISCITELLI, F. & MECHOULAM, R. 2011. Cannabinoids and Endocannabinoids in Metabolic Disorders with Focus on Diabetes. In: SCHWANSTECHER, M. (ed.) Diabetes - Perspectives in Drug Therapy. Berlin, Heidelberg: Springer Berlin Heidelberg. [DOI] [PubMed]
- di Marzo V., Silvestri C. Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome. Nutrients. 2019;11:1956–1970. doi: 10.3390/nu11081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIPATRIZIO, N. V. 2016. Endocannabinoids in the Gut. Cannabis and cannabinoid research, 1, 67-77. [DOI] [PMC free article] [PubMed]
- Dow-Edwards D., Zhao N. Oral THC produces minimal behavioral alterations in preadolescent rats. Neurotoxicol. Teratol. 2008;30:385–389. doi: 10.1016/j.ntt.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D.L., Daroff R.B., Autrup H., Bridges J., Buffler P., Costa L.G., Coyle J., McKhann G., Mobley W.C., Nadel L., Neubert D., Schulte-Hermann R., Spencer P.S. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- EFSA Conclusion on the peer review of the pesticide human health risk assessment of the active substance chlorpyrifos. Eur. Food Safety Author. J. 2014;12:3640–3674. [Google Scholar]
- EFSA EFSA Panel on Contaminants in the Food Chain: Scientific opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA J. 2015;13:4141. [Google Scholar]
- Elmazoudy R.H., Attia A.A., El-Shenawy N.S. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic. Biochem. Physiol. 2011;101:175–181. [Google Scholar]
- Elsharkawy E.E., Yahia D., El-Nisr N.A. Sub-chronic exposure to chlorpyrifos induces hematological, metabolic disorders and oxidative stress in rat: attenuation by glutathione. Environ. Toxicol. Pharmacol. 2013;35:218–227. doi: 10.1016/j.etap.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Eriksen M.B., Frandsen T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J. Med. Library Associat.: JMLA. 2018;106:420. doi: 10.5195/jmla.2018.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B., Li J.W., Zhang M., Ren F.Z., Pang G.F. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem. Toxicol. 2018;111:144–152. doi: 10.1016/j.fct.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Farokhnia M., McDiarmid G.R., Newmeyer M.N., Munjal V., Abulseoud O.A., Huestis M.A., Leggio L. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study. Transl. Psychiatry. 2020;10:1–11. doi: 10.1038/s41398-020-0756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte N., Fernández-Rilo A.C., Palomba L., di Marzo V., Cristino L. Obesity affects the microbiota–gut–brain axis and the regulation thereof by endocannabinoids and related mediators. Int. J. Mol. Sci. 2020;21:1554. doi: 10.3390/ijms21051554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDE, E. 2004. The endocannabinoid-CB1 receptor system in pre- and postnatal life. Eur. J. Pharmacol., 500, 289-297. [DOI] [PubMed]
- FRIDE, E. 2008. Multiple Roles for the Endocannabinoid System During the Earliest Stages of Life: Pre- and Postnatal Development. J. Neuroendocrinol., 20, 75-81. [DOI] [PubMed]
- Fride E., Bregman T., Kirkham T.C. Endocannabinoids and Food Intake: Newborn Suckling and Appetite Regulation in Adulthood. Exp. Biol. Med. 2005;230:225–234. doi: 10.1177/153537020523000401. [DOI] [PubMed] [Google Scholar]
- Fride E., Gobshtis N., Dahan H., Weller A., Giuffrida A., Ben-Shabat S. Academic Press; 2009. Chapter 6 The Endocannabinoid System During Development: Emphasis on Perinatal Events and Delayed Effects. Vitamins & Hormones. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Smith A.M. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperi V., Fezza F., Pasquariello N., Bari M., Oddi S., Agrò A.F., Maccarrone M. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell. Mol. Life Sci. 2007;64:219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R., Lee K., Vanin S., Laviolette S.R., Holloway A.C., Arany E., Hardy D.B. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod Toxicol. 2020;94:84–91. doi: 10.1016/j.reprotox.2020.04.070. [DOI] [PubMed] [Google Scholar]
- Ginsberg G., Hattis D., Sonawane B. Incorporating pharmacokinetic differences between children and adults in assessing children's risks to environmental toxicants. Toxicol. Appl. Pharmacol. 2004;198:164–183. doi: 10.1016/j.taap.2003.10.010. [DOI] [PubMed] [Google Scholar]
- GINSBERG , G., NEAFSEY , P., HATTIS , D., GUYTON , K. Z., JOHNS, D. O. & SONAWANE, B. 2009. Genetic Polymorphism in Paraoxonase 1 (PON1): Population Distribution of PON1 Activity. J. Toxicol. Environ. Health, Part B, ISSN: 1093-7404 (Print) 1521-6950. [DOI] [PubMed]
- Ginsberg G., Vulimiri S.V., Lin Y.-S., Kancherla J., Foos B., Sonawane B. A framework and case studies for evaluation of enzyme ontogeny in children’s health risk evaluation. J. Toxicol. Environ. Health, Part A. 2017;80:569–593. doi: 10.1080/15287394.2017.1369915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P., Mason H., Heywood K., Cocker J. Oral and dermal absorption of chlorpyrifos: a human volunteer study. Occup Environ Med. 1999;56:10–13. doi: 10.1136/oem.56.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Elbracht C. Effect of tetrahydrocannabinols on pubertal body weight spurt and sex hormones in developing male rats. Res. Exp. Med. 1983;182:95–104. doi: 10.1007/BF01851115. [DOI] [PubMed] [Google Scholar]
- Hewitt N.J., Lecluyse E.L., Ferguson S.S. Induction of hepatic cytochrome P450 enzymes: methods, mechanisms, recommendations, and in vitro–in vivo correlations. Xenobiotica. 2007;37:1196–1224. doi: 10.1080/00498250701534893. [DOI] [PubMed] [Google Scholar]
- Hirvonen J., Goodwin R.S., Li C.T., Terry G.E., Zoghbi S.S., Morse C., Pike V.W., Volkow N.D., Huestis M.A., Innis R.B. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth B., Mukhopadhyay P., Haskó G., Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012;180:432–442. doi: 10.1016/j.ajpath.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec U.T., Turner R.T. Intraperitoneal injection of ethanol results in drastic changes in bone metabolism not observed when ethanol is administered by oral gavage. Alcohol. Clin. Exp. Res. 2013;37:1271–1277. doi: 10.1111/acer.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, Y.-H., Chen, Y.-J., Chua, S. C., JR., Talmage, D. A. & Role, L. W. 2005. Integration of Endocannabinoid and Leptin Signaling in an Appetite-Related Neural Circuit. Neuron, 48, 1055-1066. [DOI] [PMC free article] [PubMed]
- Joly C., Gay-Quéheillard J., Léké A., Chardon K., Delanaud S., Bach V., Khorsi-Cauet H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and in the rat. Environ. Sci. Pollut. Res. 2013;20:2726–2734. doi: 10.1007/s11356-012-1283-4. [DOI] [PubMed] [Google Scholar]
- Joly Condette, C., Khorsi-Cauet, H., Morlière, P., Zabijak, L., Reygner, J., Bach, V. & Gay-Quéheillard, J. 2014. Increased Gut Permeability and Bacterial Translocation after Chronic Chlorpyrifos Exposure in Rats. PLOS ONE, 9, e102217. [DOI] [PMC free article] [PubMed]
- Karlsson F.H., Tremaroli V., Nookaew I., Bergström G., Behre C.J., Fagerberg B., Nielsen J., Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- KASTEN, C. R., ZHANG, Y. & BOEHM, S. L., 2ND 2017. Acute and long-term effects of Delta9-tetrahydrocannabinol on object recognition and anxiety-like activity are age- and strain-dependent in mice. Pharmacol. Biochem. Behav, 163, 9-19. [DOI] [PMC free article] [PubMed]
- Keeley R.J., Trow J., McDonald R.J. Strain and sex differences in puberty onset and the effects of THC administration on weight gain and brain volumes. Neuroscience. 2015;305:328–342. doi: 10.1016/j.neuroscience.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Knapp A.A., Lee D.C., Borodovsky J.T., Auty S.G., Gabrielli J., Budney A.J. Emerging Trends in Cannabis Administration Among Adolescent Cannabis Users. J. Adolesc. Health. 2019;64:487–493. doi: 10.1016/j.jadohealth.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopjar Nevenka, Žunec Suzana, Mendaš Gordana, Micek Vedran, Kašuba Vilena, Mikolić Anja, Lovaković Blanka Tariba, Milić Mirta, Pavičić Ivan, Čermak Ana Marija Marjanović, Pizent Alica, Lucić Vrdoljak Ana, Želježić Davor. Evaluation of chlorpyrifos toxicity through a 28-day study: Cholinesterase activity, oxidative stress responses, parent compound/metabolite levels, and primary DNA damage in blood and brain tissue of adult male Wistar rats. Chem. Biol. Interact. 2018;279:51–63. doi: 10.1016/j.cbi.2017.10.029. [DOI] [PubMed] [Google Scholar]
- Krishnan K., Mitra N.K., Yee L.S., Yang H.M. A comparison of neurotoxicity in cerebellum produced by dermal application of chlorpyrifos in young and adult mice. J. Neural Transm. 2012;119:345–352. doi: 10.1007/s00702-011-0715-5. [DOI] [PubMed] [Google Scholar]
- Lassiter T.L., Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125–130. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Leung M.C.K., Silva M.H., Palumbo A.J., Lohstroh P.N., Koshlukova S.E., Duteaux S.B. Adverse outcome pathway of developmental neurotoxicity resulting from prenatal exposures to cannabis contaminated with organophosphate pesticide residues. Reprod. Toxicol. 2019;85:12–18. doi: 10.1016/j.reprotox.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- Li J., Fang B., Pang G.-F., Zhang M., Ren F.-Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 2019;159:68–79. doi: 10.1016/j.pestbp.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Liang, Y., Zhan, J., Liu, D. D., Luo, M., Han, J., Liu, X., Liu, C., Cheng, Z., Zhou, Z. & Wang, P. 2019. Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome, 7, 19-19. [DOI] [PMC free article] [PubMed]
- Marty M.S., Andrus A.K., Bell M.P., Passage J.K., Perala A.W., Brzak K.A., Bartels M.J., Beck M.J., Juberg D.R. Cholinesterase inhibition and toxicokinetics in immature and adult rats after acute or repeated exposures to chlorpyrifos or chlorpyrifos-oxon. Regul. Toxicol. Pharmacol. 2012;63:209–224. doi: 10.1016/j.yrtph.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Marty, M. S., Domoradzki, J. Y., Hansen, S. C., Timchalk, C., Bartels, M. J. & Mattsson, J. L. 2007. The effect of route, vehicle, and divided doses on the pharmacokinetics of chlorpyrifos and its metabolite trichloropyridinol in neonatal Sprague-Dawley rats. Toxicol Sci, 100, 360-73. [DOI] [PubMed]
- Matias I., di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol. Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Mato S., del Olmo E., Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur. J. Neurosci. 2003;17:1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- Matthews A.T., Ross M.K. Oxyradical Stress, Endocannabinoids, and Atherosclerosis. Toxics. 2015;3:481–498. doi: 10.3390/toxics3040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J.L., Maurissen J.P., Nolan R.J., Brzak K.A. Lack of differential sensitivity to cholinesterase inhibition in fetuses and neonates compared to dams treated perinatally with chlorpyrifos. Toxicol Sci. 2000;53:438–446. doi: 10.1093/toxsci/53.2.438. [DOI] [PubMed] [Google Scholar]
- Medina-Cleghorn D., Heslin A., Morris P.J., Mulvihill M.M., Nomura D.K. Multidimensional profiling platforms reveal metabolic dysregulation caused by organophosphorus pesticides. ACS Chem Biol. 2014;9:423–432. doi: 10.1021/cb400796c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggs W.J., Brewer K.L. Weight gain associated with chronic exposure to chlorpyrifos in rats. J. Med. Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggs, W. J. & Brewer, K. L. 2010. Toxicant exposures and the obesity epidemic. J. Med. Toxicol., 6, 275-275. [DOI] [PMC free article] [PubMed]
- Mehrpouya-Bahrami P., Chitrala K.N., Ganewatta M.S., Tang C., Murphy E.A., Enos R.T., Velazquez K.T., McCellan J., Nagarkatti M., Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017;7:15645. doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.N., Alugubelly N., Kaplan B.L., Carr R.L. Effect of repeated juvenile exposure to Δ9-tetrahydrocannabinol on anxiety-related behavior and social interactions in adolescent rats. Neurotoxicol. Teratol. 2018;69:11–20. doi: 10.1016/j.ntt.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Motaghedi R., McGraw T.E. The CB1 endocannabinoid system modulates adipocyte insulin sensitivity. Obesity (Silver Spring, Md.) 2008;16:1727–1734. doi: 10.1038/oby.2008.309. [DOI] [PubMed] [Google Scholar]
- Muccioli G.G., Naslain D., Backhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale B.V., Gustin K.N., Lee K., Holloway A.C., Laviolette S.R., Natale D.R.C., Hardy D.B. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 2020;10:544. doi: 10.1038/s41598-019-57318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom R.J., Kelly S.J. Perinatal delta-9-tetrahydrocannabinol exposure disrupts social and open field behavior in adult male rats. Neurotoxicol Teratol. 2008;30:213–219. doi: 10.1016/j.ntt.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J.D., Aarde S.M., Vandewater S.A., Grant Y., Stouffer D.G., Parsons L.H., Cole M., Taffe M.A. Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology. 2016;109:112–120. doi: 10.1016/j.neuropharm.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J.D., Creehan K.M., Grant Y., Vandewater S.A., Kerr T.M., Taffe M.A. Explication of CB1 receptor contributions to the hypothermic effects of Δ9-tetrahydrocannabinol (THC) when delivered by vapor inhalation or parenteral injection in rats. Drug Alcohol Depend. 2020;214 doi: 10.1016/j.drugalcdep.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN, J. D., CREEHAN, K. M., KERR, T. M. & TAFFE, M. A. 2020b. Lasting effects of repeated Δ(9) -tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharmacol, 177, 188-203. [DOI] [PMC free article] [PubMed]
- NOLAN, R. J., RICK, D. L., FRESHOUR, N. L. & SAUNDERS, J. H. 1984. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicology and Applied Pharmacology 73:8-15, DPR Vol. 342-0343 # 071383 [DOI] [PubMed]
- NTP 1998. NTP Technical Report on the Toxicology and Carcinogenesis Studies of 1-Trans-Delta9-Tetrahydrocannabinol (CAS NO. 1972-08-3) in F344/N Rats and B6C3Fl Mice (Gavage Studies),. National Toxicology Program, NTP TR 466, http://ntp.niehs.nih.gov/ntp/hdocs/ft_rpts/tr446.pdf. [PubMed]
- O'Shea M., Mallet P.E. Impaired learning in adulthood following neonatal delta9-THC exposure. Behav Pharmacol. 2005;16:455–461. doi: 10.1097/00008877-200509000-00019. [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez Cristian, Morales-Navas Miguel, Aguilera-Sáez Luis Manuel, Abreu Ana Cristina, Guardia-Escote Laia, Fernández Ignacio, Garrido-Cárdenas José Antonio, Colomina María Teresa, Giménez Estela, Sánchez-Santed Fernando. Medium and long-term effects of low doses of Chlorpyrifos during the postnatal, preweaning developmental stage on sociability, dominance, gut microbiota and plasma metabolites. Environ. Res. 2020;184:109341. doi: 10.1016/j.envres.2020.109341. [DOI] [PubMed] [Google Scholar]
- Perez-Fernandez C., Morales-Navas M., Guardia-Escote L., Garrido-Cárdenas J.A., Colomina M.T., Giménez E., Sánchez-Santed F. Long-term effects of low doses of Chlorpyrifos exposure at the preweaning developmental stage: A locomotor, pharmacological, brain gene expression and gut microbiome analysis. Food Chem. Toxicol. 2020;135 doi: 10.1016/j.fct.2019.110865. [DOI] [PubMed] [Google Scholar]
- Peris-Sampedro Fiona, Basaure Pia, Reverte Ingrid, Cabré Maria, Domingo José L., Colomina Maria Teresa. Chronic exposure to chlorpyrifos triggered body weight increase and memory impairment depending on human apoE polymorphisms in a targeted replacement mouse model. Physiol Behav. 2015;144:37–45. doi: 10.1016/j.physbeh.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Peris-Sampedro F., Cabré M., Basaure P., Reverte I., Domingo J.L., Colomina M.T. Adulthood dietary exposure to a common pesticide leads to an obese-like phenotype and a diabetic profile in apoE3 mice. Environ. Res. 2015;142:169–176. doi: 10.1016/j.envres.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Pilin M.A., Robinson J.M., Dow-Fleisner S., Sanchez T.A., Krank M.D. Automatic cognitions as mediators of parental influence on adolescent cannabis use. Addict. Behav. 2021;114 doi: 10.1016/j.addbeh.2020.106728. [DOI] [PubMed] [Google Scholar]
- Qian Y., Gurley B.J., Markowitz J.S. The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications. J. Clin. Psychopharmacol. 2019;39:462–471. doi: 10.1097/JCP.0000000000001089. [DOI] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., Peng Y., Zhang D., Jie Z., Wu W., Qin Y., Xue W., Li J., Han L., Lu D., Wu P., Dai Y., Sun X., Li Z., Tang A., Zhong S., Li X., Chen W., Xu R., Wang M., Feng Q., Gong M., Yu J., Zhang Y., Zhang M., Hansen T., Sanchez G., Raes J., Falony G., Okuda S., Almeida M., Lechatelier E., Renault P., Pons N., Batto J.-M., Zhang Z., Chen H., Yang R., Zheng W., Li S., Yang H., Wang J., Ehrlich S.D., Nielsen R., Pedersen O., Kristiansen K., Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- QUARTA, C., BELLOCCHIO, L., MANCINI, G., MAZZA, R., CERVINO, C., BRAULKE, L. J., FEKETE, C., LATORRE, R., NANNI, C., BUCCI, M., CLEMENS, L. E., HELDMAIER, G., WATANABE, M., LESTE-LASSERE, T., MAITRE, M., TEDESCO, L., FANELLI, F., REUSS, KLAUS, S., SRIVASTAVA, R. K., MONORY, K., VALERIO, A., GRANDIS, A., GIORGIO, R., PASQUALI, R., NISOLI, E., COTA, D., LUTZ, B., MARSICANO, G. & PAGOTTO, U. 2010. CB1 Signaling in Forebrain and Sympathetic Neurons Is a Key Determinant of Endocannabinoid Actions on Energy Balance. Cell Metabolism, 11, 273-285. [DOI] [PubMed]
- Raber J.C., Elzinga S., Kaplan C. Understanding dabs: contamination concerns of cannabis concentrates and cannabinoid transfer during the act of dabbing. The Journal of Toxicological Sciences. 2015;40:797–803. doi: 10.2131/jts.40.797. [DOI] [PubMed] [Google Scholar]
- Radi F., Hasni M. Obesogens as an environmental risk factor for obesity. Malaysian Journal of Public Health Medicine. 2014;14:63–70. [Google Scholar]
- Rahminiwati M., Nishimura M. Effects of δ9-Tetrahydrocannabinol and Diazepam on Feeding Behavior in Mice. J. Vet. Med. Sci. 1999;61:351–355. doi: 10.1292/jvms.61.351. [DOI] [PubMed] [Google Scholar]
- Rajesh M., Bátkai S., Kechrid M., Mukhopadhyay P., Lee W.-S., Horváth B., Holovac E., Cinar R., Liaudet L., Mackie K. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygner Julie, Lichtenberger Lydia, Elmhiri Ghada, Dou Samir, Bahi-Jaber Narges, Rhazi Larbi, Depeint Flore, Bach Veronique, Khorsi-Cauet Hafida, Abdennebi-Najar Latifa, Smidt Hauke. Inulin Supplementation Lowered the Metabolic Defects of Prolonged Exposure to Chlorpyrifos from Gestation to Young Adult Stage in Offspring Rats. PLoS ONE. 2016;11(10):e0164614. doi: 10.1371/journal.pone.0164614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Mele M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanravan B., Mehrpour O., Ashrafizadeh M., Yazdanfar N., Sadighara P., Farkhondeh T., Samarghandian S. Age-dependent effect of chlorpyrifos on the hematological parameters in male rats. Toxin Reviews. 2020 [Google Scholar]
- Rubino T., Realini N., Braida D., Guidi S., Capurro V., Viganò D., Guidali C., Pinter M., Sala M., Bartesaghi R. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sadler N.C., Nandhikonda P., Webb-Robertson B.-J., Ansong C., Anderson L.N., Smith J.N., Corley R.A., Wright A.T. Hepatic Cytochrome P450 Activity, Abundance, and Expression Throughout Human Development. Drug Metab. Dispos. 2016;44:984. doi: 10.1124/dmd.115.068593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili K.S., Antonijevic T., Zurlinden T.J., Shah I., Deisenroth C., Knudsen T.B. Molecular characterization of a toxicological tipping point during human stem cell differentiation. Reprod. Toxicol. 2020;91:1–13. doi: 10.1016/j.reprotox.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler L.N., Beckerman J.L., Whitford F., Gibson K.A. Cannabis as conundrum. Crop Prot. 2019;117:37–44. [Google Scholar]
- Santos C.M.D.C., Pimenta C.A.D.M., Nobre M.R.C. The PICO strategy for the research question construction and evidence search. Revista latino-americana de enfermagem. 2007;15:508–511. doi: 10.1590/s0104-11692007000300023. [DOI] [PubMed] [Google Scholar]
- Schauer G.L., King B.A., Bunnell R.E., Promoff G., McAfee T.A. Toking, Vaping, and Eating for Health or Fun: Marijuana Use Patterns in Adults, U.S., 2014. Am. J. Prev. Med. 2016;50:1–8. doi: 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Schieve L.A., Tian L.H., Rankin K., Kogan M.D., Yeargin-Allsopp M., Visser S., Rosenberg D. Population impact of preterm birth and low birth weight on developmental disabilities in US children. Ann. Epidemiol. 2016;26:267–274. doi: 10.1016/j.annepidem.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M., Cuttler C., Finnell J.S., Mischley L.K. A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis Cannabinoid Res. 2016;1:131–138. doi: 10.1089/can.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I., Setzer R.W., Jack J., Houck K.A., Judson R.S., Knudsen T.B., Liu J., Martin M.T., Reif D.M., Richard A.M., Thomas R.S., Crofton K.M., Dix D.J., Kavlock R.J. Using ToxCast™ Data to Reconstruct Dynamic Cell State Trajectories and Estimate Toxicological Points of Departure. Environ. Health Perspect. 2016;124:910–919. doi: 10.1289/ehp.1409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey K.A., Wiley J.W. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology. 2016;151:252–266. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiplo S., Asbridge M., Leatherdale S.T., Hammond D. Medical cannabis use in Canada: vapourization and modes of delivery. Harm Reduction Journal. 2016;13:30. doi: 10.1186/s12954-016-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA, M. H. 2020. Effects of low-dose chlorpyrifos on neurobehavior and potential mechanisms: A review of studies in rodents, zebrafish, and Caenorhabditis elegans. Birth Defects Research, 112, 445-479. [DOI] [PubMed]
- Silvestri C., di Marzo V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Slotkin T.A., Brown K.K., Seidler F.J. Developmental Exposure of Rats to Chlorpyrifos Elicits Sex-Selective Hyperlipidemia and Hyperinsulinemia in Adulthood. Environ Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit E., Crespo C.J. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public Health Nutr. 2001;4:781–786. doi: 10.1079/phn2000114. [DOI] [PubMed] [Google Scholar]
- Smith J.N., Campbell J.A., Busby-Hjerpe A., Lee S., Poet T.S., Barr D.B., Timchalk C. Comparative chlorpyrifos pharmacokinetics via multiple routes of exposure and vehicles of administration in the adult rat. Toxicology. 2009;261:47–58. doi: 10.1016/j.tox.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Taffe, M. A., Creehan, K. M., Vandewater, S. A., Kerr, T. M. & Cole, M. 2020. Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Experimental and clinical psychopharmacology. [DOI] [PMC free article] [PubMed]
- Tanaka, E. 1998. In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J. Clin. Pharm. Therapeutics, 23, 247-255. [DOI] [PubMed]
- Testai, E., Buratti, F. M. & Di Consiglio, E. 2010. Chapter 70: Chlorpyrifos, Hayes’ Handbook of Pesticide Toxicology Elsevier Inc., United States of America and United Kingdom, Academic Press (Elsevier)
- Uchendu C., Ambali S.F., Ayo J.O., Esievo K.A.N. The protective role of alpha-lipoic acid on long-term exposure of rats to the combination of chlorpyrifos and deltamethrin pesticides. Toxicol Ind Health. 2017;33:159–170. doi: 10.1177/0748233715616553. [DOI] [PubMed] [Google Scholar]
- Uchendu C., Ambali S.F., Ayo J.O., Esievo K.A.N. Chronic co-exposure to chlorpyrifos and deltamethrin pesticides induces alterations in serum lipids and oxidative stress in Wistar rats: mitigating role of alpha-lipoic acid. Environ. Sci. Pollut. Res. 2018;25:19605–19611. doi: 10.1007/s11356-018-2185-x. [DOI] [PubMed] [Google Scholar]
- Upreti Vijay V., Wahlstrom Jan L. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2016;56(3):266–283. doi: 10.1002/jcph.585. [DOI] [PubMed] [Google Scholar]
- US EPA 1998. Health effect Test Guidelines (OPPS 870). Prevention, Pesticides and Toxic Substances (7101), U.S. Environmental Protection Agency, Washington, DC (https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-870-health-effects-test-guidelines; accessed 3/2020).
- US EPA 2011. Chlorpyrifos: Preliminary Human Health Risk Assessment for Registration Review. Office of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency, Washington D.C., https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0850-0025 (accessed 3-2020), 1-159.
- US EPA 2014. Chlorpyrifos: Revised Human Health Risk Assessment for Registration Review. Office of Chemical Safety and Pollution Prevention, United States Environmental Protection Agency, Washington, DC (EPA-HQ-OPP-2008-0850-0195, December 29, 2014; https://oehha.ca.gov/media/downloads/crnr/usepachlorpyrifoshhriskassessment2014_0.pdf; accessed 3-2020).
- US EPA 2020. Chlorpyrifos: Third Revised Human Health Risk Assessment for Registration Review. Office of Chemical Safety and Pollution Prevention, U.S. Environmental Protection Agency, Washington, DC., EPA-HQ-OPP-2008-0850-944.
- Velmurugan G., Ramprasath T., Swaminathan K., Mithieux G., Rajendhran J., Dhivakar M., Parthasarathy A., Babu D.D., Thumburaj L.J., Freddy A.J., Dinakaran V., Puhari S.S., Rekha B., Christy Y.J., Anusha S., Divya G., Suganya K., Meganathan B., Kalyanaraman N., Vasudevan V., Kamaraj R., Karthik M., Jeyakumar B., Abhishek A., Paul E., Pushpanathan M., Rajmohan R.K., Velayutham K., Lyon A.R., Ramasamy S. Gut microbial degradation of organophosphate insecticides induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18:8–18. doi: 10.1186/s13059-016-1134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLARES, J. 2007. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience, 145, 323-334. [DOI] [PubMed]
- Voelker R., Holmes M. Cannabis Safety Institute; Eugene, OR: 2015. Pesticide Use on Cannabis; pp. 1–19. [Google Scholar]
- Vyhlidal C.A., Gaedigk R., Leeder J.S. Nuclear Receptor Expression In Fetal And Pediatric Liver: Correlation With Cyp3a Expression. Drug Metab. Dispos. 2006;34:131. doi: 10.1124/dmd.105.005967. [DOI] [PubMed] [Google Scholar]
- Wang H.-P., Liang Y.-J., Long D.-X., Chen J.-X., Hou W.-Y., Wu Y.-J. Metabolic profiles of serum from rats after subchronic exposure to chlorpyrifos and carbaryl. Chem. Res. Toxicol. 2009;22:1026–1033. doi: 10.1021/tx8004746. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Asaka Y., Kanamura S. Postnatal development and sublobular distribution of cytochrome P-450 in rat liver: a microphotometric study. J. Histochem. Cytochem. 1993;41:397–400. doi: 10.1177/41.3.8429202. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yamaori S., Funahashi T., Kimura T., Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80:1415–1419. doi: 10.1016/j.lfs.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Weed P.F., Filipeanu C.M., Ketchum M.J., Winsauer P.J. Chronic Delta9-Tetrahydrocannabinol during Adolescence Differentially Modulates Striatal CB1 Receptor Expression and the Acute and Chronic Effects on Learning in Adult Rats. J Pharmacol Exp Ther. 2016;356:20–31. doi: 10.1124/jpet.115.227181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihrauch-Blüher S., Wiegand S. Risk Factors and Implications of Childhood Obesity. Current Obesity Reports. 2018;7:254–259. doi: 10.1007/s13679-018-0320-0. [DOI] [PubMed] [Google Scholar]
- Weldon R.H., Barr D.B., Trujillo C., Bradman A., Holland N., Eskenazi B. A pilot study of pesticides and PCBs in the breast milk of women residing in urban and agricultural communities of California. J Environ Monit. 2011;13:3136–3144. doi: 10.1039/c1em10469a. [DOI] [PubMed] [Google Scholar]
- Wiley J.L., Taylor S.I., Marusich J.A. 9-Tetrahydrocannabinol Discrimination: Effects of Route of Administration in Rats. Drug Alcohol Depend. 2021;108827 doi: 10.1016/j.drugalcdep.2021.108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jia Q., Hu C., Han M., Guo Q., Li S., Bo C., Zhang Y., Qi X., Sai L., Peng C. Effects of chlorpyrifos exposure on liver inflammation and intestinal flora structure in mice. Toxicol Res (Camb) 2021;10:141–149. doi: 10.1093/toxres/tfaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang Y., Wang G., Han R., Xie X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus) Chemosphere. 2016;153:287–293. doi: 10.1016/j.chemosphere.2016.03.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




