Abstract
Introduction
Glioblastomas are aggressive primary intracranial tumours of the central nervous system causing significant mortality and morbidity worldwide.
Objective
This study aims to evaluate the prognostic value of tissue expression by immunostaining of hypoxia-inducible factor (HIF-1α), isocitrate dehydrogenase 1 (IDH1), and tumour protein p53 in glioblastoma in Moroccan patients. The association of HIF-1α, IDH1, and p53 expression with the clinicopathological data and overall patient survival (OS) was also evaluated.
Materials and methods
Confirmed glioblastomas were included in this study. Twenty-two tissue samples were obtained by neurosurgical intervention resulting from total resection, and subtotal resection or biopsy. Karnofsky index, histological type of tumour, and the status of IDH1, p53 protein, and HIF-1α expression by immunostaining were reported.
Results
The majority of the patients were males (64%) with a sex ratio of 1.75. The average age was 54 ± 13. Median follow-up was 10.10 months and median overall survival was 10 months. The expression of HIF-1α was high in 10 samples (45%) and low in 12 (55%). There was a statistically significant difference in OS of 85% at 12 months for the subgroup of patients “HIF-1α negative IDH1 positive” p = 0.038, the unadjusted analysis showed that the group “HIF-1α positive, IDH1 positive” was a poor prognostic factor, the HR was 0.08 (95% CI: 0.009–0.756, p = 0.027).
Conclusion
Patients with negative HIF-1α expression and positive IDH1 expression have a better prognosis, suggesting that these two biomarkers may be useful in the search for new approaches for targeted therapy in glioblastoma.
Keywords: Glioblastoma, Hypoxia, HIF-1α, IDH1, Immunohistochemistry, Overall patient survival
Highlights
-
•
Brain tumours in adults, causing significant mortality and morbidity worldwide.
-
•
Glioblastomas are the aggressive primary intracranial tumours.
-
•
Glioblastomas are difficult to treat because of their inter-tumoral and intra-tumoral heterogeneity.
-
•
It is important to personalise care according to the molecular signature of glioblastoma for each patient.
-
•
The expression of HIF-1α is variable in glioblastoma, this expression is an indicator of intra-tumoral hypoxia.
-
•
A high expression of HIF-1α is associated with a poor prognosis in glioblastoma.
1. Introduction
Glioblastomas (GBM) are the main primary intracranial tumours of the central nervous system (CNS). They account for 81% of all malignant brain tumours in adults, and 60–70% of all gliomas, causing significant mortality and morbidity worldwide [[1], [2], [3]].
According to the 4th edition of the WHO classification of CNS tumours issued in 2016, the diagnosis of glioblastoma is based on four criteria: nuclear atypia, mitosis, necrosis, and endothelial capillary proliferation [4]. The mutational status of IDH1 and IDH2 genes, coding for two isoforms of the enzyme isocitrate dehydrogenase (IDH) were added to this revised 4th edition. These mutations induce the production of alpha-ketoglutarate and lead to a hypermethylation phenotype. This phenotype inhibits tumour suppressor oncogenes, inducing the development of glioblastomas [5].
Mutant IDH status is a better prognostic factor for glioblastoma. Mutation status of IDH is usually assessed by immunohistochemical (IHC) study on a section of fixed and paraffin-embedded tissue showing a mutant protein IDH1-R132H which is present in 85% of secondary glioblastomas [4].
Radiologically, the glioblastoma appears as a lesion in hyposignal T1 and hypersignal T2, with a heterogeneous irregular contrast pattern, corresponding to the hypercellular infiltrating part of the tumour, with a central zone of necrosis, associated with a peripheral hypersignal FLAIR (Fluid Attenuation Inversion Recovery) corresponding in part to the perilesional edema more or less associated with the infiltrating cells isolated around the lesion [6].
GBM are characterized by pathological vascularisation, rapid growth, intense angiogenesis, and a tendency to recur. Treatment remains difficult due to radiotherapy resistance, with a relative 5-year survival of less than 5% [2,7,8].
The standard treatment for glioblastoma has 3 pillars: a total and maximal resection, encephalic radiotherapy combined with temozolomide (Temoradiation), and semestrial cycles of adjuvant temozolomide. This therapeutic strategy has demonstrated its superiority over exclusive radiotherapy with a gain of 2.5 months in median survival and a relative reduction in the risk of death [9]. Amongst factors of radiotherapy and chemotherapy resistance, hypoxia is a predominant feature in gliomas and their microenvironment and is associated with tumour growth, progression, and resistance to conventional therapy [10]. Hypoxia leads to the stabilisation of HIF (Hypoxia Inducible Factor), of which HIF-1 is the canonical representative. This factor is composed of HIF-1alpha subunit (HIF-1α) regulated by the oxygen level and a constitutively expressed HIF-1β subunit. HIF-1α increases the expression of specific genes in the presence of low oxygen concentration [11,12].
Numerous studies have shown that mild hypoxia may decrease the level of p53 protein. This decrease may help in cell protection from apoptosis which could allow them a better adaptation in the hypoxic environment. The p53 protein is encoded by the TP53 gene, known as the most frequently mutated gene in human tumours. The p53 pathway is inactivated in most tumours because it functions as the “guardian of the genome”, acting as a tetrameric transcription factor that induces the transcription of hundreds of target genes, which are involved in the regulation of apoptosis, cell cycle, and DNA repair. Most studies have shown that the increase in p53 protein levels during hypoxia is due to a stabilisation that depends on the presence of HIF-1α protein [[13], [14], [15]].
In normoxia, the protein of HIF-1α is hydroxylated by prolyl-hydroxylase (PHD) and is then recognised by the tumour suppressor pVHL (Von Hippel-Lindau protein), leading to its ubiquitination and degradation by the proteasome [[16], [17], [18]]. Under hypoxic conditions, the PHDs are inactive, HIF-1α is no longer degraded. Before joining the nucleus, HIF-1α is accumulated and then associated with its partner HIF-1β, forming the HIF-1 factor. Thus, the dimer formed binds to the HRE (Hypoxia Responsive Element) consensus sequences contained in the promoters of target genes (more than 100 genes) including Vascular Endothelial Growth Factor (VEGF), erythropoietin, glucose transporters, and pH regulators [17,18]. HIF-1α functions as a key transcription factor for the regulation of many genes in response to hypoxia, namely the genes responsible for glucose metabolism, extracellular matrix metabolism, cell growth and proliferation, cell survival, pH regulation, and DNA repair mechanisms [12,19]. Expression and transcriptional activity of HIF-1α increases as oxygen concentration decreases [20]. The angiogenic mechanism of glioblastomagenesis is under the control of the hypoxia-induced transcription factor HIF-1α [21]. The signalling pathways involved are mainly [22]:
-
-
The Angiogenesis pathway by activating vascular endothelial growth factor (VEGF) and other promoters of the target genes: VEGF-R1, Angiopoietin-2, PDGF, FGF, COX-2.
-
-
The cell survival pathway by expressing EPO, NOS2, TGFα.
-
-
The Glucose metabolism pathway by expressing the GLUT1 and 3 genes, GAPDH, HK1, and HK2.
-
-
The integrin pathway by expressing EGF, IGF-2, TGF-β1, and TGF-β3.
This study aims to evaluate the prognostic value of tissue expression by immunostaining of HIF-1α, IDH1, and p53 on a series of glioblastomas from Moroccan patients. The association of HIF-1α, IDH1, and p53 expression with the clinicopathological data and overall patient survival (OS) was also evaluated.
2. Materials and methods
2.1. Type of study
This is a prospective observational study carried out between June 2017 and December 2019 at the Pathological Anatomy and Neurosurgery Departments of the Rabat Specialities Hospital and the Sidi Mohamed Ben Abdallah National Oncology Center in Rabat, Morocco.
2.2. Inclusion criteria
Consenting Moroccan adult patients, over 18 years of age with a histologically confirmed glioblastoma, treated at the Specialities Hospital and the National Institute of Oncology in Rabat, were included.
2.3. Exclusion criteria
Patients with other brain tumours, under 18 years old; patients with brain metastases; patients of other nationalities and patients treated in another oncology center.
3. Methodology
This study was carried out in two stages: The first stage was patient recruitment; during which an operating sheet was filled from the medical records which included clinicopathological and radiological characteristics: Sex, age, cerebral MRI, location of the tumour, extent of resection, Karnofsky index, histological type of tumour, and the status of IDH1, p53 protein and HIF-1α expression by immunostaining.
The second stage consisted of following the included patients after surgery and completing the operating sheet with therapeutic data: type of treatment received, duration of treatment, a dose of radiotherapy (in Grays), and number of the cycles of chemotherapy.
3.1. Patients
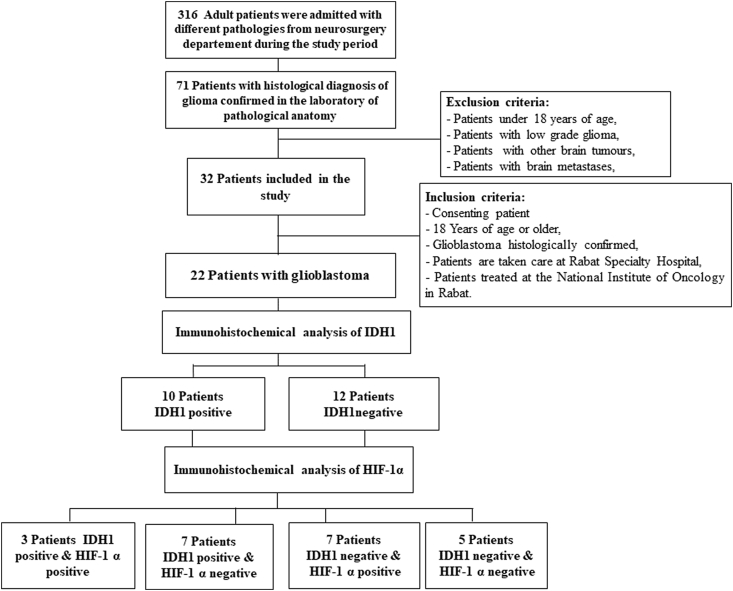
Between June 2017 and December 2018, we counted 71 patients with histologically confirmed diffuse glioma. Of which 22 glioblastomas meeting the inclusion criteria that we included in this study. Tissue samples were obtained by neurosurgical intervention leading to total resection, subtotal resection or biopsy. The anatomopathological examination was carried out by an experienced neuropathologist at the Pathological Anatomy Laboratory of the Specialities Hospital in Rabat. The histological type and grade of the tumour were determined according to 2016 WHO classification of central nervous system tumours (Fig. 1).
Fig. 1.
Flow chart recruitment of patients with glioblastoma and immunostaining by
HIF-1 α and HDI1.
3.2. Sample preparation for immunohistochemistry
The specimen received was fixed in neutral buffered formalin. The samples included in paraffin were cut to a thickness of 4 μm. The tissue ribbon obtained was spread out on pre-treated (chromo-gelatinised) slides in Bain Marie. The slides were dried in an oven at 56 °C for 1 h, then dewaxed in two toluene baths for 10 min each and then in two alcohol baths for 5 min each, and then hydrated using tap water for 5 min. An antigenic unmasking was carried out in the microwave. The blocking of endogenous peroxidase was done with 3% hydrogen peroxide or with a blocking agent for 5 min.
3.3. Immunohistochemistry
The previously prepared sections were incubated with a primary mouse monoclonal antibody anti–HIF–1α specific mouse monoclonal (clone ESEE122, 1:1000 dilution, ph6) from Abcam, a mouse monoclonal anti IDH1R132H (clone H09, 1:40 dilution, ph6) from Dia Nova, and a mouse monoclonal anti p53 (clone DO-7, pre-diluted ready to use, ph6) from Dako. The sections were incubated with a secondary antibody conjugated to biotin, and the third incubation with a tertiary antibody conjugated to peroxidase. Each incubation lasted 30 min at room temperature in a humid atmosphere, except for IDH1 sections which were incubated overnight at a temperature of 4 °C. Between each incubation, the slides are rinsed with PBS buffer and carefully wiped around the cut. The developing system was used to visualise the immunoreaction is DAB (3,3′ Diaminobenzidine Tetrahydrochloride Citrate Buffer Ph6) which gives a brown stain. Observation of the slides after immunostaining was carried out using the optical microscope.
3.4. Histological evaluation
It was performed on H&E (Hematoxylin Eosin) stained histological sections, examined by a neuropathologist and confirmed as grade IV glioblastoma according to the WHO 2016 classification.
3.5. Interpretation of immunostaining results
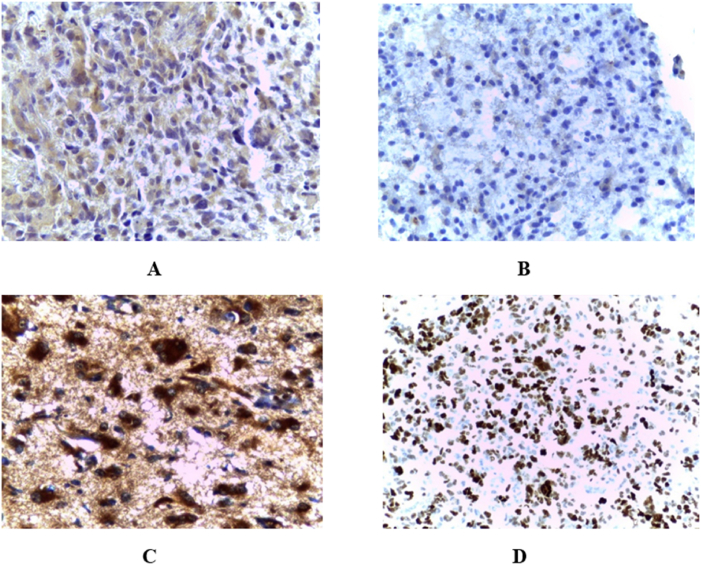
The immunoreactivity of HIF-1α was observed in the cytoplasm or nucleus of the tumour cells in our series of glioblastomas. No immunostaining of HIF-1α was observed in the parenchyma or vascular cells of normal cerebral tissue. The expression of HIF-1α was quantified under an optical microscope (x100) by evaluating the percentage of stained tissue surface area in relation to the total surface area of the sample. Staining of HIF-1α with less than 10% was considered as negative/weak staining, and more than 10% value was considered as a positive/strong staining or overexpression of HIF-1α [23].
Immunoreactivity of IDH1 was observed in the nucleus and cytoplasm of glioblastoma tumour cells with strong perinuclear staining (Fig. 2).
Fig. 2.
Immunohistochemical expression of A) Hypoxia inducible factor-1 alpha (HIF-1α), B) HIF-1α in normal cerebral tissue, C) Isocitrate dehydrogenase 1 (IDH1), Tumour protein p53.
3.6. Ethical aspect
The protocol of this study was approved (File N° 27/16) and the approbation was obtained from the Ethics Committee of the Faculty of Medicine and Pharmacy, Mohammed V University of Rabat, Morocco.
Written informed consent was signed by all patients before the use of their tissue samples and consultation of their medical records to retrieve clinicopathological and radiological data.
This study is in accordance with the Reporting Recommendations for Tumour Marker Prognostic Studies for biomarker assessment [24].
3.7. Statistical analysis
Comparison of the different prognostic and clinicopathological factors with the expression of biomarkers was done using the test of χ2 and the Fisher Exact test for a number <5.
Overall survival (OS) “survival at one year” was determined by Kaplan-Meier (KM) method. OS is defined in our study as the time from diagnosis to the date of last follow-up or death due to disease. Follow-up was censored at one year.
Follow-up was performed every 3 months by consulting medical records and by calling, adjuvant chemotherapy and radiotherapy were fully discussed with patients before the start of treatment, and all patients had received adequate chemotherapy and radiotherapy according to the treatment protocol.
Progression-free survival (PFS) has been defined as the time between surgery and tumour progression on MRI, or the death of the patient due to glioblastoma. To determine the difference in overall survival in our study, based on biomarker expression, we used the Log Rank test. The results were considered significant when p (degree of significance) is less than 0.05, very significant when p < 0.01, and highly significant when p < 0.001.
Univariate analysis using the Cox model was performed for all prognostic factors studied, multivariate analysis using Cox regression was performed for factors with p < 0.05 in the univariate analysis or representing a prognostic interest, or which may be a confounding factor.
4. Results
The 22 glioblastomas included in this study represent 6.96% of the samples received from the Neurosurgery Department and analysed at the Pathological Anatomy Laboratory. The majority of patients were male (64% men vs 36% women) with a sex ratio of 1.75.
The average age was 54 ± 13; 73% of the patients were older than 50 years. More than half of the patients (55%), had a KFS>70. Hypertension (HT) was noted in 14% of cases and 9% were diabetic. Smoking was observed in 9% of the cases. The tumour location was frontal in 41%, parietal in 23%, temporal in 18%, and multilobar in 18% of patients. Biopsy, incomplete resection, and complete resection were observed in 41%, 41%, and 18% respectively.
Radiologically, infiltration of the corpus callosum was observed in 82% of patients and edema in 73%. Histological analysis showed mitotic activity and necrosis in all patients, endotheliocapillary proliferation was observed in 64% of cases, and cell density was high in 82%.
The description of the different prognostic and clinicopathological factors is shown in Table 1. HIF-1α expression was positive in 10 patients (45%) and negative in 12 patients (55%). IDH1 expression was positive in 10 patients and negative in 12. The majority of samples expressing HIF-1α (70%) had a negative expression of IDH1. The median follow-up was 10.10 months and the median overall survival (OS) was 10 months. A total of 13 (59.1%) patients had died and 9 (40.9%) survived with the disease. The rate of OS after 6 months of diagnosis was 72.7%, but after one year of follow-up, the number of survivors was less than 39.7%.
Table 1.
Immunohistochemical biomarker expression levels in 22 GBM patients histopathologically confirmed.
| Prognostic Factor | Number of Patients n (%) | IDH-1-R132 |
HIF-1-α |
p53 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative n (%) | Positive n (%) | P-value n (%) | Positive n (%) | Negative n (%) | P-value n (%) | Positive n (%) | Negative n (%) | P-value n (%) | ||
|
Age 18–50 Years >50 Years |
6 (27) 16 (73) |
2 (33) 10 (63) |
4 (67) 6 (37) |
0.229 | 3 (50) 7 (44) |
3 (50) 9 (56) |
0.583 | 2 (40) 10 (67) |
3 (60) 5 (33) |
0.363 |
|
Sex Male Female |
14 (64) 8 (36) |
3 (38) 9 (64) |
5 (62) 5 (36) |
0.221 | 8 (57) 2 (25) |
6 (43) 6 (75) |
0.165 | 5 (62) 7 (58) |
3 (38) 5 (42) |
0.551 |
|
Karnofsky Performance status >70 ≤70 |
12 (55) 10 (45) |
7 (88) 9 (64) |
1 (12) 5 (36) |
0.026* | 5 (63) 5 (36) |
3 (37) 9 (64) |
0.546 | 5 (71) 7 (54) |
2 (29) 6 (46) |
0.663 |
|
Extent of resection Biopsy Sub-total Gross-total |
9 (41) 9 (41) 4 (18) |
6 (67) 5 (56) 1 (25) |
3 (33) 4 (44) 3 (75) |
0.257 | 4 (44) 4 (44) 2 (50) |
5 (56) 5 (56) 2 (50) |
0.699 | 6 (67) 3 (38) 3 (100) |
3 (33) 5 (62) 0 |
0.413 |
|
Corpus collosum infiltration Yes No |
18 (82) 4 (18) |
10 (56) 2 (50) |
8 (44) 2 (50) |
0.632 | 9 (50) 1 (25) |
9 (50) 3 (75) |
0.368 | 9 (56) 3 (75) |
7 (44) 1 (25) |
0.624 |
|
Edema Yes No |
16 (73) 6 (27) |
7 (44) 5 (83) |
9 (56) 1 (17) |
0.119 | 6 (38) 4 (66) |
10 (62) 2 (34) |
0.229 | 8 (53) 4 (80) |
7 (47) 1 (20) |
0.267 |
|
Mitotic activity Yes No |
22 (100) 0 |
12 (55) 0 |
10 (45) 0 |
10 (45) 0 |
12 (55) 0 |
12 (60) 0 |
8(40) 0 |
|||
|
Endothelial-capillary proliferation Yes No |
14 (64) 8 (36) |
8 (57) 4 (50) |
6 (43) 4 (50) |
0.460 | 7 (50) 3 (38) |
7 (50) 5 (62) |
0.454 | 8 (67) 4 (50) |
4 (33) 4 (50) |
0.449 |
|
Necrosis Yes No |
22 (100) 0 |
12 (55) 0 |
10 (45) 0 |
10 (45) 0 |
12 (55) 0 |
12 (60) | 8 (40) | |||
|
Cell Density Moderate High |
4 (18) 18 (82) |
2 (50) 10 (56) |
2 (50) 8 (44) |
0.632 | 2 (50) 8 (44) |
2 (50) 10 (56) |
0.632 | 2 (50) 10 (62) |
2 (50) 6 (38) |
0.574 |
*p < 0.05.
HIF-1α: Hypoxia inducible factor-1 alpha, IDH1: Isocitrate dehydrogenase 1, TP53: Tumour protein p53.
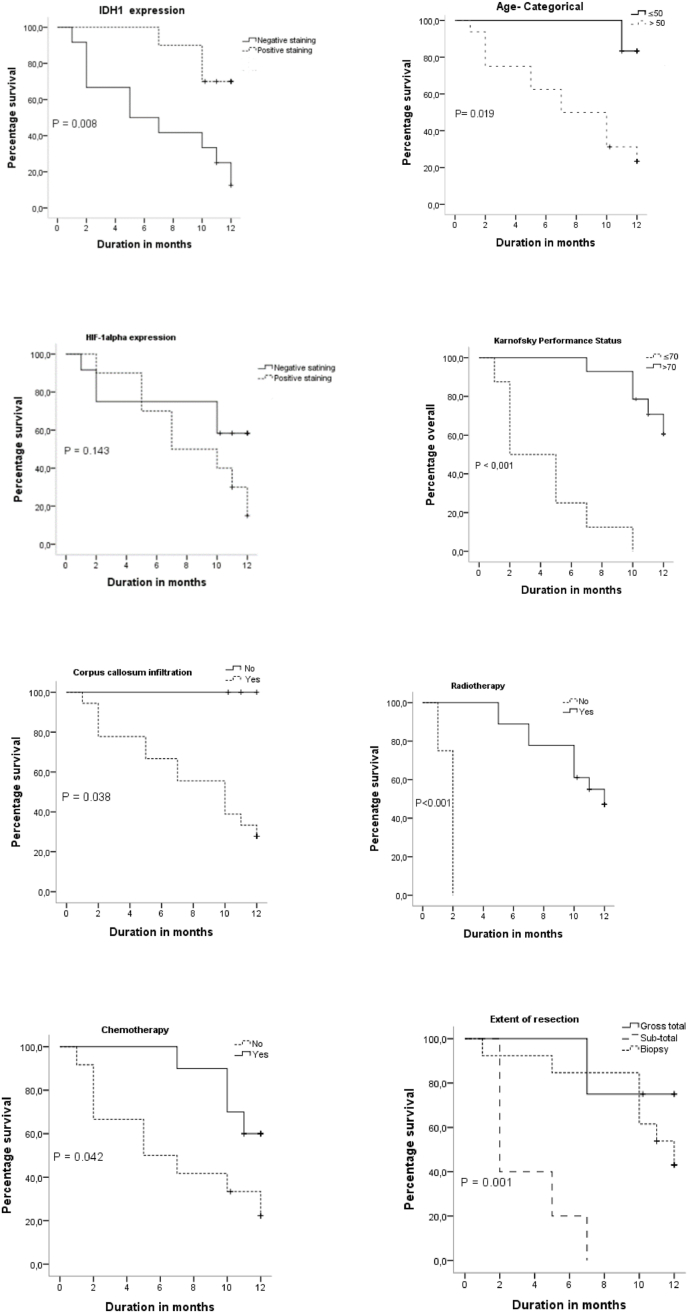
Fig. 3 illustrates the KM survival curves for age, KFS, corpus callosum infiltration, HIF-1α, IDH1, p53, Radiotherapy, and Chemotherapy. The OS of patients according to age groups showed that patients with age ≤50 years have 100% survival at 6 months and one year, however, patients with age >50 years have 60% survival at 6 months and 20% at one year. The Log Rank test showed a statistically significant difference with p = 0.019. OS in patients expressing mutant IDH1 status was high at 6 months and one year, 90% and 70%, respectively. According to the Log Rank test, this difference was very significant with p = 0.008.
Fig. 3.
KM survival diagrams for age, KFS, corpus callosum infiltration, expression of HIF-1α, IDH1, p53, Radiotherapy, Chemotherapy and the different groups according to the expression of HIF-1 α and IDH1.
Age, sex, KFS status, edema, corpus callosum infiltration, extent of resection, cell density, endotheliocapillary proliferation, expression of IDH1, HIF-1α, and p53 were all predictors of survival in the unadjusted Cox analysis (Table 2).
Table 2.
Kaplan-Meier and Cox proportional hazards analysis.
| Prognostic Factor | Kaplan-Meier |
Unadjusted Cox |
Adjusted Cox |
|---|---|---|---|
| Median OS p value Log Rank | p value HR (95% CI) | p value HR (95% CI) | |
| Age | |||
| 18–50 Years | 14.1 0.013 | R | |
| >50 Years | 7.6 | 0.054 7.52 (0.96–58.65) | 0.296 3.39 (0.34–33.48) |
| Karnofsky Performance status | |||
| >70 | 4.8 < 0.001 | R | |
| ≤70 | 11.9 | <0.001 13.98 (3.42–57.07) | 0.001 15.70 (3.09–79.78) |
| Edema | |||
| No | 9.3 0.027 | R | |
| Yes | 9.4 | 0.474 1.50 (0.48–4.65) | 0.898 1.09 (0.27–4.38) |
| Cell Density | |||
| Moderate | 10.3 0.492 | R | |
| High | 11.2 | 0.277 3.10 (0.40–23.94) | 0.276 3.12 (0.40–2.58) |
| IDH1 (R132H) | |||
| Negative | 12.7 0.013 | R | |
| Positive | 6.6 | 0,020 0.21 (0.05–0.78) | 0.103 0.24 (0.04–1.32) |
| HIF-1 α Expression | |||
| Negative | 10.3 0.15 | R | |
| Positive | 8.2 | 0,170 0.45 (0.14–1.40) | 0.823 1.17 (0.29–4.68) |
| Chemotherapy | |||
| Received | 12.4 0.039 | R | |
| Not Received | 6.8 | 0.062 0.32 (0.09–1.05) | 0.044 3.44 (1.03–11.48) |
R: reference, HR: Hazard Ratio.
Univariate Cox proportional hazard analysis showed that the mutant IDH1 is a predictive factor with a hazard ratio (HR) of 0.21 (95% Confidence Interval: 0.05–0.78, p = 0.020).
OS in patients with positive HIF-1α expression was shorter than those with a negative one, 18% versus 65% at 12 months, respectively. The log-Rank test did not give a statistically significant difference with p = 0.143. The HR of the HIF-1 alpha expression was 0.45 (95% CI: 0.14–1.40, p = 0.170) in the unadjusted Cox proportional risk analysis.
KFS status and chemotherapy were independent prognostic factors in the KM survival analysis. Log Rank test showed that KFS was a highly significant prognostic factor, p < 0.001. This significance was retained in the Cox multivariable proportional hazard analysis with an HR of 15.70 (95% CI: 3.09–79.78, p = 0.001).
Categorical age (18–50 years, >50 years), corpus callosum infiltration, edema, and extent of resection were significant in the KM analysis but found to be not significant in the multivariate Cox proportional risk analysis. Sex, risk factors, tumour location, and p53 expression had no significant effect on OS.
OS in patients who received radiotherapy was high compared to those who did not. Log Rank test showed a highly significant difference p < 0.001.
However, patients who received chemotherapy concomitantly with radiotherapy followed by adjuvant chemotherapy (6 cycles of temozolomide every 28 days) had higher OS than patients who were treated with radiotherapy alone. KM analysis and Log Rank test showed a statistically significant difference with p = 0.042. This difference was maintained in the Cox multivariate analysis with an HR of 3.44 (95% CI: 1.03–11.48, p = 0.044).
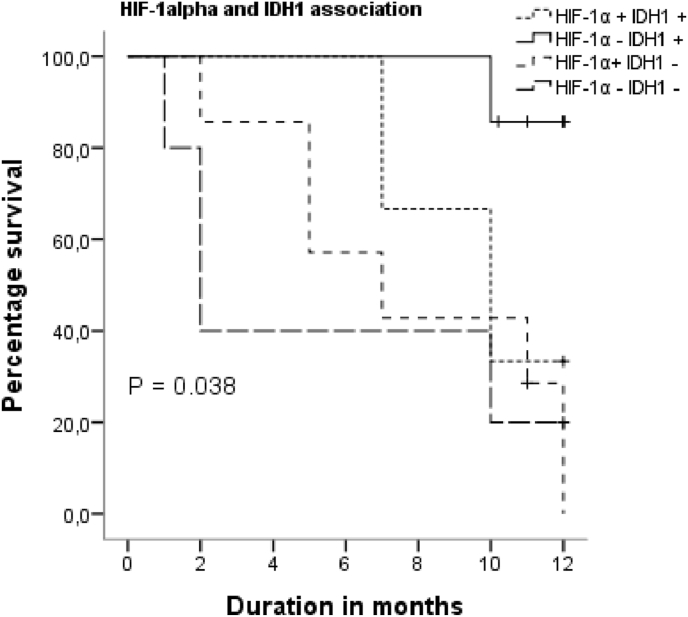
We carried out a survival analysis to show whether there is a difference in the OS between the HIF-1α and IDH1 expression groups (Table 3): “HIF-1α positive IDH1 positive” (n = 3), “HIF-1α negative IDH1positive” (n = 7), “HIF-1α positive IDH1negative” (n = 7), “HIF-1α negative IDH1 negative” (n = 5).
Table 3.
Kaplan-Meier and Cox proportional hazards analysis of association between IHC expression of HIF-1α and IDH1. Adjusted for Age-categorical, cell density, chemotherapy and KFS.
| Prognostic Factor HIF-1α vs IDH1 | Unadjusted Cox P value HR (95% CI) |
Adjusted Cox P value HR (95% CI) |
|---|---|---|
| HIF-1α positive vs IDH1 positive | 0.148 | 0.134 |
| HIF-1α negative vs IDH1 positive | 0.412 0.49 (0.08–2.69) | 0.615 0.45 (0.02–9.59) |
| HIF-1α positive vs IDH1 negative | 0.027 0.08 (0.009–0.756) | 0.37 0.009 (<0.001–0.74) |
| HIF-1α negative vs IDH1 positive | 0.762 0.82(0.22–2.94) | 0.236 0.34 (0.05–2.00) |
In Fig. 4, KM analysis and Log Rank test showed that the “HIF-1α negative HDI1 positive” group had a better OS than the other groups (85% at 12 months), with a statistically significant difference p = 0.038. Univariate unadjusted analysis showed that the “HIF-1α positive HDI1negative” group was a poor prognostic factor, with an HR of 0.08 (95% CI: 0.009–0.756, p = 0.027).
Fig. 4.
KM survival for different groups expression of HIF-1α and IDH1.
5. Discussion
Glioblastoma is the most aggressive brain tumour of the central nervous system with a median survival of 14.7 months [25]. Despite the fundamental knowledge about glioblastoma biology and the new generation of genetic sequencing “NGS”, these tumours are difficult to treat because of their inter-tumoral and intra-tumoral heterogeneity. Currently available treatments can only slow progression and reduce clinical signs and symptoms [[26], [27], [28], [29], [30], [31]].
In this study, we analysed the immunohistochemical expression of IDH1, HIF-1α, and p53 and their impact on the overall survival parameters with a special focus on the correlation of the expression of these biomarkers to the clinicopathological, radiological and therapeutic data. This work reported the positive prognostic impact of the mutant IDH1 status on the overall survival in the population at 6 months and 1 year, which confirms the important prognostic value of this biomarker for the stratification of patients with glioblastoma. A high expression of HIF-1α was associated with the extent of resection and corpus callosum infiltration. However, the expression of HIF-1α was not associated with IDH1, p53, age, sex, and tumour location. The KM analysis showed that patients with negative expression of HIF-1α had better survival at 12 months compared to patients with positive expression. The Log Rank test showed no statistically significant difference. Survival analysis comparison between the different groups of patients according to HIF-1α expression and IDH1mutated status showed that the “negative HIF-1α and positive IDH1” group had an OS of 85% at 12 months, better than the other groups. The group of patients with positive HIF-1α and negative IDH1 had a poor prognosis. These results indicate the importance of HIF-1α, and IDH1 as potential prognostic markers in glioblastomas [25,32] and as an important basis for developing personalized therapy [33].
Hypoxia and HIF-1α have been correlated with poor prognosis in several cancers such as breast cancer [34], cervical cancer [35], ovarian cancer [36], liver cancer [37], and in different patient populations. In our sample of Moroccan patients, positive expression of HIF-1α was associated with a poor prognosis, and the negative expression of HIF-1α was associated with a better prognosis, 18% versus 65% survivors at one year, suggesting that high expression of HIF-1α may lead to chemo-radioresistance and induce a poor response to conventional glioblastoma treatments. Sheehan et al., have shown that small alterations in O2 tension inside tumours may affect radiosensitivity [38]. Also, Zhao et al., have shown that high expression of HIF-1α in gliomas and glioblastomas can contribute to poor prognosis, and the deactivation of HIF-1α inhibits proliferation, invasion, and migration of glioblastoma cells in vitro and in vivo [39]. The involvement of HIF-1α in the resistance to conventional glioblastoma treatment is explained by the mechanisms of adaptation to tumour hypoxia, generally dependent on HIF-1α. These mechanisms induce the activation of genes involved in tumour angiogenesis and the reduction of tumour cells’ sensitivity to cytotoxic effectors [40,41].
The immunotherapy approach is unlikely to be effective because of the molecular heterogeneity of glioblastomas and the involvement of several pathways under the control of HIF-1α in the maintenance, progression, and resistance of these tumours. Promising perspectives include the combination of HIF-1α inhibitors with new approaches to immunotherapy, in addition to the standard treatment, for a better effect on tumour treatment, quality of life and overall survival [42,43].
In tumours, hypoxic areas are often correlated with overexpression of the mutant protein p53. The expression levels of p53 protein and HIF-1α in tumours (in vivo) can be used to discriminate between different prognostic subgroups. Several studies have shown that the immunohistological detection of functionally inactive p53 and the presence of hypoxia have no prognostic impact if analysed as single parameters, but the combination of the two parameters indicates an aggressive phenotype with an unfavourable prognosis in various types of cancers [13,44].
Inactivation of IDH1 decreases glioblastoma cell growth and promotes a more differentiated tumour cell state, increasing apoptosis in response to targeted therapies and prolonging the survival of animals with patient-derived xenografts [45].
Radiotherapy and chemotherapy treatments cause oxidative stress to the tumour cell, and the IDH1 protein expressed by the non-mutated gene protects the cell from the stress of aggressive treatment. The positive regulation of IDH1 (IDH1 wild-type) represents a metabolic adaptation that ensures macromolecular synthesis, aggressive growth, and therapeutic resistance [[45], [46], [47]]. In addition, Shimin et al. have shown that mutations in IDH1 alter the affinity of the enzyme for its substrate and dominantly inhibit the activity of wild-type IDH1 by the formation of catalytically inactive heterodimers. Forced expression of mutant IDH1 in cultured cells reduces the formation of the enzyme product α-ketoglutarate (α-KG), and increases the expression levels of HIF-1α which is stabilised by α-KG. This increase has been reversible. Expression levels of HIF-1α were higher in human gliomas with an IDH1 mutation than in tumours without a mutation. They showed that the IDH1 mutation in gliomas was correlated with overexpression of HIF-1α [48], which suggests that the mutant IDH1 status contributes to tumorigenesis, partly through induction of the HIF-1α pathway [47].
The main strength of our work is that it is a clinical study directly correlating negative HIF-1α and positive IDH1 immunohistochemical expression with OS in a group of patients with glioblastomas. This study had several limitations, the sample size was limited by the duration of the study, lack of resources to look for the methylation status of the MGMT promoter, which is a prognostic factor in glioblastomas in elderly subjects. The IDH1 mutation could not be sought by molecular sequencing. The findings of this study need to be confirmed in a larger sample.
6. Conclusion
The expression of HIF-1α is an indicator of intra-tumoral hypoxia, which plays a crucial role in the initiation, progression, and resistance to treatment. A high expression of HIF-1α is associated with a poor prognosis in several cancers including glioblastomas. Patients with negative or weak expression of HIF-1α and mutated IDH1 have a better prognosis, which allows them to be useful prognostic biomarkers in the search for new approaches to target glioblastomas.
Ethical approval
The protocol of this study was approved (file N° 27/16) and the approbation was obtained from the Ethics Committee of the Faculty of Medicine and Pharmacy, Mohamed V University of Rabat, Morocco.
Written informed consent was signed by all patients before the use of their tissue samples or consultation of their medical records to retrieve clinicopathological and radiological, and therapeutic data.
Sources of funding
This work is part of the preparation of a national thesis at laboratory of Pathological Anatomy. Research team in tumour pathology, Faculty of Medicine and Pharmacy, the basic funding for the reagents was provided by the Mohammed V University of Rabat, Morocco. No external funding was requested.
Author contribution
Fatima Sfifou: Study design, data collection, carrying out the immunostaining, writing the paper, coordination between different services. El Mehdi Hakkou: data collection in Neurosurgery Department. Abdessamad El Ouahabi: validation of clinical data of patients included in the study, correction of the paper. Redouane Abouqal: Validation of study design. Interpretation of statistical analysis. Meriem Slaoui: Data collection in Radiotherapy. Hassan Errihani: Validation of therapeutic data. EL Arbi Bouaiti: Statistical analysis. Nadia Cherradi: anatomopathological study, validation and interpretation of the immunostaining, correction and validation of the writing of the paper. Abderrahmane Al Bouzidi: Interpretation of the immunostaining.
Registration of research studies
This study was not registered in a publicly accessible database.
The protocol for this study was approved by the ethics committee before starting the work.
Guarantor
SFIFOU Fatima, CHERRADI Nadia, ABOUQAL Redouane, El OUAHABI Abdessamad, HAKKOU El Mehdi, ERRIHANI Hassan.
Consent
Signed informed consent was obtained from the patients before using the tissue samples. The same tissue sample used in the diagnosis was used for the immunohistochemical study. Only patients meeting the inclusion criteria were included in this study. All consenting patients were informed about the use of the tissue samples, clinicopathological and therapeutic data for the purpose of publication of the results while respecting the anonymity and confidentiality of the patients' data.
Ethics statement
This study was approved by the Ethics Committee of Biomedical Research of Mohammed V University on the grounds of satisfactory conditions of validity, research interests, scientific relevance, satisfactory conditions of human pretensions, ethical relevance, intelligibility of the note of information, and conformity of the modalities of collection of consent. The Ethics Committee's deliberations are based on the Helsinki Declaration (2008 version), the International Ethical Guidelines for Biomedical Research Involving Human Subjects of the Council for International Organizations of Medical Sciences (CIOMS, 2002 version), and the National Law (no. 28–13; 2015) for the Protection of Persons Participating in Biomedical Research.
Written informed consent was obtained from all participants prior to the use of their tissue sample or medical data, and all were informed that tissue samples collected in the management of their disease would be used for research projects.
Authorship and contributorship
All the authors contributed to the development of this work.
Funding information
This project is part of the preparation of a national thesis, no external funding has been received. The core funding to have the antibodies were provided by the Mohammed V University in Rabat, Morocco.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
We would like to thank all the staff of the Pathological Anatomy laboratory, the Neurosurgery Department at the Rabat Hospital for Specialities, and the National Oncology Institute in Rabat for their help.
References
- 1.Ostrom Q.T., Bauchet L., Davis F.G., Deltour I., Fisher J.L., Langer C.E., Pekmezci M., Schwartzbaum J.A., Turner M.C., Walsh K.M., Wrensch M.R., Barnholtz-Sloan J.S. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014 Jul;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi Liu, Cao Peicheng. Clinical and prognostic significance of HIF-1α in glioma patients: a meta-analysis. Int. J. Clin. Exp. Med. 2015;8(12):22073–22083. [PMC free article] [PubMed] [Google Scholar]
- 3.Jovčevska I. Sequencing the next generation of glioblastomas. Crit. Rev. Clin. Lab Sci. 2018 Jun;55(4):264–282. doi: 10.1080/10408363.2018.1462759. Epub 2018 Apr 18. [DOI] [PubMed] [Google Scholar]
- 4.Brouland J.P., Hottinger A.F. Nouvelle classification OMS 2016 des gliomes : quels changements ? Rev. Med. Suisse. 2017 Oct 18;13(579):1805–1809. [PubMed] [Google Scholar]
- 5.Reuss D.E., Mamatjan Y., Schrimpf D., Capper D., Hovestadt V., Kratz A., Sahm F., Koelsche C., Korshunov A., Olar A., Hartmann C., Reijneveld J.C., Wesseling P., Unterberg A., Platten M., Wick W., Herold-Mende C., Aldape K., von Deimling A. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015 Jun;129(6):867–873. doi: 10.1007/s00401-015-1438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vauléon E. Contribution of metabolic imaging in the management of adult patient with primary brain tumor: the neuro-oncologist’s point of view. Médicine Nucléaire. Journal ISSN. 2017 February 24;41(3):235–236. [Google Scholar]
- 7.Yang L., Lin C., Wang L., Guo H., Wang X. Hypoxia and hypoxia-inducible factors in glioblastoma multiforme progression and therapeutic implications. Exp. Cell Res. 2012 Nov 15;318(19):2417–2426. doi: 10.1016/j.yexcr.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Delgado-López P.D., Corrales-García E.M. Survival in glioblastoma: a review on the impact of treatment modalities. Clin. Transl. Oncol. 2016 Nov;18(11):1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O. European organisation for research and treatment of cancer brain tumor and radiotherapy groups; national cancer Institute of Canada clinical trials group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005 Mar 10;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 10.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol. Pharmacol. 2006 Nov;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 11.Cuvillier Olivier. La voie Sphingosine kinase-1/Sphingosine 1-phosphate dans l'hypoxie tumorale. VEGF Actu. 2012;26:6–8. [Google Scholar]
- 12.Sermeus A., Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011 May 26;2(5):e164. doi: 10.1038/cddis.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An W.G., Kanekal M., Simon M.C., Maltepe E., Blagosklonny M.V., Neckers L.M. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998 Mar 26;392(6674):405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P., Dor Y., Herbert J.M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P., Koch C.J., Ratcliffe P., Moons L., Jain R.K., Collen D., Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998 Jul 30;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 15.Chun Y.S., Kim M.S., Park J.W. Oxygen-dependent and -independent regulation of HIF-1alpha. J. Kor. Med. Sci. 2002 Oct;17(5):581–588. doi: 10.3346/jkms.2002.17.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001 Apr;13(2):167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 17.Tanimoto K., Makino Y., Pereira T., Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000 Aug 15;19(16):4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trédan O., Grantab R., Dumontet C.L. 'hypoxie tumorale peut-elle devenir un avantage pour la chimiothérapie ? Bull. Cancer. 2008 May;95(5):528–534. doi: 10.1684/bdc.2008.0637. [DOI] [PubMed] [Google Scholar]
- 19.Mobasheri A., Richardson S., Mobasheri R., Shakibaei M., Hoyland J.A. Hypoxia inducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol. Histopathol. 2005 Oct;20(4):1327–1338. doi: 10.14670/HH-20.1327. [DOI] [PubMed] [Google Scholar]
- 20.Omuro A.M., Faivre S., Raymond E. Leçons apprises dans le développement d’une thérapie ciblée pour les gliomes malins. Mol. Canc. Therapeut. 2007 Juil;6(7):1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 21.Jain R.K., di Tomaso E., Duda D.G., Loeffler J.S., Sorensen A.G., Batchelor T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007 Aug;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 22.Cao D., Hou M., Guan Y.S., Jiang M., Yang Y., Gou H.F. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Canc. 2009 Dec 10;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dales J.P., Garcia S., Carpentier-Meunier S., Andrac-Meyer L., Lavaut M.N., Allasia C., Bonnier P., Taranger-Charpin C. La surexpression immunohistochimique de HIF-1alpha est un marqueur de mauvais pronostic (suivi supérieur à 13 ans) dans le cancer du sein (n = 745 patientes) Ann. Pathol. 2004;24(Supplement 1):131. [Google Scholar]
- 24.Altman D.G., McShane L.M., Sauerbrei W., Taube S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10(1):1–39. doi: 10.1186/1741-7015-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potharaju M., Mathavan A., Mangaleswaran B., Patil S., John R., Ghosh S., Kalavakonda C., Ghosh M., Verma R.S. Clinicopathological analysis of HIF-1alpha and tert on survival outcome in glioblastoma patients: a prospective, single institution study. J. Canc. 2019 May 26;10(11):2397–2406. doi: 10.7150/jca.32909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A.P., Tirosh I., Trombetta J.J., Shalek A.K., Gillespie S.M., Wakimoto H., Cahill D.P., Nahed B.V., Curry W.T., Martuza R.L., Louis D.N., Rozenblatt-Rosen O., Suvà M.L., Regev A., Bernstein B.E. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014 Jun 20;344(6190):1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen R., Dolgalev I., Bayin N.S., Heguy A., Tsirigos A., Placantonakis D.G. Single-cell RNA sequencing of glioblastoma cells. Methods Mol. Biol. 2018;1741:151–170. doi: 10.1007/978-1-4939-7659-1_12. [DOI] [PubMed] [Google Scholar]
- 28.Frank M.O., Koyama T., Rhrissorrakrai K., Robine N., Utro F., Emde A.K., Chen B.J., Arora K., Shah M., Geiger H., Felice V., Dikoglu E., Rahman S., Fang A., Vacic V., Bergmann E.A., Vogel J.L.M., Reeves C., Khaira D., Calabro A., Kim D., Lamendola-Essel M.F., Esteves C., Agius P., Stolte C., Boockvar J., Demopoulos A., Placantonakis D.G., Golfinos J.G., Brennan C., Bruce J., Lassman A.B., Canoll P., Grommes C., Daras M., Diamond E., Omuro A., Pentsova E., Orange D.E., Harvey S.J., Posner J.B., Michelini V.V., Jobanputra V., Zody M.C., Kelly J., Parida L., Wrzeszczynski K.O., Royyuru A.K., Darnell R.B. Sequencing and curation strategies for identifying candidate glioblastoma treatments. BMC Med. Genom. 2019 Apr 25;12(1):56. doi: 10.1186/s12920-019-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y., Carter R., Natarajan S., Varn F.S., Compton D.A., Gawad C., Cheng C., Godek K.M. Single-cell RNA sequencing reveals the impact of chromosomal instability on glioblastoma cancer stem cells. BMC Med. Genom. 2019 May 31;12(1):79. doi: 10.1186/s12920-019-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Babikir H., Müller S., Yagnik G., Shamardani K., Catalan F., Kohanbash G., Alvarado B., Di Lullo E., Kriegstein A., Shah S., Wadhwa H., Chang S.M., Phillips J.J., Aghi M.K., Diaz A.A. The phenotypes of proliferating glioblastoma cells reside on a single Axis of variation. Canc. Discov. 2019 Dec;9(12):1708–1719. doi: 10.1158/2159-8290.CD-19-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z., Wang J., Gong L., Wen Z., Xu C., Huang X. Correlation of Delta-like ligand 4 (DLL4) with VEGF and HIF-1α expression in human glioma. Asian Pac. J. Cancer Prev. APJCP. 2011;12(1):215–218. [PubMed] [Google Scholar]
- 32.Chargari C., Moncharmont C., Lévy A., Guy J.B., Bertrand G., Guilbert M., Rousseau C., Védrine L., Alphonse G., Toillon R.A., Rodriguez-Lafrasse C., Deutsch E., Magné N. Facteurs de radiorésistance des cellules souches cancéreuses et perspectives de radiosensibilisation : l'exemple du glioblastome. Bull. Cancer. 2012 Dec;99(12):1153–1160. doi: 10.1684/bdc.2012.1666. [DOI] [PubMed] [Google Scholar]
- 33.Jain K.K. A critical overview of targeted therapies for glioblastoma. Front Oncol. 2018;8:419. doi: 10.3389/fonc.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W., He Y.F., Sun Q.K., Wang Y., Han X.H., Peng D.F., Yao Y.W., Ji C.S., Hu B. Hypoxia-inducible factor 1α in breast cancer prognosis. Clin. Chim. Acta. 2014 Jan 20;428:32–37. doi: 10.1016/j.cca.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Kim B.W., Cho H., Chung J.Y., Conway C., Ylaya K., Kim J.H., Hewitt S.M. Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J. Transl. Med. 2013 Aug 8;11:185. doi: 10.1186/1479-5876-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H., Yang Q., Lian X., Jiang P., Cui J. Hypoxia-inducible factor-1α (HIF-1α) promotes hypoxia-induced invasion and metastasis in ovarian cancer by targeting matrix metallopeptidase 13 (MMP13) Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019 Sep 25;25:7202–7208. doi: 10.12659/MSM.916886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L.Y., Zhu P., Jin X.P. Association between the expression of HIF-1α and VEGF and prognostic implications in primary liver cancer. Genet. Mol. Res. 2016 May 9;15(2) doi: 10.4238/gmr.15028107. [DOI] [PubMed] [Google Scholar]
- 38.Sheehan J.P., Shaffrey M.E., Gupta B., Larner J., Rich J.N., Park D.M. Improving the radiosensitivity of radioresistant and hypoxic glioblastoma. Future Oncol. 2010 Oct;6(10):1591–1601. doi: 10.2217/fon.10.123. [DOI] [PubMed] [Google Scholar]
- 39.Zhao S., Lin Y., Xu W., Jiang W., Zha Z., Wang P., Yu W., Li Z., Gong L., Peng Y., Ding J., Lei Q., Guan K.L., Xiong Y. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009 Apr 10;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasmim M., Messai Y., Noman M.Z., Chouaib S.L. 'hypoxie tumorale - un déterminant clé de la réactivité stromale et de la réponse antitumorale. Med. Sci. 2014 Apr;30(4):422–428. doi: 10.1051/medsci/20143004017. [DOI] [PubMed] [Google Scholar]
- 41.Hasmim M., Messai Y., Ziani L., Thiery J., Bouhris J.H., Noman M.Z., Chouaib S. Critical role of tumor microenvironment in shaping NK cell functions: implication of hypoxic stress. Front. Immunol. 2015 Sep 23;6:482. doi: 10.3389/fimmu.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson C.M., Choi J., Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat. Immunol. 2019 Sep;20(9):1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 43.Noch E.K., Ramakrishna R., Magge R. Challenges in the treatment of glioblastoma: multisystem mechanisms of therapeutic resistance. World Neurosurg. 2018 Aug;116:505–517. doi: 10.1016/j.wneu.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Sumiyoshi Y., Kakeji Y., Egashira A., Mizokami K., Orita H., Maehara Y. Overexpression of hypoxia-inducible factor 1alpha and p53 is a marker for an unfavorable prognosis in gastric cancer. Clin. Canc. Res. 2006 Sep 1;12(17):5112–5117. doi: 10.1158/1078-0432.CCR-05-2382. [DOI] [PubMed] [Google Scholar]
- 45.Calvert A.E., Chalastanis A., Wu Y., Hurley L.A., Kouri F.M., Bi Y., Kachman M., May J.L., Bartom E., Hua Y., Mishra R.K., Schiltz G.E., Dubrovskyi O., Mazar A.P., Peter M.E., Zheng H., James C.D., Burant C.F., Chandel N.S., Davuluri R.V., Horbinski C., Stegh A.H. Cancer-associated IDH1 promotes growth and resistance to targeted therapies in the absence of mutation. Cell Rep. 2017 May 30;19(9):1858–1873. doi: 10.1016/j.celrep.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paldor I., Drummond K.J., Kaye A.H. IDH1 mutation may not be prognostically favorable in glioblastoma when controlled for tumor location: a case-control study. J. Clin. Neurosci. 2016 Dec;34:117–120. doi: 10.1016/j.jocn.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Bayley J.P., Devilee P. Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr. Opin. Genet. Dev. 2010 Jun;20(3):324–329. doi: 10.1016/j.gde.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Zhao S., Lin Y., Xu W., Jiang W., Zha Z., Wang P., Yu W., Li Z., Gong L., Peng Y., Ding J., Lei Q., Guan K.L., Xiong Y. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009 Apr 10;324(5924):261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]