Abstract
Despite the general use of endoxylanases in poultry feed to improve broiler performance, the abundance of different endoxylanase products and the variable response to their application in the field prevent a clear understanding of endoxylanase functionality in vivo. To gain insight into this functionality, we investigated the impact of endoxylanase type (Belfeed from Bacillus subtilis versus Econase XT from Nonomuraea flexuosa) and dose (10, 100, 1,000 mg/kg) in combination with broiler age on arabinoxylan (AX) hydrolysis and fermentation in broilers (Ross 308) fed a wheat-soy based diet. In a digestibility trial and a performance trial, a total of 1,057 one-day-old chicks received the control diet or 1 of the 6 endoxylanase supplemented wheat-soy based diets with, respectively, 5 replicate cages and 8 replicate pens per dietary treatment per trial. The AX content and structure, the AX digestibility values and the short-chain fatty acids produced were analysed at the level of the ileum, caeca and excreta at d 11 and 36. Endoxylanase supplementation resulted in a more extensive solubilisation of wheat AX and a reduction in the intestinal viscosity compared to the control (P < 0.05). A high endoxylanase dose was, however, required to obtain increased hydrolysis of the dietary AX along the gastrointestinal tract against the control (P < 0.001). Depending on the type of endoxylanase, a pool of AX with distinct physicochemical properties was created. The B. subtilis endoxylanase created a large pool of soluble AX in the ileum, thereby increasing ileal viscosity compared to broilers fed an endoxylanase from N. flexuosa (P < 0.001). The N. flexuosa endoxylanase mainly triggered caecal AX fermentation in young broilers, by delivering easily fermentable AX substrates with a low degree of polymerisation (P = 0.03). The effects were particularly present in young broilers (d 11). From this study, it is clear that the type and dose of endoxylanase added to wheat-soy based diets determine the nature of AX substrates formed. These, in turn, affect the intestinal viscosity and the interplay between the dietary AX compounds and microbiota, hence dictating AX digestion at young broiler ages and performance outcomes towards slaughter age.
Keywords: Broiler, Arabinoxylan, Feed enzyme, Endoxylanase, Digestion
1. Introduction
In many European countries, wheat (Triticum aestivum L.) is by far the most important energy source in poultry diets (Austin et al., 1999). However, the nutritive value of wheat highly depends on the amount and nature of the non-starch carbohydrates (NSC) present in wheat cell walls (Amerah, 2015; Choct, 1997). These wheat cell walls contain large amounts of arabinoxylans (AX) and are known to evoke antinutritional effects in the gastrointestinal tract (GIT) of poultry (Annison and Choct, 1991; Bach Knudsen, 2014). The major part of AX in wheat cell walls is water-unextractable arabinoxylan (WU-AX), but only a small part of the AX is water-extractable arabinoxylan (WE-AX) (Maes and Delcour, 2002; Saulnier et al., 2007). Due to their multiple cross-links with other AX or cell wall components (Izydorczyk et al., 1998), the WU-AX forms a physical barrier to the endogenous digestive enzymes, and makes the enclosed nutrients unavailable for digestion (Bedford, 2002; Carré, 2004; Courtin and Delcour, 2002; Mares and Stone, 1973). On the other hand, the viscous nature of the high molecular weight (MW) WE-AX (Izydorczyk and Biliaderis, 1995) can induce a high intestinal viscosity in the bird, which further impairs nutrient digestion (Choct and Annison, 1990, 1992). To overcome these antinutritional effects, NSC-hydrolysing enzymes, particularly endo-β-1,4-xylanases (EC 3.2.1.8, endoxylanases), are commonly added to poultry feeds (Collins et al., 2005; Cowieson et al., 2006). These endoxylanases are the main depolymerising enzymes, randomly hydrolysing the internal β-1,4-linkages of wheat AX polymers (Collins et al., 2005). This solubilisation through hydrolysis of the wheat WU-AX polymers and the concomitant reduction of the MW of the solubilised AX by endoxylanases (Grootaert et al., 2007), can release AX-derived oligosaccharides, i.e. arabinoxylan-oligosaccharides (AXOS) and render them more susceptible to fermentation by the caecal microbiota (Courtin et al., 2008a; Lee et al., 2017). The resultant increase in short-chain fatty acids (SCFA) production upon endoxylanase addition can eventually lead to major improvements in the broiler's gastrointestinal health and digestive parameters (Choct et al., 1999; Kiarie et al., 2013, 2014; Lee et al., 2017).
Multiple commercial enzyme products containing endoxylanase activity are approved by the European Commission to be used as feed additives in poultry feed (European Commision, 2009). These preparations originate from different microorganisms and are characterised by different structural and biochemical properties, such as pH and temperature optima and substrate specificities (Beg et al., 2001; Polizeli et al., 2005; Sunna and Antranikian, 1997; Vandeplas et al., 2010). It can be assumed that these various endoxylanases hydrolyse the wheat AX polymers differently in the GIT. As a result, a heterogeneous pool of AX hydrolysis products could be formed, which could stimulate the digestive and fermentation processes differently. This has, however, not been experimentally demonstrated in vivo yet.
The large variability in enzyme-related factors in combination with the wide variety in broiler-specific and diet-related factors on broiler farms (Ravindran, 2013), makes it difficult to understand the full complexity and the mechanisms of action of these enzymes in broilers in vivo. This might partly explain the large inconsistencies in beneficial responses that result from endoxylanase addition that have been observed in the field and are difficult to explain (Aftab and Bedford, 2018). It is therefore of interest to analyse the changes in the AX population along the broiler's GIT induced by different types and doses of endoxylanases, commonly used in European broiler feeds. By analysing the difference in the extent of hydrolysis by various doses and types of endoxylanases in vivo, more insight can be gained in how the nature of AX substrates created upon enzyme addition can affect gastrointestinal physiology and broiler performance outcomes differently. For example, on the one hand, overdosing of endoxylanases could result in too extensive hydrolysis of AX polymers, causing the production of arabinose and xylose, which are known for their detrimental effect in the GIT when present in large amounts (Schutte, 1990). On the other hand, underdosing can slow down the MW reduction of the viscous AX polymers, resulting in an inadequate reduction of the intestinal viscosity and formation of AXOS.
The objective of this study was, hence, to investigate the contribution of the nature and the dose of 2 endoxylanase preparations in combination with broiler age, i.e. young versus older broilers, on AX hydrolysis and fermentation characteristics in the distal part of the broiler's digestive tract. By analysing the pattern and extent of hydrolysis, a better understanding of the underlying mechanisms of action of these enzymes including the reduction of the intestinal viscosity, the solubilisation of wheat cell walls and the provision of prebiotic oligosaccharides to the microbiota, was achieved.
2. Materials and methods
This broiler trial was approved by the Ethical Committee for the experimental use of animals of the KU Leuven under accession number P213/2015. The animal experiment was conducted following the appropriate EU guidelines for animal experiments (Directive 2010/63/EU, 2010).
2.1. Experimental diets
The 7 experimental diets of this broiler trial were wheat-soy based and formulated to meet Ross 308 broiler nutrient requirements based on the nutrition guidelines of Aviagen (2014a). The nutrient specifications of the wheat-soy based diets are documented in Table 1. Basal diets were supplemented with an enzyme preparations (Belfeed B1100 MP [Beldem, Andenne, Belgium] or Econase XT 25P [AB Vista, Marlborough, UK] endoxylanase preparation at a dosage rate of 10 mg/kg of complete feed or 100 mg/kg of complete feed (recommended dose by the feed manufacturer) or 1,000 mg/kg of complete feed, resulting in 6 experimental diets: Bel10, Bel100, Bel1000, Eco10, Eco100 and Eco1000. Belfeed B1100 MP contains a glycohydrolase (GH) family 11 endoxylanase from Bacillus subtilis, and Econase XT 25P contains a GH family 11 endoxylanase from Nonomuraea flexuosa. The basal diet without the addition of an endoxylanase preparation was used as the control diet (CTRL). An indigestible marker, titanium dioxide (TiO2) (5.0 g/kg) and a coccidiostat (Sacox, Huvepharma, Antwerp, Belgium) were added to all experimental diets. To more accurately assess the dose-effect of both endoxylanase preparations on gastrointestinal parameters, the formulated energy value of the basal diet was 100 kcal/kg lower than that recommended in the nutrition specifications of Aviagen (2014a), and no phytase was added to the experimental diets. Diets were formulated at Research Diet Services (Wijk bij Duurstede, The Netherlands). The wheat was milled to pass through a 3-mm screen in a hammer mill and afterwards mixed with other dietary ingredients to form pellets. Broilers were subjected to 3 feeding phases: a starter (d 1 – 11, pellets crushed into a crumble), a grower (d 11 – 25, pellets) and a finisher diet (d 25 – 36, pellets).
Table 1.
Basal diet composition of the wheat-soy based starter, grower and finisher diets.
| Item | Starter | Grower | Finisher |
|---|---|---|---|
| Ingredients, g/kg | |||
| Wheat | 602 | 618 | 651 |
| Soybean meal (48.5% CP) | 280 | 265 | 235 |
| Potato protein | 20.0 | 10.0 | 0.00 |
| Soybean oil | 43.5 | 56.0 | 67.0 |
| Premix1 | 5.00 | 5.00 | 5.0 |
| Lime fine | 15.8 | 14.2 | 12.8 |
| Monocalciumphosphate | 15.6 | 14.0 | 12.5 |
| Salt | 1.80 | 1.80 | 1.90 |
| Sodium carbonate | 3.90 | 3.90 | 3.40 |
| L-lysine HCl | 2.35 | 2.20 | 2.15 |
| DL-Methionine | 2.80 | 2.65 | 2.25 |
| L-Threonine | 1.20 | 1.05 | 1.00 |
| L-arginine | 0.30 | 0.05 | 0.00 |
| Magnesiumoxide | 1.20 | 1.30 | 1.40 |
| Calculated nutrient content | |||
| Metabolisable energy, kcal/kg | 2,924 | 2,999 | 3,075 |
| Dry matter, g/kg | 882 | 883 | 884 |
| Crude protein, g/kg | 224 | 210 | 191 |
| Crude fat, g/kg | 55.8 | 68.0 | 78.6 |
| Crude fibre, g/kg | 24.5 | 24.3 | 23.9 |
| Carbohydrates, g/kg | 507 | 514 | 528 |
| Calcium, g/kg | 9.60 | 8.70 | 7.90 |
| Total phosphorus, g/kg | 7.20 | 6.70 | 6.30 |
| Available phosphorus, g/kg | 4.80 | 4.40 | 4.00 |
| Digestible lysine, % | 1.19 | 1.09 | 0.96 |
| Digestible leucine, % | 1.44 | 1.33 | 1.18 |
| Digestible methionine + cysteine, % | 0.88 | 0.83 | 0.74 |
| Digestible arginine, % | 1.27 | 1.17 | 1.04 |
| Digestible threonine, % | 0.80 | 0.73 | 0.64 |
| Digestible tryptophan, % | 0.24 | 0.23 | 0.20 |
| Incorporation level of enzyme preparation, mg/kg | |||
| Control diet (CTRL) | 0 | 0 | 0 |
| Belfeed 1100 MP (Bel10) | 10 | 10 | 10 |
| Belfeed 1100 MP (Bel100) | 100 | 100 | 100 |
| Belfeed 1100 MP (Bel1000) | 1,000 | 1,000 | 1,000 |
| Econase XT 25P (Eco10) | 10 | 10 | 10 |
| Econase XT 25P (Eco100) | 100 | 100 | 100 |
| Econase XT 25P (Eco1000) | 1,000 | 1,000 | 1,000 |
CP = crude protein.
Mineral-vitamin premix provided per kg diet: vitamin A 10,000 IU; vitamin D3 2,500 IU; vitamin E 50 mg; vitamin K3 1.5 mg; vitamin B1 2.0 mg; vitamin B2 7.5 mg; vitamin B6 3.5 mg; vitamin B12 20 μg; niacinamide 35 mg; D-pantothenic acid 12 mg; choline chloride 460 mg; folic acid 1.0 mg; biotin 0.2 mg; iron 80 mg; copper 12 mg; manganese 85 mg; zinc 60 mg; iodine 0.8 mg; selenium 0.15 mg.
2.2. Broiler housing and experimental design
A total of 1,057 one-day-old male broilers (Ross 308) were purchased from a commercial hatchery (Belgabroed NV, Merksplas, Belgium) and upon arrival evenly and randomly assigned to 63 floor pens covered with wood shavings litter. Each dietary treatment group had 8 replicate pens with 12 broilers per pen to estimate broiler performance parameters. In addition, 55 broilers per dietary treatment were held in 7 sampling pens, which were specifically used for digestibility measurements.
Circa 7 d before sampling, broilers were randomly picked from the sampling pens and placed in digestibility cages to accurately collect their excreta during the period in which the broilers resided in the digestibility cages. The cages (39 cm × 52 cm) had a wire floor, a feed trough at the front, a drinking cup at the backside and a plastic tray underneath the cage. This tray was placed under the cage for proper excreta collection. Because the amount of digesta in young broilers, especially in the caeca, is often limited, and to have sufficient digesta samples for chemical measurements, the number of chicks residing in one cage and sampled, was varied with age. More specifically, 3, 2 and 2 chicks were placed in one digestibility cage at d 4, 15 and 28, respectively. Each dietary treatment group had five replicate digestibility cages. Samplings were scheduled when broilers were 11, 22 and 36 d of age and digesta of chicks were pooled cage-wise during these sampling days.
All broilers received water and feed ad libitum. The broilers were kept under conventional conditions for lighting, heating and ventilation (Aviagen, 2014b). The light schedule consisted of 23 h of light and 1 h of darkness during the first week of age and was changed to 18 h of light and 6 h of darkness after they were 7 d of age. Housing temperature was initially set at 34 °C and gradually decreased to 20 °C over the growing period and was kept at 20 °C until slaughter age (36 d). There was a daily inspection of housing conditions, water and feed supply as well as the health status of the broilers and chick mortality. Chicks with compromised health were excluded from the experiment.
2.3. Performance parameters
Body weights (BW) were measured individually on d 1, 11, 22, and 36. During the periods of d 1 – 11, d 1 – 22 and d 1 – 36, feed intake was recorded per pen, and the feed conversion ratio (FCR) calculated. The FCR was corrected for chick mortality.
2.4. Collection of digesta samples and weights
At 3 different ages, i.e. d 11, 22 and 36, all chicks in the digestibility cages were weighed and euthanised by electronarcosis followed by decapitation. Intestinal contents from the ileum and caeca were collected by gently finger stripping these GIT segments and subsequently pooled per cage in 1 tube, resulting in 5 replicate pools. Excreta samples were collected from the plastic tray underneath each digestibility cage. After weighing and dividing these digesta and excreta samples over different tubes, they were stored at −20 °C until further analysis of the AX and TiO2 content. Digesta samples for SCFA analysis were stored at −80 °C. The weights of the full caeca, empty gizzard and small intestine (jejunum + ileum), the pancreas, the circumference of the caecal opening and the pH of the gizzard content were measured as well.
2.5. Moisture content
Moisture contents of feed and digesta samples were analysed to calculate the dry matter (DM) content for these samples. The feed moisture content was assessed by drying the feed samples at 130 °C for 15 h in a hot air oven, whereas the moisture content of digesta samples was assessed according to a specific temperature scheme described by Bautil et al. (2019).
2.6. Analysis of arabinoxylan content and average degree of polymerisation
Total arabinoxylan (TOT-AX) and WE-AX contents (g/kg DM) were determined on feed, and freeze-dried and homogenised ileal, caecal and excreta samples using an adapted Englyst method (Englyst and Cummings, 1984; Gebruers et al., 2009). Quantification of arabinose and xylose content in aqueous extracts (containing WE-AX) and in the freeze-dried digesta and feed samples as such (containing TOT-AX) was done by gas chromatography (GC) after their hydrolysis to monosaccharides, subsequent reduction with sodium borohydride and derivatisation to alditol acetates, as previously described by Bautil et al. (2019). The extraction procedure to yield aqueous extracts containing WE-AX was performed according to the procedure described by Bautil et al. (2019). In short, 2 inactivation steps were included in the extraction procedure to prevent modification of the AX population by added or microbial-derived endoxylanases in digesta and feed samples during extraction. These inactivation steps consisted of firstly heating the samples in ethanol/water solution (800 mL/L) at 95 °C, and secondly using 10 mL of a potassium chloride/hydrogen chloride (KCl–HCl) buffer (20 mmol/L, pH 3.0) instead of distilled water during the extraction (30 min, 7 °C). After GC analysis, TOT-AX and WE-AX contents in both the feed and digesta samples themselves and their aqueous extracts were calculated by summing up 0.88 times the arabinose and xylose content. AX content is expressed on DM base (g/kg DM sample).
To determine the average degree of polymerisation (avDP) of the WE-AX, reducing end xylose contents in the aqueous extracts (2.5 mL) of ileal and caecal samples were analysed similarly to the procedure described above for the determination of WE-AX content. Prior to hydrolysis, aqueous extracts of the digesta samples were reduced, followed by acetylation of the resulting monosaccharides into alditol acetates which were separated using GC (Courtin et al., 2000). The avDP of WE-AX was calculated as the sum of the arabinose and xylose contents divided by the amount of reducing end xylose present in the aqueous extracts.
2.7. Quantification of TiO2
The indigestible marker TiO2 was measured in feed and digesta samples according to a downscaled method of Short et al. (1996) with modifications proposed by Myers et al. (2004) as previously described by Bautil et al. (2019). In short, dried digesta or feed samples (0.1 g) underwent acidic digestion using 12 mL of concentrated (18.4 mol/L) sulfuric acid and 3.5 g of a copper catalyst in macro-Kjeldahl tubes at 420 °C for 60 min. After a cooling step of 30 min, the TiO2 was precipitated by adding 10 mL of hydrogen peroxide solution (300 g/L), and subsequently, precipitates were removed by filtration. The extinction values were measured at 410 nm against a control consisting of deionised water.
2.8. Arabinoxylan digestibilities
Coefficients of TOT-AX and WE-AX digestibility at the level of the ileum, caeca and excreta (i.e. total tract digestibility coefficient) were calculated according to the following formula (Bautil et al., 2019):
with [TiO2]feed and [TiO2]digesta the measured TiO2 contents (g/kg DM) in the feed and digesta, respectively, and with [AX]feed and [AX]digesta the AX content (g/kg DM) (TOT-AX content for TOT-AX digestibility calculation and WE-AX content for WE-AX digestibility calculation) in the feed and digesta, respectively.
The meaning of the calculated AX digestibility coefficients is described in detail by Bautil et al. (2019). In short, the ingested dietary TOT-AX, consisting of WU-AX and WE-AX, would be hydrolysed into different AX substrates by endoxylanases that were added to the diet or that were produced by microorganisms in the GIT. Hydrolytic solubilisation of the WU-AX would result in the creation of enzyme-solubilised WE-AX. Hence, a relatively higher amount of WE-AX would be present in the GIT compared to the amount of native dietary WE-AX that was initially ingested by the broiler. This solubilisation process will be noticeable by the occurrence of negative WE-AX digestibility coefficients in our data. Further hydrolysis and MW reduction of this pool of WE-AX substrates result in the formation of AX oligomers, which can be fermented by the intestinal microbiota. This fermentation process will lead to an increase in the WE-AX digestibility coefficients and will finally result in positive TOT-AX digestibility coefficients in our data. AX fermentation here refers to the utilisation of AX oligo- and monosaccharides as nutrients by the resident microbiota in the GIT, which in general results in the production of different microbial metabolites such as SCFA. Hence in this article, the term AX digestion/digestibility is used in the broad sense, i.e. as a collective term to describe the combination of AX hydrolysis and fermentation processes resulting in the disappearance of AX in the broiler's GIT.
2.9. Extract and digesta viscosity
The extract viscosity of the experimental diets was determined by analysing 500 μL of the supernatant of aqueous extracts using a Brookfield DV-II + viscometer (Brookfield Engineering Laboratories Inc., Stoughton, MA, USA) with a CP40 cone and a constant shear rate of 750 s−1 at 37 °C. Feed aqueous extracts were made by suspending 1.0 g of feed in 4.0 mL KCl–HCl buffer (20 mmol/L, pH 3.0). The supernatants of these extracts were recovered after 2 centrifugation steps (2,800 × g for 10 min and 21,000 × g for 2 min at 7 °C; Himac CT15RE centrifuge, Hitachi, Japan).
Digesta viscosity was assessed immediately after sampling of the broilers according to the procedure described by Bautil et al. (2019). A total of 2.0 g of ileal content was introduced in a tube and centrifuged at 21,000 × g for 15 min at 7 °C (Himac CT15RE centrifuge, Hitachi, Japan). The resulting supernatant was kept on ice and its viscosity value immediately measured by using a Brookfield DV-II + viscometer (Brookfield Engineering Laboratories Inc.) with a CP40 cone and a constant shear rate of 90 s−1 at 37 °C.
2.10. In-feed endoxylanase activity
The endoxylanase activity (toward WU-AX) present in the feed after pelleting was determined for the 7 experimental diets using a similar procedure as the Xylazyme AX method (Megazyme, Bray, Ireland) previously described by Bautil et al. (2019). Enzymes present in feed samples were extracted by suspending 1.0 g of feed in 10 mL of a sodium acetate buffer (25 mmol/L, pH 5.0). Following shaking (30 min, 7 °C), feed suspensions were centrifuged (10 min, 4,000 × g, 7 °C) and filtered (MN 65 filter, Macherey-Nägel, Düren, Germany) to yield the aqueous feed extracts containing the enzymes. Prior to endoxylanase activity measurement, the aqueous feed extracts were diluted to an appropriate concentration for further use in the activity assay. For this assay, 1.0 mL of (diluted) aqueous feed extract was incubated with one azurine cross-linked AX tablet at 40 °C. After 60 min of incubation, the reaction was stopped by adding 10 mL of tris(hydroxymethyl)aminomethane aqueous solution (10.0 g/L), followed by filtration of the suspension. Extinction values of the blue-coloured extracts were measured at 590 nm (Ultraspec II UV/vis spectrophotometer) against a control, prepared by incubating the aqueous feed extract without an AX tablet. In-feed endoxylanase activities are expressed as endoxylanase activity units (EU) per gram DM of complete feed. One unit is defined as the amount of endoxylanase activity needed to yield a corrected extinction value of 1.0 per hour of incubation under the conditions of the assay.
2.11. SCFA analysis
Analysis of the non-branched SCFA acetic, propionic, butyric and valeric acid and branched short-chain fatty acids (BCFA) isobutyric and isovaleric acid present in fresh samples and originating from the ileum and caeca of broilers at slaughter age (d 36) was done according to the method described by Van Craeyveld et al. (2008) with slight adaptations. Before extraction, 0.1 mL of 2-ethyl butyric acid (internal standard) and 0.4 mL of NaCl solution (250 g/L) were added to test tubes containing individual ileal or caecal samples (0.5 g fresh weight). By adding 0.2 mL of 9.2 mol/L sulfuric acid, anions or salts of the SCFA were neutralised. SCFA were extracted with diethyl ether (0.8 mL) by shaking the test tubes for 2 min (60 r/min, 7 °C). After centrifugation (5 min, 1,000 × g, 7 °C; Sigma 6-16K centrifuge, Newton, UK), the ether phase containing the extracted SCFA and BCFA was recovered. Aliquots (2.0 μL) of these organic acids were injected in an Agilent 6890 Series gas chromatograph (Wilmington, DE, USA) equipped with a capillary FFA packed column [J&W DB-FFAP GC column (Bellefonte, PA, USA), 30 m × 0.53 mm, film thickness 1.0 μm], and with helium as the carrier gas. The column temperature gradually increased from 100 to 235 °C, and the injector and the flame ionisation detector temperatures were 200 and 245 °C, respectively. The concentration of SCFA was calculated using the internal standard and a calibration standard solution, i.e. a commercially available volatile free acid mix (#46975-U, Sigma–Aldrich, Bornem, Belgium) containing 9.8 mmol/L, acetic acid, 9.9 mmol/L of formic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic and hexanoic acid, and 10.0 mmol/L n-heptane acid. The concentration of each SCFA was expressed as micromoles per gram DM of digesta sample.
2.12. Statistical analysis
Data were analysed statistically by ANOVA using the JMP Pro 14.0.0 software (SAS Institute Inc., Cary, NC, USA). Performance data were presented as means (n = 8) with one pen per dietary treatment being the experimental unit. Data from the chemical analyses were pooled in a cage-wise fashion and presented as means (n = 5). Cages with either 3, 2, 2 broilers at d 11, 22, and 36, respectively, formed the experimental unit. Performance data were analysed in the periods from d 1 to 11, 1 to 22 and 1 to 36, and data of the GIT weights (log-transformed) and ileal viscosity were analysed at 3 different broiler ages (d 11, 22 and 36). Data of the other chemical analyses were only analysed at 11 and 36 d of age. Due to the limited material at young ages, production of SCFA in digesta samples was only analysed at d 36.
All chemical data were tested using two-way ANOVA with dietary treatment, age and their interaction age × dietary treatment as model effects. In addition to the global F-test, to detect significantly different means from the control group, dietary treatment means were further identified by the posthoc Dunnett's test. An additional three-way ANOVA from which the data of the control group were excluded, was conducted to detect significant differences between the dose and type of endoxylanase preparations applied. In this analysis, the type of enzyme, dose, age and their second-order interactions were used as model effects. In the three-way ANOVA, significantly different means were identified by the posthoc Tukey's HSD test. Due to the fact that SCFA were measured only at d 36, and therefore no age effect can be quantified, data from the SCFA analysis as well as performance data were analysed using one-way ANOVA with treatment and a two-way ANOVA (control group excluded) with dose, enzyme and the interaction dose × enzyme as model effects. In the one-way ANOVA, significant differences with the control group were evaluated, and the differences in-between the dose and type of preparation added were tested using the two-way ANOVA. Significantly different means were further identified by performing the posthoc Tukey's HSD test in the ANOVAs described above. Statistical significance was defined at P < 0.05, and a tendency was declared in the event 0.05 ≤ P ≤ 0.10.
3. Results
3.1. Diets, broiler performance and gastrointestinal weights
The analysed contents of TOT-AX, WE-AX, and TiO2 in the experimental diets were not expected to differ notably among each feeding phase (Table 2). However, TOT-AX and TiO2 content were slightly different in the grower and starter diets, respectively. The WE-AX content was higher in diets supplemented with a high dose of the endoxylanase preparations, and their extract viscosity was lower (Table 2).
Table 2.
Analysed contents of total arabinoxylan (TOT-AX), water-extractable arabinoxylan (WE-AX) and titanium dioxide (TiO2), and measured extract viscosity and endoxylanase activity (act.) on water-unextractable arabinoxylan (WU-AX) of the 7 experimental diets used in this broiler trial.1
| Variables | TOT-AX, g/kg DM | WE-AX, g/kg DM | TiO2, g/kg DM | Extract viscosity, cP | Endoxylanase act. (WU-AX), EU/g |
|---|---|---|---|---|---|
| Starter | |||||
| CTRL | 53.93 | 3.95 | 6.37 | 1.11 | 0.52 |
| Bel10 | 51.05 | 4.20 | 5.71 | 1.20 | 0.63 |
| Bel100 | 58.33 | 4.24 | 5.67 | 1.27 | 1.05 |
| Bel1000 | 58.22 | 4.44 | 6.13 | 1.11 | 5.33 |
| Eco10 | 50.88 | 3.96 | 6.92 | 1.25 | 3.01 |
| Eco100 | 56.71 | 4.01 | 5.86 | 1.07 | 11.66 |
| Eco1000 | 54.75 | 4.59 | 5.91 | 0.97 | 91.49 |
| Grower | |||||
| CTRL | 50.98 | 3.23 | 6.75 | 1.01 | 0.66 |
| Bel10 | 51.68 | 3.57 | 6.93 | 1.13 | 0.69 |
| Bel100 | 51.65 | 3.54 | 6.22 | 1.29 | 1.12 |
| Bel1000 | 49.79 | 3.37 | 6.50 | 1.12 | 5.18 |
| Eco10 | 54.41 | 3.40 | 6.32 | 1.11 | 1.50 |
| Eco100 | 46.07 | 3.50 | 6.32 | 1.07 | 12.12 |
| Eco1000 | 55.75 | 4.38 | 6.01 | 0.93 | 96.87 |
| Finisher | |||||
| CTRL | 56.53 | 3.11 | 6.14 | 0.99 | 0.76 |
| Bel10 | 54.64 | 2.94 | 6.09 | 1.20 | 0.77 |
| Bel100 | 56.68 | 3.05 | 5.97 | 1.33 | 1.18 |
| Bel1000 | 57.48 | 3.46 | 5.68 | 1.11 | 5.54 |
| Eco10 | 50.63 | 3.14 | 6.03 | 1.09 | 2.18 |
| Eco100 | 50.18 | 3.52 | 5.57 | 1.06 | 12.11 |
| Eco1000 | 55.27 | 4.81 | 6.27 | 0.93 | 97.35 |
| Pooled SEM | 0.61 | 0.09 | 0.06 | 0.02 | 2.82 |
EU = endoxylanase activity units; SEM = standard error of the mean; DM = dry matter.
CTRL, control diet; Bel, Belfeed supplemented diet; Eco, Econase supplemented diet. Given parameters were at least analysed in triplicate (n = 3).
Remarkably, recovered endoxylanase activities in the Bel10 and Bel100 diets were similar to that in the control diet. Higher endoxylanase activities were found in diets supplemented with Econase compared to the ones supplemented with Belfeed (Table 2).
Broiler performance in response to Econase and Belfeed addition at different doses is shown in Table 3. Body-weight gains were improved with Belfeed compared to Econase addition from 3 wk of age (P = 0.005; Table 3). On the other hand, during the starter period, feed intake was reduced for broilers receiving the Econase preparation compared to broilers receiving the Belfeed or no endoxylanase preparation (CTRL) (P = 0.016). Addition of an endoxylanase over the entire 36 d period resulted in a remarkably lower FCR compared to the control, especially for the Bel1000 treatment (P = 0.08). In addition, including 1,000 mg/kg of either endoxylanase preparation in the broiler's feed tended to positively affect the FCR over this entire period (P = 0.09; Table 3).
Table 3.
Body-weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) of broilers fed the 7 experimental diets.
| Performance parameters1 | BWG, g/broiler |
FI, g/broiler |
FCR, g/g |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 – 11 d | 1 – 22 d | 1 – 36 d | 1 – 11 d | 1– 22 d | 1 – 36 d | 1 – 11 d | 1 – 22 d | 1 – 36 d | |
| CTRL | 252 | 945 | 2,321 | 308 | 1,348a | 3,542 | 1.22 | 1.43 | 1.53 |
| Bel10 | 256 | 942 | 2,317 | 305 | 1,244 ab | 3,506 | 1.19 | 1.32 | 1.51 |
| Bel100 | 258 | 976 | 2,358 | 304 | 1,281 ab | 3,545 | 1.19 | 1.31 | 1.50 |
| Bel1000 | 255 | 950 | 2,379 | 307 | 1,259 ab | 3,445 | 1.21 | 1.32 | 1.45 |
| Eco10 | 249 | 933 | 2,358 | 303 | 1,268 ab | 3,530 | 1.22 | 1.36 | 1.51 |
| Eco100 | 247 | 902 | 2,278 | 289 | 1,201b | 3,390 | 1.17 | 1.33 | 1.49 |
| Eco1000 | 249 | 914 | 2,287 | 289 | 1,243b | 3,429 | 1.16 | 1.35 | 1.50 |
| Pooled SEM | 17 | 45 | 89 | 16 | 76 | 128 | 0.06 | 0.10 | 0.05 |
| P ANOVA (diet) | 0.86 | 0.08 | 0.34 | 0.10 | 0.03 | 0.15 | 0.34 | 0.29 | 0.08 |
| Main effects | |||||||||
| Enzyme | |||||||||
| Belfeed | 256 | 956y | 2,352 | 305y | 1,261 | 3,499 | 1.20 | 1.32 | 1.49 |
| Econase | 248 | 916z | 2,300 | 294z | 1,234 | 3,450 | 1.18 | 1.35 | 1.50 |
| Pooled SEM | 5 | 15 | 29 | 5 | 28 | 41 | 0.02 | 0.04 | 0.01 |
| Dose, mg/kg | |||||||||
| 10 | 253 | 937 | 2,327 | 304 | 1,256 | 3,518 | 1.21 | 1.34 | 1.52 |
| 100 | 252 | 939 | 2,318 | 296 | 1,241 | 3,467 | 1.18 | 1.32 | 1.50 |
| 1,000 | 252 | 932 | 2,333 | 298 | 1,246 | 3,437 | 1.19 | 1.34 | 1.47 |
| Pooled SEM | 8 | 22 | 45 | 8 | 35 | 62 | 0.02 | 0.04 | 0.02 |
| P ANOVA | |||||||||
| Enzyme | 0.12 | 0.005 | 0.08 | 0.016 | 0.24 | 0.23 | 0.50 | 0.28 | 0.41 |
| Dose | 1.00 | 0.92 | 0.91 | 0.42 | 0.87 | 0.26 | 0.39 | 0.81 | 0.09 |
| Enzyme × Dose | 0.93 | 0.15 | 0.24 | 0.30 | 0.18 | 0.17 | 0.25 | 0.95 | 0.14 |
SEM = standard error of the mean.
a,b Means within one column having different superscripts are significantly different for the effect of dietary treatment (one-way ANOVA) (P ≤ 0.05).
y,z Means within one column having different superscripts indicate the presence of a significant difference for the main effect of type of enzyme (two-way ANOVA, control excluded from the data matrix) (P ≤ 0.05).
CTRL, control diet; Bel, Belfeed supplemented diet; Eco, Econase supplemented diet. Means were obtained from 8 replicate pens per treatment of 12 broilers per replicate pen.
The caecal weight was differently affected upon addition of a different type of endoxylanase. Higher relative weights of the caeca tended to be present for broilers receiving Econase than for broilers receiving Belfeed (P = 0.07; Table 4). This difference in caecal weight between Econase (0.72 ± 0.05 g/100 g BW) and Belfeed receiving broilers (0.57 ± 0.02 g/100 g BW) was, however, only detected at slaughter age (d 36), as no difference was observed at d 11 and 22 (0.96 ± 0.04 g/100 g BW and 1.00 ± 0.04 g/100 g BW, and 0.74 ± 0.05 g/100 g BW and 0.71 ± 0.06 g/100 g BW, respectively for Econase and Belfeed receiving broilers at d 11 and 22) (P = 0.04, interaction age × enzyme). In addition to their weights, the circumference of the caecal opening was also affected by dietary treatment: intermediate and high doses of endoxylanases tended to dilate the caecal entry compared to the low doses (P = 0.08). The caecal opening also increased with broiler age (P < 0.001; Table 4). No remarkable changes in the relative weights of the empty gizzard, small intestine and pancreas were observed. The pH of the gizzard was not affected by the addition of endoxylanases in the feed.
Table 4.
Relative caecal weight and circumference of the caecal opening for broilers in digestibility cages at d 11, 22 and 36 fed the 7 experimental diets.
| Variables | Caecal weight, g/100g BW |
Caecal opening, mm |
||||
|---|---|---|---|---|---|---|
| d 11 | d 22 | d 36 | d 11 | d 22 | d 36 | |
| Treatment groups1 | ||||||
| CTRL | 0.92 | 0.71 | 0.55 | 0.72 | 1.17 | 1.59 |
| Bel10 | 1.17∗ | 0.62 | 0.62 | 0.73 | 1.08 | 1.51 |
| Bel100 | 0.93 | 0.70 | 0.55 | 0.77 | 1.18 | 1.52 |
| Bel1000 | 0.90 | 0.80 | 0.54 | 0.77 | 1.13 | 1.49 |
| Eco10 | 0.90 | 0.61 | 0.64 | 0.75 | 1.11 | 1.48 |
| Eco100 | 0.92 | 0.75 | 0.77∗ | 0.78 | 1.15 | 1.57 |
| Eco1000 | 1.05 | 0.86 | 0.75∗ | 0.76 | 1.20 | 1.58 |
| Pooled SEM | 0.18 | 0.25 | 0.16 | 0.08 | 0.12 | 0.17 |
| Main effects | ||||||
| Age | ||||||
| d 11 | 0.97 ± 0.18a | 0.76 ± 0.08c | ||||
| d 22 | 0.72 ± 0.25b | 1.15 ± 0.12b | ||||
| d 36 | 0.63 ± 0.16c | 1.53 ± 0.17a | ||||
| Enzyme | ||||||
| Bel | 0.80 ± 0.08 | 1.07 ± 0.05 | ||||
| Eco | 0.83 ± 0.08 | 1.08 ± 0.05 | ||||
| Dose, mg/kg | ||||||
| 10 | 0.80 ± 0.08 | 1.04 ± 0.07 | ||||
| 100 | 0.80 ± 0.08 | 1.11 ± 0.05 | ||||
| 1,000 | 0.84 ± 0.11 | 1.09 ± 0.05 | ||||
| P ANOVA | ||||||
| Age | <0.001 | <0.001 | ||||
| Enzyme | 0.07 | 0.34 | ||||
| Dose | 0.59 | 0.08 | ||||
| Age × Enzyme | 0.04 | 0.94 | ||||
| Age × Dose | 0.07 | 0.98 | ||||
| Dose × Enzyme | 0.014 | 0.83 | ||||
BW = body-weight; SEM = standard error of the mean.
∗Indicate treatment means which differ significantly from the control mean (P < 0.10); analysed according to the posthoc Dunnett's test.
a,b,c Means having different superscripts in a particular column for the main effect are significantly different; analysed according to the posthoc Tukey's HSD test (P < 0.05). Means are represented as means ± SEM.
CTRL, control diet; Bel, Belfeed supplemented diet; Eco, Econase supplemented diet. Reported values are means from 15, 10 and 10 individual birds for each dietary treatment group at d 11, 22 and 36, respectively.
3.2. Ileal viscosity
Addition of an endoxylanase preparation in the broiler's diet reduced the ileal viscosity compared to the control, especially when broilers were 22 d of age (P < 0.05; Table 5). However, endoxylanase supplementation in doses ten times lower than the commercially relevant ones (100 mg/kg) resulted in viscosity values that were not significantly different from those for broilers fed the control diet, especially for the Bel10 diet (P = 0.99). Comparing the types of endoxylanase added, Belfeed induced higher ileal viscosity values compared to supplementation of Econase at all broiler ages (P < 0.001; Table 5). However, this difference in ileal viscosity between Belfeed and Econase tended to be mainly present when doses of 10 mg/kg were included in the feed (P = 0.06). The higher the dose added, the greater was the reduction in ileal viscosity (P < 0.001; Table 5).
Table 5.
Ileal viscosity for the cage-wise pooled ileal digesta samples of broilers in digestibility cages at d 11, 22 and 36 fed the 7 experimental diets.
| Variable | Ileal viscosity, cP |
||
|---|---|---|---|
| d 11 | d 22 | d 36 | |
| Treatment groups1 | |||
| CTRL | 3.68 | 3.99 | 2.88 |
| Bel10 | 3.94 | 3.78 | 3.16 |
| Bel100 | 2.48∗ | 2.44∗∗ | 2.79 |
| Bel1000 | 2.55 | 2.33∗∗ | 1.95∗∗ |
| Eco10 | 2.60 | 2.78∗∗ | 2.81 |
| Eco100 | 2.43 | 2.29∗∗ | 2.00∗ |
| Eco1000 | 2.19∗∗ | 2.25∗∗ | 1.80∗∗ |
| Pooled SEM | 0.96 | 0.74 | 0.62 |
| Main effects | |||
| Age | |||
| d 11 | 2.72 ± 0.96 | ||
| d 22 | 2.64 ± 0.74 | ||
| d 36 | 2.39 ± 0.62 | ||
| Enzyme | |||
| Bel | 2.82 ± 0.39a | ||
| Eco | 2.33 ± 0.0.26b | ||
| Dose, mg/kg | |||
| 10 | 3.21 ± 0.0.46a | ||
| 100 | 2.39 ± 0.28b | ||
| 1,000 | 2.18 ± 0.29b | ||
| PANOVA | |||
| Age | 0.15 | ||
| Enzyme | <0.001 | ||
| Dose | <0.001 | ||
| Age × Enzyme | 0.78 | ||
| Age × Dose | 0.75 | ||
| Dose × Enzyme | 0.06 | ||
SEM = standard error of the mean.
∗,∗∗ indicate treatment means which differ significantly at P ≤ 0.10 and P < 0.05, respectively from the control mean; analysed according to the posthoc Dunnett's test.
a,b Means having different superscripts in a particular column for the main effect are significantly different; analysed according to the posthoc Tukey's HSD test (P < 0.05). Means are represented as means ± SEM.
CTRL, control diet; Bel, Belfeed supplemented diet; Eco, Econase supplemented diet. Reported values are means from 5 replicates from each dietary treatment group (n = 5).
3.3. Arabinoxylan hydrolysis and fermentation at the level of the ileum
TOT-AX and WE-AX content, and digestibility coefficients found in the ileum of broilers at d 11 and 36 are shown in Table 6, and Fig. 1, Fig. 2, respectively. Endoxylanase addition led to higher dietary AX solubilisation compared to the control at the level of the ileum, which was observed by an increase in the WE-AX content, especially for broilers receiving diets Bel100, Bel1000 and Eco1000 (Table 6). TOT-AX content was not affected by endoxylanase addition.
Table 6.
Analysed contents of total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) at the level of the ileum and excreta of broilers in digestibility cages at d 11 and 36 fed the 7 experimental diets.
| Variables | TOT-AX, g/kg DM |
WE-AX, g/kg DM |
||||||
|---|---|---|---|---|---|---|---|---|
| Ileum |
Excreta |
Ileum |
Excreta |
|||||
| d 11 | d 36 | d 11 | d 36 | d 11 | d 36 | d 11 | d 36 | |
| Treatment groups1 | ||||||||
| CTRL | 142.8 | 145.3 | 138.1 | 142.8 | 26.4 | 29.3 | 17.7 | 13.1 |
| Bel10 | 149.3 | 161.9 | 138.1 | 144.2 | 29.8 | 32.9 | 18 | 13.7 |
| Bel100 | 151.8 | 161.2 | 139.0 | 143.8 | 36.2∗∗ | 41.8∗∗ | 24.1 | 15.8 |
| Bel1000 | 147.9 | 138.4 | 132.1 | 123.9 | 34.7∗ | 32.1 | 29.6∗∗ | 9.9 |
| Eco10 | 154.9 | 153.2 | 138.5 | 145.8 | 31.9 | 35.0 | 25 | 15.1 |
| Eco100 | 148 | 142.2 | 139.5 | 150.0 | 33.4 | 37.3 | 31.2∗∗ | 18.2 |
| Eco1000 | 160.7∗ | 144.7 | 141.8 | 117.9 | 41.9∗∗ | 35.3 | 34.8∗∗ | 20 |
| Pooled SEM | 13.3 | 27.8 | 17.9 | 21.2 | 6.2 | 9.6 | 8.2 | 9.8 |
| Main effects | ||||||||
| Age | ||||||||
| d 11 | 152.1 ± 13.3 | 138.1 ± 17.9 | 34.7 ± 6.2 | 27.0 ± 8.2a | ||||
| d 36 | 149.8 ± 27.8 | 137.6 ± 21.2 | 35.6 ± 9.6 | 15.5 ± 9.8b | ||||
| Enzyme | ||||||||
| Bel | 151.4 ± 7.8 | 136.8 ± 6.3 | 34.3 ± 2.8 | 18.5 ± 2.8b | ||||
| Eco | 150.5 ± 5.6 | 138.9 ± 7.2 | 35.5 ± 2.3 | 23.8 ± 3.2a | ||||
| Dose, mg/kg | ||||||||
| 10 | 154.9 ± 6.1 | 141.7 ± 6.3ab | 32.3 ± 2.0 | 18.0 ± 2.9 | ||||
| 100 | 150.3 ± 7.4 | 143.2 ± 8.3a | 36.9 ± 2.9 | 21.9 ± 3.7 | ||||
| 1,000 | 147.9 ± 10.1 | 128.9 ± 7.9b | 36.0 ± 3.6 | 23.6 ± 4.2 | ||||
| P ANOVA | ||||||||
| Age | 0.70 | 0.88 | 0.52 | <0.001 | ||||
| Enzyme | 0.82 | 0.64 | 0.47 | 0.01 | ||||
| Dose | 0.47 | 0.02 | 0.06 | 0.07 | ||||
| Age × Enzyme | 0.21 | 0.73 | 0.57 | 0.67 | ||||
| Age × Dose | 0.24 | 0.06 | 0.06 | 0.13 | ||||
| Dose × Enzyme | 0.19 | 0.97 | 0.10 | 0.76 | ||||
DM = dry matter; SEM = standard error of the mean.
∗,∗∗ indicate treatment means which differ significantly P < 0.10 and P < 0.05, respectively from the control mean; analysed according to the posthoc Dunnett's test.
a,b Means having different superscripts in a particular column for the main effect are significantly different; analysed according to the posthoc Tukey's HSD test (P < 0.05). Means are represented as means ± SEM.
CTRL, control diet; Bel, Belfeed supplemented diet; Eco, Econase supplemented diet. Reported values are means from 5 replicates from each dietary treatment group (n = 5).
Fig. 1.
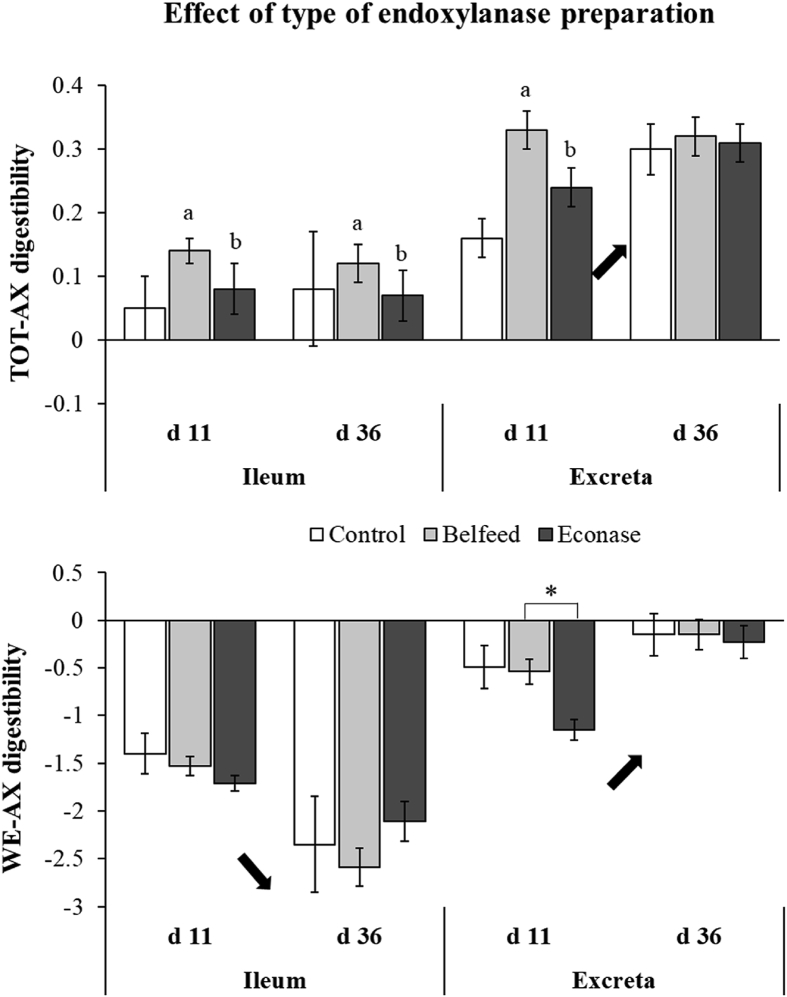
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) digestibility at the level of the ileum and excreta for broilers fed the control, Belfeed or Econase supplemented diet at 11 and 36 d of age. Small letters a,b and asterisk (∗) indicate a significant difference between the type of endoxylanase used at a particular broiler age (interaction effect enzyme × age) at P < 0.05 and P < 0.10, respectively. If present, the main effect of age is also indicated with an arrow. Error bars denote the standard error of the mean (SEM).
Fig. 2.
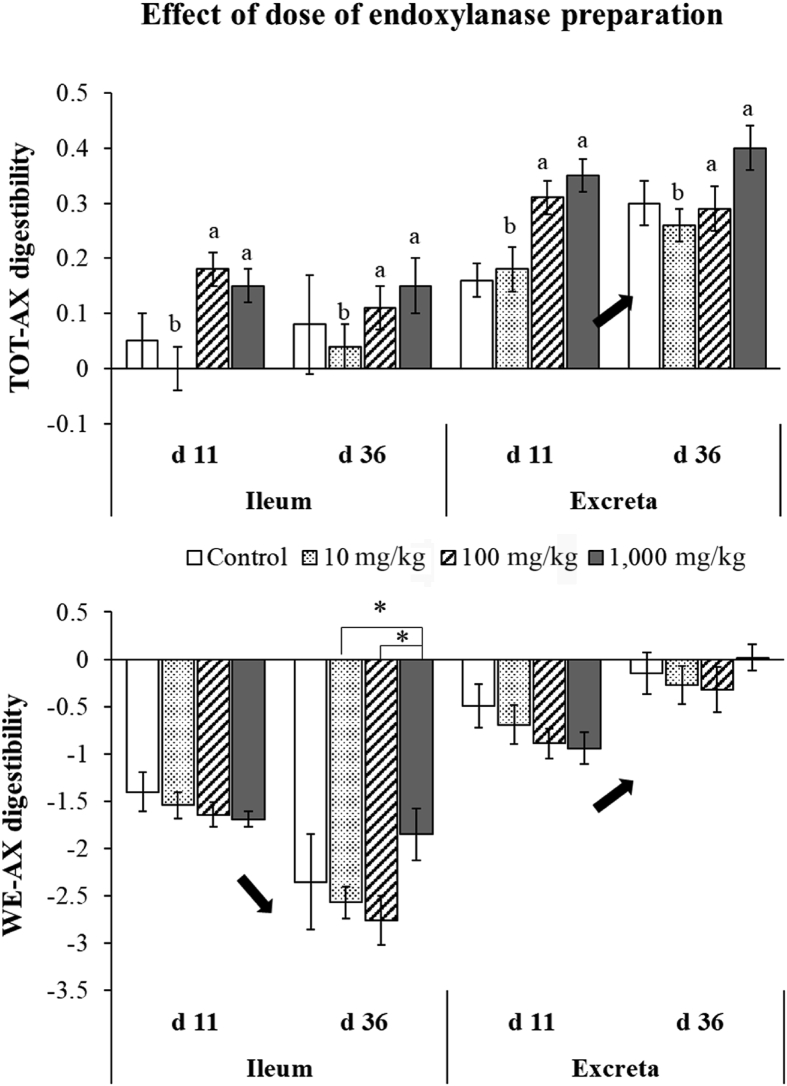
Total arabinoxylan (TOT-AX) and water-extractable arabinoxylan (WE-AX) digestibility at the level of the ileum and excreta for broilers fed the control, 10, 100 or 1,000 mg/kg of an endoxylanase preparation supplemented diet at 11 and 36 d of age. Small letters a,b and asterisk (∗) indicate a significant difference between the different doses used at a particular broiler age (interaction effect dose × age) at P < 0.05 and P < 0.10, respectively. If present, the main effect of age is also indicated with an arrow on this figure. Error bars denote the standard error of the mean (SEM).
By comparing the hydrolysis pattern and digestibility coefficients for the types of endoxylanase incorporated into the feed, it was observed that the addition of Belfeed increased the ileal digestion of the dietary AX compared to the addition of Econase (P = 0.04, Fig. 1). Incorporating high doses of endoxylanases increased ileal TOT-AX digestion at all broiler ages (P = 0.005) and tended to increase WE-AX digestibility coefficients at slaughter age (P = 0.06) (Fig. 2).
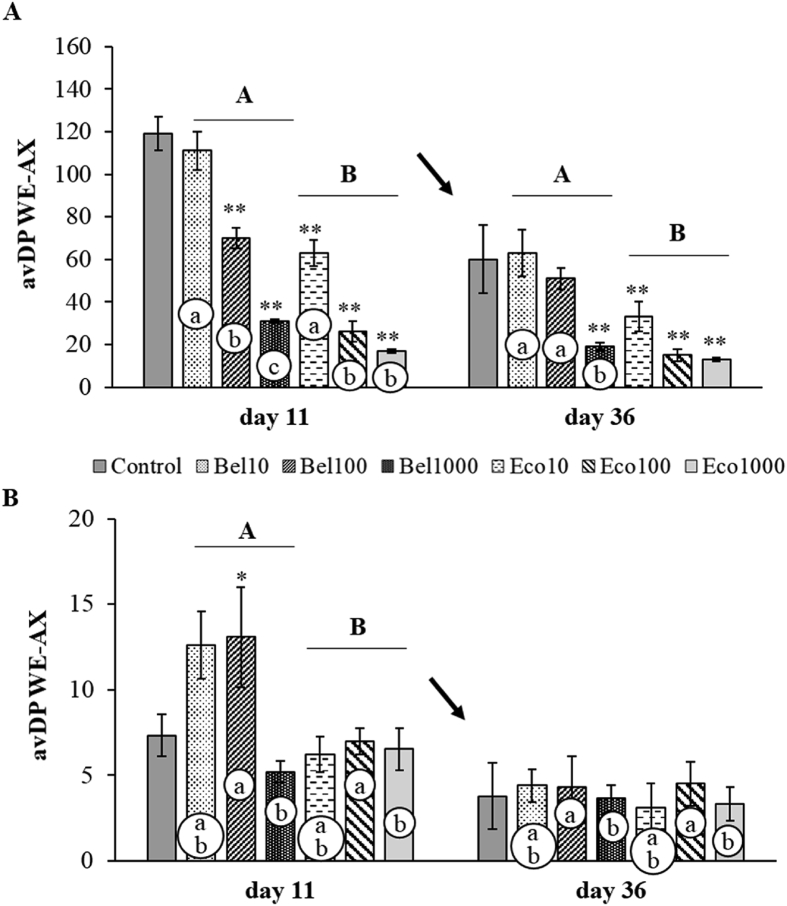
Besides improving the solubilisation of the dietary AX, the average chain length of the solubilised fragments was remarkably reduced due to endoxylanase addition (Fig. 3). Adding Econase to the feed resulted in a more significant reduction of the avDP of the ileal WE-AX than the addition of Belfeed at all broiler ages (P < 0.001, Fig. 3). However, this difference in avDP reduction only occurred when 10 or 100 mg/kg was added to the diet. Incorporating ten times the commercially relevant dose resulted in a decrease of the avDP of WE-AX in broilers of all ages, independent of the type of endoxylanase employed (P < 0.001, Fig. 3).
Fig. 3.
The average degree of polymerisation (avDP) of water-extractable arabinoxylan (WE-AX) at the level of the ileum (A) and caeca (B) for broilers fed the control diet or endoxylanase supplemented diets Bel10, Bel100, Bel1000, Eco10, Eco100 and Eco1000 at 11 and 36 d of age. ∗, ∗∗ indicate treatment means which differ significantly at P ≤ 0.10 and P < 0.05, respectively from the control mean; analysed according to the posthoc Dunnett's test (n = 5). Capital letters A, B indicate a significant difference for the effect of endoxylanase preparation at a particular broiler age (Bel vs. Eco) (P < 0.05, interaction age × enzyme). Small letters a, b, c in white circles denote a significant difference for dose (10 vs. 100 vs. 1,000 mg/kg) at a particular age and endoxylanase preparation (P < 0.05, interaction age × dose and enzyme × dose). The arrows denote a significant overall age difference (P < 0.05). Error bars denote the standard error of the represented means (SEM).
3.4. Arabinoxylan hydrolysis and fermentation at the level of the caeca
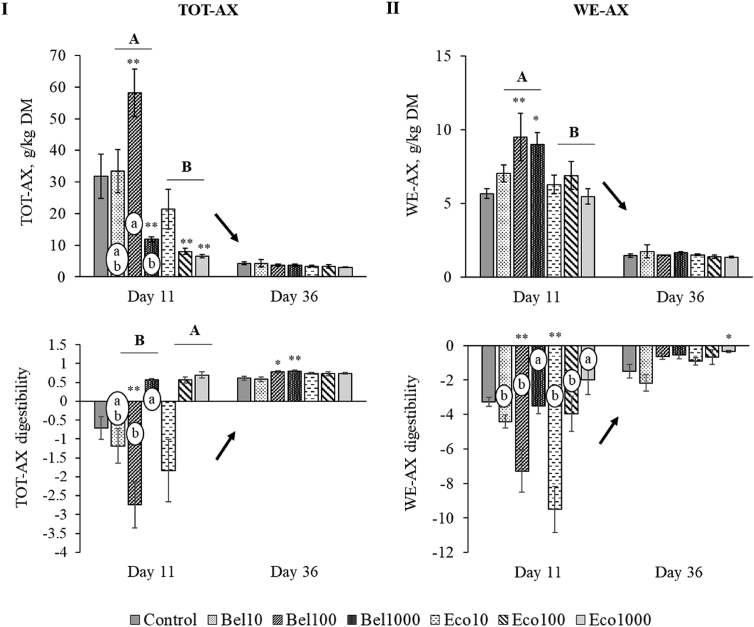
Both TOT-AX and WE-AX content significantly decreased as the broiler aged (P < 0.001, Fig. 4). As a result, the corresponding AX digestibility coefficients increased significantly toward slaughter age (P < 0.001, Fig. 4). Compared to the control, supplementing endoxylanases at 1,000 mg/kg in wheat-soy based broiler feeds lowered the amount of TOT-AX residues in the caeca at 11 but not at 36 d of age (Fig. 4; P = 0.03 and P = 0.003 for Bel1000 and Eco1000, respectively). The reduced amount of AX residues found in the caeca of young broilers receiving high dosages of endoxylanase were probably achieved by increased microbial fermentation, as suggested by the numerically higher AX digestibility coefficients at d 11 compared to the control. The structural properties of AX residues found at d 36 were similar for all dietary treatments (Fig. 3). At the same time, TOT-AX digestibility coefficients were still higher for Bel100 and Bel1000 than for the control (Fig. 4).
Fig. 4.
The total arabinoxylan (TOT-AX) content (g/kg DM) and digestibility (I) and the water-extractable arabinoxylan (WE-AX) content (g/kg DM) and digestibility (II) at the level of the caeca for broilers fed the control diet, and the diets supplemented with Bel10, Bel100, Bel1000, Eco10, Eco100 and Eco1000 at 11 and 36 d old. Capital letters A, B indicate a significant difference for the effect of endoxylanase preparation at a particular broiler age (Bel vs. Eco) (P < 0.05, interaction age × enzyme). Small letters a, b, c in white circles denote a significant difference for dose (10 vs. 100 vs. 1,000 mg/kg) at a particular age and endoxylanase preparation (P < 0.05, interaction age × dose and enzyme × dose). Error bars denote the standard error of the represented means (SEM). The arrows denote a significant overall age difference (P < 0.05). ∗, ∗∗ indicate treatment means which differ significantly at P ≤ 0.10 and P < 0.05, respectively from the control mean; analysed according to the posthoc Dunnett's test (n = 5). Error bars denote the standard error of the represented mean (SEM).
When comparing the impact of the addition of Econase and Belfeed on the amount of AX found in the caeca, it was observed that Econase lowered both TOT-AX (P < 0.001) and WE-AX content (P = 0.011) to a greater extent than Belfeed (Fig. 4). However, this difference in AX content by type of endoxylanase was only significant at 11 d of age (P < 0.001 and P = 0.001, interaction age × enzyme for TOT-AX and WE-AX respectively), and when the preparations were added at 100 mg/kg (commercial dose) (P = 0.001, interaction age × enzyme for TOT-AX). As a result of this marked difference, higher TOT-AX digestibilities were observed at young ages for broilers receiving Econase instead of Belfeed (P = 0.04, Fig. 4), especially when the enzymes were added at the commercial dose of 100 mg/kg (P = 0.003). Not only did the amount of AX entering the caeca differ between the type of endoxylanase preparation added to the diet, but also the structural characteristics of the solubilised AX were markedly different. Econase was able to reduce the avDP of WE-AX to a greater extent in the caeca of young broilers than Belfeed (P = 0.03, Fig. 3). Although WE-AX digestibilities did not differ between the 2 types of endoxylanase preparation, the dose seemed to play an important role in the young broiler. Providing more easily fermentable substrates with a low avDP (Fig. 3) resulted in a higher WE-AX digestibility coefficient for broilers receiving the high dose supplementation at young ages (P = 0.04, Fig. 4).
3.5. Total tract arabinoxylan hydrolysis and fermentation
A decrease in the WE-AX content (P < 0.001, Table 6) and a corresponding increase in total tract WE-AX and TOT-AX digestibilities were observed as the broilers aged (P < 0.001, Fig. 1). Endoxylanase doses of 1,000 mg/kg resulted in a significantly higher total tract TOT-AX digestibility coefficient compared to the control when broilers were 11 d of age (P < 0.001 and P = 0.03 for the dietary treatments Bel1000 and Eco1000, respectively) (Fig. 2).
Although no difference in WE-AX digestion was observed upon endoxylanase addition compared to the control, lower WE-AX contents (Table 6, P = 0.01) were found for broilers receiving Belfeed instead of Econase. A difference in WE-AX digestibility coefficients depending on the type of endoxylanase, tended to be present only in young broilers (P = 0.07, Fig. 1). Similar to the total tract WE-AX digestibility, TOT-AX digestion was considerably higher for young broilers (11 d) receiving Belfeed than for those receiving Econase (P = 0.04, Fig. 1). In addition to the type of endoxylanase, the dose incorporated in the diet also affected the extent of AX hydrolysis and subsequent digestion of AX substrates. Adding a tenth of the commercially relevant dose produced the lowest total tract AX digestibility coefficients, which were comparable to the AX digestibility values for broilers of the control group (P < 0.001, Fig. 2).
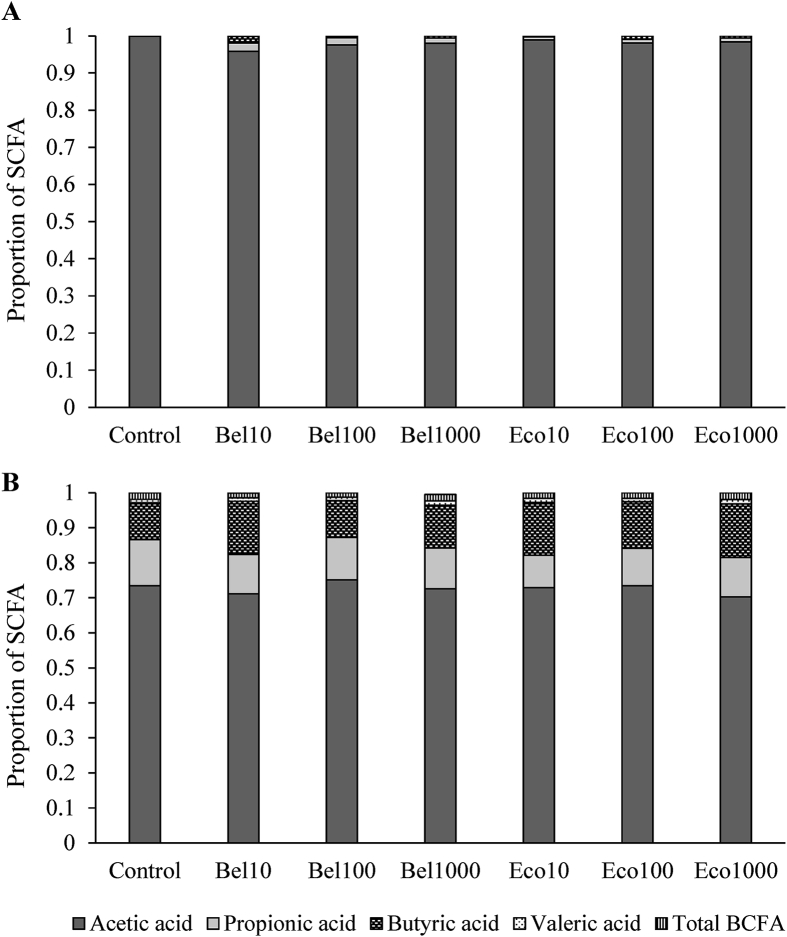
3.6. SCFA production upon AX fermentation at the level of the ileum and caeca
Total SCFA content (quantified as the sum of the acetic, propionic, butyric and valeric acid contents) and the diversity of SCFA produced at slaughter age (d 36) was considerably higher at the level of the caeca than at the level of the ileum (Fig. 5, Appendix Tables 1 and 2). Acetic acid dominated the total SCFA in both the ileum (98.1%) and caecum (73.9%). Very small proportions of propionic (1.0%), butyric (0.4%) and valeric acid (0.02%) were found here (Fig. 5 and Appendix Table 1). In the caeca, however, high contributions of butyric and propionic acid to total SCFA were observed, respectively, 13.4% and 11.5%. Negligible amounts of BCFA, i.e. isobutyric and isovaleric acid, were found in both the ileum and caeca and did not change upon endoxylanase addition (P = 0.62 and P = 0.12 for the ileum and caeca respectively, Appendix Tables 1 and 2). Endoxylanase supplementation increased the diversity of the SCFA produced along the ileum compared to broilers receiving no endoxylanase (Fig. 5). Against expectations, at the level of the caeca, a higher total SCFA content was observed for broilers receiving the control diet than for broilers receiving endoxylanase supplementation. The proportion of butyric acid increased numerically when endoxylanases were mixed into the diet (13.8% versus 10.7% of total SCFA produced for endoxylanase and control fed broilers, respectively) (Appendix Table 2).
Fig. 5.
The proportion of short-chain fatty acids (SCFA) (μmol/g DM of digesta) produced in vivo at the level of the ileum (A) and caeca (B) at slaughter age (d 36) for broilers fed the control diet or diets supplemented with Bel10, Bel100, Bel1000, Eco10, Eco100 and Eco1000 (n = 5). The total amount of branched short-chain fatty acids (BCFA) was calculated as the sum of the amounts of isobutyric and isovaleric acid.
4. Discussion
In this study, we set out to examine the effect of type and dose of commercially available endoxylanase preparations on broiler performance, and AX hydrolysis and fermentation in the distal part of the ageing broiler's digestive tract. We analysed the structural properties and content of AX, the AX digestion profile and the SCFA produced as a function of broiler age to gain more insight into the underlying mechanisms of action of these enzymes. In this section, we will discuss and integrate the results we obtained.
4.1. Hydrolysis and fermentation of dietary AX in the distal part of the GIT of broilers
4.1.1. The ileum
The presence of added endoxylanases in the hindgut resulted in an enlargement of the pool of soluble arabinoxylan-oligomers and -polymers, as noted by the increase in the WE-AX content with a reduced MW for endoxylanase receiving broilers. The more significant reduction in avDP of WE-AX upon endoxylanase addition resulted in a decreased ileal viscosity compared to the control, as previously observed by a study of Lee et al. (2017) and Liu and Kim (2017). By reducing the MW and viscosity of the WE-AX, the detrimental effects on the digestion of nutrients and, hence, broiler performance were reduced (Choct and Annison, 1992).
Broilers receiving Belfeed showed substantial solubilisation of dietary WU-AX into high MW WE-AX with a corresponding increase of the ileal viscosity values. The strong preference of B. subtilis endoxylanase (Belfeed) to bind WU-AX over WE-AX might explain the observed high ileal viscosity profile for Belfeed receiving broilers (Agrimex, 2019; Courtin and Delcour, 2001). Unlike the B. subtilis endoxylanase, the endoxylanase of Nonomuraea flexuosa (Econase) both solubilises the dietary WU-AX and reduces the MW of the dietary and solubilised WE-AX to form oligosaccharides in the hindgut (AB Vista, 2019; Courtin and Delcour, 2001; Zhang et al., 2011). Indeed, Econase reduced the MW of the WE-AX to a larger extent, which suggests that Econase addition resulted in a better modification of WE-AX substrates for microbial fermentation in the ileum. Besides this difference in substrate selectivity, the site and extent of hydrolysis might also have been different between both endoxylanase preparations. Compared to Belfeed, the higher endoxylanase activity of Econase in the diet did not result in increased intestinal viscosity and WE-AX content. Therefore, it could be that the endoxylanase originating from B. subtilis was activated earlier by the small intestinal conditions (pH 6 to 6.5, 40 °C) (Józefiak et al., 2007; Mabelebele et al., 2014), thereby solubilising the dietary AX already to a larger extent compared to the endoxylanase originating from N. flexuosa (Leskinen et al., 2005). Considering these items, the type rather than the intrinsic activity of the endoxylanase present in the commercial preparations will explain the majority of differences in amount and type of AX hydrolysis products formed along the distal part of the GIT upon endoxylanase addition. Indeed, research of Pollet et al. (2010) showed that endoxylanases belonging to the same GH family can have subtle differences in their molecular architecture, which can highly affect the substrate preferences and hydrolysis products formed. To note, however, is that the incorporated doses of endoxylanase preparations in the feed do matter, as doses incorporated at levels 10 times smaller than the commercially relevant ones did not reduce the MW of WE-AX to a large extent, as witnessed by the similar ileal AX digestibility profiles and ileal viscosity values compared to the control group.
Although Econase seemed to hydrolyse the dietary AX into substrates which seem to be more easily fermentable by the ileal microbiome, no improved TOT-AX digestibility coefficients and increased production of SCFA were observed. On the contrary, Belfeed seems to contribute positively to fermentation of AX as noted by the higher ileal TOT-AX digestibility values at all broiler ages. In spite of the higher intestinal viscosity and avDP of WE-AX measured, it seems that action of Belfeed allows the dietary AX to be better fermented by the ileal microbiome, which might have resulted in increased body weight gain at later ages. This was, however, not confirmed by an augmented SCFA production at the level of the ileum in broilers at slaughter age.
The low viscosity of the wheat-soy based diets in the current study and the concomitant low ileal viscosity values (2 – 3 cP) do not seem to pose a considerable gastrointestinal and microbial challenge anymore, as previously suggested by Aftab and Bedford (2018). This hypothesis is underpinned in the current study by the improved ileal TOT-AX digestibilities for Belfeed, in spite of its higher induced ileal viscosity compared to Econase. This observation, however, needs some further investigation.
4.1.2. The caeca
In the caeca, Econase created a higher amount of oligomers compared to Belfeed, as evidenced by the greater reduction in avDP of WE-AX for Econase receiving broilers. This increased potential of an endoxylanase originating from N. flexuosa to produce (A)XOS was also previously noted by Zhang et al. (2011) and Zhang et al. (2013). Besides this difference in AX structure, the amount of AX found in the caeca was also affected differently, depending on the type of endoxylanase added, especially at 11 d of age. Due to the considerable solubilisation activity of both endoxylanases, a high amount of soluble AX created at the level of the ileum was able to enter the caeca of young broilers (d 11). Indeed, the low viscosity values did not prevent digesta from entering the caecal pouches (Choct et al., 1999; Svihus et al., 2013). In spite of the increased supply of both WE-AX and WU-AX in the caeca of Belfeed receiving broilers compared to the control and Econase receiving broilers, this increased supply failed to increase AX fermentation by the young caecal microbiome. On the contrary, higher TOT-AX digestibilities were observed for Econase receiving broilers. Probably, the ability of the immature caecal microbiota to ferment the dietary AX was improved by an increased supply of arabinoxylan-oligomers in the broilers receiving Econase (Bautil et al., 2020). Therefore, it can be assumed that the kind of AX substrates, in terms of AX structure, rather than the amount provided to the developing caecal microbiota will markedly affect their fermentation kinetics (de Vries et al., 2016). This phenomenon was previously reported in a study of Bautil et al. (2020) in which the net addition of 5.0 g/kg AXOS in the diet improved the fermentation ability of the young caecal microbiome towards the AX fraction of the feed. In addition, a study of Bedford and Apajalahti (2018) also suggested that endoxylanase from N. flexuosa can upregulate the fermentation pathways for xylose/XOS/AXOS. Consequently, an adaptive change in the microbiome might be induced, which result in a more effective fermentation of the dietary AX. This was probably the case for Econase, but not for the Belfeed fed young broilers. The provision of more easily fermentable substrates in the caeca could also have stimulated the occurrence and diversity of bacterial spp. producing butyrate (Courtin et al., 2008b; Kiarie et al., 2013; Lee et al., 2017; Ribeiro et al., 2018). A numerical increase in butyrate production at slaughter age was indeed observed for endoxylanase supplementation in general in this study, but not specifically for Econase receiving broilers. Although no elevated concentration of butyrate was observed, the larger caeca at 36 d for Econase receiving broilers indicate the higher capacity for butyrate production than for Belfeed receiving broilers (Campbell et al., 1997). Of note, the lack in SCFA response in this study was also previously encountered in other studies (Boets et al., 2015; Gonzalez-Ortiz et al., 2019) as the amounts of SCFA measured result from the balance between SCFA production and utilisation by the microbiota or the broiler itself and the dynamics in digesta flow, especially the irregular frequency of ileal and caecal digesta evacuation. Towards slaughter age, however, Belfeed receiving broilers did catch up in their caecal AX digestibility capacity and were similar to Econase receiving broilers. The marked increase in the adaptation of the maturing caecal microbiome towards the AX substrates entering the caeca at slaughter age (d 36) as recently observed by Bautil et al. (2019), probably contributed to this improved AX digestion with age for Belfeed and control diet receiving broilers.
Irrespective of the type of endoxylanase added, a minimum of in-feed endoxylanase activity was needed to generate significant hydrolysis of the AX chain. As such, the dose incorporated in the diet needs to be significant in order to exceed the solubilisation capacity of the endogenous small intestinal microbiome. Overdosing endoxylanases (1,000 mg/kg) resulted in excessive solubilisation of the dietary AX and hydrolysis of AX polymers into oligomers, which led to very high TOT-AX digestibility values over time. Probably, the increased caecal opening with age and this high dose supplementation also contributed to the significantly improved AX digestion at the level of the caeca. The low levels of ancillary enzymes present in the background of commercial preparations might have contributed to these high AX digestibility values as well (Adeola and Cowieson, 2011; Bedford, 2018).
4.2. Total tract AX digestion
Negative total tract WE-AX digestibility coefficients indicate an excess of AX solubilisation induced by exogenously added or endogenous microbial-derived endoxylanases (i.e. for broilers receiving the control) that goes beyond the fermentative capacity of the microbiota residing in the distal part of the broiler's GIT. Despite these negative values for WE-AX, the addition of endoxylanases in the broiler's feed in the current study resulted in an on average 15% increase in total tract TOT-AX digestibility, which is in agreement with observations of Zhang et al. (2014). This improved TOT-AX digestion, compared to the control, was predominantly present at young broiler ages, and when doses were ten times higher than commercially relevant. These findings hence support the hypothesis of a large involvement of the age-related microbial development in the functionality of endoxylanases in low viscosity diets nowadays (Bautil et al., 2019; Bedford, 2018). Providing fermentable substrates with a low structural complexity to the young microbiome will be of importance to further enhance the nutritive value of the dietary fibre fraction of the feed. The kick-starter effect of AXOS on the intestinal microbiome proposed by Bautil et al. (2020) can hence also be induced by supplementation of the correct dose and type of endoxylanase.
Despite the more favourable hydrolysis products formed upon Econase addition, Belfeed supplementation resulted in a higher total tract TOT-AX digestion in the young broiler compared to Econase. This suggests that not only might the improvement in the provision of fermentable substrates to the caecal microbiota be of importance, but that precaecal digestion of the dietary AX may also have a large contribution in endoxylanase functionality and AX digestion along the GIT. Furthermore, these results emphasise the high impact of substrate specificity of the type of endoxylanases on AX hydrolysis and fermentation processes in the distal part of the young broiler's digestive tract.
4.3. Lack of improvement of broiler performance at young ages in spite of large impact on AX population with feed endoxylanase type
Despite the difference in AX digestion and the initial differing endoxylanase activities in the feed, only minor differences in enzymatic efficiency related to broiler performance were noted for the type of endoxylanase added, which is in agreement with work of Vandeplas et al. (2010). In addition, the markedly improved total tract AX digestibility values obtained with higher incorporation levels of endoxylanases in the feed did not markedly affect growth performance responses, as previously reported by Olukosi et al. (2007) and Pourreza et al. (2007). The changes we observed in the AX population upon endoxylanase supplementation were mainly present at young broiler ages. We hence would expect to observe large differences in performance between the control and the endoxylanase receiving broilers over the first 11 d, as previously observed by Lee et al. (2017) and Gonzalez-Ortiz et al. (2017). In addition, due to an incomplete functioning of the GIT (Noy and Sklan, 1995) and the immaturity of the intestinal microbiome (Lu et al., 2003; Pan and Yu, 2014), young broilers are considered to be more susceptible to antinutritional factors such as AX (Masey O'Neill et al., 2012). This was, however, not the case in the current study. From our observations, it can be speculated that the changes in AX population through endoxylanase hydrolysis will not immediately evoke a differential performance response, as these responses might need some time to develop. Indeed, providing endoxylanases throughout the broiler's entire life led to a continued enhanced degradation of antinutritional AX and a high supply of soluble low MW AX oligomers to the developing microbiome, and hence, a sustained improvement in broiler performance. As such, FCR was numerically improved from 22 d onwards when endoxylanases were added to the diet. To note, however, this improved FCR was particularly present when a high dose of Belfeed was incorporated into the feed and hence was not significantly present for a commercial relevant dose of both types of endoxylanase or the endoxylanase preparation having the highest initial feed enzyme activity. Given these results, we can state that broiler performance was only minorly affected with endoxylanase supplementation over the entire 36 d period in this study, despite lowering the nutritional quality of the feed. The latter was achieved by phytase removal from the diet and the reduction of the dietary energy value by 100 kcal/kg compared with requirements in each feeding phase. Most probably, the minor challenges posed to the birds due to the very controlled rearing conditions in our study contributed largely to overall good performance results for birds fed the control diet, thus limiting the scope for further improvements on enzyme addition (Aftab and Bedford, 2018). Indeed, all birds, even the control birds, performed very well, ahead of breeding standards (Aviagen, 2014c).
4.4. Insights in the mechanisms of action of endoxylanases in vivo
Nowadays, the positive effect of endoxylanase on broiler performance is attributed to 3 mechanisms of action: wheat cell wall degradation, intestinal viscosity reduction and the microbial feeding - prebiotic mechanism (Bedford and Cowieson, 2012; Choct, 1997; Masey O'Neill et al., 2014; Morgan et al., 2019). The data of the current study provided better insight into the relative importance of each of these mechanisms in broilers fed low viscosity wheat-soy based diets.
The high WE-AX contents and very negative WE-AX digestibility values observed with endoxylanase supplementation compared to the control indicate that significant solubilisation of wheat cell walls, probably mostly those in the endosperm (Glitsø et al., 1999), takes place. This increased solubilisation eventually led to an increase of approximately 15% in TOT-AX digestibility. An increase in the hydrolysis of the AX of wheat cell walls in vivo can be obtained with endoxylanase addition, thereby increasing the digestibility value of the dietary fibre fraction (de Vries et al., 2012). However, this improved AX digestion did not lead to superior performance compared to the control. Therefore, the importance of the cell wall degradation mechanism in the overall functionality of endoxylanase still needs further investigation.
In accordance with previous studies of Lee et al. (2017) and Liu and Kim (2017), supplementation of endoxylanases consistently reduced the intestinal viscosity at all broiler ages. However, in low viscosity diets, this reduction in intestinal viscosity might play a minor role as the creation of a pool of solubles having a relatively high avDP of WE-AX (Belfeed) did not detrimentally impact the FCR and AX digestibility values in this trial. Hence, performance outcomes will not entirely depend on a diet-related viscosity reduction.
We observed that low MW AX hydrolysis products formed upon endoxylanase addition stimulated fermentation of indigestible carbohydrates by the immature microbiota in the young broiler, an event that might be of interest in improving FCR during the growing stage. Indeed, in our study, the ileal and caecal microbiota were clearly triggered in their ability to ferment the AX substrates formed upon Belfeed and Econase addition, especially at young ages. Our data hence suggest that the age-related microbial contribution to AX digestion is of significant importance for endoxylanase functionality (Bautil et al., 2019; Bedford, 2018). By delivering the correct AX substrates to the young microbiome, early AX digestion can be boosted, which might lead to improved broiler health and performance over time. Indeed, as illustrated in our study, the beneficial AX responses initiated in the young chick can give rise to an improved broiler performance at later ages. The improved solubilisation and MW reduction of AX present in wheat cell walls and the corresponding decrease in intestinal viscosity in response to endoxylanase addition are important factors contributing to this positive interplay between the AX compounds and the young intestinal microbiome.
5. Conclusions
In conclusion, more extensive AX solubilisation was obtained with endoxylanase addition. In-feed endoxylanase activities should, however, be of significant value to realise this improved hydrolysis of the dietary AX against the control. The different types of endoxylanase present in the Belfeed and Econase preparation resulted in the formation of a heterogeneous pool of AX substrates with distinct structural properties. Addition of high doses of an endoxylanase or any dose of Econase resulted in the creation of WE-AX having a low avDP, whereas Belfeed created viscous WE-AX having a relatively high avDP. As a result, Econase profoundly improved AX fermentation in the caeca of young broilers, whereas Belfeed led to improved AX wheat cell wall digestion in the small intestine, despite its higher induced intestinal viscosity. This trial hence proved that dose and type of endoxylanase preparation can have very marked effects on the quantity and structure of WE-AX delivery in the ileum and caeca. Despite these remarkable differences in the AX population at young ages, broiler performance was mainly affected during the grower and finisher phase in this study. Because of the outstanding performance results for all diets, including the control diet, this trial could not provide evidence for the hypothesis that variation in dose and type of endoxylanase preparation can partly explain the large inconsistency in endoxylanase responses observed in the field. However, broilers receiving low viscosity diets still benefit from endoxylanase addition, as the provision of soluble and more easily fermentable AX substrates to the microbiota was considerably improved, particularly in younger broilers, which hence resulted in a sustained improvement in broiler performance. So, this study enables us to increase further our knowledge of the functionality of endoxylanases in low viscosity diets.
Author contributions
An Bautil: Conceptualization, Methodology, Formal analysis, Validation, Investigation, Writing – Original Draft, Visualization. Johan Buyse: Resources, Writing – Review & Editing. Peter Goos: Formal analysis, Writing – Review & Editing. Michael R. Bedford: Conceptualization, Writing – Review & Editing. Christophe M. Courtin: Conceptualization, Resources, Supervision, Writing – Review & Editing.
Conflic of interest
We declare that we have no financial and personal relationships with other people or organisations that can inappropriately influence our work with one potential exception. The co-author and collaborator on this project Michael R. Bedford is employed by the company that provided one of the enzymes used in this research project. We declare that this co-author did not bias the research based on his employment status.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The diligent assistance of the members of the Laboratory of Livestock Physiology is gratefully acknowledged.
Footnotes
Mention of trade names or commercial products, proprietary produce or vendor in this publication does not imply endorsement by the authors, nor criticism of similar products not mentioned.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2020.11.015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- AB Vista . AB Vista; Marlborough: 2019. The most heat resistant xylanase generates the most energy - Econase XT the hardest working xylanase.https://www.abvista.com/ABVista/media/Main/Products/Econase%20XT/EconaseXT-poultry.pdf [Google Scholar]
- Adeola O., Cowieson A.J. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J Anim Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Aftab U., Bedford M.R. The use of NSP enzymes in poultry nutrition: myths and realities. World’s Poult Sci J. 2018;74:277–286. doi: 10.1017/S0043933918000272. [DOI] [Google Scholar]
- Agrimex Belfeed, endo-1,4-beta-xylanase: one enzyme - a world of benefits. Agrimex Lille. 2019 http://www.baltivet.com/files/2214/0325/5823/Belfeed_8p__EMAIL.pdf [Google Scholar]
- Amerah A.M. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim Feed Sci Technol. 2015;199:1–9. doi: 10.1016/j.anifeedsci.2014.09.012. [DOI] [Google Scholar]
- Annison G., Choct M. Anti-nutritive activities of cereal non-starch polysaccharides in broiler diets and strategies minimizing their effects. World’s Poult Sci J. 1991;47:232–242. doi: 10.1079/WPS19910019. [DOI] [Google Scholar]
- Austin S.C., Wiseman J., Chesson A. Influence of non-starch polysaccharides structure on the metabolisable energy of U.K. wheat fed to poultry. J Cereal Sci. 1999;29:77–88. doi: 10.1006/jcrs.1998.0213. [DOI] [Google Scholar]
- Aviagen . Aviagen Group; Huntsville: 2014. Ross 308 broiler nutrition specifications.http://tmea.staging.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-308-Broiler-Nutrition-Specs-2014r17-EN.pdf [Google Scholar]
- Aviagen . Aviagen Group; Huntsville: 2014. Ross broiler management handbook.http://eu.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2014-EN.pdf [Google Scholar]
- Aviagen . Aviagen Group; Huntsville: 2014. Ross 308 broiler performance objectives.https://www.winmixsoft.com/files/info/Ross-308-Broiler-PO-2014-EN.pdf [Google Scholar]
- Bach Knudsen K.E. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Sci. 2014;93:2380–2393. doi: 10.3382/ps.2014-03902. [DOI] [PubMed] [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poultry Sci. 2019;98:4606–4621. doi: 10.3382/ps/pez159. [DOI] [PubMed] [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler. Poultry Sci. 2020;99:2555–2565. doi: 10.1016/j.psj.2019.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M.R. In: Poultry feedstuffs: supply, composition and nutritive value. McNab J.M., Boorman K.N., editors. CABI Publishing; Wallingford: 2002. The role of carbohydrases in feedstuff digestion; pp. 319–336. [Google Scholar]
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim Feed Sci Technol. 2012;173:76–85. doi: 10.1016/j.anifeedsci.2011.12.018. [DOI] [Google Scholar]
- Bedford M.R. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br Poultry Sci. 2018;59:486–493. doi: 10.1080/00071668.2018.1484074. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Apajalahti J.H.A. Exposure of a broiler to a xylanase for 35d increases the capacity of cecal microbiome to ferment soluble xylan. Poultry Sci. 2018;97(E-Suppl 1):98–99. [Google Scholar]
- Beg Q.K., Kapoor M., Mahajan L., Hoondal G.S. Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol. 2001;56:326–338. doi: 10.1007/s002530100704. [DOI] [PubMed] [Google Scholar]
- Boets E., Deroover L., Houben E., Vermeulen K., Gomand S., Delcour J.A. Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 2015;7:8916–8929. doi: 10.3390/nu7115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Fahey G.C., Wolf B.W. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- Carré B. Causes for variation in digestibility of starch among feedstuffs. World’s Poult Sci J. 2004;60:76–89. doi: 10.1079/WPS20036. [DOI] [Google Scholar]
- Choct M., Annison G. Anti-nutritive activity of wheat pentosans in broiler diets. Br Poultry Sci. 1990;31:811–822. doi: 10.1080/00071669008417312. [DOI] [PubMed] [Google Scholar]
- Choct M., Annison G. Anti-nutritive effect of wheat pentosans in broiler chickens: roles of viscosity and gut microflora. Br Poultry Sci. 1992;33:821–834. doi: 10.1080/00071669208417524. [DOI] [PubMed] [Google Scholar]
- Choct M. Feed non-starch polysaccharides: chemical structures and nutritional significance. Feed Milling Int. 1997;191:13–26. [Google Scholar]
- Choct M., Hughes R.J., Bedford M.R. Effects of a xylanase on individual bird variation, starch digestion throughout the intestine, and ileal and caecal volatile fatty acid production in chickens fed wheat. Br Poultry Sci. 1999;40:419–422. doi: 10.1080/00071669987548. [DOI] [PubMed] [Google Scholar]
- Collins T., Gerday C., Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev. 2005;29:3–23. doi: 10.1016/j.femsre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Courtin C.M., Van Den Broeck H., Delcour J.A. Determination of reducing end sugar residues in oligo- and polysaccharides by gas-liquid chromatography. J Chromatogr A. 2000;866:97–104. doi: 10.1016/S0021-9673(99)01064-X. [DOI] [PubMed] [Google Scholar]
- Courtin C.M., Delcour J.A. Relative activity of endoxylanases towards water-extractable and water-unextractable arabinoxylan. J Cereal Sci. 2001;33:301–312. doi: 10.1006/jcrs.2000.0354. [DOI] [Google Scholar]
- Courtin C.M., Delcour J.A. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci. 2002;35:225–243. doi: 10.1006/jcrs.2001.0433. [DOI] [Google Scholar]
- Courtin C.M., Broekaert W.F., Swennen K., Lescroart O., Onagbesan O., Buyse J. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. 2008;85:607–613. doi: 10.1094/CCHEM-85-5-0607. [DOI] [Google Scholar]
- Courtin C.M., Swennen K., Broekaert W.F., Swennen Q., Buyse J., Decuypere E. Effects of dietary inclusion of xylooligo- saccharides, arabinoxylooligosaccharides and soluble arabinoxylan on the microbial composition of caecal contents of chickens. J Sci Food Agric. 2008;88:2517–2522. doi: 10.1002/jsfa.3373. [DOI] [Google Scholar]
- Cowieson A.J., Hruby M., Pierson E.E.M. Evolving enzyme technology: impact on commercial poultry nutrition. Nutr Res Rev. 2006;19:90–103. doi: 10.1079/NRR2006121. [DOI] [PubMed] [Google Scholar]
- de Vries S., Pustjens A.M., Schols H.A., Hendriks W.H., Gerrits W.J.J. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: a review. Anim Feed Sci Technol. 2012;178:123–138. doi: 10.1016/j.anifeedsci.2012.10.004. [DOI] [Google Scholar]
- de Vries S., Gerrits W.J.J., Kabel M.A., Vasanthan T., Zijlstra R.T. β-glucans and resistant starch alter the fermentation of recalcitrant fibers in growing pigs. PloS One. 2016;11 doi: 10.1371/journal.pone.0167624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englyst H.N., Cummings J.H. Simplified method for the measurement of total non-starch polysaccharides by gas - liquid chromatography of constituent sugars as alditol acetates. Analyst. 1984;109:937–942. doi: 10.1039/an9840900937. [DOI] [PubMed] [Google Scholar]
- European Commision . 2009. Community register of feed additives, pursuant to regulation (EC) No 1831/2003, appendixes 3&4, annex I: list of additives.http://ec.europa.eu/food/food/animalnutrition/feedadditives/comm_register_feed_additives_1831-03.pdf [Google Scholar]
- Gebruers K., Courtin C.M., Delcour J.A. In: Healthgrain methods: analysis of bioactive components in small grain cereals. Shewry P.R., Ward J.L., editors. AACC International; St Paul: 2009. Quantification of arabinoxylans and their degree of branching using gas chromatography; pp. 177–190. [Google Scholar]
- Glitsø L.V., Gruppen H., Schols H.A., Højsgaard S., Sandström B., Bach Knudsen K.E. Degradation of rye arabinoxylans in the large intestine of pigs. J Sci Food Agric. 1999;79:961–969. doi: 10.1002/(SICI)1097-0010(19990515)79:7<961::AID-JSFA311>3.0.CO;2-1. [DOI] [Google Scholar]
- Gonzalez-Ortiz G., Sola-Oriol D., Martinez-Mora M., Perez J.F., Bedford M.R. Response of broiler chickens fed wheat-based diets to xylanase supplementation. Poultry Sci. 2017;96:2776–2785. doi: 10.3382/ps/pex092. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ortiz G., dos Santos T.T., Vienola K., Vartiainen S., Apajalahti J., Bedford M.R. Response of broiler chickens to xylanase and butyrate supplementation. Poultry Sci. 2019;98:3914–3925. doi: 10.3382/ps/pez113. [DOI] [PubMed] [Google Scholar]
- Grootaert C., Verstraete W., Van de Wiele T. Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine. Trends Food Sci Technol. 2007;18:64–71. doi: 10.1016/j.tifs.2006.08.004. [DOI] [Google Scholar]
- Izydorczyk M.S., Biliaderis C.G. Cereal arabinoxylans: advances in structure and physicochemical properties. Carbohydr Polym. 1995;28:33–48. doi: 10.1016/0144-8617(95)00077-1. [DOI] [Google Scholar]
- Izydorczyk M.S., Macri L.J., MacGregor A.W. Structure and physicochemical properties of barley non-starch polysaccharides - I. Water-extractable β-glucans and arabinoxylans. Carbohydr Polym. 1998;35:249–258. doi: 10.1016/S0144-8617(97)00137-9. [DOI] [Google Scholar]
- Józefiak D., Rutkowski A., Jensen B.B., Engberg R.M. Effects of dietary inclusion of triticale, rye and wheat and xylanase supplementation on growth performance of broiler chickens and fermentation in the gastrointestinal tract. Anim Feed Sci Technol. 2007;132:79–93. doi: 10.1016/j.anifeedsci.2006.03.011. [DOI] [Google Scholar]
- Kiarie E., Romero L.F., Nyachoti C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev. 2013;26:71–88. doi: 10.1017/S0954422413000048. [DOI] [PubMed] [Google Scholar]
- Kiarie E., Romero L.F., Ravindran V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poultry Sci. 2014;93:1186–1196. doi: 10.3382/ps.2013-03715. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Apajalahti J., Vienola K., González-Ortiz G., Fontes C.M.G.A., Bedford M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim Feed Sci Technol. 2017;234:29–42. doi: 10.1016/j.anifeedsci.2017.07.017. [DOI] [Google Scholar]
- Leskinen S., Mäntylä A., Fagerström R., Vehmaanperä J., Lantto R., Paloheimo M. Thermostable xylanases, Xyn10A and Xyn11A, from the actinomycete Nonomuraea flexuosa: isolation of the genes and characterization of recombinant Xyn11A polypeptides produced in Trichoderma reesei. Appl Microbiol Biotechnol. 2005;67:495–505. doi: 10.1007/s00253-004-1797-x. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Kim I.H. Effects of dietary xylanase supplementation on performance and functional digestive parameters in broilers fed wheat-based diets. Poultry Sci. 2017;96:566–573. doi: 10.3382/ps/pew258. [DOI] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabelebele M., Alabi O.J., Ng`ambi J.W., Norris D., Ginindza M.M. Comparison of gastrointestinal tracts and pH values of digestive organs of ross 308 broiler and indigenous Venda chickens fed the same diet. Asian J Anim Vet Adv. 2014;9:71–76. doi: 10.3923/ajava.2014.71.76. [DOI] [Google Scholar]
- Maes C., Delcour J.A. Structural characterisation of water-extractable and water-unextractable arabinoxylans in wheat bran. J Cereal Sci. 2002;35:315–326. doi: 10.1006/jcrs.2001.0439. [DOI] [Google Scholar]
- Mares D.J., Stone B.A. Studies on wheat endosperm I chemical composition and ultrastructure of the cell walls. Aust J Biol Sci. 1973;26:793–812. doi: 10.1071/BI9730793. [DOI] [Google Scholar]
- Masey O'Neill H.V., Liu N., Wang J.P., Diallo A., Hill S. Effect of xylanase on performance and apparent metabolisable energy in starter broilers fed diets containing one maize variety harvested in different regions of China. Asian-Australas J Anim Sci. 2012;25:515–523. doi: 10.5713/ajas.2011.11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masey O'Neill H.V., Smith J.A., Bedford M.R. Multicarbohydrase enzymes for non-ruminants. Asian-Australas J Anim Sci. 2014;27:290–301. doi: 10.5713/ajas.2013.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N.K., Keerqin C., Wallace A., Wu S.B., Choct M. Effect of arabinoxylo-oligosaccharides and arabinoxylans on net energy and nutrient utilization in broilers. Anim Nutr. 2019;5:56–62. doi: 10.1016/j.aninu.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers W.D., Ludden P.A., Nayigihugu V., Hess B.W. Technical Note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide. J Anim Sci. 2004;82:179–183. doi: 10.2527/2004.821179x. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poultry Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Bedford M.R., Adeola O. Xylanase in diets for growing pigs and broiler chicks. Can J Anim Sci. 2007;87:227–235. doi: 10.4141/CJAS06005. [DOI] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizeli M.L.T.M., Rizzatti A.C.S., Monti R., Terenzi H.F., Jorge J.A., Amorim D.S. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol. 2005;67:577–591. doi: 10.1007/s00253-005-1904-7. [DOI] [PubMed] [Google Scholar]
- Pollet A., Schoepe J., Dornez E., Strelkov S.V., Delcour J.A., Courtin C.M. Functional analysis of glycoside hydrolase family 8 xylanases shows narrow but distinct substrate specificities and biotechnological potential. Appl Microbiol Biotechnol. 2010;87:2125–2135. doi: 10.1007/s00253-010-2659-3. [DOI] [PubMed] [Google Scholar]
- Pourreza J., Samie A.H., Rowghani E. Effect of supplemental enzyme on nutrient digestibility and performance of broiler chicks fed on diets containing triticale. Int J Poultry Sci. 2007;6:115–117. doi: 10.3923/ijps.2007.115.117. [DOI] [Google Scholar]
- Ravindran V. Feed enzymes: the science, practice, and metabolic realities. J Appl Poultry Res. 2013;22:628–636. doi: 10.3382/japr.2013-00739. [DOI] [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poultry Sci. 2018;97:4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Saulnier L., Sado P.E., Branlard G., Charmet G., Guillon F. Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J Cereal Sci. 2007;46:261–281. doi: 10.1016/j.jcs.2007.06.014. [DOI] [Google Scholar]
- Schutte J.B. Nutritional implications and metabolizable energy value of D-xylose and L-arabinose in chicks. Poultry Sci. 1990;69:1724–1730. doi: 10.3382/ps.0691724. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 1996;59:215–221. doi: 10.1016/0377-8401(95)00916-7. [DOI] [Google Scholar]
- Sunna A., Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- Svihus B., Choct M., Classen H.L. Function and nutritional roles of the avian caeca: a review. World’s Poult Sci J. 2013;69:249–264. doi: 10.1017/S0043933913000287. [DOI] [Google Scholar]
- Van Craeyveld V., Swennen K., Dornez E., Van de Wiele T., Marzorati M., Verstraete W. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr. 2008;138:2348–2355. doi: 10.3945/jn.108.094367. [DOI] [PubMed] [Google Scholar]
- Vandeplas S., Dauphin R.D., Thonart P., Théwis A., Beckers Y. Effect of the bacterial or fungal origin of exogenous xylanases supplemented to a wheat-based diet on performance of broiler chickens and nutrient digestibility of the diet. Can J Anim Sci. 2010;90:221–228. doi: 10.4141/CJAS09067. [DOI] [Google Scholar]
- Zhang J., Siika-Aho M., Puranen T., Tang M., Tenkanen M., Viikari L. Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnol Biofuels. 2011;4:1–13. doi: 10.1186/1754-6834-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Moilanen U., Tang M., Viikari L. The carbohydrate-binding module of xylanase from Nonomuraea flexuosa decreases its non-productive adsorption on lignin. Biotechnol Biofuels. 2013;6:1–8. doi: 10.1186/1754-6834-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu J., Lei L., Jiang Y., Gao F., Zhou G.H. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different sections of the gastrointestinal tract of broilers fed wheat-Based diets. Asian-Australas J Anim Sci. 2014;27:855–861. doi: 10.5713/ajas.2014.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.