Abstract
The present study examined the microstructure as well as the physicochemical properties of human milk during the second year of lactation in an attempt to explore its applicability for the formulation of food products. It was observed that human milk fat globules (MFG) droplet size increased within 3 days of milk extraction due to coalescence, as evidenced by confocal microscopy. Furthermore, a gradual decrease of the average MFG size was noted from the sixteenth (16th) to twenty-fifth (25th) month of lactation. It was also found that the size of casein micellar structures increased upon acidification to pH 4.3 (isoelectric point of human caseins). In addition, human milk proteins enhanced the stability of oil-in-water emulsions against coalescence compared to cow, sheep, and goat milk proteins employed as macromolecular emulsifying ingredients. The cold-acid-gels of human milk proteins showed a less elastic behavior than the other milk samples, possibly due to the different structure, composition and size of human casein micelles. Furthermore, the DSC thermograms showed that human whey proteins are denatured in the same temperature range as do the cow whey proteins, but exhibit different thermal transition profiles. Overall, the findings of this research confirm that both the structure and the physicochemical properties of human milk are affected by the stage of lactation. Moreover, the particular composition and structure of human milk proteins seem to be responsible for the special functional characteristics of human milk that may lead towards the formulation of innovative products.
Keywords: Human milk, Second year of lactation, Rheology, CLSM, DSC, Zeta potential
Graphical abstract
Highlights
-
•
The physicochemical properties of human milk in the 2 nd year were examined.
-
•
Enhanced stabilization of oil-in-water emulsions with human milk proteins (HMP).
-
•
Less elastic behavior of cold-acid HMP gels compared to other milk species proteins.
-
•
HMP exhibited different thermal transition profiles than cow milk proteins.
1. Introduction
Human breast milk is an extremely complex biological fluid that contains all the nutrients necessary for the survival and development of the infant. The World Health Organization (WHO) recommends breastfeeding exclusively for the first six months and, if possible, continuing until the second year of the child life (Eidelman and Schanler, 2012). Ιn the first days postpartum, human’s colostrum is low in fat and lactose (Andreas et al., 2015), but rich in whey proteins such as immunoglobulins, lactoferrin and lysozyme (Neville et al., 2001). About 15 days postpartum, human breast milk is considered as mature and contains approximately 7.2% lactose, 3.6% fat and 0.9 % proteins (~ 0.37% caseins) (Ballard and Morrow, 2013). After the introduction of solid foods in the infant’s diet (about the sixth (6th) month postpartum), there is a gradual decrease in milk volume but no significant differences in milk composition (Mitoulas et al., 2002; Neville et al., 2001). However, it has been found that during the second year of lactation the fat content of human breast milk increases by up to 50%, when a not exclusive breastfeeding diet is employed (Mandel et al., 2005). Additionally, Kunz & Lönnerdal (1992) found that the concentration of whey proteins continues to lessen after the sixth (6th) month postpartum, resulting in a whey protein-to-casein ratio of about 50:50 in late lactation compared to a 90:10 ratio in the early lactation.
There are limited data in the literature on the physical properties of human breast milk, especially during the second year of lactation. However, most researchers agree that human milk pH is slightly alkaline (Sunarić et al., 2017). Human milk lipids (triacylglycerols) are organized in colloidal structures; i.e. the milk fat globules (MFG) are surrounded by a biological membrane, which is composed mainly of proteins, glycoproteins, enzymes, polar lipids and cholesterol (Lopez and Ménard, 2011). It has been found that the size of human milk fat globules is affected by the time postpartum (Michalski et al., 2005). Although the size of MFG in colostrum has been extensively studied, there is no relevant documentation on the size of human MFG (HMFG) during the second year of lactation.
Casein micelles make up to 20–40% of total human breast milk protein, in contrast to approximately 80% of cow’s milk protein (Liao et al., 2017). Human colostrum does not contain casein micelles. Casein micelles are observed in human breast milk after the third (3rd) day postpartum (Kunz and Lönnerdal, 1992). It is commonly accepted that human casein micelles contain only β- and κ-caseins (Kunz and Lönnerdal, 1990), although some research points to the presence of αS1-caseins in low concentrations (Altendorfer et al., 2015). It has been found that human casein micelles contain less CCP compared to cow casein micelles and only 15% of human’s milk calcium is bound to caseins (Neville et al., 1994). Human casein micelles are considered to be much smaller than cow casein micelles (Carroll et al., 1985; Rüegg and Blanc, 1981). However, the research that has been carried out so far concerns only milk from the 1st year of lactation.
Although human breast milk has about the same whey protein content as cow’s milk, there are significant differences in their protein composition. That is, β-lactoglobulin, the most abundant whey protein of cow’s milk, does not exist in human breast milk. In contrast, lactoferrin, which is found only in traces in cow’s milk, is one of the predominant proteins in human breast milk. Specifically, human breast milk contains approximately 26% α-lactalbumin, 26% lactoferrin, 16% immunoglobulins (mainly IgA), 10% lysozyme, 10% serum albumin and 12% other proteins (Guo and Hendricks, 2008). Moreover, unlike caseins, which are stable at high temperatures, whey proteins are easily denatured by heating at temperatures above 60 °C (Dannenberg and Kessler, 1988; Nikolaidis et al., 2017; Nikolaidis & Moschakis, 2017, 2018). At high temperatures, whey protein molecules unfold owing to the weakening of the bonds that stabilize their secondary, tertiary and quaternary structure (Wagner et al., 2020, Wagner, 2021). Unfortunately, there is limited knowledge regarding the thermal stability of human whey proteins and calorimetric studies have been only carried out on thermal denaturation of lactoferrin. In addition, there has been a lot of research regarding the ability of cow milk proteins and mainly caseins to stabilize oil-in-water emulsions, but the stabilizing properties of human milk proteins have not been studied so far.
Overall, it is clear from the above that the composition of human breast milk during the exclusive breastfeeding period (the first 6 months postpartum) has been extensively studied, but there is little information in the literature on the composition and structure of human breast milk during the late lactation. Furthermore, there has been little research on the physicochemical properties of human breast milk, while its functional properties have not been studied so far. However, it is known that human breast milk is still nutritious after the 1sτ year of infancy. In this context, several mothers prepare homemade meals with their own breast milk, but no attempt has ever been made to create innovative food products with human breast milk. In addition, some mothers donate their breast milk to human milk banks, which can then be used by mothers that cannot feed their own breast milk to their children. Human breast milk from those banks can also be supplied to toddlers/infants with medical conditions. Moreover, the shelf-life of breast milk is relatively short and extensive microbial analysis is required before providing it to children. The aim of the present study was a first approach to examine the microstructure as well as the physicochemical properties of human breast milk during the second year of lactation. In addition, the rheological behavior of human breast milk upon acidification as well as the ability of human milk proteins to stabilize emulsions was also investigated in an attempt to explore their applicability for the formulation of food products.
2. Materials and methods
2.1. Reagents
Trichloroacetic acid (TCA) (≥99%), calcium chloride (≥96%), glacial acetic acid (≥99.8%), imidazole (≥99%), D-(+)-gluconic acid δ-lactone (GDL) ≥99% and sodium azide (≥99,5%) were of analytical grade and purchased from Sigma-Aldrich (St. Louis, USA). Double distilled water was used for the preparation of all solutions. Kjeldahl reagents were obtained from Honeywell Riedel-de Haën (Sleeze, Germany). Powdered whey protein (WPI Bipro ™, 92.08 % w/w protein, 1.08 % w/w fat, 4.08 % w/w ash, and 1.08 % w/w lactose) was purchased from Davisco Foods International, Inc. (Le Sueur, MN, USA). D-(+)-Lactose monohydrate (≥99%) was purchased from Honeywell Riedel-de Haën. Sunflower oil was obtained from a local supermarket and used without further purification. Nile red (0.01% w/v in acetone) and Nile blue (0.1% w/v in water) were purchased from Sigma–Aldrich (St Louis, USA).
2.2. Human milk samples
Human breast milk was donated by a healthy woman (28 years old, first birth, full term pregnancy) between the 16th and 20th month postpartum. She was an urban resident (Thessaloniki, Greece), well-nourished (non-vegetarian diet) and did not use any vitamin supplements during lactation. Milk samples were collected every day at the same time by emptying both breasts, in order to minimize fat content variations. Most of the milk was defatted by centrifugal separation (6000 g, 4 °C, 15 min), freeze dried at -96 °C and 0.2 mbar for 48h (ScanVac Coolsafe 100-9 Pro freeze dryer, Labogene, Denmark) and subsequently stored at -20 °C until further use. Freeze-dried human breast milk was rehydrated by mixing it with appropriate quantities of double distilled water using a magnetic stirrer for at least 2 h at ambient temperature before usage. However, some experiments were carried out using fresh raw milk. In these cases, the samples were stored at 4 °C immediately after expression. Raw and dried human breast milk composition is shown in Table 1.
Table 1.
Chemical composition of raw human milk (mean ± standard deviation; n=5 independent milk samples) and average chemical composition of lyophilized human breast, cow, sheep and goat milk and lyophilized human milk protein concentrate (HMPC).
| Composition (%) | Raw human milk | Lyophilized human milk | Lyophilized cow milk | Lyophilized sheep milk | Lyophilized Goat milk | Lyophilized HMPC |
|---|---|---|---|---|---|---|
| Fat | 3.6–7.8a | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Solids-not-fat | 8.6 ± 0.3 | – | – | – | – | – |
| Humidity | – | <4 | <4 | <4 | <4 | <4 |
| Proteins | 1.1 ± 0.1 | 14.5 | 38.2 | 46.3 | 36.6 | 57.3 |
| Caseins | 0.43 ± 0.07 | 5.8 | 30.7 | 37.5 | 31.2 | 22.9 |
| Ash | 0.2 ± 0.1 | 1.7 | 7.7 | 8 | 7.3 | nd |
| Total sugars | 6.8 ± 0.3 | ~80 | ~50 | ~40 | ~50 | ~40 |
Minimum and maximum values of fat content.
2.3. Cow, goat and sheep milk samples
Cow milk was supplied from the Farm of Aristotle University of Thessaloniki, whereas sheep and goat milk were obtained from other local farms. All types (species) of milk were defatted, freeze dried, stored and rehydrated as described above for the human breast milk. Cow, goat and sheep dried milk composition is shown in Table 1.
2.4. Human milk protein concentrate (HMPC)
Human milk protein was concentrated by using Amicon® Ultra Centrifugal Filters with a 3000 Da molecular weight cut-off (Merck KGaA, Darmstadt, Germany) by means of dilution and standardization purposes. After the recovery of the concentrate, HMPC was lyophilized and stored in the same way as the human breast milk. Lyophilized HMPC composition is shown in Table 1.
2.5. Milk composition analysis
The protein content of all collected milk samples (human, cow, goat, sheep) was determined using the Kjeldahl method (ISO 8968-1│IDF 20–1:2001). The non-protein nitrogen (NPN) is higher in human breast milk compared to the milk of other species and a correction by the conversion factor of 6.38 leads to an overestimation of the protein content (Lönnerdal, 2003). Thus, the nitrogen content of the human breast milk was corrected for the NPN after its analysis by trichloroacetic acid precipitation (ISO 8968-4│IDF 20–4:2001). The corrected nitrogen content was then multiplied by 6.25. The determined nitrogen content of cow, goat and sheep milk samples was directly corrected by using a conversion factor of 6.38. The casein content was determined according to ISO 17997-1│IDF 29–1:2004 method with the differentiation that casein precipitation in human milk was achieved by adjusting the pH to 4.3 (instead of 4.6) and adding 60 mM CaCl2 (Kunz and Lönnerdal, 1990). Fat content was measured using the Gerber method (ISO 2446│IDF 226:2008). Solids-not-fat (S.N.F.) content of milk was estimated by subtracting fat from total solids. Total solids were determined gravimetrically as described in ISO 6731│IDF 21:2010. Ash content was estimated after incineration of the dried residue of milk in a muffle furnace at 530 °C. Finally, the carbohydrate content of milk was calculated by subtracting fat, protein and ash from the total solids.
2.6. Physicochemical measurements
The pH, water activity (aw), refractive index and freezing point of raw human breast milk were determined. The pH was measured by a glass electrode pH-meter (Hanna Instruments, Padova, Italy). The water activity was determined using the AquaLab Series 3 TE Water Activity Meter (Decagon Devices Inc., Washington, USA). The refractive index was measured using the Reichert Abbe Mark II Refractometer (Reichert Technologies, New York, USA). The freezing point was measured by a cryoscope (Advanced Milk Cryoscope 4L2, Advance Instruments Inc., Metuchen, NJ, USA). All measurements were performed in triplicate on fresh, undiluted samples at ambient temperature.
2.7. Microstructural analysis of human milk
The microstructure of raw human milk was captured by confocal laser scanning microscopy (CLSM) (Leica TCS SP5 CLSM, Leica Microsystems, Wetzlar, Germany). Confocal experiments were performed using an Argon laser operating at 488 nm excitation wavelength and an He–Ne laser operating at 633 nm. Fat globules were stained with Nile red, while proteins were stained with Nile blue. Human milk samples were observed after 3, 24, 72 and 120 h of expression and stored quiescently at 4 °C. About 0.02% sodium azide was added to the milk samples to prevent microbial growth. The microstructural analyses were performed at room temperature.
2.8. Particle size measurements
The fat globule size distribution of raw human milk was measured by laser light scattering using a Sald-7500nano (Shimadzu, Korneuburg, Austria). Size measurements were performed at most within 3 h after milk expression. The refractive index of human milk fat was taken to be 1.460 (Michalski et al., 2005). Milk was diluted in the measurement cell of the apparatus with imidazole buffer (20 mM imidazole, 5 mM CaCl2, and 30 mM NaCl, pH 7.75). The size distributions of human milk fat globules were characterized by the following parameters: volume moment-weighted mean diameter or De Broukere mean, d43, defined as Σnidi4/Σnidi3, the volume surface-weighted mean diameter or Sauter mean, d32, defined as Σnidi3/Σnidi2 where ni is the number of fat globules of diameter di. The distribution span, which describes the particle size distribution width, was calculated as Span = (d0.9 – d0.1)/d0.5, where d0.9 is the diameter below which lie 90% of the globule volume and, respectively, 10% for d0.1 and 50% for d0.5. Size measurements were repeated on the 16th, 20th, 24th and 25th month of lactation in order to investigate the effect of the lactation stage on the fat globule size. The experiments were performed in triplicate for each sample.
2.9. Dynamic light scattering measurements
Casein micelle size distribution of rehydrated freeze-dried human milk was determined by dynamic light scattering using a Zetasizer Nano (Malvern Instruments, Worcestershire, UK). The experiments were carried out at 25 °C. Rehydrated freeze-dried human milk was centrifuged (10000 g, 4 °C, 10 min) and then diluted in a 1:20 ratio. Subsequently, the samples were micro-filtrated (25 mm Syringe filter, Material-Nylon, Pore size-0.45 μm, BGB, Rheinfelden, Germany) and placed in the measurement cell of the apparatus. The experiments were performed in triplicate for each sample.
2.10. Oil-in water emulsions
Oil-in-water emulsions were prepared by mixing appropriate quantities of rehydrated freeze-dried milk (human, cow, goat and sheep) with 1% protein and sunflower oil, in order to obtain emulsions with protein to oil ratios of 1:3, 1:5, 1:7, 1:10. Homogenization of the samples was performed with the Bandelin Sonoplus HD 3200 sonicator (Berlin, Germany, VS 70T probe, ~4200 kJ), for 1 min with a 30 s interval to avoid overheating of the samples. Immediately after homogenization, the size distribution of the emulsion droplets and their microstructure were determined. For macroscopic measurements, freshly prepared emulsions were poured into glass tubes (height 40 mm, diameter 16 mm) and stored quiescently at room temperature. In order to prevent microbial growth during storage, 0.02% sodium azide was added to the emulsions. Each experiment was carried out at least in duplicate.
2.11. Rheological behavior of human milk upon acidification
The rheological behavior of human milk protein concentrate (HMPC) standardized to a 2% casein concentration was studied upon acidification and compared to that of cow’s and sheep’s milk with the same casein concentration. HMPC was prepared by mixing appropriate quantities of lyophilized HMPC and double distilled water to obtain a sample with 2% casein concentration. The other milk samples were prepared in the same way, by mixing appropriate quantities of lyophilized milk (cow and sheep milk), double distilled water, whey protein isolate (WPI) and lactose in order to obtain samples with approximately the same composition as that of the HMPC sample. Milk samples were heated to 40 °C and subsequently acidified by means of glucono-δ-lactone (GDL) at a concentration of 0.7g GDL/g of casein. Changes in pH during acidification were monitored continuously by the Bante 220 Portable pH meter (Bante Instruments, Shanghai, China). Acidification was carried out in a circulating water bath (±0,5 °C) preset at 40 °C and the pH was recorded every 15 min for a time interval of 5h to reach a pH value of <4.3. Rheological measurements of the samples were performed by a Physica MCR 300 rheometer (Physica Messtechnik GmbH, Stuttgart, Germany) using a concentric cylinder geometry (CC 27, cup and bob diameters 28.92 and 26.66 mm respectively). The preheated samples (40 °C) were placed in the rheometer immediately after the GDL addition. To avoid water evaporation, the samples were covered with a thin layer of paraffin oil. The gelation of the samples during acidification was assessed by applying small deformation oscillatory rheological testing. Specifically, storage (G′), loss (G′′) moduli and loss tangent (tan δ = G′′/G′) were monitored at fixed strain (0.5%) and frequency (1 Hz) for a 5h time interval. The temperature of the experiment (40 °C) was regulated by a Paar Physica circulating bath and a controlled Peltier system (TEZ 150P/MCR) (±0.1 °C). The structure of the samples was characterized by the following rheological parameters: Gelation time (Gt) (min) was determined as the time that a sharp increase of the value of G′ was observed; Maximum storage modulus (G′ max) (Pa) was determined as the value of G′ at the end of the rheological observation (300 min, pH ~4.2); Minimum loss tangent (tan δmin) was the value of tan δ corresponding to pH ~4.2; Elasticity increment IE [(dlogG_/dt)max] defined as the slope of log G′(t) at the inflection point and is used as a measure of gelation rate. Finally, small deformation oscillatory measurements for evaluation of the viscoelastic properties (storage G′ and loss G′′ moduli) of the samples were performed over the frequency range of 0.1 – 10 Hz at 40 °C. Each experiment was carried out at least in duplicate.
2.12. Differential scanning calorimetry
Thermal denaturation of human and cow whey proteins was studied by means of differential scanning calorimetry (DSC), using the PL DSC-Gold Calorimeter (Polymer Labs Ltd., Epsom, UK). Samples containing 15% w/w human whey protein concentration were prepared from lyophilized HMPC and HPLC grade water. The cow whey protein sample was prepared by mixing appropriate quantities of WPI, HPLC grade water and lactose in order to obtain a sample with a composition similar to that of the human whey protein sample (15% w/w cow whey protein and 18,6% w/w lactose). Samples of 50 μl were sealed hermetically into 120 μl DSC medium pressure stainless steel pans (ME-29990, Mettler, Toledo, SAE). Samples were scanned in the temperature range of about 10 - 110 °C with a 5 °C/min heating rate, using 50 μl 18.6% w/w lactose solution as reference. Onset (To), endset (Te) and peak (Tp) temperatures, i.e., the temperature values at which the denaturation starts, ends or gives the maximum signals, respectively, and the denaturation enthalpy, ΔΗD (J/g of whey protein) were evaluated from the thermograms. Reported values are means of triplicate measurements for each sample.
2.13. Statistical analysis
The data collected during measurements were analysed by ANOVA using the General Linear Model. Differences among different treatments were identified using Tukey’s test. All analyses were performed using the Minitab (Minitab Inc., USA) statistical software.
3. Results and discussion
3.1. Physical properties of human breast milk
The physical parameters (pH, aw, refractive index and freezing point) of the examined human breast milk are shown in Table 2. Generally, the aw, refractive index and freezing point values are moderately similar to those of cow’s milk. On the contrary, the pH value of human milk is higher than that of cow’s milk. The slightly alkaline pH value of human breast milk may be attributed to its low protein, casein and phosphate salts content. It is noteworthy to mention that the pH value of the milk was higher than those reported in literature. This can be attributed to the late stage of lactation. Besides, Morriss et al. (1986) found that the pH value remained between 7.0 and 7.1 until 3 months postpartum and then increased gradually to 7.4 by 10 months. According to Morriss et al. (1986), hydrogen ions and citrate may be used for the synthesis of fatty acids more rapidly than they are metabolically generated; milk samples from ruminants are usually found to have concentrations of hydrogen ions and citrate that are greater than in human milk and thus pH is less for milk of ruminants. As the fat content of human breast milk increases during the course of lactation (Mandel et al., 2005), its pH value may also increase. Τhe refractive index of the examined human breast milk is slightly lower than that reported by (Sunarić et al., 2017), which may be due to the dissimilar stage of lactation. The refractive index is affected by casein micelles and the stage of lactation, which is known to affect both the casein content of human breast milk and the casein micelle size (Holt and Baird, 1978; Kunz and Lönnerdal, 1992). Finally, the freezing point of the examined samples was within the range reported in literature (Miller and Ellis, 1953).
Table 2.
Physical parameters of the examined human breast milk compared to cow’s milk.
| Parameter | Human breast milk a | Cow milka |
|---|---|---|
| pH | 7.74 ± 0.17 (22 °C) | 6.6–6.75 (25 °C) |
| Water activity (aw) | 0.995 ± 0.001 (25 °C) | ~0.993 |
| Refractive index | 1,3465 ± 0.0005 (24 °C) | 1.3440 - 1.3485 (20 °C) |
| Freezing point | -0.523 to -0.565 °C | -0.525 to -0.565 °C |
Mean ±standard deviation; n=3 independent milk samples.
3.2. Microstructural analysis of human breast milk
As expected and evidenced from CLSM (Fig. 1), lipids are present in human breast milk in the form of spherical emulsion droplets, the fat globules, with polydisperse sizes. Three (3) hours after the expression of milk, human breast milk fat globules (HMFG) were evenly dispersed in the milk plasma (Fig. 1a). After twenty-four (24) hours of refrigeration no marginal differences were detected (Fig. 1b). However, three days (72 h) after milk expression, HMFG seemed to become larger in droplet size (Fig. 1c), whereas after 5 days of refrigeration (120 h) the HMFG’s size was further increased (Fig. 1d). This increase in the HMFG’s size may be attributed to coalescence phenomena. Coalescence is rarely observed in milk because of the protective role of the biological membrane, which covers the fat globules (Walstra, 1996). However, in some cases, partial coalescence may occur in unhomogenized milk due to the prolonged contact between fat globules during refrigeration (flocculation and creaming) and the hydrolysis of the phospholipids in the milk fat globule membrane by endogenous phospholipases (Walstra et al., 2005). As shown in Fig. 1, marginal flocculation did not occur after 5 days of refrigeration (Fig. 1), indicating a lack of “cold agglutination”, i.e. the clustering of milk fat globules at low temperatures. Cold agglutination phenomenon is mainly attributed to the interaction among immunoglobulin M (IgM), lipoprotein particles and fat globules and is usually encountered in unhomogenized raw cow milk (Euber and Brunner, 1984). Cold agglutination doesn’t occur or occurs to a considerably lower extent in goat, sheep and buffalo milk (Huppertz et al., 2020). A recent research on the creaming behavior of human milk (Meng et al., 2020) suggested that cold agglutination doesn’t occur in human milk, which is in agreement with our findings. On the other hand, phospholipases are enzymes usually of bacterial origin, mainly Gram- (Titball, 1998). Sodium azide, employed in this study as an antimicrobial agent, have inhibited the growth of Gram- bacteria and therefore, the samples probably did not contain considerable amount of phospholipases. However, raw human milk contains a lot of endogenous enzymes (Hamosh, 2001), such as lipases and proteases, which, upon storage, may have negatively contributed to the destabilization of the fat globule membrane.
Fig. 1.
Confocal micrographs of the human breast milk captured at: (a) 3; (b) 24; (c) 72; and (d) 120 h after expression. Fat globules are labeled with green color, while proteins on the fat globules’ surface are labeled with red color. Yellow color labels the overlay of fat globules and proteins together. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Human milk particle size measurements
Laser light scattering measurements showed a size distribution of the HMFGs of the examined human breast milk ranging from 0.5 to 25 μm with a characteristic first small peak in the size range ~ 0.4–2 μm (Fig. 2a). The size parameters d43, d32 and span are presented in Fig. 2b. De Brouckere mean diameter value (d43) indicates that the bulk of the milk fat was encompassed in HMFGs with a diameter ranging between 4.7 and 5.9 μm. These values are slightly higher than those reported in literature and can be attributed to the prolonged lactation. In fact, Michalski et al. (2005) observed a significant increase of HMFG size between 2nd and 18th month of lactation. Besides, during the second year of lactation human breast milk has significantly higher fat content than during the exclusive breastfeeding period (Mandel et al., 2005), while an increase in fat content results in an increase in fat globule size (Wiking et al., 2004).
Fig. 2.
Size distribution (a), volumic average diameter (d43), volume-surface average diameter (d32) and span (b) of the human milk fat globules (HMFGs) during the 16th, 20th, 24th and 25th month postpartum.
Means with different superscript letters are different (p < 0.05).
Regarding the effect of the postpartum period on the HMFGs’ size, the range of the HMFG size distribution did not change substantially between the 16th and the 25th month of lactation (Fig. 2a), which is also evidenced from the relatively constant span value during this period (Fig. 2b). However, there was a slight shift of the second peak of the distribution (mode) from almost 7 μm during the 16th month of lactation to 6 μm during the 24th and 25th month of lactation (Fig. 2a). This gradual decrease in HMFGs’ size is also reflected with the decreased values of d43 and d32 (Fig. 2b). The size of cow’s milk fat globules’ was found to decrease during weaning (Walstra et al., 2005), which has also occurred in our human milk samples.
3.4. Human milk casein micelle size measurements
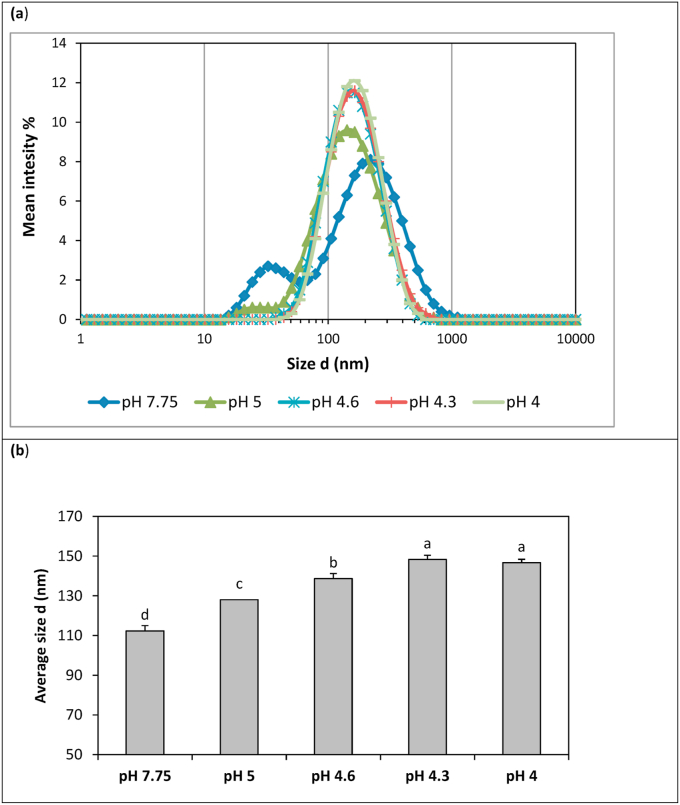
Dynamic light scattering measurements showed two modes of the casein micelles in physiological human breast milk pH (ca 7.75) with the first peak lying at ~ 35 nm and the second one at 220 nm (Fig. 3a). This type of casein micelle size distribution has also been observed in cow’s milk (Holt, 1985). The modal diameter of the second peak (220 nm) is much larger than the micelle sizes (≤50 nm) measured by electron microscopy (Carroll et al., 1985; Rüegg and Blanc, 1981) and the micelle size (~150 nm) reported in a more recent study (Inglingstad et al., 2010). Such an apparent discrepancy between the findings may be attributed to several reasons, like: (a) the different measurement technique applied. Generally, measurements based on electron micrographs are considered to be more perturbative than the dynamic light scattering measurements because casein micelles’ structure may largely change during sample preparation (De Kruif and Holt, 2003). (b) the different stage of lactation. The size values reported in literature concern human milk of the 1st year of lactation, while in the present study the examined milk samples were collected during the second year of lactation. According to Holt and Baird (1978), the stage of lactation affects the size of the cow’s casein micelles and this may be the case for the human’s casein micelles, too; (c) The high pH value of the examined milk. It has been found that by increasing milk pH in the range of 6.6 to 8.5, the casein micelles swell, probably due to the higher negative charges that strengthen the repulsive forces of the caseins chain and therefore prοduce more expanded structures in the micelles (higher hydrodymanic volume) (Sinaga et al., 2017); (d) the high lactoferrin content of human breast milk. According to Anema and De Kruif (2013) lactoferrin found in cow’s milk is partitioned between the casein micelles and the whey. Moreover, they have found that when extra lactoferrin was added to milk (up to 1.5%), the casein micelles tended to increase in size. Human breast milk is rich in lactoferrin (Guo and Hendricks, 2008) and maybe part of it is bound on the casein micelles’ surface.
Fig. 3.
Size distribution (A) and average size diameter (B) of human casein micelles at different pH values.
Means with different superscript letters are different (p < 0.05).
In addition, it should be noted that the size of the casein micelles is affected by the protein composition of the micelles. In this respect, it has been found (Pierre et al., 1995; Tziboula and Horne, 1999) that goat milk without or with low as1 protein content have larger casein micelles. This is may also be the case for our human breast milk samples. Human milk is known to contain no or very little as1 casein (Altendorfer et al., 2015). However, it should not be overlooked that the size distribution (Fig. 3a) may also include lactoferrin tetramers (~16 nm) (Bennett et al., 1981) and oligomers in the presence of calcium that are naturally found in human breast milk. Moreover, the positively charged lactoferrin may interact electrostatically with other milk proteins (e.g. caseins, β-lactoglobulin) that are mostly negatively charged around neutral pH.
Regarding the effect of pH on the size of the casein micelles, the findings are consistent with those of Sinaga et al. (2017) for cow milk; i.e. a slight increase in casein micelle average size is noted upon acidification to the pI of caseins. As observed in Fig. 3a, lowering the pH from ~ 7.7 to ~5.0 resulted in the disappearance of the first peak of the size distribution and the decrease of the modal diameter of the second peak from 220 nm to approx. 150 nm. This initial decrease in casein micelle size (modal diameter) can be attributed both to the dissolution of the CCP, release (dissociation) of caseins from the micelles and the dissociation of the lactoferrin and lactoferrin-milk casein complexes.
As known, CCP is essential for the stability of the casein micelle structure. Therefore, CCP depletion results in dissociation of the casein subunits from the micelles. The solubilization of CCP in cow milk reaches a maximum rate at pH 5.5 (Lucey et al., 1997). Additionally, the former researchers observed a growth of particle size from pH 5.5 to 5.0 due to the initiation of casein micelles aggregation.
As expected, there was a moderate increase in particle size, especially of the modal diameter, from pH 5.0 to 4.3 (Fig. 3) because of the increasing aggregation of the milk proteins at pH values close to the pI. As mentioned above, the pI of human caseins is 4.3 and this explains why the average size of the particles was higher at this pH value. At pH 4 the particle size was slightly lower as casein micelles started to dissociate from the clusters.
3.5. Oil-in water emulsions stabilized by different powdered milk species
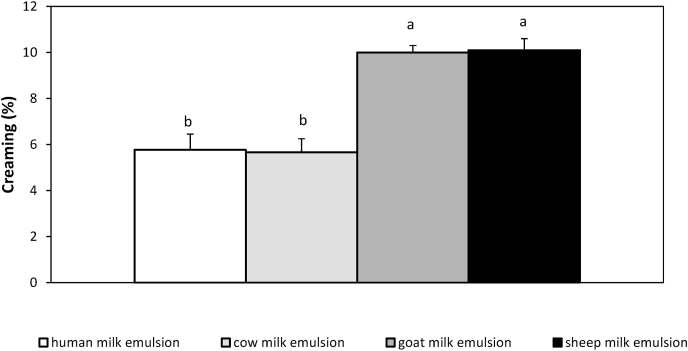
Laser light scattering measurements and confocal laser scanning microscopy showed that the emulsions prepared with human milk powder had relatively smaller droplet sizes compared to those prepared with cow, goat and sheep powdered milk (Fig. 4). Apart from the emulsions stabilized from sheep milk, the rest of the emulsions showed a bimodal size distribution with the first mode lying at around 0.24 μm and the second peak at ~1 μm Τhe emulsions prepared with human milk illustrated a higher percentage of small droplets of 0.24 μm and additionally, the second peak of the size distribution corresponded to <1 μm droplet size values. Regarding the sheep’s milk emulsion, while following the same homogenization procedure, larger emulsion droplets were noted, while droplet clusters were evidenced both by confocal microscopy and the laser diffraction technique. Visual assessment of the emulsions showed that the sample with the human’s milk proteins was as stable against creaming as the sample with the cow’s milk proteins, and with better stability upon storage than the goat’ and sheep’s milk samples (Fig. 5a). Creaming of the emulsions was observed about 14 days after their preparation. In the case of the human protein stabilized emulsion, the cream phase was particularly indistinct (Fig. 5b). Overall, human milk proteins exhibited better ability to stabilize oil-in-water emulsions against coalescence compared to that of the other milk proteins. Τhe above results were quite unexpected, in view of the low casein content of human breast milk. It is worth noting that for the preparation of the samples the same total protein was employed, but, with different casein to total protein ratios. In general, it is known that caseins are mainly adsorbed at the surface of the newly formed oil droplets rather than whey proteins. Specifically, it has been estimated that about the 93% of the oil-water interface proteins are caseins (Walstra et al., 2005). Probably, the good stability of the human’s milk protein emulsions is due to the type of the caseins that make up the human casein micelles and / or the involvement of lactoferrin and whey proteins in the formation of the protective layer surrounding the oil droplets. It is known that β-casein, the most abundant casein of human milk, is slightly more hydrophobic and surface-active than as1 casein (Dickinson, 1999) and for this reason it may be adsorbed more readily to the surface of the oil droplets. Moreover, it has been found that part of human’s β-caseins are soluble in whey (Greenberg et al., 1976), which may facilitate their interfacial adsorption. Finally, lactoferrin, an abundant whey protein of human milk, having a net positive charge at the normal pH of human milk, may be form complexes with the negatively charged β-caseins present at the oil-water interface (McCarthy, Kelly, O'Mahony and Fenelon, 2014), enhancing their steric stability (Zinoviadou, Scholten, Moschakis, & Biliaderis, 2012a, 2012b).
Fig. 4.
Microstructure of emulsions made from human breast milk (a), cow’s milk (b), goat’s milk (c), sheep’s milk (d) and sunflower oil. Pictures were taken using a confocal laser scanning microscope (CLSM). Oil droplets size distribution was determined using a laser light scattering device. Oil droplets are labeled with green color, while proteins are labeled with red color. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5a.
De Brouckere mean droplet diameter (d43), Sauter mean diameter (d32), and diameter d0.9 of the emulsions formed from different milk species.
Means with different superscript letters are different (p < 0.05).
Fig. 5b.
Effect of powdered milk species (human, cow, goat, sheep) on the macroscopic phase separation (creaming) of the emulsion samples stored quiescently at ambient temperature for 14 days.
Means with different superscript letters are different (p < 0.05).
3.6. Thermal denaturation of human whey proteins
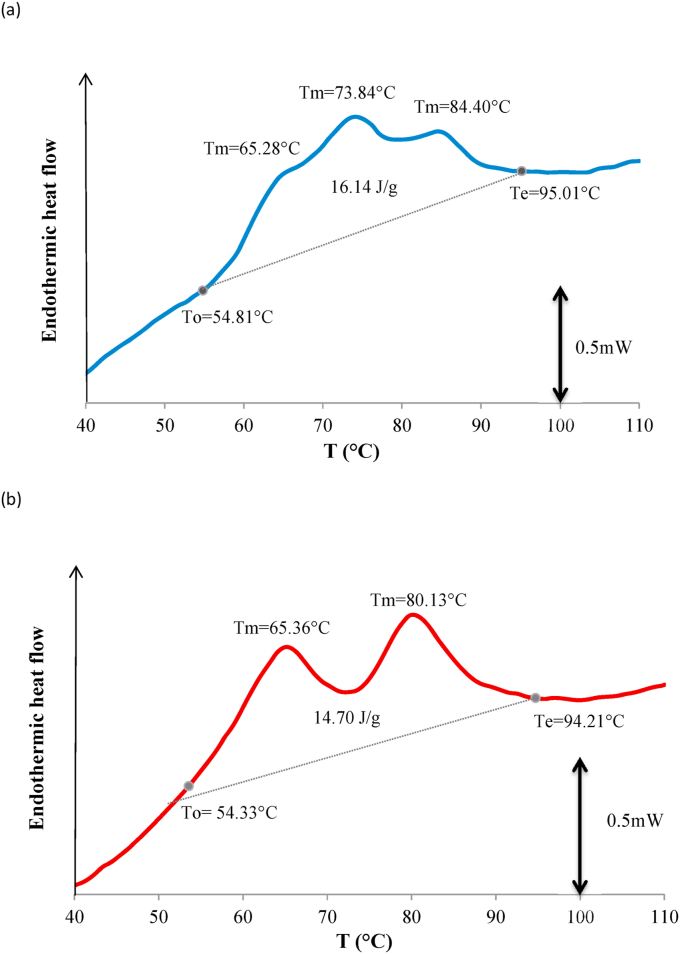
Fig. 6 (a) and (b) show denaturation thermograms of human and cow whey proteins, respectively. It should be noted, however, that the samples studied were not pure whey protein solutions–the human milk sample did also contain caseins–and the increased lactose content may also affect the protein denaturation (Boye and Alli, 2000). For this reason, a lactose solution was used as a reference instead of water. As can be seen from the Fig. 6a and b, the denaturation of both human and cow milk whey proteins started at 52–55 °C and ended at 94–95 °C. The first denaturation peak in both the cow protein and the human protein thermograms occurred at ~65 °C. At this temperature, bovine a-lactalbumin denaturation is usually observed (Boye and Alli, 2000; Nikolaidis and Moschakis, 2018; Rodríguez Arzuaga, Bosch, Añón and Abraham, 2020). However, in the temperature range of 60–65 °C, both bovine and human serum albumin are also denatured (Kosa et al., 1997; Paulsson and Dejmek, 1990). About 10% of human whey proteins is serum albumin (Guo and Hendricks, 2008). Therefore, the peak at 65 °C may be caused by the denaturation of α-lactalbumin and serum albumin (overlapping endotherm peaks).
Fig. 6.
DSC thermograms of 50 μl samples recorded at a heating rate of 5 °C/min. Samples composition (%): (a) 15% w/w human whey proteins, 10% w/w caseins, 18.6 % w/w lactose; (b) 15% w/w cow whey proteins (WPI), 18.6% w/w lactose.
Τhe second endotherm peak at ~80 °C observed in the cow’s whey protein (WPI) thermogram (Fig. 6b) could only be caused by the denaturation of β-lactoglobulin, the most abundant whey protein of cow’s milk. The denaturation temperature (TD) of this protein is highly dependent on pH; i.e., β-lactoglobulin seems to be thermally more stable at low pH, possibly due to extra hydrogen bonding (Boye and Alli, 2000; Kella and Kinsella, 1988). This fact can explain the differences between the β-lactoglobulin TD values found by various researchers. Ιn the thermogram of human whey proteins (Fig. 6a), no endotherm peak was observed at 80 °C, which was to be expected since human breast milk does not contain β-lactoglobulin. The second peak at about 74 °C (Fig. 6a) may correspond to lactoferrin denaturation. It has been previously found that native human lactoferrin denatures at about 72 °C (Conesa et al., 2008; Franco et al., 2018). Unlike bovine, sheep and goat milk lactoferrin, human milk lactoferrin exhibits only one peak in its native form, which indicates that the latter is mainly iron desaturated (Conesa et al., 2008). In fact, it has been reported that the degree of iron saturation of human milk lactoferrin is low (about 12–25%) (Bezwoda and Mansoor, 1989). On the other hand, at ~71 °C bovine lysozyme is also denatured (Iucci et al., 2007). Although no calorimetric data on human lysozyme denaturation have been found in literature, we can assume that the peak at 74 °C is the result of overlapping peaks caused by lactoferrin and lysozyme. Human breast milk also contains significant amounts of immunoglobulins, mainly IgA. There is limited knowledge about the denaturation profile of these proteins. The unfolding of immunoglobulins is a complex process in which the denatured state is obtained through several intermediate states. Ig A has been found to be less heat resistant than IgG and lysozyme (Vermeer and Norde, 2000).
Regarding the origin of the endothermic peak at 84 °C (Fig. 6a), it can be attributed to monoferric and holo-saturated lactoferrin. It has been found that the position of the denaturation peak is affected by the iron saturation of lactoferrin and the 100% iron saturated form (holo-lactoferrin) is denatured at about 94 °C (Conesa et al., 2008; Franco et al., 2018). At this point it is worthy to mention that the presence of caseins in the HMPC samples may have also affected the temperature and the apparent enthalpy of the whey proteins denaturation. It is possible that part of lactoferrin and lysozyme may be bound to casein micelles (Anema and De Kruif, 2013), thus affecting their denaturation. Finally, the heating rate during thermal analysis can affect both the temperature and the enthalpy of lactoferrin and immunogobulins denaturation.
3.7. Rheological behavior of human milk upon acidification
According to some preliminary experiments, the casein content of human breast milk (~0.5%) was not sufficient to form a three-dimensional gel network upon acidification. Thus, HMPC standardized to a 2% casein concentration (see section 2.11), was employed to examine the rheological behavior of human caseins upon acidification. Τhe evolution of storage modulus (G′) and damping factor (tanδ =G′′/G′) of the human, cow and sheep milk samples are shown in Fig. 7. At the onset of acidification, the G′ value was relatively high (~0.1 Pa) for milk samples and the tanδ value was close to 1 in all samples, indicating the existence of rather weak structured networks (Moschakis et al., 2017; Mourtzinos et al., 2018), as probed by the rheometer (small deformation mechanical tests). This is probably due to the increased solids in the samples. As the acidification proceeded further, an abrupt increase of G′ and a decrease of tanδ at <1 was noted, implying the establishment of a three-dimensional gel network (onset of gelation). Usually, gelation begins at pH 5.0–5.3 (Moschakis et al., 2018; Moschakis et al., 2017). In the present study, the gelation of all samples begun at pH ≤ 5.0 (Table 3). This may be ascribed to the increased initial viscosity of the samples, which may delay the aggregation of the casein micelles. It must be noted that in the HMPC samples the gelation initiated at even lower pH (4.8), most likely due to the lower isoelectric point of human caseins (4.3 instead of 4.6).
Fig. 7.
(a) Time dependence of storage modulus (G′) and tanδ for acidified milk gels produced from HMPC (human milk protein concentrate), cow, sheep and goat milk with casein content 2%w/w, at a frequency of 1 Hz (γ=0.5%) at 40 °C.
(b) Mechanical spectra (40 °C, γ=0.5%) of the gels at 4 °C.
Table 3.
Effect of animal origin of milk caseins on the rheological parameters of acid-coagulated milk gel products.
| HMPC | Cow milk | Sheep milk | |
|---|---|---|---|
| Gt (min) | 40±2b | 50±3a | 45±2ab |
| pH (at Gt) | 4.8 ± 0.05b | 4.9 ± 0.06ab | 5.0 ± 0.04a |
| G′max (Pa) (300 min) | 8.2 ± 2.1c | 12.2 ± 3.2b | 41.2 ± 9.4a |
| tanδmin | 0.18 ± 0.01c | 0.29 ± 0.02b | 0.33 ± 0.02a |
| IΕ (min−1) | 0.21 ± 0.1a | 0.07 ± 0.09c | 0.10 ± 0.12b |
Gt (Gelation time): time at which there was an abrupt increase of G′.
pH (at Gt): pH value at which gelation begun.
G′max: Maximum of G′ value (t = 300min).
tanδmin: Minimum tanδ value (pH ~4,2).
IΕ(Elasticity increment): [(dlogG′/dt)max].
Means within the same raw with different superscript letters are different (p < 0.05).
In the early stages of gelation, a local maximum in tanδ (tanδ peak) was noted in the sample of cow’s and sheep’s milk. However, there was not tanδ peak recorded in the HMPC sample. Since the samples in this study were not heat-treated, the tanδ peak is probably attributed to a loosening of the gel network structure as a result of solubilization of the colloidal calcium phosphate (CCP), causing partial micellar disintegration (Biliaderis et al., 1992; Moschakis et al., 2017; Mourtzinos et al., 2018). However, the tanδ values noted are much smaller than that commonly observed in acidified milk gels, perhaps due to the low amount of casein present in the samples. According to Lee and Lucey (2010), at pH ~5.0 almost all the CCP is solubilized. On the other hand, the absence of the tanδ peak from the gelation kinetic profile of the HMPC sample may be attributed to the very low content in CCP of human casein micelles; human breast milk has been found to contain 4-5 times less calcium than cow’s milk (Picciano, 2000), out of which only 15% is bound to human casein micelles, while the corresponding percentage for cow casein micelles is 65% (Neville et al., 1994). Moreover, at pH~5.0 the human casein micelle aggregation has been already initiated as can be deduced from the casein micelle size distribution (Fig. 3a). Consequently, at pH 4.8, when the gelation of the HMPC sample begins, the whole CCP is already solubilized and so an ‘uninterrupted’ process for the three-dimensional gel network structure formation is continued.
As the isoelectric point is approached, the electrostatic repulsions between casein micelles decrease and the hydrophobic interactions are enhanced (Moschakis et al., 2010). As a result, more and more casein micelles participate in the gel network structure. This leads to a further increase of the G′ value until it reaches to a “pseudo plateau” value; the higher the G′max value, the stronger the three-dimensional gel network. As can be seen from the Fig. 7a and Table 3, the sheep’s milk sample produced firmer gels, than the cow’s milk and the HMPC samples. However, none of the samples exhibited an ‘ideal’ gel behavior (Fig. 7b). As is well known, for an ideal gel which behaves elastically, the G′ value is independent of frequency and much higher than G′′ value (i.e. G′>10G′′). According to Fig. 7b in all samples G′ was slightly higher than G′′ and a moderate frequency dependence was also observed, indicating a rather weak gel structure of the samples. It should be also noted that that the HMPC samples showed the lowest tanδmin value, indicating a stronger elastic response of the HMPC gels.
Since all samples had the same casein concentration and were produced under the same experimental conditions, the differences in rheological behavior can be attributed to the structure, composition and size of the casein micelles among the different milk samples. Obviously, the β-caseins present in human breast milk are not able to form a strong three-dimensional gel network but, however, a more elastic network was measured. Α similar conclusion was reached by Domagała (2009) by studying the microstructure of goat’s yogurt compared to that of cow’s and sheep’s yogurt. In particular, they observed that the three-dimensional gel network of goat yogurt was less compact than that of cow’s and sheep’s yogurt and therefore more susceptible to deformation. In fact, sheep’s and cow’s milk have a high content of aS1 caseins (≥35% of all caseins) (Bramanti et al., 2003), which are responsible for the consistency and compact structure of yogurt. In contrast, goat’s milk, depending on the genotype of the animal, may contain 0–25% aS1 caseins (Bramanti et al., 2003), while the β caseins constitute the most abundant casein fraction of goat milk. This may also be the case for human breast milk, which in the prevailing opinion does not contain or contain very little aS1 casein but it is abundant in β caseins (Altendorfer et al., 2015; Kunz and Lönnerdal, 1990). Generally, in milks with a low percentage of as-caseins (e.g. goat milk), the gels formed upon acidification are relatively weak. However, many models have been proposed for acidified milk gels, but none has explained in an explicit manner the kinetics of network formation and growth (Horne, 2002; Lucey, 2002).
Moreover, the gelation rate, which is reflected by the Ie, is more rapid in human milk (Table 3). This parameter is a measure of the gelation rate; i.e., a high Ie value indicates a rapid gelation. The rapid gelation of human milk upon acidification may be assigned to the most abundant casein in human milk, the hydrophobic β-casein. Moreover, human milk has a lower buffering capacity, resulting in a faster reduction of pH upon acidification.
4. Conclusions
In the present study, the structure as well as the physicochemical and functional properties of human breast milk during the second year of lactation were examined in an attempt to explore its applicability for the formulation of food products. Physicochemical measurements showed that the water activity (aw), refractive index and freezing point values of the examined human breast milk were within the same range as those of cow’s milk. The pH value was slightly higher compared to values reported in the literature, probably due to the late stage of lactation. Microstructural analysis of human milk fat globules (HMFGs) using a CLSM showed that HMFGs remained dispersed after 24 h of refrigeration and no flocculation events were detected. On the other hand, merging of HMFGs was observed 3 days after milk extraction. The size of HMFGs was slightly higher compared to the literature, maybe due to the late stage of lactation. However, there was a gradual decrease in the average size of HMFGs during the period from the 16th to the 25th month of lactation. Human milk proteins also exhibited a better ability to stabilize oil-in-water emulsions against coalescence compared to that of cow’s, goat’s and sheep’s milk proteins, as evidenced by the smaller average size of oil droplets and the lower creaming rates upon storage. Additionally, HMPC formed a weaker but more elastic gel network compared to that of cow’s and sheep’s milk, obviously due to the different structure, composition and size distribution of human casein micelles. Calorimetric measurements of human and cow whey proteins showed that human whey proteins were denatured in the same temperature range as the cow whey proteins, but exhibited a different denaturation profile with multiplicity of endothermic transitions.
Overall, the findings of the present research confirm that composition, structure and physicochemical properties of human breast milk change during lactation in order to meet the nutritional needs of the infant. The dynamic composition of human breast milk makes it a unique and irreplaceable nutritional supplement even after the introduction of solid foods in infant nutrition. Furthermore, the interesting emulsifying and thermal properties of human milk proteins as well as their special rheological behavior upon acidification could be studied further in order to formulate novel food products based on human milk, especially at late lactation.
CRediT authorship contribution statement
Alexandra-Maria Βasdeki: Formal analysis, Data curation, Writing – original draft, Validation, Investigation. Dimitrios G. Fatouros: Writing – review & editing, Resources. Costas G. Βiliaderis: Writing – review & editing, Resources. Thomas Moschakis: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization, Methodology, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors CGB and TM acknowledge financial support of this work by the project “Research Infrastructure on Food Bioprocessing Development and Innovation Exploitation – Food Innovation RI” (MIS 5027222), which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund). “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
References
- Altendorfer I., König S., Braukmann A., Saenger T., Bleck E., Vordenbäumen S., Jose J. Quantification of αS1-casein in breast milk using a targeted mass spectrometry-based approach. J. Pharmaceut. Biomed. Anal. 2015;103:52–58. doi: 10.1016/j.jpba.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Andreas N.J., Kampmann B., Mehring Le-Doare K. Elsevier Ireland Ltd; 2015. Human Breast Milk: A Review on its Composition and Bioactivity. Early Human Development. [DOI] [PubMed] [Google Scholar]
- Anema S.G., De Kruif C.G. Protein composition of different sized casein micelles in milk after the binding of lactoferrin or lysozyme. J. Agric. Food Chem. 2013;61(29):7142–7149. doi: 10.1021/jf401270h. [DOI] [PubMed] [Google Scholar]
- Ballard O., Morrow A.L. Elsevier; 2013. Human Milk Composition. Nutrients and Bioactive Factors. Pediatric Clinics Of North America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.M., Bagby G.C., Davis J. Calcium-dependent polymerization of lactoferrin. Biochem. Biophys. Res. Commun. 1981;101(1):88–95. doi: 10.1016/S0006-291X(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Bezwoda W.R., Mansoor N. Lactoferrin from human breast milk and from neutrophil granulocytes. Comparative studies of isolation, quantitation, characterization and iron binding properties. Biomed. Chromatogr. 1989;3(3):121–126. doi: 10.1002/bmc.1130030307. [DOI] [PubMed] [Google Scholar]
- Biliaderis C.G., Khan M.M., Blank G. Rheological and sensory properties of yogurt from skim milk and ultrafiltered retentates. Int. Dairy J. 1992;2(5):311–323. doi: 10.1016/0958-6946(92)90035-K. [DOI] [Google Scholar]
- Boye J.I., Alli I. Thermal denaturation of mixtures of α-lactalbumin and β-lactoglobulin: a differential scanning calorimetric study. Food Res. Int. 2000;33(8):673–682. doi: 10.1016/S0963-9969(00)00112-5. [DOI] [Google Scholar]
- Bramanti E., Sortino C., Onor M., Beni F., Raspi G. Separation and determination of denatured αs1-, αs2-, β- and κ-caseins by hydrophobic interaction chromatography in cows', ewes' and goats' milk, milk mixtures and cheeses. J. Chromatogr. A. 2003;994(1–2):59–74. doi: 10.1016/S0021-9673(03)00483-7. [DOI] [PubMed] [Google Scholar]
- Carroll R.J., Basch J.J., Phillips J.G., Farrell H.M. Ultrastructural and biochemical investigations of mature human milk. Food Struct. 1985;4(2):323–331. [Google Scholar]
- Conesa C., Sánchez L., Rota C., Pérez M.D., Calvo M., Farnaud S., Evans R.W. Isolation of lactoferrin from milk of different species: calorimetric and antimicrobial studies. Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology. 2008;150(1):131–139. doi: 10.1016/j.cbpb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Dannenberg F., Kessler H. Reaction kinetics of the denaturation of whey proteins in milk. J. Food Sci. 1988;53(1):258–263. doi: 10.1111/j.1365-2621.1988.tb10223.x. [DOI] [Google Scholar]
- De Kruif C.G., Holt C. Advanced Dairy Chemistry—1 Proteins. Springer US; 2003. Casein micelle structure, functions and interactions; pp. 233–276. [DOI] [Google Scholar]
- Dickinson E. Caseins in emulsions: interfacial properties and interactions. Int. Dairy J. 1999;9(3–6):305–312. [Google Scholar]
- Domagała J. Instrumental texture, syneresis and microstructure of yoghurts prepared from goat, cow and sheep milk. Int. J. Food Prop. 2009;12(3):605–615. doi: 10.1080/10942910801992934. [DOI] [Google Scholar]
- Eidelman A.I., Schanler R.J. Breastfeeding and the use of human milk. Pediatrics. 2012 doi: 10.1542/peds.2011-3552. March. [DOI] [Google Scholar]
- Euber J.R., Brunner J.R. Reexamination of fat globule clustering and creaming in cow milk. J. Dairy Sci. 1984;67(12):2821–2832. doi: 10.3168/jds.S0022-0302(84)81642-2. [DOI] [Google Scholar]
- Franco I., Pérez M.D., Conesa C., Calvo M., Sánchez L. Elsevier Ltd; 2018. Effect of Technological Treatments on Bovine Lactoferrin: an Overview. Food Research International. April 1. [DOI] [PubMed] [Google Scholar]
- Greenberg R., Groves M.L., Peterson R.F. Amino terminal sequence and location of phosphate groups of the major human casein. J. Dairy Sci. 1976;59(6):1016–1018. doi: 10.3168/jds.S0022-0302(76)84317-2. [DOI] [PubMed] [Google Scholar]
- Guo M., Hendricks G. Chemistry and biological properties of human milk. Curr. Nutr. Food Sci. 2008;4(4):305–320. doi: 10.2174/157340108786263667. [DOI] [Google Scholar]
- Hamosh M. Bioactive factors in human milk. Pediatr. Clin. 2001;48(1):69–86. doi: 10.1016/S0031-3955(05)70286-8. [DOI] [PubMed] [Google Scholar]
- Holt C. vol. 4. SEM Inc.; 1985. The size distribution of bovine casein micelles: a review; pp. 1–10.https://digitalcommons.usu.edu/foodmicrostructureAvailableat:https://digitalcommons.usu.edu/foodmicrostructure/vol4/iss1/2 (Food Structure). AMF O’Hare. Retrieved from. [Google Scholar]
- Holt C., Baird L. Natural variations in the average size of bovine casein micelles. J. Dairy Res. 1978;45(3):339–345. doi: 10.1017/S0022029900016551. [DOI] [Google Scholar]
- Horne D.S. Casein structure, self-assembly and gelation. Curr. Opin. Colloid Interface Sci. 2002;7(5–6):456–461. [Google Scholar]
- Huppertz T., Uniacke-Lowe T., Kelly A.L. vol. 2. Springer International Publishing; 2020. Physical chemistry of milk fat globules; pp. 133–167. (Advanced Dairy Chemistry). [DOI] [Google Scholar]
- Inglingstad R.A., Devold T.G., Eriksen E.K., Holm H., Jacobsen M., Liland K.H., Vegarud G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010;90(5):549–563. doi: 10.1051/dst/2010018. [DOI] [Google Scholar]
- Iucci L., Patrignani F., Vallicelli M., Guerzoni M.E., Lanciotti R. Effects of high pressure homogenization on the activity of lysozyme and lactoferrin against Listeria monocytogenes. Food Contr. 2007;18(5):558–565. doi: 10.1016/j.foodcont.2006.01.005. [DOI] [Google Scholar]
- Kella N.K.D., Kinsella J.E. Enhanced thermodynamic stability of β-lactoglobulin at low pH. A possible mechanism. Biochem. J. 1988;255(1):113–118. doi: 10.1042/bj2550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosa T., Maruyama T., Otagiri M. Species differences of serum albumins: I. Drug binding sites. Pharmaceut. Res. 1997;14(11):1607–1612. doi: 10.1023/A:1012138604016. [DOI] [PubMed] [Google Scholar]
- Kunz C., Lönnerdal B. Casein and casein subunits in preterm milk, colostrum, and mature human milk. J. Pediatr. Gastroenterol. Nutr. 1990;10(4):454–461. doi: 10.1097/00005176-199005000-00007. [DOI] [PubMed] [Google Scholar]
- Kunz C., Lönnerdal B. Re‐evaluation of the whey protein/casein ratio of human milk. Acta Pædiatrica. 1992;81(2):107–112. doi: 10.1111/j.1651-2227.1992.tb12184.x. [DOI] [PubMed] [Google Scholar]
- Lee W.J., Lucey J.A. Formation and physical properties of yogurt. Asian-Australasian. J. Anim. Sci.Asian-Australasian Association.Anim. Prod.Soc. 2010 doi: 10.5713/ajas.2010.r.05. [DOI] [Google Scholar]
- Liao Y., Weber D., Xu W., Durbin-Johnson B.P., Phinney B.S., Lönnerdal B. Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. J. Proteome Res. 2017;16(11):4113–4121. doi: 10.1021/acs.jproteome.7b00486. [DOI] [PubMed] [Google Scholar]
- Lopez C., Ménard O. Human milk fat globules: polar lipid composition and in situ structural investigations revealing the heterogeneous distribution of proteins and the lateral segregation of sphingomyelin in the biological membrane. Colloids Surf. B Biointerfaces. 2011;83(1):29–41. doi: 10.1016/j.colsurfb.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Lucey J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002;85(2):281–294. doi: 10.3168/jds.s0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Lucey J.A., Dick C., Singh H., Munro P.A. Dissociation of colloidal calcium phosphate-depleted casein particles as influenced by pH and concentration of calcium and phosphate. Milchwissenschaft. 1997;52(11):603–606. [Google Scholar]
- Mandel D., Lubetzky R., Dollberg S., Barak S., Mimouni F.B. Fat and energy contents of expressed human breast milk in prolonged lactation. Pediatrics. 2005;116(3):e432–e435. doi: 10.1542/peds.2005-0313. [DOI] [PubMed] [Google Scholar]
- McCarthy N.A., Kelly A.L., O'Mahony J.A., Fenelon M.A. Sensitivity of emulsions stabilised by bovine β-casein and lactoferrin to heat and CaCl2. Food Hydrocolloids. 2014;35:420–428. doi: 10.1016/j.foodhyd.2013.06.021. [DOI] [Google Scholar]
- Meng F., Uniacke-Lowe T., Lanfranchi E., Meehan G., O'Shea C.A., Fox P.F., Kelly A.L. Factors affecting the creaming of human milk. Int. Dairy J. 2020;108:104726. doi: 10.1016/j.idairyj.2020.104726. [DOI] [Google Scholar]
- Michalski M.C., Briard V., Michel F., Tasson F., Poulain P. Size distribution of fat globules in human colostrum, breast milk, and infant formula. J. Dairy Sci. 2005;88(6):1927–1940. doi: 10.3168/jds.S0022-0302(05)72868-X. [DOI] [PubMed] [Google Scholar]
- Miller R.A., Ellis R.W.B. Tests for the adulteration of human milk. Arch. Dis. Child. 1953;28(139):161–169. doi: 10.1136/adc.28.139.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoulas L.R., Kent J.C., Cox D.B., Owens R.A., Sherriff J.L., Hartmann P.E. Variation in fat, lactose and protein in human milk over 24h and throughout the first year of lactation. Br. J. Nutr. 2002;88(1):29–37. doi: 10.1079/bjn2002579. [DOI] [PubMed] [Google Scholar]
- Morriss F.H., Brewer E.D., Spedale S.B., Riddle L., Temple D.M., Caprioli R.M., West M.S. Relationship of human milk pH during course of lactation to concentrations of citrate and fatty acids. Pediatrics. 1986;78(3) [PubMed] [Google Scholar]
- Moschakis T., Chantzos N., Biliaderis C.G., Dickinson E. Microrheology and microstructure of water-in-water emulsions containing sodium caseinate and locust bean gum. Food and Function. 2018;9(5):2840–2852. doi: 10.1039/c7fo01412k. [DOI] [PubMed] [Google Scholar]
- Moschakis T., Dergiade I., Lazaridou A., Biliaderis C.G., Katsanidis E. Modulating the physical state and functionality of phytosterols by emulsification and organogel formation: application in a model yogurt system. J.Funct. Foods. 2017;33:386–395. doi: 10.1016/j.jff.2017.04.007. [DOI] [Google Scholar]
- Moschakis T., Murray B.S., Dickinson E. On the kinetics of acid sodium caseinate gelation using particle tracking to probe the microrheology. J. Colloid Interface Sci. 2010;345(2):278–285. doi: 10.1016/j.jcis.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Mourtzinos I., Prodromidis P., Grigorakis S., Makris D.P., Biliaderis C.G., Moschakis T. Natural food colorants derived from onion wastes: application in a yoghurt product. Electrophoresis. 2018;39(15):1975–1983. doi: 10.1002/elps.201800073. [DOI] [PubMed] [Google Scholar]
- Neville M.C., Keller R.P., Casey C., Allen J.C. Calcium partitioning in human and bovine milk. J. Dairy Sci. 1994;77(7):1964–1975. doi: 10.3168/jds.S0022-0302(94)77142-3. [DOI] [PubMed] [Google Scholar]
- Neville M.C., Morton J., Umemura S. Lactogenesis: the transition from pregnancy to lactation. Pediatr. Clin. 2001;48(1):35–52. doi: 10.1016/S0031-3955(05)70284-4. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A., Andreadis M., Moschakis T. Effect of heat, pH, ultrasonication and ethanol on the denaturation of whey protein isolate using a newly developed approach in the analysis of difference-UV spectra. Food Chem. 2017;232:425–433. doi: 10.1016/j.foodchem.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A., Moschakis T. Studying the denaturation of bovine serum albumin by a novel approach of difference-UV analysis. Food Chem. 2017;215:235–244. doi: 10.1016/j.foodchem.2016.07.133. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A., Moschakis T. On the reversibility of ethanol-induced whey protein denaturation. Food Hydrocolloids. 2018;84:389–395. doi: 10.1016/j.foodhyd.2018.05.051. [DOI] [Google Scholar]
- Paulsson M., Dejmek P. Thermal denaturation of whey proteins in mixtures with caseins studied by differential scanning calorimetry. J. Dairy Sci. 1990;73(3):590–600. doi: 10.3168/jds.S0022-0302(90)78707-3. [DOI] [Google Scholar]
- Picciano M.F. Humana Press; 2000. Trace element and mineral nutrition during lactation; pp. 139–151. (Clinical Nutrition of the Essential Trace Elements and Minerals). [DOI] [Google Scholar]
- Pierre A., Michel F., Le Graet Y. Variation in size of goat milk casein micelles related to casein genotype. Lait. 1995;75(6):489–502. doi: 10.1051/lait:1995638. [DOI] [Google Scholar]
- Rodríguez Arzuaga M., Bosch A., Añón M.C., Abraham A.G. Heat induced conformational changes of whey proteins in model infant formulae: effect of casein and inulin. Int. Dairy J. 2020;105:104695. doi: 10.1016/j.idairyj.2020.104695. [DOI] [Google Scholar]
- Rüegg M., Blanc B. The fat globule size distribution in human milk. Biochim. Biophys. Acta Lipids Lipid. Metabol. 1981;666(1):7–14. doi: 10.1016/0005-2760(81)90085-0. [DOI] [PubMed] [Google Scholar]
- Sinaga H., Bansal N., Bhandari B. Effects of milk pH alteration on casein micelle size and gelation properties of milk. Int. J. Food Prop. 2017;20(1):179–197. doi: 10.1080/10942912.2016.1152480. [DOI] [Google Scholar]
- Sunarić S., Denić M., Lalić J., Jovanović T., Spasić A., Živković J., Kocić G. Physicochemical and biochemical parameters in milk of Serbian breastfeeding women. Turk. J. Med. Sci. 2017;47(1):246–251. doi: 10.3906/sag-1511-110. [DOI] [PubMed] [Google Scholar]
- Titball R.W. Bacterial phospholipases. J. Appl. Microbiol.Symp. Suppl. 1998;84:127S–137S. doi: 10.1128/mr.57.2.347-366.1993. [DOI] [PubMed] [Google Scholar]
- Tziboula A., Horne D.S. vol. 9. Elsevier; 1999. The role of α(s1)-casein in the structure of caprine casein micelles; pp. 173–178. (International Dairy Journal). [DOI] [Google Scholar]
- Vermeer A.W.P., Norde W. The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys. J. 2000;78(1):394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. Effect of ethanol on the microstructure and rheological properties of whey proteins: Acid-induced cold gelation. LWT. 2021;139:110518. doi: 10.1016/j.lwt.2020.110518. [DOI] [Google Scholar]
- Wagner J, Biliaderis, Moschakis T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020;60(22):3793–3806. doi: 10.1080/10408398.2019.1708263. [DOI] [PubMed] [Google Scholar]
- Walstra P. In: Encyclopedia of Emulsion Technology. Becher P., editor. vol. 4. Marcel Dekker; 1996. Emulsion stability; pp. 1–62. [Google Scholar]
- Walstra P., Wouters J.T.M., Geurts T.J. CRC press; 2005. Dairy Science and Technology. [Google Scholar]
- Wiking L., Stagsted J., Björck L., Nielsen J.H. Milk fat globule size is affected by fat production in dairy cows. Int. Dairy J. 2004;14(10):909–913. doi: 10.1016/j.idairyj.2004.03.005. [DOI] [Google Scholar]
- Zinoviadou K.G., Scholten E., Moschakis T., Biliaderis C.G. Engineering interfacial properties by anionic surfactant-chitosan complexes to improve stability of oil-in-water emulsions. Food & Function. 2012;3(3):312–319. doi: 10.1039/c2fo10197a. [DOI] [PubMed] [Google Scholar]
- Zinoviadou K.G., Scholten E., Moschakis T., Biliaderis C.G. Properties of emulsions stabilised by sodium caseinate-chitosan complexes. Int. Dairy J. 2012;26(1):94–101. doi: 10.1016/j.idairyj.2012.01.007. [DOI] [Google Scholar]