Abstract
Chytridiomycosis is a fungal disease caused by the pathogens, Batrachochytrium dendrobatidis (Bd) and B. salamandrivorans (Bsal), which has caused declines in amphibian populations worldwide. Asia is considered as a coldspot of infection, since adult frogs are less susceptible to Bd-induced mortality or morbidity. Using the next-generation sequencing approach, we assessed the cutaneous bacterial community composition and presence of anti-Bd bacteria in six frog species from India using DNA isolated from skin swabs. All the six frog species sampled were tested using nested PCR and found Bd negative. We found a total of 551 OTUs on frog skin, of which the bacterial phyla such as Proteobacteria (56.15% average relative abundance) was dominated followed by Actinobacteria (21.98% average relative abundance) and Firmicutes (13.7% average relative abundance). The contribution of Proteobacteria in the anti-Bd community was highest and represented by 175 OTUs. Overall, the anti-Bd bacterial community dominated (51.7% anti-Bd OTUs) the skin microbiome of the frogs. The study highlights the putative role of frog skin microbiome in affording resistance to Bd infections in coldspots of infection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00248-020-01669-5.
Keywords: Amphibia, Skin microbiome, Anti-Bd bacteria, 454 next-generation sequencing, Endemic frogs, Proteobacteria
Over a decade, amphibians’ (frogs, caecilians, and salamanders) skin microbiome has been studied extensively to understand their role in the amphibian panzootic disease, called chytridiomycosis [1–3]. The disease is caused by two aquatic fungal pathogens: Batrachochytrium dendrobatidis (Bd) infects frogs, salamanders, and caecilians [4], and B. salamandrivorans (Bsal) infects both frogs and salamanders [5, 6]. They have caused declines in over 500 amphibian species, and possible extinctions of 90 species, worldwide [7]. In adult frogs, the skin microbiome plays an important role in the host’s defense mechanism [8]. The frog’s skin microbiome produces a plethora of anti-fungal metabolites to protect the host [2, 8], and the bacterial community on the skin might confer resistance to pathogens [9]. Skin microbiomes of frogs have revealed some important anti-Bd bacteria, such as Janthinobacterium lividum (family Oxalobacteraceae) [2, 8] and Serratia marcescens (family: Yersiniaceae) that produce anti-fungal metabolites: “Violacein” [8] and “Prodigiosin,” respectively. Both violacein and prodigiosin compromise the integrity of the cell membrane [10, 11], and prodigiosin also inhibits RNA and protein synthesis in bacteria [12]. Consequently, these two metabolites are known to inhibit Bd fungal growth [2]. Since anti-Bd bacteria are present on frogs that show resistance to Bd [13], investigating such skin microbiomes for potential anti-Bd bacterial isolates would benefit disease mitigation strategies.
There are over 395 frog species in India, and the list is growing, as new taxa are being discovered rapidly (see, http://amphibiaweb.org/). With high species richness and endemism, the stakes are high for understanding the role of Bd on frog populations, and the mechanisms by which frogs might be able to resist Bd infection. Frog populations in the region exhibit low Bd prevalence and high Bd haplotype diversity, which points at possible historical host-pathogen co-evolution [14]. Low prevalence and low mortality could also imply that frogs show some resistance to chytridiomycosis, and this could be mediated by the skin microbiome. Investigating the role of frog’s skin microbiome in coldspots of Bd infection could reveal new pathways that inhibit the pathogen. Therefore, we studied the bacterial community composition in six frog species from two hotspots for amphibian species richness, namely the Western Ghats and the Andaman Islands. We explored the frog skin microbiome data to infer the role of bacterial community in affording them with resistance to Bd infections. (Full details on the methods are provided in the Supplementary file S1.)
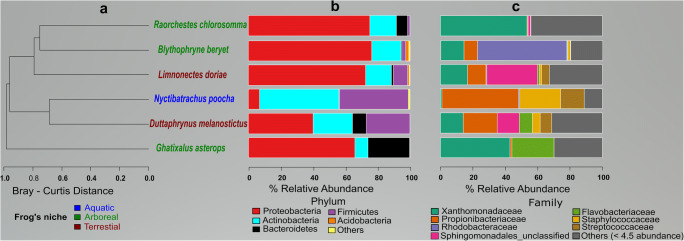
We generated a total of 18,543 good quality sequences from 18 frogs belonging to six species that had no Bd infection (Table 1). We aligned all good quality sequences in mothur and assigned them identity using SILVA v123 database. In all, we identified 1351 unique sequences (at ≥ 97% sequence similarity). These unique sequences revealed 551 operational taxonomic unit (OTU), belonging to five major bacterial phyla. Average relative abundance (ARA) of bacterial taxa in the microbiome was calculated as percentage of the total good quality bacterial sequences obtained. Proteobacteria (56.15% ARA) was the most dominant phylum, followed by Actinobacteria (21.98% ARA), Firmicutes (13.7% ARA), Bacteroidetes (7.2% ARA), and Acidobacteria (0.49% ARA). Acidobacteria was present only in B. beryet (1.99% relative abundance) and in Limnonectes doriae (0.99% relative abundance), from the Andaman Islands. In the case of aquatic Nyctibatrachus poocha, Proteobacteria (6.98% relative abundance) was found to be the least abundant, and Actinobacteria (49.12% relative abundance) was the most abundant (Fig. 1b). Rarefaction revealed that L. doriae had the highest and Ghatixalus asterops had the least number of OTUs (Table 1). Cluster analysis revealed that the bacterial community did not form clusters based on the niche occupied by the frogs (Fig. 1a).
Table 1.
Details of samples collected and the data generated on skin microbiome of frogs
| S. no. | Niche | Species | Number of samples pooled | Number of sequence reads generated | Total number of OTUs (standard error±) | Anti-Bd OTUs (%) | Sample location | Coordinates | Prevalence* | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arboreal | Blythophryne beryet | 3 | 1210 | 54.7 (3.21) | 47.7 | Little Andaman, India | 10° 44′ 47.1″ N | 92° 32′ 18.5″ E | 0% (0–48) |
| 2 | Terrestrial | Duttaphrynus melanostictus | 2 | 6148 | 46.3 (4.29) | 58.9 | Munnar, Kerala, India | 10° 04′ 24.5″ N | 77° 02′ 02.5″ E | 15.8% (5–37) |
| 1 | Little Andaman, India | 10° 35′ 07.0″ N | 92° 33′ 29.0″ E | |||||||
| 3 | Arboreal | Ghatixalus asterops | 3 | 8176 | 45.0 (4.22) | 79.1 | Munnar, Kerala, India | 10° 05′ 37.3″ N | 77° 02′ 43.2″ E | 12.1% (6–22) |
| 4 | Terrestrial | Limnonectes doriae | 3 | 545 | 86.8 (2.81) | 26.5 | Middle Andaman, India | 11° 36′ 48.5″ N | 92° 36′ 43.9″ E | 8.2% (5–12) |
| 5 | Aquatic | Nyctibatrachus poocha | 3 | 2043 | 50.5 (3.86) | 19.8 | Munnar, Kerala, India | 10° 04′ 24.5″ N | 77° 02′ 02.5″ E | 16.7% (6–40) |
| 6 | Arboreal | Raochestes chlorosomma | 3 | 421 | 57.0 (N/A) | 73.7 | Munnar, Kerala, India | 10° 04′ 24.5″ N | 77° 02′ 02.5″ E | 6.3% (0.3–28) |
N/A, not computed
*Numbers represent prevalence with 95% CI of that species population across the country. Prevalence data was obtained from a previous study by Mutnale et al. [14]
Fig. 1.
Graph represents the cluster analysis and the relative abundance of the frog skin microbiome: a cluster analysis of frog species is calculated using Bray-Curtis method. Font color of species name indicates niche of frog. b Bacterial phylum-level relative abundance in six frog species. c bacterial Family level relative abundance in six frog species (Plots were prepared using R (v3.6.3) software, and modified using Inkscape 0.91 (https://inkscape.org/en/))
Out of the 551 OTUs identified, 285 OTUs (51.7%) were anti-Bd bacterial OTUs, and they constituted 74.2% of the average relative abundance in each of the six species. The percentage of anti-Bd OTUs, represented as a fraction of the total number of OTUs recorded from each frog species, showed variation. The highest percentage of anti-Bd bacterial OTUs were present on G. asterops (171/216, 79.1% anti-Bd OTUs), followed by Raochestes chlorosomma (42/57, 73.7% anti-Bd OTUs), D. melanostictus (115/195, 58.9% anti-Bd OTUs), B. beryet (37/78, 47.4% anti-Bd OTUs), L. doriae (26/98, 26.5% anti-Bd OTUs), and N. poocha (20/101, 19.8% anti-Bd OTUs). Six anti-Bd bacterial OTUs, namely, Stenotrophomonas sp. (OTU 3), Sphingomonadales_unclassified (OTU 6), Staphylococcussp. (OTU 7), Microbacteriaceae_unclassified (OTU 18), Micrococcaceae_unclassified (OTU 21), and Caulobacteraceae_unclassified (OTU 34), were present in all six frog species. They matched with Silverstoneia flotator (inhibitory_30), Sphingomonadaceae bacterium (KU738962.1), Boophis madagascariensis (ns_6), Colostethus panamensis (inhibitory_2), Alytes obstetricans (ns_64), and Caulobacteraceae bacterium (KU738923.1), respectively, from Woodhams et al. [15] and Muletz et al. [16] database. The average relative abundance of these OTUs was 17.8%. Percentage of anti-Bd bacterial OTUs, represented as a fraction of the total anti-Bd OTUs in different bacterial phyla were: Proteobacteria (175/285, 61.4% anti-Bd OTUs), Bacteroidetes (57/285, 20% anti-Bd OTUs), Actinobacteria (36/285, 12.3% anti-Bd OTUs), and Firmicutes (17/285, 5.96% anti-Bd OTUs).
Globally, frog skin microbiomes are replete with Proteobacteria, Bacteroidetes, and Actinobacteria [17–19]. Proteobacteria is also abundant in soils [20], and thereby, associated with tropical frog skins [17, 21], and majority of them (80% of the total OTUs present) are in the anti-Bd databases [15]. Bacterial family Pseudomonadaceae (Proteobacteria) has been reported from several frogs’ skin microbiomes with varying abundance [19]. However, this bacterial family was not found in B .beryet, L. dorie, N. poocha, and R. chlorosomma (Fig. 1b). Family Xanthomonadaceae (Proteobacteria) was dominant in the bacterial community in all the frog species, except N. poocha. Actinobacteria was dominant (49.12% relative abundance), and Proteobacteria was the least represented (6.98% relative abundance) in N. poocha. Actinobacteria are associated with freshwater ecosystems [22], and N. poocha being a mountain stream-dwelling frog shares the same habitat with the bacterial phylum. The differences in the bacterial phyla composition on N. poocha could also be due to seasonal differences, or changes in pH of water in streams [23].
In terrestrial frogs (L. doriae and R. chlorosomma), OTUs were higher than in aquatic (N. poocha) and arboreal (G. asterops and B. beryet) frogs (Table 1). A similar pattern has been documented in a global comparison of frog skin microbiomes [19]. Bacterial OTUs in terrestrial frogs could be attributed to their contact with soil, which has a rich bacterial community. Based on bacterial OTUs and their abundance, there was no consistent association between the niche of the frogs and their skin bacterial community composition (Fig. 1a). This lack of association suggests that frog skin bacterial community might not be an accurate descriptor of the niche of the frog.
Anti-Bd bacterial OTUs (51.7%) were higher than those reported on frog species from Costa Rica (13%) [17] and Panama (8.45%) [23]. Salamander skin microbiome from the Eastern USA has revealed high anti-Bd bacterial OTU abundance (87% average relative abundance), which was contributed by 13% of anti-Bd OTUs [18]. Other bacterial genera with anti-Bd OTUs on the frogs were: Chryseobacterium (23/285, 8% anti-Bd OTUs), Elizabethkingia (18/285, 6.3% anti-Bd OTUs), Stenotrophomonas (18/285, 6.3% anti-Bd OTUs), and Pseudomonas (13/285, 4.5% anti-Bd OTUs; see Supplemental file S2). These bacteria have been shown to exhibit anti-Bd activity in vitro [24–27]. Anti-Bd genera, namely, Streptomyces [26], Propionibacterium, Microbacterium, and Micrococcaceae [27], belonging to the phylum Actinobacteria, also possess anti-fungal properties. They were found in the skin microbiome of the six species of frogs examined. Since a large number of anti-Bd OTUs were detected, we hypothesize that a similar community of bacteria might be present in frog populations in the region.
Low abundance of anti-Bd bacteria on frog’s skin has been linked to high prevalence of Bd in the hotspots of infection [23, 28]. However, currently, there is no evidence suggesting that a high abundance of anti-Bd bacteria on frog’s skin is associated with low prevalence of Bd infection in coldspots of infection. A rich anti-Bd bacterial community that thrives on the skin of frogs has been revealed through this study. It could be one of the reasons for the low prevalence of Bd in frog populations in the region. Anti-Bd bacteria provide frogs with protection from Bd infections in different ways: (i) by producing anti-Bd metabolite [29], (ii) by producing biofilm on frog skin surface [1], and (iii) by host-mediated selection of anti-Bd metabolite producing bacteria on its skin [30]. Since frog skin microbiome reports are scarce from Asia, future studies should focus on role of skin bacterial community in affording protection from Bd infection by attenuation of the pathogen [31]. Bacterial flora in the soil or stream might be an important source for the frog skin microbiome; therefore, we hypothesize that Bd might be experiencing strong selection pressure both in the environment, and on the frogs in coldspots of infection.
Supplementary Information
: It contains a detailed description of methods used in generating the data and analyzing it. (DOCX 29 kb)
: It provides the taxonomic identity of 551 OTUs obtained from the frog skin microbiome. (XLSX 65 kb)
Acknowledgments
We thank the State Forest Departments of Kerala and Andaman and Nicobar Islands for providing necessary permits and necessary logistic support. We thank Lilly Margaret and Sachin Anand for collecting samples from Western Ghats and Andaman Islands, respectively. A. Sreenivas assisted us in generating sequences using the Roche GS-FLX System, and we thank him for this. We thank the two anonymous reviewers of this manuscript for their valuable comments.
Author Contributions
M.C.M. and K.V. designed the research; M.C.M. and K.V. conducted the field surveys; M.C.M. conducted all laboratory analyses; G.S.N. R. performed 454 DNA sequencing experiment. M.C.M. and K.V. analyzed the data and wrote up the manuscript.
Funding
The study was funded by the Council for Scientific and Industrial Research (BSC 0207_ConservE).
Data Availability
Sequencing files have been submitted at the NCBI Sequence Read Archive (SRA) repository (BioProject ID: PRJNA639992; Temporary Submission ID: SUB7618771).
Compliance with Ethical Standards
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Piovia-Scott J, Rejmanek D, Woodhams DC, Worth SJ, Kenny H, McKenzie V, Lawler SP, Foley JE. Greater species richness of bacterial skin symbionts better suppresses the amphibian fungal pathogen Batrachochytrium dendrobatidis. Microb Ecol. 2017;74:217–226. doi: 10.1007/s00248-016-0916-4. [DOI] [PubMed] [Google Scholar]
- 2.Woodhams DC, LaBumbard BC, Barnhart KL, et al. Prodigiosin, Violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb Ecol. 2018;75:1049–1062. doi: 10.1007/s00248-017-1095-7. [DOI] [PubMed] [Google Scholar]
- 3.Kruger A. Functional redundancy of Batrachochytrium dendrobatidis inhibition in bacterial communities isolated from Lithobates clamitans skin. Microb Ecol. 2020;79:231–240. doi: 10.1007/s00248-019-01387-7. [DOI] [PubMed] [Google Scholar]
- 4.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. Et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. doi: 10.2307/3761366. [DOI] [Google Scholar]
- 5.Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci. 2013;110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegen G, Pasmans F, Schmidt BR, Rouffaer LO, van Praet S, Schaub M, Canessa S, Laudelout A, Kinet T, Adriaensen C, Haesebrouck F, Bert W, Bossuyt F, Martel A. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature. 2017;544:353–356. doi: 10.1038/nature22059. [DOI] [PubMed] [Google Scholar]
- 7.Scheele BC, Pasmans F, Skerratt LF, et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science (80- ) 2019;363:1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 8.Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT, Minbiole KPC. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009;3:818–824. doi: 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- 9.Becker MH, Walke JB, Cikanek S, Savage AE, Mattheus N, Santiago CN, Minbiole KPC, Harris RN, Belden LK, Gratwicke B. Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc R Soc B Biol Sci. 2015;282:20142881. doi: 10.1098/rspb.2014.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danevcic T, Vezjak MB, Tabor M et al (2016) Prodigiosin induces autolysins in actively grown Bacillus subtilis cells. Front Microbiol 7. 10.3389/fmicb.2016.00027 [DOI] [PMC free article] [PubMed]
- 11.Cauz ACG, Carretero GPB, Saraiva GKV, Park P, Mortara L, Cuccovia IM, Brocchi M, Gueiros-Filho FJ. Violacein targets the cytoplasmic membrane of bacteria. ACS Infect Dis. 2019;5:539–549. doi: 10.1021/acsinfecdis.8b00245. [DOI] [PubMed] [Google Scholar]
- 12.Danevčič T, Borić Vezjak M, Zorec M, Stopar D. Prodigiosin - a multifaceted Escherichia coli antimicrobial agent. PLoS One. 2016;11:e0162412. doi: 10.1371/journal.pone.0162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bataille A, Lee-Cruz L, Tripathi B, Kim H, Waldman B. Microbiome variation across amphibian skin regions: implications for chytridiomycosis mitigation efforts. Microb Ecol. 2016;71:221–232. doi: 10.1007/s00248-015-0653-0. [DOI] [PubMed] [Google Scholar]
- 14.Mutnale MC, Anand S, Eluvathingal LM, Roy JK, Reddy GS, Vasudevan K. Enzootic frog pathogen Batrachochytrium dendrobatidis in Asian tropics reveals high ITS haplotype diversity and low prevalence. Sci Rep. 2018;8:10125. doi: 10.1038/s41598-018-28304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodhams DC, Alford RA, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz M, Daskin JH, Davis LR, Flechas SV, Lauer A, Gonzalez A, Harris RN, Holden WM, Hughey MC, Ibáñez R, Knight R, Kueneman J, Rabemananjara F, Reinert LK, Rollins-Smith LA, Roman-Rodriguez F, Shaw SD, Walke JB, McKenzie V. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology. 2015;96:595–595. doi: 10.1890/14-1837.1. [DOI] [Google Scholar]
- 16.Muletz-Wolz CR, DiRenzo GV, Yarwood SA, Campbell Grant EH, Fleischer RC, Lips KR. Antifungal bacteria on woodland salamander skin exhibit high taxonomic diversity and geographic variability. Appl Environ Microbiol. 2017;83:e00186–e00117. doi: 10.1128/AEM.00186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abarca JG, Vargas G, Zuniga I, Whitfield SM, Woodhams DC, Kerby J, McKenzie VJ, Murillo-Cruz C, Pinto-Tomás AA. Assessment of bacterial communities associated with the skin of costa rican amphibians at La Selva biological station. Front Microbiol. 2018;9:1–12. doi: 10.3389/fmicb.2018.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muletz Wolz CR, Yarwood SA, Campbell Grant EH, Fleischer RC, Lips KR. Effects of host species and environment on the skin microbiome of Plethodontid salamanders. J Anim Ecol. 2018;87:341–353. doi: 10.1111/1365-2656.12726. [DOI] [PubMed] [Google Scholar]
- 19.Kueneman JG, Bletz MC, McKenzie VJ, et al. Community richness of amphibian skin bacteria correlates with bioclimate at the global scale. Nat Ecol Evol. 2019;3:381–389. doi: 10.1038/s41559-019-0798-1. [DOI] [PubMed] [Google Scholar]
- 20.Spain AM, Krumholz LR, Elshahed MS. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009;3:992–1000. doi: 10.1038/ismej.2009.43. [DOI] [PubMed] [Google Scholar]
- 21.Belden LK, Hughey MC, Rebollar EA, Umile TP, Loftus SC, Burzynski EA, Minbiole KPC, House LL, Jensen RV, Becker MH, Walke JB, Medina D, Ibáñez R, Harris RN. Panamanian frog species host unique skin bacterial communities. Front Microbiol. 2015;6:1171. doi: 10.3389/fmicb.2015.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghai R, Rodriguez-Valera F, Mcmahon KD, et al. Metagenomics of the water column in the pristine upper course of the Amazon river. PLoS One. 2011;6:23785. doi: 10.1371/journal.pone.0023785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela BJ, Lesbarrères D, Ibáñez R, Green DM. Environmental and host effects on skin bacterial community composition in Panamanian frogs. Front Microbiol. 2018;9:1–13. doi: 10.3389/fmicb.2018.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flechas SV, Sarmiento C, Cárdenas ME, Medina EM, Restrepo S, Amézquita A. Surviving chytridiomycosis: differential anti-Batrachochytrium dendrobatidis activity in bacterial isolates from three lowland species of Atelopus. PLoS One. 2012;7:e44832. doi: 10.1371/journal.pone.0044832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madison JD, Berg EA, Abarca JG, Whitfield SM, Gorbatenko O, Pinto A, Kerby JL. Characterization of Batrachochytrium dendrobatidis inhibiting bacteria from amphibian populations in Costa Rica. Front Microbiol. 2017;8:290. doi: 10.3389/fmicb.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park ST, Collingwood AM, St-Hilaire S, Sheridan PP. Inhibition of Batrachochytrium dendrobatidis caused by bacteria isolated from the skin of boreal toads, Anaxyrus (Bufo) boreas boreas, from Grand Teton National Park, Wyoming, USA. Microbiol Insights. 2014;7:MBI.S13639. doi: 10.4137/mbi.s13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De León ME. Comparison of in vitro methods to inhibit growth of a virulent strain of Batrachochytrium dendrobatidis (Longcore , Pessier , and Nichols 1999) Amphib Reptile Conserv. 2020;14:12–23. [Google Scholar]
- 28.Kolby JE, Ramirez SD, Berger L, Richards-Hrdlicka KL, Jocque M, Skerratt LF. Terrestrial dispersal and potential environmental transmission of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) PLoS One. 2015;10:e0125386. doi: 10.1371/journal.pone.0125386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loudon AH, Holland JA, Umile TP, Burzynski EA, Minbiole KPC, Harris RN. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front Microbiol. 2014;5:1–8. doi: 10.3389/fmicb.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loudon AH, Venkataraman A, Van Treuren W, et al. Vertebrate hosts as islands: dynamics of selection, immigration, loss, persistence, and potential function of bacteria on salamander skin. Front Microbiol. 2016;7:333. doi: 10.3389/fmicb.2016.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voyles J, Woodhams DC, Saenz V, et al. Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Science (80- ) 2018;359:1517–1519. doi: 10.1126/science.aao4806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
: It contains a detailed description of methods used in generating the data and analyzing it. (DOCX 29 kb)
: It provides the taxonomic identity of 551 OTUs obtained from the frog skin microbiome. (XLSX 65 kb)
Data Availability Statement
Sequencing files have been submitted at the NCBI Sequence Read Archive (SRA) repository (BioProject ID: PRJNA639992; Temporary Submission ID: SUB7618771).