Abstract
Background
The contribution of BRCA1 and BRCA2 to the incidence of male breast cancer (MBC) in the United Kingdom is not known, and the importance of these genes in the increased risk of female breast cancer associated with a family history of breast cancer in a male first-degree relative is unclear.
Methods
We have carried out a population-based study of 94 MBC cases collected in the UK. We screened genomic DNA for mutations in BRCA1 and BRCA2 and used family history data from these cases to calculate the risk of breast cancer to female relatives of MBC cases. We also estimated the contribution of BRCA1 and BRCA2 to this risk.
Results
Nineteen cases (20%) reported a first-degree relative with breast cancer, of whom seven also had an affected second-degree relative. The breast cancer risk in female first-degree relatives was 2.4 times (95% confidence interval [CI] = 1.4–4.0) the risk in the general population. No BRCA1 mutation carriers were identified and five cases were found to carry a mutation in BRCA2. Allowing for a mutation detection sensitivity frequency of 70%, the carrier frequency for BRCA2 mutations was 8% (95% CI = 3–19). All the mutation carriers had a family history of breast, ovarian, prostate or pancreatic cancer. However, BRCA2 accounted for only 15% of the excess familial risk of breast cancer in female first-degree relatives.
Conclusion
These data suggest that other genes that confer an increased risk for both female and male breast cancer have yet to be found.
Keywords: BRCA1, BRCA2, family history, male breast cancer
Introduction
Male breast cancer (MBC) is a rare disease and little is known about its aetiology. However, female first-degree relatives of MBC cases are at increased risk of breast cancer [1,2,3,4,5,6], which suggests that there is an inherited component to the disease. Several genes that are associated with a high lifetime risk of breast cancer in women have been identified during the past decade. One of these, BRCA2, has also been shown to confer a significant risk of breast cancer in men, and a recent study found the risk of breast cancer in male BRCA2 mutation carriers from multiple case breast/ovarian cancer families to be 80-fold higher than in the general population [7]. This equates to a 7% risk of breast cancer by age 80. The prevalence of BRCA2 mutations in MBC cases unselected for family history has been estimated in several studies from Europe and the United States [8,9,10,11,12,13,14]. The estimated mutation carrier frequencies varied from 4% to 40%, but were higher in the three studies carried out in populations in which founder mutations are known to occur [9,12,13]. Two other studies have screened for the BRCA2 founder mutation 6174delT in male breast cancer cases of Jewish origin unselected for family history. Struewing et al. identified 15 mutation carriers in 100 cases [15] and Sverdlov et al. found no mutation carrier in 20 cases [16].
A putative association between BRCA1 and MBC is less clear. MBC is known to occur in multiple breast/ovarian cancer case families with mutations in BRCA1 [17], but a study of 22 MBC families found strong evidence against linkage to the BRCA1 locus [18]. The two studies of the Jewish BRCA2 founder mutation described above also screened for the BRCA1 founder mutation 185delAG. Sverdlov et al. identified no mutation carrier in 20 cases [16] and Struewing et al. identified four mutation carriers out of 110 cases [15]. However, in two other studies of MBC in populations without common founder mutations, no mutation carriers were identified in a total of 72 cases [10,13]. Together, these data suggest that the MBC risk associated with BRCA1 is, at best, very small.
The purpose of this study was to estimate the breast cancer risks in women with a family history of male breast cancer and to establish the prevalence of BRCA1 and BRCA2 mutations in a male breast cancer cases series from the UK.
Materials and methods
Cases were identified from an ongoing population-based study of male breast cancer. All men diagnosed with breast cancer in the areas served by the East Anglia, Trent and West Midlands cancer registries who were alive on 31 December 1998 were eligible to take part, excluding those whose general practitioner thought them unsuitable for the study because of severe concurrent illness. Participants were asked to complete a detailed epidemiological questionnaire, which includes details of a family history of cancer, and to provide a blood sample for genetic analysis. The study has approval from the Anglia and Oxford multi-centre research ethics committee. One hundred and sixty-five eligible patients were identified, of whom 137 have so far agreed to take part. The first 94 patients who enrolled in the study were included in the current analysis.
Pedigree data from the 94 index cases were used to estimate the risk of breast cancer associated with a family history of breast cancer in a male first-degree relative. The program PERSON YEARS [19] was used to calculate the expected number of breast cancers in female first-degree relatives based on age and period specific breast cancer incidence rates. Individuals entered the at risk cohort on 1 January 1960 and were censored on diagnosis of cancer, on death, or on the date the family history questionnaire was completed. Relatives born before 1890 and those who died or developed cancer before 1960 were excluded because reported data for these individuals was likely to be less reliable.
BRCA1 and BRCA2 mutation analysis was performed as previously described [20]. The whole coding sequence, including intron–exon boundaries, was screened using a combination of single strand conformation analysis/ heteroduplex analysis (SSCA/HA) and direct fluorescent sequencing. DNA from six patients could not be reliably amplified by PCR and these were excluded from the results.
Results
The mean age of diagnosis of the cases was 67.3 years (range 36–89 years). Forty-five cases reported a history of cancer in at least one first-degree relative. Of these, 18 reported a history of breast cancer in a female first-degree relative, and one reported a brother with breast cancer. In 8125 person years of observation of first-degree female relatives 16 breast cancers were observed compared to 6.63 expected, which equates to a relative risk of 2.4 (95% CI = 1.4–4.0).
We considered deleterious mutations to be those that are predicted to result in protein truncation (frameshift, splice site and nonsense mutations), or those missense mutations that have been previously shown to be disease associated on the basis of their co-segregation with disease in families. No disease-associated mutations were identified in BRCA1. Five disease-associated variants were identified in BRCA2 (Table 1). These were all in the coding region and are predicted to lead to a truncated protein, and all but one (2192delC) has been previously reported on the Breast Information Core database [21]. The mean age at diagnosis in the mutation carriers was 58.8 years (range 48–69 years), which was 9 years younger than in non-mutation carriers (67.9 years, range 36–89 years). The five mutation carriers all reported a family history of cancer (Table 1). One had a single second-degree relative affected with breast cancer, and the other four had at least one first-degree relative affected with one of the cancers thought to be associated with BRCA2 (breast, prostate or pancreas). We also identified rare missense mutations of unknown significance in five patients (Table 1) and several common polymorphisms in both BRCA1 and BRCA2.
Table 1.
Details of rare gene variants
| Family history (age at diagnosis) | ||||||
| Patient | Gene | Variant | Effect | Age* | First-degree relatives | Second-degree relatives |
| Protein truncating mutations | ||||||
| M0271 | BRCA2 | 253delC | Frameshift | 48 | None | MGM Br(?age) |
| M0041 | BRCA2 | 2192delC | Frameshift | 55 | F Pa(74) | PU Pa(42), PA Br(48), PA Pa(63) |
| M0040 | BRCA2 | 5974delCT | Frameshift | 60 | F Pr(61) | None |
| M0293 | BRCA2 | 7928delCT | Frameshift | 69 | M Br(67) | None |
| M0238 | BRCA2 | 8474delAG | Frameshift | 62 | F Pr + Ki(75) | None |
| Missense variants of unknown significance | ||||||
| M0383 | BRCA1 | 2640C > T | R841W | 63 | None | None |
| M0016 | BRCA2 | 5972T > C | T1915M | 74 | F HN(85), B Lu(50's) | None |
| M0021 | BRCA2 | 5972T > C | T1915M | 72 | None | None |
| M0288 | BRCA2 | 4486G > T | D1420Y | 55 | M Br(77) | None |
*Age at diagnosis (years). F, father; M, mother; B, brother; MGM, maternal grandmother; PU, paternal uncle; PA, paternal aunt; Br, breast cancer; HN, head and neck; Ki, kidney cancer; Lu, lung cancer; Pa, pancreatic cancer; Pr, prostate cancer.
Discussion
Our estimate of the familial risk of breast cancer associated with a family history of male breast cancer is similar to other published estimates. However, our estimate is based on a reported family history of breast cancer that was not independently confirmed. It is known that a family history of breast cancer is generally accurately reported by women [22], but it may be less accurate for men, for whom some degree of under-reporting is likely. This may result in an underestimate of the familial risk.
As with other studies, we found no BRCA1 mutation carriers in our case series. The carrier frequency of BRCA2 mutations was 6% (95% CI = 2–13%), which is broadly comparable with that of previous estimates, but likely to be an underestimate of the true carrier frequency. For example, some of the missense variants that we identified may disrupt the protein structure substantially, but in the absence of a good functional assay for BRCA1 or BRCA2 these variants were not classified as disease causing. In addition, the sensitivity of SSCP/HA is known to be less than 100%, and for nonsense mutations may be as low as 50%. Finally, large genomic rearrangements which will not be identified by PCR-based mutation detection methods are known to occur in BRCA1 and BRCA2. A study combining linkage and mutation data in multiple case families has estimated the sensitivity of the most commonly used mutation detection techniques for these genes to be approximately 70% [23]. Based on this sensitivity, the frequency of BRCA2 mutations in this series could be as high as 8%. Survival bias may also affect the estimate of carrier mutation frequency. The participants in this study were prevalent cases and carrier frequency may be either underestimated or overestimated if male breast cancer associated with a BRCA1 or BRCA2 mutation carries a different prognosis from breast cancer in men without a mutation. However, there are no published data on outcome in BRCA-associated MBC, and in women the effect of BRCA status on outcome is not clear [24,25].
The risk of female breast and ovarian cancer is thought to vary depending on the location of mutations throughout the BRCA2 gene [7]. Mutations in a central portion of the gene, referred to as the ovarian cancer cluster region, are associated with either an increase in the risk of ovarian cancer, a decrease in the risk of breast cancer, or a combination of both, compared with mutations elsewhere in the gene. Figure 1 shows the location of BRCA2 mutations identified by this and other studies of male breast cancer. This illustrates that there appears to be no clustering of mutations that would indicate a genotype–phenotype correlation analogous to that observed for female breast and ovarian cancer.
Figure 1.
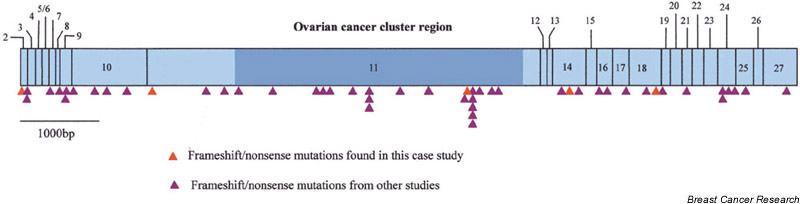
Summary of the location of published mutations in BRCA2 identified in male breast cancer cases (red triangles, this study; purple triangles, other published studies).
Our data show that BRCA2 accounts for only a small proportion of the excess familial risk of female breast cancer associated with MBC. There were 9.37 excess cases of breast cancer in female first-degree relatives (16 observed – 6.63 expected), of which only one was accounted for by BRCA2. Thus, allowing for a mutation detection sensitivity of 70%, BRCA2 accounts for approximately 15% of the excess risk.
Conclusion
The majority of male breast cancer is not accounted for by BRCA1 and BRCA2, and these genes account for only 15% of the excess familial risk of breast cancer in female first-degree relatives. This suggests that other genes that confer an increased risk for both female and male breast cancer have yet to be found.
Abbreviations
CI = confidence interval; MBC = male breast cancer; PCR = polymerase chain reaction; SSCA/HA = single strand conformation analysis/hetero-duplex analysis.
Acknowledgments
Acknowledgements
This study was funded by the Cancer Research Campaign (CRC). BAJP is a Gibb Fellow of the CRC, DFE is a Principal Research Fellow of the CRC and PDPP is a Senior Clinical Research Fellow of the CRC. The authors thank the Anglian Cancer Registry, the Trent Cancer Registry, the West Midlands Cancer Registry, the general practitioners and hospital consultants who have helped recruit patients into the study, and the patients and their families.
References
- Casagrande JT, Hanisch R, Pike MC, Ross RK, Brown JB, Henderson BE. A case-control study of male breast cancer. Cancer Res. 1988;48:1326–1330. [PubMed] [Google Scholar]
- Rosenblatt KA, Thomas DB, McTiernan A, Austin MA, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG, Satariano W, Austin DF, Isacson P, Greenberg RS, Key C, Kolonlel L, West D. Breast cancer in men: aspects of familial aggregation. J Natl Cancer Inst. 1991;83:849–854. doi: 10.1093/jnci/83.12.849. [DOI] [PubMed] [Google Scholar]
- Anderson DE, Badzioch MD. Breast cancer risks in relatives of male breast cancer patients. J Natl Cancer Inst. 1992;84:1114–1117. doi: 10.1093/jnci/84.14.1114. [DOI] [PubMed] [Google Scholar]
- Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. A systematic population based assessment of cancer risk in first degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- Storm HH, Olsen J. Risk of breast cancer in offspring of male breast-cancer patients [letter]. Lancet. 1999;353:209. doi: 10.1016/S0140-6736(05)77219-6. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Vaittinen P. Male breast cancer: risk to daughters. Lancet. 1999;353:1186–1187. doi: 10.1016/S0140-6736(05)74408-1. [DOI] [PubMed] [Google Scholar]
- Thompson D, Easton D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch FJ, Farid LM, Deshano ML, Tavtigian SV, Calzone K, Campeau L, Peng Y, Bogden B, Chen Q, Neuhausen S, Shattuck Eidens D, Godwin A, Daly M, Radford DM, Sedlacek S, Rommens J, Simard J, Garber J, Merajver S, Weber BL. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13:123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- Thorlacius S, Olafsdottir G, Tryggvadottir L, Neuhausen S, Jonasson JG, Tavtigian SV, Tulinius H, Ogmundsdottir HM, Eyfjord J. A single BRCA2 mutation in male and female breast cancer families from Iceland with varied cancer phenotypes. Nat Genet. 1996;13:117–119. doi: 10.1038/ng0596-117. [DOI] [PubMed] [Google Scholar]
- Friedman LS, Gayther SA, Kurosaki T, Gordon D, Noble B, Casey G, Ponder BA, Anton Culver H. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60:313–319. [PMC free article] [PubMed] [Google Scholar]
- Mavraki E, Gray IC, Bishop DT, Spurr NK. Germline BRCA2 mutations in men with breast cancer. Br J Cancer. 1997;76:1428–1431. doi: 10.1038/bjc.1997.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, Borg A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58:1367–1371. [PubMed] [Google Scholar]
- Csokay B, Udvarhelyi N, Sulyok Z, Besznyak I, Ramus S, Ponder B, Olah E. High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Res. 1999;59:995–998. [PubMed] [Google Scholar]
- Kwiatkowska E, Teresiak M, Lamperska KM, Karczewska A, Breborowicz D, Stawicka M, Godlewski D, Krzyzosiak WJ, Mackiewicz A. BRCA2 germline mutations in male breast cancer patients in the Polish population [letter]. Hum Mutat. 2001;17:73. doi: 10.1002/1098-1004(2001)17:1<73::AID-HUMU12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Coriaty ZM, Ron E, Livoff A, Konichezky M, Cohen P, Resnick MB, Lifzchiz-Mercerl B, Lew S, Iscovich J. Founder BRCA1/2 mutations among male patients with breast cancer in Israel. Am J Hum Genet. 1999;65:1800–1802. doi: 10.1086/302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov RS, Barshack I, Bar Sade RB, Baruch RG, Hirsh Yehezkel G, Dagan E, Feinmesser M, Figer A, Friedman E. Genetic analyses of male breast cancer in Israel. Genet Test. 2000;4:313–317. doi: 10.1089/10906570050501579. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Brody LC, Erdos MR, Kase RG, Giambarresi TR, Smith SA, Collins FS, Tucker MA. Detection of eight BRCA1 mutations in 10 breast/ovarian cancer families, including 1 family with male breast cancer. Am J Hum Genet. 1995;57:1–7. [PMC free article] [PubMed] [Google Scholar]
- Stratton MR, Ford D, Neuhasen S, Seal S, Wooster R, Friedman LS, King MC, Egilsson V, Devilee P, McManus R, Daly PA, Smyth E, Ponder BAJ, Peto J, Cannon-Albright L, Easton DF, Goldgar DE. Familial male breast cancer is not linked to the BRCA1 locus on chromosome 17q. Nat Genet. 1994;7:103–107. doi: 10.1038/ng0594-103. [DOI] [PubMed] [Google Scholar]
- Coleman M, Douglas A, Hermon C, Peto J. Cohort study analysis with a FORTRAN computer program. Int J Epidemiol. 1986;15:134–137. doi: 10.1093/ije/15.1.134. [DOI] [PubMed] [Google Scholar]
- Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, van Rensburg EJ, Dunning AM, Love R, Evans G, Easton D, Clayton C, Stratton MR, Ponder BAJ. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype–phenotype correlation. Nat Genet. 1995;11:428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Breast Information Core Database http://www.nhgri.nih.gov/Intra-mural_research/Lab_transfer/Bic/Member/index.html
- Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol. 1997;146:244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkadottir R, Eyfjord J, Lynch H, Ponder BAJ, Gayhter SA, Birch JM, Lindblom A, Stoppa-Lyonnet D, Bignon Y, Borg A, Hamann U, Haites N, Scott RJ, Maugard CM, Vasen H, Seitz S, Cannon-Albright LA, Schofield A, Hedman MZ, Breast Cancer Linkage Consortium Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson M. Are BRCA1- and BRCA2-associated breast cancers different? Prognosis of BRCA1-associated breast cancer. J Clin Oncol. 2000;18(suppl):113s–118s. [PubMed] [Google Scholar]
- Verhoog LC, Berns EM, Brekelmans CT, Seynaeve C, Meijers Heijboer EJ, Klijn JG. Prognostic significance of germline BRCA2 mutations in hereditary breast cancer patients. J Clin Oncol. 2000;18(suppl):119s–124s. [PubMed] [Google Scholar]


