Abstract
Infections due to triazole-resistant Aspergillus fumigatus are increasingly reported worldwide and are associated with treatment failure and mortality. The principal class of azole-resistant isolates is characterized by tandem repeats of 34 bp or 46 bp within the promoter region of the cyp51A gene. Loop-mediated isothermal amplification (LAMP) is a widely used nucleic acid amplification system that is fast and specific. Here we describe a LAMP assay method to detect the 46 bp tandem repeat insertion in the cyp51A gene promoter region based on novel LAMP primer sets. It also differentiated strains with TR46 tandem repeats from those with TR34 tandem repeats. These results showed this TR46-LAMP method is specific, rapid, and provides crucial insights to develop novel antifungal therapeutic strategies against severe fungal infections due to A. fumigatus with TR46 tandem repeats.
Subject terms: Fungal genomics, Environmental impact, Fungal infection
Introduction
Antimicrobial resistance (AMR) was defined by the World Health Organization (WHO) as one of the most critical threats to human health. AMR can compromise our ability to treat infectious diseases, as well as undermining other advances in health care1. Although bacterial resistance remains the most common finding in the clinical setting, fungal resistance, especially to azole drugs among filamentous fungi, is relentlessly increasing worldwide2–4. Resistance to azole antifungals can be due, in general, to two major genetic mechanisms; point mutation(s) in the cyp51A open reading frame with or without tandem repeat (TR) of 34 or 46 base pair (bp) in the promoter region of the gene (TR34 or TR46) and overexpression of oligonucleotide sequence in the cyp51A gene5. These mutations and overexpression of the gene confer different levels of resistance6. Point mutations result from previous exposure to azole drugs in a clinical setting, such as prophylaxis or therapeutic purposes7,8. On the other hand, TR with a point mutation(s) is the aftermath of previous exposure to azole fungicides. In an agricultural setting, azole fungicides are widely used to prevent fungal contamination in a large variety of crop and plant protection, allowing the TR-type resistant strains to emerge in the environments, and the conidia disperse to the air8,9. In Brazil, agribusiness represents about 25% of the Brazilian Gross National Product (GNP) (http://www.agricultura.gov.br), and in this scenario, fungicide use has steadily increased over the years. The consumption of pesticides in Brazil grew 190% in 2010, and fungicides corresponded to 14% of this (https://www.ipessp.edu.br).
In the Netherlands, the prevalence of TR-type resistant strains was high. Of 952 clinical A. fumigatus strains were collected and included 225 and 98 had TR34 and TR46, respectively10. In another study, TR34 L98H and TR46 Y121F T289A mutation that occur in patients without previous azole exposure have been reported in Europe, Asia, the Middle East, Africa, and Australia11.
Additionally, there are several reports of fatal invasive aspergillosis caused by A. fumigatus carrying the TR46 in acute myeloid leukemia (AML) patients and hematopoietic stem cell transplant recipients12. Higher mortality of patients with invasive aspergillosis caused by azole-resistant strains has been reported12,13. Thus, a rapid and specific method to identify the presence of TR would contribute to faster therapeutic decision-making14.
As one of the promising diagnostic tools for the azole-resistant A. fumigatus, loop-mediated isothermal amplification (LAMP) for the development of improved DNA-based diagnostic kits has been reported15. In general, the LAMP method was found to be similar or superior to the standard PCR method, more specific, lower-cost, and easier to perform. LAMP-based approaches have been applied to a wide range of samples, such as whole blood, paraffin-embedded tissues, and various microbial pathogens16,17. In this paper, we report a novel LAMP assay method that selectively detects triazole resistant A. fumigatus strains due to the presence of double TR46(TR462)or triple TR46(TR463)in the cyp51A promoter region.
Results
Antifungal susceptibility tests
Drug susceptibilities of 41 A. fumigatus strains against azole drugs itraconazole and voriconazole are shown in Table 1. Thirty strains designated as wild type were isolated from clinical specimens, and they were confirmed to be susceptible to itraconazole and voriconazole. The remaining 11 strains (TR34 and TR46) were resistant to voriconazole, and most of them showed MIC values of > 8 μg/mL against voriconazole. Among the 11 strains, 2 strains (IFM64460 with TR34/L978H and IFM64733 with TR34/LH98H) were resistant to itraconazole, and the remaining 9 strains were susceptible to itraconazole (Table 1).
Table 1.
Aspergillus fumigatus strains used in this experiment and their drug susceptibility profiles against itraconazole and voriconazole.
| Strain no | Isolation sources | cyp51A genotypes | MIC values (μg/ml) | |
|---|---|---|---|---|
| ITCZ | VRCZ | |||
| IFM63432a | Clinic | TR462/Y121F/T289A | 4 | > 8 |
| BE1-2 | Environment (bulb)b | TR462/Y121F/T289A | 2 | > 8 |
| BE1-4 | Environment (bulb) | TR462/Y121F/S363P/I364V/G448S | 2 | > 8 |
| BE 3-5 | environment (bulb | TR462/Y121F/T289A | 2 | > 8 |
| BE 3-6 | Environment (bulb) | TR462/Y121F/T289A | 2 | > 8 |
| BE 1-1 | Environment (bulb) | TR463/Y121F/M172I/T289A/G448S | 2 | > 8 |
| W1-4 | Environment (bulb) | TR463/Y121F/M172I/T289A/G448S | 2 | > 8 |
| W2-12-1 | Environment (bulb) | TR463/Y121F/M172I/T289A/G448S | 2 | > 8 |
| IFM64460 | Clinic | TR34/L98H | > 8 | > 8 |
| IFM64733 | Environment | TR34/L98H | > 8 | > 8 |
| 3-1-B | Environment (bulb) | TR34/L98H/T289A/I364V/G448S | 2 | > 8 |
| IFM62918c | Clinic | Wild | 0.5 | 1 |
| IFM62799 | Clinic | Wild | 0.5 | 1 |
| IFM60516 | Clinic | Wild | 1 | 1 |
| IFM58402 | Clinic | Wild | 0.5 | 0.5 |
| IFM51977 | Clinic | Wild | 0.25 | 0.25 |
| IFM60065 | Clinic | Wild | 1 | 0.5 |
| IFM61960 | Clinic | Wild | 0.5 | 0.5 |
| IFM51748 | Clinic | Wild | 0.125 | 0.125 |
| IFM63666 | Clinic | Wild | 1 | 2 |
| IFM62520 | Clinic | Wild | 1 | 0.5 |
| IFM50999 | Clinic | Wild | 0.5 | 0.5 |
| IFM50268 | Clinic | Wild | 0.25 | 0.125 |
| IFM55548 | Clinic | Wild | 0.25 | 0.25 |
| IFM63311 | Clinic | Wild | 1 | 0.5 |
| IFM63355 | Clinic | Wild | 2 | 2 |
| IFM60901 | Clinic | Wild | 0.5 | 0.5 |
| IFM62674 | Clinic | Wild | 1 | 2 |
| IFM62709 | Clinic | Wild | 0.5 | 0.5 |
| IFM52659 | Clinic | Wild | 1 | 1 |
| IFM57130 | Clinic | Wild | 0.25 | 0.125 |
| IFM60814 | Clinic | Wild | 0.5 | 0.5 |
| IFM49435 | Clinic | Wild | 0.25 | 0.25 |
| IFM61572 | Clinic | Wild | 0.5 | 0.5 |
| IFM50669 | Clinic | Wild | 0.5 | 0.25 |
| IFM59832 | Clinic | Wild | 0.5 | 0.5 |
| IFM55044 | Clinic | Wild | 0.25 | 0.25 |
| IFM47670 | Clinic | Wild | 0.5 | 0.5 |
| IFM51978 | Clinic | Wild | 0.5 | 0.25 |
| IFM58328 | Clinic | Wild | 0.5 | 0.5 |
| IFM60369 | Clinic | Wild | 0.5 | 0.5 |
ITCZ itraconazole, VRCZ voriconazole.
aLAMP positive control strain.
bobtained from plant bulbs.
cLAMP negative control strain.
Primer design
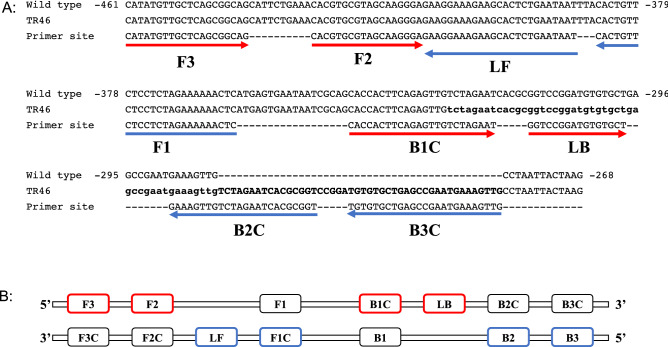
The most crucial step in the LAMP assay is the design of primers. In the LAMP assay, six primers are necessary to amplify the targeted region under isothermal condition. First, we inspected the promoter region (− 461 bp to − 296 bp counted from start codon) of the cyp51A gene to select a set of primer sequences that specifically amplify the repeated 46 bp sequence in strains with a TR46 mutation (Figs. 1 and 2). To enable specific amplification against repeated TR46 sequences, B2 was set on the joint of two 46 bp sequences. Then, another five sequences for primer sets were chosen in the target region, according to the standard criteria, to obtain a specific and rapid LAMP primer set in the LAMP assay. Namely, six primers (F1, F2, F3, B1, B2, B3) that target six specific regions of a DNA template of the TR46 gene of cyp51A were selected, and in addition, two loop primers (LF, LB) were also chosen to accelerate the reaction (Fig. 1). Several new candidates of LAMP primers were designed based on the above information and their utility tested. From those, one useful LAMP primer set based on the detection of TR46 regions in the cyp51A gene was selected (Table 2). In this LAMP method, the primers were selected based on the criteria that amplification started within about 30–50 min, and maximum amplification was completed within 70–80 min. Nucleotide sequence of promoter region for resistance gene of LAMP primer sets to detect the resistance gene was shown in Fig. 3. This primer amplifies between consecutive 46 bp sequences between TR46-1 bp and TR46-2 bp. The base sequence in this part corresponds to the B2 sequence in Table 2.
Figure 1.
Genetic information for the design of the LAMP primer sets. (A) Schematic illustration of cyp51A gene showing LAMP primer positions and corresponding sequences of TR46 bp promoter tandem repeat compared to wild-type sequences. (B) Primers F3, F2, F1, B1, B2, and B3 show primer sequence positions. Sequences of some primers are complementary, as shown in Table 2. See LAMP primer and methods, which are shown in Refs.19,20.
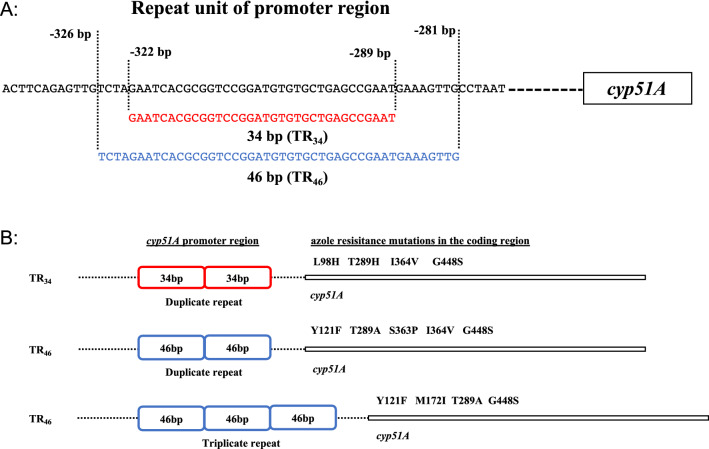
Figure 2.
Illustration of tandem repeat regions of cyp51A genes used in this experiment. (A) Tandem repeat unit of promoter genes of TR34 and TR46. (B) Tandem repeat: 34 bp (double) and 46 bp (double or triple), and cyp51A gene associated point mutation place.
Table 2.
Sequence information of newly designed TR46-LAMP primer sets in the present experiment.
| LAMP primer names | Sequence (5′ to 3′) |
|---|---|
| F3 | CATATGTTGCTCAGCGGCAG |
| B3 | CAACTTTCATTCGGCTCAGCA |
| FIP (F1 complementary + F2) | GAGTTTTTTCTAGAGGAGAACAGTG-CACGTGCGTAGCAAGGGA |
| BIP (B1 + B2 complementary) | CACCACTTCAGAGTTGTCTAGAAT-ACCGCGTGATTCTAGACAACTTTC |
| LF | ATTATTCAGAGTGCTTCTTTCCTTC |
| LB | GGTCCGGATGTGTGCTG |
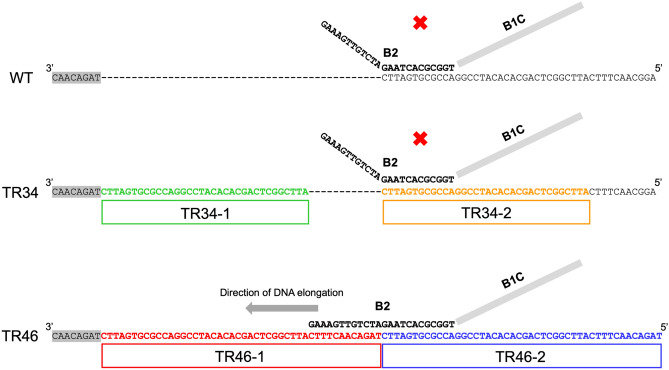
Figure 3.
Schematic figure of TR46 LAMP primer amplification site in comparison with those of wild type and TR34. The nucleotide sequence is targeted for the promoter region for the TR46 resistance gene (between TR46-1 and TR46-2). The sequence in this part corresponds to the B2 sequence in Table 2.
Validation of LAMP primer sets for TR46
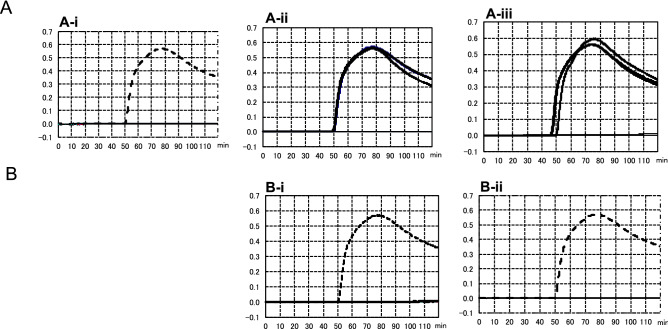
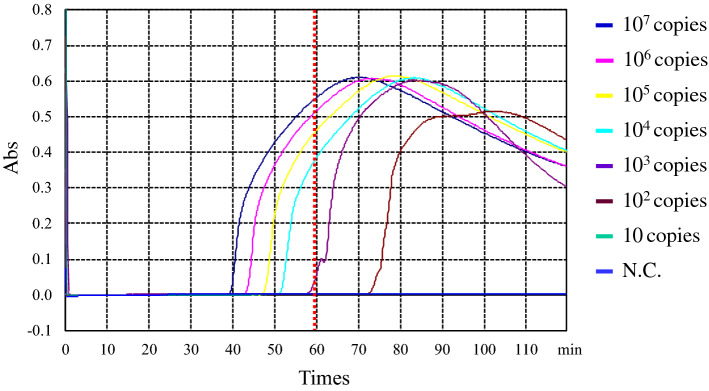
The specificity of the primer sets was tested using various types of A. fumigatus strains, such as wild isolates and environmental or clinical azole resistance isolates (Fig. 4). In this study, IFM63432 and IFM62918 strains were used as positive and negative control strains, respectively. As shown in Fig. 4A-i, TR46 LAMP primer could not amplify the DNA from 30 strains of azole drug-susceptible clinical isolates of A. fumigatus. However, the start of the LAMP amplification in the positive control strain of A. fumigatus strains (IFM 63432) was at around 50 min. On the other hand, TR46 LAMP primer could amplify DNA from A. fumigatus strains carrying the duplicate 46 bp promoter repeat in cyp51A gene (IFM63432, BE1-2, BE1-4, BE3-5, BE3-6) as shown in Fig. 4A-ii. This result suggests that the present LAMP primer could amplify the four TR462 strains harboring the TR46 resistant mutation (TR46/Y121F/T289A). It was also confirmed that TR46 LAMP primer could amplify DNA of A. fumigatus strains carrying three tandem repeats (TR463) (BE1-1, W1-4, W2-12-1) (Fig. 4A-iii). When this TR46 LAMP primer was tested for three TR342 strains (Table 1), namely strains IFM64460 and IFM64733 (with mutation of TR34/L98H) and strain 3-1-B (with mutations of TR34/L98H/Y289/T289A/I364V/G448S), DNA amplification was not observed (Fig. 4B-i,B-ii). These results also suggested that the present LAMP primer could not detect TR342 drug-resistant strains regardless of their point mutation site in the cyp51A gene (Fig. 4B-i,B-ii). These studies confirmed that the newly established TR46 LAMP primer set was specific for A. fumigatus strains with TR of double or triple 46-bp promoter tandem repeats in the cyp51A gene. The sensitivity of the TR46 LAMP assay was verified. The detection limit was 1 × 104 copies per reaction in 60 min. In the 80 min reaction, 102 copies per reaction were also detected (Fig. 5).
Figure 4.
Comparative amplification profiles of A. fumigatus wild type and environmental or clinical azole-resistant isolates with or without TR46 double or triple 46 bp promoter repeats in cyp51A gene by a newly developed LAMP primer sets. The dotted curve shows the amplification by control strain (IFM 63432). (A-i) DNA amplification profiles using 30 strains of A. fumigatus wild type. DNA amplification was not confirmed in all wild-type strains tested (30 strains). Among 30 wild-type strains, IFM 62918 strain was used as a negative control strain (no amplification). (A-ii) DNA amplification was confirmed by five TR462 strains (IFM63432, BE1-2, BE1-4, BE3-5, BE3-6), which have double 46 bp promoter repeats. (A-iii) DNA amplification was confirmed by three TR463 strains (BE1-1, W1-4, W2-12-1), which have triple 46 bp promoter repeats. (B-i) DNA amplification was not confirmed by two TR342 strains (IFM64460, IFM64733), which have duplicate 34 bp promoter repeats with one mutation in the one coding region (L98H). The dotted line shows amplification by the control strain. (B-ii) DNA amplification was not confirmed by one TR342 strain (3-1-B), which has duplicate 34 bp promoter repeats with multi-mutations in the four coding regions (L98H/T289A/I364V/G448S). The dotted line shows amplification by the control strain.
Figure 5.
Experimental results of the detection limit of the TR46 LAMP assay. The detection limit reaction was carried out using 107 to 10 copies of plasmid DNA per reaction. The detection limit was measured within 60 min.
Discussion
Azole antifungals mainly inhibit the ergosterol biosynthetic pathway by targeting the cytochrome P450-dependent enzyme lanosterol 14-α-demethylase, encoded by cyp51A in molds. Resistance to this class of drugs in the major human pathogen A. fumigatus is emerging and reaching levels to prevent their clinical use6. Advances in recent molecular genetic technologies such as real-time PCR have introduced various proper diagnostic assay methods into the fields in azole-resistant mechanism analysis. The LAMP assay described here has advantages of high sensitivity and specificity, low costs, and short amplification time. In addition, there have been no reports using LAMP techniques to study azole-resistant mechanisms in A. fumigatus by the strains with TR46 in the cyp51A promoter region.
Recently Yu Shan-Ling et al.18 reported a similar rapid technique to detect azole-resistant strains due to amplification of a TR of a 34 bp (TR34) and a 46 bp (TR46) within the promoter region of cyp51A of A. fumigatus. However, here we used a newly designed TR46 LAMP primer set different from those reported by Yu Shan-Ling et al.18. Compared to experiments such as Yu Shan-Ling et al.18, our method used adjusted genomic DNA (2 ng/μL) and can detect TR46 strains when resistance is confirmed, and this leads to a simple identification method of A. fumigatus carrying the TR46 in the cyp51A promoter region, in routine clinical practice.
There is a difference in MIC values between strains with TR46 and strains with TR3419. Therefore, the importance of detecting TR46 lies in the fact that strains of A. fumigatus harboring TR46 are resistant to voriconazole but not to itraconazole. Two TR34 strains (IFM64460: TR34/L98H and IFM64733: TR34/L98H) are highly resistant to voriconazole19 but not to itraconazole. Further detailed drug susceptibility mechanism study against TR34 strain (3-1-B: TR34/L98H/T289A/I364V/G448S) is of interest.
The high specificity and rapidity of the LAMP assay are achieved by applying four primers that target six regions of a DNA template, and two loop primers (LF, LB) to accelerate the reaction. In this study, we succeeded in designing valuable TR46 LAMP primer sets to detect specifically a TR46 within the promoter regions of azole-resistant A. fumigatus. Furthermore, the designed primer sets could differentiate azole-resistant TR46 strains from the TR34 strains and wild type strains. There was no cross reaction of the assay with neither the TR34 type nor the wild type. Further studies on the preparation of specific primers that can distinguish between TR34 strains and wild type strains regardless of the results of drug susceptibility testing are needed. To our knowledge, this is a new and helpful report of a detection method for one of the most prevalent cyp51A resistant gene TR46 in A. fumigatus azole-resistant strains.
Recently, the strain consisting of the four repeats of 46 bp of the promoter region was reported in the Netherlands (TR464)20. The LAMP primer we designed was able to detect both two copies of the TR46 tandem repeat and three copies of the TR46. Moreover, these amplification curves (as well as the starting point) were similar. The BIP (B1 + B2 complementary; Fig. 1) of the primer we designed is TR-specific. B1 is designed at the boundary where the repeat unit is inserted, and B2 is designed at the boundary between the repeat units. In addition, B3 is designed on a repeat unit. Based on the results of strains having double repeat and triple repeat, it was suggested that the primer used this time may be able to detect even if the number of repeats increases, such as TR464.
It is widely known that exposure to azole fungicides resulted in the emergence of azole-resistant strains with tandem repeats in the promoter region of cyp51A gene8,9. For this reason, epidemiological studies such as the incidence of azole-resistant strains in the environment are essential. Many environmental and clinical isolates need to be screened to generate epidemiological data, such as the frequency of detection of azole-resistant A. fumigatus. The method developed in this study would be an easy-to-use screening procedure.
Since the LAMP assay developed in the present study is a one-step and rapid detection method, coupled with its high reliability and ease of use, it can prompt detect specific drug-resistant genes due to TR46 in A. fumigatus in the clinical laboratory setting. Thus, early detection of infections due to TR46 drug-resistant strains in A. fumigatus might be helpful to guide the early start of corrective and effective antifungal therapy.
Methods
Aspergillus isolates and MIC determination by broth microdilution test
Forty-one strains, including thirty-three from the clinical setting and eight environmental (plant bulbs) isolates21 of A. fumigatus, were provided through the National Bio-Resource Project (NBRP), Japan (http://www.nbrp.jp/); source and drug susceptibility are shown in Table 1.
DNA preparation and extraction
Fungal strains were cultured on Sabouraud dextrose agar. Genomic DNA was extracted from overnight cultures of A. fumigatus mycelia by the urea-phenol method. Mycelia were mixed with 0.5 mm size glass beads, 0.5 ml of PCI (phenol/chloroform/Isoamyl alcohol) solution and 0.5 ml DNA extraction buffer (50 mM Tris–HCl, pH 8.0, 20 mM EDTA, 0.3 M NaCl, 0.5% SDS, 5 M urea), and disrupted by Fast Prep FP100A (MP-Biomedicals, Santa Ana, USA) for 3 cycles of 30 s each at a speed of 4.0 m/s. After centrifugation, the upper layer was transferred to a new tube and subjected to ethanol precipitation. The resulting DNA pellet was suspended in 100 μL TE buffer. DNA concentration was determined by the methods described in our previous paper22.
All A. fumigatus strains were submitted to antifungal susceptibility tests according to the CLSI M38 protocol (https://clsi.org/standards/products/microbiology/documents/m38/), using Eiken Dried Plates (9DEF47, Eiken Chemical Co., Tokyo, Japan).
LAMP-method
LAMP was performed as described in our previous studies23. TR46 LAMP primers were designed based on the target promoter region sequences of the cyp51A gene of A. fumigatus, which includes tandem repeats in the promoter region containing TR46 mutant alleles. The sequence of the cyp51A gene was downloaded from NCBI Gen-Bank (https://www.ncbi.nlm.nih.gov/genebank, accession numbers AF222068 for wild type, MH231595.1 for TR34, and MH040305.1 for TR46). In total, a 184-bp nucleotide alignment (Fig. 1) was used for TR46 LAMP primer design by the protocol of the Eiken Company (Primer Explorer V5, Eiken Chemical Co. Ltd, Tokyo. Japan). LAMP primers are composed of six primers recognizing eight distinct regions. LAMP reactions were performed with a Loopamp DNA amplification kit using reaction mixtures composed of 40 pmol each of primers FIP and BIP, 5 pmol each of primers F3 and B3, 20 pmol each of primers LF and LB, 12.5 mL × 2 reaction mixture, 1 μl Bst DNA polymerase, 2 μL DNA sample and distilled water up to a final volume of 25 μL (Eiken Chemical Co., Ltd., Tokyo, Japan). The LAMP reactions were analyzed by a real-time turbidimeter (Loopamp EXIA; Eiken Chemical Co.) and were conducted at 63 °C, for 120 min and then heated at 80 °C for 2 min to terminate the reaction. The start of amplification of LAMP products at 30 to 50 min in the graph suggested the positive reaction due to the presence of corresponding 46 bp tandem repeats of cyp51A gene. Since overall reaction can be obtained within 2 h, prompt drug therapy can be deployed within a short time. To check the detection limit of TR46 specific LAMP primers, the plasmid DNA was used. To construct plasmid contained TR46 and cyp51A gene sequences, we cloned the alleles using the shuttle vector pCB1004. Genomic DNA of IFM63432 was used as template to clone the alleles. The cyp51 coding region including approximately 1 kb fragments upstream and downstream were amplified by PCR using the primers pCB1004_Hind_cyp51A-F (5’-aggaattcgatatcaTAGAATGAGTGAGCTGATTT-3’) and pCB1004_Kpn_cyp51A-R (5’-gggcgaattgggtacCAGGTTTTCGCACGAGCTTCTCC-3’). Amplified DNA fragments were fused into pCB1004 digested with HindIII and KpnI, by In-Fusion H Cloning Kit (Takara Bio, Otsu, Japan). The size of plasmid DNA was 8164 bp.
Acknowledgements
This study was supported by Japan Agency for Medical Research and Development (AMED) under Grant Number JP21jm0110015 and Japan International Cooperation Agency (JICA) through the collaborative research project Science and Technology Research Partnership for Sustainable Development (SATREPS), Japan under Grant Number 02-P-9427/2018.
Author contributions
P.T.: conception; methodological design; laboratory work; data collection; data analysis; writing; revising. T.M.: conception; methodological design; laboratory work; data collection; data analysis; writing, revising. T.A.:conception; methodological design; laboratory work; data collection; data analysis; writing, revising. D.H.: methodological design; laboratory work; data collection; writing. Y.M.: methodological design; writing; supervision. M.L.M.: critical review; team leadership; funding. A W.: critical review; team leadership; funding.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Revie NM, Lyer KR, Robbins N, Cowen LE. Antifungal drug resistance: evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018;45:70–76. doi: 10.1016/j.mib.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowdhary A, Sharma C, Hagen F, Meis JF. Exploring azole antifungal drug resistance in Aspergillus fumigatus with particular reference to resistance mechanisms. Future Microbiol. 2014;9:697–711. doi: 10.2217/fmb.14.27. [DOI] [PubMed] [Google Scholar]
- 3.Beer KD, et al. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure: Three states, 2010–2017. MMWR Morb. Mortal Wkly. Rep. 2018;67:1064–1067. doi: 10.15585/mmwr.mm6738a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson TE, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;63:438–442. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagiwara D, et al. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front. Microbiol. 2016;7:1382. doi: 10.3389/fmicb.2016.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enserink M. Infectious diseases: Farm fungicides linked to resistance in a human pathogen. Science. 2009;326(5957):1173. doi: 10.1126/science.326.5957.1173. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016;47:45–68. doi: 10.1183/13993003.00583-2015. [DOI] [PubMed] [Google Scholar]
- 8.Gsaller F, et al. Sterol biosynthesis and azole tolerance is governed by the opposing actions of SrbA and the CCAT binding complex. PLOS Pathog. 2016;12(7):e1005775. doi: 10.1371/journal.ppt.1005775.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nywening AV, Rybak JM, Rogers PD, Fortwendel JR. Mechanisms of triazole resistance in Aspergillus fumigatus. Environ. Microbiol. 2020;22:4924–4952. doi: 10.1111/1462-2920.15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Ingen J, et al. Azole, polyene and echinocandin, MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J. Antimicrob. Chemother. 2015;70:178–181. doi: 10.1093/jac/dku364. [DOI] [PubMed] [Google Scholar]
- 11.Nathan PW, et al. First detection of TR34 L98H and TR46 Y121F T289A cyp51 mutations in Aspergillus fumigatus isolated in the United States. J. Clin. Microbiol. 2016;54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rößler S, et al. Progressive dispersion of azole resistance in Aspergillus fumigatus: Fatal invasive aspergillosis in a patient with acute myeloid leukemia infected with an A. fumigatus strain with a cyp51A TR46 Y121FM1721 T289A allele. Antimicrob. Agents Chemother. 2017;61(8):e00270. doi: 10.1128/AAC.00270-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rybak JM, Fortwendel JR, Rogers PD. Emerging threat of triazole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2019;74:835–842. doi: 10.1093/jac/dky517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharpe AR, Lagrou K, Meis JF, Chowdhary A, Lockhart SR, Verweij PE. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018;56:S38–S92. doi: 10.1093/mmy/myx144. [DOI] [PubMed] [Google Scholar]
- 15.Inacio J, Flores O, Spencer-Martins I. Efficient identification of clinical relevant Candida yeast species by use of an assay combining panfungal loop-mediated isothermal DNA amplification with hybridization to species-specific oligonucleotide probes. J. Clin. Microbiol. 2008;46:713–729. doi: 10.1128/JCM.00514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrice F, et al. Rubustness of a loop-nediated isothermal amplification reaction for diaganostic applification. FEMS Immunol. Med. Microbiol. 2011;62:41–48. doi: 10.1111/j.1574-695X.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 17.Shirato K. Detecting amplification of loop-mediated isothermal amplification. Microbiol. Immunol. 2019;63:407–412. doi: 10.1111/1348-0421.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling SY, et al. Rapid detection of azole-resistant Aspergillus fumigatus in clinical and environmental isolates by use of a lab-on-a-chip diagnostic system. J. Clin. Microbiol. 2020;58:e00843. doi: 10.1128/JCM.00843-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudakova A, et al. Molecular tools for the detection and deduction of azole antifungal drug resistance phenotypes in Aspergillus species. Clin. Microbiol. Rev. 2017;30:1065–1091. doi: 10.1128/CMR.00095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. MBio. 2017;8(3):e00791. doi: 10.1128/mBio.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagiwara D. Isolation of azole-resistant Aspergillus fumigatus from imported plant bulbs in Japan and the effect of fungicide treatment. J. Pestic. Sci. 2020;45:147–150. doi: 10.1584/jpestics.D20-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabasso P, et al. Isolation and drug susceptibility of Candida parapsilosis sensu lato and other species of C. parapsilosis complex from patients with blood stream infections and proposal of a novel LAMP identification method for the species. Mycopathology. 2015;179:53–62. doi: 10.1007/s11046-014-9830-9. [DOI] [PubMed] [Google Scholar]
- 23.de Souza M, et al. Comparison of DNA microarray, loop-mediated isothermal amplification (LAMP) and real-time PCR with DNA sequencing for identification of Fusarium spp. obtained from patients with hematologic malignancies. Mycopathology. 2017;182:625–632. doi: 10.1007/s11046-017-0129-5. [DOI] [PubMed] [Google Scholar]