Abstract
Expressive communication impairment is associated with haploinsufficiency of SETBP1, as reported in small case series. Heterozygous pathogenic loss-of-function (LoF) variants in SETBP1 have also been identified in independent cohorts ascertained for childhood apraxia of speech (CAS), warranting further investigation of the roles of this gene in speech development. Thirty-one participants (12 males, aged 0; 8–23; 2 years, 28 with pathogenic SETBP1 LoF variants, 3 with 18q12.3 deletions) were assessed for speech, language and literacy abilities. Broader development was examined with standardised motor, social and daily life skills assessments. Gross and fine motor deficits (94%) and intellectual impairments (68%) were common. Protracted and aberrant speech development was consistently seen, regardless of motor or intellectual ability. We expand the linguistic phenotype associated with SETBP1 LoF syndrome (SETBP1 haploinsufficiency disorder), revealing a striking speech presentation that implicates both motor (CAS, dysarthria) and language (phonological errors) systems, with CAS (80%) being the most common diagnosis. In contrast to past reports, the understanding of language was rarely better preserved than language expression (29%). Language was typically low, to moderately impaired, with commensurate expression and comprehension ability. Children were sociable with a strong desire to communicate. Minimally verbal children (32%) augmented speech with sign language, gestures or digital devices. Overall, relative to general development, spoken language and literacy were poorer than social, daily living, motor and adaptive behaviour skills. Our findings show that poor communication is a central feature of SETBP1 haploinsufficiency disorder, confirming this gene as a strong candidate for speech and language disorders.
Subject terms: Behavioural genetics, Genetics research, Development
Introduction
Clinical investigations of individuals with disruptions of SET binding protein 1 (SETBP1) on 18q12.3 have suggested the gene as a candidate for expressive speech disorder [1]. Haploinsufficiency of SETBP1 was first associated with expressive language ‘delay’ or ‘impairment’ in descriptive single cases [1, 2] and case series [3]. Other presenting features were mild-to-severely impaired intellect, gross and fine motor delays and/or deficits, hypotonia, distinctive facial features, attention deficits, and less commonly, autistic traits [1, 3].
A novel disorder encompassing this symptomatology was later confirmed in a cohort selected for intellectual disability (ID) [4]. Five individuals with LoF variants and one with a de novo deletion encompassing SETBP1 were identified [4]. The authors combined these cases with two further novel cases with truncating variants from a separate ID screen, together with the previously published small deletions [1, 3] and de novo variants [2] to examine the phenotype across all the identified individuals. Only retrospective clinical data were available, limiting the scope of the investigation. Most of the individuals were reported to have intelligence quotient and language deficits, with completely absent or significantly impaired speech in 92% of this group [4]. The nature of the speech deficits was not described, although apraxia was noted previously in one of the cases [1]. The specific speech and language phenotype in individuals with SETBP1 LoF variants remains unclear.
From a clinical genetics perspective, there is a need to identify genes that contribute to severe and persistent communication deficits, such as childhood apraxia of speech (CAS). Parental concern for speech development is a common reason for referral to paediatricians, yet the aetiology and prognosis for CAS are poorly understood and children are largely managed with a ‘watch-and-wait’ approach [5]. Until recently, few additional candidates for CAS had been revealed, since the unearthing of FOXP2 almost 20 years ago [6]. A number of new contenders have now been identified through next-generation sequencing screens of genomes/exomes in two cohorts ascertained on the basis of CAS [7, 8]. Notably, whilst modest in cohort size (n = 18 and n = 34, respectively), each study independently identified an individual with a heterozygous pathogenic SETBP1 LoF variant, suggesting disruptions of this gene as a recurrent cause for CAS; which occurs at a rate of only 1 or 2 cases per 1000 in the general population [9]. The findings add weight to the premise that SETBP1 may play an important role in speech and language development.
Two studies examining common single-nucleotide polymorphisms (SNPs) further support the potential relevance of SETBP1 variation for communication abilities. Associations between SETBP1 and scores on a test examining syntactic complexity (mean length of sentences and use of complex sentence structures) were reported in a Genome Wide Association Study of developmental language disorder in a geographically isolated Russian cohort aged 3–18 years [10]. More tentatively, SNPs in SETBP1 have been reported to show association with phonological working memory; just one of many reading-related traits examined across a reading-impaired cohort of modest size for complex trait analyses (n = 135) [11]. Whether SETBP1 is more closely linked with variations in speech, language and/or reading ability in the general population is not yet clear.
Thus, nascent evidence from a range of sources suggests SETBP1 as a gene of relevance to speech and language development. Yet information concerning the clinical phenotype associated with SETBP1 haploinsufficiency has so far been drawn only from descriptive case series, relying largely on retrospective examination of medical records. Here, we performed in-depth examination of speech, language and literacy abilities in a cohort of 31 individuals with SETBP1 LoF variants and deletions, using standardised tests, to precisely characterise the communication phenotype of this syndrome. Linguistic performance was considered relative to other areas of neurodevelopment (e.g. motor abilities, social skills), to determine whether communication was differentially affected.
Methods
Inclusion criteria were a molecular diagnosis of heterozygous SETBP1 truncating (stop-gain or frameshift) variants or 18q12.3 deletions in individuals aged ≥6 months. Participants were recruited globally via the SETBP1 Society (http://www.setbp1.org) and clinician referral. Ethics approval was obtained from the Royal Children’s Hospital, Melbourne, Human Research Ethics Committee (HREC 37353A).
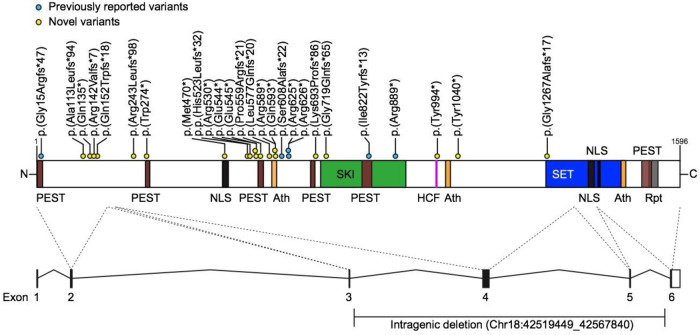
Thirty-one participants were recruited (Table 1; 20 males, average age 9 years, range 0; 8–23; 2 years). Genotypes included single-nucleotide variants (28 participants), a 18q12.3 intragenic deletion and two larger deletions encompassing SETBP1 (Fig. 1). Twenty four participants were novel cases, not previously reported in the literature. Nineteen participants also participated in a separate study on the broader medical phenotype (see the companion paper by Jansen et al., Eur J Hum Genet, submitted). Deletions and phenotypic data were submitted to Decipher (https://decipher.sanger.ac.uk/) and sequence variants were submitted to Leiden Open Variation Database (Database ID: #chr18_002464-002468, #SETBP1_000018-000020, #SETBP1_000033, #SETBP1_000078, #SETBP1_000083, #SETBP1_000085, #SETBP1_000103, #SETBP1_000106, #SETBP1_000108-000111, #SETBP1_00014, #SETBP1_000116-000117, #SETBP1_000119-000120, #SETBP1_000123-000125, #SETBP1_000127, #SETBP1_000129).
Table 1.
Developmental history and medical features.
| IND | Age | Sex | Variant (NM_ 015559.2) | History of feeding difficulties | Intellectual impairment | Speech therapy | Fine motor abilitya | Gross motor abilitya | OT/PT | ADHD/ attention issues |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0y8m | M | c.453_454insTGGG (p.(Lys152Trpfs*18)) | Y | # | N | Mod. Low | Mod. Low | Y | N |
| 2 | 1y3m | M | arr[GRCh37] 18q12.3(42519449_42567840)x1 | Y | # | N | Mod. Low* | Mod. Low | Y | N |
| 3 | 2y11m | F | c.1588C>T (p.(Arg530*)) | Y | # | Y | Mod. Low | Mod. Low | Y | Y |
| 4 | 3y2m | M | arr 18q12.2q12.3(34576844_43015201)x1 | Y | # | Y | Mod. Low | Mod. Low | N | N |
| 5 | 3y4m | F | c.1633G>T (p.(Glu545*)) | N | # | Y | Adequate | Mod. Low | Y | N |
| 6 | 3y7m | F | c.1630C>T (p.(Arg544*)) | Y | # | Y | Y~ | Y~ | Y | N |
| 7 | 3y8m | F | c.1873C>T (p.(Arg625*)) | Y | Mild | Y | Low | Mod. Low | Y | Y |
| 8 | 4y6m | F | c.1777C>T (p.(Gln593*)) | N | Mild | Y | Adequate | Mod. Low | Y | Y |
| 9 | 5y7m | F | c.1876C>T (p.(Arg626*)) | Y | Moderate | Y | Y~ | Y~ | Y | Y |
| 10 | 6y1m | M | c.1408delA (p.(Met470*)) | Y | Moderate~ | Y | Mod. Low | Mod. Low | Y | N |
| 11 | 6y2m | M | c.726_732del (p.(Arg243Leufs*98)) | N | Borderline | Y | Mod. Low* | Adequate | Y | Y |
| 12 | 6y10m | M | c.1676delC (p.(Pro559Argfs*21)) | N | Mild | Y | Adequate | Mod. Low | Y | Y |
| 13 | 7y0m | F | c.3799_3800insC (p.(Gly1267Alafs*17)) | Y | Moderate | Y | Low | Mod. Low | Y | Y |
| 14 | 7y2m | M | c.3120C>A (p.(Tyr1040*)) | N | Borderline | Y | Mod. Low | Low | Y | Y |
| 15 | 7y6m | M | c.337delG (p.(Ala113Leufs*94)) | Y | Mild–moderate | Y | Mod. Low | Mod. Low | Y | N |
| 16 | 8y1m | M | c.1568delA (p.(His523Leufs*32)) | Y | Borderline | Y | Mod. Low | Mod. Low | Y | Y |
| 17 | 8y5m | M | c.821G>A (p.(Trp274*)) | N | Borderline | Y | Adequate | Adequate | Y | Y |
| 18 | 8y11m | F | c.2665C>T (p.(Arg889*)) | Y | Borderline | Y | Y | Y | Y | N |
| 19 | 9y4m | F | c.1765C>T (p.(Arg589*)) | N | Mild | Y | Low | Mod. Low | Y | N |
| 20 | 11y0m | M | c.1730_1749del (p.(Leu577Glnfs*20)) | Y | Moderate | Y | Low* | Adequate* | Y | Y |
| 21 | 11y7m | M | c.1821del (p.(Ser608Alafs*22)) | Y | Mild | Y | Low* | Mod. Low* | Y | Y |
| 22 | 12y0m | F | c.422dup (p.(Arg142Valfs*7)) | Y | Mild | Y | Mod. Low* | Mod. Low* | Y | Y |
| 23 | 12y1m | M | c.1873C>T (p.(Arg625*)) | N | Mild | Y | Low* | Adequate* | Y | Y |
| 24 | 13y0m | M | arr[hg19] 18q12.3q21.1(39600614_45460709)x1 | N | Moderate | Y | Y~ | NA | N | N |
| 25 | 13y5m | M | c.403C>T (p.(Gln135*)) | N | Severe~ | Y | Low* | Adequate* | N | N |
| 26 | 13y7m | M | c.1765C>T (p.(Arg589*)) | N | Mild | Y | Low* | Mod. Low* | Y | Y |
| 27 | 14y2m | F | c.2076_2092delinsC (p.(Lys693Profs*86)) | Y | Mild | Y | Mod. Low* | Adequate* | Y | Y |
| 28 | 14y10m | M | c.2156del (p.(Gly719Glufs*65)) | Y | Mild–moderate | Y | Low* | Mod. Low* | Y | Y |
| 29 | 15y10m | M | c.39_40del (p.(Gly15Argfs*47)) | N | Moderate | Y | Low* | Mod. Low* | N | N |
| 30 | 19y10m | M | c.2464del (p.(Ile822Tyrfs*13)) | N | Moderate | Y | N~ | N~ | Y | N |
| 31 | 23y2m | M | c.2982C>G (p.(Tyr994*)) | Y | Average | Y | Mod. Low* | Mod. Low* | Y | N |
Severity of cognitive impairment was based on standard ID severity levels: borderline (71–85), mild (50/55–70), moderate (35–49/54), severe [20–34], profound (<20); ~ = parent report; # = no clinical cognitive assessment due to young age.
y years, m months, F Female, M male, Y feature present, N feature absent, OT occupational therapy received, PT physiotherapy received, ADHD attention deficit hyperactivity disorder.
aMotor scores on Vineland Adaptive Behaviour Scale.
*Performance compared to peers aged 9y11m which is the age limit of the normative data for this motor tool.
Fig. 1. Location of truncating variants in relation to SETBP1 protein.
Schematic representation of the SETBP1 protein (UniProt: Q9Y6X0) indicating loss-of-function variants included in this study. Five exons (black bars) encode isoform A of the protein (1596 amino acids). Five exons (black bars) encode isoform A of the protein (1596 amino acid residues). The SETBP1 protein sequence contains three AT-hook domains (Ath; orange; amino acids 584–596, 1016–1028, 1451–1463), a SKI homologous region (SKI; green; amino acids 706–917), a HCF1-binding motif (HCF; magenta; amino acids 991–994), a SET-binding domain (SET; blue; amino acids 1292–1488), three bipartite NLS motifs (black; amino acids 462–477, 1370–1384, 1383–1399), six PEST sequences (brown; amino acids 1–13, 269–280, 548–561, 678–689, 806–830, 1502–1526) and a repeat domain (Rpt; grey; amino acids 1520–1543) [31–34]. Blue circles represent previously reported variants and yellow circles indicate novel variants. Two individuals with larger deletions (IND 4, 24) are not shown here. For cDNA annotation of the variants see Table 1.
Health and development
Health and medical information, including data on neurodevelopmental conditions, intellectual ability and intervention (e.g. speech therapy, physiotherapy), was collected via an established survey [12, 13] (Table 1), translated into multiple languages. Health professional reports (e.g. psychology, neurology, clinical genetic, speech pathology) and telehealth consults confirmed questionnaire responses. Feeding (Child Oral and Motor Proficiency Scale) [14] and drooling (Drooling Impact Scale) [15] measures were collected where age appropriate.
Speech
Verbal children completed the Diagnostic Evaluation of Articulation and Phonology to examine articulation and phonological errors [16]. A 5-minute speech sample was analysed for diagnoses of CAS [17] and dysarthria using established methods [18, 19]. The Intelligibility in Context Scale [20] examined how often the individual is understood, with a 5 point scale of responses ranging from never to always.
Language
Verbal children were assessed with the Children’s Communication Checklist (CCC-2) [21]. Minimally verbal children (defined as <50 spoken words), and those <4 years of age were assessed with the Macarthur Bates Communicative Development Inventory (MB-CDI) [22] and Communication and Symbolic Behaviour Scales Developmental Profile (CSBS-DP) [23]. The MB-CDI measures understanding and use of gesture, vocabulary and sentences. The CSBS-DP provides social communication, speech and symbolic communication scores for children aged 6–24 months, or for chronologically older children with limited linguistic abilities [23].
Adaptive behaviour
The Vineland Adaptive Behaviour Scales-Parent/Caregiver [24], provided domain scores for communication, socialisation, daily living and motor skills, with an overall adaptive behaviour composite. Wilcoxon signed-rank sum tests were used to determine the relative involvement of language compared to other Vineland subdomain scores.
Results
Health and development
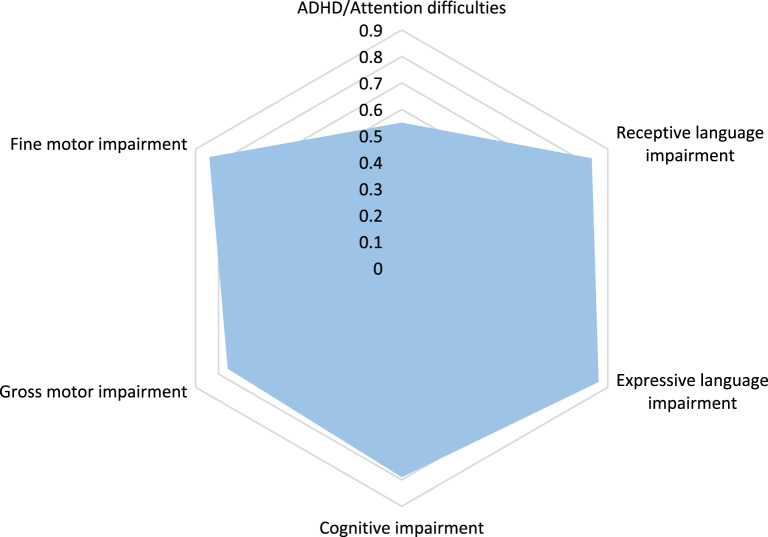
The cohort consisted of one infant, seven pre-school children, 21 school-aged children and adolescents and two adults (n = 31 (20 males, 11 females); Table 1). Participants were recruited from the US (n = 12), Netherlands (n = 8), United Kingdom (n = 3), France (n = 3), Canada (n = 2), Israel (n = 2) and Australia (n = 1). Developmental issues relevant to speech and language development included early feeding difficulties (58%), and excessive drooling (35%). Almost all participants (94%) had generalised motor delay or disorder that required occupational therapy and/or physiotherapy (87%; Table 1 and Fig. 2). Motor deficits included difficulties with personal care (managing buttons and zippers, teeth brushing, washing), writing, drawing, using scissors, riding a bike and toilet training. Intellectual impairment was reported in the majority of individuals aged >4 years. In the seven individuals aged <4 years, six parents reported that their child was experiencing developmental delay compared to same-aged peers. Seven patients had seizures; four had febrile seizures, one had generalised tonic-clonic seizures, one had absence seizures and one reported a history of seizures despite a normal EEG. Hearing impairment was infrequent (3/31, 10%) and all presentations were mild (25–39 dBHL) and bilateral, with two cases of mixed (IND 8, 12) and one of conductive (IND 27) hearing loss, although periodic conductive losses due to otitis media were also common (58%). Visual impairments (42%) were addressed with glasses and hypermetropia was the most common diagnosis (62%). Palatal abnormalities included cleft lip and palate (IND 26), submucous cleft palate (IND 23) and a high arch palate (IND 31). Micrognathia (3/31, 10%) was noted in few participants.
Fig. 2. Radar plot showing core neurodevelopmental features (n = 29).
2 children <2 years and hence too young for reliably determining the presence of these comorbid features.
Attention issues were common (55%; including nine with formal ADHD diagnoses, see Fig. 2). All but the 8-month old participant had been directly assessed for autism spectrum disorder (ASD), but only three (10%) received a clinical ASD diagnosis. Other diagnoses included developmental coordination disorder (19%) and sensory processing disorder (23%).
Academically, eight of the 21 (38%) school-aged participants attended mainstream schools and the remaining 13 (62%) attended special education schools. Of the pre-school participants, three attended mainstream, and four specialised, childcare or pre-school settings, and one was cared for at home. Learning support was common (86%) across all settings. The two young adults had completed school and were engaged in supported employment. Most parents of school-aged children and young adults (21/23, 91%) reported that their child’s academic progress had been limited by their speech and language difficulties.
Speech
Speech development was characterised by limited babbling and a reduced phonetic (sound) inventory relative to peers across the first 7 years of life; when a full inventory is typically acquired. Most participants had acquired first spoken words by 18 months of age (52%) (Table 2). For the majority of the verbal children, short phrases or sentences were developed by 6–7 years (protracted relative to typical developmental milestone of 2–3 years) (Table 2). Most (94%) had accessed speech therapy, with the exception of two young participants (aged 8 months and 1 year 3 months). The dose of speech therapy increased during the pre-school period; typically once per week/fortnight, but up to five times per week where available. Intelligibility across the group ranged from never understood (32%), to rarely (23%), sometimes (61%) and usually understood (13%), based on the Intelligibility in Context Scale [20] scores.
Table 2.
Speech and language performance.
| IND | Age | Communication milestones (y; m) | Minimally verbal | Speech diagnosis~ | Language abilitya | ||||
|---|---|---|---|---|---|---|---|---|---|
| Spoken words | Short sentences | Expressive | Receptive | Written | Social skillsa | ||||
| 1 | 0y8m | ^ | ^ | ^ | ^ | Mod. Low | Mod. Low | NA | Adequate |
| 2 | 1y3m | NYA^ | ^ | ^ | N~ | Low | Low | NA | Mod. Low |
| 3 | 2y11m | NYA | NYA | Y | CAS | Low | Mod. Low | NA | Adequate |
| 4 | 3y2m | 15–18m | NYA | Y | NA | Low | Mod. Low | Mod. Low | Mod. Low |
| 5 | 3y4m | <12m | NYA | Y | Phon, CAS | Mod. Low | Adequate | Adequate | Mod. Low |
| 6 | 3y7m | <12m | NYA | Y | N~ | NA | NA | NA | NA |
| 7 | 3y8m | >18m | NYA | Y | CAS | Low | Low | Low | Mod. Low |
| 8 | 4y6m | <12m | 4–5y | N | Phon, CAS | Low | Low | Mod. Low | Adequate |
| 9 | 5y7m | >18m | NYA | Y | N~ | Low | Low | Low | Low |
| 10 | 6y1m | 15–18m | 4–5y | N | Phon, CAS | Low | Adequate | Low | Adequate |
| 11 | 6y2m | >18m | 4–5y | N | Artic, CAS, Phon | Mod. Low | Mod. Low | Mod. Low | Mod. Low |
| 12 | 6y10m | 12–15m | 6–7y | N | CAS | Low | Mod. Low | Low | Mod. Low |
| 13 | 7y0m | >18m | NYA | Y | Phon, CAS, Dysarthria~ | Low | Low | Low | Mod. Low |
| 14 | 7y2m | >18m | 4–5y | N | Phon, CAS, Dysarthria | Mod. Low | Mod. Low | Low | Low |
| 15 | 7y6m | >18m | 4–5y | N | Phon, CAS, Dysfluency | Low | Mod. Low | Low | Mod. Low |
| 16 | 8y1m | 12–15m | 4–5y | N | Phon, CAS | Mod. Low | Adequate | Mod. Low | Mod. Low |
| 17 | 8y5m | >18m | 4–5y | N | CAS | Adequate | Adequate | Adequate | Adequate |
| 18 | 8y11m | >18m | 6–7y | N | CAS, Dysarthria | Severeb | Severeb | Severeb | – |
| 19 | 9y4m | >18m | 6–7y | N | Phon, CAS | Low | Low | Mod. Low | Mod. Low |
| 20 | 11y0m | 12–15m | NYA | Y | N~ | Low | Low | Low | Mod. Low |
| 21 | 11y7m | <12m | 6–7y | N | Phon, CAS, Dysarthria | Low | Low | Low | Mod. Low |
| 22 | 12y0m | 15–18m | 6–7y | N | Artic, Phon, CAS | Adequate | Mod. Low | Low | Adequate |
| 23 | 12y1m | <12m | 4–5y | N | Phon, CAS | Adequate | Adequate | Mod. Low | Mod. Low |
| 24 | 13y0m | NYA | NYA | Y | Artic, CAS | Severeb | NA | NA | NA |
| 25 | 13y5m | NYA | NYA | Y | CAS | Low | Mod. Low | Low | NA |
| 26 | 13y7m | >18m | 4–5y | N | CAS | Mod. Low | Mod. Low | Mod. Low | Low |
| 27 | 14y2m | 12–15m | 6–7y | N | CAS | Mod. Low | Mod. Low | Low | Low |
| 28 | 14y10m | >18m | >8y | N | CAS, Dysarthria | Mod. Low | Mod. Low | Low | Mod. Low |
| 29 | 15y10m | 15–18m | NYA | Y | Phon, CAS~ | Mod. Low | Mod. Low | Low | Mod. Low |
| 30 | 19y10m | <12m | 4–5y | N | Phon, CAS | NA | NA | NA | NA |
| 31 | 23y2m | <12m | 2–3y | N | Phon, CAS | Adequate | Low | Mod. Low | Low |
~ = Based on parent report; ^ = patient is too young for these developmental milestones.
m months, y years, NYA not yet achieved, NA not assessed, Phon phonological disorder, CAS childhood apraxia of speech, Artic articulation disorder.
aRated using Vineland-3 standard scores and severity ratings.
bSeverity rated according to other clinical language assessment results provided.
Verbal children presented with a complex motor speech disorder, best characterised as CAS (80%; Table 2) with: inconsistency of phoneme production; increased errors with increasing word length; simplified syllable structures relative to age, as well as vowel and prosodic errors. A small proportion (16%) had dysarthria, typically characterised here by low pitch, hypernasality, monotonous, monoloud and flaccid, slow speech. Other speech diagnoses of phonological disorder (48%), articulation impairment (specifically a lisp) (9%) and stuttering (3%) were reported alongside CAS.
Minimally verbal children (11/31, 35%; Table 2) had few spoken words but had communicative intent, and used gesture, sign and/or communication devices for expression. Of this group, six were young children (aged 3–5 years of age) and five were older (aged 7–15 years). Speech intervention for the younger group focused on language stimulation, non-verbal gestures and verbal speech production. The older group was producing single words or short phrases using their digital devices. Relative strengths in social and symbolic language abilities (average standard scores 9.29 and 9.57, where mean = 10, standard deviation = 3) relative to speech (average composite standard score 6.57) were revealed on the CSBS.
Language (expressive, receptive, written, social)
For most participants, expressive and receptive language abilities were commensurate with each other (18/28; 64%) on the VABS. Poorer expressive than receptive performance was the next most common profile (8/28; 29%). For children of reading age, written language ranged from typical (2/25; 8%) to moderately low (8/25; 32%) and low to severe (15/25; 60%). Many had difficulty with writing tasks, such as copying letters or their name, although a few older patients were able to write in longer sentences. A large proportion (10/23; 43%) had received a formal diagnosis of a reading and/or writing disorder from a health professional.
The CCC-2 enabled further comparison within and across general communication (e.g. semantics, syntax, coherence) and social interaction domains. All children assessed with the CCC-2 (n = 16), had poor communication abilities (Table 3). Pragmatic language and social skills were relative strengths overall, compared to speech, language structure, vocabulary and discourse, based on individual scaled scores (Fig. 3). Autistic traits were reported in half this group (8/16, 50%), including poor social skills and restricted interests compared to peers. Yet only three had a clinical diagnosis of ASD, as noted earlier. Participants showed a desire to communicate and share interests, with intact basic social skills and non-verbal gestures. Whilst data are limited, a widening gap in social skills was suggested, relative to peers, with increasing age (Table 2).
Table 3.
Children’s Communication Checklist scores (N = 16)a.
| Children’s Communication Checklist domains | Average standard score Mean = 10, SD = 3 |
|---|---|
| Speech | 1.9 |
| Syntax | 1.4 |
| Semantic | 1.9 |
| Coherence | 2.0 |
| Inappropriate initiation | 3.6 |
| Stereotyped (scripted language) | 4.8 |
| Use of context | 2.1 |
| Non-verbal communication | 4.2 |
| Social relations | 3.1 |
| Interests | 4.9 |
aEnglish speaking children aged >4 years.
Fig. 3. Performance across sub-domains of language in the children’s communication checklist (n = 16) (English speaking children aged >4 years).
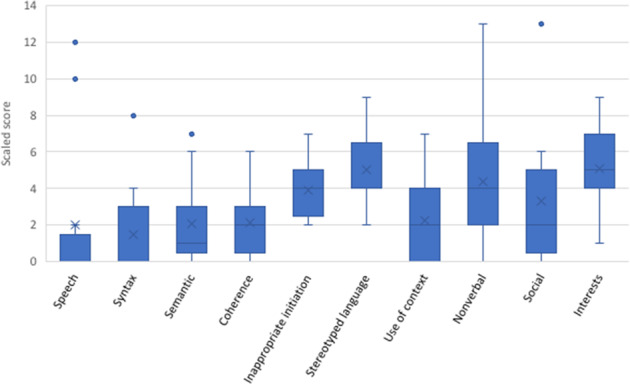
Lines denote median scores and X denotes the mean scores; • indicates an outlier. Scaled scores between 7 and 13 are within the average range.
Adaptive behaviour: language relative to daily functioning, social and motor skills
Overall adaptive behaviour scores were in the moderately low range (Table 4). This was commensurate with daily living skills and socialisation (Table 4 and Fig. 4). Motor skills were stronger than communication abilities (p = 0.0021), although these data represent participants aged <9; 11 only, with information about normative motor skills unavailable for older children. Fine motor skills were poorer relative to gross motor in most cases, confirming parent reported motor abilities from the questionnaire data. Performance in the communication domain was substantially lower than that for socialisation (p = 0.0055) and daily living skills (p = 0.0023) domains (Table 4).
Table 4.
Adaptive Behaviour Scores (N = 27).
| Adaptive Behaviour (Vineland-3) sub-domains | Average standard score Mean = 100, SD = 15 |
|---|---|
| Adaptive behaviour composite | 72.5 |
| Communication domain | 65.4 |
| Daily living skills domain | 75.6 |
| Socialization domain | 76.7 |
| Motor skillsa | 77.9 |
Averages calculated based on data from the Vineland-3.
aBased on scores for patients aged <9y11m, as normative data are unavailable on the Vineland Adaptive Behaviour Scale-3 for older ages.
Fig. 4. Performance across domains of the Vineland adaptive behavior scale-3 (n = 27).
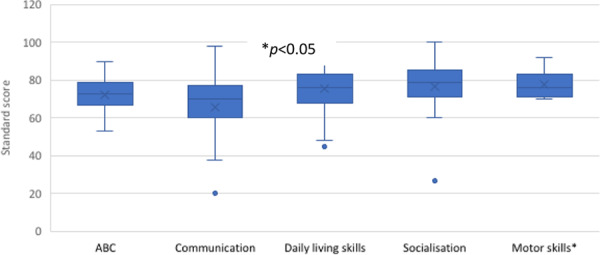
Lines denote median scores; X denotes mean scores; • indicates an outlier; ABC adaptive behaviour composite, that is overall combined score. Standard scores between 85 and 115 are considered within the average range.
Discussion
Here we report the speech and language phenotype of individuals selected for pathogenic SETBP1 LoF variants. Our findings indicate that articulatory, spoken and written language (reading, writing) deficits are distinctive features of the broader neurodevelopmental profile. We expand the phenotype of this disorder beyond ‘expressive speech’ difficulties, to reveal specific sub-types of speech disorder and highlight difficulties with the understanding as well as expression of language.
Protracted and impoverished speech, language and literacy (reading, writing) development was seen across the group, regardless of cognitive ability. Although first words were developed at the typical 12-month milestone for some, the ongoing trajectory of linguistic development was markedly protracted. Verbal children displayed a complex range of speech diagnoses implicating perturbed motor (CAS, articulation impairment, dysarthria) and linguistic deficits (phonological errors) that have not previously been recognized as features of SETBP1 haploinsufficiency disorder. Yet CAS was the most common finding in our cohort, in line with the recent identification of pathogenic SETBP1 LoF variants in gene discovery cohorts ascertained for CAS [7, 8]. The co-morbid articulation, phonological and dysarthric impairments seen alongside CAS were more notable in older children in the middle school years. Changes in speech profile across the lifespan are recognised in other neurogenetic conditions [8, 12, 25] and confirm the need for regular speech surveillance to enable precisely targeted therapies at particular ages.
A subset of children remained minimally verbal at ages 7–15 years. How to extricate the relative cognitive-linguistic from motor contributions in children with minimally verbal presentations is an area of ongoing debate in other neurodevelopmental conditions such as ASD [26], and no simple algorithm is available. All minimally verbal children here had communicative intent and used augmentative approaches alongside speech, such as sign language, gesture or digital devices to convey messages. This strong desire to communicate was also reported in the histories of children who became verbal, showing little differentiation between verbal and minimally verbal speakers in this regard. One hypothesis to explain the minimally verbal presentation in some is that they may have more severe involvement on the speech-motor continuum, described as anarthria and/or significant speech praxis. Early speech intervention appears to be critical for all with SETBP1 LoF variants who present with severe speech disorder, with best evidenced approaches for speech apraxia known to involve intensive therapy as often as four sessions per week [27]. Future clinical trials of intensive speech therapies in individuals with SETBP1 LoF variants are warranted.
In terms of language performance; previous case descriptions of children with SETBP1 LoF variants have implied that language comprehension is more intact than language production [1, 3]. Yet administration of standardised language tests in our cohort revealed that understanding of language is largely commensurate with expression. This highlights potential for clinical bias in making subjective assessments of language comprehension in children with speech production disorders. Further, language deficits appeared ubiquitous without clear disparity across sub-domains of vocabulary, syntax and coherence. Similarly, there was corresponding involvement of written (reading, spelling) and spoken language, without clear dissociations between these skills. Spoken and written language abilities were in turn, generally commensurate with cognitive abilities.
In terms of broader neurodevelopmental profile, clinical reports of gross and fine motor deficits affecting motor planning, programming and execution occurred with equivalent frequency to the speech-motor deficits seen here. Attention deficits and cognitive impairment were also prevalent. These are recognisable features previously reported as concomitant with CAS [8].
Differentiating severe communication deficits from ASD can be challenging [28] and for some individuals, the negative cycle of communication breakdown leads to further social withdrawal over time [29, 30]. A number of children in our cohort had ‘autistic features’ represented by limited social skills and restricted interests relative to peers, yet only three had a formal ASD diagnosis. Further, concern over limited speech development, rather than autistic features, was the core presenting concern for parents, and all had a strong desire to communicate, despite their recognised social skill deficits. Overall, we found limited evidence for a distinct ASD signature associated with SETBP1 LoF variants.
Clinical implications
We show that aberrant communication development is a central feature of the SETBP1 LoF syndrome. Children with heterozygous pathogenic SETBP1 LoF variants or deletions should be enrolled in speech therapy in the first year of life. Given the markedly delayed verbal communication trajectory, multi-modal communication, such as sign language or communication devices would support language acquisition prior to speech developing. The complex and widespread linguistic deficits signal that children will need speech-motor therapies to develop verbal speech, but also phonological interventions focused on early literacy awareness and approaches targeting language comprehension as well as production. Whilst children demonstrate a strong desire to communicate, social skills warrant therapeutic attention. Given this pervasive communication profile, we confirm SETBP1 as a strong candidate for speech and language disorders.
Acknowledgements
Sincere thanks to the children, families and clinicians who took part in this project. A special thanks to Haley Oyler, president of the SETBP1 Society for assistance with recruitment and for providing unique parental insights into children with SETBP1 haploinsufficiency disorder.
Funding
Funding was provided by National Health and Medical Research Council (NHMRC) Practitioner Fellowship #1105008 (AM); NHMRC Investigator Grant #1195955. NHMRC Centre of Research Excellence in Speech and Language Neurobiology #1116976 (AM, DA, SEF); and the Max Planck Society (MMKW, SEF). This work was supported by the Victorian Government’s Operational Infrastructure Support Program.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Angela Morgan, Ruth Braden
References
- 1.Marseglia G, Scordo MR, Pescucci C, Nannetti G, Biagini E, Scandurra V, et al. 372 kb microdeletion in 18q12.3 causing SETBP1 haploinsufficiency associated with mild mental retardation and expressive speech impairment. Eur J Med Genet. 2012;55:216–21. doi: 10.1016/j.ejmg.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–82. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 3.Filges I, Shimojima K, Okamoto N, Röthlisberger B, Weber P, Huber AR, et al. Reduced expression by SETBP1 haploinsufficiency causes developmental and expressive language delay indicating a phenotype distinct from Schinzel-Giedion syndrome. J Med Genet. 2011;48:117–22. doi: 10.1136/jmg.2010.084582. [DOI] [PubMed] [Google Scholar]
- 4.Coe BP, Witherspoon K, Rosenfeld JA, van Bon BWM, Vulto-van Silfhout AT, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014;46:1063–71. doi: 10.1038/ng.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan AT, Eecen KT, Pezic A, Brommeyer K, Mei C, Eadie P, et al. Who to refer for speech therapy at 4 years of age versus who to “watch and wait”? J Pediatr. 2017;185:200–4. doi: 10.1016/j.jpeds.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 6.Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–23. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 7.Eising E, Carrion-Castillo A, Vino A, Strand EA, Jakielski KJ, Scerri TS, et al. A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry. 2018;24:1065–78. doi: 10.1038/s41380-018-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildebrand MS, Jackson VE, Scerri TS, Van Reyk O, Coleman M, Braden RO, et al. Severe childhood speech disorder: gene discovery highlights transcriptional dysregulation. Neurology. 2020;94:e2148–67. doi: 10.1212/WNL.0000000000009441. [DOI] [PubMed] [Google Scholar]
- 9.Shriberg LD. Five subtypes of developmental phonological disorders. Clin Commun Disord. 1994;4:38–53. [PubMed] [Google Scholar]
- 10.Kornilov SA, Rakhlin N, Koposov R, Lee M, Yrigollen C, Caglayan AO, et al. Genome-wide association and exome sequencing study of language disorder in an isolated population. Pediatrics. 2016;137:e20152469. doi: 10.1542/peds.2015-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perdue MV, Mascheretti S, Kornilov SA, Jasińska KK, Ryherd K, Mencl WE, et al. Common variation within the SETBP1 gene is associated with reading-related skills and patterns of functional neural activation. Neuropsychologia. 2019;130:44–51. doi: 10.1016/j.neuropsychologia.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan AT, van Haaften L, van Hulst K, Edley C, Mei C, Tan TY, et al. Early speech development in Koolen de Vries syndrome limited by oral praxis and hypotonia. Eur J Hum Genet. 2018;26:75–84. doi: 10.1038/s41431-017-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei C, Fedorenko E, Amor DJ, Boys A, Hoeflin C, Carew P, et al. Deep phenotyping of speech and language skills in individuals with 16p11.2 deletion. Eur J Hum Genet. 2018;26:676–86. doi: 10.1038/s41431-018-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pados BF, Thoyre SM, Park J. Age-based norm-reference values for the Child Oral and Motor Proficiency Scale. Acta Paediatrica. 2018;107:1427–32. doi: 10.1111/apa.14299. [DOI] [PubMed] [Google Scholar]
- 15.Reid SM, Johnson HM, Reddihough DS. The Drooling Impact Scale: a measure of the impact of drooling in children with developmental disabilities. Dev Med Child Neurol. 2010;52:e23–8. doi: 10.1111/j.1469-8749.2009.03519.x. [DOI] [PubMed] [Google Scholar]
- 16.Dodd B, Hua Z, Crosbie S, Holm A, Ozanne A. Diagnostic evaluation of articulation and phonology. London: Psychological Corporation; 2002.
- 17.American-Speech-Language-Hearing-Association. Childhood apraxia of speech. 2007. http://www.asha.org.
- 18.Duffy JR. Motor speech disorders: substrates, differential diagnosis and management. 3rd ed. St. Louis: Mosby; 2013. [Google Scholar]
- 19.Morgan AT, Masterton R, Pigdon L, Connelly A, Liegeois FJ. Functional magnetic resonance imaging of chronic dysarthric speech after childhood brain injury: reliance on a left-hemisphere compensatory network. Brain. 2013;136:646–57. doi: 10.1093/brain/aws355. [DOI] [PubMed] [Google Scholar]
- 20.McLeod S, Crowe K, Shahaeian A. Intelligibility in context scale: normative and validation data for English-speaking preschoolers. Lang, speech, hearing Serv Sch. 2015;46:266–76. doi: 10.1044/2015_LSHSS-14-0120. [DOI] [PubMed] [Google Scholar]
- 21.Bishop DV. Children’s communication checklist, Second Edition. 2nd ed. London: Pearson; 2003. [Google Scholar]
- 22.Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick S, Bates E. The MacArthur-Bates communicative development inventories user’s guide and technical manual. 2nd ed. Baltimore: Brookes; 2006.
- 23.Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales Developmental Profile™ Infant-Toddler Checklist. Baltimore: Brookes; 2002.
- 24.Sparrow SS, Cicchetti DV, Saulnier CA. Vineland Adaptive Behaviour Scales, third edition. 3rd ed. Bloomington: Pearson; 2016. [Google Scholar]
- 25.Morgan AT, Fisher SE, Scheffer IE, Hildebrand MS. GeneReviews(R) [Internet]. Seattle: University of Washington; 2017. FOXP2-Related Speech and Language Disorders. [Google Scholar]
- 26.Chenausky KV, Brignell A, Morgan A, Gagne D, Norton A, Tager-Flusberg H, et al. Factor analysis of signs of childhood apraxia of speech. J Commun Disord. 2020:106033. 10.1016/j.jcomdis.2020.106033. [DOI] [PMC free article] [PubMed]
- 27.Morgan AT, Murray E, Liegeois FJ. Interventions for childhood apraxia of speech. Cochrane Database Syst Rev. 2018;5:CD006278. doi: 10.1002/14651858.CD006278.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dereu M, Roeyers H, Raymaekers R, Meirsschaut M, Warreyn P. How useful are screening instruments for toddlers to predict outcome at age 4? General development, language skills, and symptom severity in children with a false positive screen for autism spectrum disorder. Eur Child Adolesc Psychiatry. 2012;21:541–51. doi: 10.1007/s00787-012-0280-y. [DOI] [PubMed] [Google Scholar]
- 29.Fujiki M, Brinton B, Hart CH, Olsen J, Coombs M. Using measurement invariance to study social withdrawal in children with developmental language disorders. Lang, Speech, Hear Serv Sch. 2019;50:253–66. doi: 10.1044/2018_LSHSS-18-0071. [DOI] [PubMed] [Google Scholar]
- 30.Durkin K, Toseeb U, Botting N, Pickles A, Conti-Ramsden G. Social confidence in early adulthood among young people with and without a history of language impairment. J Speech Lang Hear Res. 2017;60:1635–47. doi: 10.1044/2017_JSLHR-L-16-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coccaro N, Tota G, Zagaria A, Anelli L, Specchia G, Albano F. SETBP1 dysregulation in congenital disorders and myeloid neoplasms. Oncotarget. 2017;8:51920–35. doi: 10.18632/oncotarget.17231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minakuchi M, Kakazu N, Gorrin-Rivas MJ, Abe T, Copeland TD, Ueda K, et al. Identification and characterization of SEB, a novel protein that binds to the acute undifferentiated leukemia-associated protein SET. Eur J Biochem. 2001;268:1340–51. doi: 10.1046/j.1432-1327.2001.02000.x. [DOI] [PubMed] [Google Scholar]
- 33.Piazza R, Magistroni V, Redaelli S, Mauri M, Massimino L, Sessa A, et al. SETBP1 induces transcription of a network of development genes by acting as an epigenetic hub. Nat Commun. 2018;9:2192. doi: 10.1038/s41467-018-04462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piazza R, Valletta S, Winkelmann N, Redaelli S, Spinelli R, Pirola A, et al. Recurrent SETBP1 mutations in atypical chronic myeloid leukemia. Nat Genet. 2013;45:18–24. doi: 10.1038/ng.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]