Abstract
Background
The prevalence of diabetes mellitus is on the inexorable rise despite the promises of a wide range of conventional medications. Thus, there is a need to scientifically investigate plants for antidiabetic effect.
Methods
After the Rubus Erlanrige Engl (Rosaceae) leaf has been decocted, the plant extract's antidiabetic activity was first investigated in vitro and then in vivo. The in vitro activity was assessed using 3, 5-Dinitrosalicylic acid, and 2,2-diphenyl-1-picrylhydrazine method for α-amylase inhibition and antioxidant effect respectively. On the other hand, the in vivo antidiabetic activity was carried out in normoglycemic, glucose loaded (2.5 g/kg) and single dose streptozotocin (200 mg/kg) induced diabetic mice.
Results
Acute toxicity study showed the extract is safe with ≥2 g/kg. The in vitro results demonstrated the extract has an IC50 of 7.34 ± 0.02 and 10.38 ± 0.0.62 μg/ml for antioxidant and α-amylase inhibition activity respectively. On the other hand, the in vivo study revealed that the extract significantly reduced blood glucose level following glucose loading. The extract did not, however, produce a significant reduction of glucose level in normal mice indicating low risk of hypoglycemia. The extract also significantly decreased blood glucose levels in streptozotocin-induced diabetic mice. In the single dose study, the extract lowered blood glucose level all except by lower dose at the 3rd and 4th h (p < 0.05). In repeated dose studies, the reduction in fasting blood glucose was significant with all doses of the extract from the 2nd week onwards. In addition, the extract produced less reduction in body weight after diabetic induction.
Conclusion
The findings collectively indicate that the extract has an antidiabetic activity, with low risk of hypoglycemia, probably mediated by various secondary metabolites that act in synergy.
Keywords: Diabetes mellitus, Rubus erlangeri, In vitro, Streptozotocin, In vivo
Abbreviations: BGL, blood glucose level; DM, Diabetes mellitus; DNS, Dinitrosalicylic acid; DPPH, diphenyl-1-picrylhydrazine; FBG, Fasting blood glucose; GAE, Gallic acid equivalent weight; IP, intraperitoneal; TFC, Total flavonoid content; NO, nitric oxide; OGTT, Oral glucose tolerance; STZ, Streptozotocin
1. Background
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion and/or insulin action [1]. The chronic hyperglycemia in diabetes is associated with long-term damage, dysfunction, and failure of different organs, especially the eyes, kidneys, nerves, heart, and blood vessels. Failure of these organs is frequently associated with development of macro- and microvascular diseases [2]. Now a days, DM is becoming a disease of major concern both globally and regionally and is a leading cause of death in most countries [3]. It is one of the four major non-communicable diseases comprising, cardiovascular diseases, cancers and chronic respiratory diseases, which jointly contributes to 63% of non-communicable deaths worldwide [4].
The autoimmune destruction of β cells, which is a key factor for insulin deficiency leading to hyperglycemia, could result from genetic, environmental and immunologic factors [5]. Moreover, DM is caused by other numerous mechanisms, including increased oxidative stress, postprandial hyperglycemia, glucose autoxidation and non-enzymatic protein glycation. Oxidative stress plays important role in the impairment of insulin action and exacerbation of DM complications. Antioxidants including ascorbic acid have shown to have a prospect in the treatment of DM [6]. Postprandial hyperglycemia which invigorates insulin resistance is associated with increased polysaccharide breakdown. α-amylase enhances this phenomenon through converting oligosaccharides and disaccharides to monosaccharides. Therefore, lowering the digestion and breakdown of starch may have beneficial effects on insulin resistance and glycemic index control in people with diabetes [7].
Despite the availability of many convetional prescription medications, numerous side effects, which are intolerable for many patients is on the rise. In recent years, the search for alternative therapeutic agents in the treatment of diabetes has been the focus of scientific research, as medicinal plants with diverse actions have been used traditionally for the control, management and/or treatment of DM in many parts of the world [8].
Rubus erlangeri Engl (Rosaceae) is an endemic plant to Ethiopia and belongs to the genus of Rubus [9]. The family is mostly found in temperate regions and comprises more than 100 genera and about 3000 species [10]. In traditional medicine, many species of this genus are used for the treatment of DM. For instance, R. caesius in Iran [11]; R. sanctus in Turkey [12]; R. ulmifolius in Moroco [13]; leaves of R. fruticosu in India [14] and infusion of leaves of R. apetalus poir in Tanzania [15] are widely used for this purpose. In Ethiopia, the leaves of R. steudneri and R. apetalus are used for DM as decoction [16]. The antidiabetic effects of some of the plants in this species have also been evaluated experimentally and showed a promise in reducing blood glucose level (BGL) [[17], [18], [19], [20]]. Little or no information is, however, available regarding the use of R. erlangeri for DM in the literature, many of the species from this genus have been claimed or shown to have antidiabetic property. From chemotaxonomic knowledge and molecular phylogenetic data, if some species from genera or families known to have an effect such as the above mentioned against certain disease conditions, the others too are believed to be associated with a certain bioactivity or therapeutic potential. The reason behind this is, plants within the same phylogenetic groups may share similar genetics, thus possess similar chemical-producing potentials, which in turn exhibit similar therapeutic effects. Thus selection of species from these hot taxa would lead to higher success rates in drug discovery [21,22]. The experimental plant, Rubus erlangeri, was selected based on this notion. Therefore the present study attempted to evaluate antidiabetic activity of the leaves of R. erlangeri first in vitro and then in vivo.
2. Materials and methods
2.1. Drug chemicals and materials
STZ (Streptozotocin) and 3,5-Dinitrosalicylic acid (DNSA) (Sisco Research Laboratories Pvt. Ltd, India), α-amylase (Blulux Laboratories Pvt. Ltd., Faridaban, India), Acarbose (Bayer, Germany), Aluminum chloride, sodium carbonate, sodium nitrate, starch, sodium chloride, sodium hydroxide, potassium sodium tartrate tetrahydrate, disodium hydrogen phosphate and sodium dihydrogen phosphate (BDH Laboratory Supplies Ltd, England), 2,2-diphenyl-1-picrylhydrazine (DPPH), Galic acid, qurcetin and Folin-Ciocalteu's reagent (Sigm Chemicals Co, St. Louis, MO, USA), glibenclamide (Sanof–Aventis, USA) and glucose (Munchen, Germany).
2.2. Plant material
The leaves of plant were collected in March 2019 from Tocha Wereda, Dawro Zone, Southern Nations, Nationalities, and peoples Region, which is about 500 km southwest of Addis Ababa, Ethiopia. Identification and authentication of the plant specimen was performed by a taxonomist Ato Melaku Wondafrash and a voucher specimen was deposited (voucher number of AG001) at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference.
2.3. Experimental Animals
Healthy Swiss albino mice (body weight, 25–35 g; age 8–10 weeks old) were used for the experiments. Female mice were used for acute oral toxicity and male mice for STZ induced diabetic model. In addition, randomly selected mice with either sex were used for normoglycemic and oral glucose tolerance test (OGTT). All mice were obtained from the animal house of School of Pharmacy, College of Health Sciences, Addis Ababa University. Mice were maintained under standard conditions and fed on standard pellet diet and water ad libitum. They were acclimatized to the environment for one week before commencement of the experiment. Animal handling procedure and killing methods were done according to international accepted guidelines [23,24]. Moreover, each procedure was approved by IRB of the School of Pharmacy.
2.4. Preparation of plant extract
The leaves of R. erlangeri were dried under shade and then pulverized using a mortar and pestle to get a coarse powder. Decoction method was used for extraction. A portion of R. erlangeri powder (150 g) was boiled with 1.2 L of Distilled Water [25] for 15 min [26,27] and was allowed to cool. After cooling, it was filtered through muslin cloth followed by Whatman grade No 1 filter paper (Schleicher and Schuell Microscience Gmbh, Germany). The filtrate was then frozen in a deep freezer and dried in a lyophilizer (Operon, Korea). Finally, The extract was then packed in a bottle and stored in a desiccator until use.
2.5. Preliminary phytochemical screening
Screening of secondary metabolites such as saponins, polyphenols, flavonoids, alkaloids, tannins [28], steroids, glycosides, and terpenoids was done following the standard procedures with some modifications [29].
2.6. Determination of total phenol content
The total phenolic content of the R. erlangeri leaf extract was determined using Folin-Ciocalteu reagent [30]. One ml of aliquots and standard gallic acid (6.25, 12.5, 25, 50, 100 μg/ml) was placed into the test tubes, and shortly after adding 5 ml of distilled water and 0.5 ml of Folin Ciocalteu's reagent the mixture was shaken. Five minutes later, 1.5 ml of 20% sodium carbonate was added and volume made up to 10 ml with distilled water following this it was allowed to incubate for 2 h at room temperature. The intense blue color was developed. After incubation, absorbance was measured at 750 nm (Jenway, model 6500). The extract (sample) was initially prepared with a concentration of 1mg/1 ml in distilled water. Then, the same procedure was followed similar to the standard gallic acid mixture preparation to make a volume of 10 ml. The blank was performed using reagent blank with solvent. The calibration curve was plotted using standard gallic acid. The data for total phenolic contents of the extract expressed as mg of gallic acid equivalent weight (GAE)/g of dry mass. All the analyses were repeated three times and the mean value of absorbance was obtained.
2.7. Determination of total flavonoids
The aluminium chloride colorimetric assay method was used for the determination of the total flavonoid content of the sample [31]. One ml of the standard quercetin solution (0.065, 0.125, 0.25, 0.5, and 1 mg/ml) was placed into test tubes and 0.3 ml of 5% sodium nitrite solution was added into each. After 5 min, 0.3 ml of 10% aluminum chloride was added. At 6th minute, 2 ml of 1 M sodium hydroxide was added. Finally, volume was made up to 10 ml with distilled water and shaken well. The absorbance of each quercetin solution was determined at 510 nm (Jenway, model 6500) after 30 min of incubation. Similarly, 1 ml having a concentration of 1mg/1 ml of plant extracts was mixed instead of 1 ml gallic acid with the same reagents as described above in three different test tubes. The absorbance was measured against a reagent blank, which was composed of the same reagents except for test extract or the standard quercetin.
The total flavonoid content (TFC) in the extract was calculated using Eq. (1). The result was expressed as mg quercetin equivalents per g of dried weight sample (mg QE/g d.w.). All the analyses were repeated three times and the mean value of absorbance was obtained.
| (1) |
Where C is the sample concentration from the calibration curve (mg/ml), V is the volume (ml) of the solvent used to dilute the extract, and M represents the weight (g) of the dried extract used.
2.8. In-vitro antidiabetic activity
2.8.1. Determinations of α-amylase inhibition activity
α-amylase inhibition assay was performed using the 3,5-Dinitrosalicylic acid (DNSA) (Sisco Research Laboratories Pvt. Ltd. Mumbai, India) method with a slight modification [32]. Accordingly, different concentrations (10–500 μg/ml) of both extract and acarbose (Bayer, Germany) were prepared. Following this, a volume of 200 μl of α-amylase solution (2 units/ml) was mixed with 200 μl of each concentration of the extract and acarbose and incubated for 10 min at 30 °C. Thereafter, 200 μl of starch solution (1% in water (w/v)) was added to each test tube. The reaction was terminated after 3 min by adding 200 μl of DNSA (DNSA reagent was made by mixing 12 g of sodium potassium tartrate tetrahydrate in 8.0 mL of 2 M NaOH with 96 mM of DNSA dissolved in 20 mL of DW). Test tubes containing all the above added solution were then boiled for 10 min in a water bath at 85 °C. The mixture, which has orange color was then cooled to ambient temperature and diluted with 5 ml of distilled water (DW), and the absorbance was measured at 540 nm using a UV–Visible spectrophotometer (Jenway, model 6500). The blank with 100% enzyme activity was prepared by replacing the plant extract/acarbose solution with 200 μl of buffer. In addition, buffer without enzyme solution, and each concentrations of inhibitor (extract or acarbose) without enzyme was prepared as the same procedure mentioned above. The α-amylase inhibitory activity is expressed as percent inhibition and was calculated using the equation given below. Finally, the % α-amylase inhibition was plotted against the extract and acarbose concentration and the IC50 value was obtained from the graph for both extract and standard drug [33]. Measurement was carried out three times and the average IC50 was taken.
Inhibition (%) = x100,where Ac refers to the absorbance of control (enzyme and buffer); Acb refers to the absorbance of control blank (buffer without enzyme); As refers to the absorbance of sample (enzyme and inhibitor); and Asb is the absorbance of sample blank (inhibitor without enzyme).
2.8.2. Determinations of antioxidant activity
Free radical scavenging activity of R. erlangeri was examined using 2,2-diphenyl-1-picrylhydrazine (DPPH) (Sigma Chemicals Co, St. Louis, MO, USA) assay [34]. Both the plant extract and the standard, ascorbic acid (Vitamin C), were dissolved in methanol and different concentrations (1000, 500, 250, 125 and 62.5 μg/ml) were prepared. DPPH (5 ml of 0.004% methanol solution) was mixed with 50 μl of each extract and ascorbic acid solution. The mixture which has purple to yellow color was then incubated at a temperature of 37 °C for 30 min in hot oven. The blank solution was prepared by replacing 5 ml of DPPH with 50 μl methanol in place of extract or positive standard. Finally, the absorbance of each sample was measured at 517 nm using UV-spectrophotometer (Jenway, model 6500). For each mixture, absorbance was measured trice and the average value was taken. Percent inhibition was calculated using the equation below and the IC50 value of each sample, was obtained from dose vs. inhibition curve.
X100. Where, Ao is absorbance of the negative control (0.004% methanol solution of DPPH without test sample) and As is the absorbance of the solution in the presence of sample extract or ascorbic acid.
2.9. Acute toxicity study
Acute toxicity for R. elangeri aqueous leaf extract was performed according to Organization for Economic Co-operation and Development guideline 425 [35]. For the study, five female mice of 6–8 weeks old were used. All mice were fasted for 4 h before and 2 h after administration of the extract. Test was first performed with a dose of 2000 mg/kg on a single mouse and this was followed by repeating the test on the remaining 4 mice as per the guideline.
2.10. In-vivo antidiabetic activity
2.10.1. Grouping and dosing of animals
In both normoglycemic and OGTT, animals of either sex were randomly divided into five groups (negative control, positive control and three test groups), each comprising of 6 animals per group. Group I (negative control, NOC) and Group II (positive control, GL5). Group III, IV and V were treated with 100 mg/kg (RELE100), 200 mg/kg (RELE200) and 400 mg/kg (RELE400) doses of R. erlangeri leaf extract, respectively. For STZ induced diabetic model, after inductions of diabetes, mice were divided into 6 Groups (6 mice per group), one group was normal control without STZ injection (Group I, NOC). The others were: Group II (negative control, NC, dosed with DW), Group III (positive control, GL5). Group IV (RELE100), V (RELE200), and VI (RELE400). In all three models, the negative and normal controls received DW, whereas the positive control group was treated with 5 mg/kg glibenclamide (GL5) (Sanofi-Aventis, USA). All administrations were performed through oral route with a maximum volume of 10 mL/kg.
2.10.2. Measurement of blood glucose level
Blood samples were withdrawn from the tail vein of mouse by cutting the tip of the tail using scissors in all animal models. To prevent infection at the tip of the tail, 70% ethanol using cotton was applied during each measurement. BGL was measured using glucometer and glucose standard strip/kits (Smart lab,Gmbh, Germany). Measurement was carried out three times and the average value was taken. For STZ model, particularly in studying the repeated dose effect BGL from animals without food for 8 h was considered as FBG level [36]. During fasting time in all in vivo models, animals were placed in a bare cage to prevent feeding of sawdust for specified fasting time.
2.10.3. Induction of diabetes
Diabetes was induced using single high dose of STZ. The dose of this agent required for inducing diabetes depends on the animal species, route of administration and nutritional status [37]. Therefore, to determine at what dose STZ would induce DM in this experiment, a pilot study was carried out at three dose levels (150 mg/kg, 180 mg/kg and 200 mg/kg). Accordingly, 200 mg/kg was selected as it produced better results. Prior to STZ injection, mice were fasted for 6 h. STZ was first dissolved in freshly prepared 0.1 M citrate buffer and the pH was adjusted to 4.5. It was then given to each mouse immediately. They were then provided with food and 5% glucose in place of water for the next 24 h to prevent death associated with hypoglycemia. After 72 h, FBG was assayed and animals with FBG ≥200 mg/dl were considered diabetic [38,39].
2.10.4. Assessment of hypoglycemic activity in normal mice
Hypoglycemic effect of the extract was assessed on normoglycemic mice. Mice were fasted for 6 h with free access to water and randomly divided into 5 groups as described in grouping and dosing section. Blood samples were collected from animal tail veins at 0, 1, 2, 3 and 4 h after treatment [40] for BGL determination using a one touch glucometer and glucose standard strip/kits (Smart lab,Gmbh, Germany).
2.10.5. Oral glucose tolerance test
An antihyperglycemic effect of extract was done on overnight fasted mice (14 h). After fasting, mice were randomly divided into 5 groups (6 mice per group). Baseline BGL was measured (just immediately before giving each agent based on their grouping). Thereafter DW, extract and standard drug were administered. Thirty mins post administrations of each agent; animals were loaded with 2.5 g/kg of glucose solution orally. Blood glucose levels were then measured after 30, 60 and 120 min [41].
2.10.6. Streptozotocin induced diabetic mice
2.10.6.1. Single dose study
Effect of single dose of aqueous extract was carried out on STZ induced diabetic mice. After fasting for 14 h, blood sample was collected at 0 h (just before treatment), 1, 2, 3, and 4 h after treatment as per the respective grouping [40].
2.10.6.2. Repeated dose studies
Anti-hyperglycemic activity of repeated dose of the extract was carried out in STZ induced diabetic mice. Based on their grouping, diabetic mice were given DW, standard drug or extract for 3 weeks. The non-diabetic group (NOC) was also administered DW for the same time. The blood glucose lowering effects of extract was then determined by measuring FBG every seven days for three weeks. BGL of diabetic mice were measured just before starting treatment on the 1st day of treatment (3 days after STZ injection) as baseline, and then on the first, second and third week following fasting for 8 h [42].
2.10.6.3. Body weight determination
Body weight reduction effects of STZ and improvement in body weight change by extract and standard were determined. The body weight of all the treated and control group of mice were recorded before treatment (on day 0) and during treatment period i.e. at the end of first, second and third week using an electronic balance.
2.11. Statistical analysis
All statistical analyses were performed using international business machine of statistical package for the social Sciences, (IBM SPSS), version 25 for windows (SPSS inc, Chicago, Illinois, USA). For in vitro Studies, independent sample t-test was employed. And, for in vivo antidiabetic effect, statistical differences between groups was analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test. A two-way ANOVA followed by Tuckey's tests was also used. Results were expressed as mean ± standard error mean (SEM) and P-values less than 0.05 were considered as statistically significant.
3. Results
3.1. Percentage yield
From 150 g of dried leaves, 14 g of brown coarse powder with a percentage yield of 9.3% was obtained.
3.2. Acute toxicity study
Physical and behavioral observations of the experimental mice revealed that there is no visible sign of toxicity with a dose of 2 g/kg. This indicates that Median Lethal Dose (LD50) of the extract is greater than 2 g/kg.
3.3. Preliminary phytochemical screening
The preliminary phytochemical screening of aqueous leaf extract of R. erlangeri revealed the presence of saponins, polyphenols, flavonoids, alkaloids, tannins, and glycosides.
3.4. Determination of total phenol content
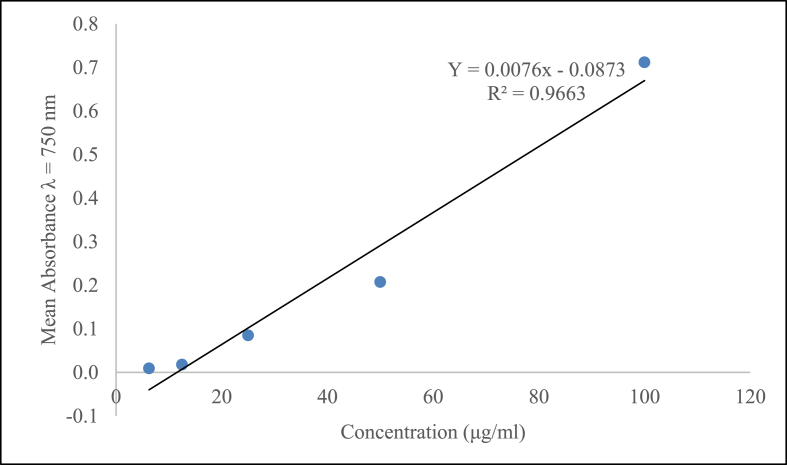
The total phenolic content was derived from the calibration curve (Y = 0.0076x - 0.0873, R2 = 0.9663) of quercetin (6.25–100 μg/ml) and expressed in mg gallic acid equivalence (mg GAE/g) per g dry extract weight (Fig. 1). From the calibration curve the total phenolic content of R. erlaneri aqueous leaf extract was found to be 693 mg of GAE/g of extract.
Fig. 1.
Total phenolic content for standard gallic acid, R2 values represented mean data set of n = 3.
3.5. Determination of total flavonoids
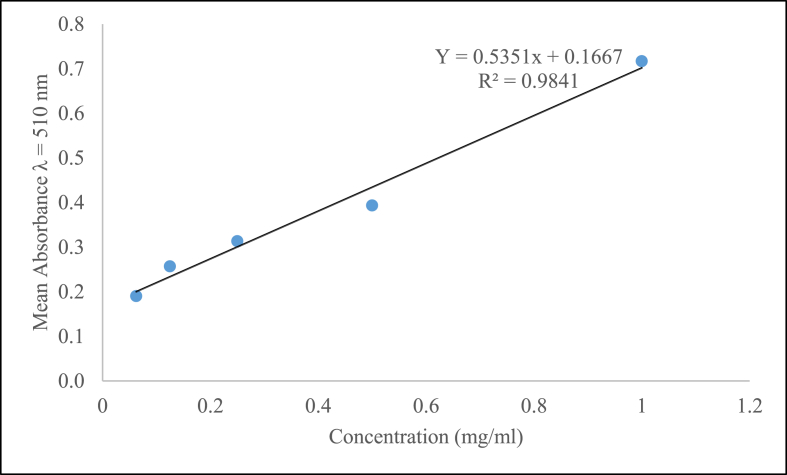
The content of total flavonoids in R. erlangeri aqueous leaf extract was determined from the regression equation of the calibration curve (y = 0.5351x+ 0.1667, R2 = 0.9841) and expressed as mg of quercetin equivalents (QE) (Fig. 2). In this study, the total flavonoid content of the extract was found to be 77 mg QE/g.
Fig. 2.
Total flavonoid content for standard quercetin, R2 values represented mean data set of n = 3.
3.6. In vitro antidiabetic effect
3.6.1. α-amylase inhibition activity
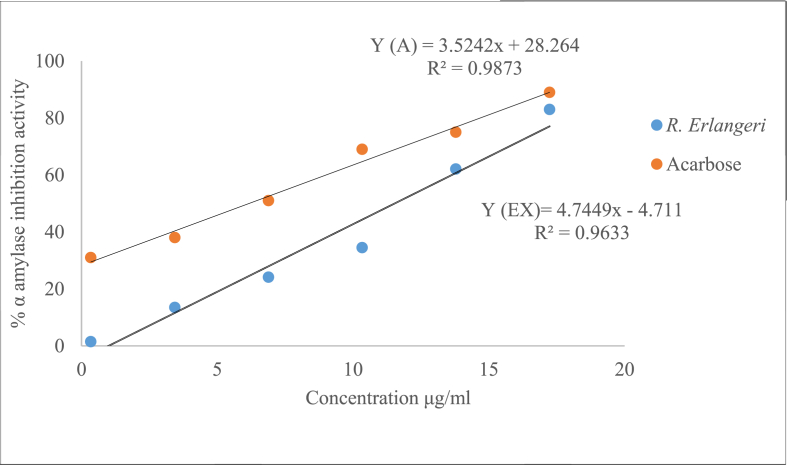
The test showed that the extract has an appreciable α-amylase inhibitory effects when compared with acarbose. Fig. 3 depicts how IC50 was derived from the standard curve just by taking the values of first measurment. The formula is expressed as a function of Y(AC) for acarbose and Y(EX) for extract. The average IC50 value for the triplicate measurements was 7.51 ± 0.96 for acarbose and 10.38 ± 0.62 μg/ml for aqueous leaf extract of R. erlangeri. Comparing the IC50 values obtained from each triplicate measurement, there was no apparent difference between acarbose and the extract (Fig. 3).
Fig. 3.
Percentage α-amylase inhibitory effects of the aqueous leaf extract of Rubus elangeri. Data was expressed as IC50 ± S.E.M for each IC50. Analysis was performed by independent sample t-test for (n = 3).
3.6.2. Antioxidant activity
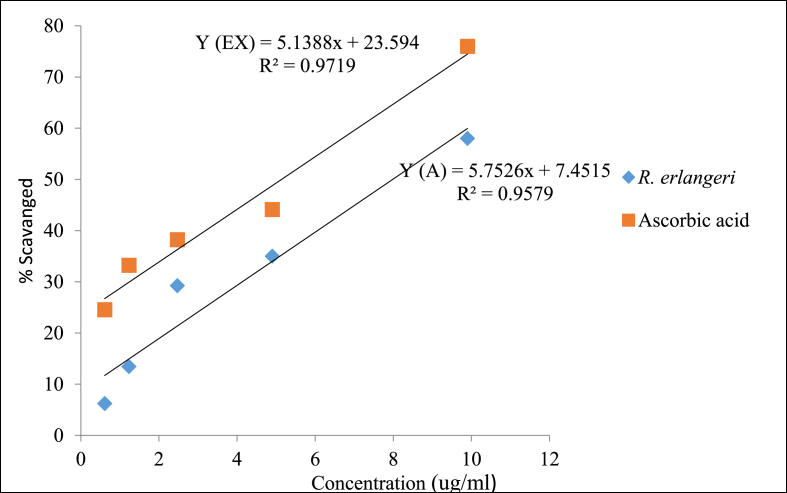
The IC50 values of ascorbic acid and aqueous leaf extract were obtained from the equations represented by a function of Y(A) and Y(EX) for ascorbic acid and extract, respectively (Fig. 4). Once again, Fig. 4 shows how IC50 values were derived jut by taking the first measurement. The IC50 value for ascorbic acid and extract were found to be 5.91 ± 0.39 μg/ml and 7.34 ± 0.02 μg/ml respectively. Comparison between the two values did not produce a significant difference.
Fig. 4.
Radical scavenging activity of the aqueous leaf extract of Rubus erlangeri; Data was expressed as IC50 ± S.E.M for each; Analysis was performed by independent sample t-test for (n = 3).
3.7. Acute toxicity study
The acute toxicity test result of this study revealed that the aqueous leaf extract of R. erlangeri was safe by oral route at a dose of 2000 mg/kg. After 24 h, animals were found to tolerate the administered dose and there was no sign of toxicities displayed in any of the mice within two weeks.
3.8. In-vivo antidiabetic activity
3.8.1. Hypoglycemic effects of the extract
The FBG level was not significantly different among groups before giving each agent (t = 0) (Table 1). GL5 produced a significant BGL reduction at the 3rd and 4th h (p < 0.01) compared to the negative control. By contrast, the extract did not cause a decline in BGL at the doses used in all time points compared to controls. There was no apparent difference in BGL neither between the standard and extract nor amongst the different doses of the extract (Table 1).
Table 1.
Effect of aqueous leaf extract of Rubus erlangeri on blood glucose level of normoglycemic mice.
| Groups | Blood glucose Level (mg/dl) |
||||
|---|---|---|---|---|---|
| 0 min | 1 h | 2 h | 3 h | 4 h | |
| NOC | 99.4 ± 2.74 | 85.40 ± 5.28 | 89.20 ± 7.91 | 79.20 ± 6.11 | 76.8 ± 5.24 |
| GL5 | 101.00 ± 11.58 | 72.00 ± 8.67 | 65.00 ± 8.05 | 55.20 ± 2.20 Ab | 50.60 ± 0.87 Ab |
| RELE100 | 96.20 ± 3.35 | 83.60 ± 4.06 | 73.60 ± 2.11 | 66.00 ± 4.33 | 63.40 ± 4.87 |
| RELE200 | 96.60 ± 5.04 | 83.20 ± 3.27 | 74.80 ± 4.58 | 65.60 ± 2.20 | 62.73 ± 1.86 |
| RELE400 | 98.40 ± 2.95 | 78.40 ± 6.37 | 76.60 ± 5.73 | 67.80 ± 5.72 | 63.00 ± 2.34 |
Each value represents mean ± S.E.M; n = 6; Analysis was performed by one way ANOVA; Acompared to negative control; number followed by RELE and GL indicates dose in mg/kg; bp < 0.01.
Abbreviations: NC, negative control; GL, glibenclamide; RELE, Rubus erlangeri leaf extract.
3.8.2. Antihyperglycemic effect of extract in oral glucose tolerance test
Glucose loading produced hyperglycemia after 30 min of administration in all groups, with a maximum increase achieved with the negative control (164%) (Table 2). Unlike the standard, which significantly reduced BGL (p < 0.05), no appreciable effect was observed with the extract at any of the doses after 30 min of glucose loading. Effect of the extract started to be apparent as time went by and significant decline was noted at 60 min by all doses, which was maintained by the middle (REL200) and higher (REL400) doses until the end of the experiment (Table 2).
Table 2.
The Effect of aqueous leaf extract of Rubus erlangeri on oral glucose tolerance test.
| Groups | Blood glucose Level (mg/dl) |
% BGL increment |
|||
|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 120 min | at 30 min | |
| NC | 94.40 ± 3.93 | 249.80 ± 34.63 | 209.45 ± 34.95 | 123.20 ± 15.72 | 164.60 |
| GL5 | 96.20 ± 6.38 | 110.60 ± 18.85 Aa | 72.40 ± 9.24 Ac | 56.80 ± 3.02 Ac | 15.00 |
| RELE100 | 90.40 ± 8.31 | 166.8 ± 12.35 | 119.80 ± 8.57Aa | 89.80 ± 5.03 | 84.50 |
| RELE200 | 84.80 ± 6.41 | 137.40 ± 9.76 | 103.80 ± 3.69 Ab | 70.20 ± 3.07 Ab | 62.02 |
| RELE400 | 90.00 ± 2.30 | 170.00 ± 12.41 | 109 ± 10.39a2 | 81.00 ± 9.76 Ab | 88.80 |
Note: Each value represents mean ± S.E.M; n = 6; Analysis was performed by one way ANOVA; Acompared to negative control; number followed by RELE and GL indicates dose in mg/kg; ap < 0.05; bp < 0.01; cp < 0.001. Time refers after glucose loading.
Abbreviations: NC, negative control; GL, glibenclamide; RELE, Rubus erlangeri leaf extract.
3.8.3. Antihyperglycemic effect of the extract in streptozotocin-induced diabetic mice
Of the 120 mice used for diabetic induction (60 mice for each single and repeated dose studies), 76 mice were found to be diabetic, producing a success rate of 63.3%. Among the diabetic mice, 10 were excluded from the experiment, since their FBG level was above the detection limits of the glucometer. Although no death was observed at the time of induction, four mice died at the end of the first week (2 from negative control, 1 from RELE200, and 1 from RELE400). However, this was compensated by inducing other mice as per protocol.
3.8.3.1. Single dose studies
After DM was induced, there was no significant difference in the initial BGL among the diabetic groups (Table 3). NC mice had significantly higher (p < 0.001) FBG levels with mean value > 200 mg/dl at all-time points compared to NOC mice. Group analysis revealed that once again GL5 had a rapid onset of action, as it produced a significant decline (p < 0.05) in the 2nd h, which continued till the end of the experiment. RELE200 and RELE400 reduced FBG level significantly (p < 0.05) at the 3rd and 4th h, but this effect was not shared by RELE100. Although there was no detectable difference between the extract and standard at all timepoints, RELE400 and GL5 were the only regimen that brought values to NOC levels at the 4th h (Table 3).
Table 3.
Antihyperglycemic effects of single dose aqueous leaf extract in streptozotocin-induced diabetic mice.
| Groups | FBG level (mg/dl) |
||||
|---|---|---|---|---|---|
| 0 h | 1st h | 2nd h | 3rd h | 4th h | |
| NC | 286.00 ± 12.9a3 | 284.00 ± 13.60Ac | 277.80 ± 13.88 Ac | 274.60 ± 13.80Ac | 269.00 ± 14.20Ac |
| NOC | 91.6 ± 1.16 | 90.00 ± 0.94 | 87.80 ± 1.31 | 84.80 ± 0.66 | 83.44 ± 0.87 |
| GL5 | 280.60 ± 18.10Ac | 239.20 ± 12.80Ac | 186.60 ± 13.55 AaBa | 175.00 ± 11.26 AaBb | 133.00 ± 8.56 Bc |
| RELE100 | 284.00 ± 21.38Ac | 266.00 ± 20.96 Ac | 259.20 ± 18.02 Ac | 230.00 ± 21.05 Ac | 202.60 ± 29.80 Ab |
| RELE200 | 298.80 ± 24.92Ac | 258.60 ± 26.70 Ac |
239.20 ± 36.87 Ac | 190.80 ± 27.93 AbBa |
186.40 ± 26.50 AbBa |
| RELE400 | 297.80 ± 13.70a3 | 252.00 ± 17.42 Ac | 200 ± 13.50Ab | 173.20 ± 12.99AaBa | 154.20 ± 12.06Ba |
Each value represents mean ± S.E.M; n = 6; Analysis was performed by one way ANOVA; Acompared to normal control; Bcompared to negative control; number followed by RELE and GL indicates dose in mg/kg; ap < 0.05; bp < 0.01; cp < 0.001.
Abbreviations: NOC, normal control; NC, negative control; GL, glibenclamide; RELE, Rubus erlangeri leaf extract.
3.8.3.2. Repeated dose studies
Compared to NOC, NC group had significantly higher (p < 0.001) FBG level with mean value > 200 mg/dl at all-time points. Neither the extract nor the standard produced a significant reduction in BGL in the first week. RELE100 (P < 0.01) and the two other regimens (p < 0.001) in the 2nd week and all doses in the third week (p < 0.001) however, produced a significant (Table 4). Though RELE100 was able to reduce BGL compared to NC group, it did not bring down BGL levels to NOC values unlike the other groups.
Table 4.
Antihyperglycemic activity of aqueous leaf extract of Rubus erlangeri on weekly fasting blood glucose level in streptozotocin-induced diabetic mice.
| Groups | Weekly FBG level (mg/dl) |
|||
|---|---|---|---|---|
| Initial | 1st | 2nd | 3rd | |
| NOC | 96.40 ± 0.67 | 97.80 ± 0.97 | 97.60 ± 0.51 | 96.80 ± 0.86 |
| NC | 357.00 ± 23.01 Ac | 374.00 ± 27.72 Ac | 393.60 ± 29.61 Ac | 394.80 ± 26.19 Ac |
| GL5 | 323.60 ± 45.47 Ac | 223.00 ± 42.09 Aa | 158.20 ± 28.31 Bc | 86.40 ± 5.99 Bc |
| RELE100 | 320.40 ± 25.80Ac | 312.60 ± 54.41A1 | 232.40 ± 40.26 AaBb | 205.20 ± 31.47 AcBc |
| RELE200 | 307.60 ± 33.26Ac | 288.00 ± 35.25Aa | 197.00 ± 21.86 Bc | 188.00 ± 20.40 Bc |
| RELE400 | 315.00 ± 35.12Ac | 256.60 ± 29.49 Aa | 197.20 ± 22.69 Bc | 179.60 ± 15.14Bc |
Each value represents mean ± S.E.M; n = 6; Analysis was performed by one way ANOVA; Acompared to normal control; Bcompared to negative control; number followed by RELE and GL indicates dose in mg/kg; ap < 0.05; bp < 0.01; cp < 0.001.
Abbreviations: NOC, normal control; NC, negative control; GL, glibenclamide; RELE, Rubus erlangeri leaf extract.
3.8.4. Effect of aqueous leaf extract on body weight change
Body weight reduction was noted with the NC in the 2nd (p < 0.001) and 3rd (p < 0.001) week compared to the NOC group. Treatment with the extract as well as the standard attenuated body weight reduction at the 3rd week compared to the NC group (Table 5).
Table 5.
Effect of the aqueous leaf extract of Rubus erlangeri on weekly body weight change of diabetic mice.
|
Groups |
Body Weight in g |
|||
|---|---|---|---|---|
| Initial | 1st Week | 2nd Week | 3rd Week | |
| NOC | 30.00 ± 1.41 | 31.10 ± 1.46 | 32.00 ± 1.21 | 32.87 ± 1.12 |
| NC | 29.20 ± 1.46 | 26.10 ± 1.63 | 22.82 ± 1.08Ac | 19.6 ± 1.28 Ac |
| GL5 | 29.70 ± 1.47 | 28.60 ± 1.48 | 28.10 ± 1.50 | 28.00 ± 1.58Bc |
| RELE100 | 29.18 ± 1.58 | 27.40 ± 1.28 | 26.52 ± 1.29Aa | 26.04 ± 1.42 BaAa |
| RELE200 | 29.7 ± 0.99 | 28.32 ± 0.98 | 27.28 ± 1.31 | 27.2 ± 1.27 BaAa |
| RELE400 | 27.80 ± 0.86 | 26.40 ± 0.83 | 26.00 ± 0.83Aa | 25.9 ± 0.71 BaAa |
Note: Each value represents mean ± S.E.M; n = 6; Analysis was performed by one way ANOVA; acompared to normal control; bcompared to negative control; number followed by RELE and GL indicates dose in mg/kg; ap < 0.05; bp < 0.001.
Abbreviations: NOC, normal control; NC, negative control; GL, glibenclamide; RELE, Rubus erlangeri leaf extract.
4. Discussion
In the present study, before proceeding to in vivo experiment, the antidiabetic effect of the leaves of R. erlangeri was first evaluated in vitro by determining α-amylase inhibition activity. This enzyme is found in the brush borders of the intestine, whose function is hydrolyzing complex polysaccharides with the help of α-glycosidase. The later enzyme converts oligosaccharides and disaccharides to monosaccharides and increases postprandial glucose levels. Thus, one therapeutic approach in DM treatment is the prevention of carbohydrate absorption after food intake by inhibition of these enzymes. Moreover, lowering the digestion and breakdown of starch may have beneficial effects on insulin resistance and glycemic index control in people with diabetes [32]. The result from α amylase inhibitory effect suggested that the plant is endowed with antidiabetic activity since there was no significant difference between IC50 values of the standard drug and extract (7.51 ± 0.96 for vs 10.38 ± 0.62 μg/ml). Rubus species are potential sources of cyanidin-3-rutinoside, which is an anthocyanin polyphenolic compound [43]. Combinations of cyanidin-3-rutinoside have been shown to reduce the postprandial blood glucose after maltose and sucrose loading in rats [44]. Therefore, the activity of this plant extract against α-amylase might be due to the content of this phenolic compound. In addition, this in vitro result demonstrated that one of the mechanisms for the antidiabetic activity of the extract might be through delaying carbohydrate digestion and reducing the postprandial plasma glucose rise.
It is believed that oxidative stress plays important role in the impairment of insulin action and exacerbation of DM complications. Antioxidants including ascorbic acid have been shown to have a prospect in the treatment of diabetes [45]. Hence, the use of antioxidants along with antidiabetic drugs is frequently recommended to avoid such problems [46]. The extract is shown to have a good antioxidant profile, which is comparable with vitamin C. Once again, no significant difference was observed between the IC50 values of Vitamin C and the extract (5.91 ± 0.39 vs. 7.34 ± 0.02 μg/ml), suggesting that the extract is endowed with radical scavenging activity, a beneficial effect in treating DM. The experimental plant might also exert antidiabetic activity through the enhancement of endogenous free radical scavenging enzymes. There is evidence that the administration of leaf extracts of R. apetalus and R. steudneri, which are both in the same genus as the experimental plant, increased levels of endogenous free radical scavengers such as superoxide dismutase, glutathione peroxidase, total thiols, and catalase in diabetic mice [47].
The above findings called for the need to do the antidiabetic activity in in vivo model. Therefore, the effect of the extract on BGL in normal mice for hypoglycemic and in oral glucose loaded normal mice, as well as STZ induced diabetic mice for antihyperglycemic activity, was initiated. The normoglycemic studies revealed that the aqueous extract of R. erlangeri had no hypoglycemic activity compared to normal control, suggesting a lower risk of hypoglycemia. The slight reduction in BGL in negative control is attributed to extended fasting time in between measurements.
A drug that is effective in diabetes will have the ability to control the rise in glucose level by different mechanisms and the ability of the extracts to prevent hyperglycemia could be determined by glucose loaded hyperglycemic mode [48]. For the antihyperglycemic activity, in oral glucose tolerance test, mice first fasted for 14 h before glucose loading. This method is referred to as the physiological induction of DM because the blood glucose level of the animal is transiently increased with no damage to the pancreas [48]. Fasting is required before glucose administration to provide stable baseline glucose measurements and to eliminate fluctuations in BGL by food intake [49]. It also stimulates glucose-induced insulin sensitivity [50]. Since there is a discrepancy among researches regarding sex preference to the sensitivity of glucose-induced insulin secretion, either sex was used for OGTT as well as for hypoglycemic test in normal mice [51,52]. The effect of extract in lowering BGL following OGTT might point that it is capable of stimulating insulin release. The secreted insulin would then stimulate peripheral glucose consumption and controls the production of glucose through different mechanisms. In animal models of OGTT, secreted insulin requires ≥ 2 h to bring back the glucose level to normal [52]. In this study, whilst >2 h was required to bring back BGL to normal level in NOC, the extract and GL5 brought down BGL to baseline within or less than 2 h. GL5 and all doses of the aqueous extract started to decrease BGL with an onset of around 1 h and 90 min, respectively. And, the effect lasted for ≥ 1 h for GL5, middle and high dose of the extract, and 30 min for RELE100. Since the extract lowered BGL following glucose loading and glucose induces insulin release, the effect behind this antihyperglycemic activity of the extract might involve an insulin-like action, probably, through peripheral glucose consumption or enhancing the sensitivity of β-cells to glucose. From the results of in vitro α-amylase inhibition activity and in vivo OGTT, antidiabetic activity of the extract might be attributed to inhibition of glucose absorption.
STZ administration causes partial destruction of the β-cell population, leading to a transient increase in blood glucose levels. The selective pancreatic β-cell toxicity and diabetic condition result from the structural similarity between STZ and glucose, allowing GLUT-2 mediate transport [53]. The toxic effector mechanism of STZ starts with its decomposed products and free radicals generated, which damage the pancreatic β-cells by alkylating DNA, impairing mitochondrial activity by inhibiting O-GlcNAcase [54], NAD + depletion through methylation of DNA, and self-destruction by NO [55]. Single-dose studies showed that the middle and larger doses of the extract are as effective as the standard, although the onset is a bit delayed. The lower dose was devoid of any effect, suggesting that adequate concentration was not reaching to the site of action, as this deficiency was overcome with the repeated dose studies.
Studies show that a single IP injection of a high dose of STZ can produce sustained hyperglycemia in mice at least for 8 weeks [56]. Similarly, STZ induced persistent hyperglycemia in this study with no significant change in BGL during the study period of three weeks as observed in NC. On the contrary, daily oral administrations of R. erlangeri leaf extract reduced FBG. Particularly, the middle and high doses of the extract lowered BGL below 200 mg/dl at the second and third week and there was no significant difference compared to NOC at these time points. This suggests that repeated administration of the extract up to 2–3 weeks could reverse BGL to a normal level. A single dose IP administration of STZ at a dose of 200 mg/kg did not cause complete destruction of β-cells. Studies demonstrated that an increase in β-cell density per area of islet in diabetic rats, suggesting initiation of a compensatory response to STZ damage by surviving pancreatic β-cell [57]. Therefore, it is plausible to assume that the antihyperglycemic activity of the R. erlangeri aqueous leaf extract in STZ-induced diabetic mice might also involve potentiation of insulin effect probably by increasing secretion of insulin from the remaining β–cells or by increasing the peripheral glucose uptake.
In addition to persistent hyperglycemia, STZ induced diabetes is associated with significant weight loss, possibly as a result of the catabolic effects of insulin deficiency and severe hyperglycemia as well as the volume depletion associated with osmotic diuresis [58]. Similarly, significant body weight loss was observed in the negative control group starting from the second week which continued until the end of the experiment. But, oral administration of R. erlangeri leaf extract and GL5 attenuated this loss, which could probably be attributed to the improvement of glycemic control and structural protein synthesis [59].
Interrms of lowering BGL at the end of 3rd week since DM was induced, R. erlangeri brought maximum reduction (44%) compared to other specieses of Rubus such as R. steudneri (23.6%) and R. apetalus (38%) [47]. But it showed lower reduction compared to R. ellipticus (71%) [20].
The antidiabetic effects of plants are due to the presence of secondary metabolites such as phenols, flavonoids, tannins, alkaloids, and saponins [60]. Polyphenols and flavonoids are known for their natural antidiabetic effect. They interfere with the production of free radicals, reduce oxidative stress, and inhibit a digestive enzyme, thus lowering postprandial glucose. They also promote the uptake of glucose in tissues, and therefore improving insulin sensitivity [30]. From the quantitative estimations, R. erlangeri aqueous leaf extract contains total phenols (693 mg of GAE/g of extract) and flavonoids (77 mg QE/g of extract).
Plants in Rubus species contain resveratrol, which is a polyphenol. Resveratrol increases pancreatic β-cell function and has the potential of protecting β-cell dysfunction. It also increases the glucose induced incretin effect through inhibiting phosphodiesterases [61]. On the other hand, tannins work through decreasing glycogen content and inducing glucose transport via insulin mediated signaling pathway in adipocytes [62]. The activities of saponin are linked to regulating BGL and prevent diabetic complications due to their antioxidant activity [63]. Alkaloids exert their effect through inhibiting protein tyrosine phosphatase-1B and their activity is also because of their antioxidant effect [63].
5. Conclusion
The study revealed that the aqueous leaf extract of R. erlangeri have shown a significant lowering of blood glucose level on oral glucose loaded and STZ induced diabetic mice without permitting bodyweight loss and risk of hypoglycemia. The results also found that inhibition of intestinal alpha-amylase and the extracts' free radical scavenging activity can contribute to the antihyperglycemic activity of the plant extract.
Declaration
The Authors would like to declare that this article is part of a thesis work and the copy of the manuscript is found at Addis Ababa University institutional repository for the purpose of public access with the following link (http://213.55.95.56/bitstream/handle/123456789/21097/Akeberegn%20Gorems.pdf?sequence=1&isAllowed=y).
Ethics approval
The protocol was approved by institutional review board of the School of Pharmacy, College of Health sciences, Addis Ababa University with Reference no, ERB/SOP/212/03/2019.
Consent for publication
Not applicable.
Data availability
The datasets used for this study are available from the corresponding author on reasonable request.
Funding
The study was done with funding obtained from Addis Ababa University for Msc dissertation research. The role of the funder on the research was providing animals used in the experiment and financial support for purchasing materials and chemicals.
Author's contributions
All authors made significant contributions starting from conceiving the idea to developing and engaged in writing the article or critically revising. Moreover, all authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no any financial and/or non-financial competing interests.
Acknowledgements
The authors would like to thank Addis Ababa University, College of health science, department of pharmacology and clinical pharmacy for the financial support of this study.
Contributor Information
Akeberegn Gorems Ayele, Email: Akeberegn.gorems@aau.edu.et.
Prem Kumar, Email: premkrupandih@gmail.com.
Ephrem Engidawork, Email: ephrem.engidawork@gmail.com.
References
- 1.Riddle M.C. Standards of medical care in diabetes. , S1-S193. Standards of medical care in diabetes. American Diabetes Association. American Diabetes Association. 2019;42:S1–S193. 2019. [Google Scholar]
- 2.Papatheodorou K., Banach M., Bekiari E., Rizzo M., Edmond M. Complications of diabetes 2017. Journal of Diabetes Research. 2018;2018 doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmet P., Magliano D.J., Herman W.H., Shaw J. Diabetes: a 21st century challenge. The lancet Diabetes endocrinology. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed S.F., Mwangi M., Mutua M.K., Kibachio J., Hussein A., Ndegwa Z. Prevalence and factors associated with pre-diabetes and diabetes mellitus in Kenya: results from a national survey. BMC Publ Health. 2018:1215. doi: 10.1186/s12889-018-6053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaccardi F., Webb D.R., Yates T., Davies M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med. 2016;92(1084):63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- 6.Sowndhararajan K., Kang S. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci. 2013;20(4):319–325. doi: 10.1016/j.sjbs.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickramaratne M.N., P J.C., Wdb M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Compl Alternative Med. 2016;16(24):466. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran S., Rajasekaran A., Manisenthilkumar K. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed. 2012;2(4):262. doi: 10.1016/S2221-1691(12)60020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awas T. Institute of Biodiversity Conservation; 2016. Endemic plants of Ethiopia: preliminary working list to contribute to National plant conservation target; p. 14. [Google Scholar]
- 10.Hummer K.E., Janick J. Springer; 2009. Rosaceae: taxonomy, economic importance, genomics. Genetics and genomics of Rosaceae; pp. 1–17. [Google Scholar]
- 11.Bahmani M., Zargaran A., Rafieian-Kopaei M., Saki K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med. 2014;7:S348–S354. doi: 10.1016/S1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- 12.Durmuskahya C., Ozturk M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malays. 2013;42(10):1431–1438. [Google Scholar]
- 13.Katiri A., Barkaoui M., Msanda F., Boubaker H. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in the Tizi n'Test region (Taroudant Province, Morocco) J pharmacogn nat prod. 2017;3(1) doi: 10.1016/j.jep.2017.01.023. 2472-992. [DOI] [PubMed] [Google Scholar]
- 14.Swargiary A., HankhrayBoro Kumar Brahma B., Rahman S. Ethno-botanical study of anti-diabetic medicinal plants used by the local people of Kokrajhar District of Bodoland Territorial Council, India. Journal of Medicinal Plants Studies. 2013;1(5):5–58. [Google Scholar]
- 15.Ruffo C.K., Birnie A., Tengnäs B. Regional Land Management Unit/Sida Nairobi; 2002. Edible wild plants of Tanzania. [Google Scholar]
- 16.Meresa A., Gemechu W., Basha H., Fekadu N., Teka F., Ashebir R. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. J American Journal of Advanced Drug Delivery. 2017;5(1):40–58. [Google Scholar]
- 17.Schädler V., Dergatschewa S. Rubus caesius L. Leaves: pharmacognostic analysis and the study of hypoglycemic activity. NJPPP. 2017;7(5):501. [Google Scholar]
- 18.Lemus I., Garcia R., Delvillar E., Knop G. Hypoglycaemic activity of four plants used in Chilean popular medicine. Phytother Res. 1999;13(2):91–94. doi: 10.1002/(SICI)1099-1573(199903)13:2<91::AID-PTR350>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Kanegusuku M., Benassi J.C., Pedrosa R.C., Yunes R.A., Cechinel Filho V., Delle Monache F. Cytotoxic, hypoglycemic activity and phytochemical analysis of Rubus imperialis (Rosaceae) Z Naturforsch B Chem Sci. 2002;57(3–4):272–276. doi: 10.1515/znc-2002-3-412. [DOI] [PubMed] [Google Scholar]
- 20.Sharma U., Kumar A. Anti-diabetic effect of Rubus ellipticus fruit extracts in alloxan induced diabetic rats. Journal of Diabetology. 2011;2(2):4. [Google Scholar]
- 21.Alonso R., Cadavid I., Calleja J. A preliminary study of hypoglycemic activity of Rubus fruticosus. PLANT MED. 1980;40(S 1):102–106. doi: 10.1055/s-2008-1075012. [DOI] [PubMed] [Google Scholar]
- 22.Hao D-c, Xiao P-g. Chinese Herbal Medicines; 2020. Pharmaceutical resource discovery from traditional medicinal plants: pharmacophylogeny and pharmacophylogenomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2011. A N. Guide for the Care and Use of Laboratory Animals. 8th edition National Research Council. [Google Scholar]
- 24.Association A.V.M. American Veterinary Medical Association; Schaumburg, IL: 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. [Google Scholar]
- 25.Hedberg I., Edwards S. vol. 3. Addis Abeba University, Addis Abeba, Ethiopia and Uppsala University; Uppsala, Sweden: 1989. (Flora of Ethiopia). [Google Scholar]
- 26.Remington J.P. Lippincott Williams & Wilkins; 2006. Remington: the science and practice of pharmacy. [Google Scholar]
- 27.EVANS W.C. Saunders. Elsevier; Edinburgh/New York: 2009. Trease and evans pharmacognosy. [Google Scholar]
- 28.Debella A. Preliminary screening techniques of secondary metabolites. Manual for Phytochemical Screening of Medicinal Plants. 2002:38–57. [Google Scholar]
- 29.Njoku V.O., Obi C. Phytochemical constituents of some selected medicinal plants. AJPAC. 2009;3(11):228–233. [Google Scholar]
- 30.Kamtekar S., Keer V., Patil V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J Appl Pharmaceut Sci. 2014;4(9):61. [Google Scholar]
- 31.Patel A., Patel A., Patel A., Patel N. Estimation of flavonoid, polyphenolic content and in vitro antioxidant capacity of leaves of Tephrosia purpurea Linn.(Leguminosae) Int J Pharma Sci Res. 2010;1(1):66–77. [Google Scholar]
- 32.Wickramaratne M., Punchihewa N., Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Compl Alternative Med. 2016;16(24):466. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sama K., Sivaraj R., Rajiv P. In vitro antidiabetic activity of anthocyanin extract of Asystasia gangetica (Chinese violet) flower. Asian J Plant Sci Res. 2013;3(2):88–92. [Google Scholar]
- 34.Tadesse S., Asres K., Veeresham C. Antioxidant activities of three Rubus species growing in Ethiopia. Ethiop Pharmaceut J. 2007;25:103–110. [Google Scholar]
- 35.OECD . The Organization of Economic Co-operation and Development; Paris, France: 2008. Guidelines for testing of chemicals: guideline 425 : acute oral toxicity. [Google Scholar]
- 36.Sun C., Li X., Liu L., Conet M., Guan Y., Fan Y. Effect of fasting time on measuring mouse blood glucose level. Int J, Clin Exp, mecl. 2016;9(2):4186–4189. [Google Scholar]
- 37.Mythili M.D., Vyas R., Akila G., Gunasekaran S. Effect of streptozotocin on the ultrastructure of rat pancreatic islets. Microsc Res Tech. 2004;63(5):274–281. doi: 10.1002/jemt.20039. [DOI] [PubMed] [Google Scholar]
- 38.Brosius F. Animal models of diabetic complications consortium protocols. 2009. High-dose streptozotocin induction protocol (Mouse)/Low-Dose streptozotocin induction protocol (mouse) pp. 1–3. [Google Scholar]
- 39.Furman B.L. Streptozotocin‐induced diabetic models in mice and rats. Current protocols in pharmacology. 2015;70(1) doi: 10.1002/0471141755.ph0547s70. 5.47. 1-5. 20. [DOI] [PubMed] [Google Scholar]
- 40.Gebreyohannis T., Shibeshi W., Asres K. Effects of solvent fractions of Caylusea abyssinica (fresen.) fisch. And mey. On blood glucose levels of normoglycemic, glucose loaded and streptozotocin-induced diabetic rodents. J Nat Med. 2013;14(1):67–75. [Google Scholar]
- 41.Tesfaye A., Makonnen E., Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113. [Google Scholar]
- 42.Sun C., Li X., Liu L., Conet M.J., Guan Y., Fan Y. Effect of fasting time on measuring mouse blood glucose level. Int J Clin Exp Pathol. 2016;9(2):4186–4189. [Google Scholar]
- 43.Lim T., Jung H., Hwang K.T. Bioconversion of cyanidin-3-rutinoside to cyanidin-3-glucoside in Black raspberry by Crude α-L-rhamnosidase from Aspergillus species. J Microbiol Biotechnol. 2015;25(11):1842–1848. doi: 10.4014/jmb.1503.03098. [DOI] [PubMed] [Google Scholar]
- 44.Adisakwattana S., Yibchok-Anun S., Charoenlertkul P., Wongsasiripat N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J Clin Biochem Nutr. 2011;49(1):36–41. doi: 10.3164/jcbn.10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sowndhararajan K., Kang S.C. Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J Biol Sci. 2013;20(4):319–325. doi: 10.1016/j.sjbs.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wickramaratne M.N., Punchihewa J., Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Compl Alternative Med. 2016;16(1):466. doi: 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raghavendra H., Upashe S.P., Reyes D.R.A., Floriano J.F. Antidiabetic and antioxidant activity of Rubus apetalus poir. And Rubus steudneri schweinf. Leaf extract on alloxan induced diabetes mellitus. J Bioanal Biomed. 2019;11(2):1–6. [Google Scholar]
- 48.Jarald E., Joshi S.B., Jain D.C. Biochemical study on the hypoglycaemic effects of extract and fraction of Acacia catechu willd in alloxan-induced diabetic rats. Int J Diabetes Metabol. 2009;17:63–69. [Google Scholar]
- 49.Bowe J.E., Franklin Z.J., Hauge-Evans A.C., King A.J., Persaud S.J., Jones P.M. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. Internet J Endocrinol. 2014;222(3):G13–G25. doi: 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
- 50.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. DIS MODEL MECH. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson C., Nikolic A., Kentish S., Shalini S., Hatzinikolas G., Page A. Sex-specific alterations in glucose homeostasis and metabolic parameters during ageing of caspase-2-deficient mice. Cell Death Dis. 2016;2:16009. doi: 10.1038/cddiscovery.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vital P., Larrieta E., Hiriart M. Sexual dimorphism in insulin sensitivity and susceptibility to develop diabetes in rats. Internet J Endocrinol. 2006;190(2):425–432. doi: 10.1677/joe.1.06596. [DOI] [PubMed] [Google Scholar]
- 53.Eleazu C.O., Eleazu K.C., Chukwuma S., Essien U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. JDMDC. 2013;12(1):60. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goud B.J., Dwarakanath V., Chikka B. Streptozotocin-a diabetogenic agent in animal models. Int J Pharm Pharmaceut Res. 2015;3(1):253–269. [Google Scholar]
- 55.Spinas G.A. The dual role of nitric oxide in islet β-cells. PHYSIOL INT. 1999;14(2):49–54. doi: 10.1152/physiologyonline.1999.14.2.49. [DOI] [PubMed] [Google Scholar]
- 56.Tian H.-L., Wei L., Xu Z., Zhao R., Jin D., Gao J. Correlations between blood glucose level and diabetes signs in streptozotocin-induced diabetic mice. Int J Pharmacol. 2010;4(3):111–116. [Google Scholar]
- 57.Schmeltz L., Metzger B. Comprehensive medicinal chemistry II. Elsevier Science; Amsterdam: 2007. Therapeutic areas I: Central nervous system, pain, metabolic syndrome, urology, gastrointestinal and cardiovascular. [Google Scholar]
- 58.Deeds M., Anderson J., Armstrong A., Gastineau D., Hiddinga H., Jahangir A. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eleazu C., Iroaganachi M., Okafor P., Ijeh I., Eleazu K. Ameliorative potentials of ginger (Z. officinale Roscoe) on relative organ weights in streptozotocin induced diabetic rats. IJBS. 2013;9(2):82. [PMC free article] [PubMed] [Google Scholar]
- 60.Kooti W., Farokhipour M., Asadzadeh Z., Ashtary-Larky D., Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician. 2016;8(1):1832. doi: 10.19082/1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aryaeian N., Sedehi S.K., Arablou T. Polyphenols and their effects on diabetes management: a review. MJIRI. 2017;31:134. doi: 10.14196/mjiri.31.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babby A., Elanchezhiyan C., Suhasini S., Chandirasegaran G. Antihyperglycemic effect of tannic acid in streptozotocin induced diabetic rats. Int J Curr Res. 2014;6(3):5396–5398. [Google Scholar]
- 63.El Barky A.R., Hussein S.A., Alm-Eldeen A.-E. Saponins and their potential role in diabetes mellitus. Diabetes Manag. 2017;7(1):148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for this study are available from the corresponding author on reasonable request.