Abstract
Otosclerosis is a pagetoid proliferation of bone remodeling, vascular proliferation, bone resorption and new bone formation in the tympanic region of the temporal bone. The resulting anklyosis of the stapes footplate as it articulates with the oval window is the most common cause of conductive hearing loss in young to middle aged, predominantly Caucasian individuals. The characteristic histologic features have been well documented by autopsy studies of the temporal bone. Although stapedectomy is the surgical treatment for otosclerosis, the stapes specimen may be submitted for gross examination only or not examined at all. A retrospective study of 73 stapedectomy specimens (2008–2019) not including the stapes footplate. Clinical features from the electronic medical record as well as standard histologic sections from surgical specimens were reviewed. Neither the stapedal head nor crura showed histologic features of otosclerosis. There was mild osteoarthritis affecting the head, possibly as a consequence of persistent ossicular vibration superimposed on the ankylosed rigidity. The most common changes were surface fissuring (65%), cartilaginous erosion (49%) and irregularity of the osteochondral interface (51%). An occasional osteophyte (8%) was observed. The ear ossicles, embryologically analogous to long bones of the extremities, develop via endochondral ossification and exhibit articular surfaces of hyaline cartilage. The present observations suggest that a consequence of otosclerotic ankylosis is osteoarthritis of the stapedal head. In this study, the histological features could not be correlated with the severity of hearing loss or duration of clinical disease.
Keywords: Otosclerosis, Stapes, Ankylosis, Osteoarthritis
Introduction
Otosclerosis is a pagetoid process of bone remodeling specifically affecting one or more regions of the otic capsule. The vascular proliferation, bone resorption and new bone formation noted histologically most commonly involve the region of the oval window. This pathologic process results in ankylosis of the stapes footplate as it articulates with the oval window and clinically produces conductive hearing loss. Although less frequent, otosclerosis may exhibit either contiguous or multifocal involvement of the round window, stapes footplate and cochlea [1–3], which may account for mixed conductive and sensorineural hearing loss. Surgical intervention, i.e., stapedectomy, is a mainstay of treatment, which involves removal of the stapes crura and head with retention of the footplate into which a prosthesis is inserted.
The histopathology of otosclerosis has been studied in the temporal bone in autopsy specimens [1–3]. However, the extension of these characteristic changes into the ear ossicles, specifically the stapes, has not been systematically investigated. The present study was undertaken to assess (1) the possible extent of histologic otosclerosis beyond the temporal bone and (2) the pathologic effects of ankylosis of the stapes footplate on the resected surgical specimen.
Materials and Methods
Following approval from the Institutional Review Board, a retrospective search of the NorthShore University HealthSystem surgical pathology accessions between 2008 and 2019 was undertaken for stapedectomy specimens from patients with a clinical diagnosis of hearing loss, either conductive or mixed conductive-sensorineural. Clinical features as documented in the electronic medical record were reviewed for each patient: age, sex, type of hearing loss, laterality and family history. While audiograms were available for most patients, a detailed analysis of those studies is beyond the scope of this report. Stapedectomy specimens were decalcified and submitted intact for routine paraffin embedding. The hematoxylin and eosin sections were reviewed, additional deeper sections examined as appropriate, and assessed for the following histological parameters: (1) presence of otosclerosis in the stapes (2) surface fissuring of articular cartilage (3) erosion of articular cartilage (4) irregularity of the osteochondral junction and (5) osteophyte formation.
Results
Ninety-one specimens were retrieved, of which 18 specimens were excluded due to poor embedding or specimen orientation which could not be satisfactorily remedied by deeper sections. Of the remaining 73 specimens, ten were from patients who underwent bilateral stapedectomy and for whom both specimens were available for review. Three of these ten were excluded for technical reasons as described above. For the final total of 64 patients, the relevant clinical information was assessed via the electronic medical records (Table 1). There were 39 females and 25 males (1.5:1), ranging from 8 to 76 years (average 41), with 47 (74%) between ages 26–65. Bilateral hearing loss was identified in 49 (77%) with an almost equal conductive and mixed hearing loss in 30 (47%) and 34 (53%) respectively. Twenty-two (34%) patients had a positive family history for hearing loss, suggesting a possible genetic predisposition; further clinical investigation was not done.
Table 1.
Clinical features (n = 64 patients): hearing loss and stapedectomy
| Clinical parameter | N | PCT (%) |
|---|---|---|
| Age | ||
| 8–25 years | 6 | 9 |
| 26–45 years | 19 | 30 |
| 46–65 year | 28 | 44 |
| 66–76 years | 11 | 17 |
| Sex | ||
| Male | 25 | 39 |
| Female | 39 | 61 |
| Hearing loss | ||
| Unilateral | 15 | 23 |
| Bilateral | 49 | 77 |
| Type of hearing loss | ||
| Conductive | 30 | 47 |
| Mixed | 34 | 53 |
| Family history of hearing loss | ||
| Present | 22 | 34 |
| Absent | 42 | 66 |
N number, PCT percentage
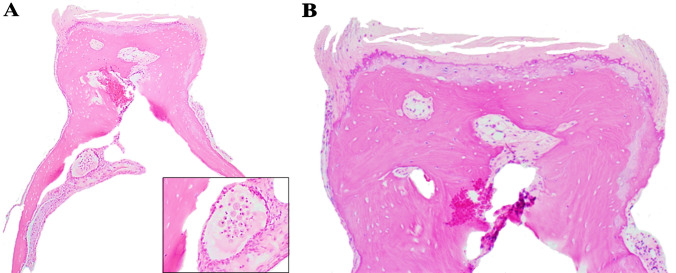
The histologic features of osteoarthritis assessed in this study are summarized (Table 2). The pagetoid proliferation characteristic of otosclerosis was not identified in any specimen. Osteoarthritic changes affecting the stapedal head were graded as mild or marked. Figure 1a shows a specimen with mild/minimal change, most closely approximating the normal anatomic and histologic features of the stapes demonstrating the head with an articular surface, neck and crura; the inset shows a collection of subchondral histiocytes. The articular surface of that specimen illustrates the most common histological abnormality encountered, that of mild fissuring of the articular cartilage accompanied by mild flaking and separation of cartilage fragments from the surface. Varying degrees of this were seen in 36 specimens (65%) (Fig. 1b). The severity of cartilaginous erosion of the stapes head showed mild and marked changes in 49% and 8% of specimens (Fig. 2a and b); mild and marked osteochondral interface changes were respectively seen in 51% and 8% of specimens (Fig. 2a and b). Occasional osteophyte formation (Fig. 3a) was present in 8% and there was a single instance of an intra-cartilaginous cyst (Fig. 3b).
Table 2.
Histopathologic features (n = 73 specimens): stapedectomy specimens
| Pathologic parameter | N | PCT (%) |
|---|---|---|
| Otosclerosis in the stapes | 0 | 0 |
| Erosion of articular cartilage | ||
| Mild | 36 | 49 |
| Moderate | 6 | 8 |
| Irregularity of the osteochondral junction | ||
| Mild | 37 | 51 |
| Moderate | 6 |
8 8 |
| Surface fissuring | 49 | 65 |
| Osteophyte formation | 6 | 8 |
| Other | ||
| Intra-cartilaginous cyst | 1 | 1.4 |
| Subchondral histiocytes | 1 | 1.4 |
N number, PCT percentage
Fig. 1.
Normal histology of the stapes. a Stapes including the stapes head, neck and crus. Inset: shows a collection of subchondral histiocytes. b Stapes head with cartilage showing mild surface fissuring (H&E*). *Hematoxylin and eosin
Fig. 2.
Degree of cartilaginous erosion and osteochondral junction irregularity. a Stapes head with mild surface cartilage erosion and irregularity of the osteochondral junction. b Marked surface cartilaginous erosion and irregularity of the osteochondral junction. Inset: Near eburnation of the cartilaginous surface (H&E*). *Hematoxylin and eosin
Fig. 3.
Other histological features. a Right side of the image shows osteophyte formation. b Stapes head with intra-cartilaginous cyst (H&E*). *Hematoxylin and eosin
In the ten patients with bilateral specimens available for review, one specimen in each of three patients had to be excluded for technical reasons. The demographic and pathological features of the seven remaining patients are highlighted (Table 3). Both surgeries were performed within a year of each other in four; in three, the second operation occurred between approximately 1 to 3 years. No significant histologic differences were noted in the seven patients where bilateral specimens were available for review).
Table 3.
Clinicopathologic features and interval between bilateral stapedectomy procedures
| Demographics | Interval between procedures | 1st stapedectomy | 2st stapedectomy | ||||
|---|---|---|---|---|---|---|---|
| Age | Sex | Type of hearing loss | Erosion of articular cartilage | Irregularity of the osteochondral junction | Erosion of articular cartilage | Irregularity of the osteochondral junction | |
| 9 | F | Mixed | 2 years 8 months | Mild | None | Mild | Mild |
| 45 | F | Mixed | 4 months | Mild | Mild | None | Mild |
| 64 | M | Mixed | 6 months | Marked | Marked | Marked | None |
| 34 | F | Conductive | 5 months | Mild | None | Mild | Mild |
| 55 | F | Conductive | 3 months | None | Mild | Mild | Mild |
| 51 | M | Conductive | 1 year 3 months | Mild | Mild | Mild | None |
| 35 | F | Conductive | 3 years 3 months | Mild | Mild | None | None |
Discussion
Otosclerosis is the most common cause of deafness among Caucasians, uncommon in Blacks, Asians and Native Americans [4–6]. Approximately 10% of the U.S. population is affected, women twice as often as men. It principally occurs in younger to middle aged individuals between 10 and 45 years and is bilateral in about 80% of cases. While there is no generally agreed common etiology, a familial occurrence with an autosomal dominant pattern is alleged in about 60% of cases; some assert that up to half the cases are sporadic [5, 6]. Other etiologic possibilities suggested in previous literature have included poorly defined inflammatory conditions, autoimmunity to various collagen genes and a post-infectious condition related to measles virus [5–9]. None of these latter etiologies was pursued in the evaluation of the patients reported here.
The cases reported here show demographic features compatible with previous reports. There was a predominant female population (1.5:1), a broad age range averaging 41 years; a positive family history of hearing loss, not otherwise specified, was recorded in 34%. Bilateral hearing loss affected 77% of the present cases; however, specimens from both sides were available and suitable for review in only seven patients. While definitive conclusions related to bilaterality are difficult to draw from the present cohort, two speculations may be offered. First, a reason for unilateral presentation of symptoms is unclear since it is possible that the pathologic alterations in the temporal bone and therefore affecting the stapes are already present bilaterally. This suggests some inherent predisposition of the otic capsule to the pagetoid reactive bone changes. Second, in the admittedly few cases available for review, the time separation of the surgical procedures seems to have no effect on any morphologic progression of the disease.
The term “otosclerosis” was not popularized until the late 19th and early 20th centuries. Since then, there have been multiple histologic studies of the temporal bone [1, 2, 10, 11]. These autopsy studies have demonstrated combinations of osteoblastic and osteoclastic activity with vascular proliferation and bone thickening simulating a pagetoid process. In the region of the otic capsule, the oval window is the predominant focus of involvement producing a conductive hearing loss. Less frequently, the round window and cochlea are affected. These regions being more closely approximated to the auditory nerve may account at least in part for the added sensorineural component to the hearing loss. Overall, the major anatomic consequence of this sclerotic process is ankylosis of the stapes footplate.
The histologic features of otosclerosis in the ear ossicles have not been systematically studied, although rare involvement of the incus [12] and the crura of the stapes [4, 13] has been reported. In the present study, the classic histologic features of otosclerosis were absent in the resected stapes specimens. However, the possibility of otosclerotic changes in the stapes footplate remains, since it is not resected as part of the surgical procedure and was not subject to histologic review. The retained footplate becomes the receptacle for the prosthesis which in turn re-establishes the integrity of the ossicular chain and is the rationale upon which surgical therapy is based.
Embryologically, the ear ossicles originate from the mesenchyme of the first and second branchial arches and mature by endochondral ossification, a process similar to that occurring in the long bones of the extremities [14]. Each ossicle is covered by a rim of articular (hyaline) cartilage in the formation of the interossicular joints. The function of the ear ossicles is to transmit sound via vibration of the bones, from the tympanic membrane to the otic capsule and auditory nerve. The transmission of sound depends on the ability of the ossicles to vibrate. It seems reasonable to presume that the ankylosis of the footplate at the oval window contributes to a rigidity of the ossicular chain and inhibits effective vibration. Over time, the ongoing mechanical injury to the head of the stapes results in the development of degenerative joint changes, i.e., fissuring and erosion of the articular surface, exposure of subchondral bone, irregularity of the osteochondral interface and eventual, albeit uncommon, osteophyte formation. The mild osteoarthritic changes in the stapedal head at the incudostapedial joint as demonstrated here, represent a morphological aspect of hearing loss that has at least been underappreciated. It is not clear from simple histologic examination whether the duration of symptoms and degree of clinical hearing loss were related to the severity of the histologic changes. A correlation with audiometric data may provide additional information regarding this point but such an investigation is beyond the scope of this report.
Although 77% of patients in the present series manifested bilateral hearing loss, a clinical -pathologic correlation relating the severity of hearing loss and the severity of the histopathologic features could not be reached. Time intervals between operations may be only partially contributory in assessing the severity of pathologic changes, since symptoms of hearing loss may range from a few months to many years. It is possible that for some patients there is a bilateral stabilization of the pathologic process at the time of clinical presentation and for others there is continuous progression. Since both specimens were available for review in only seven patients, and the histologic changes between both sides were similar, no definitive conclusion can be reached in relating the severity of clinical disability to those histologic changes.
There is a dearth of information related to the pathologic changes in stapedectomy specimens. It is common for certain specimens, e.g., palatine tonsils in elective tonsillectomy, cataracts, cosmetic skin resections, liposuction, as well as surgical hardware to be submitted for gross examination only or not to be accessioned. The reasons for this practice may be intuitive for some specimens, debatable for others. However, in recent years there has also been a trend to cease the examination of major joint replacement specimens for osteoarthritis and other non-neoplastic orthopedic conditions. It is perhaps consistent with those practices that a stapedectomy specimen may be submitted to the surgical pathology laboratory for gross examination only or not submitted at all. The seriousness of the morbidity of hearing loss notwithstanding, hearing loss is admittedly not a life-threatening condition. In addition, as shown here, the pathologic changes in the stapes are innocuous, i.e., no tumor, no serious infection. Nevertheless, the justification of no examination on these grounds is an unfortunate thought process as it reflects a lack of interest in the pathology of a major clinical disorder, an erroneous presumption that the pathologic alterations are irrelevant and that all that is necessary to know about a given condition is already extant.
Osteoarthritis in the head of the stapes is an unanticipated and novel pathologic finding in the examination of these tiny specimens. It is as yet of uncertain clinical significance. The goal of the present study has been to examine the morphologic changes in the stapes specimens, since this has not been previously undertaken. Audiometry introduces a separate area of clinicopathologic correlation which is outside the morphologic scope of this paper, yet the absence of audiometric analysis is an admitted limitation of this study. In the future, the correlation of audiometric data with the severity of osteoarthritic changes in resected surgical specimens may provide additional information related to the surgical treatment of hearing loss.
Funding
No outside funding was used in this project.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All the clinico-pathological investigations detailed in the manuscript have been conducted in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent for publication data and images was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna-Lee Clarke-Brodber, Email: aclarke-brodber@northshore.org.
Jerome B. Taxy, Email: jtaxy@northshore.org
References
- 1.Nager GT. Histopathology of otosclerosis. Arch Otolaryngol. 1969;89:157–79. doi: 10.1001/archotol.1969.00770020343022. [DOI] [PubMed] [Google Scholar]
- 2.Brondbo K, Hawke M, Abel SM, Alberti PW. The natural history of otosclerosis: a correlation of the volume and activity of the otosclerotic lesion with age. J Otolaryngol. 1983;12:163–8. [PubMed] [Google Scholar]
- 3.Wiet RJ, Raslan W, Shambaugh GE., Jr Otosclerosis 1981–1985: our four-year review and current perspective. Am J Otol. 1986;7:221–8. [PubMed] [Google Scholar]
- 4.Huang TS, Lee FP. Surgically confirmed clinical otosclerosis among the Chinese. Arch Otolaryngol Head Neck Surg. 1988;114:538–44. doi: 10.1001/archotol.1988.01860170068021. [DOI] [PubMed] [Google Scholar]
- 5.Cureoglu S, Schachern PA, Ferlito A, Rinaldo A, Tsuprun V, Paparella MM. Otosclerosis: etiopathogenesis and histopathology. Am J Otolaryngol. 2006;27:334–40. doi: 10.1016/j.amjoto.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Rudic M, Keogh I, Wagner R, Wilkinswon E, Kiros N, Ferrary E, Sterkers O, Bozorg-Grayeli A, Zarkovic K, Zarkovic N. The pathophysiology of otosclerosis: review of current research. Science Direct (Hear Res) 2015;330:51–6. doi: 10.1016/j.heares.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Niedermeyer HP, Arnold W, Neubert WJ, Sedlmeier R. Persistent measles virus infection as a possible cause of otosclerosis: atate of the art, ENT-Ear. Nose Throat J. 2000;79:552–61. doi: 10.1177/014556130007900807. [DOI] [PubMed] [Google Scholar]
- 8.Karosi T, Csomor P, Petko M, Liktor B, Szabo LZ, Pytel J, Jori J, Sziklai I. Histopathology of nonotosclerotic stapes fixations. Otol Neurotol. 2009;30:1058–66. doi: 10.1097/MAO.0b013e31819fe802. [DOI] [PubMed] [Google Scholar]
- 9.Flores-Garcia ML, Colin-Castro CA, Hernandez-Palestina MS, Sanchez-Larios R, Franco-Cendejas R. Absence of measles virus detection from stapes of patients with otoslcerosis. Otol Neurotol. 2018;158:158–62. doi: 10.1177/0194599817733674. [DOI] [PubMed] [Google Scholar]
- 10.Quesnel AM, Ishai R, Cureoglu S, Linthicum F, Lopez IA, Nadol JB, Jr, McKenna MJ. Lack of evidence for nonotosclerotic stapes fixation in human temporal bone histopathology. Otol Neurotol. 2016;37:316–20. doi: 10.1097/MAO.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quesnel A, Ishai R, McKenna MJ, Otosclerosi Temporal bone pathology. Science Direct (Otolaryngol Clin N Am) 2018;51:291–303. doi: 10.1016/j.otc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Escada PA, Capucho C, Chorao M, DaSilva JFM. Otosclerosis of the incus. Otol Neurotol. 2007;28:301–3. doi: 10.1097/01.mao.0000265186.37592.78. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho B, Hamerschmidt R, Telles JE, Richter N. Anatomopathology of the superstructure of te stapes in patients with otosclerosis. Int Arch Otolaryngol. 2015;19:1–4. doi: 10.1055/a-0034-1382096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallo M. Embryological and geneticaspects of middle ear development. Int J Dev Biol. 1998;42:11–22. [PubMed] [Google Scholar]