Abstract
Unicystic ameloblastoma (UA) is an uncommon variant of ameloblastoma and behaves totally different from the solid multicystic variant of ameloblastoma (SMA); furthermore the histological subgroups of UA also show varied behavior regarding proliferation. The present multi-centric study was designed to present the clinicopathological features of unicystic ameloblastoma (UA) and to compare the two popular histological classifications systems. 80 satisfactory cases of UA were retrieved and evaluated for clinicopathological parameters from four teaching dental schools of North India. The cases were classified using modified Reichart and Philipsen system and Marx and Stern system followed by comparison of inter-observer variability. The results were analyzed using SPSS software. The mean age of occurrence was 30.79 ± 16.49 years. Males outnumbered females (M:F::1.67:1). The majority of cases occurred in the third decade irrespective of the gender. Most cases were found in body–angle–ramus region of the mandible. The modified Reichart and Philipsen classification yielded better interobserver agreement (kappa value 0.845). The modified Reichart and Philipsen classification yields better inter-rater agreement and is easy to reproduce amongst oral pathologists. Being simpler it may easily be understood by the operating surgeon for better treatment outcome.
Keywords: Ameloblastoma, Intraluminal, Luminal, Mural, Unicystic
Clinicopathological profile of 80 cases of unicystic ameloblastoma aided by a histopathological comparison using modified Philipsen–Reichart classification and Marx–Stern classification.
Introduction
Generated at any stage in the life of an individual, odontogenic tumors (OTs) are heterogeneous group of lesions derived from odontogenic apparatus comprised of the odontogenic epithelium, the ectomesenchyme and/or the mesenchymal elements [1]. Ameloblastoma is a benign odontogenic tumor that most commonly presents as a radiolucency of the posterior mandibular region [1–3]. It has been considered as a locally aggressive neoplasm that may rarely demonstrates metastasis. According to the latest WHO classification of head and neck tumors ameloblastomas are sub-categorized into solid multicystic, unicystic, peripheral and metastasizing types [4].
Unicystic ameloblastoma (UA) is an uncommon, biologically less aggressive cystic variant that shows features of a typical mandibular cyst clinically, radiographically and grossly, but on histopathological examination shows a typical ameloblastomatous epithelium lining part of the cyst cavity, with or without luminal and/or mural tumor growth [5]. UA behaves totally different from solid multicystic variant of ameloblastoma (SMA); furthermore the histological subgroups of UA also show varied behavior regarding proliferation. Ackermann et al. in a clinicopathological study of 57 cases classified UA into 3 histological groups. The classification was further modified by Philipsen and Reichart [6, 7]. Another histological sub-grouping was put forwarded by Marx and Stern [8].
No previous attempts have been made in the literature to compare the most commonly used classification systems of unicystic ameloblastoma. The aim of the present multi-centric study was thus to present the clinicopathological profile of unicystic ameloblastoma and to classify them according to different histological classification systems available in the literature followed by analysis of inter-rater agreement between these commonly used sub-grouping systems. This comparison could be beneficial for pathologists and operating surgeons alike.
Methodology
Data files were retrieved retrospectively from the archives of four dental teaching institutions of North India. Eighty satisfactory cases of unicystic ameloblastoma were included in the present study after reassessing the diagnosis. Slides without histopathological criteria for the definitive diagnosis of UA and cases without slides and corresponding paraffin-embedded tumor specimens were not included. Furthermore, the cases showing invasion beyond the cyst wall (i.e., invading into the bone) were excluded as they were no longer considered as UA and managed akin to solid multicystic ameloblastoma (Fig. 1).
Fig. 1.
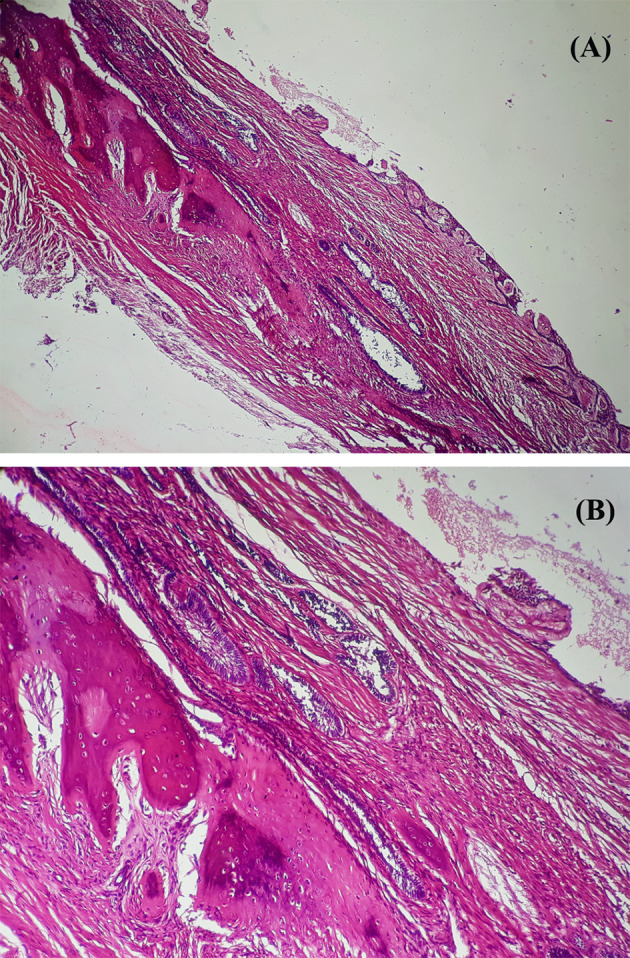
Photomicrograph of an H and E stained sections from a case of maxillary unicystic ameloblastoma showing invasion of mural follicles into the bone; a × 40 and b × 100
Data was analyzed for age, gender, site, radiological features, symptoms and treatment. The maxilla and mandible were divided into four quadrants each as follows: (a) quadrant I: involved tooth number 11 to tooth number 18 including maxillary tuberosity region, (b) quadrant II: involved tooth number 21 to tooth number 28 including maxillary tuberosity region, (c) quadrant III: involved tooth number 31 to tooth number 38 including angle and ramus region and (d) quadrant IV: involved tooth number 41 to tooth number 48 including angle and ramus region. The lesions crossing the midline were recorded separately. Histological evaluation was done independently by two observers using Modified Philipsen and Reichart histological sub-grouping and Marx and Stern classification [6, 7]. Any disagreement regarding diagnosis was resolved by uniform consensus using a penta-headed microscope.
The results were entered in Microsoft excel spreadsheet 2013 and final statistical analysis was done on IBM SPSS software (version 21.0, IBM Analytics, Armonk, New York, US). Descriptive statistics was done for frequency counts. Inter-observer variability was determined by kappa statistics [9].
Results
Clinico-demographic Profile
The present multi-institutional study yielded 80 cases of unicystic ameloblastoma. The mean age of occurrence was 30.79 ± 16.49 years (range 7–70 years). Males outnumbered females by a ratio of 1.67:1 (M50: F30). The majority of cases occurred in the third decade irrespective of the gender.
Most cases were located in the mandible (74 cases; 92.5%) in contrast to only 6 maxillary cases (7.5%). In mandibular region, 41 cases (51.2%) involved the left quadrant and 26 cases (32.5%) were seen in the right quadrant. 7 Mandibular cases were seen in the anterior region (8.8%) crossing the midline. In maxillary segments, 5 cases (6.3%) involved the second quadrant and a single case was recorded that crossed the midline (1.3%). As shown in Fig. 2 most cases were found in body–angle–ramus region of the mandible.
Fig. 2.
Figure depicting number wise distribution of maxillary and mandibular cases. Most cases were noted in the body–angle–ramus of the mandible
For 21 cases the exact radiographic description was not available in the archival files. Of remaining 59 cases, 37 cases were explained as unicystic/unilocular and rest 22 cases were described as multilocular on radiologic examination. Root resorption of one or more associated teeth was mentioned in 33 cases. For 14 cases only incisional biopsy was done while 66 cases were excised in toto and none of the excised cases recurred.
Histopathological Data and Interobserver Agreement Between Two Classification Systems
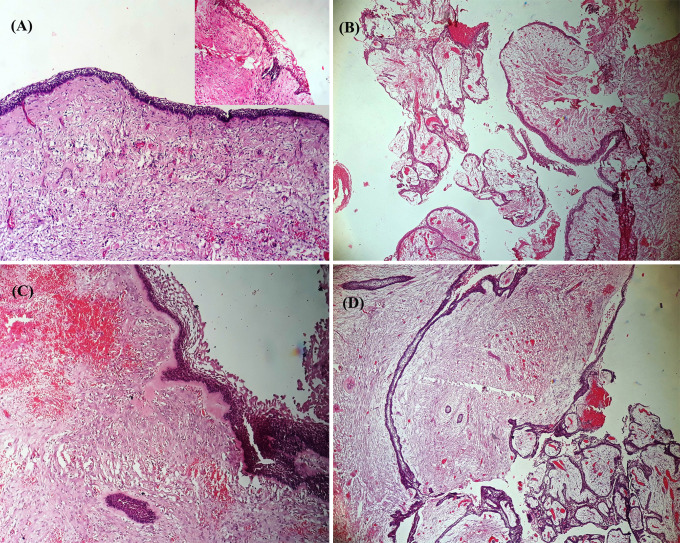
The cyst lining in all 80 cases was composed of ameloblastomatous epithelium, major portions however, lacked the typical appearance and resembled dentigerous cyst, inflamed radicular cyst or was non-specific (Fig. 3). Serial sections and extensive grossing aided in the identification of ameloblastomatous epithelium. Figure 4 shows the histological subgroups of UA. Juxtaepithelial hyalinization was noted in nearly half of the cases (Fig. 4c). All cases were classified and compared using two histopathological grading systems.
Fig. 3.

Photomicrograph of an H and E stained sections showing a dentigerous cyst like lining (× 40), b nonspecific non-keratinized epithelium (× 40) and c radicular cyst like epithelium with arcade formation (× 40)
Fig. 4.
Photomicrograph of an H and E stained sections showing a luminal unicystic ameloblastoma (× 100, inset shows abrupt transition to parakeratin), b luminal and intraluminal variant of unicystic ameloblastoma (× 40), c luminal and mural unicystic ameloblastoma with juxtaepithelial and perifollicular hyalinization (× 100) and d luminal, intraluminal and mural unicystic ameloblastoma (× 40)
Modified Philipsen and Reichart Classification System
33.8% of the cases (n = 27) were categorized under 1.2.3 category (luminal, intraluminal and mural), followed by 31.3% in 1.3 (luminal, mural; n = 25), luminal + intraluminal (n = 15; 18.8%) and luminal UA (n = 13; 16.3%). Kappa value of 0.845 suggested a very strong inter-observer agreement.
Marx and Stern Classification System
In Marx and Stern system, IIA category comprised the maximum cases (n = 33, 41.3%), followed by categories IB (n = 15), IA and IIB (n = 10 each), IIIA (n = 9) and I (n = 3) in the same descending order. Kappa value of 0.522 suggested a moderate agreement between the observers. (Table 1).
Table 1.
Inter-observer agreement between two observers as deciphered by kappa value (P value ≤ 0.05 is considered as statistically significant)
| Classification system | Number of valid cases | Kappa value | Asymptotic standard error | Approximate T value | Approximate significance |
|---|---|---|---|---|---|
| Modified Philipsen and Reichart classification | 80 | 0.845 | 0.049 | 12.717 | 0.000 |
| Marx and Stern classification | 80 | 0.522 | 0.066 | 9.913 | 0.000 |
Table 2 shows the detailed clinicopathological profile of 80 cases of UA included in the present study.
Table 2.
Detailed clinicopathological profile of 80 cases of unicystic ameloblastoma
| Parameter | |
|---|---|
| Gender | |
| Male | 50 |
| Female | 30 |
| Mean age | |
| Overall | 30.79 ± 16.49 years |
| Site | |
| Maxilla | 6 |
| Mandible | 74 |
| Radiographic features | |
| Not available | 21 |
| Multilocular | 22 |
| Unicystic/unilocular | 37 |
| Histopathology | |
| Philipsen and Reichart classification | |
| • Subgroup 1 | 13 |
| • Subgroup 1.2 | 15 |
| • Subgroup 1.3 | 25 |
| • Subgroup 1.2.3 | 27 |
| Marx and Stern classification | |
| • Subgroup I | 3 |
| • Subgroup IA | 10 |
| • Subgroup IB | 15 |
| • Subgroup IIA | 33 |
| • Subgroup IIB | 10 |
| • Subgroup IIIA | 9 |
Discussion
The term ‘unicystic ameloblastoma’ was coined owing to the macroscopic and microscopic presentation of the pathology and the subsequent histopathological features describing a large cystic cavity lined partially or completely by odontogenic epithelium. Much of the perplexity regarding the terminology and histologic classification stems from the fact that the pathology presents as a unilocular or multilocular intrabony lesion on radiographs. The two most popular classification systems followed worldwide are by Marx and Stern and the other one by Philipsen and Reichert [7, 8].
The present study was an attempt to describe the clinicopathological features of unicystic ameloblastoma cases retrieved from multiple teaching dental schools of Northern India. Further we aimed to compare the two most commonly used classification systems i.e. Philipsen and Reichart system [7] (modified from Ackerman et al.) and Marx and Stern classification system [8]. The first systematic sub-categorization of UA was done by Ackerman et al. in 1988 in their classic paper comprising of details from 57 cases reported over a period of 30 years [6].
Odontogenic tumors have peeked interest amongst oral pathologists for several decades which has resulted in varying classifications and categorization of these tumors. These odontogenic tumors constitute primarily of hamartomatous, non-neoplastic lesions, benign tumors and malignant neoplasms. Ameloblastoma is one such benign odontogenic tumor for which different classifications have been devised for its better categorization and understanding.
Unicystic ameloblastoma is a far less frequent growth pattern encountered and comprises 6% of all intraosseous ameloblastomas [2, 10]. The present study depicted that most cases affected the mandibular posterior region which can be owed to the fact that the odontogenic epithelium could still be active in the molar region developing comparatively later and thus might act as a potential source for the pathology. These findings are in concordance with the published data [6, 11, 12]. The present study revealed a mean age of 30.79 ± 16.49 years which was comparatively higher than previously reported studies [6, 11]. The recent systematic review comprising of 132 UA cases also demonstrated a lower mean age than the present study [13]. Concordant with the published literature, a male predilection was noted (1.67:1) [6, 11]. In contrast to these findings, almost equal preponderance was noted in many other published papers [12–14].
A slightly higher mean age was established in our study with most cases affecting the fourth decade of life. Literature illustrates a higher average age of initial diagnosis in developed nations as compared to developing nations. Dodge insinuated that accelerated ageing process in developing countries could be due to poor healthcare measures which could stand true in the present study as well [15]. In the systematic review on UA in children conducted by Seintou A et al., the authors concluded that painless swelling is universally reported as the chief complaint [14]. This may be correlated with the presentation of patients to hospital only in the presence of debilitating symptoms in developing nations complicated by lack of regular dental/medical checkups. On the contrary, ameloblastomas are frequently detected during routine dental check-ups in developed countries with better healthcare norms [16]. It has been reported that UA tends to occur at a lower age as compared to solid multicystic variants of ameloblastoma [11]. A logical speculation made by Ord RA et al. could explain the prevalence of UA at younger age. They opined that development of UA represents a continuum from odontogenic cyst to cyst with ameloblastoma developing in the lining only and ultimately to mural unicystic ameloblastoma and local bony invasion akin to SMA with advanced age [17]. The de novo cases may however not be explained with this speculation. Thus, a higher mean age of patients from North India in the present study could collectively be due to lack of symptoms of UA, no regular dental visits, and late self presentation.
Clearly, the mandible was affected more commonly than maxilla (12.3:1) similar to previous other studies [6, 11, 18]. The same holds true for solid multicystic variant of ameloblastoma. Observations from the present series showed that the most common radiographic description was unicystic/unilocular. Multilocular appearance was not uncommon (22/80). We could not classify UA cases into dentigerous or non-dentigerous radiographic variants, as the observations were collected from archival medical records. As found in the present series, presence of root resorption of associated teeth should alert to the possibility of ameloblastoma [17]. In a detailed examination of internal morphology of ameloblastomas, Ngwenya et al. supported the usage of the terms ‘unilocular’ and ‘multilocular’ rather than ‘unicystic’ and ‘multicystic’ to describe the radiological appearances of ameloblastoma [19]. A real distinction can however be made after microscopic examination of the resected specimen [14]. Then again, one might not always be able to get the specimen as a whole, which may further jeopardize the diagnosis. Therefore, it must always be correlated clinically and radiologically. The concept of a ‘unicystic multilocular ameloblastoma’ suggested by Reichart et al. may not be thus completely overlooked [2]. Gardner DG did not support the aforementioned view [20]. Multilocularity could be ascribed to pseudopod like cystic extension with in the cyst wall encapsulating a common cavity [11], unequal growth potential or dissimilar rate of bone resorption.
Categorization of this benign yet sometimes aggressive pathology is vital from the pathologists and surgeon’s perspective as the treatment could differ drastically depending upon the classification which is centered on the extent of tumor invasion. The difference not just lies histopathologically but also in expression of various immunomarkers amongst subtypes. Previous studies have found lowest PCNA and Ki-67 index in the luminal component as compared to intraluminal and mural areas depicting differences in proliferative potential between different components of unicystic ameloblastoma [21]. Similar findings for Ki-67 were reported in another study [22]. It may thus be prudent to believe that histological sub-typing of UA may not only be of academic interest but also dictates the treatment modality.
In this maiden attempt to compare two universally accepted classification systems, we found a very strong inter-observer agreement using the modified Reichart and Philipsen classification system (kappa value − 0.845). The classification appears to be simple and easy to reproduce amongst pathologists in contrast to Marx and Stern classification which generated a moderate agreement between two observers (kappa value − 0.522). There is a need of a universal system which is easily reproducible amongst pathologists and surgeons alike, since the treatment modality largely depends on the histological subtype of unicystic ameloblastoma.
Juxtaepithelial hyalinization as noted in around half of the cases was previously reported by Li et al. [11]. They suggested sub-epithelial hyalinization as a distinguishing feature from other cyst except calcifying odontogenic cyst. Recent literature showed juxtaepithelial hyalinization as an additional factor in odontogenic keratocysts [23]. The authors suggest that same may be applicable to UA, but we could not correlate this feature with patient outcome limited by lack of long term follow-up. Future prospective studies are warranted to elicit this unexplored yet intriguing feature for better patient outcome.
The operating surgeon needs to know the extent of proliferation of tumor islands, as the luminal or intraluminal variant are managed conservatively compared to the lesions showing mural proliferation. The extent beyond cyst wall into the bone demands more aggressive surgical procedures. Surgical implantation of ameloblastomatous foci deep into the bone has been suggested by vigorous curettage following limited resection (enucleation). When comparing the different treatment modalities, Lau et al., reported lowest recurrence rate following resection (3.6%) [13]. Enucleation alone yielded a recurrence rate of 30.5%, which has been noted to drop drastically to 16% and 18% when Carnoys’ solution and marsupialization were respectively used following limited resection (enucleation) [13, 24].
There were some inherent limitations of the present study. Firstly, the data may not be representative of the entire population as some cases might have been lost to general practitioners and not subsequently submitted for histopathological examination. Secondly, there was lack of a long term follow-up. However, it does provide a baseline data for planning intervention programs for better patient outcome.
Conclusions
The present multi-institutional study compared the two most common classification systems of UA, and it was found that the modified Reichart and Philipsen classification yields better interobserver agreement and is easy to reproduce amongst pathologists. Being simpler it may easily be understood by the operating surgeon for better treatment outcome.
Funding
There was no funding involved with this project.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The manuscript includes the data from the archival medical records of four teaching schools with no additional psychological, physical and financial burden for the patient.
Informed Consent
Further, the informed and written consents were taken from the patients at the time of admission for treatment.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pandiar D, Shameena PM, Sudha S, Varma S, Manjusha P, Banyal VS, et al. Odontogenic tumors: a 13-year retrospective study of 395 cases in a South Indian Teaching Institute of Kerala. Oral Maxillofac Pathol J. 2015;6:602–8. [Google Scholar]
- 2.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B. 1995;31B:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 3.Agbaje JO, Olumuyiwa Adisa A, Ivanova Petrova M, Adenike Olusanya A, Osayomi T, Ajibola Effiom O, et al. Biological profile of ameloblastoma and its location in the jaw in 1246 Nigerians. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:424–31. doi: 10.1016/j.oooo.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 4.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. Odontogenic and maxillofacial tumors. In: WHO classification of tumours of the head and neck, 4th ed. Lyon: IARC Press; 2017. pp. 203–60.
- 5.Chaudhary Z, Sangwan V, Pal US, Sharma P. Unicystic ameloblastoma: a diagnostic dilemma. Natl J Maxillofac Surg. 2011;2:89–92. doi: 10.4103/0975-5950.85863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann GL, Altini M, Shear M. The unicystic ameloblastoma: a clinicopathological study of 57 cases. J Oral Pathol. 1988;17:541–6. doi: 10.1111/j.1600-0714.1988.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 7.Philipsen HP, Reichart PA. Unicystic ameloblastoma. a review of 193 cases from the literature. Oral Oncol. 1998;34:317–25. doi: 10.1016/S1368-8375(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Mark RE, Stern D, editors. Odontogenic tumors. In: Oral and maxillofacial pathology: a rationale for diagnosis and treatment. 2nd ed. Hanover Park: Quintessence Publishing Co, Inc.; 2012. pp 667–84.
- 9.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler HP. Intraosseous ameloblastoma. Oral Maxillofac Surg Clin N Am. 2004;16:309–22. doi: 10.1016/j.coms.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Li TJ, Wu YT, Yu SF, Yu GY. Unicystic ameloblastoma: a clinicopathologic study of 33 Chinese patients. Am J Surg Pathol. 2000;24:1385–92. doi: 10.1097/00000478-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstein T, Pogrel MA, Smith RA, Regezi JA. Cystic ameloblastoma—behavior and treatment of 21 cases. J Oral Maxillofac Surg. 2001;59:1311–6. doi: 10.1053/joms.2001.27522. [DOI] [PubMed] [Google Scholar]
- 13.Lau SL, Samman N. Recurrence related to treatment modalities of unicystic ameloblastoma: a systematic review. Int J Oral Maxillofac Surg. 2006;35:681–90. doi: 10.1016/j.ijom.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Seintou A, Martinelli-Kläy CP, Lombardi T. Unicystic ameloblastoma in children: systematic review of clinicopathological features and treatment outcomes. Int J Oral Maxillofac Surg. 2014;43:405–12. doi: 10.1016/j.ijom.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Dodge OG. Tumors of the jaw, odontogenic tissues and maxillary antrum (excluding Burkitt lymphoma) in Uganda Africans. Cancer. 1965;18:205–15. doi: 10.1002/1097-0142(196502)18:2<205::AID-CNCR2820180212>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: a study of 1,088 cases from Northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg. 2006;64:1343–52. doi: 10.1016/j.joms.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Ord RA, Blanchaert RH Jr, Nikitakis NG, Sauk JJ. Ameloblastoma in children. J Oral Maxillofac Surg. 2002;60:762–70; discussion 770–1. [DOI] [PubMed]
- 18.Tatapudi R, Samad SA, Reddy RS, Boddu NK. Prevalence of ameloblastoma: a three-year retrospective study. J Indian Acad Oral Med Radiol. 2014;26:145–51. doi: 10.4103/0972-1363.143687. [DOI] [Google Scholar]
- 19.Ngwenya SP, Raubenheimer EJ, Noffke CE. Internal morphology of ameloblastomas: a study of 24 resected specimens. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:754–62. doi: 10.1016/j.tripleo.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DG. Critique of the 1995 review by Reichart et al. of the biologic profile of 3677 ameloblastomas. Oral Oncol. 1999;35:443–9. doi: 10.1016/S1368-8375(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 21.Li TJ, Browne RM, Matthews JB. Expression of proliferating cell nuclear antigen (PCNA) and Ki-67 in unicystic ameloblastoma. Histopathology. 1995;26:219–28. doi: 10.1111/j.1365-2559.1995.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 22.Sah P, Menon A, Kamath A, Chandrashekar C, Carnelio S, Radhakrishnan R. Role of immunomarkers in the clinicopathological analysis of unicystic ameloblastoma. Dis Markers. 2013;35:481–8. doi: 10.1155/2013/517834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottom HE, Bshena FI, Speight PM, Craig GT, Jones AV. Histopathological features that predict the recurrence of odontogenic keratocysts. J Oral Pathol Med. 2012;41:408–14. doi: 10.1111/j.1600-0714.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 24.Al Qahtani K, Alkhudhayri AF, Islam T, Al Mufargi K, Al Shakweer W, Otaibi F. Recurrent unicystic maxillary ameloblastoma presenting as unilateral proptosis. Saudi J Ophthalmol. 2019;33:94–8. doi: 10.1016/j.sjopt.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]