Abstract
Fine-needle aspiration (FNA) biopsy reliably diagnoses parotid gland lesions preoperatively, whereas intraoperative frozen section (FS) has the additional benefit of assessing surgical margins and refining diagnoses; however, the role of FS in the setting of prior FNA diagnosis is not well established. Our aim was to determine whether FS should still be performed after a prior FNA/ CNB diagnosis. Parotid gland resections from January 2009 to January 2020 were identified; however, only patients who had both FNA and FS constituted our study population. For the purpose of statistical analysis, FNA diagnoses were classified into non-diagnostic (ND), non-neoplastic (NN), benign neoplasm (BN), indeterminate, and malignant. FS diagnoses were classified into benign, indeterminate, or malignant. Resections were dichotomized into benign and malignant and regarded as the gold standard to subsequently calculate diagnostic accuracy of FNA and FS. A total of 167 parotid gland resections were identified, but only 76 patients (45.5%) had both FNA and FS. In 35 cases deemed as benign preoperatively, three (8.6%) were reclassified as malignant on FS. Out of 18 lesions reported as malignant on FNA, four (22.2%) were interpreted as benign on FS, with three of these benign lesions confirmed on permanent slides. In addition, in patients with both FNA and FS, compared to FNA, FS was able to provide a definitive diagnosis in all five ND cases and in 61.1% (11/18) of indeterminate tumors. Intraoperative assessment provided a relative increase of 33.3% in specificity and 38.5% in positive predictive value when compared to preoperative FNA. The addition of FS to FNA was helpful to further refine the diagnoses of parotid gland lesions, which may provide better guidance for surgical intervention.
Keywords: Parotid cancer, Salivary gland, Fine needle aspiration, Frozen section, Cytology
Introduction
Salivary gland (SG) tumors are rare in the United States. While most of these neoplasms originate in the parotid gland and are benign, it is estimated that one per 100,000 adults will be diagnosed with salivary gland cancer each year. Clinical stage is the primary determinant of prognosis; therefore, early detection of parotid gland neoplasms is critical [1–3].
In current clinical practice, diagnostic workup of parotid masses includes a fine-needle aspiration (FNA) biopsy. This modality has become widely accepted as a rapid, cost-effective, and reliable diagnostic test in the assessment of these lesions [4–7]. Pitfalls in salivary gland FNA, however, are well documented [8–10]. In particular, considerable cytomorphological overlap exists among parotid tumors with significantly different biological behaviors.
Intraoperative frozen section (FS) has the potential to assess surgical margins and refine the diagnosis of SG lesions; however, the role of such technique in clarifying the diagnosis of parotid tumors with pre-operative FNA and/ or core needle biopsy (CNB) is controversial and contemporary data is lacking. While some advocate FS and FNA have similar diagnostic performance [11], others claim FS to be superior [12–15].
Since surgical management may drastically differ for benign and malignant lesions [16], it is important to determine the best diagnostic approach. Our goal was to determine whether the use of intraoperative assessment significantly altered the pre- operative FNA and/or CNB diagnosis of primary parotid gland neoplasms.
Materials and Methods
This retrospective study was approved by the Institutional Review Board (IRB) of the Mayo Clinic. The pathology database at Mayo Clinic Arizona was searched for all parotid gland resections performed from January 2009 to January 2020. We then identified patients who had both preoperative biopsies and frozen section diagnoses and these patients constituted the study population.
Since the purpose of this study was to determine whether the use of FS significantly altered the diagnosis of FNA in primary parotid lesions, intraoperative consultation performed solely for margin evaluation were excluded from the study. For similar reasons, cases in which preoperative assessment determined secondary involvement or metastases to the parotid gland were also excluded.
Preoperative evaluation of parotid lesions consisted of fine needle aspiration with and without cell block and/or core needle biopsy (FNA/CNB) specimens. These procedures were routinely performed by surgeons, cytopathologists, or radiologists. FNA was carried out by percutaneous route with a 23- or 25-gauge needle, often under ultrasound guidance. Conventional smears were produced and stained with Diff-Quick and Papanicolaou stains and part of the sample was collected for cell block (CB). 18-gauge core-needle biopsies were occasionally obtained after FNA passes were performed. These were fixed in 10% neutral buffered formalin, processed, and stained with hematoxylin and eosin. Slides from the FNA, CB, and CNB (when available) were examined and reported together.
A subset of patients had preoperative evaluation performed at outside institutions, but in-house review of outside slides was performed, and these patients were also included in our cohort.
For the purpose of statistical analysis, FNA/CNB diagnoses were classified into five categories: non-diagnostic (ND), non-neoplastic (NN), benign neoplasm (BN), indeterminate, and malignant based on institutional criteria. Of note, nodules classified as indeterminate included both samples that were indefinite for a neoplastic condition, such as cases reported as atypical cells seen, and those in which the diagnosis of a neoplasm was possible, but its malignant potential was uncertain. The malignant category included specimens suspicious or diagnostic for malignancy, as both of these scenarios would result in the same clinical management in our institution.
Frozen section diagnoses were classified into benign, indeterminate or malignant. Indeterminate lesions represented those where a definitive entity could not be recognized, and the pathologist would defer the appropriate diagnosis to permanent section. Frozen sections were performed by general surgical pathologists with and without cytopathology expertise.
Permanent section (PS) diagnoses were dichotomized into benign (negative) or malignant (positive) and regarded the gold standard used to subsequently calculate diagnostic accuracy measures for FNA/CNB and FS. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated using only the patients that underwent both procedures. For these calculations, among FNA/CNB cases, ND were excluded, NN or BN were considered a negative test, and indeterminate or malignant were grouped as positive. On the other hand, benign and indeterminate cases on FS were considered negative, as only a malignant diagnosis in this setting has the potential to dictate the extent of surgery [11].
Exact Binomial Test for Differences in Sensitivity and Specificity of two binary diagnostic tests in a paired study design was used to compare these parameters between FNA/CNB and FS [17]. Similarly, a test for differences in (positive and negative) predictive values of two binary diagnostic tests using a generalized score statistic was applied [18].
Statistical analyses were performed using R software version 3.6 (R Foundation for Statistical Computing, Vienna, Austria). The significance level was fixed at 5% for all tests.
Results
A total of 167 patients with parotid resections were retrieved. Of these, 98 (58.7%) underwent prior FNA/CNB, 142 (85%) had intraoperative frozen section diagnosis and 76 (45.5%) had both (Table 1). Patients in this study were predominantly female (n = 104, 62.3%) and the median age was 60 years (range 19–91 years).
Table 1.
Distribution of diagnostic categories across FNA/CNB, frozen sections and permanent sections
| FNA/CNB (n = 98) | |
|---|---|
| Non-diagnostic | 6 (3.6%) |
| Scant/absent cellularity | 6/6 |
| Non-neoplastic | 4 (2.4%) |
| Inflammatory/reactive | 3/4 |
| Cyst | 1/4 |
| Benign neoplasm | 37 (22.2%) |
| Pleomorphic adenoma | 20/37 |
| Warthin tumor | 11/37 |
| Other | 6/37 |
| Indeterminate | 20 (12%) |
| Basaloid | 11/20 |
| Oncocytoid/clear cell | 8/20 |
| Other | 1/20 |
| Malignant | 31 (18.6%) |
| Carcinoma | 29/31 |
| Other | 2/31 |
| Frozen section (n = 142) | |
| Benign | 89 (53.3%) |
| Pleomorphic adenoma | 39/89 |
| Warthin tumor | 20/89 |
| Non-neoplastic | 14/89 |
| Other | 16/89 |
| Indeterminate | 21 (12.6%) |
| Malignant | 32 (34.1%) |
| Carcinoma | 28/32 |
| Other | 4/32 |
| Permanent section (n = 167) | |
| Benign | 98 (58.7%) |
| Pleomorphic adenoma | 50/98 |
| Warthin tumor | 20/98 |
| Non-neoplastic | 13/98 |
| Other | 15/98 |
| Malignant | 69 (41.3%) |
| Carcinoma | 62/69 |
| Other | 7/69 |
FNA/CNB fine-needle aspiration/core-needle biopsy
The diagnoses of the 98 FNA/CNB cases were as follows: six (3.6%) were ND, four (2.4%) non-neoplastic, 37 (22.2%) benign neoplasms, 20 (12%) indeterminate, and 31 (18.6%) malignant (Table 1). Of the 142 FS diagnoses, 89 (53.3%) were benign, 21 (12.6%) indeterminate, and 32 (34.1%) malignant. Final diagnoses on permanent sections resulted in 98 (58.7%) benign and 69 (41.3%) malignant lesions (Table 1).
The comparison of the diagnostic categories among the 76 patients with both FNA/CNB and FS is shown in Table 2. Of note, FS was able to provide a definitive diagnosis in all five ND cases, and in 61.1% (11/18) of tumors initially deemed as indeterminate on preoperative assessment. In addition, shifts in diagnoses were noted in a subset of cases with initial definitive FNA/CNB diagnoses: of the 35 cases deemed as benign (NN or BN) preoperatively, three (8.6%) were reclassified as malignant on FS. All three subsequently confirmed malignancies on permanent sections (Table 3 and Figure 1); of the 18 lesions reported as malignant on FNA/CNB, four (22.2%) were interpreted as benign on intraoperative assessment, with three of these benign lesions confirmed on PS (Table 3 and Figure 1).
Table 2.
Comparison of FNA/CNB and frozen section diagnoses (n = 76) in patients with both previous FNA/CNB and FS
| FNA/CNB | Frozen section | Total | ||
|---|---|---|---|---|
| Benign | Indeterminate | Malignant | ||
| Non-diagnostic | 4 (80%) | 1 (20%) | 5 | |
| Non-neoplastic | 2 (50%) | 2 (50%) | 4 | |
| Benign neoplasm | 28 (90.3%) | 2 (6.5%) | 1 (3.2%) | 31 |
| Indeterminate | 6 (33.3%) | 7 (38.9%) | 5 (27.8%) | 18 |
| Malignant | 4 (22.2%) | 14 (77.8%) | 18 | |
FNA/CNB fine-needle aspiration/core-needle biopsy
Table 3.
Diagnostic discrepancies from FNA to FS and clinical consequences
| FNA | FS | Final | Clinical Consequences |
|---|---|---|---|
| 1. Reactive lymph node | Poorly differentiated carcinoma | Poorly differentiated carcinoma | Total parotidectomy, neck dissection |
| 2. Abscess | SCC with cystic degeneration and abscess | Suspicious for primary SCC in the setting of abscess | Neck dissection |
| 3. Atypical basaloid SGN, favor AdCC | PA | PA | Superficial parotidectomy with facial nerve preservation |
| 4. Suspicious for cystic SCC | Bland cystic lesion, favor benign | Benign salivary duct cyst | No neck dissection, no deep parotid lobe excision |
| 5. Suspicious for SCC | WT | WT with extensive squamous metaplasia | No neck dissection, no deep parotid lobe excision |
| 6. Myoepithelial carcinoma | Scar | Residual cells suggestive of myoepithelial carcinoma | None |
| 7. Suggestive of WT | Fibrosis, ductal proliferation, chronic inflammation, suspicious for MEC | Low grade MEC | Total parotidectomy |
SCC squamous cell carcinoma, SGN salivary gland neoplasm, AdCC adenoid cystic carcinoma, PA pleomorphic adenoma, WT warthin tumor, MEC mucoepidermoid carcinoma
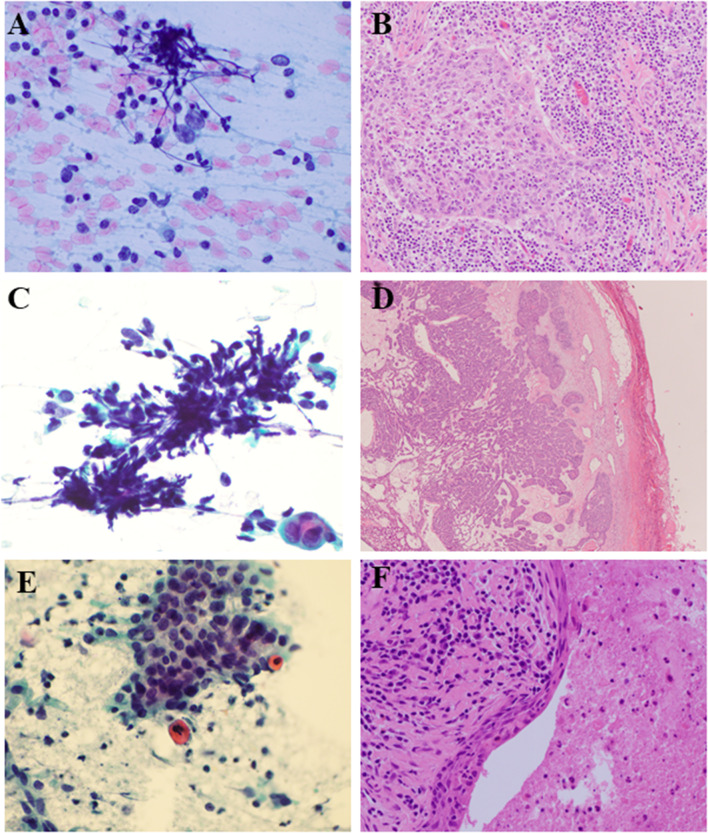
Fig. 1.
Discrepant cases. a Case 1 FNA 60X PAP stain- very rare large malignant cells in a background of abundant lymphocytes. b Case 1 Resection 20X HE- poorly differentiated carcinoma with abundant admixed lymphocytes. c Case 3 FNA 60X PAP stain- basaloid cells with crush artifact and no stroma. d Case 3 Resection 4X HE- well circumscribed cellular pleomorphic adenoma. e Case 4 FNA 60X PAP stain- scant atypical cells with squamous differentiation and bland appearing basaloid cells. f Case 4 Resection 40X HE- benign salivary duct cyst with squamous metaplasia
Calculated diagnostic test measures for the use of FS in comparison to FNA/CNB alone are shown in Table 4. Of note, nondiagnostic FNA cases (5) were excluded from the calculations. Significant differences were noted in the comparison of such measures between these two scenarios (Table 4). In particular, intraoperative assessment provided a relative increase of 33.3% (CI 13.1–62.4%) in specificity and 38.5% (CI 15.1–73.6%) in PPV when compared to preoperative FNA/CNB alone.
Table 4.
Comparison of sensitivity, specificity, positive predictive value, and negative predictive value between FNA/CNB and frozen section (n = 71)
| Statistics | FNA/CNB | Frozen section | p value | ||||
|---|---|---|---|---|---|---|---|
| Estimate (%) | CI(95%) | Estimate (%) | CI(95%) | ||||
| Lower (%) | Upper (%) | Lower (%) | Upper (%) | ||||
| Sensitivity | 83.87 | 70.92 | 96.82 | 70.97 | 54.99 | 86.95 | 0.344* |
| Specificity | 75.00 | 61.58 | 88.42 | 100.00 | 100.00 | 100.00 | 0.002* |
| PPV | 72.22 | 57.59 | 86.85 | 100.00 | 100.00 | 100.00 | 0.0003** |
| NPV | 85.71 | 74.12 | 97.31 | 81.63 | 70.79 | 92.47 | 0.518** |
FNA/CNB fine-needle aspiration/core-needle biopsy, CI confidence interval, PPV positive predictive value, NPV negative predictive value
*Exact Binomial Test for Differences in Sensitivity and Specificity: Performs an exact binomial test for differences in sensitivity and specificity of two binary diagnostic tests in a paired study design
**Generalized Score Statistic for Comparison of Predictive Values: Performs a test for differences in (positive and negative) predictive values of two binary diagnostic tests using a generalized score statistic proposed by Leisenring, Alonzo and Pepe (2000)
Discussion
It has been long recognized that most tumors that arise in the parotid gland are benign. As illustrated by our results, pleomorphic adenoma, followed by Warthin tumor, are the two most common benign neoplasms to arise in the parotid gland [9, 19–22]. These two entities are usually readily identifiable on cytology, which makes FNA a valuable diagnostic tool in the evaluation of parotid nodules. However, pitfalls are well documented [8, 23, 24], and false-negative rates of up to 20% have been reported [20]. In addition, preoperative evaluation cannot always yield a definitive diagnosis. In our cohort, 3.6% of cases were deemed as ND and 12%, as indeterminate on FNA/CNB. In a meta-analysis including 5647 patients with parotid masses, the probabilities of ND and indeterminate cytology were reported at 5.3% and 14.7%, respectively [25].
The use of intraoperative frozen section in refining diagnoses has been suggested as a solution to potentially overcome the limitations of FNA. [11, 13, 15] The majority of cases initially deemed as ND or indeterminate on FNA/CNB in our study were able to achieve a definitive diagnosis on FS (100% and 61.1%, respectively). This is in agreement with the results from a similar cohort of 260 patients who underwent parotidectomy, to whom intraoperative evaluation was also able to provide a definitive diagnosis in the majority of cases: 73.7% (14/19) and 61.1% (18/29) of lesions with a previous ND and indeterminate FNA result, respectively [26]. Furthermore, in our cohort, a subset of cases with initial definitive FNA/CNB results had changes in diagnosis on FS evaluation, with most of these confirmed on permanent sections (Table 3).
Discrepant cases from FNA to FS included cases in which the neoplastic cells were obscured by a dense lymphoid component (cases 1, 2, and 7) and cases in which tissue architecture was required for diagnosis (cases 3, 4, and 5). Basaloid lesions can be challenging to subclassify both on FNA as well as on FS, especially when stroma is scant or absent (case 3). Squamous atypia misinterpreted on FNA as squamous cell carcinoma (SCC) is also a common pitfall (cases 4 and 5). Sampling error during frozen section was noted in a patient with a prior history of myoepithelial carcinoma (case 6). Selected images of available cases are provided to illustrate the above diagnostic challenges (Fig. 1).
As noted on Table 3, FS guides immediate surgical approach with a more complete local resection for malignant lesions, which may include removing the deep parotid lobe, facial nerve sacrifice, and neck dissection. A more radical surgical approach is usually reserved for high grade SG tumors, but in selected cases it may also apply to low grade neoplasms [16].
As noted above, both FNA and FS have technical limitations, but in our cohort FS of parotid gland lesions was able to provide a definitive diagnosis in the majority of patients with preoperative non-diagnostic and indeterminate diagnoses. Moreover, the use of FS in addition to FNA/CNB was able to provide a significant increase in specificity and PPV, with relative increases of 33.3% in specificity and 38.5% in PPV, respectively, while differences in sensitivity and NPV were not significant. It is difficult to compare our results to those of prior studies that have reported on the diagnostic accuracy of FNA and intraoperative assessment of parotid lesions.
First, we acknowledge that the use of paired FNA/CNB in the preoperative evaluation of parotid lesions is not a routine practice in many centers, and this approach might have lowered our incidence of ND and indeterminate preoperative results in comparison to studies that used only FNA. The occasional presence of CNB in addition to FNA smears was not well documented in many older reports, therefore we cannot evaluate if there was any difference in sensitivity or specificity for cases in which a core was available. Moreover, preoperative samples in our cohort were reported according to institutional criteria, which may slightly differ from the ones listed in the current standardized reporting system for salivary gland specimens [27].
Furthermore, the calculation of accuracy assumes that a test has only two possible results: benign or malignant. However, the evaluation of parotid lesions is more complex, as it includes diagnostic categories that go beyond the above two categories, ND and indeterminate. Different authors have used distinct approaches on converting multiple reporting categories into a dichotomous system for these analyses. In particular, while some excluded indeterminate results from these calculations [9, 14, 25, 26, 28], others have opted to incorporate them into either benign or malignant groups [11, 20]. We find the latter approach to be more appropriate, as a significant proportion of parotid samples routinely fall into indeterminate categories, and excluding them from analyses would not accurately represent a real-practice scenario.
In the meta-analysis by Liu et al, the estimated sensitivity and specificity for cytology are 78% (CI 74–82%) and 98% (CI 97–98%), respectively [25]. While our calculated sensitivity for FNA/CNB alone is similar [83.9% (CI 70.9–96.8%)], our specificity is considerably lower [75% (CI, 61.6–88.4%)]. We believe the decision to exclude indeterminate results from their pooled analysis might have contributed to this discrepancy. Notably, in a different meta-analysis, the authors conclude that it is not possible to address the clinical usefulness of parotid FNA owing to the large variability in the included study results [28]. Similar differences in numbers can be seen when looking at frozen section data. A group of researchers evaluated the accuracy of intraoperative assessment for parotid lesions in a meta-analysis with 1880 cases. Their estimates for FS sensitivity and specificity were 99% (CI 98–100%) and 90% (CI 81–94%), respectively [14]. These numbers do not considerably overlap with our estimated 71% (CI 55–87%) sensitivity and 100% (CI 100–100%) specificity for the use of intraoperative FS. Their decision to exclude inconclusive FS results from these calculations might have contributed to the differences. On the other hand, in a cohort of 220 cases, incorporating indeterminate diagnoses into their analyses, the authors reported 77% sensitivity and 100% specificity of FS, similar to our estimates. Irrespective of methodology used, several authors agree that parotid FS has higher specificity than sensitivity [12, 13, 15, 26, 29].
Statistical comparisons of test performance between salivary gland pre- and intraoperative assessments must be examined with caution. In our study, to evaluate the role of FS in clarifying FNA/CNB diagnoses, and to allow for subsequent statistical comparisons, accuracy measures were calculated only for paired cases, that is, those patients who underwent both procedures. Different cohorts, however, may have included lesions that had only FNA, only FS and both in the same pooled analysis, mixing paired and unpaired cases in the same calculations. Acknowledging this potential pitfall, published studies seem to agree there is a role for intraoperative assessment in the diagnostic workup of parotid lesions [12–15, 30]. Confronting results between a parotid FNA and a parotid FS meta-analysis, a group of authors conclude, using area under the summary receiver operating characteristic curve (AUSROC), that the diagnostic accuracy of FS [AUSROC = 0.99 (CI 0.98–1.00)] is significantly higher than the one from FNA [AUSROC = 0.96 (CI 0.94–0..97)] [14, 28]. We believe that the sensitivity reported for FNA/CNB herein supports its use in the initial assessment of parotid lesions. The additional use of FS, however, provided a significant increase in specificity and PPV.
The feasibility of using routine parotid FS in addition to preoperative evaluation, although proven herein able to refine FNA/CNB diagnoses, depends on other factors not reported in this study. First, intraoperative assessment is associated with supplementary costs and we understand these extra charges are not trivial issues, which may represent one of the major hurdles to performing FS evaluation. Second, intraoperative consultation might be associated with extended operation time and potential surgical complications. This association, however, was not statistically significant in a previous study [15].
A limitation of our study is the lack of clarity regarding the surgeon’s specific intent to request FS in the setting of a prior FNA/ CNB diagnosis. Usually, a more specific reason for requesting a FS other than “obtain diagnosis” or “assess margins” is rarely provided on operative reports or medical records. Since this was a retrospective review that included cases at least a decade old, molecular testing did not play a significant role in diagnostic clarification in this cohort. Lastly, we understand the retrospective nature of this study comes with inherent bias; therefore, our findings should be correlated with large, prospective efforts.
In conclusion, the addition of intraoperative FS to preoperative FNA/CNB evaluation was helpful to further refine the diagnoses of parotid gland lesions and provide better guidance for the surgical intervention. The routine use of this double-assessment approach merits further investigation in prospective cohorts.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This research did not directly involved human participants and/or animals. This retrospective study was approved by the Institutional Review Board (IRB) of the Mayo Clinic and informed consent was not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guzzo M, Locati LD, Prott FJ, et al. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74(2):134–48. doi: 10.1016/j.critrevonc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Gandolfi MM, Slattery W., 3rd Parotid Gland Tumors and the Facial Nerve. Otolaryngol Clin North Am. 2016;49(2):425–34. doi: 10.1016/j.otc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Salivary Gland Cancer: Statistics | Cancer.Net [Internet]. [cited 2020 Oct 16]. https://www.cancer.net/cancer-types/salivary-gland-cancer/statistics
- 4.Rivera Rolon M, Schnadig VJ, Faiz S, Nawgiri R, Clement CG. Salivary gland fine-needle aspiration cytology with the application of the Milan system for risk stratification and histological correlation: A retrospective 6-year study. Diagn Cytopathol. 2020. https://doi.org/10.1002/dc.24478. Epub ahead of print. PMID: 32452653. [DOI] [PubMed]
- 5.Leite AA, Vargas PA, dos Santos Silva AR, Galvis MM, de Sá RS, Lopes Pinto CA, Kowalski LP, Saieg M. Retrospective application of the Milan System for reporting salivary gland cytopathology: a Cancer Center experience. Diagn Cytopathol. 2020;48(9):821–826. doi: 10.1002/dc.24464. [DOI] [PubMed] [Google Scholar]
- 6.Rohilla M, Singh P, Rajwanshi A, Gupta N, Srinivasan R, Dey P, Vashishta RK. Three-year cytohistological correlation of salivary gland FNA cytology at a tertiary center with the application of the Milan system for risk stratification. Cancer Cytopathol. 2017;125(10):767–775. doi: 10.1002/cncy.21900. [DOI] [PubMed] [Google Scholar]
- 7.Rossi ED, Wong LQ, Bizzarro T, Petrone G, Mule A, Fadda G, Baloch ZM. The impact of FNAC in the management of salivary gland lesions: Institutional experiences leading to a risk-based classification scheme. Cancer Cytopathol. 2016;124(6):388–396. doi: 10.1002/cncy.21710. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JH, Volk EE, Wilbur DC. Pitfalls in salivary gland fine-needle aspiration cytology: lessons from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytology. Arch Pathol Lab Med. 2005;129(1):26–31. doi: 10.5858/2005-129-26-PISGFC. [DOI] [PubMed] [Google Scholar]
- 9.Hanege FM, Tuysuz O, Sakallioglu O, Arslan Solmaz O. Diagnostic value of preoperative fine needle aspiration cytology in parotid gland tumors. Diagn Cytopathol. 2020. https://doi.org/10.1002/dc.24514. Epub ahead of print. PMID: 32562515. [DOI] [PubMed]
- 10.Griffith CC, Pai RK, Schneider F, Duvvuri U, Ferris RL, Johnson JT, Seethala RR. Salivary gland tumor fine-needle aspiration cytology: a proposal for a risk stratification classification. Am J Clin Pathol. 2015;143(6):839–853. doi: 10.1309/AJCPMII6OSD2HSJA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27(3):217–223. doi: 10.1002/hed.20142. [DOI] [PubMed] [Google Scholar]
- 12.Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK. Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol - Head Neck Surg. 2007;136(5):788–792. doi: 10.1016/j.otohns.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Zbaren P, Guelat D, Loosli H, Stauffer E. Parotid tumors: fine-needle aspiration and/or frozen section. Otolaryngol Head Neck Surg. 2008;139: 811-815. http://doi.org/10.1016/j.otohns.2008.09.013 PMID: 19041508 [DOI] [PubMed]
- 14.Schmidt RL, Hunt JP, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am J Clin Pathol. 2011;136(5):729–738. doi: 10.1309/AJCP2SD8RFQEUZJW. [DOI] [PubMed] [Google Scholar]
- 15.Grasl S, Kadletz L, Janik S, Riedl A, Erlacher B, Formanek M, Grasl MC, Erovic BM. Fine-needle aspiration cytology and intraoperative frozen section in parotid gland tumour surgery: A retrospective multicenter analysis of 417 cases. Clin Otolaryngol. 2019;44(3):461–465. doi: 10.1111/coa.13314. [DOI] [PubMed] [Google Scholar]
- 16.Moore MG, Yueh B, Lin DT. Controversies in the workup and surgical management of parotid neoplasms. Otolaryngol Head Neck Surg. 2020;164(1):27–36. doi: 10.1177/0194599820932512. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Obuchowski N, McClish D. Statistical Methods in Diagnostic Medicine. Wiley Series in Probability and Statistics. 2nd ed. Hoboken, New Jersey: John Wiley & Sons; 2011.
- 18.Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs wendy. Biometrics. 2000;56:345–351. doi: 10.1111/j.0006-341X.2000.00345.x. [DOI] [PubMed] [Google Scholar]
- 19.Perkins C, Toll E, Reece P. Fine-needle aspiration cytology and radiological imaging in parotid gland tumours: our experience in 103 patients. Clin Otolaryngol. 2019;44(6):1124–1127. doi: 10.1111/coa.13410. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, Kawata R, Higashino M, Nishikawa S, Terada T, Haginomori SI, Kurisu Y, Hirose Y. Values of fine-needle aspiration cytology of parotid gland tumors: a review of 996 cases at a single institution. Head Neck. 2019;41(2):358–365. doi: 10.1002/hed.25503. [DOI] [PubMed] [Google Scholar]
- 21.Shkedy Y, Alkan U, Mizrachi A, Shochat T, Dimitstein O, Morgenstern S, Shpitzer T, Bachar G. Fine-needle aspiration cytology for parotid lesions, can we avoid surgery? Clin Otolaryngol. 2018;43(2):632–637. doi: 10.1111/coa.13038. [DOI] [PubMed] [Google Scholar]
- 22.Eytan DF, Yin LX, Maleki Z, Koch WM, Tufano RP, Eisele DW, Boahene KDO, Fakhry C, Bishop JA, Westra WH, Gourin CG. Utility of preoperative fine needle aspiration in parotid lesions. Laryngoscope. 2018;128(2):398–402. doi: 10.1002/lary.26776. [DOI] [PubMed] [Google Scholar]
- 23.Salehi S, Maleki Z. Diagnostic challenges and problem cases in salivary gland cytology: A 20-year experience. Cancer Cytopathol. 2018;126(2):101–111. doi: 10.1002/cncy.21949. [DOI] [PubMed] [Google Scholar]
- 24.Dostalova L, Kalfert D, Jechova A, Koucky V, Novak S, Kuchar M, Zabrodsky M, Novakova Kodetova D, Ludvikova M, Kholova I, Plzak J. The role of fine-needle aspiration biopsy (FNAB) in the diagnostic management of parotid gland masses with emphasis on potential pitfalls. Eur Arch Otorhinolaryngol. 2020;277(6):1763–1769. doi: 10.1007/s00405-020-05868-1. [DOI] [PubMed] [Google Scholar]
- 25.Liu CC, Jethwa AR, Khariwala SS, Johnson J, Shin JJ. Sensitivity, Specificity, and Posttest Probability of Parotid Fine-Needle Aspiration. Otolaryngol Neck Surg [Internet]. 2016 Jan;154(1):9–23. http://journals.sagepub.com/doi/10.1177/0194599815607841 [DOI] [PMC free article] [PubMed]
- 26.Patel KR, Scognamiglio T, Kutler DI, Kuhel WI, Gromis J, Phillips CD, Cohen MA. Retrospective assessment of the utility of imaging, fine-needle aspiration biopsy, and intraoperative frozen section in the management of parotid neoplasms: The weill cornell medical college experience. Orl. 2015;77(3):171–179. doi: 10.1159/000381678. [DOI] [PubMed] [Google Scholar]
- 27.Faquin WC, Rossi ED, Baloch Z, Barkan GA, Foschini MP, Kurtycz DFI, Pusztaszeri M, Vielh P. The Milan system for reporting salivary gland cytopathology. The Milan System for Reporting Salivary Gland Cytopathology. 2018.
- 28.Schmidt RL, Hall BJ, Wilson AR, Layfield LJ. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol. 2011;136(1):45–59. doi: 10.1309/AJCPOIE0CZNAT6SQ. [DOI] [PubMed] [Google Scholar]
- 29.Badoual C, Rousseau A, Heudes D, Carnot F, Danel C, Meatchi T, Hans S, Bruneval P, Brasnu D, Laccourreye O. Evaluation of frozen section diagnosis in 721 parotid gland lesions. Histopathology. 2006;49(5):538–540. doi: 10.1111/j.1365-2559.2006.02527.x. [DOI] [PubMed] [Google Scholar]
- 30.Mianroodi AAA, Sigston EA, Vallance NA. Frozen section for parotid surgery: Should it become routine? ANZ J Surg. 2006;76(8):736–739. doi: 10.1111/j.1445-2197.2006.03844.x. [DOI] [PubMed] [Google Scholar]