Abstract
The dopamine transporter (DAT) mediates the inactivation of released dopamine (DA) through its reuptake, and thereby plays an important homeostatic role in dopaminergic neurotransmission. Amphetamines exert their stimulant effects by targeting DAT and inducing the reverse transport of DA, leading to a dramatic increase of extracellular DA. Animal models have proven critical to investigating the molecular and cellular mechanisms underlying transporter function and its modulation by psychostimulants such as amphetamine. Here we establish a behavioral model for amphetamine action using adult Drosophila melanogaster. We use it to characterize the effects of amphetamine on sleep and sleep architecture. Our data show that amphetamine induces hyperactivity and disrupts sleep in a DA-dependent manner. Flies that do not express a functional DAT (dDAT null mutants) have been shown to be hyperactive and to exhibit significantly reduced sleep at baseline. Our data show that, in contrast to its action in control flies, amphetamine decreases the activity of dDAT null mutants and restores their sleep by modulating distinct aspects of sleep structure. To begin to explore the circuitry involved in the actions of amphetamine on sleep, we also describe the localization of dDAT throughout the fly brain, particularly in neuropils known to regulate sleep. Together, our data establish Drosophila as a robust model for studying the regulatory mechanisms that govern DAT function and psychostimulant action.
Introduction
The dopamine transporter (DAT) mediates the inactivation of released dopamine (DA) through its reuptake[1, 2], and thereby plays a critical role in modulating the physiological functions of DA, which include motor control, arousal, motivation and reward-seeking behavior[3]. DAT is also the main molecular target for psychostimulants, including amphetamines (AMPHs), methylphenidate, and cocaine[4]. AMPHs are transported across the plasma membrane by DAT and then into synaptic vesicles by the vesicular monoamine transporter (VMAT), thereby causing the release of vesicular DA into the cytoplasm[4–7]. Non-exocytic efflux of this cytoplasmic DA through DAT-mediated reverse transport results in a dramatic increase of extracellular DA and is believed to play a major role in the psychostimulatory and rewarding properties of AMPHs[4, 5, 8]. In contrast, cocaine and methylphenidate are competitive inhibitors of DAT function, leading to the accumulation of extracellular DA after its exocytic release[4]. AMPH and methylphenidate are the most commonly prescribed drugs for the treatment of attention deficit hyperactivity disorder (ADHD). While psychostimulants can cause restlessness and hyperactivity in healthy subjects[9], they can effectively decrease hyperactivity in patients with ADHD[10], although dosing may play a role in this paradoxical response[11]. The psychomotor effects of psychostimulants have been extensively modeled in rodents, where they induce hyperactivity in wild-type animals. Intriguingly, DAT knockout (KO) mice and rats, which are hyperactive at baseline due to their inability to clear released DA, decrease their activity in response to both AMPH and cocaine[12–15]. However, the precise mechanism of action of these drugs in the absence of DAT remains poorly understood.
DAT is widely conserved across vertebrate and invertebrate species. The Drosophila DAT gene (dDAT) was cloned in 2001 and classified based on sequence homology, cellular expression, and functional properties[16]. Comparative analysis showed that dDAT and human DAT share 50% overall sequence identity, with ~80% similarity in the transmembrane domains. dDAT was found to efficiently transport DA and tyramine, as has been shown for human DAT[16]. Pharmacologically, dDAT diverges somewhat from its mammalian homologs and more closely mirrors the C. elegans homolog, ceDAT, in that its profile is a hybrid of that of the mammalian DAT and of the norepinephrine transporter (NET)[16]. Like the mammalian DATs, dDAT is stereoselective for (+)-AMPH over (-AMPH)[16]. It is also sensitive to cocaine, the NET-selective inhibitor nisoxetine, and the tricyclic antidepressants desipramine, imipramine, and amitriptyline. In contrast, the DAT-selective GBR-compounds 12909 and 12935 are at least 30 times less potent against dDAT than they are against the mammalian DATs[16]. This suggests the possibility that dDAT and ceDAT represent primordial monoamine transporter genes that existed before the emergence of the mammalian catecholamine carrier subtypes, DAT and NET.
With its accessibility to genetic and molecular analyses, Drosophila has emerged as a powerful and tractable model system for studying the regulation of DA signaling in general and of DAT function in particular. The fly homologs of many molecules involved in DA neurotransmission have been identified, including the synthesis enzymes tyrosine hydroxylase (TH)[17] and dopa-decarboxylase (DDC)[18], the vesicular transporter VMAT[7, 19], and DA receptors[20]. DA regulates similar functions in flies to those in mammals[21], including sleep and circadian rhythm[22], aggression[23], attention[24], reward[25, 26], and learning and memory[27, 28]. Sleep behaviors have been thoroughly documented and characterized in flies[22, 29–32]. Episodes of quiescence meet several criteria used to identify sleep in mammals, including circadian control, increased arousal thresholds, reduced brain activity, and a homeostatic response to sleep deprivation[31–34]. Sleep has been shown to be promoted by serotonin and gamma-aminobutyric acid (GABA) in flies, whereas DA has been shown to promote arousal[22]. Critically, a dDAT null mutant named fumin, which means sleepless in Japanese, was serendipitously identified as a sleep mutant[35]. Similarly to DAT-KO mice and rats, mutation[35, 36] or knockdown[37] of dDAT leads to heightened levels of activity and reduced sleep, consistent with increased levels of extracellular DA caused by impairment of reuptake.
Psychostimulants that act at DAT, including AMPH[38, 39], methamphetamine[40], methylphenidate[37, 39, 41], and cocaine[42, 43] have been shown to alter locomotor activity and/or sleep in wild-type flies; methylphenidate was further found to ameliorate sleep deficits caused by knockdown of dDAT[37]. We previously demonstrated that Drosophila larvae increase their activity in response to AMPH in a DA-dependent manner[39]. We further showed that while dDAT null larvae do not display an increase in locomotion when fed AMPH, the response can be rescued upon expression of the human DAT transgene in DA neurons, using the UAS/GAL4 binary expression system[39]. We and others have since used this “humanized” fly model to investigate the distinct mechanisms by which different genetic and pharmacological manipulations modulate the actions of DAT, including the phosphorylation of the amino terminus of DAT[38, 39], the interaction of DAT with the membrane raft protein Flotillin-1[39], and the electrostatic interactions between specific regions of the DAT amino terminus and PIP2 lipids[44, 45].
The locomotor assay we previously used to study AMPH action in larvae[38, 39] presented a number of limitations, including a lack of high-throughput potential and difficulty in studying the modulation of DAT-mediated sleep behavior by psychostimulants. For this reason, we sought to establish a model for AMPH-induced behavior in adult flies. We show that flies exhibit heightened locomotor activity and disrupted sleep when fed AMPH, in a DA-dependent manner. In contrast, we show that AMPH exerts the opposite effect on dDAT null mutants, ameliorating their sleep deficit. Further analysis of sleep structure suggests that AMPH exerts this paradoxical effect by primarily acting on pathways that promote sleep initiation in the dDAT null brain. To begin to explore the distinct circuits where AMPH acts at dDAT to modulate behavior, we also examined the localization of endogenous dDAT in the brain using immunofluorescence analysis and show, for the first time, the extensive breadth of dDAT localization and dopaminergic innervation of various structures throughout the fly brain. Together, our data establish Drosophila as a robust model for studying the regulatory mechanisms that govern DAT function and its modulation by psychostimulants.
Results
Differential regulation of activity and sleep by AMPH in the presence or absence of dDAT
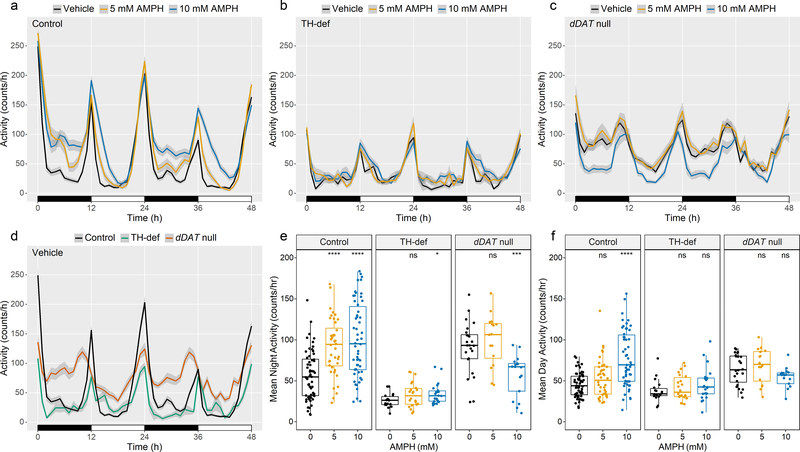
To establish a genetic model for AMPH-induced arousal in Drosophila, we quantified the effect of AMPH treatment on locomotor activity and sleep in the Drosophila isogenic background strain white1118 (w1118) using the TriKinetics Drosophila Activity Monitoring (DAM) system (Fig. 1)[46]. Flies were placed individually in tubes containing food made of agar and sucrose, delivered in water (vehicle) or AMPH solution. Flies were continuously monitored for movement by infrared beam arrays. Activity was measured as the number of times the fly crossed the beam per minute. In 12:12 h light:dark (LD) conditions, flies exhibited a typical bimodal activity pattern, with activity peaks occurring in the morning (lights on) and in the evening (lights off) (Fig. 2, Control). Consistent with published data[47, 48], we found that TH-deficient flies that display severely diminished levels of DA in the brain, exhibited significantly lower levels of activity (Fig. 2, TH-def). In contrast, flies that carry a homozygous null mutation in the gene encoding dDAT were hyperactive at baseline (Fig. 2, dDAT null), as previously described[35, 36]. Treatment of w1118 (control) flies with AMPH led to a significant increase in activity (Fig. 2a, e, f, Control), and this response was dependent in large part on DA, as it was significantly blunted in TH-deficient flies (Fig. 2b, e, f, TH-def). Notably, the stimulatory effect of AMPH was more prominent during nighttime, consistent with previous studies showing that the wake-promoting effect of DA in flies is buffered by light[49]. dDAT null flies did not increase their activity to 5 mM AMPH, a concentration that elicited a significant response in control flies, consistent with DAT being the main molecular target for AMPH. Furthermore, in contrast to our observations in control flies, we found that treatment with 10 mM AMPH led to a dramatic decrease in the activity of dDAT null mutants, similar to what has been observed in DAT-KO mice and rats[12–14] (Fig. 2c, e, f, dDAT null).
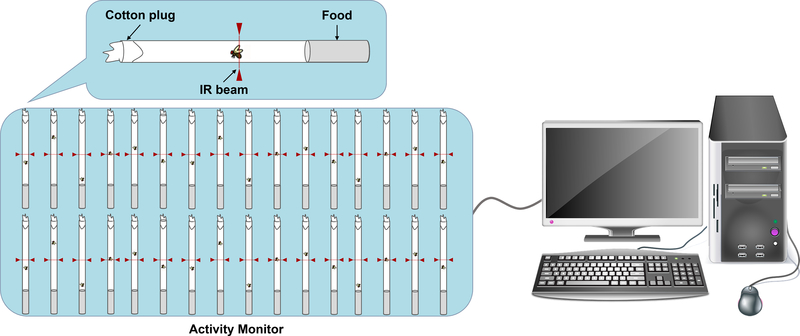
Fig. 1. Drosophila Activity Monitoring System.
Flies are placed individually in polycarbonate tubes containing food on one side and a cotton plug on the other, and monitored using Drosophila Activity Monitors (DAMs). Each monitor is capable of recording up to 32 animals simultaneously. Monitors are housed in a designated behavior chamber under 12 h light:dark conditions at 25°C and ~50% humidity. Locomotor activity is measured by recording crossings through an infrared (IR) beam at the center of the tube. Data collection is automated via the DAMSystem software, and output files are analyzed in R using the Rethomics framework[87].
Fig. 2. AMPH induces hyperactivity in an isogenic fly strain but ameliorates hyperactivity in dDAT null mutants.
(a-c) Activity profiles upon exposure to Vehicle, 5 mM AMPH or 10 mM AMPH for (a) w1118 (control, n = 60, 40, 54), (b) TH-deficient (TH-def, n = 18, 22, 22), or (c) dDAT null (n = 24, 16, 17) flies. Profiles represent the first 48 h of data collection in 12:12 h light:dark (LD) conditions. Night and day are depicted by black and white bars, respectively. Shaded area around the mean indicates a 95% confidence interval (CI). (d) Baseline activity profiles for control, TH-def, and dDAT null flies (n= 60, 18, 24) exposed to vehicle. Profiles represent the first 48 h of data collection in LD conditions. Night and day are depicted by the black and white bars, respectively. Shaded area around the mean indicates a 95% CI. (e-f) Mean baseline and AMPH-induced activity during (e) the first two nights of recording (0 – 12 h and 24 – 36 h) and (f) the first two days of recording (12 – 24 h and 36 – 48 h) for individual flies of each genotype, indicated by dots. Box plot signifies upper and lower quartiles, and the center line indicates the median. Asterisks indicate significance, Mann-Whitney-Wilcoxon, ****p <0.0001, ***p <0.001, **p < 0.01, *p <0.05, ns = not significant (compared to Vehicle).
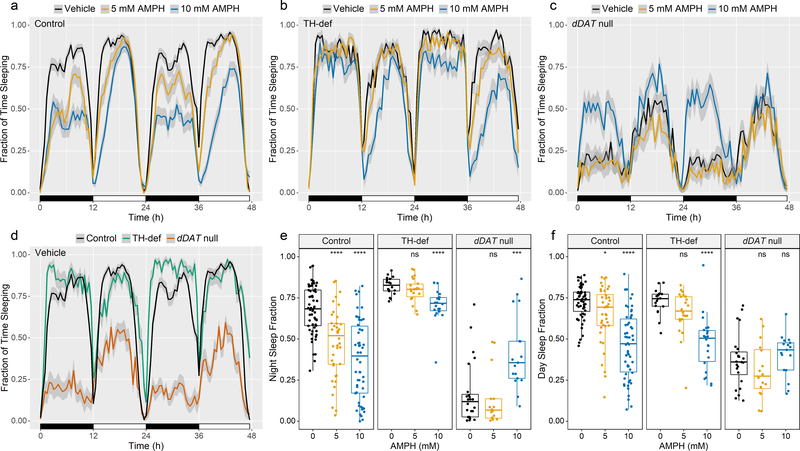
We next analyzed the effects of AMPH on sleep, which is commonly defined in Drosophila as 5 or more minutes of inactivity, based on the fact that the arousal threshold of resting flies is significantly elevated after 5 minutes of inactivity[31]. Our data showed that control flies spent significantly less time sleeping when fed either concentration of AMPH during both daytime and nighttime (Fig. 3a, e, f, Control). TH-deficient flies spent significantly more time sleeping than control at night (p < 0.0001) and exhibited a blunted response to AMPH (Fig. 3b, d, e, TH-def), suggesting that DA mediates most of the wake-promoting effects of AMPH during nighttime. dDAT null mutants, on the other hand, spent significantly more time sleeping when fed 10 mM AMPH compared to vehicle (Fig. 3c, e, f, dDAT null), in stark contrast to the severe AMPH-induced sleep disruption observed in control flies.
Fig. 3. AMPH inhibits sleep in an isogenic fly strain but restores it in dDAT null mutants.
(a-c) Sleep profiles upon exposure to Vehicle, 5 mM AMPH or 10 mM AMPH for (a) w1118 (control, n = 60, 40, 54), (b) TH-deficient (TH-def) (n = 18, 22, 22), or (c) dDAT null (n = 24, 16, 17) flies. Profiles represent the first 48 h of recording during LD conditions. Night and day are depicted by black and white bars, respectively. Shaded area around the mean indicates a 95% CI. (d) Baseline sleep profiles for control, TH-def, and dDAT null flies (n= 60, 18, 24) exposed to vehicle. Profiles represent the first 48 h of data collection in LD conditions. Night and day are depicted by the black and white bars, respectively. Shaded area around the mean indicates a 95% CI. (e-f) Mean baseline and AMPH-induced changes in sleep fractions during (e) the first two nights of recording (0 – 12 h and 24 – 36 h) and (f) the first two days of recording (12 – 24 h and 36 – 48 h) for individual flies of each genotype indicated by dots. Box plot signifies upper and lower quartiles, and the center line indicates the median. Asterisks indicate significance, Mann-Whitney-Wilcoxon, ****p <0.0001, ***p <0.001, **p < 0.01, *p <0.05, ns = not significant (compared to Vehicle).
Differential regulation of sleep architecture by AMPH in the presence or absence of dDAT
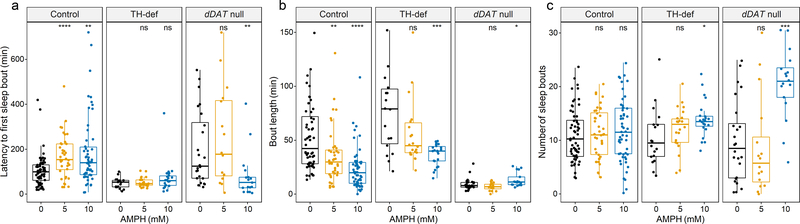
In Drosophila, sleep consists of a series of episodes (bouts), with long sleep bouts at night and shorter sleep bouts during the daytime[50]. In order to better understand the paradoxical effect of AMPH on sleep we sought to examine different aspects of sleep structure during the night in control, TH- deficient, and dDAT null flies, including sleep latency (time between lights-off and the first sleep bout), the average length of sleep bouts, and sleep bout number (Fig. 4). We found that AMPH-induced arousal in control flies was characterized by an increase in sleep latency (Fig. 4a) and a decrease in average length of sleep bout (Fig. 4b), without a significant effect on the number of sleep bouts (Fig. 4c). In other words, the flies took longer to fall asleep and slept for shorter periods of time, but the number of sleep episodes remained unchanged. TH-deficient flies experienced longer sleep bouts at baseline (p < 0.05), compared to control (Fig. 4b). When fed AMPH, they exhibited shorter sleep bouts (Fig. 4b), similar to control flies fed AMPH. However, in contrast to control, AMPH at 10 mM increased the number of sleep bouts in these flies (Fig. 4c). Thus, even though TH-deficient flies experienced less disruption in total amount of sleep loss during the night in response to AMPH (Fig. 3b, e, TH-def), their sleep was more fragmented. At baseline, dDAT null mutants did not display significant changes in sleep latency (Fig. 4a) or sleep bout number (Fig. 4c), compared to control. Rather, sleep structure analysis suggests that their sleep disruption was marked by severely shortened sleep bouts (Fig. 4b), the signature of AMPH response in control flies. Interestingly, AMPH primarily restored sleep in dDAT null mutants (Fig. 3c, e, dDAT null) by promoting a significant 2-fold increase in the number of sleep bouts (Fig. 4c), in addition to a more moderate but significant increase in the average bout length (Fig. 4b).
Fig. 4. Modulation of sleep structure by AMPH in the presence or absence of dDAT.
(a) Mean sleep latency, (b) mean sleep bout length, and (c) mean number of sleep bouts at baseline and after treatment with AMPH (5 or 10 mM) for the first two nights (0 – 12 h and 24 – 36 h) for control, TH-def, and dDAT null flies. Individual flies indicated by dots. Box plot signifies upper and lower quartiles, and the center line indicates the median. In (b) for average sleep bout length, outliers (n = 5) with sleep bouts beyond 150 min are not shown, but were included in the generation of box plots and statistical analysis. Asterisks indicate significance, Mann-Whitney-Wilcoxon, ****p <0.0001, ***p <0.001, **p < 0.01, *p <0.05, ns = not significant (compared to Vehicle).
Expression pattern of dDAT in the adult Drosophila brain
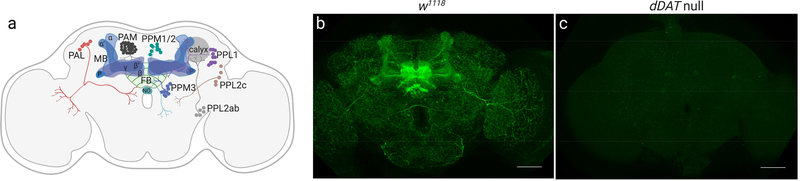
To enable circuit analysis of the actions of AMPH at dDAT, we determined the localization pattern of the transporter in the central nervous system. The adult Drosophila brain has approximately 280 TH-positive dopaminergic neurons, subdivided into distinct clusters that extend their processes to defined structures throughout the brain. The innervation patterns of two major anterior clusters of DA neurons (PAL and PAM) and six major posterior clusters (PPL1, PPL2ab, PPL2c, PPM1/2, PPM3) have been extensively mapped[51–54], and have been shown to innervate multiple centers of sleep–wake circuitry, including the mushroom bodies and the central complex[55] (Fig. 5a). Multiple studies have probed for dDAT expression in larval and adult brains using in situ hybridization[16, 56, 57] or single-cell (sc)-RNAseq[58], and have found dDAT mRNA to be expressed primarily in TH-positive neurons, as predicted by its function. However, the specific localization of the dDAT protein in the fly brain has not been mapped previously.
Fig. 5. Expression pattern of dDAT in the adult Drosophila brain.
(a) Schematic demonstrating DA neuron clusters in the anterior (PAL and PAM) and posterior (PPM1/2, PPM3, PPL1, PPL2ab, PPL2c) regions of the adult fly brain along with their major axonal projections to the mushroom body lobes (MB, blue), fan-shaped body (FB, green), and calyx (gray). (b-c) Whole-mount adult brain immunostaining of (b) the isogenic strain w1118 and (c) dDAT null flies using anti-dDAT antibody (green). (b) w1118 brains show prominent staining in the MB, FB, and other neuropils innervated by DA neurons. (c) dDAT null brains lack anti-dDAT staining, confirming the specificity of the antibody. Maximum intensity projections through the whole brain are shown. Scale bars are 50 μm.
We performed immunofluorescence analysis of dDAT throughout the fly brain, using whole-mount staining with a monoclonal antibody previously raised against a dDAT variant used for functional characterization and crystallization of dDAT for structural analysis[59]. In addition to discernable localization in dopaminergic cell bodies (Supplementary Fig. 1), we found considerable dDAT immunoreactivity at all sites of known dopaminergic innervation, including many substructures of the mushroom bodies and the central complex (Figs. 5b, 6a–e, and Supplementary Videos 1–4), consistent with the predicted localization of dDAT at presynaptic DA terminals. We did not detect immunoreactivity in brains from dDAT null mutants, confirming the specificity of the antibody (Fig. 5c).
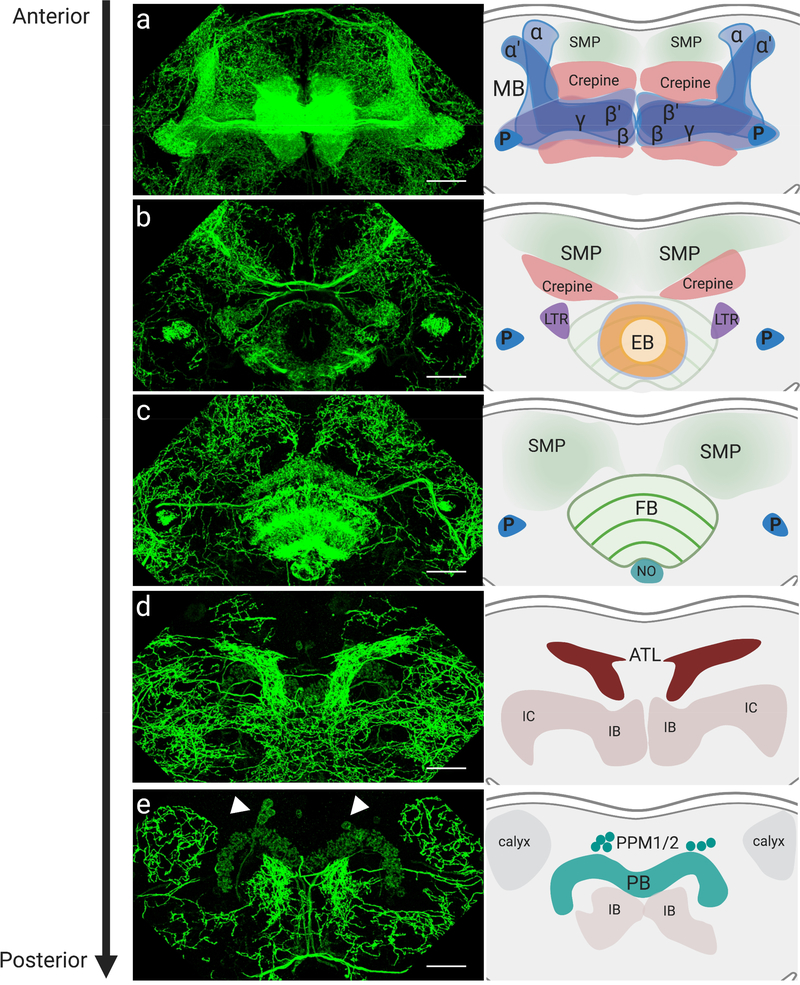
Fig. 6. Localization of dDAT in distinct neuropils of the adult Drosophila brain.
(a-e) Anterior to posterior maximum intensity projections of whole-mount brain showing anti-dDAT immunostaining (green) in the (a) mushroom body lobes, (b) ellipsoid body, (c) fan-shaped body, (d) antlers, (e) protocerebral bridge. Arrowheads indicate anti-dDAT staining in PPM1/2 DA cell bodies (refer to Supplementary Fig. 1 for higher magnification image). Schematics show neuropils with dDAT innervation. MB: mushroom body, P: peduncle, SMP: superior medial protocerebrum, EB: ellipsoid body, FB: fan-shaped body, NO: paired noduli, ATL: antlers, IB: inferior bridge, IC: inferior clamp, PB: protocerebral bridge. Scale bars are 25 μm.
We observed dDAT to be prominently localized to the mushroom bodies (Fig. 6a), an associative learning network known to be important for sleep control[60, 61]. The dendrites of ~2000 Kenyon cells per hemisphere form the calyx in the posterior of the brain, and their parallel axons extend anteriorly through the peduncle to form distinct mushroom body lobes (α/β, α’/β’ and γ lobes) (Fig. 5a), where they make synaptic connections with the dendrites of glutamatergic, cholinergic, and GABAergic mushroom body output neurons (MBONs), in a highly compartmentalized manner[62]. The Kenyon cells have been shown to regulate sleep through synaptic activation of MBONs, which then convey sleep control signals to downstream target regions. Sleep-promoting Kenyon cells activate cholinergic MBONs, whereas wake promoting Kenyon cells activate glutamatergic MBONs[60, 61]. Approximately 130 DA neurons from two different clusters, the protocerebral anterior medial (PAM) and protocerebral posterior lateral cluster neuron 1 (PPL1), project their axons into the mushroom body lobes[22] and converge onto Kenyon cell-MBON synapses, where DA is believed to act locally to modulate the efficacy of the Kenyon cell-MBON synapse[25, 63]. PAM and PPL1 neurons have also been found to synapse directly onto MBON dendrites, in addition to forming axo-axonic reciprocal synapses with Kenyon cells[64, 65]. All 4 fly DA receptors are known to be expressed in the Kenyon cells[20, 58, 66, 67], supporting a role for dopaminergic innervation in modulating their function. Activation of a subset of PAM and PPL1 neurons leads to the activation of wake-promoting MBONs and, consequently, suppression of sleep. In addition, two types of PPL2ab neurons have been shown to innervate the dendrites of Kenyon cells in the mushroom body calyx[51, 68], where we also observed prominent dDAT immunolabeling (Fig. 6e). Projecting from the mushroom bodies, MBON axons converge onto five discrete neuropils where DA neuron dendrites also project, including the lateral horn (LH), the superior intermediate protocerebrum (SLP), the superior intermediate protocerebrum (SIP), the superior medial protocerebrum (SMP), and crepine neuropil (CRE), in possible feedback and feed-forward loops that modulate behavior[62] (Fig. 6a–c). dDAT has also been shown to be expressed in α′β’ Kenyon cells[58, 66], suggesting a novel mechanism by which neurons post-synaptic to DA neurons might also regulate the duration and magnitude of DA signal they receive. Further analysis will be needed to distinguish between immunolabeled dDAT that is expressed intrinsically in Kenyon cells as opposed to dDAT that is in presynaptic DA neuron axons projecting onto the Kenyon cells and MBONs.
We also found robust and extensive immunostaining of dDAT in most neuropils of the central complex, a major locomotion and navigation center in the fly brain (Fig. 6). The central complex is made up of 5 neuropils, the ellipsoid body (EB, Fig. 6b), the fan-shaped body (FB, Fig. 6c), the paired noduli (NO, Fig. 6c), the protocerebral bridge (PB, Fig. 6e), and the asymmetrical body (AB)[69–72]. Both the dorsal FB (dFB) and ring 2 neurons (R2) of the EB have been shown to play a role in sleep. DA neurons from the PPL1 and the protocerebral posterior medial cluster neuron 3 (PPM3) clusters have been shown to project axons onto the central complex (Fig. 5a), where DA receptors can be found expressed in most neuropils[20, 67]. Activation of either PPL1[73] or PPM3[74] neurons mediates arousal by inhibiting sleep-promoting neurons in the dFB. A bilateral pair of PPM3 neurons was also shown to mediate ethanol-induced locomotor activity through action at the EB[75]. The axons of PPL1 neurons ramify throughout the dFB, whereas PPM3 neurons branch out and innervate different compartments within the central complex, including the FB and NO, as well as the EB and lateral triangles[76]. dDAT can also be seen in the PB (Fig. 6e), previously shown to be innervated by a pair of DA neurons located in the tritocerebrum (T1), found to be involved in modulating aggressive behavior[23].
Dense projections can be found in the antlers (ATL, Fig. 6d). These neuropils have not been extensively studied to date, but were recently found to receive projections from wind-sensing wedge projection neurons (WPNs)[77]. Furthermore, a recent study reconstructed the wiring of a single serotonergic neuron and found that it projects dendritic processes onto the ATL and receives input from WPNs[78]. WPNs in turn receive mechano-sensory information regarding wind orientation from the fly’s antennae[77], suggesting a new role for DA and serotonin in modulating this process.
Discussion
Animal models provide powerful tools for the investigation of transporter function and pharmacology. In recent years, Drosophila has emerged as an ideal model to study DAT function, its alteration by disease-associated mutations, and its regulation by psychostimulants. In this study, we established a model for psychostimulant-induced changes in sleep and sleep structure, in the presence or absence of dDAT. We found that while AMPH severely disrupts sleep in flies that express a functional dDAT, it paradoxically restores sleep in dDAT null mutants that exhibit major sleep defects at baseline. To identify brain circuits where dDAT functions to mediate behavior, we also examined the distribution of dDAT in the adult fly brain and showed an extensive pattern of localization, delineating the breadth of dopaminergic innervation throughout the brain.
Our data are consistent with a number of studies in both mice and rats in which psychostimulants have been shown to exert a calming effect and reduce hyperactivity caused by knockout of DAT[13, 14, 79]. These studies have suggested varied explanations for the underlying mechanisms. Studies in mice indicate that the anti-hyperkinetic effects of AMPH in DAT-KO animals are mediated by changes in serotonergic signaling, presumably mediated by action of AMPH at SERT[12, 15]. AMPH was also found to rescue long-term potentiation (LTP) defects in prefrontal cortex pyramidal neurons of DAT-KO mice at a dose that led to the erosion of LTP in controls, and this effect was found to be mediated primarily by activation of β- adrenergic receptors, suggesting that the actions of AMPH at NET mediate this response[80]. AMPH also restored cognitive function of DAT-KOs in a maze task, whereas treatment of wild-type animals replicated the behavior observed in untreated DAT-KO mice. In this same assay, treatment with the NET blocker, atomoxetine, restored cognitive performances in DAT-KO mice without affecting hyperactivity, whereas treatment with a nonselective serotonin receptor agonist rescued the hyperactivity with no effect on cognitive function[81]. Taken together, these data suggest that in the absence of DAT, other neurotransmitter systems can gain functional significance in response to hyperdopaminergic conditions. The model we have established will allow us to probe these mechanisms further in flies. In addition to input from dopaminergic neurons, the mushroom bodies, FB, and EB integrate synaptic inputs from serotonergic and octopaminergic neurons, and it will be critical to determine the effect of AMPH at these systems in the absence of dDAT. The fly homolog of SERT has been identified[82] and has been shown to localize to many of the same neuropils where we observe dDAT localization[83]. In flies tyramine and octopamine are not trace amines but rather act as main neuromodulators with functions that parallel those of epinephrine and norepinephrine in mammals[84], but their transporters have yet to be identified[57] and it therefore remains unclear whether AMPH can act directly to modulate those pathways. It will also be interesting to explore the compensatory mechanisms that arise in response to the hyperdopaminergic state in control flies fed AMPH and in dDAT null mutants. Notably, increasing DA levels by feeding flies L-DOPA has been shown to lead to reduced connectivity of serotonergic neurons to their target neurons in the mushroom bodies[85].
Our data showed that AMPH disrupts sleep in flies by delaying sleep onset after the lights go off and by decreasing the length of sleep bouts per sleep episode, suggesting that it disrupts both sleep initiation and maintenance. The effect of AMPH in TH-deficient flies was blunted; even at the higher concentration they maintained sleep levels that were comparable to the untreated control. However, their sleep was fragmented, with more sleep bouts of shorter length, suggesting an inability to enter into long sleep episodes that may correspond to deep sleep states. This is interesting in light of the recent finding that activation of a subset of serotonergic neurons in the EB fragments sleep without major changes in the total amount of sleep, and is consistent with the hypothesis that in the event of diminished DA signaling the effect of AMPH at other systems may play a more dominant role. Remarkably, in contrast to its effect in control flies, AMPH restores sleep in dDAT null mutants by inducing a much quicker sleep onset and dramatically increasing the number of sleep episodes, with only a moderate effect on sleep bout length. Taken together, our data suggest that AMPH has distinct effects on sleep structure that may offer clues to where it is acting in the absence of dDAT, likely modulating a pathway that promotes sleep initiation but not sleep consolidation and maintenance. Such “uncoupling” has been previously described and is speculated to occur in situations where sleep-promoting signals effectively silence wake-promoting neurons to initiate sleep, but fail to disinhibit sleep-maintenance neurons[86].
Our imaging analyses delineate, for the first time to our knowledge, the extent of dDAT localization in the adult fly brain. Limitations inherent to confocal microscopy, combined with the high density of dDAT antibody staining in the brain, preclude us from determining the precise subcellular distribution and relative enrichment of dDAT throughout DA neurons. Still, we were able to discern dDAT immunoreactivity at many previously described dopaminergic processes and cell bodies, as well as to identify new regions where DA signaling may be acting to modulate physiology and behavior.
Using the robust and high-throughput behavioral model we have established, and guided by our imaging studies, we can now investigate the underlying mechanisms and circuits in depth, by taking advantage of recently refined genetic tools that allow rational targeting of small subsets of DA neurons to manipulate dDAT function and DA signaling in select neural circuits[52]. Our findings can also more efficiently guide parallel investigation in mammalian systems that are not as amenable to the high-throughput studies possible in flies, to achieve a comprehensive understanding of the effects of AMPH on sleep and sleep architecture in various dopaminergic states.
Materials and methods
Fly stocks and transgenic Drosophila lines
All fly strains were reared on a standard corn meal, yeast, molasses, agar medium at 25°C and 45–47% humidity under a 12:12 h light:dark cycle. An isogenic w1118 fly strain (Exelixis strain A5001, BL-6326) was used as the control.
The dDATfmn null mutants were a gift from Dr. K. Kume (Kumamoto, Japan) and were back-crossed to the w1118 isogenic strain for 7 generations. These mutants have the 5’ portion of a roo transposon inserted into intron 6 of the dDAT gene resulting in an in-frame stop codon[35].
The TH-def flies (DTHgFS±; ple)[48] were a gift from Dr. S. Birman (Paris, France). DTHg FS± is a splice variant of dTH that allows for the rescue of the null dTH mutation (ple) only in non-neuronal cells using both TH-GAL4 and Ddc-GAL4[48] (also a gift from Dr. S. Birman).
Behavioral assay
Flies were aged for 7 days after eclosion, housed in vials containing standard medium, and entrained to a 12:12 h light:dark regime under rearing conditions. Individual aged male flies were then anesthetized briefly with CO2 and placed in polycarbonate tubes [65 mm x 5 mm x 3 mm] (length x external diameter x inside diameter) containing food consisting of 1% agar and 3% sucrose delivered in water (vehicle) or AMPH solution (5 mM or 10 mM) (Sigma, A5880). Flies were habituated to the experimental environment for 8–10 h prior to the start of data collection, which began at the onset of the first dark period.
Data acquisition and analysis
Flies were continuously monitored for movement by a single infrared beam in the center of the tube using Trikinetics Drosophila Activity Monitors (DAM 5) (TriKinetics, Waltham, MA). Each monitor is capable of recording up to 32 animals at a time. Locomotor activity was measured by recording infrared beam crossings (activity counts) by individual flies totaled in 1 min bins for ~60 h. All experiments were carried out in a designated behavior room under LD conditions at 25°C and ~45–50% humidity with ad libitum access to food (Vehicle or AMPH). Animals that died within the first 12 h of the experiment were excluded from the analysis.
Output files were analyzed in R using the previously published Rethomics framework, which seamlessly joins experimental metadata and DAM locomotion data for rapid analysis[87]. Sleep was defined as periods of inactivity lasting 5 min or longer; therefore, all immobility bouts longer than 300 s were calculated as sleep bouts (including the first 300 s). Mean activity was calculated by binning activity counts over 60 min and averaging across the first two nights of recording (0 – 12 h and 24 – 36 h) and the first two days of activity (12 – 24 h and 36 – 48 h) for each individual animal. Sleep fractions were calculated for each individual animal for the first two nights and the first two days of recording, as listed above. Sleep structure graphs, including mean sleep latency, mean sleep bout length, and mean number of sleep bouts, were calculated for each of the first two nights of recording (0 – 12 h and 24 – 36 h) and subsequently averaged for each individual. Sleep latency for each night was calculated as the time (in minutes) before the first sleep bout after lights off; flies that did not experience any sleep bouts for the entire duration of a particular night were assigned a latency value of 720 min (12 h) for that night.
Statistical analysis
Activity and sleep profiles were generated using ggplot2 and the Rethomics framework[87, 88]. Shaded areas around the mean (eg. Figs. 1a–d, 2a–d) represent a 95% confidence interval.
Boxplots were generated using ggpubr[89] and represent the median as the middle line, with lower and upper edges of the boxes representing the 25% and 75% quartiles, respectively. All outliers are shown unless otherwise noted. Asterisks indicate the statistical significance of the difference between the mean response to AMPH compared to vehicle as calculated by the non-parametric Mann-Whitney-Wilcoxon test.
Brain dissection and immunohistochemistry
Brain dissection and immunohistochemistry were performed according to the Janelia Flylight protocols available online at https://www.janelia.org/project-team/flylight/protocols. Adult fly brains were dissected in cold Schneider’s Insect Medium (S2) and fixed for 24 h in 1.2% paraformaldehyde (PFA) in S2 at 4°C. After four 10 min washes in 0.5% Triton-X100 in PBS (PBT) at 4°C, samples were blocked and permeabilized in 5% normal goat serum (NGS) in 0.5% PBT for 1.5 h at room temperature (RT). Samples were then incubated in a primary antibody solution of mouse anti-dDAT (1:1000; gift from Dr. Eric Gouaux) in 5% NGS for 3.5 h at RT and 2 overnights at 4°C. After a rinse and three 30 min washes in PBT at RT, brains were incubated in a secondary antibody solution of Alexa Fluor 488 goat anti-mouse IgG (1:500; Invitrogen) in 5% NGS in PBT for 3.5 h at RT and 2 overnights at 4°C. Secondary antibodies were then removed, samples were rinsed and then washed for three 30 min washes at RT in PBT. For pre-embedding fixation, PBT was replaced with 1.75 mL of 4% PFA in PBS for 4 h at RT. Fix was then removed and samples were rinsed and then washed for four 15 min washes at RT.
To mount the samples for confocal imaging, glass microscope slides (Fisher Scientific, Premium Superfrost, 75 × 25 mm) were prepared with two glass coverslips (Fisher Scientific, No. 1, 22 × 22 mm) placed 1 cm apart and secured with glycerol to prevent flattening of the brain sample when mounted. Whole-mount brains were then mounted to a coverslip (Thermofisher Scientific, No. 1.5, 20 × 20 mm) with ProLong Gold antifade mountant (Invitrogen) and the slide was placed on top. Slides were allowed to cure for 48 h in the dark prior to imaging.
Confocal imaging
Immunolabeled adult brains were imaged under 20x dry and 63x oil immersion objective lenses using a Leica TCS SP8 confocal microscope. Whole brain images were captured with a 20x objective and a zoom factor of 1.2 to yield a pixel size of 0.473 × 0.473 × 0.685 μm. Neuropil images were captured at 63x with a pixel size of 0.18 × 0.18 × 0.299 μm. All z-stack images were scanned sequentially with a 488 nm HyD and a 633 nm PMT laser at 1% intensity and at a resolution of 1024 × 1024 pixels.
Supplementary Material
Supplementary Video 4. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) captured at 63x.
Supplementary Image 1. Maximum intensity projection of neuronal cell bodies, as well as parts of the PCB and IB, in whole adult fly brains stained with anti-dDAT antibody (green), imaged under 63x with a 3.5x optical zoom. Scale bar indicates 10 μm. LIGHTNING adaptive deconvolution was used to obtain maximum resolution[90].
Supplementary Video 1. 3D reconstruction of an anterior segment consisting of 122 sections of 0.3-μm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63x.
Supplementary Video 2. 3D reconstruction of a posterior segment consisting of 175 sections of 0.3-μm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63x.
Supplementary Video 3. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) in Drosophila adult brain captured at 40x.
Acknowledgements
This work was supported by R01 DA041510 (JAJ and CSK), U01 DA042233 (JAJ, CSK and BLW), and T32 MH018870 (SKJ). We thank Dr. Eric Gouaux for providing the anti-dDAT antibody, Dr. Stephen Thornquist for advice on using the antibody for fluorescence imaging, Dr. Claudia Ma for her help with the initial experimental setup, and Dr. Mimi Shirazu-Hiza for sharing her Trikinetic monitors during the initial setup of the assay.
References
- 1.Aggarwal S, Mortensen OV (2017) Overview of monoamine transporters. Curr Protoc Pharmacol 79:12.16.1–12.16.17. doi: 10.1002/cpph.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hovde MJ, Larson GH, Vaughan RA, Foster JD (2019) Model systems for analysis of dopamine transporter function and regulation. Neurochem Int 123:13–21. doi: 10.1016/j.neuint.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30:188–193. doi: 10.1016/j.tins.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. doi: 10.1016/j.neuron.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433. doi: 10.1016/j.pneurobio.2005.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Reith MEA, Gnegy ME (2020) Molecular mechanisms of amphetamines. Handb Exp Pharmacol 258:265–297. doi: 10.1007/164_2019_251 [DOI] [PubMed] [Google Scholar]
- 7.Freyberg Z, Sonders MS, Aguilar JI, et al. (2016) Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun 7:10652. doi: 10.1038/ncomms10652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karam CS, Javitch JA (2018) Phosphorylation of the amino terminus of the dopamine transporter: regulatory mechanisms and implications for amphetamine action. Adv Pharmacol 82:205–234. doi: 10.1016/bs.apha.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asser A, Taba P (2015) Psychostimulants and movement disorders. Front Neurol 6:75. doi: 10.3389/fneur.2015.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortese S (2020) Pharmacologic Treatment of Attention Deficit-Hyperactivity Disorder. N Engl J Med 383:1050–1056. doi: 10.1056/NEJMra1917069 [DOI] [PubMed] [Google Scholar]
- 11.Arnsten AFT (2006) Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383. doi: 10.1038/sj.npp.1301164 [DOI] [PubMed] [Google Scholar]
- 12.Gainetdinov RR, Wetsel WC, Jones SR, et al. (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283:397–401. doi: 10.1126/science.283.5400.397 [DOI] [PubMed] [Google Scholar]
- 13.Leo D, Sukhanov I, Zoratto F, et al. (2018) Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J Neurosci 38:1959–1972. doi: 10.1523/JNEUROSCI.1931-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efimova EV, Gainetdinov RR, Budygin EA, Sotnikova TD (2016) Dopamine transporter mutant animals: a translational perspective. J Neurogenet 30:5–15. doi: 10.3109/01677063.2016.1144751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall FS, Sora I, Hen R, Uhl GR (2014) Serotonin/dopamine interactions in a hyperactive mouse: reduced serotonin receptor 1B activity reverses effects of dopamine transporter knockout. PLoS One 9:e115009. doi: 10.1371/journal.pone.0115009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pörzgen P, Park SK, Hirsh J, et al. (2001) The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: a primordial carrier for catecholamines. Mol Pharmacol 59:83–95. doi: 10.1124/mol.59.1.83 [DOI] [PubMed] [Google Scholar]
- 17.Neckameyer WS, White K (1993) Drosophila tyrosine hydroxylase is encoded by the pale locus. J Neurogenet 8:189–199. doi: 10.3109/01677069309083448 [DOI] [PubMed] [Google Scholar]
- 18.Livingstone MS, Tempel BL (1983) Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature 303:67–70. doi: 10.1038/303067a0 [DOI] [PubMed] [Google Scholar]
- 19.Greer CL, Grygoruk A, Patton DE, et al. (2005) A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol 64:239–258. doi: 10.1002/neu.20146 [DOI] [PubMed] [Google Scholar]
- 20.Karam CS, Jones SK, Javitch JA (2020) Come Fly with Me: An overview of dopamine receptors in Drosophila melanogaster. Basic Clin Pharmacol Toxicol 126 Suppl 6:56–65. doi: 10.1111/bcpt.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, Seto ES (2014) Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim 63:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nall A, Sehgal A (2014) Monoamines and sleep in Drosophila. Behav Neurosci 128:264–272. doi: 10.1037/a0036209 [DOI] [PubMed] [Google Scholar]
- 23.Alekseyenko OV, Chan Y-B, Li R, Kravitz EA (2013) Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci USA 110:6151–6156. doi: 10.1073/pnas.1303446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Swinderen B (2011) Attention in Drosophila. Int Rev Neurobiol 99:51–85. doi: 10.1016/B978-0-12-387003-2.00003-3 [DOI] [PubMed] [Google Scholar]
- 25.Waddell S (2013) Reinforcement signalling in Drosophila; dopamine does it all after all. Curr Opin Neurobiol 23:324–329. doi: 10.1016/j.conb.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaun KR, Azanchi R, Maung Z, et al. (2011) A Drosophila model for alcohol reward. Nat Neurosci 14:612–619. doi: 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waddell S (2010) Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci 33:457–464. doi: 10.1016/j.tins.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaun KR, Rothenfluh A (2017) Dopaminergic rules of engagement for memory in Drosophila. Curr Opin Neurobiol 43:56–62. doi: 10.1016/j.conb.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckwith EJ, French AS (2019) Sleep in drosophila and its context. Front Physiol 10:1167. doi: 10.3389/fphys.2019.01167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ly S, Pack AI, Naidoo N (2018) The neurobiological basis of sleep: Insights from Drosophila. Neurosci Biobehav Rev 87:67–86. doi: 10.1016/j.neubiorev.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G (2000) Correlates of sleep and waking in Drosophila melanogaster. Science 287:1834–1837. doi: 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- 32.Hendricks JC, Finn SM, Panckeri KA, et al. (2000) Rest in Drosophila is a sleep-like state. Neuron 25:129–138. doi: 10.1016/s0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- 33.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ (2002) Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol 12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3 [DOI] [PubMed] [Google Scholar]
- 34.Bushey D, Tononi G, Cirelli C (2015) Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc Natl Acad Sci USA 112:4785–4790. doi: 10.1073/pnas.1419603112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kume K, Kume S, Park SK, et al. (2005) Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno T, Kume K (2014) Functional characterization of dopamine transporter in vivo using Drosophila melanogaster behavioral assays. Front Behav Neurosci 8:303. doi: 10.3389/fnbeh.2014.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Voet M, Harich B, Franke B, Schenck A (2016) ADHD-associated dopamine transporter, latrophilin and neurofibromin share a dopamine-related locomotor signature in Drosophila. Mol Psychiatry 21:565–573. doi: 10.1038/mp.2015.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizzo AB, Karam CS, Zhang Y, et al. (2014) Amphetamine-induced behavior requires CaMKII-dependent dopamine transporter phosphorylation. Mol Psychiatry 19:279–281. doi: 10.1038/mp.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pizzo AB, Karam CS, Zhang Y, et al. (2013) The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Mol Psychiatry 18:824–833. doi: 10.1038/mp.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andretic R, van Swinderen B, Greenspan RJ (2005) Dopaminergic modulation of arousal in Drosophila. Curr Biol 15:1165–1175. doi: 10.1016/j.cub.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 41.van Swinderen B, Brembs B (2010) Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci 30:1003–1014. doi: 10.1523/JNEUROSCI.4516-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bainton RJ, Tsai LT, Singh CM, et al. (2000) Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol 10:187–194. doi: 10.1016/s0960-9822(00)00336-5 [DOI] [PubMed] [Google Scholar]
- 43.McClung C, Hirsh J (1998) Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol 8:109–112. doi: 10.1016/s0960-9822(98)70041-7 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton PJ, Belovich AN, Khelashvili G, et al. (2014) PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat Chem Biol 10:582–589. doi: 10.1038/nchembio.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belovich AN, Aguilar JI, Mabry SJ, et al. (2019) A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol Psychiatry. doi: 10.1038/s41380-019-0620-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffenberger C, Lear BC, Keegan KP, Allada R (2010) Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc 2010:pdb.prot5518. doi: 10.1101/pdb.prot5518 [DOI] [PubMed] [Google Scholar]
- 47.Cichewicz K, Garren EJ, Adiele C, et al. (2017) A new brain dopamine-deficient Drosophila and its pharmacological and genetic rescue. Genes Brain Behav 16:394–403. doi: 10.1111/gbb.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riemensperger T, Isabel G, Coulom H, et al. (2011) Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci USA 108:834–839. doi: 10.1073/pnas.1010930108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang Y, Haynes P, Pírez N, et al. (2011) Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 14:889–895. doi: 10.1038/nn.2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andretic R, Shaw PJ (2005) Essentials of sleep recordings in Drosophila: moving beyond sleep time. Meth Enzymol 393:759–772. doi: 10.1016/S0076-6879(05)93040-1 [DOI] [PubMed] [Google Scholar]
- 51.Mao Z, Davis RL (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3:5. doi: 10.3389/neuro.04.005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie T, Ho MCW, Liu Q, et al. (2018) A genetic toolkit for dissecting dopamine circuit function in drosophila. Cell Rep 23:652–665. doi: 10.1016/j.celrep.2018.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheffer LK, Xu CS, Januszewski M, et al. (2020) A connectome and analysis of the adult Drosophila central brain. Elife. doi: 10.7554/eLife.57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartenstein V, Cruz L, Lovick JK, Guo M (2017) Developmental analysis of the dopamine-containing neurons of the Drosophila brain. J Comp Neurol 525:363–379. doi: 10.1002/cne.24069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Artiushin G, Sehgal A (2017) The Drosophila circuitry of sleep-wake regulation. Curr Opin Neurobiol 44:243–250. doi: 10.1016/j.conb.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meissner GW, Nern A, Singer RH, et al. (2019) Mapping Neurotransmitter Identity in the Whole-Mount Drosophila Brain Using Multiplex High-Throughput Fluorescence in Situ Hybridization. Genetics 211:473–482. doi: 10.1534/genetics.118.301749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thimgan MS, Berg JS, Stuart AE (2006) Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J Exp Biol 209:3383–3404. doi: 10.1242/jeb.02328 [DOI] [PubMed] [Google Scholar]
- 58.Croset V, Treiber CD, Waddell S (2018) Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. Elife. doi: 10.7554/eLife.34550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penmatsa A, Wang KH, Gouaux E (2013) X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503:85–90. doi: 10.1038/nature12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joiner WJ, Crocker A, White BH, Sehgal A (2006) Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441:757–760. doi: 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- 61.Pitman JL, McGill JJ, Keegan KP, Allada R (2006) A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441:753–756. doi: 10.1038/nature04739 [DOI] [PubMed] [Google Scholar]
- 62.Aso Y, Hattori D, Yu Y, et al. (2014) The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3:e04577. doi: 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aso Y, Siwanowicz I, Bräcker L, et al. (2010) Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol 20:1445–1451. doi: 10.1016/j.cub.2010.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cervantes-Sandoval I, Phan A, Chakraborty M, Davis RL (2017) Reciprocal synapses between mushroom body and dopamine neurons form a positive feedback loop required for learning. Elife. doi: 10.7554/eLife.23789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takemura S-Y, Aso Y, Hige T, et al. (2017) A connectome of a learning and memory center in the adult Drosophila brain. Elife. doi: 10.7554/eLife.26975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng B, Li Q, Liu X, et al. (2019) Chemoconnectomics: mapping chemical transmission in drosophila. Neuron 101:876–893.e4. doi: 10.1016/j.neuron.2019.01.045 [DOI] [PubMed] [Google Scholar]
- 67.Kahsai L, Carlsson MA, Winther AME, Nässel DR (2012) Distribution of metabotropic receptors of serotonin, dopamine, GABA, glutamate, and short neuropeptide F in the central complex of Drosophila. Neuroscience 208:11–26. doi: 10.1016/j.neuroscience.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 68.Boto T, Stahl A, Zhang X, et al. (2019) Independent contributions of discrete dopaminergic circuits to cellular plasticity, memory strength, and valence in drosophila. Cell Rep 27:2014–2021.e2. doi: 10.1016/j.celrep.2019.04.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strausfeld NJ, Hirth F (2013) Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340:157–161. doi: 10.1126/science.1231828 [DOI] [PubMed] [Google Scholar]
- 70.Wolff T, Rubin GM (2018) Neuroarchitecture of the Drosophila central complex: A catalog of nodulus and asymmetrical body neurons and a revision of the protocerebral bridge catalog. J Comp Neurol 526:2585–2611. doi: 10.1002/cne.24512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Liu Q, Tabuchi M, Wu MN (2016) Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell 165:1347–1360. doi: 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donlea JM, Pimentel D, Miesenböck G (2014) Neuronal machinery of sleep homeostasis in drosophila. Neuron 81:1442. doi: 10.1016/j.neuron.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Q, Liu S, Kodama L, et al. (2012) Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol 22:2114–2123. doi: 10.1016/j.cub.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueno T, Tomita J, Tanimoto H, et al. (2012) Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci 15:1516–1523. doi: 10.1038/nn.3238 [DOI] [PubMed] [Google Scholar]
- 75.Kong EC, Woo K, Li H, et al. (2010) A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One 5:e9954. doi: 10.1371/journal.pone.0009954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omoto JJ, Nguyen B-CM, Kandimalla P, et al. (2018) Neuronal constituents and putative interactions within the drosophila ellipsoid body neuropil. Front Neural Circuits 12:103. doi: 10.3389/fncir.2018.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suver MP, Matheson AMM, Sarkar S, et al. (2019) Encoding of wind direction by central neurons in drosophila. Neuron 102:828–842.e7. doi: 10.1016/j.neuron.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coates KE, Calle-Schuler SA, Helmick LM, et al. (2020) The wiring logic of an identified serotonergic neuron that spans sensory networks. J Neurosci 40:6309–6327. doi: 10.1523/JNEUROSCI.0552-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barr AM, Lehmann-Masten V, Paulus M, et al. (2004) The selective serotonin-2A receptor antagonist M100907 reverses behavioral deficits in dopamine transporter knockout mice. Neuropsychopharmacology 29:221–228. doi: 10.1038/sj.npp.1300343 [DOI] [PubMed] [Google Scholar]
- 80.Xu T-X, Ma Q, Spealman RD, Yao W-D (2010) Amphetamine modulation of long-term potentiation in the prefrontal cortex: dose dependency, monoaminergic contributions, and paradoxical rescue in hyperdopaminergic mutant. J Neurochem 115:1643–1654. doi: 10.1111/j.1471-4159.2010.07073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del’Guidice T, Lemasson M, Etiévant A, et al. (2014) Dissociations between cognitive and motor effects of psychostimulants and atomoxetine in hyperactive DAT-KO mice. Psychopharmacology 231:109–122. doi: 10.1007/s00213-013-3212-8 [DOI] [PubMed] [Google Scholar]
- 82.Demchyshyn LL, Pristupa ZB, Sugamori KS, et al. (1994) Cloning, expression, and localization of a chloride-facilitated, cocaine-sensitive serotonin transporter from Drosophila melanogaster. Proc Natl Acad Sci USA 91:5158–5162. doi: 10.1073/pnas.91.11.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giang T, Ritze Y, Rauchfuss S, et al. (2011) The serotonin transporter expression in Drosophila melanogaster. J Neurogenet 25:17–26. doi: 10.3109/01677063.2011.553002 [DOI] [PubMed] [Google Scholar]
- 84.Roeder T (2005) Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol 50:447–477. doi: 10.1146/annurev.ento.50.071803.130404 [DOI] [PubMed] [Google Scholar]
- 85.Niens J, Reh F, Çoban B, et al. (2017) Dopamine modulates serotonin innervation in the drosophila brain. Front Syst Neurosci 11:76. doi: 10.3389/fnsys.2017.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agosto J, Choi JC, Parisky KM, et al. (2008) Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci 11:354–359. doi: 10.1038/nn2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geissmann Q, Garcia Rodriguez L, Beckwith EJ, Gilestro GF (2019) Rethomics: An R framework to analyse high-throughput behavioural data. PLoS One 14:e0209331. doi: 10.1371/journal.pone.0209331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wickham H (2011) ggplot2. WIREs Comp Stat 3:180–185. doi: 10.1002/wics.147 [DOI] [Google Scholar]
- 89.Kassambara A (2020) ggpubr: “ggplot2” Based Publication Ready Plots. CRAN [Google Scholar]
- 90.Reymann J (2018) LIGHTNING: Image Information Extraction by Adaptive Deconvolution [White paper]. Leica Microsystems [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 4. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) captured at 63x.
Supplementary Image 1. Maximum intensity projection of neuronal cell bodies, as well as parts of the PCB and IB, in whole adult fly brains stained with anti-dDAT antibody (green), imaged under 63x with a 3.5x optical zoom. Scale bar indicates 10 μm. LIGHTNING adaptive deconvolution was used to obtain maximum resolution[90].
Supplementary Video 1. 3D reconstruction of an anterior segment consisting of 122 sections of 0.3-μm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63x.
Supplementary Video 2. 3D reconstruction of a posterior segment consisting of 175 sections of 0.3-μm thickness from a whole-mount Drosophila brain fluorescently labeled with anti-dDAT antibody (green), captured by confocal microscopy at 63x.
Supplementary Video 3. Raw fluorescent Z-stack images showing antibody-labeled dDAT (green) in Drosophila adult brain captured at 40x.