Abstract
Background and Aims:
We conducted a retrospective cohort study comparing the risk of serious infections between patients treated with tumor necrosis factor-α (TNFα) antagonists vs. vedolizumab in patients with inflammatory bowel diseases (IBD).
Methods:
Using an administrative claims database, we identified patients with IBD who were new-users of either TNFα antagonists or vedolizumab between 2014–2018 and had insurance coverage for at least 1y before and after treatment initiation. We compared the risk of serious infections (infections requiring hospitalization) between patients treated with vedolizumab or TNFα antagonists using marginal structural Cox proportional hazard models adjusted for baseline disease characteristics, healthcare utilization, comorbidities, and time-varying use of corticosteroids, immunomodulators and opiates.
Results:
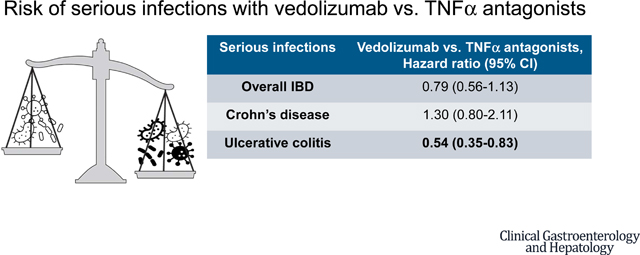
We included 4881 patients treated with TNFα antagonists (age, 41±15y, 60% with Crohn’s disease [CD]) of whom 434 developed serious infections over 5786 person-year [PY] follow-up, and 1106 patients treated with vedolizumab (age, 44±16y, 39% with CD) of whom 86 developed serious infections over 1040-PY follow-up. Vedolizumab was associated with 46% lower risk of serious infections as compared with TNFα antagonists in patients with ulcerative colitis (HR,0.54 [95% CI,0.35–0.83), but no significant differences were observed in patients with CD (HR,1.30 [0.80–2.11]). Vedolizumab was associated with lower risk of extra-intestinal serious infections in patients with UC, but higher risk of gastrointestinal serious infections in patients with CD.
Conclusions:
In an observational study of patients with IBD, vedolizumab was associated with lower risk of serious infections as compared with TNFα antagonists, in patients with UC, but not in patients with CD.
Keywords: infections, safety, choice, colitis, biologics
Graphical Abstract
With expanding treatment options for the management of inflammatory bowel diseases (IBD), comparative efficacy and safety are two key considerations in choosing optimal therapy. While a recent head-to-head trial, indirect treatment comparison network meta-analyses and multiple observational studies have reported on comparative effectiveness of different biologic agents for the management of Crohn’s disease (CD) and ulcerative colitis (UC), there has been limited assessment of the comparative safety of modern therapies.1–6
Tumor necrosis factorα (TNFα) antagonists have been associated with an increased risk of serious infections in clinical registries and real-world observational studies.7–10 In contrast, vedolizumab, a gut-specific anti-integrin agent, is presumed to be associated with lower risk of serious infections, although there is paucity of registry or large real-world observational studies. In an open-label extension study of six trials of vedolizumab, Colombel and colleagues observed that incidence rates of serious infections were similar for vedolizumab vs. placebo (4.3 vs. 3.8 per 100 person years [PY]).11 However, open-label extension studies, which selectively include patients responding to the treatment of interest, may not provide reliable estimates of the risk of serious infections.7 There has been limited head-to-head comparison of the safety of TNFα antagonists vs. vedolizumab. In the recent VARSITY trial in patients with moderate-severe UC, no significant differences were observed in the risk of serious infections between vedolizumab vs. adalimumab (incidence rate, 1.6 vs. 2.2 per 100-PY).1 Network meta-analysis of randomized trials suggest no significant differences in the risk of infections in TNFα antagonists vs. vedolizumab-treated patients.12,13 In recent multi-center propensity-score matched studies, vedolizumab was associated with lower risk of serious infections in patients with UC as compared with TNFα antagonists (odds ratio [OR], 0.41; 95% confidence intervals [CI], 0.23–0.73) but not in patients with CD (OR, 1.18; 95% CI, 0.79–1.80).6,14 However, these studies have been limited by low rates of serious infections.
We conducted a real-world observational study comparing the risk of serious infections in patients treated with vedolizumab vs. TNFα antagonists in a de-identified administrative claims database. We hypothesized that vedolizumab would be associated with a lower risk of serious infections as compared with TNFα antagonists, particularly in patients with UC. We also hypothesized that use of vedolizumab would be associated with a lower risk of extra-intestinal infections, not directly related to underlying disease.
METHODS
Data Source
We conducted a retrospective analysis of de-identified medical and pharmacy administrative claims from a large database, OptumLabs® Data Warehouse (online supplement).15
Study Population
We identified all patients who filled a prescription (or received an infusion) for TNFα antagonists (infliximab, adalimumab, certolizumab pegol and/or golimumab) and/or vedolizumab between January 1, 2014 and December 31, 2018. From this cohort, we included adult patients (18–89 years) with: (a) at least one diagnosis code for IBD (CD: ICD-9 555.x or ICD-10 K50; UC: ICD-9 556.x or ICD-10 K51) prior to index date (date of first filled prescription or infusion for TNFα antagonists or vedolizumab), either from an inpatient or outpatient visit, (b) continuous health plan enrollment with pharmacy benefits, with no prescription for candidate biologic in the 12 months prior to index date (new-user design), and minimum 12-month enrollment in health plan after index date (patients who received candidate for <12m, and discontinued due to intolerance or non-response, but still remained in the health plan were included). In case a patient received diagnostic codes for both CD and UC, then the patient was classified as having CD if the majority of diagnostic codes were for CD.
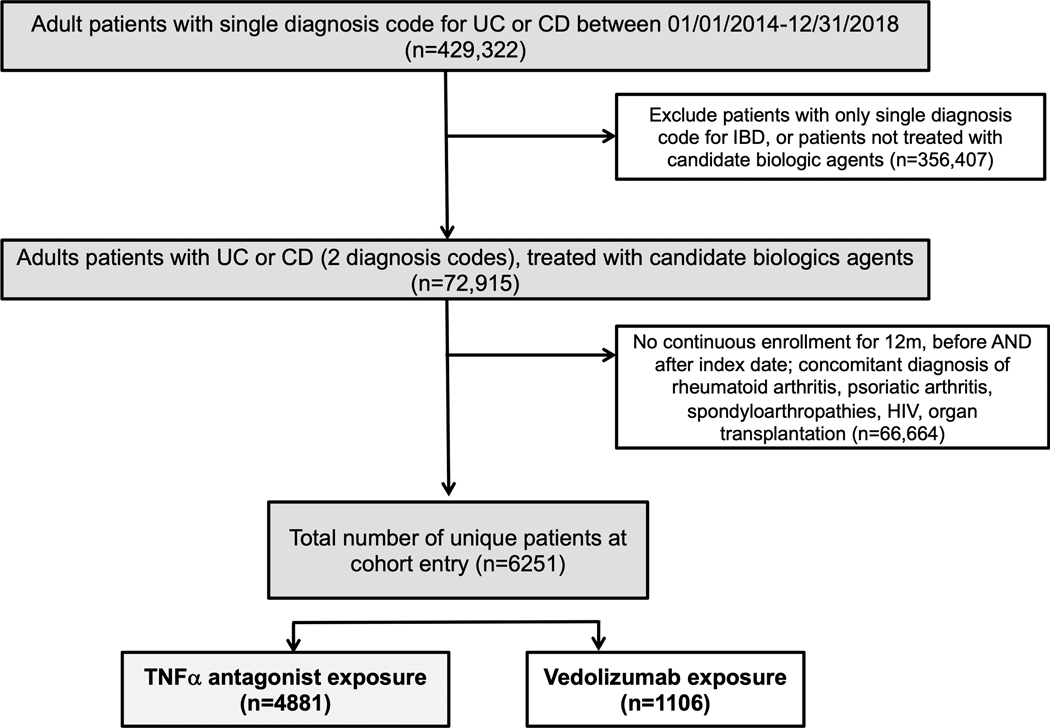
We excluded patients with (a) human immunodeficiency virus infection, congenital immunodeficiency, or organ transplantation, or (b) concomitant diagnosis of rheumatoid arthritis, ankylosing spondylitis, psoriasis or psoriatic arthritis within the baseline 12-month period prior to prescription of TNFα antagonists. Figure 1 shows the flow of patients for identification of the cohort.
Figure 1.
Flow of patients for identification of TNFα antagonist- or vedolizumab-treated patients with inflammatory bowel diseases
Exposure
The primary exposures of interest were TNFα antagonists and vedolizumab (online supplement). We considered patients as being continuously exposed from the index date for the duration of their prescription. Patients could contribute to both exposure groups sequentially (TNFα antagonists and vedolizumab) as long as they were new-users of specific biologic exposure (12 months drug-free period without same biologic); patients who switched from one TNFα antagonist to another contributed person-time to TNFα antagonist group for both exposures combined. Patients were followed until occurrence of the outcome of interest (see below), disenrollment from healthcare plan, treatment discontinuation (absence of new prescription or fill for a period of >4 months, without switching to alternative agent), or last date of follow-up (December 31, 2019).
Outcome
The primary outcome of interest was time to serious infections, defined as infection requiring hospitalization. These infections were identified based on principal discharge diagnoses (ICD-9 or ICD-10 codes) and included infections of the respiratory tract, skin and soft tissue, genitourinary tract, gastrointestinal tract, central nervous system, and septicemia/sepsis (eTable 1).16 In prior studies, considering medical chart reviews as the reference, this definition for serious infection requiring hospitalization has consistently shown positive predictive values of 80% or higher.17,18 Due to low event rate for opportunistic infections requiring hospitalization, we did not perform separate comparative analyses for risk of opportunistic infections.
Covariates
Time-fixed (baseline) covariates (at time of biologic exposure or in preceding 12m) included demographics: age, sex, race, census region, calendar year, comorbidity burden measured using the Elixhauser index, frailty,19 health care utilization (hospitalization or emergency department visits), serious and/or opportunistic infections, as well as IBD phenotype (CD or UC), abdominal surgery and receipt of endoscopy and/or abdominal imaging.20 We did not have access to individual patient medical records, endoscopy reports or biochemical parameters.
Since concomitant exposure to immunomodulators, corticosteroids and opiates influence risk of serious infections in biologic-treated patients with IBD, these medication exposures were included as time-varying covariates updated every 30 days.
Statistical Analysis
The association between treatment (vedolizumab vs. TNFα antagonists) and outcomes of interest (serious and opportunistic infections) were estimated using marginal structural Cox proportional hazard models. Marginal structural models are appropriate in the presence of time-dependent covariates (such as exposure to corticosteroids and immunomodulators) that might be associated with both exposure and outcomes and could also be affected by past exposure to biologic agents. Briefly, weights were constructed from the inverse probability of treatment and the probability of being censored; these probabilities were derived from logistic regression models which adjusted for the time-fixed and time-varying covariates listed above; these weight calculations were performed as suggested by Cole and Hernán.21 Final weights were trimmed at the 1st and 99th percentile to improve model estimation and to reduce the impact of extreme outliers on final effect estimates.
Pre-planned stratified analyses were performed, including: (a) CD and UC and (b) age at time of biologic exposure (<60y vs. ≥60y). Due to comparable to slightly higher efficacy of vedolizumab vs. TNFα antagonists in patients with UC, and potentially lower efficacy of vedolizumab in patients with CD, we hypothesized that vedolizumab would be associated with lower risk of serious infections as compared with TNFα antagonists in patients with UC, but not CD. We also performed sensitivity analyses excluding: (a) patients with serious infections and (b) patients who received immunomodulators, in the 12m baseline period. Since gastrointestinal infections may be related to disease (for example, intra-abdominal or perianal abscesses in patients with CD) or to treatment, we performed additional analyses comparing risk of gastrointestinal and extra-intestinal infections between vedolizumab vs. TNFα antagonists. We hypothesized that vedolizumab would be associated with lower risk of extra-intestinal infections (less likely to be directly related to IBD), but not gastrointestinal infections (potentially directly related to underlying IBD), as compared with TNFα antagonists.
RESULTS
Our cohort included 4881 new users of TNFα antagonists, who contributed 5786-PY follow-up, and 1106 new users of vedolizumab, who contributed 1040-PY follow-up. Baseline characteristics of TNFα antagonists and vedolizumab-treated patients with IBD are shown in Table 1; baseline characteristics in patients with CD and UC are shown in eTable 2. Among TNFα antagonist-treated patients, 60% had CD, 29.4% were hospitalized and 12.2% underwent abdominal surgery in the 12m prior to initiation of TNFα antagonist. Only 0.9% had received vedolizumab in the 12m prior to starting TNFα antagonist; 61.5% received corticosteroids and 13.2% received immunomodulators in the 3 months prior to starting TNFα antagonist. Adalimumab and infliximab were the most common TNFα antagonists used (CD: adalimumab [56.3%], infliximab [36.3%], certolizumab pegol (7.4%); UC: adalimumab [52.6%], infliximab [38.9%] and golimumab [8.5%]). Among vedolizumab-treated patients, 61% had UC, 26.2% were hospitalized and 6.5% underwent abdominal surgery in the 12m prior to initiation of vedolizumab. Approximately 20% patients had received TNFα antagonist in the 12m prior to starting vedolizumab (only 3.5% in 3m prior); 60.2% received corticosteroids and 13.5% received immunomodulators in the 3 months prior to starting vedolizumab. Overall, 8.2% TNFα antagonist- and 6.3% vedolizumab-treated patients experienced serious infections in the baseline 12m prior to starting candidate biologic. TNFα antagonist-treated patients carried a lower burden of comorbidities.
Table 1.
Baseline demographic characteristics, healthcare utilization and IBD-related medication use in the 12 months prior to initiation of index biologic, in the entire cohort.
| Variable | TNFα antagonists (n=4881) | Vedolizumab (n=1106) |
|---|---|---|
| Demographic variables | ||
| Mean age ± SD, years | 41 ± 15 | 44 ± 16 |
| Sex (% males) | 50.8 | 49.3 |
| Race/Ethnicity (%) • Caucasians • African American • Asian • Hispanic • Unknown |
71.9 12.6 3.6 7.3 4.6 |
73.0 10.8 3.7 7.1 5.4 |
| IBD phenotype • Crohn’s disease (%) • Ulcerative colitis (%) |
60.0 40.0 |
39.3 60.7 |
| Mean (± SD) follow-up after starting biologic, months | 15.0 ± 13.7 | 12.0 ± 10.8 |
| Healthcare utilization and comorbidities (in 12 months prior to starting candidate biologic) | ||
| Emergency room visits (% pts with ≥1) | 48.5 | 45.6 |
| Inpatient hospitalization (% pts with ≥1) | 29.4 | 26.2 |
| Abdominal Imaging (% of pts with ≥1) | 56.2 | 45.1 |
| Endoscopic procedures (% pts with ≥1) | 75.0 | 73.5 |
| Abdominal surgery (% pts with ≥1) | 12.2 | 6.5 |
| Mean (± SD) Elixhauser score • Elixhauser score 2–3 • Elixhauser score 4 or more |
1.8 ± 1.9 29.1 15.4 |
2.1 ± 2.1 31.8 19.9 |
| Major comorbidities • Chronic obstructive lung disease • Diabetes with or without complication • Hypertension with or without complication • Obesity • Anemia |
12.6 9.3 22.0 8.3 7.2 |
13.2 12.9 26.1 10.8 10.0 |
| Serious infection (% pts with ≥1) | 8.2 | 6.3 |
| IBD-related medication use (in 3 or 12 months prior to starting candidate biologic) | ||
| TNFα antagonists (in baseline 12m) (%) | 0 | 19.8 |
| Vedolizumab (in baseline 12m) (%) | 0.9 | 0 |
| Oral corticosteroids • Prior use (in baseline 12m), % • Recent use (in baseline 3m), % |
74.2 61.5 |
77.7 60.2 |
| Immunomodulators • Prior use (in baseline 12m), % • Recent use (in baseline 3m), % |
16.3 13.2 |
18.2 13.5 |
| Opiates (in baseline 12m), % | 42.7 | 39.9 |
[Abbreviations: n=number of patients, SD=standard deviation, TNF=tumor necrosis factor]
After initiating biologic therapy, 435 TNFα antagonist- and 85 vedolizumab-treated patients experienced serious infection requiring hospitalization, corresponding to an incidence rate (IR) of 5.6 (5.0–6.2) and 5.5 (4.3–7.1), per 100-PY exposure, respectively. The most common serious infections were sepsis (244 infections, 205 in TNFα antagonist-treated patients vs. 39 in vedolizumab-treated patients), gastrointestinal infections (172 infections, 136 in TNFα antagonist-treated patients vs. 36 in vedolizumab-treated patients) and pulmonary infections (153 infections, 128 in TNFα antagonist-treated patients vs. 25 in vedolizumab-treated patients). Fifty four TNFα antagonist- and 13 vedolizumab-treated patients experienced opportunistic infections requiring hospitalization, corresponding to IR 0.7 (95% CI, 0.5–0.9) and 0.7 (95% CI, 0.3–1.4), per 100-PY exposure, respectively. Incidence rate ratio (IRR) of serious infections for vedolizumab vs. TNFα antagonists for all patients with IBD, CD and UC was 0.99 (95% CI, 0.74–1.31), 1.40 (95% CI, 0.90–2.09) and 0.70 (95% CI, 0.46–1.05), respectively (Table 2).
Table 2.
Incidence rate of serious infections and opportunistic infections by treatment exposure during follow-up, overall, and in groups stratified by IBD phenotype.
| Incidence rate (95% CI), per 100 person-years | Incidence rate ratios (95% CI), vedolizumab vs. TNFα antagonists | ||
|---|---|---|---|
| All Patients with IBD | |||
| Vedolizumab (n=1106, 1040 p-y) | TNFα antagonists (n=4881, 5786 p-y) | ||
| Serious infections • Overall • Extra-intestinal • Gastrointestinal |
5.2 (4.0–6.8) 2.6 (1.8–3.8) 2.6 (1.8–3.8) |
5.3 (4.8–5.9) 3.7 (3.2–4.2) 1.6 (1.3–1.9) |
0.99 (0.74–1.31) 0.76 (0.50–1.11) 1.55 (0.97–2.39) |
| Opportunistic infections requiring hospitalization | 0.4 (0.1–3.2) | 0.6 (0.5–0.9) | 0.97 (0.37–2.20) |
| Patients with Crohn’s disease | |||
| Vedolizumab (n=435, 394 p-y) | TNFα antagonists (n=2931, 3703 p-y) | ||
| Serious infections • Overall • Extra-intestinal • Gastrointestinal |
7.0 (4.8–10.1) 5.5 (3.6–8.4) 1.8 (0.9–3.7) |
5.0 (4.3–5.8) 3.9 (3.3–4.6) 1.0 (0.8–1.4) |
1.40 (0.90–2.09) 1.39 (0.83–2.21) 1.69 (0.64–3.82) |
| Opportunistic infections requiring hospitalization | 1.0 (0.4–2.7) | 0.5 (0.3–0.8) | 1.88 (0.47–5.62) |
| Patients with ulcerative colitis | |||
| Vedolizumab (n=671, 646 p-y) | TNFα antagonists (n=1950, 2083 p-y) | ||
| Serious infections • Overall • Extra-intestinal • Gastrointestinal |
4.6 (3.2–6.6) 1.4 (0.7–2.7) 3.1 (2.0–4.8) |
6.5 (5.5–7.7) 3.7 (3.0–4.7) 2.8 (2.1–3.6) |
0.70 (0.46–1.05) 0.37 (0.16–0.74) 1.11 (0.63–1.88) |
| Opportunistic infections requiring hospitalization | 0.5 (0.1–1.4) | 0.9 (0.6–1.5) | 0.48 (0.09–1.63) |
[Abbreviations: CI=confidence interval, n=number of patients, p-y=person-years, TNF=tumor necrosis factor]
In analysis using marginal structural Cox proportional hazard models, accounting for time-fixed and time-varying exposure to corticosteroids, immunomodulators and opiates, no significant differences were observed in the risk of serious infections between vedolizumab and TNFα antagonists in the full cohort of patients with IBD (HR, 0.79; 95% CI, 0.56–1.13). However, on pre-planned stratified analysis, we observed that vedolizumab was associated with 46% lower risk of serious infections as compared with TNFα antagonists in patients with UC (HR, 0.54; 95% CI, 0.35–0.83) (eFigure 1), while no significant differences were observed between vedolizumab and TNFα antagonists in patients with CD (HR, 1.30; 95% CI, 0.80–2.11) (Table 3, eFigure 2). No significant differences were observed between vedolizumab and TNFα antagonists by age at initiation of medications (<60y vs. 60y or more), or in a subset of patients starting biologic monotherapy (HR, 0.89; 95% CI, 0.63–1.25) or without serious infections during the preceding 12m (HR, 0.78; 95% CI, 0.53–1.14). Vedolizumab was associated with an increased risk of gastrointestinal serious infections (HR, 1.82; 95% CI, 1.08–3.07), but not extra-intestinal serious infections (HR, 0.81; 95% CI, 0.45–1.43), in all patients with IBD. On post-hoc analysis additionally adjusting for presence of perianal disease in the baseline period in patients with CD, prior exposure to TNFα antagonists (in vedolizumab-treated patients) and vedolizumab (in TNFα antagonist-treated patients), and combination therapy with biologic and immunomodulators as a time varying covariate, results were unchanged (eTable 3). The statistical model for the primary analysis has been presented in the online supplement (eTable 4).
Table 3.
Risk of serious infections, overall, and by organ type in patients treated with vedolizumab vs. TNFα antagonists (reference), using marginal structural models. Estimates highlighted in bold are statistically significant.
| Vedolizumab vs. TNFα antagonists (reference), | All serious infections | Extra-intestinal serious infections | Gastrointestinal serious infections |
|---|---|---|---|
| adjusted HR (and 95% CI) | |||
| All patients with IBD | 0.79 (0.56–1.13) | 0.81 (0.45–1.43) | 1.82 (1.08–3.07) |
| IBD phenotype • Crohn’s disease • Ulcerative colitis |
1.30 (0.80–2.11) 0.54 (0.35–0.83) |
1.43 (0.73–2.79) 0.41 (0.15–1.12) |
2.90 (1.21–6.94) 1.20 (0.57–2.53) |
| Age at biologic initiation • ≥60y • <60y |
0.79 (0.38–1.62) 0.77 (0.52–1.16) |
0.68 (0.19–2.38) 0.74 (0.37–1.51) |
2.92 (0.90–9.43) 1.04 (0.44–2.43) |
| Excluding patients with serious infection in preceding 12m | 0.78 (0.53–1.14) | 0.75 (0.41–1.36) | 1.87 (1.07–3.28) |
| Excluding patients with immunomodulator exposure in preceding 12m | 0.89 (0.63–1.25) | 0.98 (0.51–1.88) | 1.80 (0.89–3.62) |
[Abbreviations: CI=confidence interval, HR=hazard ratio, TNF=tumor necrosis factor]
In subgroup analyses of patients with UC, consistent trends were observed with lower risk of serious infections with vedolizumab being observed in younger and older adults, in patients on biologic monotherapy at index date, and in patients without serious infections in preceding 12m (Table 4). Lower risk of serious infections with vedolizumab was driven by lower risk of extra-intestinal infections (HR, 0.41; 95% CI, 0.15–1.12) and not gastrointestinal infections (HR, 1.20; 95% CI, 0.57–2.53). The most common gastrointestinal serious infections in patients with UC were Clostridiodes difficile colitis (71.6%), infectious gastroenteritis (13.1%) and cholangitis (7.6%). On subgroup in patients with CD, no significant differences were observed in risk of vedolizumab vs. TNFα antagonists, in younger and older adults, in patients on biologic monotherapy at index date, and in patients without serious infections in preceding 12m (Table 4). Vedolizumab-treated patients with CD experienced higher risk of gastrointestinal infections as compared with TNFα antagonist-treated patients (HR, 2.90; 95% CI, 1.21–6.94), without significant differences in risk of extra-intestinal serious infections (HR, 1.43; 95% CI, 0.73–2.79). The most common gastrointestinal serious infections in patients with CD were Clostridiodes difficile colitis (69.0%), cholangitis (7.1%), peritonitis (5%) and infectious gastroenteritis (4.3%).
Table 4.
Comparative risk of serious infections in patients with (A) Crohn’s disease and (B) ulcerative colitis, treated with vedolizumab vs. TNFα antagonists (reference), using marginal structural models.
| Vedolizumab vs. TNFα antagonists (reference), adjusted HR (and 95% CI) | A. Crohn’s disease | B. Ulcerative colitis |
|---|---|---|
| Type of serious infections • Gastrointestinal • Extra-intestinal |
2.90 (1.21–6.94) 1.43 (0.73–2.79) |
1.20 (0.57–2.53) 0.41 (0.15–1.12) |
| Age at biologic initiation • ≥60y • <60y |
1.36 (0.75–2.44) 0.59 (0.18–1.88) |
0.38 (0.12–1.19) 0.53 (0.31–0.91) |
| Excluding patients with serious infection in preceding 12m | N/A | 0.51 (0.31–0.84) |
| Excluding patients with immunomodulator exposure in preceding 12m | N/A | 0.74 (0.47–1.17) |
[Abbreviations: CI=confidence interval, HR=hazard ratio, N/A=Not available since models did not converge due to low event rates; TNF=tumor necrosis factor]
DISCUSSION
In a large administrative claims database of 4888 new users of TNFα antagonists and 1106 new users of vedolizumab, followed over 6800-PY, using marginal structural models to account for propensity to be prescribed either biologic class and accounting for time-varying use of immunomodulators, corticosteroids and opiates, we made several key observations. First, overall, no significant differences were observed in the risk of serious infections between vedolizumab- and TNFα antagonist-treated patients with IBD. However, vedolizumab was associated with 46% lower risk of serious infections as compared with TNFα antagonists in patients with UC, without a significant difference observed in patients with CD. Second, safety of vedolizumab may be driven by a lower risk of extra-intestinal infections which may not be directly related to underlying IBD activity; however, vedolizumab was associated with a higher risk of gastrointestinal serious infections, particularly in patients with CD. Clostridiodes difficile colitis was the most common gastrointestinal infection, besides infectious complications related to penetrating and/or perianal CD. Third, no specific differences were observed in the comparative safety of vedolizumab vs. TNFα antagonists in older patients with IBD, who may be at higher risk of serious infections with immunosuppressive therapy.
These findings provide robust, real-world evidence on the comparative safety of vedolizumab vs. TNFα antagonist in patients with IBD and can directly inform decision-making. In patients with UC, in light of comparable to higher effectiveness of vedolizumab vs. TNFα antagonists from prior studies, and now evidence suggesting lower risk of serious infections with vedolizumab, our findings support the use of vedolizumab over TNFα antagonists.1,5,22,23 In contrast, in patients with CD, in light of potentially lower effectiveness of vedolizumab vs. TNFα antagonist from prior studies, and now, lack of a safety advantage and potentially a higher risk of gastrointestinal infections with vedolizumab, our findings support the use of TNFα antagonists over vedolizumab.6,24,25 Our findings were recently confirmed in another claims based study by Kirchgesner et al. In their study, based on two U.S. nationwide commercial insurance databases and the French nationwide health insurance database, using propensity score methods, they observed that the overall risk of serious infections was not different between vedolizumab and TNFα antagonists in the overall IBD cohort (HR, 0.95; 95% CI, 0.79–1.13); however, the risk was decreased for vedolizumab users in patients with UC (HR, 0.68; 95% CI, 0.50–0.93), but not CD (HR, 1.10; 95% CI, 0.87–1.38).26
Two key factors determine the safety of biologic therapy in patients with IBD. First, the intrinsic immunosuppressive effect of the agent, and second, its effectiveness in controlling disease, achieving corticosteroid-free remission and avoiding disease-related complications.7 Vedolizumab’s gut specificity was confirmed in a vaccination study in healthy volunteers, in which it selectively reduced response to orally administered antigens, but not to parenterally administered antigens.27 This suggests that vedolizumab is less immunosuppressive as compared to TNFα antagonists. The recent VARSITY trial demonstrated that vedolizumab is more effective than adalimumab in achieving clinical and endoscopic remission in patients with moderate-severe UC.1 Indirect treatment comparison network meta-analyses suggest that it may be as effective as infliximab, particularly in biologic-naïve patients.5,22,23 Hence, the high efficacy of vedolizumab in achieving and maintaining remission, combined with lesser degree of immunosuppression may explain why vedolizumab was safer than TNFα antagonists in patients with UC. In contrast, vedolizumab may be less effective than TNFα antagonists in patients with CD, particularly in biologic-exposed patients, and in patients with high-risk phenotype such as perianal disease and high inflammatory burden. As a result, despite lesser degree of direct immunosuppression due to vedolizumab, no safety advantage was observed with vedolizumab vs. TNFα antagonists in patients with CD. In fact, vedolizumab was associated with 2.9-fold higher risk of gastrointestinal serious infections, which may arise directly from disease complications in patients with CD.
While we adopted a meticulous approach, applied marginal structural models to account for treatment selection and time varying covariates, a priori defined subgroup analyses, we acknowledge several important limitations to our study. First, as an administrative claims database study, we did not have access to subjective or objective measures of disease activity or endoscopy reports and did not have accurate details of disease location and behavior. However, our measurement of treatment exposure and outcomes was robust. Second, as with any observational study, we cannot rule out unobserved confounders, especially those due to treatment selection; however, our analytical approach, with a new user design, accounting for time-fixed and time-varying covariates, including corticosteroid exposure which may serve as a surrogate of disease activity, provides some protection against bias. Third, we were unable to compare the risk of serious infections in patients treated with TNFα antagonist or vedolizumab monotherapy or their use in combination with immunomodulators. However, sensitivity analyses focusing on patients who did not receive immunomodulators within 12m prior initiation of biologics, suggesting likely intention to use biologic monotherapy, did not identify differences in risk of serious infections between vedolizumab and TNFα antagonist. We also accounted for time-varying exposure to immunomodulators after starting biologic. Fourth, due to low event rate, we were unable to examine the risk of opportunistic infections; we therefore opted to focus on serious infections, as infections requiring hospitalization, rather than capturing all infections. Ideally, infections would be adjudicated by medical record review and microbiology data, but this level of data is unavailable in claims databases. However, our definition of serious infections requiring hospitalization has been validated with a high positive predictive value.17,18
In summary, in a large administrative claims database study of approximately 6000 patients with IBD between 2014 to 2018, we observed that vedolizumab is associated with lower risk of serious infections as compared with TNFα antagonists, in patients with UC, but not in patients with CD. This lower risk was driven primarily by lower risk of extra-intestinal serious infections which may not be directly related to underlying IBD; risk of gastrointestinal serious infections, which may be directly related to IBD complications particularly in patients with CD, was higher in vedolizumab-treated patients. Future prospective registry and real-world observational studies are warranted to confirm these findings and to contextualize risk of serious infections with other non-TNFα antagonists biologics like ustekinumab and janus kinase inhibitors. The interplay of effectiveness and relative safety of different agents, in patients who respond vs. do not respond to therapy also merits close evaluation to understand risk-benefit trade-offs of novel therapies. These findings will inform optimal choice of different biologics depending a patient’s risk of disease- and treatment-related complications.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background:
With expanding treatment options for the management of inflammatory bowel diseases, treatment safety is an important consideration in choosing optimal therapy. We compared the risk of serious infections between patients treated with tumor necrosis factor-α antagonists vs. vedolizumab.
Findings:
In a retrospective cohort study of 4881 patients treated with TNFα antagonists and 1106 patients treated with vedolizumab, vedolizumab was associated with 46% lower risk of serious infections as compared with TNFα antagonists in patients with ulcerative colitis, but no significant differences were observed in patients with Crohn’s disease (hazard ratio,1.30; 95% confidence interval, 0.80–2.11).
Implications for patient care:
Vedolizumab may be associated with a lower risk of serious infections compared with TNFα antagonists in patients with ulcerative colitis, but not Crohn’s disease. Combining this with data on comparative efficacy informs positioning biologics agents in clinical practice.
Acknowledgments
Funding: This project was supported by the NIH/NIDDK K23DK117058 (Siddharth Singh) and IOIBD Operating Grant 2019 (Siddharth Singh). Dr. Sandborn is partially supported by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest:
• Siddharth Singh reports research grants from AbbVie, Janssen
• Herbert Heien – None
• Jeph Herrin – reports working under contract to Centers for Medicare and Medicaid Services to develop quality measures
• Parambir S. Dulai reports research support from Takeda, Pfizer, Abbvie, Janssen, Polymedco, ALPCO, Buhlmann, Prometheus, and consulting fees from Takeda, Pfizer, Abbvie and Janssen.
• Lindsey Sangaralingham – None to declare
• Nilay D. Shah – None to declare
• William J. Sandborn reports research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences. Spouse: Iveric Bio - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - consultant, stock options; Escalier Biosciences – prior employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories) - employee, stock options; Shoreline Biosciences – stock options; Ventyx Biosciences – stock options; Vimalan Biosciences – stock options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sands BE, Peyrin-Biroulet L, Loftus EV Jr., , et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N Engl J Med 2019;381:1215–1226. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Andersen NN, Andersson M, et al. Comparison of Infliximab and Adalimumab in Biologic-Naive Patients With Ulcerative Colitis: A Nationwide Danish Cohort Study. Clin Gastroenterol Hepatol 2017;15:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Andersen NN, Andersson M, et al. Comparison of infliximab with adalimumab in 827 biologic-naive patients with Crohn’s disease: a population-based Danish cohort study. Aliment Pharmacol Ther 2018;47:596–604. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Heien HC, Sangaralingham LR, et al. Comparative Effectiveness and Safety of Anti–Tumor Necrosis Factor Agents in Biologic-Naive Patients With Crohn’s Disease. Clin Gastroenterol Hepatol 2016;14:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Murad MH, Fumery M, et al. First- and Second-Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:2179–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohm M, Xu R, Zhang Y, et al. Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn’s disease. Aliment Pharmacol Ther 2020;52:669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmer A, Singh S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev Clin Immunol 2019;15:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with crohn’s disease: More than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Haens G, Reinisch W, Colombel JF, et al. Five-year Safety Data From ENCORE, a European Observational Safety Registry for Adults With Crohn’s Disease Treated With Infliximab [Remicade] or Conventional Therapy. J Crohns Colitis 2017;11:680–689. [DOI] [PubMed] [Google Scholar]
- 10.D’Haens G, Reinisch W, Panaccione R, et al. Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients With Crohn’s Disease With up to 6 Years of Follow-Up in the Pyramid Registry. Am J Gastroenterol 2018;113:872–882. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonovas S, Fiorino G, Allocca M, et al. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol 2016;14:1385–1397. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Facciorusso A, Dulai PS, et al. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukin D, Faleck D, Xu R, et al. Comparative Effectiveness and Safety of Vedolizumab and Tumor Necrosis Factor-Antagonist Therapy in Ulcerative Colitis. Clin Gastroenterol Hepatol 2020; doi: 10.1016/j.cgh.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace PJ, Shah ND, Dennen T, et al. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–94. [DOI] [PubMed] [Google Scholar]
- 16.Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018;155:337–346. [DOI] [PubMed] [Google Scholar]
- 17.Grijalva CG, Chung CP, Stein CM, et al. Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Saf 2008;17:890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patkar NM, Curtis JR, Teng GG, et al. Administrative codes combined with medical records based criteria accurately identified bacterial infections among rheumatoid arthritis patients. J Clin Epidemiol 2009;62:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 21.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Allegretti JR, Siddique SM, et al. AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020;158:1465–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018;48:394–409. [DOI] [PubMed] [Google Scholar]
- 25.Macaluso FS, Ventimiglia M, Fries W, et al. A propensity score weighted comparison of vedolizumab and adalimumab in Crohn’s disease. J Gastroenterol Hepatol 2021;36:105–111. [DOI] [PubMed] [Google Scholar]
- 26.Kirchgesner J, Desai RJ, Beaugerie L, et al. Risk of serious infections with vedolizumab versus tumor necrosis factor antagonists in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2020;doi: 10.1016/j.cgh.2020.12.030 [DOI] [PubMed] [Google Scholar]
- 27.Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut 2015;64:77–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.