Abstract
Objectives
Detrusor underactivity is diagnosed using urodynamic testing. We hypothesized that nocturia is associated with detrusor underactivity.
Methods
We performed a retrospective chart review of all women who underwent urodynamic testing at our institution between 2016 and 2018. Uroflowmetry and pressure-flow study parameters were compared between women with nocturia (≥ 2 voids/night) and without nocturia (0–1 void/night). Detrusor underactivity was diagnosed using three different criteria: Bladder voiding efficiency < 90%, bladder contractility index < 100, and a composite of three urodynamic measures (Gammie criteria).
Results
Of 358 women, 172 (48%) were in the nocturia group and 186 (52%) in the no nocturia group. On uroflowmetry, median postvoid residual volume was similar (20 mL) in both groups. Median maximum flow rate (15 versus 17 mL/s, p < 0.05) and average flow rate (6 mL/s versus 7 mL/s, p < 0.05) were significantly lower in the nocturia compared to the no nocturia group. During pressure-flow study, a significantly greater proportion of women with nocturia were unable to void around the catheter (30% versus 27%, p < 0.01). The overall rate of detrusor underactivity varied with the criteria used: bladder voiding efficiency (54%), bladder contractility index (41%), and Gammie criteria (7%). The rate of detrusor underactivity using bladder voiding efficiency criteria was significantly higher in the nocturia group (63% versus 48%, p < 0.01), but no significant differences were noted using the other criteria.
Conclusion
Nocturia is associated with reduced voiding efficiency in women. The diagnosis of detrusor underactivity using urodynamics is challenging.
Keywords: voiding dysfunction, nocturia, pressure flow study, urodynamics, detrusor underactivity
INTRODUCTION
Nocturia, characterized by waking at night to void, is a common problem that has an immense impact on quality of life.1–3 Nocturia of two or more episodes, also called pathological nocturia,4 has been associated with increased daytime fatigue, fractures, falls and even increased mortality.2,5–7 Despite this, the pathophysiology of nocturia in women remains poorly understood.
While advancing age is one of the most well-known risk factors for nocturia, the mechanism through which it causes nocturia has not yet been fully elucidated. Prior studies have implicated aging related detrusor contraction abnormalities including detrusor overactivity (DO), detrusor underactivity (DU), and bladder outlet obstruction (BOO) in the pathogenesis of nocturia. While the relationship between detrusor overactivity and nocturia is well-known,8–10 two recent studies also reported an increased prevalence of detrusor underactivity in women with nocturia as high as 48% to 77%.11,12 Resnick et al. described coexisting storage and voiding symptoms as a part of geriatric syndrome (known as detrusor hyperactivity with impaired contractility).13 These findings suggest that DO can co-exist with DU in older women. However, in a recent large study investigating the relationship between DU and lower urinary tract symptoms, subjects with DO were excluded.14 Finally, some studies have reported an association between bladder outlet obstruction (BOO) and nocturia in women.15,16 Thus, the relationship between nocturia and detrusor contraction abnormalities remains unclear.
Detrusor contraction abnormalities are diagnosed using pressure-flow studies. While there is broad consensus on the criteria for the diagnosis of DO on cystometry,17 precise diagnostic criteria for DU and BOO have not been established for women. Table 1 lists a series of criteria used in the literature to define DU and BOO. The two composite criteria, by Hoag and Gani (BCI criteria)11 and Gammie et al.14 are both complex and require multiple measures obtained during pressure-flow studies. The recently convened 2nd International Congress on Underactive Bladder (CURE-UAB), pointed to the critical need for a simple diagnostic criteria for DU to facilitate research on the contribution of detrusor contraction abnormalities to lower urinary tract symptoms.18 Traditionally, postvoid residual volume (PVR) has been used as an indicator of incomplete bladder emptying. However, an elevated PVR is neither sensitive nor specific for detecting DU.19–22 The proportion of the bladder volume that is emptied during voiding, i.e. bladder voiding efficiency or BVE may be a more clinically meaningful measure of bladder emptying. Since prior studies have shown that BVE <90% is associated with symptoms of incomplete emptying,14,20 CURE-UAB proposed using BVE <90% as a simple diagnostic criteria for DU.11,14,18
Table 1.
Urodynamic criteria for detrusor underactivity, bladder outlet obstruction, and normal pressure flow based on recent studies in women
| Study | Detrusor Underactivity | Bladder Outlet Obstruction | Normal Pressure Flow |
|---|---|---|---|
| Hoag & Gani, 201511 | BCI <100 and absence of identifiable BOO | -- | -- |
| Gammie et al., 201614 | PdetQmax < 20 cmH2O Qmax < 15 mL/s BVE < 90% |
PdetQmax ≥ 40 cmH2O Qmax < 12 mL/s BVE ≥ 90% |
PdetQmax ≥ 20 cmH2O Qmax ≥ 20 mL/s BVE > 90%† |
| CURE-UAB, 201718 | BVE < 90% | -- | -- |
BCI: Bladder Contractility Index = PdetQmax + 5Qmax
BOO: Bladder Outlet Obstruction
PdetQmax: Detrusor pressure at maximum flow; Qmax: maximum flow rate
BVE: Bladder Voiding Efficiency = (Voided volume/(voided volume + residual volume)) × 100
Modified Gammie criteria
The primary aim of this study was to describe different types of detrusor contraction abnormalities as diagnosed by urodynamic testing in women with nocturia. Our secondary aim was to compare the rate of detrusor contraction abnormalities between women with and without nocturia. We hypothesized that nocturia would be associated with detrusor underactivity.
MATERIALS AND METHODS
Following approval by our institutional review board, we performed a retrospective chart review of women who presented for evaluation of lower urinary tract symptoms to a single tertiary care urogynecology clinic between March 2016 and July 2018.
Patients were included if they were ≥ 18 years old and had undergone complete evaluation using a structured format and complete urodynamic evaluation (including uroflowmetry, cystometry, and pressure-flow studies). Exclusion criteria included a history of neurologic disorders (such as dementia, stroke, multiple sclerosis, etc.), pelvic surgery or childbirth within the previous six months, prior neuromodulation or pelvic radiation. Patients with missing data on nocturia, or who could not complete urodynamic testing (e.g., due to infection or vasovagal episode) were also excluded.
Study design
We adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement criteria for performing observational studies.23 Clinical data including nocturia had been collected in the electronic record using standardized intake forms. We used a de-identified data form to extract relevant demographic and clinical data.
We defined nocturia as two or more voids at night. Two or more nocturnal voids have been shown to be a clinically meaningful threshold associated with significant negative consequences on health and well-being.2,4–6 Prolapse stage was defined according to the Pelvic Organ Prolapse Quantitative system.24 Clinical diagnoses of overactive bladder, stress urinary incontinence and mixed urinary incontinence as documented by the consulting physician were extracted.
Urodynamic testing
Multi-channel urodynamic tests for all subjects had been performed by trained technicians using Laborie Dorado® equipment and an 8Fr T-Doc® catheter. Urodynamic testing included uroflowmetry, cystometry, and pressure-flow studies. All studies were performed according to the International Continence Society (ICS) guidelines.25–27 Bladder voiding efficiency (BVE) was calculated using uroflow volumes and bladder contractility index (BCI) using pressure-flow data (Table 1). Since flow rates are dependent on voided volume, we only analyzed pressure flow curves for women who voided at least 100 mL.25,28 Urodynamic tracings were reviewed by two research assistants who had received extensive training from a Urogynecology faculty member and were blinded to clinical findings.
Definitions
Detrusor overactivity was as defined by ICS/IUGA,17 and was diagnosed when involuntary detrusor contractions were present on filling cystometry.17 Standardized numerical criteria for the diagnosis of normal pressure flow, DU, and BOO in women are not available. We defined normal pressure flow using modified Gammie criteria (Table 1). We diagnosed detrusor underactivity using three different criteria outlined in Table 1: 1) Bladder contractility index (BCI) = PdetQmax + 5Qmax,11 2) combination criteria proposed by Gammie et al,14 and 3) bladder voiding efficiency (BVE) = [(voided volume/voided volume + postvoid residual volume)] x100 measured on uroflowmetry.18 Bladder outlet obstruction was also diagnosed as proposed by Gammie et al. Combined DO and DU was diagnosed based on the presence of DO on cystometry and BVE < 90%.
Statistical analysis
Statistical analysis was performed using Stata15 (Stata Corp., College Station, TX). Variables were compared between nocturia and no nocturia groups using Wilcoxon rank-sum test for continuous data and the chi-square test for categorical data. Missing data was considered missing at random. Two-sided p-value of ≤ 0.05 was used to determine statistical significance.
RESULTS
Of the 358 patients, 172 (48%) were in the nocturia group and 186 (52%) in the no nocturia group. Uroflowmetry data was missing in 25% (90/358) and pressure flow data in 23% (84/358) of the cohort. The rate of missing uroflowmetry was higher in the nocturia than the no nocturia group (30% versus 21%, p= 0.06). The rate of missing pressure flow data was also significantly higher in the nocturia than the no nocturia group (30% versus 17%, p < 0.01).
Table 2 summarizes the demographic and clinical data. Women with nocturia were significantly older, had higher BMI and had higher rates of falls compared to those without nocturia. The rates of clinically diagnosed overactive bladder, stress urinary incontinence and mixed urinary incontinence were higher in the nocturia group.
Table 2.
Demographic data and clinical variables in subjects with and without nocturia
| All Patients N=358 | No Nocturia (0–1 void per night) N=186 | Nocturia (≥ 2 voids per night) N=172 | P-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 58 (47,68) | 55 (45,65) | 63 (51,72) | <0.001 |
| BMI (kg/m2), median (IQR) | 28 (24,32) | 26 (23,31) | 29 (25,33) | < .01 |
| Self-reported Race, n(%) | ||||
| Black | 87 (24%) | 24 (13%) | 63 (37%) | |
| Asian | 8 (2%) | 6 (3%) | 2 (1%) | |
| Current Smoker, n (%) | 26 (7%) | 13 (7%) | 13 (8%) | 0.8 |
| Alcohol Use, n (%) | 183 (52%) | 105 (58%) | 78 (46%) | < 0.05 |
| Prior Hysterectomy | 69 (19%) | 32 (17%) | 37 (22%) | 0.3 |
| Prior Anti-incontinence surgery | 19 (5%) | 9 (5%) | 10 (6%) | 0.6 |
| Vaginal Atrophy, n (%) | 188 (53%) | 86 (47%) | 102 (60%) | < 0.05 |
| History of falls, n (%) | 42 (12%) | 13 (7%) | 29 (18%) | < 0.01 |
| History of recurrent UTI | 49 (13%) | 21 (11%) | 28 (16%) | 0.17 |
| Overactive Bladder¶ | 151 (42%) | 64 (34%) | 87 (51%) | < 0.01 |
| Stress urinary incontinence¶ | 133 (37%) | 56 (30%) | 77 (45%) | < 0.01 |
| Mixed Urinary Incontinence¶ | 148 (41%) | 91 (49%) | 57 (33%) | < 0.01 |
| Anterior vaginal prolapse, n (%) | ||||
| Stage 2 (−1 ≤ Ba ≤ +1) | 61 (17%) | 39 (21%) | 22 (13%) |
IQR: Interquartile range
Clinical diagnosis
Urodynamic findings are summarized in Table 3. On uroflowmetry, the nocturia group had significantly lower maximum flow rate (Qmax) and lower average flow rate (Qavg) than the no nocturia group. The median PVR was not significantly different between groups. During cystometry, maximum capacity was significantly lower for the nocturia group. A total of 274 women (77%) provided interpretable pressure flow tracings of ≥ 100 ml. During pressure -flow studies, women in the nocturia group were significantly less likely to void around the catheter than those in the no nocturia group.
Table 3.
Urodynamic parameters in women with and without nocturia
| All Patients N=358 | No Nocturia (0–1 void per night) N=186 | Nocturia (≥ 2 voids per night) N=172 | P-value | |
|---|---|---|---|---|
| Uroflowmetry (n = 268) | ||||
| Voided volume (mL), median (IQR) | 136 (66,262) | 139 (69,281) | 123 (58,229) | 0.4 |
| PVR (mL), median (IQR) | 20 (10,50) | 20 (10,50) | 20 (10,50) | 0.6 |
| Qmax (mL/s), median (IQR) | 16 (10,26) | 17 (11,27) | 15 (8,23) | < 0.05 |
| Qavg (mL/s), median (IQR) | 6 (4,10) | 7 (4,12) | 6 (3,10) | < 0.05 |
| BVE, Median (IQR) (%) | 86 (77,94) | 88 (76,95) | 86 (78,94) | 0.4 |
| Filling Cystometry (mL) (n = 358) | ||||
| First sensation, median (IQR) | 112 (76,160) | 114 (79,160) | 108 (75,160) | 0.5 |
| First desire, median (IQR) | 144 (101,209) | 149 (104,225) | 136 (100,194) | 0.1 |
| Strong urge, median (IQR) | 180 (127,264) | 187 (131,290) | 169 (122,246) | 0.1 |
| Maximum cystometric capacity, median (IQR) | 238 (160,352) | 245 (170,377) | 228 (142,323) | < 0.05 |
| Pressure Flow Studies (n = 274) | ||||
| Void < 100 mL, n (%) | 84 (23%) | 32 (27%) | 52 (30%) | < 0.01 |
| Qmax (mL/s), median (IQR) | 16 (12,21) | 16 (12,21) | 15 (11,21) | 0.3 |
| Qavg (mL/s), median (IQR) | 6 (4,8) | 6 (5,8) | 6 (4,8) | 0.9 |
| PdetQmax (cm H2O), median (IQR) | 26 (18,39) | 26 (17,38) | 25 (18,41) | 0.6 |
| Peak Pdet (cm H2O), median (IQR) | 46 (32,63) | 47 (32,65) | 45 (32,60) | 0.6 |
BVE: Bladder Voiding Efficiency = (voided volume/voided volume + residual volume) × 100
PVR: Post void residual volume, Pdet: Detrusor pressure; Qmax: Maximum flow rate, Qavg: Average flow rate, PdetQmax: Detrusor pressure at maximum flow
IQR: Interquartile range
Table 4 shows the rates of various detrusor contraction abnormalities diagnosed by urodynamic testing for the cohort and the nocturia and no nocturia groups. The overall rate of DO on cystometry for the cohort was 54% with significantly higher rates for the nocturia group.
Table 4.
Detrusor contraction abnormalities associated with nocturia
| Urodynamic Diagnoses | All Patients N=274 | No Nocturia (0–1 void per night) N=154 | Nocturia (≥ 2 voids per night) N=120 | P-value |
|---|---|---|---|---|
| Normal Pressure Flow | ||||
| Gammie et al., 201614 | 26 (9%) | 17 (14%) | 9 (11%) | 0.5 |
| Detrusor Overactivity, n (%) | 149 (54%) | 73 (47%) | 76 (63%) | < 0.01 |
| Detrusor Underactivity, n (%) | ||||
| CURE-UAB (BVE < 90%)18 | 147 (54%) | 66 (48%) | 81 (63%) | < 0.01 |
| Bladder Contractility Index < 100*,11 | 111 (41%) | 59 (39%) | 52 (45%) | 0.3 |
| Gammie et al., 2016 | 19 (7%) | 9 (6%) | 10 (8%) | 0.4 |
| Bladder Outflow Obstruction, n (%) | ||||
| Gammie et al., 201614 | 6 (2%) | 3 (2%) | 3 (4%) | 0.6 |
| Combined Detrusor Overactivity and Underactivity± | 84 (31%) | 47 (24%) | 37 (38%) | < 0.05 |
BVE: Bladder Voiding Efficiency = (voided volume/voided volume + residual volume) × 100
IQR: interquartile range
Bladder contractility index < 100 and absence of bladder outlet obstruction
Detrusor overactivity on cystometry and BVE < 90%
The overall rate of DU for the cohort varied widely based on the diagnostic criteria used (Figure 1; Table 4). Rates of DU were high (54%) when single criteria such as BVE proposed by CURE-UAB were used but declined to 41% with the Hoag and Gani criteria that combines BCI with absence of BOO, and 7% when the Gammie criteria (composite of three measurements) were used (Table 4). When using composite criteria proposed by Gammie et al., 154 of the 274 subjects (56%) did not fit into any diagnostic group (normal pressure flow, BOO, or DU).
Figure 1. Rates of detrusor underactivity by three different criteria of all 274 patients analyzed with pressure flow data.
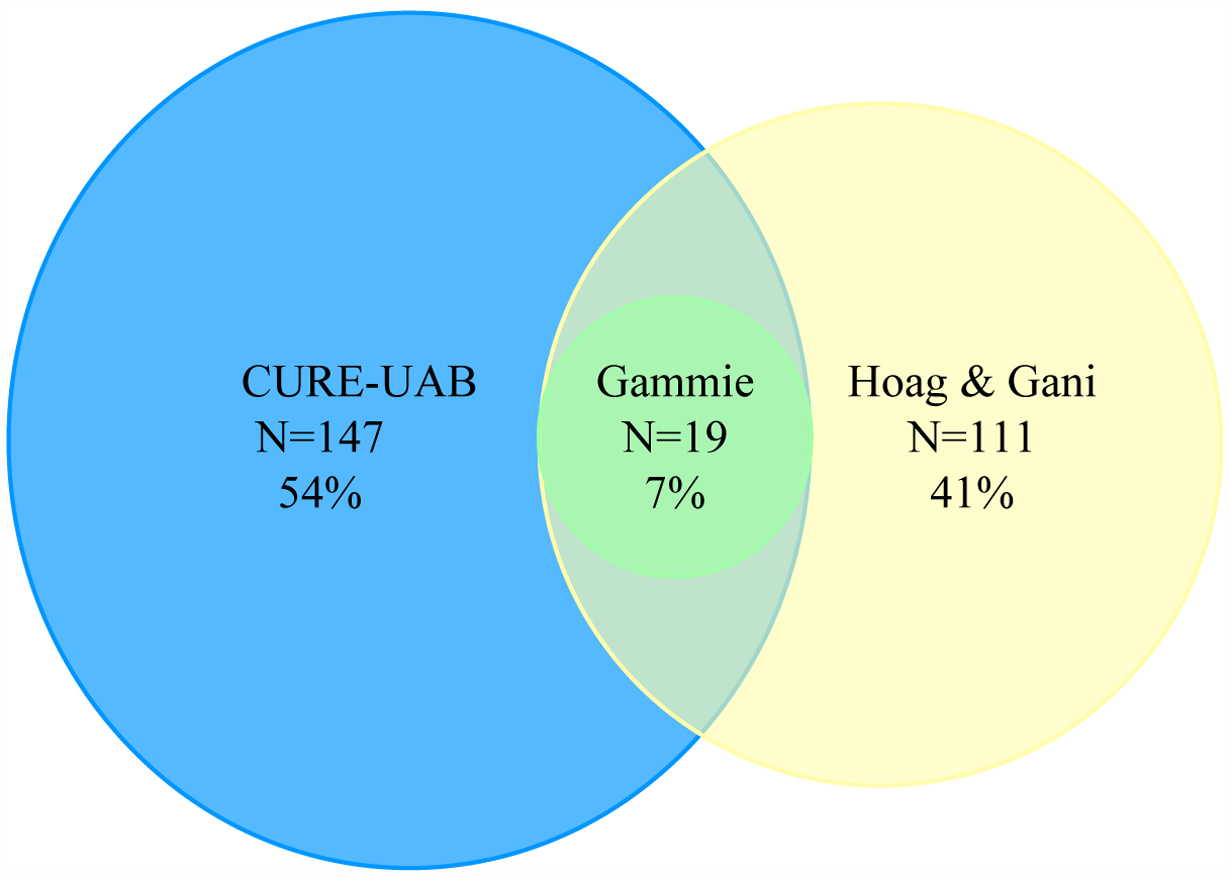
CURE-UAB: Bladder voiding efficiency (BVE) < 90%
BCI (Hoag & Gani): Bladder contractility index (BCI) < 100 with absence of identified bladder outlet obstruction
Gammie: PdetQmax < 20 cmH2O + Qmax < 15 mL/s + BVE < 90%
The rate of DU was significantly higher in the nocturia group using the BVE criteria as proposed by CURE-UAB (63% versus 48%, p < 0.01), however, rates were similar when the BCI or the Gammie criteria were used. The rate of co-existing DO and DU was also significantly higher in the nocturia group (38% versus 24%, p < 0.05).
DISCUSSION
Detrusor contraction abnormalities are currently diagnosed using urodynamic testing.17,25 In a large cohort of women who underwent urodynamic studies at our institution, we noted that detrusor contraction abnormalities are common in women with nocturia, ranging from 63% for DO, 63% for DU (using BVE criteria) to 4% for BOO. The rate of DO and the rate of DU using the BVE criteria was significantly higher in the nocturia than the no nocturia group. The rate of DU using the modified Gammie and the Hoag and Gani criteria was not significantly different between those with and without nocturia. The rate of BOO was low in women with nocturia and did not significantly differ from the no nocturia group.
Several findings in our study suggest that nocturia is associated with inefficient voiding despite the median PVR being similar between groups. First, significantly higher proportion of women with nocturia were diagnosed with DU based on BVE < 90% compared to those without nocturia. Prior studies suggest that BVE is likely a better indicator of incomplete emptying than PVR alone.14,18,20 Next, the average and maximum flow rate on uroflowmetry were lower in the nocturia group. Average and maximum flow rate are age dependent and precise cut-offs for abnormal values based on nomograms for women have not been established.29–35 The median maximum flow rate in the nocturia group (15 mL/sec) was close to the cut-off of <15mL/sec proposed as indicative of DU in the modified Gammie criteria. Finally, a significantly greater proportion of women with nocturia were unable to void around the catheter during pressure-flow study, a finding more likely to occur in women with DU. Taken together, these findings suggest that nocturia maybe associated with reduced voiding efficiency.
Strengths and limitations of using BVE based definition of DU proposed by CURE-UAB must be considered. The advantage of BVE is that it is simple to calculate. However, studies correlating BVE measurements with patient reported voiding symptoms are lacking. Another limitation of the CURE-UAB definition of DU is that it could potentially include women with BOO. However, common causes of BOO in women such as prior anti-incontinence surgery and advanced prolapse were similar between groups. These findings suggest that the observed higher rate of reduced BVE in the nocturia group may be due to underlying DU, however, prospective studies that combine BVE measurements with validated symptom questionnaires are required to confirm our findings.
In our study, the rate of DO and combined DO and DU (using the BVE criteria) were significantly higher in the nocturia group. Prior studies have reported that DO and DU frequently co-exist.36–38 We did not perform a multivariable analysis to determine whether DU was independently associated with nocturia because animal studies suggest that DU and DO are biologically linked to each other such that a detrusor muscle affected by severe overactivity is unable to generate an efficient contraction during voiding.29,39–41 Similarly, women in the nocturia group were older, had higher BMI, and higher rates of falls, all of which have been previously associated with DO and DU.37,42–44
An important finding of our study is that detrusor contraction abnormalities are difficult to diagnose using urodynamic parameters, and the rate varies with the diagnostic criteria used. Specifically, the rate of DU decreased as the number of components in the diagnostic criteria used to define DU increased. In our study, the rate of diagnosis of DU was 54% for the CURE-UAB criteria that is based on a single measure, 41% for Hong & Gani criteria that consists of two components, and 7% for the Gammie criteria that comprises of three components (Table 4; Figure 1). In the absence of a “gold standard” criteria to diagnose DU, the sensitivity and specificity of each technique is difficult to assess. An additional clinical problem with the diagnosis of DU using the invasive pressure-flow study is that subjects with DU may not be able to void around the catheter. In our study, 23% of subjects overall were unable to void during pressure-flow and the rate was significantly higher in the nocturia than the no nocturia group. Interestingly, this problem that leads to a non-diagnostic pressure-flow study has rarely been discussed in the literature but has important implications for both clinical practice and research. First, the non-diagnostic pressure-flow studies may have contributed to the widely different rates of DU using the three different diagnostic criteria. Second, the lack of significant differences in rates of DU between the nocturia and no nocturia groups using the composite Gammie et al. and Hong & Gani criteria may be due to a failure to diagnose DU. These findings demonstrate the challenges of diagnosing detrusor contraction abnormalities using invasive urodynamics and support the CURE-UAB recommendations that diagnoses of DU should be based on combination of patient reported symptoms and non-invasive measurements such as the BVE.
Our study has several limitations. We did not have patient reported data on voiding symptoms using validated questionnaires. However, the primary goal of our study was to describe the rate of urodynamic diagnoses of detrusor contraction abnormalities. Because of the retrospective design, we run into the risk of selection biases. To minimize this, we assessed all patients during the study period who met our inclusion criteria regardless of the indication for urodynamics. Women in the no nocturia group included women with one episode of nocturia, however, this likely had minimal influence on our results because including women with nocturia in the “control” group would have biased our findings towards the null. The rate of missing uroflowmetry and missing pressure flow data was higher in the nocturia than the no nocturia group, this too would have biased our findings towards the null. Our study also has several strengths. We diagnosed detrusor contraction abnormalities meticulously using standardized criteria. For example, we excluded patients who voided less than 100 ml during the pressure-flow study, and thus avoided over diagnosing DU. We also reported on rates of missing pressure-flow data, a common occurrence when patients are unable to void around the catheter but which few prior studies on detrusor contraction abnormalities have reported.
In conclusion, our findings that nocturia is associated with reduced bladder efficiency contributes to a better understanding of the pathophysiology of nocturia and provides valuable direction for future research. Our findings also highlight the challenges of using urodynamic testing to diagnose detrusor contraction abnormalities and the urgent need to develop non-invasive criteria for diagnosing detrusor underactivity.
Financial Support:
This research was supported in part through a FOCUS Medical Student Fellowship in Women’s Health supported by the Bertha Dagan Berman Award. Dr. Andy’s effort was supported in part by NIH grant R03-AG-053277.
REFERENCES:
- 1.Van Kerrebroeck P, Andersson K. Terminology, epidemiology, etiology, and pathophysiology of nocturia. Neurourol Urodyn. 2014;33(1):S2–S5. doi:doi: 10.1002/nau.22595. [DOI] [PubMed] [Google Scholar]
- 2.Coyne K, Zhou Z, Bhattacharyya S, et al. The prevalence of nocuria and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92(9):948–954. doi: 10.1046/j.1464-410X.2003.04527.x [DOI] [PubMed] [Google Scholar]
- 3.Hashim H, Blanker MH, Drake MJ, et al. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol Urodyn. 2019;38(2):499–508. doi: 10.1002/nau.23917 [DOI] [PubMed] [Google Scholar]
- 4.Gopal M, Sammel MD, Pien G, et al. Investigating the Associations Between Nocturia and Sleep Disorders in Perimenopausal Women. J Urol. 2008;180(5):2063–2067. doi: 10.1016/j.juro.2008.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funada S, Tabara Y, Setoh K, et al. Impact of Nocturia on Mortality: The Nagahama Study. J Urol. 2020;204(5):996–1002. doi: 10.1097/JU.0000000000001138 [DOI] [PubMed] [Google Scholar]
- 6.Kupelian V, Fitzgerald MP, Kaplan SA, et al. Association of nocturia and mortality: Results from the third national health and nutrition examination survey. J Urol. 2011;185(2):571–577. doi: 10.1016/j.juro.2010.09.108 [DOI] [PubMed] [Google Scholar]
- 7.Asplund R Mortality in the elderly in relation to nocturnal micturition. BJU Int. 1999;84(3):297–301. [DOI] [PubMed] [Google Scholar]
- 8.Daan NMP, Schweitzer KJ, Van Der Vaart CH. Associations between subjective overactive bladder symptoms and objective parameters on bladder diary and filling cystometry. Int Urogynecol J. 2012;23(11):1619–1624. doi: 10.1007/s00192-012-1774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haylen BT, Chiu TL, Avery D, et al. Improving the clinical prediction of detrusor overactivity by utilizing additional symptoms and signs to overactive bladder symptoms alone. Int Urogynecol J Pelvic Floor Dysfunct. 2014;25(8):1115–1120. doi: 10.1007/s00192-014-2362-5 [DOI] [PubMed] [Google Scholar]
- 10.Jeong S, Lee S, Jeong C, et al. Clinical and urodynamic differences among women with overactive bladder according to the presence of detrusor overactivity. Int Urogynecol J Pelvic Floor Dysfunct. 2013;24(2):255–261. doi: 10.1007/s00192-012-1817-9 [DOI] [PubMed] [Google Scholar]
- 11.Hoag N, Gani J. Underactive bladder: Clinical features, urodynamic parameters, and treatment. Int Neurourol J. 2015;19(3):185–189. doi: 10.5213/inj.2015.19.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uren AD, Drake MJ. Definition and symptoms of underactive bladder. Investig Clin Urol. 2017;58:S61–S67. doi: 10.4111/icu.2017.58.S2.S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick N, Yalla S Detrusor hyperactivity with impaired contractile function: An unrecognized but common cause of incontinence in elderly patients. JAMA. 1987;257(22):3076–3081. [DOI] [PubMed] [Google Scholar]
- 14.Gammie A, Kaper M, Dorrepaal C, et al. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests from a Large Group of Patients Undergoing Pressure Flow Studies. Eur Urol. 2016;69(2):361–369. doi: 10.1016/j.eururo.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 15.Al-Zahrani AA, Gajewski J. Urodynamic findings in women with refractory overactive bladder symptoms. Int J Urol. 2016;23(1):75–79. doi: 10.1111/iju.12954 [DOI] [PubMed] [Google Scholar]
- 16.Klijer R, Bar K, Białek W. Bladder outlet obstruction in women: Difficulties in the diagnosis. Urol Int. 2004;73(1):6–10. doi: 10.1159/000078795 [DOI] [PubMed] [Google Scholar]
- 17.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798 [DOI] [PubMed] [Google Scholar]
- 18.Dewulf K, Abraham N, Lamb LE, et al. Addressing challenges in underactive bladder: recommendations and insights from the Congress on Underactive Bladder (CURE-UAB). Int Urol Nephrol. 2017;49(5):777–785. doi: 10.1007/s11255-017-1549-3 [DOI] [PubMed] [Google Scholar]
- 19.Jeong S, Kim H, Lee Y, et al. Prevalence and clinical features of detrusor underactivity among elderly with lower urinary tract symptoms: A comparison between men and women. Korean J Urol. 2012;53(5):342–348. doi: 10.4111/kju.2012.53.5.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong S, Lee J, Kim K, et al. How do we diagnose detrusor underactivity? Comparison of diagnostic criteria based on an urodynamic measure. Investig Clin Urol. 2017;58(4):247–254. doi: 10.4111/icu.2017.58.4.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown E, Cohn J, Kaufman M, et al. Phenotyping women with detrusor underactivity by presumed etiology: is it plausible? Neurourol Urodyn. 2017;36(4):1151–1154. doi:doi: 10.1002/nau.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khayyami Y, Klarskov N, Lose G. Post-void residual urine under 150 ml does not exclude voiding dysfunction in women. Int Urogynecol J. 2016;27(3):467–473. doi: 10.1007/s00192-015-2854-y [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 24.Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. http://www.ncbi.nlm.nih.gov/pubmed/8694033 [DOI] [PubMed] [Google Scholar]
- 25.Rosier P, Schaefer W, Lose G, et al. International Continence Society Good Urodynamic Practices and Terms 2016: Urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36(5):1243–1260. doi: 10.1002/nau.23124 [DOI] [PubMed] [Google Scholar]
- 26.Drake MJ, Doumouchtsis SK, Hashim H, et al. Fundamentals of urodynamic practice, based on International Continence Society good urodynamic practices recommendations. Neurourol Urodyn. 2018;37(July):S50–S60. doi: 10.1002/nau.23773 [DOI] [PubMed] [Google Scholar]
- 27.Schfer W, Abrams P, Liao L, et al. Good Urodynamic Practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21(3):261–274. doi: 10.1002/nau.10066 [DOI] [PubMed] [Google Scholar]
- 28.Pauwels E, Laet K, Wachter S, et al. Healthy, Middle-Aged, History-Free, Continent Women—Do They Strain to Void? J Urol. 2006;175:1403–1407. [DOI] [PubMed] [Google Scholar]
- 29.Smith PP. Aging and the Underactive Detrusor: A Failure of Activity or Activation? Neurourol Urodyn. 2010;29:408–412. [DOI] [PubMed] [Google Scholar]
- 30.Leitner L, Walter M, Sammer U, et al. Urodynamic investigation: A valid tool to define normal lower urinary tract function? PLoS One. 2016;11(10):1–15. doi: 10.1371/journal.pone.0163847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahfouz W, Al Afraa T, Campeau L, et al. Normal urodynamic parameters in women. Int Urogynecol J. 2011;23(3):269–277. doi: 10.1007/s00192-011-1585-y [DOI] [PubMed] [Google Scholar]
- 32.Dain L, Auslander R, Rosen T, et al. Urodynamic findings in women with pelvic organ prolapse and obstructive voiding symptoms. Int J Gynecol Obstet. 2010;111(2):119–121. doi: 10.1016/j.ijgo.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 33.Li X, Liao LM, Chen GQ, et al. Clinical and urodynamic characteristics of underactive bladder. Medicine (Baltimore). 2018;97(3):1–4. doi: 10.1097/MD.0000000000009610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Digesu GA, Hutchings A, Salvatore S, et al. Reproducibility and reliability of pressure flow parameters in women. BJOG An Int J Obstet Gynaecol. 2003;110(8):774–776. doi: 10.1111/j.1471-0528.2003.02081.x [DOI] [PubMed] [Google Scholar]
- 35.Valentini F, Robain G, Marti B. Urodynamics in Women from Menopause to Oldest Age: What Motive? What Diagnosis? Int Braz J Urol. 2011; Vol. 37 (1(1):100–107. doi: 10.1590/S1677-5538201100020001 [DOI] [PubMed] [Google Scholar]
- 36.Mancini V, Tarcan T, Serati M, et al. Is coexistent overactive–underactive bladder (with or without detrusor overactivity and underactivity) a real clinical syndrome? ICI-RS 2019. Neurourol Urodyn. 2020; (October2019):1–10. doi: 10.1002/nau.24311 [DOI] [PubMed] [Google Scholar]
- 37.Gammie A, Kaper M, Steup A, et al. What are the additional signs and symptoms in patients with detrusor underactivity and coexisting detrusor overactivity? Neurourol Urodyn. Published online2018:1–6. doi: 10.1002/nau.23565 [DOI] [PubMed] [Google Scholar]
- 38.Drake M, Kanai A, Bijos D, et al. The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU Int. 2017;119:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbadawi A, Yalla S, Resnick NM. Structural Basis of Geriatric Voiding Dysfunction. III. Detrusor Overactivity. J Urol. 1993;150(5):1668–1680. [DOI] [PubMed] [Google Scholar]
- 40.Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: A new clinical entity? a review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65(2):389–398. doi: 10.1016/j.eururo.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 41.van Koeveringe G, Vahabi B, Andersson K, et al. Detrusor Underactivity: A Plea for New Approaches to a Common Bladder Dysfunction. Neurol Urodynamics. 2011;30:723–728. [DOI] [PubMed] [Google Scholar]
- 42.Malone-Lee J, Wahedna I. Characterisation of Detrusor Contractile Function in Relation to Old Age. Br J Urol. 1993;72(6):873–880. doi: 10.1111/j.1464-410X.1993.tb16289.x [DOI] [PubMed] [Google Scholar]
- 43.Griffiths D, Tadic S, Schaefer W, et al. Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage. 2007;37(1):1–7. doi: 10.1016/j.neuroimage.2007.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abarbanel J, Marcus EL. Impaired Detrusor Contractility in Community-Dwelling Elderly Presenting with Lower Urinary Tract Symptoms. Urology. 2007;69(3):436–440. doi: 10.1016/j.urology.2006.11.019 [DOI] [PubMed] [Google Scholar]


