Abstract
We describe the heretofore unreported case of an HPV-related carcinoma of the palatine tonsil with distinct areas of squamous cell- and adenoid cystic carcinoma-like differentiation in a 54-year old patient. The morphological, immunophenotypic and molecular findings of the tumor are illustrated. We discuss the parallels between the tumor and HPV-related multiphenotypic sinonasal carcinoma (HMSC) which is well-known to exhibit adenoid cystic carcinoma-like features. A review of the literature of high-risk HPV-associated non-squamous carcinomas of the oropharynx is presented.
Keywords: HPV-related, Adenoid-cystic like features, HPV mRNA in situ hybridization, Multiphenotypic, Biphenotypic carcinoma, HMSC, HPV16
Introduction
The morphologic spectrum and clinical characteristics of human papillomavirus (HPV) associated nonkeratinizing squamous cell carcinoma of the oropharynx are now well understood. In the initial decade(s), pathologists and researchers elucidated the essential features of this tumor [1, 2] and its distinction from typical keratinizing squamous cell carcinoma of the oral cavity. A strong association with high-risk HPV type 16 [3, 4] was demonstrated, followed by a search (still ongoing) for other upper aerodigestive tract tumors that showed an association with transcriptionally active HPV infection. Though HPV-associated tumors of the oropharynx with other morphologies have been described these have likewise been predominantly either variants of squamous cell carcinoma [1, 5] or rare entities such as ciliated adenocarcinoma with only a few reported instances [6, 7]. Among these, the HPV-related multiphenotypic sinonasal carcinoma (HMSC) is the most recent well- characterized neoplasm [8] with consistent if variegated non-squamous differentiation, most notably including adenoid cystic carcinoma-like differentiation [9, 10]. Here we describe a hitherto undescribed case of an HPV type 16-related primary tumor with biphenotypic squamous-and adenoid cystic carcinoma (AdCC) differentiation involving the palatine tonsillar oropharynx. We review the occurrence of HPV-associated adenocarcinoma involving the oropharynx in the literature and summarize parallels between the present case and HMSC.
Case Report
The patient is a 54-year old man who initially presented with a history of a left-sided neck mass for 4 months. Physical examination performed a month after the neck mass became evident had showed a lesion in the left tonsil which was biopsied under direct laryngoscopy. His past medical history was unremarkable. The patient was self-employed and was a former user of chewing tobacco (duration/amount unknown) and consumed 8 drinks of alcohol per week. Family history was notable for metastatic lung carcinoma in his mother. The patient was referred to the University of Iowa Hospitals and Clinics for further management. Oral, pharyngeal and laryngoscopic exams showed a ~ 2 cm mass in the left palatine tonsil with biopsy site changes, and no other primary lesions. On examination of the neck, there was a 3–4 cm left level II lymph node that was hard, mobile and non-tender. A positron emission tomography (PET) imaging study showed activity throughout the Waldeyer’s ring that was increased on the left side, and a left level 2 lymph node with increased 2′-fluorodeoxyglucose avidity. There was no other evidence of metastatic disease. The patient was managed with a staged surgical approach, with left neck dissection followed by transoral robotic assisted left radical tonsillectomy and right tonsillectomy. He received adjuvant radiotherapy to the neck (60 Gy); no chemotherapy was administered. At last follow up, the patient showed no evidence of disease at the primary site or the neck, nor distant metastasis, 2 years after the initial surgical treatment.
Histopathologic Examination
The diagnostic biopsy of the left tonsil showed a typical p16-positive nonkeratinizing squamous cell carcinoma. The subsequent left radical tonsillectomy specimen showed an ulcerated mass measuring 1.6 × 1.5 × 1.3 cm located in the central portion. Microscopic examination of routine H&E stained sections showed a nonkeratinizing squamous cell carcinoma (NKSCC), admixed with distinct areas of tumor showing adenoid cystic carcinoma -like morphology (Fig. 1a). The former was composed of the typical oval to spindled tumor cells with hyperchromatic nuclei in sheets and trabeculae, with a ‘syncytial’ appearance (Figs. 1b, 2a). The adenoid cystic carcinoma -like component was composed of cellular basophilic tumor cells in solid sheets arranged in lobules separated by fibrous bands, interspersed freely with areas exhibiting cribriform and nested architecture, with variably sized microcysts filled with acellular mucoid material (Figs. 1c, 2b). In scattered areas, these exhibited a discernible dual population, with outer smaller myoepithelial-like tumor cells with clear cytoplasm arranged around inner luminal cells with more abundant eosinophilic cytoplasm. There was focal intraepithelial extension of adenoid cystic carcinoma with replacement of portions of crypt epithelium (Fig. 2c). Areas of comedo-necrosis were prominently seen in both components of the tumor.
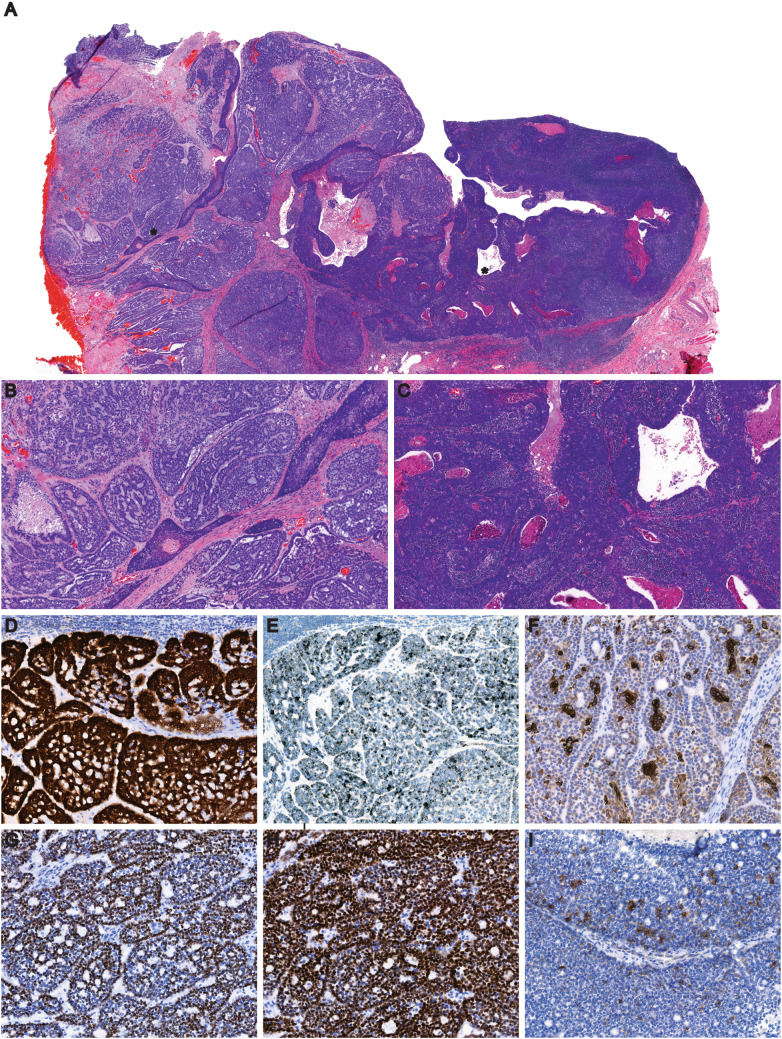
Fig. 1.
(numbers in parenthesis indicates magnification level): a Panoramic view of tumor from the left radical tonsillectomy (3 ×). AdCC and NKSCC areas are identified by asterisks, b Higher magnification view of the AdCC component of the tumor (10 ×), c Higher magnification view of the nonkeratinizing squamous cell carcinoma component (10 ×), d p16 shows diffuse, strong nuclear and cytoplasmic (3 +) positivity in AdCC areas in both ductal and myoepithelial cells (11.8 ×), e HPV type 16 E6/E7 mRNA ISH: positive in tumor cells (11.8 ×), f AE1/AE3 highlights luminal ductal epithelium at places with weak focal positivity in myoepithelial cells, g p40 highlights mainly myoepithelial cells, h p63 shows greater myoepithelial cell positivity, i c-kit highlights scattered ductal cells in tumor (panels f–i: all 20 × magnification)
Fig. 2.
Higher magnification views of the distinct squamous cell- and adenoid cystic carcinoma- like areas of tumor. a squamous differentiation with oval to spindled tumor cells with foci of maturation (keratin production) with a fairly rich intratumoral lymphoid infiltrate (20 ×), b Adenoid cystic carcinoma areas consisting of ‘blue’ tumor cells with hyperchromatic, molded nuclei and scant cytoplasm arranged in lobules and infiltrative cords with punched-out cribriform spaces with basophilic secretions (20 ×). c Surface aspect showing intraepithelial extension of adenoid cystic carcinoma focally replacing it in portions of crypt epithelium indicated by arrows (20 ×)
The left neck dissection (performed a week preceding the tonsillectomy) showed a single enlarged level 2A lymph node measuring 4.5 cm on gross examination. On microscopy, metastatic carcinoma was noted, with adenoid cystic carcinoma like areas, extensive tumor necrosis, and areas with prominent intratumoral basement membrane -like hyaline deposits in the enlarged node. No extranodal extension of tumor was identified. The remaining lymph nodes (n = 31) showed no involvement by metastatic carcinoma.
Immunohistochemistry
Immunohistochemistry was performed with standard techniques on 4-µm thick paraffin-embedded sections from both primary tumor and the metastasis. The tumor showed diffuse and strong nuclear and cytoplasmic p16 positivity (> 90%) in both the NKSCC and AdCC components (Fig. 1d). The NKSCC component was strongly positive for AE1/AE3, which was immunopositive in the luminal epithelial cells in the AdCC component and showed scattered weak expression in abluminal tumor cells (Figs. 1f, 4c, f). Both p63 and p40 were predominantly positive in the myoepithelial basaloid cells, with greater p63 positivity than p40 (Fig. 1g, h). The AdCC component was positive for LEF1 and SOX10 (nuclear) but both were negative in SCC (Fig. 3b, c). S100 was negative (not shown). C-kit highlighted ductal epithelial elements but was negative in the myoepithelial outer cells and in SCC (Fig. 1i). Beta-catenin immunostains showed membranous expression and no nuclear positivity in both tumor areas (not shown). WT-1 showed moderate to strong cytoplasmic positivity in AdCC-like areas and was negative in SCC. Additionally, foci of tumor with solid AdCC-like morphology were identified that displayed a greater intensity of cytoplasmic AE1/AE3 positivity, but not as much as the squamous component (Fig. 4b, e). Table 1 presents a list of antibodies used in the characterization of the tumor.
Fig. 4.
Variable cytoplasmic expression of pan-keratin AE1/AE3 (numbers in parenthesis indicate magnification level). Panels a, d: HE and AE1/AE3 immunostain, respectively, showing typical NKSCC areas and strong cytoplasmic cytokeratin expression in SCC component (both 11.5 ×), b, e HE and AE1AE3 in solid AdCC-like areas, with intermediate cytoplasmic immunopositivity (both 10 ×), and, c, f AdCC-like areas with clear, defined duct-like structures, with AE/AE3 immunoreactivity predominantly confined to ducts (both 16.8 ×)
Fig. 3.

(All panels 20 × magnification): a Part of tumor showing AdCC (left half) and NKSCC (right half) areas, b LEF1 shows strong nuclear immunoreactivity and is negative in NKSCC component, c SOX10 shows similar pattern of expression, d HPV mRNA ISH shows numerous positive signals (black dots) in both components
Table 1.
Antibodies used in the present study (HIER = heat induced epitope retrieval)
| Antibody | Vendor | Clone | Dilution | Antigen retrieval |
|---|---|---|---|---|
| AE1/AE3 | DAKO (Agilent) | AE1/AE3 | 1:200 | 9.0 HIER |
| P40 | Biocare | BC28 | 1:250 | 9.0 HIER |
| P63 | Biocare | 4A4 | 1:100 | 9.0 HIER |
| S100 | DAKO (Agilent) | Polyclonal | – | None |
| Calponin | DAKO (Agilent) | CALP | 1:25 | Proteinase K |
| p16 | Ventana (Roche) | E6H4 | 1:5 | 9.0 HIER |
| SOX10 | CellMarque | EP268 | 1:400 | 6.0 HIER |
| LEF1 | ABCAM | EPR2029Y | 1:500 | 9.0 HIER |
| Β-catenin | DAKO (Agilent) | B-CATENIN-1 | 1:500 | 9.0 HIER |
| Kit | DAKO (Agilent) | Polyclonal | 1:300 | 9.0 HIER |
In Situ Hybridization
HPV16 mRNA in situ hybridization (ISH) was performed on formalin-fixed, paraffin-embedded tissue using the Roche Ventana Benchmark Ultra automated platform. In this assay, bound DNP-labeled ISH probes to HPV type 16 E6/E7 mRNA are recognized by a rabbit anti-DNP antibody, which is subsequently recognized by a multi-HRP labeled goat anti-rabbit antibody. This is followed by HQ-labeled tyramide amplification and addition of a multi-HRP labeled mouse anti-HQ antibody, which develops a set of silver chromogen substrates. Cases with multiple black, punctate dots present in tumor cell cytoplasm and/or nucleus, generally throughout the sampled tumor, are scored positive. HPV mRNA ISH performed on section from both metastatic and primary tumor showed numerous positive signals in tumor cells in both squamous and AdCC components. (Figs. 1e, 3d, respectively). Testing for other high-risk HPV types was not performed.
Molecular Analysis
Tumor mRNA fusion transcripts were molecularly analyzed using techniques as described previously [11]. In brief, RNA was extracted from selected metastatic tumor on 6-µm thick paraffin-embedded sections, reverse transcribed into cDNA followed by PCR amplification to generate a library in which each specimen is uniquely identified by ligation of molecular bar codes. Sequencing was performed using anchored multiplexed PCR for targeted next-generation sequencing. The panel targets gene fusion products of 14 genes commonly associated with salivary gland tumors, including multiple exons from genes ETV 6, EWSR1, HMGA2, MAML2, MYB1, NFIB, NTRK3, PLAG1, and single nucleotide variants in PRKD1 codon 710 and HRAS codon 61. No fusion transcripts were detected in the present case.
Discussion
The differential diagnosis of a malignant basaloid neoplasm with salivary gland differentiation and cribriform and solid architecture includes polymorphous adenocarcinoma/cribriform adenocarcinoma of salivary glands, basal cell adenocarcinoma, basaloid squamous cell carcinoma and adenoid cystic carcinoma. The pattern of p40/p63 positivity, negative S100 expression, heterogenous expression of AE1/AE3, and the lack of specific morphologic features such as slate-grey tumor stroma largely excludes polymorphous adenocarcinoma (PAC). Nuclear expression of beta-catenin has been reported consistently in basal cell adenomas and less frequently in basal cell adenocarcinoma [12] whereas the present case exhibits uniform membranous expression of beta-catenin in both the glandular and squamous components. The biphasic pattern of p40 expression and the lack of uniform AE1/AE3 positivity are not in favor of basaloid squamous cell carcinoma. Furthermore, though not confirmatory, the lack of specific molecular markers such as MYB-NFIB fusion, PRKD1 E710D mutation, CTNNB1 mutation lends support in exclusion of the diagnostic considerations. Interestingly, WT1 positivity has been described in PAC and much less commonly in AdCC. Nevertheless, the sum of the features support a diagnosis of adenoid cystic carcinoma.
Adenoid cystic carcinoma (AdCC) constitutes ~ 1% of malignant epithelial neoplasms of the oral cavity and is well-described in this location. On the other hand, reports of oropharyngeal tonsillar tissue involvement by pure adenoid cystic carcinoma are exceedingly rare. Azizli et al. describe an unusual case of bilateral palatine tonsil AdCC, with tonsillar parenchymal involvement on the left and peritonsillar involvement on the right side [13]. Azarpira et al.described a 70-year old man with adenoid cystic carcinoma involving the right palatine tonsil with surface ulceration [14]. No p16 immunohistochemistry or HPV in situ hybridization studies were performed in either case. From older literature, we were able to find a report of a ‘cylindroma of the palatine tonsil’ from 1949 [15] that describes a pure adenoid cystic carcinoma with photomicrographs. No squamous differentiation is described in any of these reports. To the extent these are adenoid cystic carcinoma originating in tonsillar tissue and not peritonsillar minor salivary glands, they could represent other cases of tonsillar HPV-associated neoplasms with divergent differentiation analogous to HMSC.
Several parallels to HPV-related multiphenotypic sinonasal carcinoma are seen in the present case. A lack of nuclear expression of beta-catenin was identified in a series of 10 HMSC [16], similar to our finding. Strong nuclear LEF1 and SOX10 expression has been independently described [16, 17] in HMSC and the present case shows similar diffuse and strong expression of LEF1 and SOX10 in the ACC-like component but not in adjacent SCC (Fig. 3). Interestingly, p16 showed diffuse and strong expression in both NKSCC and AdCC components unlike a negative p16 in the SCC component found by Rupp et al. in their case of HMSC [18]. However, nearly identical to their case, we found areas exhibiting heterogenous expression of AE1/AE3, with a small portion of solid AdCC-like tumor exhibiting greater AE1/AE3 expression [18]. Surface epithelial spread of tumor has been frequently identified in HMSC, and the tumor in the present case showed multifocal attachment to the overlying squamous mucosa and focal but distinct intraepithelial extension (Fig. 2c) with the remainder of the surface epithelium overlying the adenoid cystic carcinoma component being sloughed off. These findings illustrate the variable extent of salivary gland differentiation and corresponding marker expression in this type of tumor, including the present case. HMSC has been described to have an indolent clinical course despite locoregional metastasis, and only rare cases with distant metastasis have been identified. The present case shows no evidence of local or distant metastasis after definitive treatment at 2 years follow-up but the long-term outcome remains to be determined.
Though uncommon, malignant tumors of minor salivary gland origin have been reported in the peritonsillar region [19–28]. In these reports, p16 and/or HPV status is not investigated, and the tumors are thought to arise from minor salivary glands present in the region, though some are described as directly intratonsillar [25]. p16 is known to be positive in salivary clear cell carcinoma in the absence of an association with HPV [29]. In addition, non-salivary non-intestinal adenocarcinomas with transcriptionally active high-risk HPV or HPV DNA have rarely been described involving oropharyngeal tonsillar tissue, with two reports in the palatine tonsils. Damato et al.described an adenocarcinoma with glandular and undifferentiated areas that was positive for p16 and HPV type 16 DNA in-situ hybridization [30]. Alexiev et al.recently described an adenocarcinoma of the palatine tonsil in a 51-year old man with glandular and signet ring cell features that was strongly p16-positive and showed transcriptionally active high-risk HPV (type 16) mRNA by in-situ hybridization [31]. The remainder are reports of HPV-ISH-positive adenocarcinomas in the base of tongue region, with varying morphologies [20, 32–34] though only two of them demonstrated presence of transcriptionally active HPV with mRNA ISH and/or PCR. Interestingly, one of the cases described by Hanna et al.was reported to show a minor component of squamous cell carcinoma, though their findings are not illustrated in the report. The tumor described by Perez-Ordonez et al.[34] showed surface epithelial involvement analogous to HMSC. Table 2 presents a summary of the findings of primary non-squamous carcinomas with glandular differentiation in the oropharynx (i.e., base of tongue and palatine tonsils).
Table 2.
Non-squamous malignant tumors of the oropharynx (base of tongue, palatine tonsil) with demonstrated high-risk HPV
| Report | Age (years) | Sex | Site | P16 IHC | HPV status | Histologic features |
|---|---|---|---|---|---|---|
| Damato et al.[30] | 53 | Male | 4.2 cm mass in palatine tonsil. Metastatic carcinoma in 2 Level II lymph nodes | Positive | High-risk HPV type 16 DNA ISH | High-grade adenocarcinoma and undifferentiated areas. No squamous component identified |
| Alexiev et al.[31] | 51 | Male | 4.5 cm mass in palatine tonsil. Metastatic carcinoma in six lymph nodes | Positive | Strong expression of high-risk HPV E6/E7 mRNA by ISH. Type unknown | High-grade adenocarcinoma with cribriform and tubular areas; focal signet ring cell growth. No squamous differentiation |
| Chang et al.[32] | 70 | Male | 2.8 cm tumor in left base of tongue | Positive | High-risk HPV DNA shown by multiple probe ISH. Type unknown | Non-specific high-grade adenocarcinoma |
| Perez-Ordonez et al.[33] | 64 | Male | Tongue base, glossotonsillar sulcus, vallecula and lateral pharyngeal wall on the left | Positive, predominantly cytoplasmic | HPV type 16 detected by DNA ISH and qPCR and E6/E7 transcript qRT-PCR | High-grade adenocarcinoma with cribriform areas |
| Hanna et al.[34] | 53 | Male | 2 cm mass in base of tongue, pretracheal and bilateral cervical lymph node involvement, lung metastasis | Positive |
HPV type 16 DNA PCR positive HPV type 16 DNA ISH: positive, moderate signal in illustration |
Cribriform and papillary architecture with luminal mucin; no undifferentiated areas. Minor squamous component identified |
| Hanna et al.[34] | 57 | Male | 3.3 cm mass in base of tongue/vallecula | Positive |
HPV type 16 DNA PCR positive HPV type 16 DNA ISH: ‘positive’, low signal in illustration |
Glandular and non-specific undifferentiated areas. Luminal mucin identified |
To date, the divergent differentiation observed in HMSC has shown to be predominantly associated with high-risk HPV types other than type 16: only two are reported in association with HPV 16 [8, 35]. The glandular and squamous differentiation in HMSC coupled with its uniquely restrictive site of occurrence lead authors to propose [8] the junctional/transitional epithelium of excretory ducts of subepithelial glands of sinonasal mucosa as a likely compartment of origin of the tumor. Studies of tonsillar anatomy focused on peritonsillar abscess formation have shown that minor salivary glands are present throughout peritonsillar tissue [36, 37]. Additionally, peritonsillar abscess (pus) analysis [38] has shown presence of high levels of amylase and presence of the same organisms in tonsillar tissues and abscess [37]. Overall, these findings point to a connection between squamous-lined tonsillar crypts and peritonsillar gland excretory ducts, providing a virotrophic junctional compartment niche in the palatine tonsils [30].
Our findings extend these conjectures. First, the present case shows all high-risk HPV—including type 16—is capable of driving transformation to a HMSC-like tumor, of which, we hypothesize, typical oropharyngeal NKSCC is one subtype with exclusive squamous differentiation. Second, the occurrence of an HMSC-like tumor in the palatine tonsil suggests the existence of a similar junctional/transitional epithelium at this site. The pluripotency seen in HMSC is not restricted to HPV-affected sites other than the palatine tonsils. Alternatively, it implies that the putative stem cells that give rise to NKSCC in the tonsils are capable to divergent differentiation just as in the sinonasal tract. Additionally, the present case leads us to think whether a minor/underrecognized component of a basaloid/adenoid cystic-like adenocarcinoma may lurk undetected in oropharyngeal carcinoma more commonly than appreciated.
Conclusion
In summary, we report, to our knowledge, the first case of a biphenotypic carcinoma with squamous- and adenoid cystic- like differentiation in association with transcriptionally active HPV type 16 involving the palatine tonsil. The tumor exhibits several properties similar to HPV-associated multiphenotypic carcinoma but occurs outside the sinonasal tract. Diligent tumor sampling at gross examination and judicious use of HPV testing may uncover more such examples in routine practice.
Author Contributions
All authors attest that they meet the current ICMJE criteria for Authorship.All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CKH, RAR and ARKD. The first draft of the manuscript was written by ARKD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding or grant support.
Compliance with Ethical Standards
Conflict of interest
All the authors have no financial disclosures.
Informed Consent
This report does not contain any personal identifying information. The University of Iowa Human Subjects Office determined that the study did not meet regulatory definition of human subjects’ research as it is a case report on a single case and exempted review by the IRB.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mourad M, Jetmore T, Jategaonkar AA, Moubayed S, Moshier E, Urken ML. Epidemiological Trends of Head and Neck Cancer in the United States: a SEER Population Study. J Oral Maxillofac Surg. 2017;75(12):2562–2572. doi: 10.1016/j.joms.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35(5):747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Mofty SK. Human papillomavirus-related head and neck squamous cell carcinoma variants. Semin Diagn Pathol. 2015;32(1):23–31. doi: 10.1053/j.semdp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Bishop JA, Westra WH. Ciliated HPV-related carcinoma: a well-differentiated form of head and neck carcinoma that can be mistaken for a benign cyst. Am J Surg Pathol. 2015;39(11):1591. doi: 10.1097/PAS.0000000000000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radkay-Gonzalez L, Faquin W, McHugh JB, Lewis JS, Tuluc M, Seethala RR. Ciliated adenosquamous carcinoma: expanding the phenotypic diversity of human papillomavirus-associated tumors. Head Neck Pathol. 2016;10(2):167–175. doi: 10.1007/s12105-015-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop JA, Andreasen S, Hang J-F, Bullock MJ, Chen TY, Franchi A, et al. HPV-related multiphenotypic sinonasal carcinoma: an expanded series of 49 cases of the tumor formerly known as HPV-related carcinoma with adenoid cystic carcinoma-like features. Am J Surg Pathol. 2017;41:1690–1701. doi: 10.1097/PAS.0000000000000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brzezinska K, Hammad A. Human papillomavirus-related multiphenotypicsinonasal carcinoma: a recent discovery. A case report and literature review. Head Neck Pathol. 2020;14(2):473–479. doi: 10.1007/s12105-019-01069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guseva NV, Tanas MR, Stence AA, Sompallae R, Schade JC, Bossler AD, et al. The NAB2-STAT6 gene fusion in solitary fibrous tumor can be reliably detected by anchored multiplexed PCR for targeted next-generation sequencing. Cancer Genet. 2016;209(7–8):303–312. doi: 10.1016/j.cancergen.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 12.Kawahara A, Harada H, Abe H, Yamaguchi T, Taira T, Nakashima K, et al. Nuclear β-catenin expression in basal cell adenomas of salivary gland. J Oral Pathol Med. 2011;40(6):460–466. doi: 10.1111/j.1600-0714.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 13.Azizli E, Akpinar M, Gunver F, Yigit O. Bilateral tonsillar adenoid cystic carcinoma. J Craniofac Surg. 2011;22(6):2408–2409. doi: 10.1097/SCS.0b013e318231fdf8. [DOI] [PubMed] [Google Scholar]
- 14.Azarpira N, Ashraf MJ, Shishegar M. Fine-needle aspiration biopsy of adenoid cystic carcinoma of the palatine tonsil. Indian J Pathol Microbiol. 2011;54(2):424–425. doi: 10.4103/0377-4929.81613. [DOI] [PubMed] [Google Scholar]
- 15.Giagnoni A. Cylindroma of the palatal tonsil. Boll Mal Orecch Gola Naso. 1949;67(11–12):238–246. [PubMed] [Google Scholar]
- 16.Shah AA, Oliai BR, Bishop JA. Consistent LEF-1 and MYB immunohistochemical expression in human papillomavirus-related multiphenotypic sinonasal carcinoma: a potential diagnostic pitfall. Head Neck Pathol. 2019;13(2):220–224. doi: 10.1007/s12105-018-0951-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh MS, Lee YH, Jin YT, Huang WC. Strong SOX10 expression in human papillomavirus-related multiphenotypic sinonasal carcinoma: report of 6 new cases validated by high-risk human papillomavirus mRNA in situ hybridization test. Hum Pathol. 2018;82:264–272. doi: 10.1016/j.humpath.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Rupp NJ, Camenisch U, Seidl K, Rushing EJ, Anderegg N, Broglie MA, et al. HPV-related multiphenotypic sinonasal carcinoma: four cases that expand the morpho-molecular spectrum and include occupational data. Head Neck Pathol. 2019;14:623. doi: 10.1007/s12105-019-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li JX. Clinico-pathologic studies on 143 cases of tonsillar malignancies with special reference to lymphomas. Zhonghua Zhong Liu Za Zhi. 1992;14(6):433–436. [PubMed] [Google Scholar]
- 20.Veit JA, Reichelt U, Tesche S. Signet ring cell adenocarcinoma of the oropharynx: presentation of a rare case and review of the literature. Auris Nasus Larynx. 2009;36(6):717–720. doi: 10.1016/j.anl.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Pittman CB, Zitsch RP. Polymorphous low-grade adenocarcinoma of the tonsil: report of a case and review of the literature. Am J Otolaryngol. 2002;23(5):297–299. doi: 10.1053/ajot.2002.124194. [DOI] [PubMed] [Google Scholar]
- 22.Colmenero CM, Patron M, Burgueño M, Sierra I. Polymorphous low-grade adenocarcinoma of the oral cavity: a report of 14 cases. J Oral Maxillofac Surg. 1992;50(6):595–600. doi: 10.1016/0278-2391(92)90440-b. [DOI] [PubMed] [Google Scholar]
- 23.Horky JK, Chaloupka JC, Putman CM, Roth TC, Weaver EM, Sasaki CT. True malignant mixed tumor (carcinosarcoma) of tonsillar minor salivary gland origin: diagnostic imaging and endovascular therapeutic embolization. Am J Neuroradiol. 1997;18(10):1944. [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis SJ, Giangrande V, Brennan PA. Mucoepidermoid carcinoma of the tonsil: a very rare presentation. Acta Otorhinolaryngol Ital. 2013;33(4):286–288. [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess B, Ananthanarayanan V, Charous S. Hyalinizing clear cell carcinoma of the tonsil: a case report. Head Neck Pathol. 2017;11(4):580–583. doi: 10.1007/s12105-017-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hijjawi SB, Abdullah SE, Abdelhadi K, Eyzaguirre E, Willis M, Abdulla NE. Hyalinizing clear cell carcinoma of the tonsil and its treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):e32–e36. doi: 10.1016/j.oooo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Pagano A, Dennis K. Cribriform adenocarcinoma of the minor salivary gland arising in the tonsil with metastasis to a cervical lymph node: a case report with description of fine needle aspiration cytology. Diagn Cytopathol. 2017;45(5):468–471. doi: 10.1002/dc.23687. [DOI] [PubMed] [Google Scholar]
- 28.Ferlito A. Acinic cell carcinoma of minor salivary glands. Histopathology. 1980;4(3):331–343. doi: 10.1111/j.1365-2559.1980.tb02926.x. [DOI] [PubMed] [Google Scholar]
- 29.Bishop JA, Rooper LM, Chiosea SI, Westra WH. Clear cell carcinoma of salivary glands is frequently p16 positive: a pitfall in the interpretation of oropharyngeal biopsies. Am J Surg Pathol. 2018;42(3):367–371. doi: 10.1097/pas.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damato S, Thavaraj S, Winter S, Shah KA. Human papillomavirus-associated adenocarcinoma of the palatine tonsil. Hum Pathol. 2014;45(4):893–894. doi: 10.1016/j.humpath.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Alexiev BA, Behdad A, Lu X, Cheng E, Samant S. High-risk human papillomavirus-mediated adenocarcinoma of palatine tonsil. Pathol Res Pract. 2020;216:152924. doi: 10.1016/j.prp.2020.152924. [DOI] [PubMed] [Google Scholar]
- 32.Chang AM, Nikiforova MN, Johnson JT, Bauman JE, Perez-Ordonez B, Seethala RR, et al. Human papillomavirus-associated adenocarcinoma of the base of tongue: potentially actionable genetic changes. Head Neck Pathol. 2014;8(2):151–156. doi: 10.1007/s12105-013-0508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Ordonez B, Irish JC, Yu ES, Gillison ML. Human papillomavirus-16 associated adenocarcinoma NOS of base of tongue. Head Neck Pathol. 2013;7(3):268–273. doi: 10.1007/s12105-012-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna J, Reimann JD, Haddad RI, Krane JF. Human papillomavirus-associated adenocarcinoma of the base of the tongue. Hum Pathol. 2013;44(8):1516–1523. doi: 10.1016/j.humpath.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Ching D, Pirasteh S, Ly C. HPV-related multiphenotypic sinonasal carcinoma: a unique case. Int J Surg Pathol. 2019;27(8):888–892. doi: 10.1177/1066896919866508. [DOI] [PubMed] [Google Scholar]
- 36.Kraitrakul S, Sirithunyaporn S, Yimtae K. Distribution of minor salivary glands in the peritonsillar space. J Med Assoc Thai. 2001;84(3):371–378. [PubMed] [Google Scholar]
- 37.Kaltiainen E, Wikstén J, Aaltonen L-M, Ilmarinen T, Hagström J, Blomgren K. The presence of minor salivary glands in the peritonsillar space. Eur Arch Oto-Rhino-Laryngol. 2017;274(11):3997–4001. doi: 10.1007/s00405-017-4738-x. [DOI] [PubMed] [Google Scholar]
- 38.El-Saied S, Puterman M, Kaplan DM, Cohen-Lahav M, Joshua B-Z. Involvement of minor salivary glands in the pathogenesis of peritonsillar abscess. Otolaryngology. 2012;147(3):472–474. doi: 10.1177/0194599812445552. [DOI] [PubMed] [Google Scholar]