Abstract
Background
Syphilis is a sexually-transmitted infectious disease caused by Treponema pallidum. Cases of primary and secondary syphilis are on the rise in the United States, with a 14.4% increase in new cases noted from 2017 to 2018 and an escalation of 71% between the years 2014 and 2018. Fulfilling its nickname of “the great imitator,” oral manifestations of syphilis may mimic a variety of infectious, neoplastic, or immune-mediated processes, both clinically and histopathologically. This large spectrum of appearances can create a diagnostic challenge to the clinician and/or pathologist, leading to delay in diagnosis or misdiagnosis.
Methods
A database of oral syphilis cases was created from archives at the University of Kentucky, University of Pittsburgh, LIJMC, Columbia University MC, and University of Tennessee. The age, sex, race, location, duration, and clinical description were recorded. Cases without positive reaction upon immunohistochemistry or serologic tests were excluded.
Results
We identified 19 new cases of oral syphilis (17 males, one female, and one case unknown sex) and described the clinical and histopathological features of this re-emerging and potentially fatal disease. All cases demonstrated dense lymphoplasmacytic inflammation, often with inflammatory exocytosis or ulceration at the surface, and perivascular inflammation.
Conclusions
Early recognition of the histopathologic and clinical manifestations of oral syphilis is imperative for prompt diagnosis, improved patient outcomes, and disease prevention.
Keywords: Syphilis, Chancre, Gumma, Mucous patch, Oral, Treponema pallidum, Oral cavity
Introduction
Syphilis is an infectious disease caused by the tightly-coiled, anaerobic, filamentous bacterial spirochete Treponema pallidum [1–4]. Although cases may be transmitted from pregnant mother to child or through hematogenous spread, the infection mainly is spread through direct contact with a syphilitic lesion [4, 5]. This contact most often occurs during vaginal, anal, or oral sex. While the origin of syphilis is uncertain, two theories prevail: “The New World Theory” in which the infection was taken from the Americas to Europe by the shipmates aboard Christopher Columbus’ voyage and the “Old World Theory” in which the infection already existed in Europe prior to Columbus’ expedition and that it was the cause of the “Great Pox” epidemic in the late 15th/early sixteenth century [6].
In 2001 the incidence of syphilitic infection was the lowest reported since the initiation of public health surveillance of the disease began in 1941; however, an observable increase of cases has occurred in recent years. All cases of syphilis increased 13.3% in the United States (U.S.) from 2017 to 2018, and the rates of primary and secondary cases increased 14.4% during that same period, particularly in men who have sex with men (MSM) [7]. In 2018, 35,063 new cases of primary and secondary syphilis and 115,045 total cases were reported in the U.S. [7]. This number reflects a 71% increase in the number of primary and secondary syphilis from 2014 to 2018 [7].
Humans are the only natural host for syphilis, and re-infection of the host is possible, as primary infection does not confer immunity [8–10]. In fact, approximately 15–20% of individuals diagnosed with syphilis in the U.S. each year have had a previous infection [5]. The infection progresses through four overlapping stages: primary, secondary, latent, and tertiary [1, 2, 4, 5, 11]. Primary, secondary, and tertiary syphilis can all present with oral manifestations [1, 2, 4]. Oral manifestations often are one of the first signs of infection. The head and neck healthcare clinician or pathologist may play a critical role in early recognition, diagnosis, and the initiation of necessary therapy [8]. While syphilis is curable with antibiotics, many cases go undiagnosed for long periods of time and progress to the tertiary stage of syphilis in 30% of patients, which may result in potentially fatal neurologic and cardiovascular diseases [2]. Furthermore, because the first and second stages of the disease are highly contagious, prompt awareness of the clinical and histopathological features is imperative [12]. Because some patients with undiagnosed syphilis may only present with oral lesions, early recognition of the oral manifestations with potential biopsy will prove useful during these times of syphilis resurgence [13].
Methods
Following proper protocol from the respective Institutional Review Boards, a database of oral syphilis cases was created using cases diagnosed within the Division of Oral and Maxillofacial Pathology of the University of Kentucky (2005–2020). Additional cases were obtained from the oral and maxillofacial pathology departments at the University of Pittsburgh School of Dental Medicine, Northwell Health Long Island Jewish Medical Center, Columbia University Medical Center, and University of Tennessee College of Dentistry. When known, the age, sex, race, location of lesion, duration, and clinical description were recorded (Table 1). The H&E slides were reviewed by at least one board-certified oral and maxillofacial pathologist, and the histopathological characteristics of each case were examined. Cases without positive reaction upon immunohistochemical staining with anti-Treponema pallidum or positive serologic tests were excluded. Due to the nature of the biopsy services, follow-up and serologic testing information is unavailable.
Table 1.
Clinical characteristics of oral syphilis cases 1–19
| Case | Age | Sex | Race | Location | Duration | Clinical description | Clinical impression | Syphilitic lesion |
|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | C | Lower lip | > 5 weeks | 1.5 cm, Rapidly growing, painful, ulcerated mass with firm borders | Squamous Cell Carcinoma; Salivary Gland Malignancy | Chancre |
| 2 | 30 | M | C | Ventral tongue, midline | At least 1 week | White lesion | Focal Epithelial Hyperplasia | Mucous Patch |
| 3 | 50 | M | N/a | Lateral tongue | N/a | N/a | N/a | Unknown |
| 4 | 61 | F | N/a | Lateral tongue | N/a | Erythemous lesion with hyperplastic tissue | N/a | Unknown |
| 5 | 64 | M | C | Lateral tongue | N/a | White lesion | Lichen Planus | Unknown |
| 6 | 63 | M | N/a | Palate | N/a | Ulceration with indurated borders | N/a | Chancre |
| 7 | 47 | M | C | Mandibular vestibule/gingiva | 2–3 weeks | White Lesion | Hyperkeratosis, Dysplasia, Carcinoma In Situ, Squamous Cell Carcinoma | Unknown |
| 8 | 23 | M | C | Tongue and lip | N/a | N/a | Autoimmune disease | Unknown |
| 9 | 38 | M | C | Lateral tongue | N/a | N/a | Focal Fibrous Hyperplasia | Unknown |
| 10 | 41 | M | C | Hard/soft palate/tonsillar pillars | At least 2 months | White, red, and ulcerative lesions; painful; no skin rash | Chronic Ulcerations | Mucous Patch |
| 11 | 40 | M | N/a | Soft palate/tonsillar pillar/floor of mouth | Red/white | Squamous cell carcinoma; hyperkeratosis | Mucous Patch | |
| 12 | N/a | N/a | N/a | Ventral tongue | N/a | N/a | Papilloma, condyloma | Mucous Patch |
| 13 | N/a | M | N/a | Lateral tongue | N/a | N/a | Squamous Cell Carcinoma | Unknown |
| 14 | N/a | M | N/a | Dorsal tongue | N/a | N/a | Lymphoid Hyperplasia | Unknown |
| 15 | 36 | M | C | Ventrolateral tongue | N/a | N/a | Leukoplakia | Mucous Patch |
| 16 | 28 | M | N/a | Upper lip | ~ 1 week | N/a | Ulceration | Chancre |
| 17 | 25 | M | N/a | Lateral tongue | 6 weeks | 1.0 cm Non-healing ulceration | Squamous cell carcinoma | Chancre |
| 18 | 28 | M | N/a | N/a | N/a | Painful, odorous, red/white lesions | N/a | Mucous Patch |
| 19 | 50 | M | AA | Buccal mucosa, ventral tongue, labial mucosa | N/a | Tan, white plaques; slightly elevated and focally ulcerated; painful | Infection vs. granulomatous inflammation | Mucous Patch |
Results
Nineteen cases of oral syphilis were identified within our combined database (Table 1)—17 males, one female, and one case of unknown sex. The average patient age was 42 years with a range of 23–64 years. The clinical impressions from the submitting clinicians varied from benign entities (including focal epithelial/fibrous hyperplasia, hyperkeratosis, autoimmune disease, papilloma/condyloma, lymphoid hyperplasia) to malignancies, namely squamous cell carcinoma or salivary gland malignancies. The most common location was the tongue.
In all cases, the histologic sections showed a dense, mixed inflammatory cell infiltrate comprised mainly of plasma cells and lymphocytes. In cases that did not demonstrate complete ulceration of the surface, the epithelium often was hyperplastic and exhibited pronounced inflammatory exocytosis. The majority of cases also showed a prominent perivascular arrangement of chronic inflammation, particularly composed of plasma cells. In three cases, a Periodic Acid-Schiff fungal stain was ordered, and in two cases, immunohistochemical staining for lambda and kappa light chains was performed to prove polyclonality of the dense plasmacytic infiltrate. In both cases, immunohistochemical staining did not demonstrate restriction of either light chain. In case number one, immunohistochemical staining for CD3, CD20, CD30, kappa, lambda, and pan-cytokeratin were performed due to the presence of enlarged lymphocytes, abundance of plasma cells, and concern to exclude hematopoietic malignancies and a CD30/EBER-positive mucocutaneous ulceration.
Discussion
For both clinicians and pathologists alike, the importance of recognizing the oral manifestations of syphilis cannot be understated, as misdiagnosis of this curable disease may have tragic consequences for the patients. Syphilis has been termed the “great imitator” since its signs, symptoms, and even histopathological characteristics look like many other diseases [5]. The clinical characteristics depend greatly on the disease stage. In a recent large case series of 40 cases, 37 cases were diagnosed in the secondary stage, and 3 cases were consistent with the primary stage [4]. As in our series, no cases of tertiary syphilis were identified. This preponderance of secondary syphilis cases is of great interest, as the most varied clinical presentations occur in this stage. Schuch et al. also found that the mean age of cases was 39.5 years, with the third and fourth decades of life being most represented [4]; however, it is noteworthy that cases have been reported in teenagers, and, in increasing numbers, the elderly [14, 15]. Like our series, Schuch et al. reported the tongue to be the most common site affected in 33.9% of cases [4] and males as the gender most affected (60.0% within their series of 40 patients and 78.9% in their review of the literature). It is possible to hypothesize that the higher prevalence in males is due to the higher risk of infection among MSM [2, 4, 5, 15]. Given the retrospective nature of this review and fact the cases were submitted from outside providers, we were unable to obtain a sexual history from our patients.
Clinical Characteristics
Because the histopathological features of oral syphilis may be vague or mimic other inflammatory processes of the mouth, clinical correlation often is imperative for proper diagnosis of the biopsy sample; frequently, it is the clinical correlation in combination with the histopathological features that prompts immunohistochemical staining with anti-T. pallidum. Primary syphilis manifests within 3 to 90 days—typically within 2–3 weeks—after exposure to another person’s lesion at the site of direct inoculation [4, 5]. The hallmark of primary syphilis is the chancre [1, 2, 4, 16] (Fig. 1). Chancres may be solitary or rarely multiple and often appear as firm and asymptomatic ulcerations, although occasional cases may be painful [1, 2, 4] (Table 1—case 1). Importantly, chancres are highly infectious [2]. Because chancres occur at the site of inoculation, the most commonly affected area is the genital mucosa. Most extra-genital chancres—40–75%—occur in the mouth [1, 2]. Approximately 4–12% of patients with primary syphilis present with oral chancres [1], which commonly are seen on the lip, tongue, buccal mucosa, palate, gingiva, or tonsillar pillar. In addition to the chancre, patients often present with lymphadenopathy, which may or may not be tender [5, 8, 15, 16]. Chancres typically remit spontaneously in 3 to 8 weeks [1, 7, 10]. Due to the asymptomatic nature of the primary lesion, only 30–40% of patients in the primary stage are diagnosed [8, 9, 16, 17].
Fig. 1.
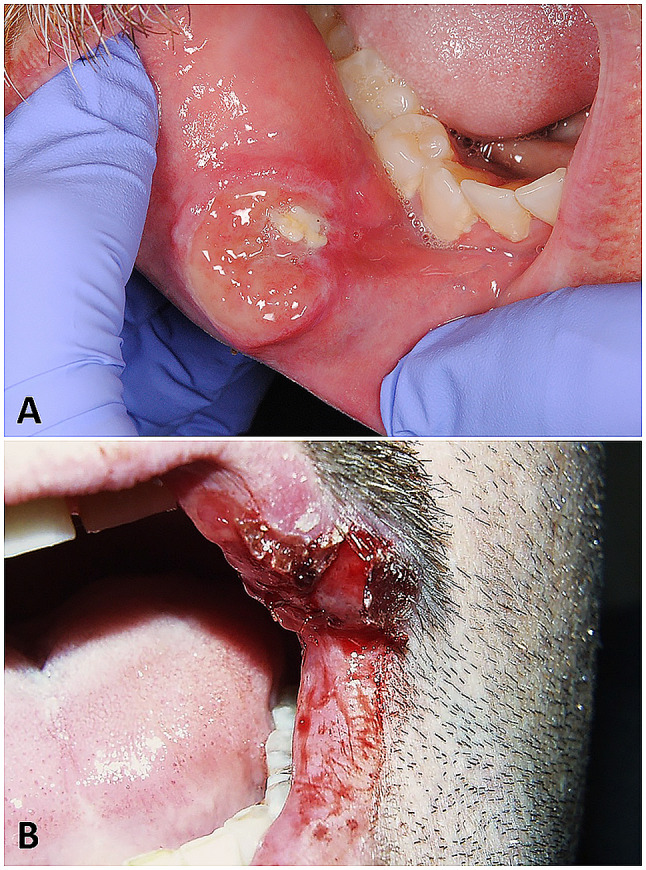
Chancres of oral syphilis for cases 1 (a) and 16 (b)
Unlike primary syphilis and its typically solitary lesion, multiple lesions are usually encountered in patients with secondary syphilis [4]. Secondary syphilis presents approximately 2–12 weeks after the primary stage with maculopapular skin rashes which may affect one or more areas of the body or mucous membrane lesions [1, 2, 5, 10, 16]. The rashes may be subtle and often are not pruritic. In addition to the rash, patients may present with other systemic symptoms including malaise, fatigue, myalgia, sore throat, fever, and headache [5, 16]. Mucous patches present in approximately 30% and demonstrate a white/pink change of the mucous membrane which may exhibit a serpentine or snail-track pattern [1, 2, 4, 18] (Fig. 2g). Superficial necrosis of the epithelium may occur, leaving denuded and raw underlying connective tissue. Mucous patches most often are found on the lip, tongue, buccal mucosa, and palate. When they are centered over the commissures of the mouth, they are referred to as split papules [3]. Another small percentage of patients with secondary syphilis may demonstrate raised, papillary gray/white lesions known as condyloma lata [16]. These lesions often develop in warm, moist areas, such as the underarm, groin, or oral regions.
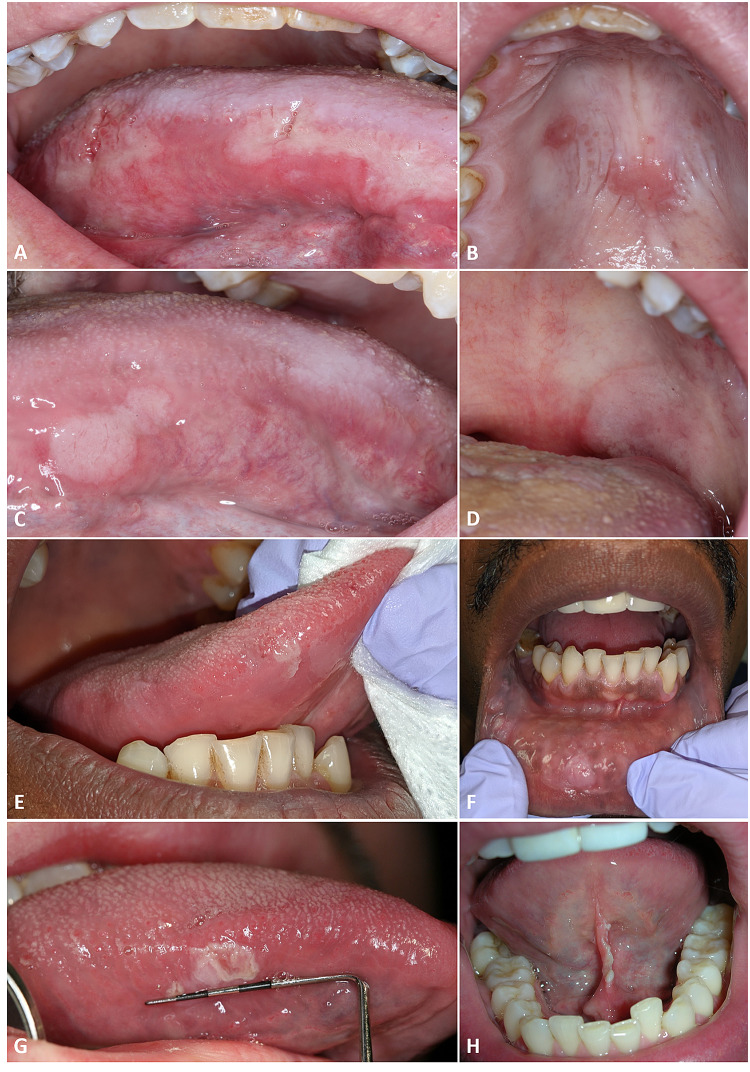
Fig. 2.
Mucous patches of secondary oral syphilis. Case 18 demonstrates painful red, tan, and white ulcerations throughout the oral cavity (a–d), case 19 exhibits white patches of the lateral tongue, buccal mucosa, and labial mucosa which mimic leukoplakias (e, f) case 15 imitates a leukoplakia on the lateral tongue (g), and case 12 depicts a linear ulceration affecting the lingual frenum (h)
The disease may progress to tertiary syphilis up to 30 years after primary infection (the asymptomatic years being termed “latent syphilis”). Tertiary syphilis occurs in approximately 30% of patients, and it has serious complications that may affect numerous organ systems, including nerves, brain, eyes, heart, liver, blood vessels, joints, and bones. When syphilis affects the central nervous system, it is termed “neurosyphilis,” and it may cause paralysis, sensory deficits, difficulty coordinating muscle movements, dementia, paresis, tabes dorsalis, or death. It is noteworthy that neurosyphilis can occur at any stage of infection, not just the tertiary stage. Patients with tertiary syphilis may also demonstrate sites of granulomatous inflammation on the skin or mucosa. These sites are termed “gumma” and present as ulcerated, nodular, or firm lesions that may cause significant destruction of the tissues. When gummas are found inside the mouth, the most common location is the tongue or palate. Occasionally, gummas that affect the tongue can produce diffuse atrophy (termed “luetic glossitis”) or a lobulated and irregular pattern (referred to as “interstitial glossitis”) [3].
When syphilis is transmitted from a pregnant woman to her unborn baby, the baby demonstrates “congenital syphilis.” Approximately 988,000 pregnant women were infected worldwide with syphilis in 2016, and although preventable, mother-to-child transmission of syphilis still remains problematic [19, 20]. The classic triad of diagnostic characteristics, known as “Hutchinson triad,” includes Hutchinson teeth, ocular interstitial keratitis, and eighth nerve deafness. Hutchinson teeth are teeth altered in utero by the infection. Patients with congenital syphilis may also demonstrate a multitude of other signs, including a saddle nose deformity, frontal bossing, a high-arched palate, enlargement of the clavicle near the sternum (termed “Higoumenaki sign”), premature perioral fissuring (“rhagades”), painless synovitis and enlargement of the joints (“clutton joints”), or anterior bowing of the tibia (termed “saber shin”). Over 50% of untreated syphilis cases in pregnant mothers result in adverse birth outcomes, including infant mortality; however, effective perinatal screening and subsequent treatment of the infection in the pregnant mother can result in prevention of congenital syphilis [21].
Also important in the epidemiology and pathology of syphilis is the well-established connection between syphilis and human immunodeficiency virus (HIV). Syphilis, in addition to other sexually transmitted infections (STI), has been shown to facilitate the transmission of HIV [22]. Syphilis increases the acquisition of HIV in MSM by two-to-threefold [23]. Additionally, syphilis has been shown to decrease the CD4 count and increase the HIV viral load, although antiretroviral therapy may diminish that effect [22, 24]. In pregnant women, syphilis has been shown to increase the risk of mother-to-child transmission of HIV [25]. While almost every country in the world has implemented antenatal screening processes for both HIV and syphilis, rates of mother-to-child transmission of syphilis and HIV continue to be significant causes of perinatal morbidity and mortality [19].
Clinical Differential Diagnosis of Oral Syphilis
The oral lesions of syphilis can be nonspecific; and, because syphilis may mimic a variety of other diseases, the differential diagnosis is long [1, 2, 4, 8, 9, 12]. In general, the differential diagnosis consists of lesions/processes which present as ulcerations with a firm border or multifocal ulcerations with pseudomembranes. These entities include traumatic granulomas/ulcerations, atypical/major aphthous ulcerations, geographic tongue, deep fungal infections (e.g. histoplasmosis, blastomycosis), tuberculosis, squamous cell carcinoma, Crohn disease, pyostomatitis vegetans, subepithelial vesiculoerosive diseases (e.g. erosive lichen planus), drug-related ulcerations, or granulomatosis with polyangiitis. Typically, condyloma lata may be confused clinically with human papilloma virus-related papillomas/condyloma accuminata. “Leukoplakia-like” lesions have been reported, and rare cases have even been reported where the oral lesions clinically mimic other uncommon pathologies such as oral hairy leukoplakia and pemphigus vulgaris [11, 26, 27].
Histopathologic and Immunohistochemical Characteristics
For oral syphilis, an incisional biopsy may prove most useful to (a) eliminate other entities in the differential diagnosis and (b) obtain a positive result upon specific immunohistochemistry with anti-T. pallidum. A positive test for T. pallidum on direct immunofluorescence [28] or biopsy with immunohistochemistry may provide valuable information, especially in the initial stages when antibodies may be undetectable via serologic examination; however, just as syphilis mimics a variety of diseases clinically, the histopathological appearance may mimic a plethora of diagnoses, as well. The histopathological evaluation of syphilis, particularly in the primary and secondary stages, often demonstrates a dense plasmacytic/lymphocytic infiltrate in the superficial lamina propria and occasionally in the deeper stroma [1, 29, 30] (Fig. 3). The infiltrate characteristically surrounds blood vessels and nerves (Fig. 3d). Epithelial hyperplasia and obliterative endarteritis with swollen endothelial cells may also be observed [1, 29, 30]. Ulceration with fibrinopurulent exudate and/or extensive areas of neutrophilic exocytosis and abscesses may be present (Fig. 3c). All cases in our series demonstrated many or all the above histopathologic characteristics. Lesions of tertiary syphilis may demonstrate chronic granulomatous inflammation, and the organism may be difficult to find on tissue samples during this stage [1].
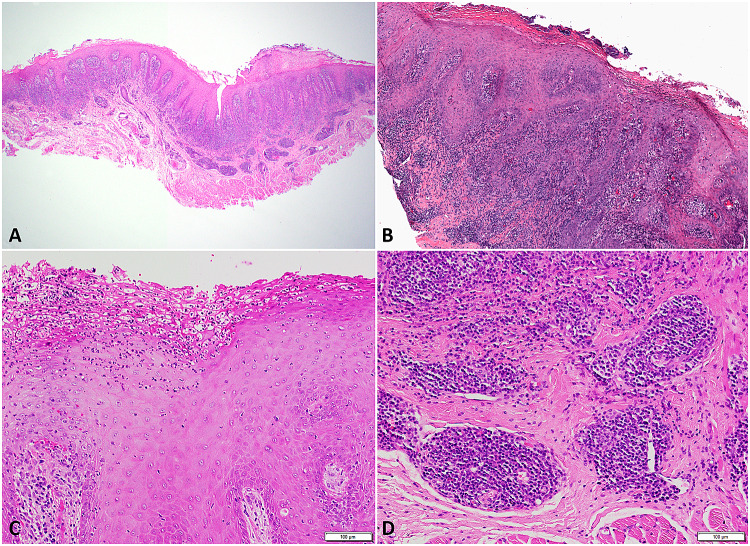
Fig. 3.
Microscopic examination of oral syphilis demonstrates a dense lymphoplasmacytic inflammatory cell infiltrate in the superficial lamina propria which may extend into the deeper stroma (a). Focal ulceration (b), extensive neutrophilic exocytosis (c), and perivascular inflammation (d) also are often present
While the treponemes are positive upon histochemical staining with a Warthin-Starry stain, most cases are confirmed via immunohistochemistry for anti-T. pallidum [30, 31]. Immunohistochemistry has been shown to have greater sensitivity than Warthin-Starry staining [1, 30–32] and eliminates confusion with another common oral entity, Treponema denticola. Immunohistochemistry reveals the presence of numerous corkscrew-shaped spirochetes that infiltrate the epithelium and may demonstrate a perivascular pattern in the underlying fibrous stroma (Fig. 4). It is noteworthy, however, that cross-reactivity of anti-T. pallidum has been reported with other bacterial organisms, including Borrelia burgdorferi and spirochetes of the Brachyspira family [33]. Strong clinical correlation is of major significance in these cases of misleading cross-reactivity.
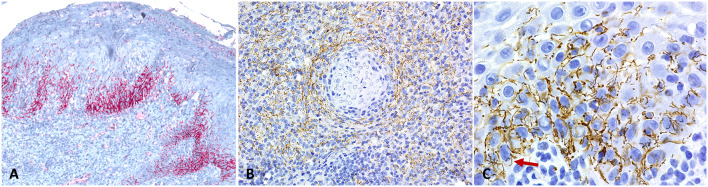
Fig. 4.
Immunohistochemistry for Treponema pallidum (a–c) reveals dense positive staining (red or brown) predominantly in the lower basal and spinous cell layers of the epithelium (a) as well as surrounding blood vessels in the deeper stroma (b). High power image (C-1000 × original magnification) demonstrates tightly coiled spirochetes, positive upon staining with immunohistochemistry
Histopathologic Differential Diagnosis
Due to the heavy plasmacytic and/or lymphocytic infiltrate with scattered atypical morphology, the histomorphology of syphilis may mimic a variety of hematopoietic processes on microscopic examination, including lymphoma, myeloma, or atypical lymphoproliferative processes (e.g. EBER-positive mucocutaneous ulceration). Additionally, oral syphilis may imitate geographic tongue (erythema migrans) or a variety of vesiculoerosive processes, including lichen planus, a lichenoid reaction, chronic ulcerative stomatitis, or lupus erythematosus, further emphasizing the need to correlate the histopathological features with the clinical scenario. Significant clinical and histopathological suspicion for syphilis often prompts immunohistochemical staining for anti-T. pallidum.
Diagnosis and Management
Due to extensive variability in testing results, a diagnosis of syphilis often is made upon the combined evaluation of the clinical characteristics, social history, serological tests, and histopathological features. Misdiagnosis often occurs when a proper evaluation is not undertaken. Because syphilis tends to be stigmatized in society, the patient may provide a misleading medical and social history, making the diagnosis increasingly difficult.
Serology includes nontreponemal tests (venereal disease research laboratory- VDRL and rapid plasma reagin-RPR) which are relatively inexpensive and often used for screening purposes, as well as Treponema tests, including Treponema pallidum particle agglutination assay (TP-PA), fluorescent treponemal antibody absorption (FTA-ABS), rapid treponemal assays, chemiluminescence immunoassays, and immunoblots. Because those infected with syphilis produce antibodies that persist throughout life, patients with a previous positive test may obtain nontreponemal titers to evaluate for the presence of disease or response to treatment.
Luckily, the susceptibility of T. pallidum to antibiotics has not diminished over time. Syphilis is treated with antibiotics in the penicillin family: penicillin G benzathine or penicillin G procaine [10]. In patients with an allergy to penicillin, doxycycline or azithromycin are used, although resistance to azithromycin has been observed [10].
Conclusions
Due to the significant diversity of the clinical and histopathologic manifestations of oral syphilis, both pathologists and clinicians must demonstrate a high degree of suspicion in order to diagnose syphilis. It is prudent that pathologists are familiar with the variable clinical and histopathological presentations of this re-emerging infection.
Acknowledgements
The authors thank Drs. Kevin Andrus, Matthew Conquest, Craig Fowler, P. Shawn Stopperich, Anthony Tortorich, and Shane Wilson for their contribution of case information and/or clinical photographs.
Author Contributions
MHS: Main author, corresponding author and contributor of most cases. RJV: Critical review, contribution of cases, wrote a portion of the manuscript. EAB: Critical review, contribution of cases. MA: Critical review, contribution of cases. AT: Critical review, wrote a portion of the manuscript. CRC: Critical review, contribution of cases. JEF: Critical review, contribution of cases. YBR: Critical review, contribution of cases.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This retrospective review study involving de-identified human participants was performed in accordance with the principles of the Declaration of Helsinki.
Informed Consent
Consent was waived or the cases were exempt in accordance with the institutional review boards at the institutions from where the cases originated (the University of Kentucky, the University of Pittsburgh School of Dental Medicine, Northwell Health Long Island Jewish Medical Center, Columbia University Medical Center, and University of Tennessee College of Dentistry).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ficarra G, Carlos R. Syphilis: the renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3(3):195–206. doi: 10.1007/s12105-009-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leuci S, Martina S, Adamo D, Ruoppo E, Santarelli A, Sorrentino R, Favia G, Mignogna M. Oral Syphilis: a retrospective analysis of 12 cases and a review of the literature. Oral Dis. 2013;19(8):738–746. doi: 10.1111/odi.12058. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, et al. Oral and maxillofacial pathology. 4. St. Louis: Elsevier; 2016. [Google Scholar]
- 4.Schuch LF, da Silva KD, de Arruda JAA, Etges A, Gomes APN, Mesquita RA, Vasconcelos ACU, Tarquinio SBC. Forty cases of acquired oral syphilis and a review of the literature. Int J Oral Maxillofac Surg. 2018;48(5):635–643. doi: 10.1016/j.ijom.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Hook EW., III Syphilis. Lancet. 2017;389:1550–1557. doi: 10.1016/S0140-6736(16)32411-4. [DOI] [PubMed] [Google Scholar]
- 6.Tramont EC. Syphilis in adults: from Christopher Columbus to Sir Alexander Fleming to AIDS. Clin Infect Dis. 1995;21(6):1361–1371. doi: 10.1093/clinids/21.6.1361. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Syphilis. https://www.cdc.gov/std/syphilis/stdfact-msm-syphilis.htm. Accessed on 13 Apr 2020.
- 8.de Andrade RS, de Freitas EM, Rocha BA, Gusmão ES, Filho MR, Júnior HM. Oral findings in secondary syphilis. Med Oral Patol Oral Cir Bucal. 2018;23(2):e138–e143. doi: 10.4317/medoral.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelner N, Rabelo GD, da Cruz Perez DE, Assunção JN, Jr, Witzel AL, Migliari DA, Alves FA. Analysis of nonspecific oral mucosal and dermal lesions suggestive of syphilis: a report of 6 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):1–7. doi: 10.1016/j.oooo.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Seibt CE, Munerato MC. Secondary syphilis in the oral cavity and the role of the dental surgeon in STD prevention, diagnosis and treatment: a case series study. Braz J Infect Dis. 2016;20(4):393–398. doi: 10.1016/j.bjid.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compilato D, Amato S, Campisi G. Resurgence of syphilis: a diagnosis based on unusual oral mucosa lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):e45–e49. doi: 10.1016/j.tripleo.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Hertel M, Matter D, Schmidt-Westhausen AM, Bornstein MM. Oral syphilis: a series of 5 cases. J Oral Maxillofac Surg. 2014;72(2):338–345. doi: 10.1016/j.joms.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Eyer-Silva WA, Freire MAL, Horta-Araujo CA, Almeida Rosa da Silva G, Francisco da Cunha Pinto J, Raphael de Almeida Ferry F. Secondary syphilis presenting as glossodynia, plaques en Prairie Fauchée, and a split papule at the oral commissure: case report and review. Case Rep Med. 2017;217:1980798. doi: 10.1155/2017/1980798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman J, Garcia B, Horrall S. Syphilis at age 15 years. Proc (Bayl Univ Med Cent) 2018;31(1):105–106. doi: 10.1080/08998280.2017.1401838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minicucci EM, Vieira RA, Oliveira DT, Marques SA. Oral manifestations of secondary syphilis in the elderly—a timely reminder for dentists. Aust Dent J. 2013;58(3):368–370. doi: 10.1111/adj.12085. [DOI] [PubMed] [Google Scholar]
- 16.Carbone PN, Capra GG, Nelson BL. Oral secondary syphilis. Head Neck Pathol. 2016;10(2):206–208. doi: 10.1007/s12105-015-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautenschlager S. Diagnosis of syphilis: clinical and laboratory problems. J Dtsch Dermatol Ges. 2006;4:1058–1075. doi: 10.1111/j.1610-0387.2006.06072.x. [DOI] [PubMed] [Google Scholar]
- 18.de Paulo LF, Servato JP, Oliveira MT, Durighetto AF, Jr., Zanetta-Barbosa D. Oral manifestations of secondary syphilis. Int J Infect Dis. 2015;35:40–42. doi: 10.1016/j.ijid.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Mabey D, Peeling RW. Syphilis, still a major cause of infant mortality. Lancet Infect Dis. 2011;11:654–655. doi: 10.1016/S1473-3099(11)70150-5. [DOI] [PubMed] [Google Scholar]
- 20.WHO-WPRO. Elimination of mother-to-child transmission of HIV and syphilis. www.wpro.who.int/hiv/topics/pmtct/en/. Accessed 5 May 2020.
- 21.Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect. 2017;93(S4):S3–S15. doi: 10.1136/sextrans-2016-053069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart-Malloy R, Rosenthal M, Patterson W, Currenti S, O'Donnell T, Gunn JK. Syphilis among adult males with a history of male-to-male sexual contact living with diagnosed HIV in New York State (excluding New York City): The challenge of intersecting epidemics. PLoS ONE. 2019;14(12):e0226614. doi: 10.1371/journal.pone.0226614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An Q, Wejnert C, Bernstein K, Paz-Bailey G, Group NS Syphilis screening and diagnosis among men who have sex with men, 2008–2014, 20 U.S. Cities. J Acquir Immune Defic Syndr. 2017;75(Suppl 3):S363–S369. doi: 10.1097/QAI.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 24.Buchacz K, Patel P, Taylor M, Kerndt PR, Byers RH, Holmberg SD, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18(15):2075–2079. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwapasa V, Rogerson SJ, Kwiek JJ, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS. 2006;20:1869–1877. doi: 10.1097/01.aids.0000244206.41500.27. [DOI] [PubMed] [Google Scholar]
- 26.Aquilina C, Viraben R, Denis P. Secondary syphilis simulating oral hairy leukoplakia. J Am Acad Dermatol. 2003;49(4):749–751. doi: 10.1067/S0190-9622(03)00484-5. [DOI] [PubMed] [Google Scholar]
- 27.Mignogna AM, Fortuna G, Leuci S, Mignoga C, Delfino M. Secondary syphilis mimicking pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2009;23:479–480. doi: 10.1111/j.1468-3083.2008.02926.x. [DOI] [PubMed] [Google Scholar]
- 28.Theel ES, Katz SS, Pillay A. Molecular and direct detection tests for Treponema pallidum subspecies pallidum: a review of the literature. Clin Infect Dis. 2020;71(Supplement_1):S4–S12. doi: 10.1093/cid/ciaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett AW, Villarroel Dorrego M, Hodgson TA, Porter SR, Hopper C, Argiriadou AS, Speight PM. The histopathology of syphilis of the oral mucosa. J Oral Pathol Med. 2004;33(5):286–291. doi: 10.1111/j.0904-2512.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 30.Flamm A, Parikh K, Xie Q, Kwon EJ, Elston DM5 Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73(6):1025–1030. doi: 10.1016/j.jaad.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 31.Martín-Ezquerra G, Fernandez-Casado A, Barco D, Jucglà A, Juanpere-Rodero N, Manresa JM, de Almeida LM, Rodríguez-Peralto JL, Kutzner H, Cerroni L, Barranco C, Lloreta J, Requena L, Pujol RM. Treponema pallidum distribution patterns in mucocutaneous lesions of primary and secondary syphilis: an immunohistochemical and ultrastructural study. Hum Pathol. 2009;40(5):624–630. doi: 10.1016/j.humpath.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Strieder LR, León JE, Carvalho YR, Kaminagakura E. Oral syphilis: report of three cases and characterization of the inflammatory cells. Ann Diagn Pathol. 2015;19(2):76–80. doi: 10.1016/j.anndiagpath.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Pettit C, McMurray S, Randall MB, Jones A, Fisher K. Highlighting a potential pitfall: positive Treponemapallidum immunohistochemical stain in a patient without syphilis. Am J Dermatopathol. 2019;41(12):924–926. doi: 10.1097/DAD.0000000000001443. [DOI] [PubMed] [Google Scholar]