Abstract
Ameloblastoma, a benign but locally aggressive odontogenic tumor, often demonstrates metastasis despite benign histological features and this variant is termed as metastasizing ameloblastoma (METAM). It was classified under the malignant category in the 2005 WHO but has been re-classified under benign epithelial odontogenic tumors in the latest 2017 WHO classification. The present review aimed at gathering the available data on METAM to update the current cognizance about the pathology. Comprehensive search of the databases (viz., PubMed, Medline, SCOPUS, Web of Science, EMBASE and Google Scholar) was done for published articles on METAM following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A total of 42 cases were extracted. The mean age of occurrence was 42.71 ± 15.87 years. A slight male predilection was noted. Mandibular cases showed more metastasis than maxillary cases. Follicular ameloblastoma was most frequently encountered at secondary site followed by plexiform type. Lungs were the most commonly affected secondary sites. METAM is a rare odontogenic tumor and the diagnosis is usually made in retrospect. Inadequate treatment may result in multiple recurrences and metastasis in rare instances. Metastasis in ameloblastoma appears to be multi-factorial in nature and needs further investigation in untapped territory like exploration of quantum effects at cellular and molecular levels.
Keywords: Ameloblastoma, Follicular, METAM, Metastasis, Metastasizing, Plexiform, Quantum
Introduction
Although metastasis is not a usual feature of benignancy, various benign tumors such as pleomorphic adenoma, tuberous sclerosis, and giant cell tumor of bone have been reported to metastasize to distant locations [1–3]. Ameloblastoma, a benign but locally aggressive odontogenic tumor every so often demonstrates metastasis despite benign histological features and this variant is termed as metastasizing ameloblastoma (METAM).
Previous reviews on METAM were published by Dissanayake et al. and Van Dam et al. who audited the cases from 1923 through 2009 [4, 5]. The data was however, presented and analyzed based on 2005 WHO classification of head and neck tumors where METAM was categorized under the malignant category [6]. The tumor has been reclassified under the category of benign epithelial odontogenic tumors in the latest 2017 WHO classification of head and neck tumors but with ICD-O code of malignant tumors i.e. 9310/3 [7]. No justification is howbeit presented for this recategorization [8].
In the present review, we aimed to present the clinicopathological features of METAM reported in the last decade and to compare the data with the previous literature. An attempt for possible pathogenesis is also made for metastasis in ameloblastoma.
Methodology
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were undertaken to design the present clinicopathological review. Two investigators (DP and RA) independently searched the electronic databases viz. PubMed, Medline, SCOPUS, Web of Science, EMBASE and Google Scholar for the following keywords singly or in combination: ‘metastasizing ameloblastoma’, ‘metastatic ameloblastoma’, ‘malignant ameloblastoma’, and ‘METAM’. Subsequent citations were identified through the reference lists of included papers; bibliographical linkages were also included. Any discordance was resolved by discussion to reach a consensus on the inclusion of the study.
Inclusion and Exclusion Criteria
All the papers of METAM published from 2010 through December 2019 were included in the present comprehensive review. Reviews, mini-reviews and systematic reviews were excluded from the study. Papers published in ‘non English’ language and articles where full text could not be obtained were not included. The extracted data were analyzed and tabulated for the following parameters: age, gender, site and laterality of lesion, histopathology of primary lesion, secondary metastatic site, histopathology of secondary lesion, number of recurrences, time interval between primary and secondary lesion, treatment and overall survival.
The data were presented on Microsoft excel spreadsheet 2013. Descriptive statistics was done for frequency counts. Death due to any cause of the patient was considered as an event of interest. IBM SPSS statistics software version 25 (IBM Analytics, Armonk, New York, U.S.) was used to analyze the data.
Results
Clinicopathological Profile
Forty-two satisfactory cases of metastasizing ameloblastoma were included in the present study [4, 5, 9–43]. The mean age of occurrence was 42.71 ± 15.87 years (range 8–74). Males outnumbered females slightly (males-23; females-19) with a ratio of 1.21:1. Evidently, the mandibular cases (n = 33) showed more metastasis than maxillary cases (n = 8). For one case the exact site was not mentioned. No data was available regarding laterality of primary tumor in 7 cases. Of the remaining, 20 cases affected the left side, 14 cases were right sided and a single case was seen involving the anterior segment.
According to WHO 2017, ameloblastoma is histologically categorized into follicular, plexiform, acanthomatous, granular, basaloid and desmoplastic types [7]. Unicystic ameloblastoma is regarded as a separate entity. All variants have tendency to invade the adjacent bone (Fig. 1). In the present review, the information about histological subtype of both primary and secondary sites was found to be non-uniform. At the primary site, the histological subtype was not mentioned in 47.6% of the cases; follicular ameloblastoma was the most common subtype (8 cases, 19%) followed by plexiform (5 cases, 11.9%). Two cases of acanthomatous ameloblastoma were seen, and one case each of basaloid, granular and unicystic type. The histological pattern was allegedly mixed in the remaining four patients. Discordance was noticed in the histological type between primary and metastatic site. Follicular ameloblastoma was most frequently encountered at secondary site followed by plexiform type.
Fig. 1.
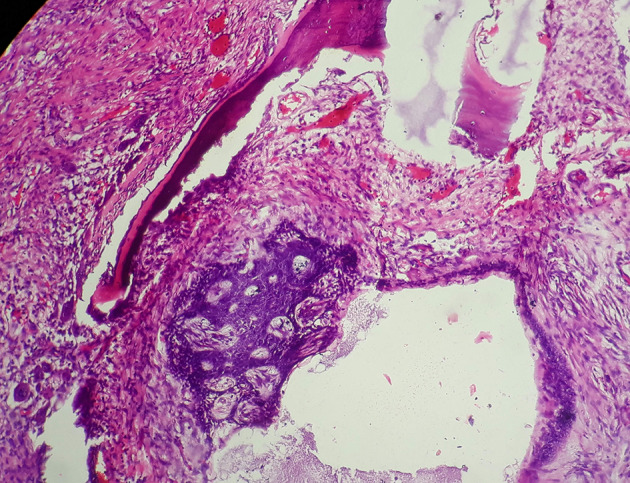
Photomicrograph of H&E stained section showing follicular ameloblastoma showing local invasion to adjacent bone (×100)
Lungs were the most commonly affected secondary sites and in 16 cases the metastatic process was bilateral. Lymph nodes were the next commonly involved sites of metastasis. Table 1 shows the detailed description of site distribution of METAM.
Table 1.
Site-wise distribution of metastasizing ameloblastoma, the total number of cases and the total number of sites affected are marked in bold
| Sites | No. of cases (42) |
Number of sites affected (57) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung (29) |
LN (15) |
Bone (3) |
Brain/intracranial (3) | Liver (2) | Scalp (1) | Chest wall (1) | Peri-cardium (1) | Abdomen (1) | Para renal spaces (1) | ||
| Single site (34) | |||||||||||
| Lung | 22 | 22 | |||||||||
| LN | 10 | 10 | |||||||||
| Distant sites | 2 | 1 | 1 | ||||||||
| Two sites (4) | |||||||||||
| Lung + LN | 2 | 2 | 2 | ||||||||
| Lung + 1 distant site | 1 | 1 | 1 | ||||||||
| LN + 1 distant site | 1 | 1 | 1 | ||||||||
| > 2 sites (4) | |||||||||||
| Lung + distant sites | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | |||
| Lung + distant site + LN | 2 | 2 | 2 | 1 | 1 | ||||||
LN lymph node
Treatment and Outcome
The number of recurrences ranged between 0 and 7; for one of the case, recurrence status was described as ‘multiple’. Mean interval between the primary and the secondary tumor was 11.453 ± 9.59 years (median 10 years; range 0–35). The treatment details of METAM cases included in the present review and their corresponding overall survival is shown in Table 2. The follow-up of only 20 cases was mentioned, the overall mean survival of these twenty patients was 4.934 ± 6.33 years (median-2.5 years; range 0.17–27 years).
Table 2.
Detailed treatment for metastasizing ameloblastoma with corresponding follow-up and survival
| Treatment of secondary site | Total no. of cases | No. of cases with follow up | Mean overall survival (in years) |
|---|---|---|---|
| Surgery only | 21 [4, 5, 9, 10, 12, 13, 16, 18, 20–24, 26, 27, 29, 30, 33, 38, 40] | 9 [5, 10, 12, 13, 16, 20, 21, 24, 33] | 4.74 |
| Chemotherapy only | 6 [5, 17, 32–34, 39] | 4 [5, 17, 34] | 4.125 |
| Surgery + chemotherapy | 1 [41] | 1 [41] | 1.5 |
| Surgery + radiotherapy | 2 [37, 43] | – | – |
| Radiotherapy + chemotherapy | 1 [42] | – | – |
| Alternative | 1 [35] | 1 [35] | 27 (no treatment for metastasis) |
| No treatment | 4 [11, 14, 31, 36] | 2 [11, 14] | 0.67 |
| No data available | 7 [15, 19, 25, 28] | 3 [28] | 3.31 |
Discussion
METAM is considered to be a rare, benign but metastasizing odontogenic tumor. The primary and secondary lesions must show histological features of benign ameloblastoma, for making a diagnosis of METAM [7]. Notably, the diagnosis is only made in retrospect and there are no specific histological features to predict the metastasis. Using Surveillance, Epidemiology and End-Results (SEER) database, an overall incidence of 1.79 per million/person was reported for malignant ameloblastoma by Rizzitelli et al. however this incidence was combined for metastasizing ameloblastoma and ameloblastic carcinoma under the umbrella term ‘malignant ameloblastoma’ [44]. This term was used synonymously with metastasizing ameloblastoma in the previous 2005 WHO classification of odontogenic tumors and METAM was categorized as a malignant odontogenic tumor. METAM always show benign histological features even at the metastatic site in contrast to ameloblastic carcinoma which displays malignant features such as increased nucleo-cytoplasmic ratio, pleomorphism, nuclear hyperchromatism, increased mitotic activity and perineural/vascular invasion (Fig. 2) [7].
Fig. 2.
Photomicrograph of H&E stained section from a case of ameloblastic carcinoma showing follicles of odontogenic epithelium with marked pleomorphism and hyperchromatism. Occasion mitotic figures are seen (a ×40, b ×100)
Independent systematic reviews on (METAM) were performed and presented by Dissanayake et al. [4] and Van Dam et al. [5]. Their reviews included data from 1923 to 2009 and reported a mean age of 33.85 years and 30 years respectively. The present review included the published cases of the past decade (2010–2019), the mean age of occurrence was found to be 42.72 ± 15.68 years (range 8–74 years), which is relatively higher than previous reviews in the literature, depicting a change in the trend over the past decade. The range has however been seen to be more or less constant [15, 16, 45]. The average age of initial diagnosis of solid multicystic ameloblastoma in industrial countries was reported to be 39.1 years which is 10–15 years higher than developing nations [46]. Dodge OG proposed that this could be attributed to the accelerated aging process in developing nations owing to poor nutrition and healthcare [47]. The same could be applicable to metastasizing ameloblastoma also, as the treatment modalities and overall lifestyle are improving positively. Another valid reason could be late presentation of patients due to lack of awareness and infrequent routine dental checkup in underdeveloped nations.
Concordant with literature, a slight male predominance was noted in the present review [4]. Other studies documented no gender predilection or definitive male preponderance [16, 23]. Mandibular ameloblastoma definitely show more tendency to metastasize as depicted by the present review and previous reports [4, 5]. The simple explanation for this trend could be the actual relative incidence of solid multicystic ameloblastoma in gnathic bones, mandible being far more commonly affected than maxilla. The latent period between the initial diagnosis of such tumors has been reported in various papers as: 2 months–42 years [4], 3–45 years [5] and 9–14 years [41]. We found a mean of 11.453 ± 9.59 years (range 0–35 years), as in four cases primary lesion and metastatic lesions were diagnosed simultaneously [12, 13, 15, 27].
Ameloblastoma was seen most commonly metastasizing to lungs, in 16 reports the process was seen bilaterally, followed by lymph nodes. Metastasis to bone [5, 19, 25], brain [30, 37, 42], scalp [43], liver [19, 42], chest wall [5], pericardium [5], abdomen [5], and para-renal region [5] was infrequent. Histologically, it has been reported that plexiform ameloblastoma exhibits most metastases [4]. Plexiform ameloblastoma histologically shows growth pattern recapitulating normal odontogenesis prior to histo- or morpho- differentiation with numerous bud like structures (Fig. 3). These structures may get easily dislodged and implanted at another site during surgical procedures. However, in the present review follicular ameloblastoma (n = 8) was the most common metastasizing variant. Remarkably, same subtype was encountered more frequently seeded at secondary site (n = 20). There was a variation in the number of cases, regarding the type, in primary and secondary site which is attributable to the fact that the diagnosis is usually made retrospectively and in as many as eighteen cases the histo-type of primary tumor was unspecified. Rare cases of unicystic and granular cell ameloblastoma have also been reported which maintained their histology at secondary site [12, 20, 30]. Few cases revealed a mixed histological pattern, authors believe that such a diagnosis should however be avoided, as reporting should be done based on the major histological pattern noted.
Fig. 3.
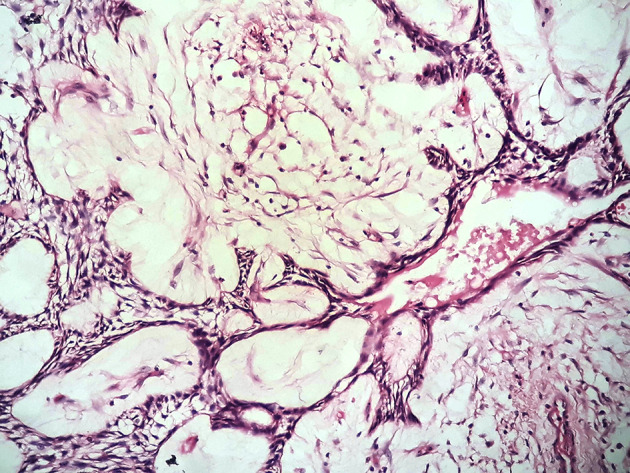
Photomicrograph of H&E stained section showing plexiform ameloblastoma with interconnected strand like pattern and bud like structures recapitulating dental lamina stage of normal odontogenesis (×40)
The most common proposed theories for metastasis are lung aspiration theory, surgical implantation theory and the phenomenon of heterotopias. Lai et al. suggested that despite being metastatic, it is wise to regard this entity as benign because METAM shows benign histological appearance, low grade biological behavior and limited damage at metastatic site in contrast to malignant tumors [11]. The lymphatogenous spread of METAM tumors cells is well accepted amongst investigators but hematogenous route is more favored [5]. A query arises that if we consider surgical implantation or exploration/multiple recurrences as driving factors for seeding of tumor cells, could there be any other factors responsible for metastasis? The answer may lie in provenance. That is, the inherent property of odontogenic cells to migrate as a derivative of neural crest cells (NCCs) or under their influence occurring during odontogenesis as discussed in the sections below.
Ameloblastoma arises from the remnants of dental lamina or cell rest of Malassez. NCCs migrate from the future forebrain region to the site of primitive stomodeum to develop into parts of the future dental apparatus. These cells arise from the neuroectoderm and are capable to migrate and show pleuripotency. During differentiation, NCCs undergo epithelial–mesenchymal transformation, which facilitates their migration. It has been found that snail and slug zinc-finger transcription factor family is responsible for repression of E-cadherin, a cell adhesion molecule [48]. Fibroblast growth factor (FGF), bone morphogenetic proteins (BMP) and Wnt play a significant role in this cascade [49]. NCCs developing into the ectomesenchyme inversely interact with the oral ectoderm known as epithelial mesenchymal interaction. During odontogenesis, odontoblasts differentiating from dental papilla (NCC origin), align themselves in a perpendicular orientation to inner enamel epithelium and undergo a mitotic division to form an odontoblast and a sub-odontoblastic layer. At the same time the cells replicate by mitosis in enamel organ. Thus, all cells of dental papilla and enamel organ cells inversely expose each other to signaling molecules and growth factors which in turn are the determinants of differentiation.
An important protein to be acknowledged here is Wnt5a, whose increased expression has been demonstrated in various studies suggesting a role in development of ameloblastoma [50, 51]. Qiao et al. found up-regulation of Wnt5a in ameloblastomas and suggested that it leads to an enhanced mitochondrial energy production and altered calcium homeostasis [50]. Raised calcium levels directly trigger alteration in mitochondrial dynamics and interactions between the mitochondria and the cytoskeleton. Further, the migratory capacity of ameloblastoma cell lines was found to be impaired when Wnt5a or its downstream cytoskeleton-associated protein (Coro1A) were knocked down [50].
Recently, the phenomenon of ‘quantum coherence and quantum entanglement’ among microtubule-based centrioles and mitotic spindles for organization of normal mirror-like mitosis was proposed in normal living cells. This spectacle ensures precise, complementary duplication of daughter cell genomes and recognition of daughter cell boundaries [52]. We suggest that this ‘twinning’ results in daughter cells bearing all the properties of the parent cell. It has also been suggested that any impairment during this state of quantum entanglement can result in abnormal differentiation, abnormal distribution of chromosomes, uncontrolled growth, eventually accounting for all aspects of malignancy. We hereby opine that whether it concerns normal cells, malignant cells or benign cells at metastatic sites, behaviour corresponds to the biology of the parent cell owing to quantum entanglement/coherence.
Thus, the remnants of dental lamina once exposed to all signaling growth factors/molecules have a tendency to show the feature of cell migration so as the odontogenic tumors derived from it. It may however be varied in various tumors. For e.g. some tumors are more aggressive and despite being benign may show metastasis. Pindborg tumor, a benign odontogenic neoplasm, shows marked nuclear pleomorphism and may also show vascular emboli [53, 54]. Ameloblastomas do metastasize. Thus, it is prudent to believe that cell migration may be an inherent property of tumor cells.
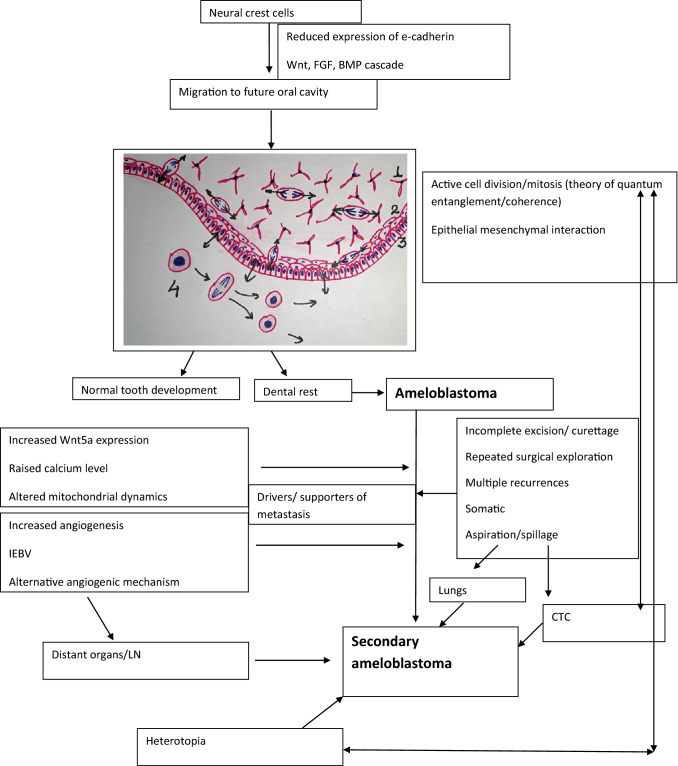
Further, it has been shown that there is release of angiogenic growth factors by the tumor cells in ameloblastoma which promote proliferation of endothelial cells and induce angiogenesis. The angiogenesis is seen in the stroma, and as recently demonstrated intra-tumorally. The concept is better known as intraepithelially entrapped blood vessels (IEBV) [55]. It was demonstrated that these vessels were highly altered in morphology as compared to normal blood vessels. IEBVs show tight junctions and pericytes around but the basement membrane is often incomplete or absent. This abnormality may provide easy access to close abnormal blood vessels of benign tumor cells which may have a very limited ability to migrate in contrast malignant tumor cells. We speculate that apart from IEBVs alternate non angiogenic mechanism may further aid in the process such as vasculogenic mimicry or mosaic vessels. The field deserves further exploration. Additional factors which may play an important role are heterotopic/ectopic cell proliferation, perineural invasion and circulating tumor cells [16, 30]. A proposed mechanism in the pathogenesis of metastasis in ameloblastoma is demonstrated in the flowchart (Table 3).
Table 3.
Flowchart depicting the plausible multi-factorial pathogenesis of METAM; (1) stellate reticulum, (2) stratum intermedium, (3) inner enamel epithelium, (4) odontoblastic layer. CTC Circulating tumor cells, LN Lymph node
The number of recurrence in the review presented by Van Dam et al. ranged from 0 to 11 [5]. We found a maximum of seven times recurrences. In one case the exact number was not mentioned [25]. The overall survival in the present review was found to be 4.934 ± 6.33 years. Previous studies have reported a 5 years survival of 44% [4]. Five year survival of 70% has been stated elsewhere in the literature [7]. Surgery alone appears to be the optimal treatment of both primary and secondary sites. A long term follow-up is mandatory to get a better perspective.
Conclusion
METAM is a rare odontogenic tumor which has been reclassified under benign category in the latest 2017 WHO classification. The diagnosis is made in retrospect and exhibits benign histological features. Ameloblastoma is a locally aggressive tumor with a potential to invade the adjacent vital structures ultimately leading to demise of the patient. Inadequate treatment results in multiple recurrences and metastasis in rare instances. The usage of the term ‘inherent low grade malignancy’ by Kunze cannot be completely ruled out and requires a revisit [56]. Metastasis in ameloblastoma appears to be multi-factorial in nature and needs further investigation in untapped territory like exploration of quantum effects at cellular and molecular levels.
Funding
There was no funding involved with this project.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knight J, Ratnasingham K. Metastasising pleomorphic adenoma: systematic review. Int J Surg. 2015;19:137–145. doi: 10.1016/j.ijsu.2015.04.084. [DOI] [PubMed] [Google Scholar]
- 2.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003;38(4):376–381. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 3.Tubbs WS, Brown LR, Beabout JW, Rock MG, Unni KK. Benign giant-cell tumor of bone with pulmonary metastases: clinical findings and radiologic appearance of metastases in 13 cases. AJR Am J Roentgenol. 1992;158(2):331–334. doi: 10.2214/ajr.158.2.1729794. [DOI] [PubMed] [Google Scholar]
- 4.Dissanayake RK, Jayasooriya PR, Siriwardena DJ, Tilakaratne WM. Review of metastasizing (malignant) ameloblastoma (METAM): pattern of metastasis and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(6):734–741. doi: 10.1016/j.tripleo.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Van Dam SD, Unni KK, Keller EE. Metastasizing (malignant) ameloblastoma: review of a unique histopathologic entity and report of Mayo Clinic experience. J Oral Maxillofac Surg. 2010;68(12):2962–2974. doi: 10.1016/j.joms.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 6.Barnes L, Everson J, Reichart P, Sidransky D. World Health Organization classification of tumors. Pathology and genetics of head and neck tumors. Lyon: IARC press; 2005. p. 285. [Google Scholar]
- 7.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. 4th ed., Chapt. 8. Lyon: IARC; 2017. p. 204–60.
- 8.Sivapathasundharam B, Biswas PG, Preethi S. The World Health Organization classification of odontogenic and maxillofacial bone tumors: an appraisal. J Oral Maxillofac Pathol. 2019;23(2):178–186. doi: 10.4103/jomfp.JOMFP_211_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rameshkumar A, Ramyamalini V, Sriram V, Kannadasan KK. Metastasizing ameloblastoma—a rare entity. SRM Univ J Dent Sci. 2010;1(1):112–115. [Google Scholar]
- 10.Ricard AS, Majoufre-Lefebvre C, Siberchicot F, Laurentjoye M. A multirecurrent ameloblastoma metastatic to the lung. Rev Stomatol Chir Maxillofac. 2010;111(2):98–100. doi: 10.1016/j.stomax.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Lai H, Wang J. Benign metastasizing ameloblastoma or malignant ameloblastoma? J Craniofac Surg. 2011;22(3):995–997. doi: 10.1097/SCS.0b013e31821015de. [DOI] [PubMed] [Google Scholar]
- 12.Bansal A, Bhatnagar A, Saxena S. Metastasizing granular cell ameloblastoma. J Oral Maxillofac Pathol. 2012;16(1):122–124. doi: 10.4103/0973-029X.92988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger AJ, Son J, Desai NK. Malignant ameloblastoma: concurrent presentation of primary and distant disease and review of the literature. J Oral Maxillofac Surg. 2012;70(10):2316–2326. doi: 10.1016/j.joms.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Georgakas I, Lazaridou M, Dimitrakopoulos I, Tilaveridis I, Sekouli A, Papakosta D, Kontakiotis T. Pulmonary metastasis in a 65-year-old man with mandibular ameloblastoma: a case report and review of the literature. J Oral Maxillofac Surg. 2012;70(5):1109–1113. doi: 10.1016/j.joms.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Golubović M, Petrović M, Jelovac DB, Nenezić DU, Antunović M. Malignant ameloblastoma metastasis to the neck—radiological and pathohistological dilemma. Vojnosanit Pregl. 2012;69(5):444–448. doi: 10.2298/VSP1205444G. [DOI] [PubMed] [Google Scholar]
- 16.Luo DY, Feng CJ, Guo JB. Pulmonary metastases from an Ameloblastoma: case report and review of the literature. J Craniomaxillofac Surg. 2012;40(8):e470–e474. doi: 10.1016/j.jcms.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Ghiam A, Al Zahrani A, Feld R. A case of recurrent metastatic ameloblastoma and hypercalcaemia successfully treated with carboplatin and paclitaxel: long survival and prolonged stable disease. Ecancermedicalscience. 2013;4(7):323. doi: 10.3332/ecancer.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klee C, Lindskog S, Hirsch JM, Thor A. Recurrent ameloblastoma of the mandible: surgical seeding or metastasis of malignant ameloblastoma? Case Rep Clin Med. 2013;2:154. doi: 10.4236/crcm.2013.22042. [DOI] [Google Scholar]
- 19.Niu N, Chen L, Liu Y, Li F. Multiple organ metastases from ameloblastoma detected by FDG PET/CT imaging. Clin Nucl Med. 2013;38(12):1009–1011. doi: 10.1097/RLU.0b013e3182a7597f. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi K, Kishimoto H, Yamanegi K, Moridera K, Takaoka K, Urade M. Unicystic ameloblastoma metastasizing to multiple cervical lymph nodes. J Surg Case Rep. 2013;2013(5):rjt033. doi: 10.1093/jscr/rjt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibric Cioranu V, Iorgulescu D, Petrescu Seceleanu V, Ibric Cioranu S, Toma C, Fronie AI, Făgeţan IM, Nicolae V. Malignant ameloblastoma in an 8-year-old child with metastasis to the lung: case report with a clinicopathologic analysis. Rom J Morphol Embryol. 2014;55(1):183–187. [PubMed] [Google Scholar]
- 22.Chou YH, Jhuang JY, Chang MH, Huang WC, Hsieh MS. Metastasizing ameloblastoma with localized interstitial spread in the lung: report of two cases. Int J Surg Pathol. 2014;22(4):343–346. doi: 10.1177/1066896913491321. [DOI] [PubMed] [Google Scholar]
- 23.Jayaraj G, Sherlin HJ, Ramani P, Premkumar P, Natesan A, Ramasubramanian A, Jagannathan N. Metastasizing ameloblastoma—a perennial pathological enigma? Report of a case and review of literature. J Craniomaxillofac Surg. 2014;42(6):772–779. doi: 10.1016/j.jcms.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Choi SW, Lee JH, Ahn KM. A single cervical lymph node metastasis of malignant ameloblastoma. J Craniomaxillofac Surg. 2014;42(8):2035–2040. doi: 10.1016/j.jcms.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, He JF, Li ZY, Liu JH. Ameloblastoma with varied sites of metastasis: report of two cases and literature review. J Craniomaxillofac Surg. 2014;42(5):e301–e304. doi: 10.1016/j.jcms.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Yun JS, Kim DW, Kim SS, Choi YD, Song SY, Na KJ. Metastatic pulmonary ameloblastoma misdiagnosed as primary squamous cell carcinoma preoperatively. Korean J Thorac Cardiovasc Surg. 2014;47(1):63–65. doi: 10.5090/kjtcs.2014.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atun JM, Carante J. Metastasizing ameloblastoma. Philipp J Otolaryngol Head Neck Surg. 2015;30(2):67–68. doi: 10.32412/pjohns.v30i2.365. [DOI] [Google Scholar]
- 28.Bi R, Shen L, Zhu X, Xu X. Malignant ameloblastoma (metastatic ameloblastoma) in the lung: 3 cases of misdiagnosis as primary lung tumor with a unique growth pattern. Diagn Pathol. 2015;10:123. doi: 10.1186/s13000-015-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godbole CR, Pathak S. Metastasizing ameloblastoma: a case report. Ann Appl Bio-Sci. 2015;2(3):C32–C34. [Google Scholar]
- 30.Li CH, Huang PH, Chen YF, Chen HC, Huang SY, Huang SJ, et al. Metastasizing granular cell type ameloblastoma with intracranial perineural spread along the abducens nerve. J Cancer Res Pract. 2015;2(3):261–269. [Google Scholar]
- 31.Saheer S, Enose P, Thangakunam B, Irodi A, Korula A. Cavitating lung metastasis secondary to ameloblastoma. Lung India. 2015;32(5):527–528. doi: 10.4103/0970-2113.164181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang RN, Wang XS, Ren J, Xie YF, Zhou D, Ge DF, Feng XS, Gao SG. Mandible ameloblastoma with lung metastasis: a rare case report. Int J Clin Exp Pathol. 2015;8(6):6793–6799. [PMC free article] [PubMed] [Google Scholar]
- 33.Deb S, Iseli TA, Wong T, Phal PM. Imaging characteristics of nodal metastases in paraganglioma, ameloblastoma and olfactory neuroblastoma: case reports and literature review. BJR Case Rep. 2016;2(3):20150096. doi: 10.1259/bjrcr.20150096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A, Kumar R, Kumar K, Bakhshi S. Multiple nodular lung metastases with no obvious primary showing low positive 18-fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET) scan: a unique case of metastatic ameloblastoma. Cancer Treat Commun. 2016;5:11–13. doi: 10.1016/j.ctrc.2015.11.006. [DOI] [Google Scholar]
- 35.Ikai A, Suzuki S, Hayashi K. A case of ameloblastoma with extensive pulmonary metastasis survived for 14 years without treatment of the lung. J Oral Maxillofac Surg Med Pathol. 2017;28(2):138–142. doi: 10.1016/j.ajoms.2015.09.004. [DOI] [Google Scholar]
- 36.Rabo JIS, Carpela AB, Guevara ES, Romualdez JA. Mandibular ameloblastoma with lung metastasis 10 years after resection. Philipp J Otolaryngol Neck Surg. 2016;31:53–56. doi: 10.32412/pjohns.v31i1.321. [DOI] [Google Scholar]
- 37.Rotellini M, Maggiore G, Trovati M, Saraceno MS, Franchi A. Metastasizing maxillary ameloblastoma: report of a case with molecular characterization. J Oral Maxillofac Res. 2016;7(1):e5. doi: 10.5037/jomr.2016.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanagarajah P, Ciment LM, Ciment AJ, Clum SR, Rumbak MJ. Metastatic endobronchial ameloblastoma. J Bronchol Interv Pulmonol. 2017;24(4):307–309. doi: 10.1097/LBR.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 39.Salami A, Ezenkwa U, Salami M, Ajani M, Okolo C. Malignant ameloblastoma: a challenging diagnosis. Autops Case Rep. 2018;8(4):e2018043. doi: 10.4322/acr.2018.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valkadinov I, Conev N, Dzhenkov D, Donev I. Rare case of ameloblastoma with pulmonary metastases. Intractable Rare Dis Res. 2017;6(3):211–214. doi: 10.5582/irdr.2017.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacin S, Dogrul A, Dikmen E, Kertmen N, Turker A, et al. Metastatic ameloblastoma to the liver: rare presentation of a rare disease. J Clin Case Rep. 2019;9:1207. [Google Scholar]
- 42.Li D, Xu S, Sun M, Qiao L, Wang L, Liu Y. MAID chemotherapy regimen as a treatment strategy for metastatic malignant ameloblastoma: a case report. Medicine (Baltimore) 2019;98(25):e15873. doi: 10.1097/MD.0000000000015873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geanny M-R. Malignant ameloblastoma: multiple local recurrence and metastasis in the scalp. Case Rep. 2018;5(1):36–45. [Google Scholar]
- 44.Rizzitelli A, Smoll NR, Chae MP, Rozen WM, Hunter-Smith DJ. Incidence and overall survival of malignant ameloblastoma. PLoS One. 2015;10(2):e0117789. doi: 10.1371/journal.pone.0117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichart PA, Philipsen HP. Metastasizing, malignant ameloblastoma. In: Reichart PA, Philipsen HP, editors. Odontogenic tumours and allied lesions. London: Quintessence Publishing Co. Ltd;; 2004. [Google Scholar]
- 46.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B(2):86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 47.Dodge OG. Tumors of the jaw, odontogenic tissues and maxillary antrum (excluding burkitt lymphoma) in Uganda Africans. Cancer. 1965;18(2):205–215. doi: 10.1002/1097-0142(196502)18:2<205::AID-CNCR2820180212>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 48.Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, Helms JA. Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A. 2011;155A(2):270–279. doi: 10.1002/ajmg.a.33702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137(16):2605–2621. doi: 10.1242/dev.040048. [DOI] [PubMed] [Google Scholar]
- 50.Qiao X, Niu X, Shi J, Chen L, Wang X, Liu J, Zhu L, Zhong M. Wnt5a regulates ameloblastoma cell migration by modulating mitochondrial and cytoskeletal dynamics. J Cancer. 2020;11(18):5490–5502. doi: 10.7150/jca.46547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukarawan W, Simmons D, Suggs C, Long K, Wright JT. WNT5A expression in ameloblastoma and its roles in regulating enamel epithelium tumorigenic behaviors. Am J Pathol. 2010;176(1):461–471. doi: 10.2353/ajpath.2010.090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hameroff SR. A new theory of the origin of cancer: quantum coherent entanglement, centrioles, mitosis, and differentiation. Biosystems. 2004;77(1–3):119–136. doi: 10.1016/j.biosystems.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Pandiar D, Anand R, Kamboj M. Nuclear atypia in Pindborg tumor: an unexplored phenomenon of a well recognized entity. Oral Oncol. 2020;100:104481. doi: 10.1016/j.oraloncology.2019.104481. [DOI] [PubMed] [Google Scholar]
- 54.Cheng YS, Wright JM, Walstad WR, Finn MD. Calcifying epithelial odontogenic tumor showing microscopic features of potential malignant behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(3):287–295. doi: 10.1067/moe.2002.121991. [DOI] [PubMed] [Google Scholar]
- 55.Siar CH, Ishak I, Ng KH. Intra-epithelially entrapped blood vessels in ameloblastoma. J Oral Pathol Med. 2015;44(5):378–385. doi: 10.1111/jop.12247. [DOI] [PubMed] [Google Scholar]
- 56.Kunze E, Donath K, Luhr HG, Engelhardt W, De Vivie R. Biology of metastasizing ameloblastoma. Pathol Res Pract. 1985;180(5):526–535. doi: 10.1016/S0344-0338(85)80017-0. [DOI] [PubMed] [Google Scholar]