Abstract
Primary sarcomas of the larynx are rare and are associated with diagnostic and treatment challenges. Studies of these tumors are limited, and most examples have been reported as small series. To further increase our understanding of laryngeal sarcomas, we reviewed our experience of an adult cohort. A retrospective search for laryngeal sarcomas from our pathology archives and consultation files of one of the authors was performed. We studied 27 primary laryngeal sarcomas that included 25 males, and 2 females, with a mean age of 60 years (range 33–85). The cases included conventional chondrosarcoma (16), well-differentiated liposarcoma (2), clear cell chondrosarcoma (1), leiomyosarcoma (2), high grade myxofibrosarcoma (2), high grade myofibroblastic sarcoma (1), low-grade myofibroblastic sarcoma (1), malignant granular cell tumor (1), and Kaposi sarcoma (1). Data on treatment and follow-up was available in 17 and 16 cases, respectively. 12 patients underwent partial laryngeal resection; five had total laryngectomy, and the patient with Kaposi sarcoma received combined highly active antiretroviral therapy and chemotherapy. Three patients developed local recurrence, and two patients developed metastases. The remaining patients with follow up had a favorable outcome and were disease-free after treatment. The important differential diagnosis of spindle cell sarcoma is sarcomatoid squamous cell carcinoma, and their distinction often requires extensive sampling of the mucosal surface and immunohistochemical analysis. The mainstay of treatment for laryngeal sarcomas is surgical removal, with the extent dictated by tumor type and grade. Adjuvant therapy is reserved for high-grade sarcomas and may be given in a neoadjuvant or adjuvant setting.
Keywords: Larynx, Chondrosarcoma, Liposarcoma, Myxofibrosarcoma, Myofibroblastic sarcoma, Kaposi sarcoma, Malignant granular cell tumor
Introduction
The larynx is an anatomically complex structure composed of a variety of different cell types. Neoplasms arising in the larynx usually recapitulate the tissues that comprise its structure, and most are benign, epithelial, and squamous in differentiation [1]. In contrast, mesenchymal tumors of the larynx are uncommon, and the majority are malignant [2–5]. Sarcomas arising in the larynx are rare, representing less than 1% of malignant laryngeal neoplasms, and the majority are chondrosarcomas [6–9]. To further increase our understanding of laryngeal sarcomas, we reviewed our experience and discuss the differential diagnosis and biological potential of these unusual neoplasms.
Material and Methods
With prior approval from our Institutional Review Board, a retrospective search for laryngeal sarcomas from the pathology archives of the University of Miami and Jackson Memorial Hospital from the 2007 to 2020 period and consultation files of one of the authors (A.E.R) was performed. Nine cases were obtained from the consultation service of A.E.R. The remaining cases were retrieved from our pathology archives. The clinical data were obtained from available medical records, and the histological slides and ancillary studies were retrieved and reviewed. A total of 827 adult patients diagnosed with non-sarcomatous malignant neoplasms of the larynx during this time period were identified by using terms such as carcinoma, lymphoma, and malignant in combination with larynx or laryngectomy. Tumors with surface dysplasia or diffuse and strong expression of epithelial markers (keratins, EMA) and squamous markers (p40 or p63) were excluded from our cohort. 27 primary laryngeal sarcomas were identified and form the study cohort.
Results
We identified a total of 854 adult patients with malignant neoplasms arising in the larynx, 27 of which were primary laryngeal sarcomas, and they form the study cohort. These cases represented 3.1% of total laryngeal malignancies in our pathology archives, including consultation cases. 25 patients were male, and two patients were female, with a mean age of 60 (range of 33–85 years). The tumors included 16 (59%) conventional chondrosarcomas, one (3.7%) clear cell chondrosarcoma, 2 (7.4%) well-differentiated liposarcomas, 2 (7.4%) leiomyosarcomas, 2 (7.4%) myxofibrosarcomas, 1 (3.7%) high-grade myofibroblastic sarcoma, 1 (3.7%) low-grade myofibroblastic sarcoma, 1 (3.7%) malignant granular cell tumor, and 1 (3.7%) Kaposi sarcoma. All patients were symptomatic at the time of diagnosis and presented with hoarseness (9), dysphonia/voice changes (8), dysphagia/difficulty swallowing (4), shortness of breath (5), cough (1), stridor (1), or hemoptysis (1). The clinicopathologic features are summarized in Table 1.
Table 1.
Clinicopathologic features of laryngeal sarcomas
| Case | Diagnosis | Age (years)/Gender | Symptoms | Size (cm) | Grade | Site | Recurrence (months) | Treatment/Margin status | Status & Total follow up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Myxofibrosarcoma | 62/M | Stridor and shortness of breath | 4.0 | 3 | Vocal cords, bilateral | No | Total laryngectomy and bilateral neck dissection/Negative margins | LOST; 1 month |
| 2 | Leiomyosarcoma | 65/M | Difficulty swallowing | 5.3 | 2 | Cricoid cartilage | Yes; (13 months after resection) | Total laryngectomy, partial pharyngectomy, and partial thyroidectomy/Negative margins | DOD; 48 months |
| 3 | Conventional chondrosarcoma | 33/M | Voice changes | 2.5 | 1 | Cricoid cartilage | No | Segmental wide resection of the cricoid/Negative margins | NED; 40 months |
| 4 | Myofibroblastic sarcoma | 79/M | Cough and voice changes, hoarseness, fatigue, and fainting episodes | 1.2 | 3 | Left vocal cord | No | Left partial laryngectomy/Negative margins | LOST; 24 months with NED |
| 5 | Conventional chondrosarcoma, hyaline and myxoid type | 85/M | Voice changes | 5.5 | 2 | Left thyroid and cricoid cartilage | No | Total laryngectomy/radical resection of soft tissue tumor/left hemithyroidectomy/Negative margins | NED; 24 months |
| 6 | Well-differentiated liposarcoma, lipoma-like | 54/M | Progressive shortness of breath | 5.1 | 1 | Left epiglottis | No | Left partial laryngectomy with limited pharyngectomy/Negative margins | LOST; 12 months with NED |
| 7 | Conventional chondrosarcoma, arising from chondroma | 52/M | Hoarseness | 2.5 | 1 | Left cricoid cartilage | No | Left hemilaryngectomy/Negative margins | LOST; 1 month |
| 8 | Conventional chondrosarcoma | 72/M | Left vocal cord paralysis | 4.1 | 1 | Left posterior thyroid cartilage | NA | NA | NA |
| 9 | Conventional chondrosarcoma, hyaline type | 50/M | Dysphonia | NA | 1 | Cricoid cartilage | NA | NA | NA |
| 10 | Conventional chondrosarcoma, hyaline type | 48/M | Hoarseness | 2.0 | 2 | Subglottis | NA | NA | NA |
| 11 | Conventional chondrosarcoma, hyaline type, arising from a chondroma | 69/M | NA | NA | 1 | Larynx | NA | NA | NA |
| 12 | Conventional chondrosarcoma, hyaline type | 50/M | Voice changes | 2.8 | 2 | Left cricoid cartilage | No | Left hemicricoid resection/Negative margins | NED; 18 months |
| 13 | Kaposi sarcoma | 44/M | Voice changes and hemoptysis | 1.5 | – | Left false vocal cord | No | Six cycles of chemotherapy and highly active antiretroviral therapy | NED; 24 months |
| 14 | Conventional chondrosarcoma | 75/M | Dysphagia | 6.0 | 1 | Cricoid cartilage | No | Total laryngectomy/Negative margins | NED; 79 months |
| 15 | Clear cell chondrosarcoma, hyaline type | 72/M | Voice changes | 3.2 | 2 | Thyroid cartilage | Yes; 204 months | Partial laryngectomy × 2/ Positive margins | NED; 90 months |
| 16 | Well-differentiated liposarcoma, mixed lipoma, and sclerosing type | 71/M | Progressive shortness of breath | 3 | 1 | Left supraglottis | Yes;4 times (1995, 2002, 2004, and 2013) | Supraglottic laryngectomy/Positive margins | LOST; 1 month |
| 17 | Low-grade myofibroblastic sarcoma | 69/F | Dysphonia and globus sensation | 1.6 | 1 | Left false and true vocal cords | No | Supracricoid partial laryngectomy/Negative margins | NED; 12 months |
| 18 | Conventional chondrosarcoma, hyaline type | 45/M | Hoarseness | NA | 1 | Left larynx | NA | NA | NA |
| 19 | Conventional chondrosarcoma, hyaline type | 65/M | Hoarseness | NA | 1 | Cricoid cartilage, posterior aspect | NA | NA | NA |
| 20 | Conventional chondrosarcoma, hyaline type | 42/M | Hoarseness, dysphonia | 1.3 | 1 | Left arytenoid cartilage | NA | NA | NA |
| 21 | Conventional chondrosarcoma, hyaline type | 49/M | Progressive dysphagia, hoarseness | NA | 2 | Left posterior cricoid | NA | Partial excision/subtotal tumor removal/ Margin status NA | NA |
| 22 | Conventional chondrosarcoma, mixed hyaline and myxoid type, arising from chondroma | 68/M | Hoarseness | 4.0 | 1 | Right cricoid cartilage | NA | Left partial cricoidectomy/Positive peripheral margins | NED; 1 month |
| 23 | Conventional chondrosarcoma, myxoid and hyaline type, arising from chondroma | 72/M | NA | 3.5 | 2 | Cricoid cartilage | No | Total laryngectomy/Negative margins | DOC; 116 months |
| 24 | Malignant granular cell tumor | 38/F | Dysphagia and odynophagia | 6.8 | 2 | Left lateral pharynx, pyriform sinus and left aryepiglottic fold | NA | Left partial pharyngectomy and partial vertical laryngectomy/Positive margin | NED; 14 months |
| 25 | Conventional chondrosarcoma, hyaline type | 48/M | Shortness of breath | 2.9 | 1 | Left thyroid cartilage | NA | NA | NA |
| 26 | Leiomyosarcoma | 81/M | Shortness of breath | 1.5 | 2 | Left arytenoid | NA | NA | NA |
| 27 | Myxofibrosarcoma | 78/M | Shortness of breath | 4.0 | 3 | Right vocal fold | NA | NA | NA |
LOST Lost to follow up; DOD Died of disease; NED No evidence of disease; AWD Alive with disease; DOC Died of other causes; NA Not available
The 17 chondrosarcomas accounted for 62.9% of the tumors; sixteen were conventional chondrosarcoma, and one was the clear cell variant. Eight conventional chondrosarcomas were retrieved from consultation files of AER. All patients were male (n = 17); their mean age was 58 (range of 33–85 years). The majority of tumors were grade 1/3 (11 cases; 65%) followed by grade 2/3 (6 cases; 35%); four tumors arose in association with a preexisting chondroma, and none exhibited dedifferentiation. The most common site of origin was the cricoid cartilage, 9 (53%), followed by the thyroid cartilage, 3 (18%), thyroid and cricoid cartilage, 1 (6%), and, lastly, the arytenoid cartilage, 1 (6%) (Fig. 1a). Nine patients had documented surgical resection (1 wide, 1 subtotal, 4 partial, and 3 total laryngectomies); information regarding the remaining cases is not available. The tumor size known in 12 cases was 1.3–6.0 cm, average of 3.2 cm (Fig. 1b). Histologically, conventional chondrosarcomas were composed of delineated nodules of well-formed hyaline cartilage that exhibited increased cellularity and mild to moderate cytological atypia that varied according to the sarcoma grade (Fig. 1c). The clear cell chondrosarcoma was composed of large polygonal tumor cells with abundant clear to pale eosinophilic cytoplasm closely admixed with trabeculae of metaplastic woven bone focally lined by osteoblasts and also contained small foci of conventional grade 2/3 chondrosarcoma (Fig. 1d). Follow-up was available on 8 patients and ranged from 1 to 116, average 43 months. Local recurrence occurred in one patient after 17 years—the original tumor had been treated with a partial laryngectomy. None of the patients developed metastases or died of disease.
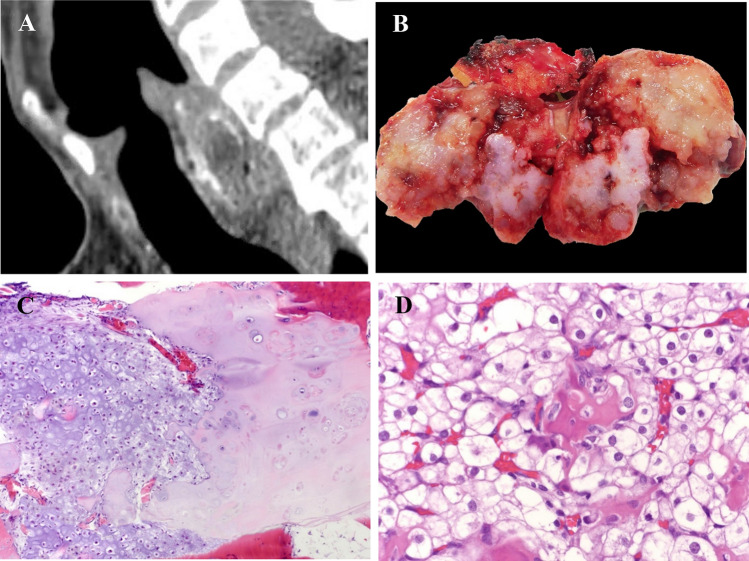
Fig. 1.
a Reformatted sagittal computed tomography image of the larynx demonstrates a focally calcified mass located posteriorly, causing stenosis of the lumen. b Excised chondrosarcoma is solid, lobular, and has an area that is blue-white and regions that are glistening pale tan-yellow. c Low-grade chondrosarcoma arising from chondroma. The chondrosarcoma shows increased cellularity and mild nuclear atypia juxtaposed to the chondroma that is less cellular and lacks atypia. (HES × 10). d Clear cell chondrosarcoma. Sheets of large polygonal tumor cells with abundant clear to pale eosinophilic cytoplasm closely admixed with trabeculae of metaplastic woven bone focally lined by osteoblasts (HES × 40)
Two cases of well-differentiated liposarcoma, lipoma like subtype, were identified and comprised 7.4% of cases—both patients were male, and their ages were 54 and 71 years. One patient had a 5.1 cm tumor excised from the left aryepiglottic fold (Fig. 2a), and at 1 year follow up, there was no local recurrence or metastatic disease. The other patient had a previous diagnosis of well-differentiated liposarcoma 25 years prior and had four subsequent recurrences. Histologically, the tumors were composed of lobules of white adipocytes that varied in size, and scattered cells had enlarged hyperchromatic nuclei. The septae dividing the tumors into lobules were thick, collagenous, and contained spindle cells, some of which had enlarged hyperchromatic nuclei (Fig. 2b). No areas of dedifferentiation were identified. Immunohistochemistry was performed in one case, and the tumor was positive for MDM2 and CDK4, and amplification of MDM2 by fluorescence in-situ hybridization was positive.
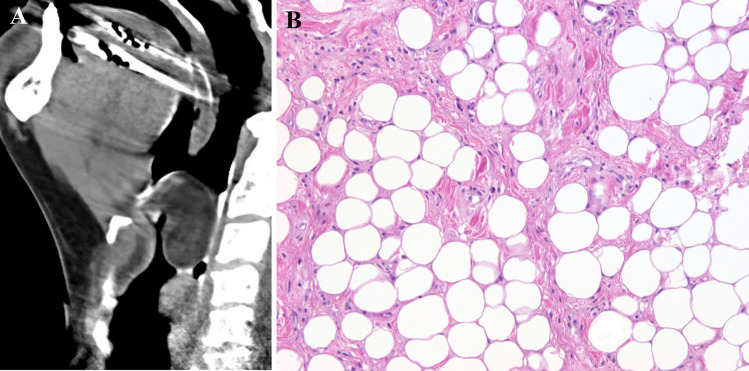
Fig. 2.
a Reformatted sagittal computed tomography. Fat density mass seen extends from the inferior oropharynx to the larynx just above the level of the false vocal cords. b Well differentiated liposarcoma is composed of lobules of white adipocytes that vary in size, and scattered cells had enlarged hyperchromatic nuclei. The septae are thick, collagenous, and also contain spindle cells, some of which had enlarged hyperchromatic nuclei (HES × 20)
The Kaposi sarcoma occurred in a 44-year-old male patient with HIV/AIDS and Kaposi sarcoma of the skin (chest, arms, toes), cervical lymph node, and oropharyngeal and laryngeal mucosa (base of tongue, tonsils, and left false vocal cord). The 1.5 cm pedunculated vocal cord lesion was excised (Fig. 3a); histologically, it was composed of hypercellular fascicles of spindle cells with intervening slit-like vascular spaces containing numerous red blood cells, scattered plasma cells, and hemosiderin deposits (Fig. 3b). Immunohistochemistry showed that the tumor cells exhibited positive nuclear staining for HHV-8 (Fig. 3c). The patient was treated with highly active antiretroviral therapy and six cycles of doxorubicin with resolution of the disease in the head and neck, chest, and abdomen. No evidence of recurrence was seen after 2 years of follow-up.
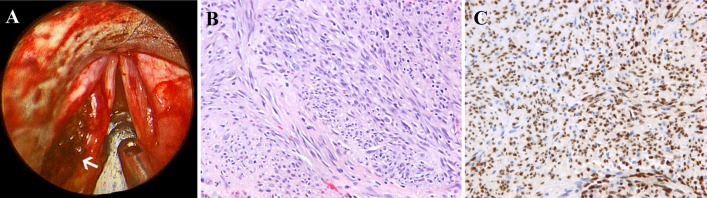
Fig. 3.
a Direct laryngoscopy shows a 1.5 cm pedunculated mass growing in the left false vocal cord. b Kaposi sarcoma is composed of hypercellular fascicles of spindle cells with intervening slit-like vascular spaces containing numerous red blood cells, scattered plasma cells, and hemosiderin deposits (HES × 40). c Immunohistochemistry shows that the tumor cells exhibit positive nuclear staining for HHV-8
Two leiomyosarcoma cases were identified and comprised 7.4% of cases- both patients were male, and their ages were 65 and 81 years. The 65-year-old patient had a past medical history of Hodgkin lymphoma treated 41 years previously with chemotherapy and radiation to the submental area and presented with a 5.3 cm laryngeal mass. The biopsy revealed leiomyosarcoma grade 2/3. Immunohistochemistry showed that the tumor cells were positive for actin and negative for keratin, DOG-1, CD34, and S100. 13 months after resection, the patient had a hypopharyngeal recurrence and was treated with several cycles of systemic chemotherapy. Regardless, the disease progressed, pulmonary metastases developed, and the patient died four years after the initial diagnosis. The other patient presented to the emergency room with shortness of breath and was found to have a 1.5 cm broad base pedunculated submucosal mass centered in the left arytenoid region. The laryngeal mass was excised and revealed a leiomyosarcoma grade 2/3. Immunohistochemistry showed that the tumor cells were positive for SMA, HCD, and focally positive for desmin and p63 (weak, focal) and negative for CD34, S100, p40, pancytokeratin, and CK5/6. The Ki-67 nuclear labeling index was approximately 20%. No follow up was available for this patient. Histologically, both tumors were composed of spindle cells containing elongate nuclei with coarse chromatin and blunted ends and eosinophilic cytoplasm arranged in fascicles that intersected one another at right angles (Fig. 4a and b).
Fig. 4.
a Leiomyosarcoma is composed of spindle cells with elongate nuclei that have coarse chromatin and blunted ends and eosinophilic cytoplasm. The tumor cells are arranged in fascicles that intersected one another at right angles (HES × 10). b Scattered cells have enlarged pleomorphic hyperchromatic nuclei (HES × 40)
A 78-year-old male presented with a 1.2 cm left vocal cord mass, and the biopsy revealed a cytologically malignant pleomorphic spindle cell neoplasm with hyalinized collagenous stroma. Immunohistochemistry showed that the tumor cells were strongly positive for SMA, focally positive for desmin and SATB2, and negative for S100, pancytokeratin, MyoD1, myogenin, and CD34. Amplification of MDM2 by chromogenic in situ hybridization was negative in the tumor cells. The neoplasm was classified as high-grade myofibroblastic sarcoma, grade 3/3. On resection, there was no evidence of mucosal dysplasia or carcinoma. The patient completed post-operative radiation therapy with no evidence of disease after 2 years of follow-up.
Two cases of myxofibrosarcoma were identified and comprised 7.4% of cases- both patients were male, and their ages were 62 and 78 years. The 62-year-old male presented with 4.0 cm mass in the anterior hypopharynx at and below the level of the true vocal cords. The biopsy and resection revealed high-grade myxofibrosarcoma. Immunohistochemistry showed that the tumor cells were positive for SMA, focally positive for EMA, and negative for desmin, pancytokeratin, CK7, myogenin, p63, p40. Amplification of MDM2 by chromogenic in situ hybridization was negative in the tumor cells. There was no morphologic evidence of mucosal dysplasia or invasive carcinoma or spread to regional lymph nodes. After surgery, adjuvant radiation was recommended, but the patient transferred his care to another facility and was lost to follow-up. The 78-year-old patient was found to have a 4.0 cm obstructive right vocal fold mass. The tumor was completely removed and sent to pathology. The resection specimen revealed a high-grade myxofibrosarcoma. Immunohistochemistry showed that the tumor cells were positive for SMA, desmin, and negative for pancytokeratin, CK 5/6, p40, CK 8/18, p63, CD34, S100, Sox-10, myogenin, and MYOD1. Amplification of MDM2 by chromogenic in situ hybridization was negative in the tumor cells. No follow was available for this patient. Histologically both tumors were composed of malignant pleomorphic spindle cells enmeshed in a myxoid stroma that contained arching small-caliber blood vessels (Fig. 5).
Fig. 5.
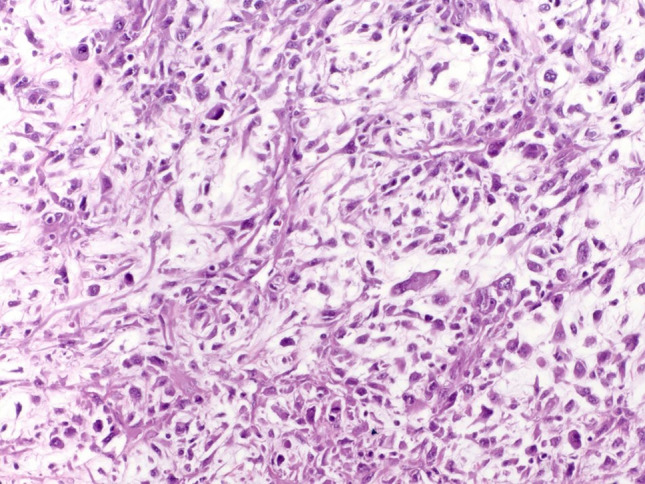
a Myxofibrosarcoma is composed of malignant pleomorphic spindle cells enmeshed in a myxoid stroma that contains arching small-caliber blood vessels (HES × 20)
A 69-year-old female with a past medical history of melanoma 12 years prior had locally recurred and metastasized to an axillary lymph node presented with a 1.6 cm mass involving the left vocal and vestibular folds that impaired movement of the hemilarynx. A biopsy showed a low-grade myofibroblastic sarcoma composed of spindle cells that exhibited mild atypia and had elongate, vesicular nuclei that contained small nucleoli. The mitotic rate was < 2 per 10 high-power fields, there was no necrosis, and the tumor infiltrated deeply into the striated muscle fibers of the larynx. Immunohistochemistry showed that the tumor cells were focally positive for desmin and negative for smooth muscle actin, keratin, S100, and Alk-1. The overlying laryngeal mucosa was normal. No further treatment was given, and the patient was free of disease 1 year after resection. This case was previously published by Covello et al. and is from AER consultation files.
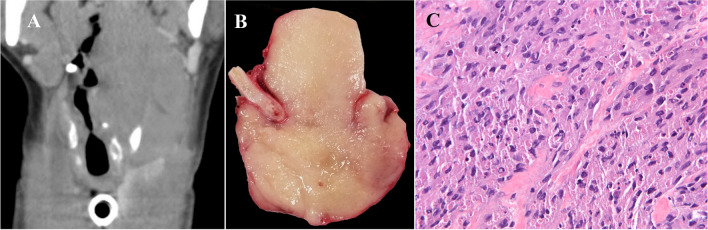
The malignant granular cell tumor occurred in a 39-year-old female (Fig. 6a and b). The tumor cells were large, polygonal with abundant eosinophilic granular cytoplasm and showed spindling, cellular pleomorphism, increased nuclear to cytoplasmic ratio, and single-cell necrosis (Fig. 6c). Immunohistochemistry showed that the tumor cells were positive for Sox-10 and S100. A biopsy of a newly enlarged contralateral lymph node identified on a 4-week post-operative CT scan revealed metastatic malignant granular cell tumor, and the patient completed a course of adjuvant radiation therapy with no evidence of disease after fourteen months of follow-up.
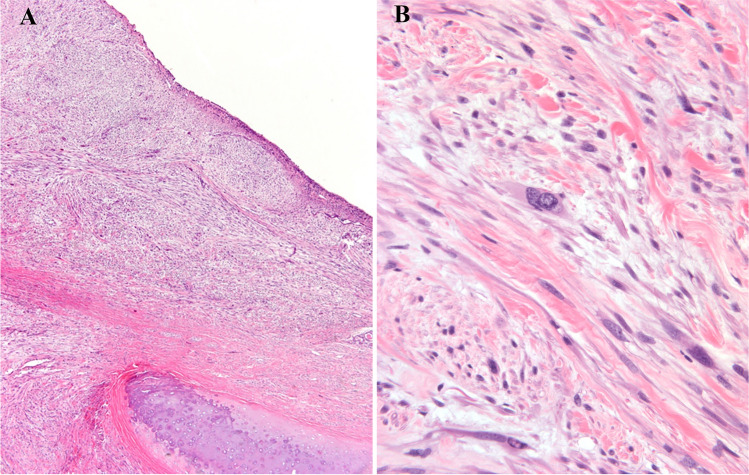
Fig. 6.
a Reformatted coronal computed tomography demonstrates a large mass involving the left pharyngeal wall, the base of tongue, left true vocal cord, and subglottic region. b Excised malignant granular cell tumor shows glistening tan-yellow cut surface. c Sheets of spindle and polygonal cells with granular cytoplasm (HES × 40)
Discussion
Malignant neoplasms of the larynx are uncommon. Approximately 95% are carcinomas, most showing squamous differentiation, followed by non-epithelial tumors, including sarcomas, lymphomas, and melanomas. Malignant non-epithelial neoplasms are more common than their benign counterparts, and the percentage of malignant versus benign tumors varies from 55.6 to 75% depending on the series [2–5, 10]. This study reviews a large series of laryngeal sarcomas in adults, and it is evident that they have lower mortality and recurrence rates than their counterparts developing in other anatomic sites, and this may be due to the early onset of symptoms, their small size, and location [6, 7, 9]. In our experience, imaging studies are not very helpful in rendering a definitive diagnosis; the identification of fat or calcifications indicative of cartilage are helpful for fatty and cartilaginous tumors but do not discern between benign and malignant tumors, which must be determined by pathological analysis.
In our experience, chondrosarcomas of the larynx are the most common primary laryngeal sarcoma. In this series, they accounted for 62.9% of cases, and all patients were male (n = 17). Chondrosarcomas of the larynx constitute up to 1% of all primary laryngeal tumors, and affected patients have a mean age of 63 years (range of 25–91 years), the male-to-female ratio is 3.6:1 [4, 7–9, 11–14]. Our series included 16 conventional chondrosarcomas and one clear cell chondrosarcoma. Up to 60% of laryngeal chondrosarcomas arise in association with preexisting chondromas, and there is an abrupt transition from chondroma to chondrosarcoma, as seen in four of our cases [9]. It has been reported that up to 11.8% of laryngeal chondrosarcomas harbor a low IDH1 mutation rate, a finding that suggests a different mode of tumorigenesis from chondrosarcomas that arise in bone [15]. Prior reports have shown that these tumors usually have an excellent overall long-term prognosis, and distant metastasis are rare [9, 13]. Approximately 18–50% of laryngeal chondrosarcomas develop local recurrences, and it often results from incomplete resection [9, 11]. In our series, one patient had recurrence of disease seventeen years after prior resection.
The differential diagnosis of laryngeal chondrosarcoma includes chondroma, and their distinction from one another can be difficult. Although, it has been reported that chondromas are usually less than 2.0 cm in diameter, and chondrosarcomas have a mean diameter of 3.5 cm [16, 17]. Histological analysis is required to distinguish them from one another. Chondromas are distinguished from normal laryngeal cartilaginous structures in that they show increased cellularity, and the chondrocytes are often arranged in clusters. The cartilage in chondroma, like conventional chondrosarcoma, is well-formed but is hypocellular, contains chondrocytes with small round dark nuclei with no nuclear atypia, and the matrix may calcify.
In contrast, low-grade conventional chondrosarcoma exhibits increased cellularity, and the chondrocytes demonstrate mild cytological atypia in the form of nuclear enlargement, irregular nuclear contours, and small nucleoli. In grade 2 chondrosarcomas, there is greater cellularity and moderate atypia in the form of nuclear enlargement and hyperchromasia. In most instances, the distinction between chondroma and conventional chondrosarcoma does not affect treatment as both are managed in a similar fashion.
One of our patients with a biopsy diagnosis of conventional hyaline chondrosarcoma was reclassified to clear cell chondrosarcoma after reviewing the resection specimen in which the majority of the tumor was composed of clear cell chondrosarcoma with a minor component of grade 2/3 conventional chondrosarcoma. This highlights the issue of sampling error in small biopsy specimens and the importance of careful evaluation of resection specimens. Clear cell chondrosarcoma is a rare variant of chondrosarcoma that accounts for up to 2% of all chondrosarcomas [18]. Clear cell chondrosarcoma in the head and neck is exceedingly rare as fewer than 10 cases have been reported; 6 cases developed in the larynx, and others arose in the maxilla and nasal septum [14, 18–22]. Most conventional and clear cell chondrosarcomas behave as low-grade tumors and have a prolonged clinical course characterized by local recurrence and very low risk of dissemination [18]. The current recommended treatment is conservative surgery with preservation of laryngeal function and removal of gross disease, and obtaining negative margins, if possible [8, 9]. This recommendation for treatment of laryngeal clear cell chondrosarcoma should be viewed with caution as the number of reported cases is very small, and in the appendicular skeleton, they have a high rate of local recurrence if curetted rather than excised en bloc with negative margins [23].
There were two well-differentiated liposarcoma cases; both patients were male and presented with progressive shortness of breath. Previous reports have shown that liposarcomas of the head and neck are usually well-differentiated lipoma-like and sclerosing subtypes, represent approximately 5.6% of all head and neck sarcomas, and most arise in the soft tissues of the neck, followed by the larynx [24–27]. These tumors are locally aggressive and have a high rate of local recurrence (up to 51% of cases) [24, 26]. The current recommended treatment of choice is wide surgical excision with negative margins; however, this may be difficult to achieve because of the size and extent of the neoplasms [28]. The differential diagnosis of well-differentiated liposarcoma is lipoma, and they can be distinguished because lipoma lacks thickened fibrous bands and adipocytes and spindle cells with large hyperchromatic nuclei. In difficult cases, immunohistochemistry, chromogenic in-situ hybridization, or fluorescence in-situ hybridization for amplification of MDM2 can be used to distinguish between these neoplasms. We perform tests for amplification of MDM2 when the tumor shows histological features that raise the possibility of well-differentiated liposarcoma or is deep-seated and greater than 10 cm.
Kaposi sarcoma is an uncommon locally aggressive endothelial neoplasm associated with human herpesvirus 8 and represents 20–25% of all head and neck sarcomas [29, 30]. It is estimated that 66% of mucosal KS involves the head and neck sites, with the hard palate as the most common location, followed by the gingiva and tongue [31]. Kaposi sarcoma infrequently affects the larynx; however, it has been reported that patients with cutaneous and multiple oral cavity lesions have a higher risk of laryngeal involvement, and this should be excluded by direct laryngoscopy [32]. The current recommended treatment for Kaposi sarcoma in HIV patients is highly active antiretroviral therapy and single-agent chemotherapy for advanced disease [33, 34]. The differential diagnosis includes sarcomatoid carcinoma and a variety of spindle cell sarcomas. Sarcomatoid carcinoma usually shows a greater degree of cytological atypia, does not contain a prominent lymphoplasmacytic infiltrate or hemosiderin deposits, and unlike Kaposi sarcoma, is often positive for epithelial markers and negative for HHV8 and endothelial markers. Also, included in the differential is angiosarcoma which is composed of a proliferation of malignant epithelioid or spindled endothelial cells that line vascular spaces and have an infiltrative growth pattern. Angiosarcoma, unlike Kaposi sarcoma, shows significant cytological atypia, is negative for HHV8 and lacks the characteristic lymphoplasmacytic inflammatory infiltrate present in Kaposi sarcoma.
Head and neck leiomyosarcomas account for 3% of all leiomyosarcomas, and the most common affected regions are the oral cavity, sinonasal area, scalp, neck, orbit, and cervical esophagus [35, 36]. In the larynx, the tumor arises in the glottis (48%), supraglottis (32%), and supraglottis-glottis (6.5%) [37]. Treatment is surgical resection with widely negative margins. These patients are at increased risk of distant metastases (up to 19%); therefore, adjuvant chemotherapy may be considered as a part of therapy [36]. The differential diagnosis in the larynx is sarcomatoid carcinoma and other spindle cell sarcomas (e.g., rhabdomyosarcoma). The distinguishing features of leiomyosarcoma are the blunt-ended spindle-shaped nuclei, fibrillar eosinophilic cytoplasm, and fascicles of tumor cells intersecting one another at right angles. Additionally, leiomyosarcoma is usually positive for one or more myogenic markers. Although sarcomatoid squamous cell carcinomas may stain with smooth muscle actin, they are also usually positive for keratin and p40. Also, leiomyosarcoma and primary laryngeal sarcomas lack significant dysplasia of the overlying squamous mucosa, which is often present in association with sarcomatoid squamous cell carcinoma. Lastly, rhabdomyosarcomas show diffuse expression for MyoD1 and myogenin which are negative in leiomyosarcoma.
The incidence of fibroblastic/myofibroblastic sarcoma, and myxofibrosarcoma, is reported to be less than 2% of all malignancies in the larynx. It accounts for approximately 5% of all sarcomas of the head and neck [10]. High-grade variants were previously known as malignant fibrous histiocytoma and are also known as undifferentiated pleomorphic sarcoma. They are rare in the larynx and occur more commonly in males (M: F = 3:1). The age of affected patients is 6–68 years, and the glottis is the most common site [38]. The prognosis of high grade fibroblastic/myofibroblastic sarcoma is associated with tumor size, age, and gender; tumors less than 3 cm in female patients over 60 years of age have a better prognosis [39]. The diagnosis of high grade fibroblastic/myofibroblastic sarcoma should only be rendered after extensive tumor sampling and immunohistochemistry. In cases where the differential diagnosis includes granulation tissue with reactive stromal changes, the presence of cellular pleomorphism and atypical mitotic activity are features of malignancy. The diagnostic approach for these spindle cell sarcomas should include careful evaluation of surface epithelium to exclude the presence of dysplasia. Immunohistochemical testing for cytokeratins, p40 or p63 can be helpful in detecting epithelial or squamous differentiation. Lastly, the possibility of dedifferentiated liposarcoma (in our experience very rare in this anatomic location) should be considered given the morphologic overlap with other spindle cell sarcomas and testing for amplification of MDM2 can be performed in this setting. Three of our spindle cell sarcomas (myxofibrosarcoma and myofibroblastic sarcoma) were tested for amplification of MDM2 by chromogenic in-situ hybridization and had a negative result, excluding the possibility of dedifferentiated liposarcoma. Hematogenous dissemination to the lungs is frequent, with no tendency to develop cervical lymph node metastases. Wide surgical margins are recommended because of the high local recurrence rates (44–73%). Partial laryngectomy to preserve function may be performed for early-stage tumors [10, 40–42]. Chemotherapy and radiation may be used in patients with large tumors and close or positive margins. Close follow-up is essential due to the aggressive nature of this tumor [38].
Low-grade myofibroblastic sarcoma is an uncommon sarcoma that usually affects middle-aged patients (range 4–75 years) with a slight male predominance and has a predilection for the head and neck region, particularly the tongue, oral cavity, and rarely the larynx [43, 44]. The prognosis is usually good; they have a high propensity to recur but rarely metastasize. Surgical excision with negative margins has been the optimal therapy [44]. The differential diagnosis includes spindle cell sarcomatoid squamous cell carcinoma and inflammatory myofibroblastic tumor. Low-grade myofibroblastic sarcoma is distinguished from sarcomatoid squamous cell carcinoma by its limited cytological atypia combined with the expression of markers of myogenic differentiation. Sarcomatoid carcinoma may show overt squamous differentiation or have accompanying high-grade dysplasia of the overlying squamous mucosa. Inflammatory myofibroblastic tumor is distinguished from low-grade myofibroblastic sarcoma because the latter is usually accompanied by an inflammatory infiltrate of plasma cells with or without lymphocytes and eosinophils and frequently expresses ALK and cytokeratin (13.6–81% of cases) [45].
Malignant granular cell tumors of the larynx are rare, and at least four cases have been reported in the literature, including our case [46–49]. The histologic criteria for malignancy include necrosis, spindling, vesicular nuclei with prominent nucleoli, increased mitotic activity (greater than 2 mitoses per 10 HPF), high nuclear to cytoplasmic ratio, and pleomorphism. Tumors with three or more of these features are considered malignant [49]. Patients with laryngeal tumors reportedly developed nodal and distant metastasis (lung and bone) and were alive after an average follow-up of 17 months [46, 47, 49]. The current recommended treatment is complete surgical resection with negative margins [47]. The differential diagnosis includes alveolar soft part sarcoma and metastatic renal cell carcinoma. The immunophenotype of these tumors facilitates their distinction from one another.
Finally, there are other smaller series of primary laryngeal sarcomas that have been published with a slightly different spectrum of sarcomas. The series published by Mantilla et al. [6] and AbdullGaffar et al. [50] identified a total of 15 primary laryngeal sarcomas. They included (5) chondrosarcomas, (2) osteosarcomas, (2) Kaposi sarcomas, (1) well-differentiated liposarcoma, (1) embryonal rhabdosarcoma, (1) synovial sarcoma, (1) angiosarcoma, (1) leiomyosarcoma, and (1) undifferentiated spindle cell sarcoma. The difference observed between subtypes of sarcomas between our series, and those series may be related to both referral patterns and coincidence.
Conclusion
Primary laryngeal sarcomas are rare and are classified according to their phenotype and histological grade. The majority have specific histologic features and distinct growth patterns. As sarcomatoid carcinoma is often in the differential diagnosis, careful evaluation of the mucosal epithelium is essential in rendering the correct diagnosis and permitting the institution of appropriate patient management. Immunohistochemical and molecular studies are useful to precisely classify some of these tumors. Also, correlation with imaging studies can provide valuable information regarding tumor location and the relationship of the tumor to surrounding structures.
The approach to the treatment of sarcomas of the larynx depends on the site and extent of the tumor, histology, and resectability. In the majority of cases, extensive resections may not be necessary, and laryngeal preservation should be considered when possible for low grade or small and circumscribed tumors. The management of these patients should be done in a multidisciplinary fashion because treatment and outcome depend on the expertise of the multidisciplinary team. Long-term clinical follow-up is essential to monitor for recurrence and disease-free survival.
Author Contributions
JVT, AER, JADP, and EMD contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JVT, EMD, JL, DW, GT, FC, DA, CGF, and JADP. JVT and AER wrote and edited the manuscript. JVT and AER provided radiographs and micrographs. All authors read and approved the final manuscript.
Funding
No funding was obtained.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Narozny W, Mikaszewski B, Stankiewicz C. Benign neoplasms of the larynx. Auris Nasus Larynx. 1995;22(1):38–42. doi: 10.1016/s0385-8146(12)80180-5. [DOI] [PubMed] [Google Scholar]
- 2.Saraydaroglu O, Narter S, Ozsen M, Coskun H. Non-epithelial tumors of the larynx: case series of 12 years. Eur Arch Otorhinolaryngol. 2019;276(10):2843–2847. doi: 10.1007/s00405-019-05527-0. [DOI] [PubMed] [Google Scholar]
- 3.Rzepakowska A, Osuch-Wojcikiewicz E, Bruzgielewicz A, Niemczyk K. Non-epithelial neoplasms of the larynx and hypopharynx—12-years of experience. Otolaryngol Pol. 2015;69(5):9–15. doi: 10.5604/00306657.1170420. [DOI] [PubMed] [Google Scholar]
- 4.Karatayli-Ozgursoy S, Bishop JA, Hillel AT, Akst LM, Best SR. Non-epithelial tumors of the larynx: a single institution review. Am J Otolaryngol. 2016;37(3):279–285. doi: 10.1016/j.amjoto.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Friedman AD, Burns JA, Lutch MJ, Zeitels SM. Submucosal neoplasms of the laryngeal introitus. J Laryngol Otol. 2012;126(7):706–713. doi: 10.1017/S0022215112000928. [DOI] [PubMed] [Google Scholar]
- 6.Mantilla JG, Xu H, Ricciotti RW. Primary sarcomas of the larynx: a single institutional experience with ten cases. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Acenero MJ, Larach F, Ortega-Fernandez C. Non-epithelial lesions of the larynx: review of the 10-year experience in a tertiary Spanish hospital. Acta Otolaryngol. 2009;129(1):108–112. doi: 10.1080/00016480802008207. [DOI] [PubMed] [Google Scholar]
- 8.Casiraghi O, Martinez-Madrigal F, Pineda-Daboin K, Mamelle G, Resta L, Luna MA. Chondroid tumors of the larynx: a clinicopathologic study of 19 cases, including two dedifferentiated chondrosarcomas. Ann Diagn Pathol. 2004;8(4):189–197. doi: 10.1053/j.anndiagpath.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Thompson LD, Gannon FH. Chondrosarcoma of the larynx: a clinicopathologic study of 111 cases with a review of the literature. Am J Surg Pathol. 2002;26(7):836–851. doi: 10.1097/00000478-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Cao X, Liu J, Zheng Y, Li Q, Teng Y, Li Y. Simultaneous squamous cell carcinoma with primary malignant fibrous histiocytoma of the larynx: a case report. Mol Med Rep. 2012;5(4):971–973. doi: 10.3892/mmr.2012.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubal PM, Svider PF, Kanumuri VV, Patel AA, Baredes S, Eloy JA. Laryngeal chondrosarcoma: a population-based analysis. Laryngoscope. 2014;124(8):1877–1881. doi: 10.1002/lary.24618. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JE, Olsen KD, Inwards CY. Cartilaginous tumors of the larynx: clinicopathologic review of 47 cases. Ann Otol Rhinol Laryngol. 1997;106(2):94–100. doi: 10.1177/000348949710600202. [DOI] [PubMed] [Google Scholar]
- 13.Coca-Pelaz A, Rodrigo JP, Triantafyllou A, Hunt JL, Fernandez-Miranda JC, Strojan P, de Bree R, Rinaldo A, Takes RP, Ferlito A. Chondrosarcomas of the head and neck. Eur Arch Otorhinolaryngol. 2014;271(10):2601–2609. doi: 10.1007/s00405-013-2807-3. [DOI] [PubMed] [Google Scholar]
- 14.Alexander J, Wakely PE., Jr Primary laryngeal clear cell chondrosarcoma: report of a case and literature review. Head Neck Pathol. 2014;8(3):307–310. doi: 10.1007/s12105-014-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallegas M, Miquelestorena-Standley E, Labit-Bouvier C, Badoual C, Francois A, Gomez-Brouchet A, Aubert S, Collin C, Tallet A, de Pinieux G. IDH mutation status in a series of 88 head and neck chondrosarcomas: different profile between tumors of the skull base and tumors involving the facial skeleton and the laryngotracheal tract. Hum Pathol. 2019;84:183–191. doi: 10.1016/j.humpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Baatenburg de Jong RJ, van Lent S, Hogendoorn PC. Chondroma and chondrosarcoma of the larynx. Curr Opin Otolaryngol Head Neck Surg. 2004;12(2):98–105. doi: 10.1097/00020840-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Heffner DK, Venegas R, French SW. Atypical chondroma of the cricoid cartilage: fine-needle aspiration cytologic and histopathologic findings. Diagn Cytopathol. 1999;20(3):164–166. doi: 10.1002/(sici)1097-0339(199903)20:3<164::aid-dc10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Mokhtari S, Mirafsharieh A. Clear cell chondrosarcoma of the head and neck. Head Neck Oncol. 2012;4:13. doi: 10.1186/1758-3284-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks T, Cardemil F, Sader C. Clear cell chondrosarcoma of the larynx. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-226541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slootweg PJ. Clear-cell chondrosarcoma of the maxilla: report of a case. Oral Surg Oral Med Oral Pathol. 1980;50(3):233–237. doi: 10.1016/0030-4220(80)90376-x. [DOI] [PubMed] [Google Scholar]
- 21.Euteneuer S, Sudhoff H, Stein H, Delling G, Dazert S. Clear cell chondrosarcoma (CCCS) of the nasal septum. Laryngorhinootologie. 2004;83(4):232–235. doi: 10.1055/s-2004-814243. [DOI] [PubMed] [Google Scholar]
- 22.Kim TJ, Lee AH, Choi YJ, Jung ES, Kim MS, Lee KY. Clear cell chondrosarcoma in the nasal septum. Otolaryngol Head Neck Surg. 2007;137(6):972–973. doi: 10.1016/j.otohns.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Bjornsson J, Unni KK, Dahlin DC, Beabout JW, Sim FH. Clear cell chondrosarcoma of bone. Observations in 47 cases. Am J Surg Pathol. 1984;8(3):223–230. doi: 10.1097/00000478-198403000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Yang LH, Liu TT, Wang J, Li H, Yu G, Wang Z, Lv JJ, Zhao HY, Wang EH. Liposarcoma of the larynx: report of a case and review of literature. Int J Clin Exp Pathol. 2015;8(1):1068–1072. [PMC free article] [PubMed] [Google Scholar]
- 25.Golledge J, Fisher C, Rhys-Evans PH. Head and neck liposarcoma. Cancer. 1995;76(6):1051–1058. doi: 10.1002/1097-0142(19950915)76:6<1051::aid-cncr2820760620>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Wenig BM, Weiss SW, Gnepp DR. Laryngeal and hypopharyngeal liposarcoma. A clinicopathologic study of 10 cases with a comparison to soft-tissue counterparts. Am J Surg Pathol. 1990;14(2):134–141. doi: 10.1097/00000478-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wenig BM, Heffner DK. Liposarcomas of the larynx and hypopharynx: a clinicopathologic study of eight new cases and a review of the literature. Laryngoscope. 1995;105(7 Pt 1):747–756. doi: 10.1288/00005537-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Powitzky R, Powitzky ES, Garcia R. Liposarcoma of the larynx. Ann Otol Rhinol Laryngol. 2007;116(6):418–424. doi: 10.1177/000348940711600605. [DOI] [PubMed] [Google Scholar]
- 29.Peng KA, Grogan T, Wang MB. Head and neck sarcomas: analysis of the SEER database. Otolaryngol Head Neck Surg. 2014;151(4):627–633. doi: 10.1177/0194599814545747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavrakas M, Nixon I, Andi K, Oakley R, Jeannon JP, Lyons A, McGurk M, Urbano TG, Thavaraj S, Simo R. Head and neck sarcomas: clinical and histopathological presentation, treatment modalities, and outcomes. J Laryngol Otol. 2016;130(9):850–859. doi: 10.1017/S0022215116008604. [DOI] [PubMed] [Google Scholar]
- 31.Thariat J, Kirova Y, Sio T, Choussy O, Vees H, Schick U, Poissonnet G, Saada E, Thyss A, Miller RC. Mucosal Kaposi sarcoma, a Rare Cancer Network study. Rare Tumors. 2012;4(4):e49. doi: 10.4081/rt.2012.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mochloulis G, Irving RM, Grant HR, Miller RF. Laryngeal Kaposi’s sarcoma in patients with AIDS. J Laryngol Otol. 1996;110(11):1034–1037. doi: 10.1017/s0022215100135698. [DOI] [PubMed] [Google Scholar]
- 33.Dupont C, Vasseur E, Beauchet A, Aegerter P, Berthe H, de Truchis P, Zucman D, Rouveix E, Saiag P. Long-term efficacy on Kaposi’s sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d’information et de soins de l’immunodeficience humaine. AIDS. 2000;14(8):987–993. doi: 10.1097/00002030-200005260-00010. [DOI] [PubMed] [Google Scholar]
- 34.Bower M, Dalla Pria A, Coyle C, Andrews E, Tittle V, Dhoot S, Nelson M. Prospective stage-stratified approach to AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2014;32(5):409–414. doi: 10.1200/JCO.2013.51.6757. [DOI] [PubMed] [Google Scholar]
- 35.Khadivi E, Taziky MH, Jafarian AH, Nasseri Sadr M. Laryngeal leiomyosarcoma, a case report and review of articles. Iran J Otorhinolaryngol. 2013;25(73):253–258. [PMC free article] [PubMed] [Google Scholar]
- 36.Skoulakis CE, Stavroulaki P, Moschotzopoulos P, Paxinos M, Fericean A, Valagiannis DE. Laryngeal leiomyosarcoma: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2006;263(10):929–934. doi: 10.1007/s00405-006-0092-0. [DOI] [PubMed] [Google Scholar]
- 37.Marioni G, Bertino G, Mariuzzi L, Bergamin-Bracale AM, Lombardo M, Beltrami CA. Laryngeal leiomyosarcoma. J Laryngol Otol. 2000;114(5):398–401. doi: 10.1258/0022215001905698. [DOI] [PubMed] [Google Scholar]
- 38.Cambruzzi E, Cruz RP, Gava VG, Pegas KL. Undifferentiated high-grade pleomorphic sarcoma of the larynx treated with partial laringectomy. Braz J Otorhinolaryngol. 2016 doi: 10.1016/j.bjorl.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberal I, Samim E, Astarci M, Ozeri C. Radiation-induced malignant fibrous histiocytoma of the neck in a patient with laryngeal carcinoma. Am J Otolaryngol. 2001;22(2):146–149. doi: 10.1053/ajot.2001.22579. [DOI] [PubMed] [Google Scholar]
- 40.Karkos PD, Dova S, Sotiriou S, Markou K, Kostopoulos I. Double primary malignant fibrous histiocytoma and squamous cell carcinoma of the larynx treated with laser laryngeal conservation surgery. Ecancermedicalscience. 2016;10:636. doi: 10.3332/ecancer.2016.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabesan T, Xuexi W, Yongfa Q, Pingzhang T, Ilankovan V. Malignant fibrous histiocytoma: outcome of tumours in the head and neck compared with those in the trunk and extremities. Br J Oral Maxillofac Surg. 2006;44(3):209–212. doi: 10.1016/j.bjoms.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Soh KB, Westmore GA, Moir AA, Colloby PS. Malignant fibrous histiocytomas of the larynx–report of two cases. Ann Acad Med Singapore. 1996;25(6):878–881. [PubMed] [Google Scholar]
- 43.Kordac P, Nikolov DH, Smatanova K, Kalfert D. Low-grade myofibroblastic sarcoma of the larynx: case report and review of literature. Acta Medica (Hradec Kralove) 2014;57(4):162–164. doi: 10.14712/18059694.2015.82. [DOI] [PubMed] [Google Scholar]
- 44.Covello R, Licci S, Pichi B, Spriano G, Vidiri A, Morelli L, Rosenberg AE. Low-grade myofibroblastic sarcoma of the larynx. Int J Surg Pathol. 2011;19(6):822–826. doi: 10.1177/1066896910393958. [DOI] [PubMed] [Google Scholar]
- 45.Qiu X, Montgomery E, Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum Pathol. 2008;39(6):846–856. doi: 10.1016/j.humpath.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Chiang MJ, Fang TJ, Li HY, Chen IH, Lee KF. Malignant granular cell tumor in larynx mimicking laryngeal carcinoma. Am J Otolaryngol. 2004;25(4):270–273. doi: 10.1016/j.amjoto.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Bradford Bell EJ, Thomas GR, Leibowitz J, Velez Torres JM. Benign and malignant granular cell tumor of the hypopharynx: two faces of a rare entity. Head Neck Pathol. 2020 doi: 10.1007/s12105-020-01157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aksoy S, Abali H, Kilickap S, Harputluoglu H, Erman M. Metastatic granular cell tumor: a case report and review of the literature. Acta Oncol. 2006;45(1):91–94. doi: 10.1080/02841860500341132. [DOI] [PubMed] [Google Scholar]
- 49.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22(7):779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 50.AbdullGaffar B, Keloth T. Laryngeal sarcomas: a case series of 5 cases. Ann Diagn Pathol. 2018;37:35–41. doi: 10.1016/j.anndiagpath.2018.09.007. [DOI] [PubMed] [Google Scholar]