Abstract
Human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is related to improved treatment outcomes. What remains unclear is whether all HPV DNA genotypes carry similar prognostic relevance. We aimed to evaluate disease control and survival outcomes by HPV DNA genotype. Patients with primary OPSCC without distant metastases treated with curative intent were retrospectively identified from an IRB-approved institutional database. Patients that underwent HPV DNA polymerase chain reaction (PCR) testing with available genotype were included and dichotomized by the presence of HPV type 16 (HPV-16) or other high-risk HPV genotype (HPV-non16). Overall survival (OS), disease-free survival (DFS), locoregional control (LRC) and distant control (DC) were determined using the Kaplan–Meier method and compared using the log-rank test. In our cohort of 193 patients treated from 2012 to 2018 with HPV DNA PCR, 10% were detected as HPV-non16 high-risk types. Patients with HPV-16 were significantly younger than those with HPV-non16, but no other baseline factors were associated with HPV-non16. With a median follow-up of 42.9 months, there were no significant differences in outcomes between the HPV-16 and HPV-non16 groups for 3-year OS (87.7% v. 73.6%), DFS (82.9% v. 68.7%), LRC (92.8% v. 88.5%) or DC (91% v. 89.2%). There is no statistically significant difference in outcomes between OPSCC with HPV-16 and HPV-non16 high-risk genotypes in our cohort, though trends of overall worse survival and disease-free survival in HPV-non 16 OPSCC were seen. Further studies with larger cohorts of patients with HPV-non 16-associated OPSCC are required to make definitive conclusions regarding the prognostic and clinical significance of HPV type.
Keywords: Oropharyngeal carcinoma, Human papillomavirus, Head and neck neoplasms, Squamous cell carcinoma, HPV testing
Introduction
Human papillomavirus (HPV) is well known to be a major cause of various types of cancer in humans, including in the head and neck, and more specifically, as a major etiology of oropharyngeal cancer [1, 2]. The historical risk factors for oropharyngeal squamous cell carcinoma (OPSCC) such as smoking, alcohol use, and poor oral hygiene have since become less significant compared to the rising incidence of HPV associated cancers over the past decade [3, 4]. Since the discovery of the oncogenic nature of HPV, there have been over 150 genotypes recognized and further dichotomized into high-risk and low-risk, with high-risk being closely associated with causing cancer. The most common high-risk type of HPV in OPSCC is type 16, accounting for almost 90% of all HPV-positive cases [3]. Other high-risk genotypes such as 18, 31, and 33 are less commonly identified [5].
In clinical practice, it is important to identify whether a newly diagnosed OPSCC is HPV-associated, as HPV-positivity is prognostic of improved overall survival [6]. HPV-associated OPSCC are a unique entity based on the presentation of the disease, affected population, and responsiveness to treatment [7]. Based on the recently released AJCC 8th Edition guidelines, prognosis is overall better for those with HPV-positive OPSCC [8]. The established favorable prognosis of HPV-associated OPSCC has led to clinical trials investigating the efficacy of de-escalation of radiation treatment for this cohort [9]. It is hypothesized that patients who have HPV-associated disease may respond better to radiation treatment and current regimens are over-treating this population of patients.
The pathogenesis of HPV-associated cancer begins with abnormal epithelial cell growth that may be caused by either low or high-risk types [7]. However, infection with a high-risk type has the potential to induce carcinogenic transformation of the affected cells [10, 11]. The virology and mechanism in which HPV is carcinogenic has been described in detail [12, 13], but it is unknown whether oncogenic potential varies among the different high-risk types. In the present study, we aim to investigate the outcomes of disease control and survival of HPV-positive OPSCC based on viral genotype.
Methods
Patient Population and Treatment
Patients with oropharyngeal squamous cell carcinoma treated with definitive intent between 2012 and 2018 were retrospectively identified from an institutional head and neck cancer database. Study data were collected and managed using REDCap electronic data capture tools hosted by the Wake Forest Clinical and Translational Science Institute [14]. Patients with recurrent or second primary head and neck cancer, distant metastatic disease, those treated with re-irradiation or with palliative intent, or those treated with radiotherapy to an incomplete dose (defined as less than 60 Gy) were excluded. Patients with tumor information on HPV status were selected for further analysis of outcomes based on HPV genotype. Patient demographics, reported tumor pathology, and treatment characteristics were abstracted from the electronic medical record.
Patients were managed based on the recommendations of a multidisciplinary tumor board. Patients treated with definitive radiotherapy were treated with concurrent chemotherapy for stage III (AJCC 7th Ed.) or greater disease unless contraindicated. When chemotherapy was delivered concurrently with radiotherapy, high-dose cisplatin was preferred, with alternate options including low-dose weekly cisplatin or other radiosensitizing chemotherapy regimens at the discretion of the medical oncologist. According to institutional practice, unilateral neck irradiation was considered for patients with tonsillar cancer with a lateralized primary tumor (> 1 cm from midline) and limited neck involvement (N2b or less, size < 3 cm); patients with base of tongue primary tumors routinely received bilateral neck irradiation. Surgical management was performed via transoral robotic surgery (TORS) or an open approach at the discretion of the surgeon. Postoperative radiotherapy was recommended according to established guidelines for high-risk pathologic features such as advanced tumor stage, lymphovascular invasion, perineural invasion, multiple positive nodes, or close margins. Adjuvant chemoradiotherapy was recommended (unless contraindicated) in the case of positive surgical margin or extracapsular extension. Radiotherapy was performed as previously described [15].
Pathologic Assessment for HPV
HPV testing was performed on formalin-fixed paraffin-embedded (FFPE) tissues obtained from biopsy or excision specimens, or from FFPE cell block material from fine needle aspirations. Genomic DNA was extracted using the QIAamp DSP DNA FFPE Tissue kit (QIAGEN, Venlo Netherlands), in which proteinase K digestion of FFPE tissue is followed by silica-membrane technology for the isolation and purification of DNA.
Following DNA extraction, qualitative HPV testing, with HPV genotyping for positive samples, was performed. Prior to June 2015, HPV testing was performed by PCR amplification using primers specific for the L1 region of HPV, with concurrent amplification of beta-globin as an internal control. HPV DNA positive PCR products were subject to restriction fragment length polymorphism analysis by digestion with restriction endonucleases Hae III, Pst I, and Rsa I. Digested DNA fragments were separated on a 5% polyacrylamide gel and visualized by ethidium bromide intercalation. The restriction fragment pattern of the sample determined the specific genotype(s) present. From June 2015 onward, HPV testing was performed using the Roche COBAS 4800 system (Roche Molecular Diagnostics, Pleasanton, CA) which uses real-time PCR to detect 14 high-risk HPV types including 16, 18, and grouped other high-risk types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68).
For some cases, immunohistochemistry (IHC) for p16 was performed on FFPE tissue sections using clone E6H4 (Ventana Medical Systems, Tucson, Arizona). Data on p16 positivity by IHC was collected from the pathology reports. Of note, after publication of the CAP recommendations, tumor was deemed positive if tumor showed nuclear and cytoplasmic staining in at least 70% of tumor cells; however, prior to this publication, no consensus standards existed for pathologist determination of positivity.
Assessment of Outcomes
Overall survival (OS) was defined as the duration to death from any cause. Disease-free survival (DFS) was defined as the time from treatment completion to any disease failure (local, regional, or distant), or death. Locoregional control (LRC) was defined as freedom from local (at the primary site) or regional (within the regional lymph node basins) recurrence. Distant control (DC) was defined as freedom from distant metastasis elsewhere in the body.
Statistical Analysis
Patients were stratified by HPV type 16 (HPV-16) versus non-type 16 (HPV-non16). Categorical data were summarized with count (frequency) and compared using the Chi-square test or Fisher’s exact test as appropriate. Continuous variables were compared using t-tests or the Wilcoxon rank-sum test for normal and non-normally distributed variables, respectively. Time to event outcomes (OS, DFS, and LRC) were calculated from the date of diagnosis, estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Multiple comparisons where utilized were corrected using the Bonferroni method. In order to account for potential confounding of the impact of HPV type by imbalanced baseline predictors, multivariable Cox proportional hazards models including HPV type and any factors found to be significantly imbalanced between groups were generated. Proportional hazards assumptions were tested for all models. An alpha of 0.05 was utilized to define statistical significance for all tests. All analyses were performed using R version 3.6 (R Foundation for Statistical Analysis, Vienna, Austria).
Results
Prevalence of HPV-16 and HPV-non16
In total, 377 patients were identified. After exclusion of patients with recurrent/second primary disease (n = 33), those treated with re-irradiation (n = 3), those with metastatic disease (n = 1), those without available HPV PCR testing (n = 117), and patients treated with incomplete radiotherapy (n = 2), a total of 233 patients were included in the analysis. In total, 173 (75.1%) patients were positive for HPV-16, 20 patients were positive for HPV-non16 (8.6%), and 38 (16.3%) tested negative for HPV DNA. Of patients with HPV testing (n = 193), 90.7% were positive for HPV-16 and 10.4% were positive for HPV-non16.
Clinical and Treatment Factors by HPV Type
Patient baseline characteristics stratified by HPV type are summarized in Table 1. Generally, all patients regardless of HPV type presented with similar demographics and characteristics. Patients with HPV-16 were younger than those with HPV-non16 (mean age 58.9 v. 63.7 years, p = 0.01). There were no significant differences noted between gender, race, ECOG performance status, AJCC 7th and 8th Edition T-stage, N-stage and overall stage, and smoking status. A numeric but nonsignificant increase in baseline pack-years smoking history was evident in patients with HPV-non16 compared to HPV-16. Fifty-eight out of 62 HPV-16 tumors and 6 of 6 HPV-non16 patients tested positive for p16 by IHC.
Table 1.
Baseline patient characteristics
| Overall | HPV-16 | HPV-non16 | p-value | |
|---|---|---|---|---|
| Patients (n) | 193 | 173 | 20 | |
| Age (mean (SD)) | 59.3 (8.0) | 58.8 (7.8) | 63.7 (8.6) | 0.01 |
| Gender | ||||
| Female | 31 (16.1) | 27 (15.6) | 4 (20.0) | 0.54 |
| Male | 162 (83.9) | 146 (84.4) | 16 (80.0) | |
| Race | ||||
| Black | 12 (6.2) | 12 (6.9) | 0 (0.0) | 0.66 |
| Caucasian | 180 (93.3) | 160 (92.5) | 20 (100.0) | |
| Other | 1 (0.5) | 1 (0.6) | 0 (0.0) | |
| ECOG (%) | ||||
| 0 | 75 (39.3) | 67 (39.2) | 8 (40.0) | 0.44 |
| 1 | 106 (55.5) | 96 (56.1) | 10 (50.0) | |
| 2 | 10 (5.2) | 8 (4.7) | 2 (10.0) | |
| OPSCC sub site (%) | ||||
| Tonsil | 103 (53.4) | 96 (55.5) | 7 (35.0) | 0.12 |
| Base of tongue | 86 (44.6) | 74 (42.8) | 12 (60.0) | |
| Other | 4 (2.1) | 3 (1.7) | 1 (5.0) | |
| T stage (AJCC 7th ed) | ||||
| T0 | 3 (1.6) | 2 (1.2) | 1 (5.0) | 0.14 |
| T1 | 65 (33.7) | 62 (35.8) | 3 (15.0) | |
| T2 | 85 (44.0) | 73 (42.2) | 12 (60.0) | |
| T3 | 20 (10.4) | 19 (11.0) | 1 (5.0) | |
| T4a | 13 (6.7) | 11 (6.4) | 2 (10.0) | |
| T4b | 7 (3.6) | 6 (3.5) | 1 (5.0) | |
| N stage (AJCC 7th ed) | ||||
| N0 | 19 (9.8) | 15 (8.7) | 4 (20.0) | 0.13 |
| N1 | 32 (16.6) | 30 (17.3) | 2 (10.0) | |
| N2 | 1 (0.5) | 0 (0.0) | 1 (5.0) | |
| N2a | 23 (11.9) | 20 (11.6) | 3 (15.0) | |
| N2b | 73 (37.8) | 68 (39.3) | 5 (25.0) | |
| N2c | 41 (21.2) | 36 (20.8) | 5 (25.0) | |
| N3 | 4 (2.1) | 4 (2.3) | 0 (0.0) | |
| T stage (AJCC 8th ed) | ||||
| T0 | 3 (1.6) | 2 (1.2) | 1 (5.0) | 0.12 |
| T1 | 65 (33.7) | 62 (35.8) | 3 (15.0) | |
| T2 | 85 (44.0) | 73 (42.2) | 12 (60.0) | |
| T3 | 20 (10.4) | 19 (11.0) | 1 (5.0) | |
| T4 | 20 (10.4) | 17 (9.8) | 3 (15.0) | |
| N stage (AJCC 8th ed) | ||||
| N0 | 19 (9.8) | 15 (8.7) | 4 (20.0) | 0.27 |
| N1 | 123 (63.7) | 113 (65.3) | 10 (50.0) | |
| N2 | 47 (24.4) | 41 (23.7) | 6 (30.0) | |
| N3 | 4 (2.1) | 4 (2.3) | 0 (0.0) | |
| Overall stage (AJCC 7th ed) | ||||
| I | 7 (3.6) | 7 (4.0) | 0 (0.0) | 0.36 |
| II | 11 (5.7) | 8 (4.6) | 3 (15.0) | |
| III | 32 (16.6) | 30 (17.3) | 2 (10.0) | |
| IVA | 132 (68.4) | 118 (68.2) | 14 (70.0) | |
| IVB | 11 (5.7) | 10 (5.8) | 1 (5.0) | |
| Overall stage (AJCC 8th ed) | ||||
| I | 127 (65.8) | 114 (65.9) | 13 (65.0) | 0.88 |
| II | 42 (21.8) | 38 (22.0) | 4 (20.0) | |
| III | 24 (12.4) | 21 (12.1) | 3 (15.0) | |
| Smoking status | ||||
| Current/former smoker | 126 (65.6) | 115 (66.9) | 11 (55.0) | 0.42 |
| Never smoker | 66 (34.4) | 57 (33.1) | 9 (45.0) | |
| 10-year smoking history | ||||
| ≤10 pack-years | 32 (25.0) | 32 (27.4) | 0 (0.0) | 0.06 |
| > 10 pack-years | 96 (75.0) | 85 (72.6) | 11 (100.0) | |
| Pack Years (median [IQR]) | 25.0 [13.8, 40.5] | 24.0 [10.0, 40.0] | 40.0 [20.0, 46.0] | 0.11 |
| p16 status (% available) | ||||
| Negative | 4 (5.6) | 4 (6.2) | 0 (0.0) | 1.00 |
| Positive | 67 (94.4) | 61 (93.8) | 6 (100.0) |
ECOG Eastern cooperative oncology group, IQR interquartile range, OPSCC oropharyngeal squamous cell carcinoma, SD standard deviation
The primary treatment method was surgical in 115 (59%) of patients and radiotherapy in the remaining 80 (41%). There were no differences between treatment paradigm, RT neck target, chemotherapy utilization, or surgical technique between the two groups. Treatment details are summarized in Table 2. Seventy-seven patients were treated nonsurgically with definitive radiotherapy. 48 patients were treated with surgery alone and thus had no neck irradiation and 36 patients were treated with primary surgery at our institution but referred for adjuvant radiotherapy at an outside facility. Postoperative pathologic risk factors were similar between groups for HPV-16 and HPV-non16.
Table 2.
Treatment characteristics
| Overall | HPV-16 | HPV-non16 | p-value | |
|---|---|---|---|---|
| Patients (n) | 193 | 173 | 20 | |
| Primary treatment (%) | ||||
| RT | 77 (39.9) | 69 (39.9) | 8 (40.0) | 1.00 |
| S | 116 (60.1) | 104 (60.1) | 12 (60.0) | |
| Treatment type (%) | ||||
| CRT | 76 (39.4) | 68 (39.3) | 8 (40.0) | 0.88 |
| RT | 1 (0.5) | 1 (0.6) | 0 (0.0) | |
| S | 48 (24.9) | 44 (25.4) | 4 (20.0) | |
| S + CRT | 38 (19.7) | 34 (19.7) | 4 (20.0) | |
| S + RT | 30 (15.5) | 26 (15.0) | 4 (20.0) | |
| Neck RT target (%) | ||||
| Unilateral | 3 (1.6) | 3 (1.7) | 0 (0.0) | 0.55 |
| Bilateral | 106 (54.9) | 96 (55.5) | 10 (50.0) | |
| No RT | 48 (24.9) | 44 (25.4) | 4 (20.0) | |
| Not available | 36 (18.7) | 30 (17.3) | 6 (30.0) | |
| Concurrent chemotherapy with RT (%) | ||||
| No | 79 (40.9) | 71 (41.0) | 8 (40.0) | 1.00 |
| Yes | 114 (59.1) | 102 (59.0) | 12 (60.0) | |
| Induction chemotherapy (%) | ||||
| No | 182 (94.3) | 163 (94.2) | 19 (95.0) | 1.00 |
| Yes | 11 (5.7) | 10 (5.8) | 1 (5.0) | |
| Transoral Robotic surgery (%) | ||||
| No | 17 (14.7) | 15 (14.4) | 2 (16.7) | 0.69 |
| Yes | 99 (85.3) | 89 (85.6) | 10 (83.3) | |
| Neck dissection (%) | ||||
| None | 5 (2.6) | 4 (2.3) | 1 (5.0) | 0.29 |
| Ipsilateral | 81 (42.2) | 75 (43.6) | 6 (30.0) | |
| Bilateral | 29 (15.1) | 24 (14.0) | 5 (25.0) | |
| Nonsurgical | 77 (40.1) | 69 (40.1) | 8 (40.0) | |
| RT Dose (median [range]) (Gy) | 70.00 [60.00, 70.00] | 70.00 [60.00, 70.00] | 70.00 [66.00, 70.00] | 0.14 |
CRT chemoradiotherapy, Gy Gray, RT radiation therapy, S surgery
Survival and Disease Control in Patients with HPV-16 versus HPV-non16
Median follow-up for the entire cohort is 42.9 months (95% CI 37.5–46.5), 42.9 months for HPV-16 patients and 36.2 months for HPV-non16 patients. Three-year OS was 81.0% (95% CI 75.5–86.9), 3-year DFS was 74.9% (95% CI 69.0–81.2), and 3-year LRC was 89.5% (95% CI 85.4–93.9).
Overall, there were no significant differences between OS, DFS, locoregional or distant control. Overall survival at 3 years was 87.7% (95% CI 82.1–93.6) for the HPV-16 group and 73.6% (56.0–96.6) for the HPV-non16 group (log-rank p = 0.11) (Fig. 1a). Disease-free survival at 3 years was 82.9% (95% CI 76.9–89.4) for the HPV-16 group and 68.7% (50.7–93.3) for the HPV-non16 group (log-rank p = 0.16) (Fig. 1b). Locoregional control at 3 years was 92.8% (95% CI 88.7–97.0) for the HPV-16 group and 88.5% (74.8–100) for the HPV-non16 group (log-rank p = 0.55) (Fig. 1c). Three-year distant control was 91.0% (95% CI 86.4–95.9) and 89.2% (76.0–100), respectively (log-rank p = 0.73) (Fig. 1d). Cox proportional hazards models adjusting for age found that HPV-non16 was not associated with significantly worse OS (adjusted hazard ratio [aHR] 1.83, 95% CI 0.74–4.52, p = 0.19) or DFS (aHR 1.00, 95% CI 0.96–1.04, p = 0.93).
Fig. 1.
Kaplan–Meier plots of overall survival (a), disease-free survival (b), locoregional control (c) and distant control (d) for patients with HPV-16 versus HPV-non16 disease
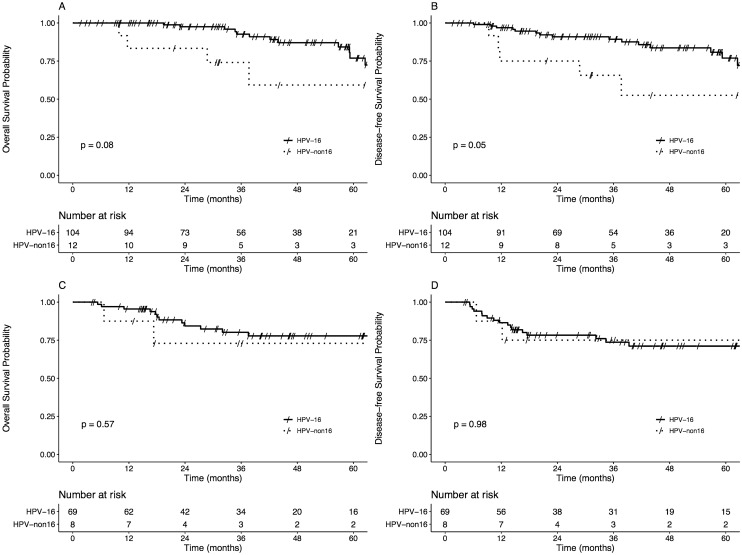
When stratified by primary treatment (surgical versus non-surgical), there were no significant differences in OS or DFS between groups (Fig. 2). There was a nonsignificant trend of worse overall survival and disease-free survival in HPV-non16 patients for primary surgical patients (p = 0.08 and p = 0.05, respectively) (Fig. 2a and b).
Fig. 2.
Kaplan–Meier plots of overall survival and disease-free survival for primary surgical (surgery alone, surgery + radiotherapy, or surgery + chemoradiotherapy; a–b) and primary radiotherapy (radiotherapy alone or chemoradiotherapy) patients (c–d)
Discussion
The main purpose of this study was to determine the impact of HPV genotype in the setting of HPV-associated OPSCC. Consistent with prior literature, most of the patients with HPV-positive disease in our cohort were positive for HPV-16, with only approximately 10% of patients with other high-risk HPV types detected [1, 16]. This HPV-non 16 positivity frequency is similar to that seen in previous reports (9%) [17] and somewhat higher than seen other studies (~ 5%) [18, 19]. The clinical characteristics and prevalence of HPV-non16 OPSCC had been recently described by Varier et al. in a metropolitan setting, including 27 HPV-non16 and 119 HPV-16 patients [17]. In this retrospective review, HPV-non16 types presented with more advanced disease staging, but otherwise similar characteristics to HPV-16 types. There continues to be a scarcity in data that analyzes the behavior of HPV-non16 types in OPSCC in comparison to HPV-16, or even HPV-negative OPSCC.
An interesting finding was noted in a study by Misiukiewicz et al. that HPV-non16 types may have a worse prognosis overall [20]. In this 23-patient clinical trial for radiation de-escalation following induction chemotherapy, it was suggested that for future clinical trials, DNA PCR may be clinically necessary. Although our analysis did not appreciate a significant difference in survival, the nonsignificant trends of worse overall survival and disease-free survival, especially in primarily surgically treated patients, may suggest that HPV-non16 types indeed carry variable prognoses compared to HPV-16. While the emerging de-escalation trials for HPV-positive OPSCC continue to develop, it is imperative to understand if there remains a clinical difference with HPV-non16 types.
The optimal method of defining HPV association had been controversial, with prior studies using a variety of techniques (detection of surrogate maker p16, HPV DNA PCR, HPV RNA PCR, or DNA/RNA in situ hybridization) to correlate with clinical outcomes [21–23]. Current College of American Pathologists (CAP) guidelines recommend testing of all oropharyngeal tissue specimens by p16 IHC, and “additional HPV-specific testing may be done at the discretion of the pathologist and/or treating clinician, or in the context of a clinical trial” [24]. Of note, our current study included cases from 2012–2018, so only the later subset were triaged and evaluated according to current CAP guidelines. During the time prior to these guidelines, HPV-association of OPSCC was typically assessed at our institution by PCR testing for HPV DNA, with p16 IHC performed in only a subset of cases. Molecular testing for HPV with genotyping at our institution has markedly decreased since publication of the CAP guidelines, which will make further evaluation of genotype effect on clinical outcome more difficult outside of a research setting. Of interest, though not the focus of this study, no histologic findings stood out as distinguishing HPV-16 and HPV-non 16 disease by review of pathology reports. HPV-non 16 OPSCC were moderately to poorly differentiated, some with basaloid features, and tended to be non-keratinizing, similar to that described generally for HPV-associated OPSCC [24–26].
Concordance between p16 surrogate marker staining and PCR/ISH is generally high [27, 28], though discordance between these methodologies remain. In one prospective French study of 71 patients with OPSCC, both p16 immunohistochemistry and HPV-PCR was performed on tumor biopsies, with p16 positivity on 43.7% and DNA-PCR positivity on 31% of samples [27]. The study ascertains that it would be more reliable to test for both in clinical practice based on those results. In a meta-analysis of patients with head and neck squamous cell carcinomas with survival reported by p16/HPV PCR status, the relative risk of discordance was approximately 7% [29]. Those with p16+/PCR+ oropharyngeal tumors were found to have improved 5-year survival in comparison with p16+/PCR- or p16-/PCR+. In our study, 100% of the DNA-PCR HPV-non16 patient tumors tested for p16 IHC were positive (6/6), however 93.5% of HPV-16-positive tumors were p16 positive (58/62). A new classification first described by Weinberger et al. [21], asserts a system based on both p16 expression and the presence of HPV DNA. In this study, p16+/HPV+ had the most favorable prognosis. This has continued to be validated in other studies [30], however, it is prudent to recognize the platform that each institution uses to assess for HPV.
It is well known that HPV is a major driver of other cancers such as cervical cancer. In the setting of cervical cancer in which carcinogenesis is largely driven by HPV, there have been very few studies examining the role of HPV-non16 types. In a recent Swedish study that followed the risk of developing high grade cervical intraepithelial neoplasia (CIN) in 96 patients, those with baseline HPV16 or 18 were at a higher risk of developing CIN than other high-risk HPV types [31]. However, it has been noted in literature that HPV-non16 or –non18 subtypes account for about 44% of cervical cancer cases, which is much higher than what is observed in our study and supporting studies [32]. As the prevalence of vaccinations against HPV increases in the United states using vaccines such as Cervarix (GlaxoSmithKline, Brentford, London, England) and Gardasil (Merck & Co., Kenilworth, New Jersey, USA), it is anticipated that the incidence of high-risk HPV infections will decrease. Most importantly, in June 2020, Merck and the FDA officially announced that Gardasil is indicated for people aged 9–45 as a preventive measure against head and neck cancers. It will be exceedingly important to continue to advocate and support cancer prevention efforts such as HPV-vaccination, especially to our most vulnerable populations.
There are several limitations to this study. As a retrospective review, there are limits to the data that can be abstracted from the medical records, including data that has been omitted or missing from electronic medical records. This is particularly relevant in this patient cohort that spans an era with changing standards for HPV testing, resulting in variations in HPV status available within the medical record (PCR, p16, or both). There is a lack of p16 IHC testing for all patients in our database, as well as HPV DNA-PCR testing particularly for the more recent patients (since the publication of the CAP guidelines). Although both p16 IHC and DNA-PCR are both acceptable methods of tumor testing, it provides difficulty in comparisons in retrospective review. Since there does not appear to be a significant interaction between HPV type and disease extent at diagnosis or treatment modality, selection biases may not greatly impact these findings. This study is also limited by the small sample size of the HPV-non16 group. Lastly, many patients included in this study have a reported history of smoking and alcohol use, which may confound these data. Smoking has carcinogenic effects that over time, may outweigh the HPV oncogenic effects. Therefore, caution must be taken with patients with > 10 pack year smoking history and HPV+OPSCC [9]. As a result of these limitations, the findings of this work are limited to hypothesis generation. Additional prospective, multicenter studies investigating the impact of high-risk HPV types in HPV-associated OPSCC are needed.
Conclusion
HPV-16 versus HPV-non16 high-risk genotype is not associated with significant differences in disease characteristics and outcomes among patients with HPV-associated OPSCC. While data suggested trends of worse overall survival and disease-free survival in HPV-non16 patients, the low number of patients prohibits definitive conclusions but may be hypothesis-generating for future research. HPV-16 remains the most common high-risk type in OPSCC, perhaps with the best prognosis. Co-testing with p16 immunohistochemistry and HPV DNA PCR in order to identify patients with these rare, high-risk types may be clinically impactful.
Funding
This work was supported by the Wake Forest Baptist Medical Center and National Center for Advancing Translational Sciences (NCATS), National Institutes of Health funded Wake Forest Clinical and Translational Science Institute (WF CTSI) through Grant Award Number UL1TR001420.
Compliance with Ethical Standards
Conflict of interest
There are no conflicts of interest to be declared.
Ethical Approval
This study was reviewed by the Wake Forest Clinical and Translational Science Institute IRB and approved by ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gillison ML. Human papillomavirus-related diseases: oropharynx cancers and potential implications for adolescent HPV vaccination. J Adolesc Health. 2008;43(4 Suppl):S52–60. doi: 10.1016/j.jadohealth.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dayyani F, et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6(Suppl 1):S48–54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, et al. Eurogin Roadmap: comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix. Int J Cancer. 2014;134(3):497–507. doi: 10.1002/ijc.28201. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallen-St Clair J, et al. Human papillomavirus in oropharyngeal cancer: the changing face of a disease. Biochim Biophys Acta. 2016;1866(2):141–150. doi: 10.1016/j.bbcan.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al, editors. AJCC cancer staging manual. 8th ed. Springer International Publishing, American Joint Commission on Cancer; 2017.
- 9.Chera BS, Amdur RJ. Current status and future directions of treatment deintensification in human papilloma virus-associated oropharyngeal squamous cell carcinoma. Semin Radiat Oncol. 2018;28(1):27–34. doi: 10.1016/j.semradonc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Keck MK, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 11.Grce M, Mravak-Stipetic M. Human papillomavirus-associated diseases. Clin Dermatol. 2014;32(2):253–258. doi: 10.1016/j.clindermatol.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.de Villiers EM, Gissmann L, Zur Hausen H. Molecular cloning of viral DNA from human genital warts. J Virol. 1981;40(3):932–935. doi: 10.1128/jvi.40.3.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger K, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagatsuka M, et al. Omitting elective irradiation of the contralateral retropharyngeal nodes in oropharyngeal squamous cell carcinoma treated with intensity-modulated radiotherapy. Cureus. 2019;11(1):e3825. doi: 10.7759/cureus.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elrefaey S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299–309. [PMC free article] [PubMed] [Google Scholar]
- 17.Varier I, et al. Clinical characteristics and outcomes of oropharyngeal carcinoma related to high-risk non-human papillomavirus16 viral subtypes. Head Neck. 2016;38(9):1330–1337. doi: 10.1002/hed.24442. [DOI] [PubMed] [Google Scholar]
- 18.St Guily JL, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France-The EDiTH VI study. J Clin Virol. 2011;51(2):100–104. doi: 10.1016/j.jcv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi AK, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 20.Misiukiewicz K, et al. Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: the quarterback trial. Oral Oncol. 2019;95:170–177. doi: 10.1016/j.oraloncology.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger PM, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 22.Semrau R, et al. Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head Neck. 2013;35(9):1339–1344. doi: 10.1002/hed.23126. [DOI] [PubMed] [Google Scholar]
- 23.Driessen CM, et al. Toxicity and efficacy of accelerated radiotherapy with concurrent weekly cisplatin for locally advanced head and neck carcinoma. Head Neck. 2016;38(Suppl 1):E559–E565. doi: 10.1002/hed.24039. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JS, Jr, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the college of American pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 25.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Westra WH. The pathology of HPV-related head and neck cancer: implications for the diagnostic pathologist. Semin Diagn Pathol. 2015;32(1):42–53. doi: 10.1053/j.semdp.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Fonmarty D, et al. Study of the concordance between p16 immunohistochemistry and HPV-PCR genotyping for the viral diagnosis of oropharyngeal squamous cell carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(3):135–139. doi: 10.1016/j.anorl.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Pannone G, et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 immunohistochemistry, consensus PCR HPV-DNA, and In Situ Hybridization. Infect Agent Cancer. 2012;7:4. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albers AE, et al. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep. 2017;7(1):16715. doi: 10.1038/s41598-017-16918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JC, et al. High prevalence of discordant human papillomavirus and p16 oropharyngeal squamous cell carcinomas in an African American cohort. Head Neck. 2016;38(Suppl 1):E867–E872. doi: 10.1002/hed.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froberg M, et al. Impact of the human papillomavirus status on the development of high-grade cervical intraepithelial neoplasia in women negative for intraepithelial lesions or malignancy at the baseline: a 9-year Swedish nested case-control follow-up study. Cancer. 2019;125(2):239–248. doi: 10.1002/cncr.31788. [DOI] [PubMed] [Google Scholar]
- 32.Hopenhayn C, et al. Prevalence of human papillomavirus types in invasive cervical cancers from 7 US cancer registries before vaccine introduction. J Low Genit Tract Dis. 2014;18(2):182–189. doi: 10.1097/LGT.0b013e3182a577c7. [DOI] [PMC free article] [PubMed] [Google Scholar]