Abstract
Introduction
The administration of medications targeting type 2 diabetes mellitus (T2D) has evolved over time. As injection delivery systems continue to evolve, it is necessary to understand patients’ perceptions of currently available treatments. The objective of this study was to examine the patient perspective of injectable treatment for T2D and identify characteristics of these treatments that are most important to patients.
Methods
Data were collected via an online survey study with a sample of individuals in the UK and US who were treated for T2D with injectable medication. The survey was designed to elicit perceptions of the treatment process for injectable glucagon-like peptide 1 (GLP-1) receptor agonists and insulin.
Results
The sample included 504 participants (251 UK, 253 US). Approximately half (50.4%) were treated with a GLP-1 receptor agonist and half (49.6%) were treated with insulin. Respondents were presented with a list of 17 characteristics of injectable medication and asked to indicate which were most important to them. Respondents most frequently selected confidence in administering the correct dose (n = 300, 59.5%); ease of selecting the correct dose (n = 268, 53.2%); overall ease of using the injection device (n = 239, 47.4%); frequency of injections (n = 223, 44.2%); and ease of carrying the device when necessary to inject away from home (n = 190, 37.7%). Characteristics least frequently cited as important included dose escalation (n = 79, 15.7%); handling the needle (n = 74, 14.7%); connectivity to an electronic device (n = 70, 13.9%); and the time required to prepare and inject each dose (n = 62, 12.3%).
Conclusion
Results of this survey suggest that patients prioritize some attributes of injectable treatments over others. These findings may have implications for clinical practice and development of injection devices.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01097-9.
Keywords: Type 2 diabetes, Patient preferences, Injectable medication, Injection device, Injection pen, Insulin, GLP-1 receptor agonist
Key Summary Points
| Why carry out this study? |
| As injection delivery systems for medications targeting type 2 diabetes (T2D) continue to evolve, it is necessary to understand patients’ perceptions of currently available treatments. |
| The objective of this study was to examine the patient perspective of injectable treatment for T2D and identify characteristics of these treatments that are most important to patients. |
| What was learned from the study? |
| Attributes of injection delivery systems that patients most frequently cited as important include confidence in delivering the correct dose, ease of administering the correct dose, ease of using the injection device, and dose frequency. |
| Attributes that appeared to be less important to patients include dose escalation, handling the needle, electronic connectivity, and dose preparation time. |
| Patient preferences, such as those reported in this study, can inform clinical decision-making and the development of injection devices with features more likely to enhance adherence and maximize treatment benefit. |
Introduction
Globally, the number of individuals diagnosed with diabetes has increased at an alarming rate for decades [1–8], and projections indicate that as many as 578 million people will be living with the disease by 2030 [7]. Type 2 diabetes mellitus (T2D), which accounts for about 90–95% of all diabetes cases, impacts over 30 million Americans [9]. Although somewhat lower in the United Kingdom (UK), prevalence of T2D in that country has doubled in recent years, exceeding 5% of the population [10], and it is considered one of the country’s fastest growing health issues [11, 12].
Although lifestyle changes and metformin continue to be the first-line approach for most patients with T2D, use of second-line glucose-lowering therapies, including injectable medications, has increased dramatically in recent years [13, 14]. Over time, the process of taking medications for T2D has evolved, particularly the developments in injection delivery systems for insulin and glucagon-like peptide 1 (GLP-1) receptor agonists [15–19]. For example, several convenient attributes of the newer insulin pens include their availability in prefilled designs (e.g., NovoLog® FlexPen® [20], Humalog KwikPen® [21]), more precise dosing selection options (e.g., NovoPen Echo® [22], HumaPen® Luxura™ HD [23], Humalog® Junior KwikPen® [24]), and memory functionality to revert to previous dose settings (e.g., HumaPen® Memoir™ [25], NovoPen Echo® [22]). Other features designed to improve the patient experience of injection devices for insulin and GLP-1 receptor agonists include autoinjector capabilities (e.g., Bydureon BCise® [26], Autopen® Classic [27]); hidden needles (e.g., Trulicity® Single-Dose Pen [28], NovoPen® 3 PenMate® [29], BD AutoShield™ Duo [30]); spring-loaded mechanisms that require less force while injecting (e.g., NovoLog® FlexPen® [31], Autopen Classic® [27]); single-dose, single-use devices (e.g., Trulicity® Single-Dose Pen [28]); and audible clicks to confirm dose delivery (e.g., NovoPen® 4 [32]).
Changes to dosing and timing requirements of certain medications have been implemented to enhance convenience and further improve the overall patient experience. For example, a “super-long-acting” basal insulin (insulin icodec) intended to be dosed as a once-weekly injection (rather than once-daily) is under development [33, 34], while recently developed fast-acting insulins have no premeal dosing requirements [35, 36]. Similarly, several GLP-1 receptor agonists are now available as once-weekly injections, including exenatide [26], dulaglutide [28], and semaglutide [37].
While efficacy and safety are the primary factors to consider when selecting a medication, aspects of the treatment process can also contribute to treatment outcomes. Treatment process attributes that enhance convenience and ease of use have been shown to influence patient preference [38–43], and previous studies have demonstrated that patients tend to prefer simpler treatment regimens [44–49]. The patient perspective is important because patients may have better treatment adherence with preferred treatments, and adherence can affect patient outcomes [15, 50–56]. A recent consensus report issued by the American Diabetes Association (ADA) and European Association for the Study of Diabetes emphasized the importance of patient preference when discussing and selecting a diabetes treatment regimen [57]. Therefore, as injection delivery systems continue to evolve, it is necessary to survey patients with T2D to understand their perceptions of currently available injection devices.
The objective of this study was to examine the patient perspective of injectable treatment for T2D, including aspects of the injection treatment process. One area of focus was to determine which treatment process characteristics of injectable T2D medications are most important to patients. Data were collected via an online survey with a geographically diverse sample in the US and UK.
Methods
Study Design
This was an observational, online survey study with a sample of individuals living with T2D in the UK and US who were treated with injectable medication (GLP-1 receptor agonist or insulin). Approximately half of the sample was recruited from the US, and the other half was recruited from the UK. Within each country, efforts were made to recruit approximately 50% who were treated with an injectable GLP-1 receptor agonist and approximately 50% using an insulin pen.
The study proceeded in three phases: (1) “pre-testing” qualitative interviews; (2) a pilot online survey; and (3) the main online data collection survey. The qualitative interviews in phase 1 were conducted by telephone and were designed to evaluate the clarity of the survey and determine whether revisions were needed prior to launching the survey in phase 2. Phase 2 data were reviewed to confirm that there were no problems with the online survey before proceeding with the larger data collection in phase 3. Individuals were not permitted to participate in more than one phase of the study.
Participants were required to provide written informed consent before completing study procedures, and all procedures and materials were approved by an independent institutional review board (Ethical & Independent Review Services; Initial approval on March 31, 2020 [Protocol # 20021–01]; Modification approval on May 24, 2020 [Protocol # 20021-01B]). This study was conducted in accordance with the Declaration of Helsinki. A license agreement was obtained to use the electronic version of the EQ-5D-5L in this study. Other than the EQ-5D-5L, the content of the survey was developed by the authors. Therefore, no external permissions were required to use or implement the survey.
Participants
Individuals who met the following criteria were eligible for participation in the study: adult (18 years or older) resident of the US or UK; self-report of T2D diagnosis by a medical professional (duration of T2D at least 6 months); currently being treated for T2D with either an injectable GLP-1 receptor agonist or insulin pen; and able to read and understand English sufficiently to participate in the study and complete all assessments. The following criteria excluded individuals from participating in the study: diagnosed with type 1 diabetes or latent autoimmune diabetes; currently taking an injectable combination treatment that includes both a GLP-1 receptor agonist and an insulin; currently taking both a GLP-1 receptor agonist and insulin; taking insulin via vial and syringe injections; diagnosed with gestational diabetes; pharmaceutical employees; or employed in a position where they have a direct role in treating patients with diabetes. In addition, potential participants currently treated with a GLP-1 receptor agonist were excluded if they had previously taken insulin. Similarly, individuals treated with insulin were excluded if they had previously taken a GLP-1 receptor agonist.
To recruit participants for phase 1, potential study participants were identified from a database of patients who self-reported diabetes and who had participated in previous studies. Potential participants were invited by email to participate in the study. The email included information about the study and a telephone number that interested individuals could call to be screened for eligibility. After screening by phone, individuals who met study inclusion/exclusion criteria were scheduled for an interview. A weblink was sent to participants so they could complete the online survey immediately prior to their scheduled interview. In the phase 1 interview, participants provided feedback on the survey.
In phases 2 and 3, participants were recruited through online patient panels that included individuals who self-reported diabetes. These online patient panels consisted of patients who had completed extensive screening surveys, including sociodemographic and healthcare data. To bolster recruitment efforts in phase 3, additional participants were identified through the same database of patients utilized in phase 1 of this study. Individuals were invited by email to complete the online survey. The invitation email included a brief description of the study and a survey link.
Survey Procedures
Once potential participants clicked on the link provided in the invitation email, they were taken to a screening questionnaire to confirm eligibility for the study. Respondents who were eligible were taken to an electronic informed consent page, which they were asked to review. After providing consent to participate in the study, participants were directed to the survey. The survey took approximately 15–20 min to complete, including confirmation of eligibility and the electronic informed consent process. Participants were remunerated for their participation.
Measures
The survey included the measures described below.
Sociodemographic and Clinical Questions
Sociodemographic information was collected including age, gender, living situation, employment, education level, and racial/ethnic background. Questions on clinical information asked participants to report their height, weight, current T2D medication, diabetes-related complications, other health conditions, glycated hemoglobin (HbA1c), and use of continuous glucose monitoring devices. Details about their injection devices were gathered with separate questions for GLP-1 receptor agonist users and insulin device users.
Treatment Administration and Device Characteristics Questions
Questions focusing on treatment administration and injection device characteristics were designed to identify which characteristics of the injection treatment process were most important to participants. Seventeen attributes of injectable medication were included as response options. These attributes were selected based on published literature on insulin and GLP-1 receptor agonist injection delivery systems [17, 58–61], as well as consideration of the injection devices used with currently available treatments [20, 26, 28, 32]. Respondents were asked to select the five attributes that were most important to them. The survey also included several questions focusing on participants’ history of injectable treatment for their T2D. Key questions from the survey are presented in the supplementary material.
EQ-5D-5L
The EQ-5D-5L is a self-administered, generic, preference-weighted measure designed to assess current health status [62–65]. The first section consists of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). There are five levels to assess each dimension of health-related quality of life and these levels correspond to 1 (no problems), 2 (slight problems), 3 (moderate problems), 4 (severe problems), or 5 (unable). The second section of the EQ-5D-5L consists of a 20-cm vertical visual analogue scale, with anchors of 0 (“worst imaginable health state”) and 100 (“best imaginable health state”). Participants were asked to choose a number along the scale that reflected their current health. The appropriate language version of the EQ-5D-5L was administered to the UK and US participants. The EQ-5D-5L descriptive system was scored as outlined by van Hout et al. [66].
Pre-testing Qualitative Interviews for Phase 1 Participants
Participants in phase 1 participated in qualitative pre-testing interviews conducted by telephone. In these interviews, they were asked a series of open-ended questions immediately following completion of the online survey. These questions assessed the extent to which the survey was clear and easy to understand to determine if the survey should be refined prior to launching phase 2.
Statistical Analysis
Analyses were performed with data from the combined samples in phase 2 and phase 3, as well as separately by country. Descriptive statistics (e.g., mean, median, standard deviation; range for continuous variables; frequency and percentages for categorical variables) were used to summarize participants’ responses and characterize the sample in terms of sociodemographic and clinical characteristics. Statistical tests were run to compare UK patients to US patients; chi-squared tests were performed for categorical variables, while t tests were run for continuous variables. Statistical significance for p values was 0.05, and all tests were two-tailed. Statistical analyses were completed using SAS version 9.4 (SAS Institute 1979–2013).
Results
Phase 1 Results
In phase 1, 10 patients with T2D, five from the UK and five from the US, completed the draft survey followed by a qualitative interview. Half of these patients were being treated with insulin at the time they completed the survey, while the other half were receiving a GLP-1 receptor agonist. The primary goal of the interviews, each of which took approximately 30 min to conduct, was to determine whether any revisions to the survey were necessary prior to administering it to a larger number of respondents in the next phase. Patients generally reported that the survey questions and response options were clear and understandable, and most patients said the survey was easy to complete. Relatively minor edits were made to the survey on the basis of respondents’ recommendations. For example, the response option “computer app” was changed to “desktop/laptop” for the item that asked about the connectivity of one’s continuous glucose monitoring device. For the item about health conditions other than T2D, the response option “substance abuse” was changed to “drug abuse.” The slightly revised version of the survey was subsequently administered in phase 2.
Sample Selection for Phases 2 and 3
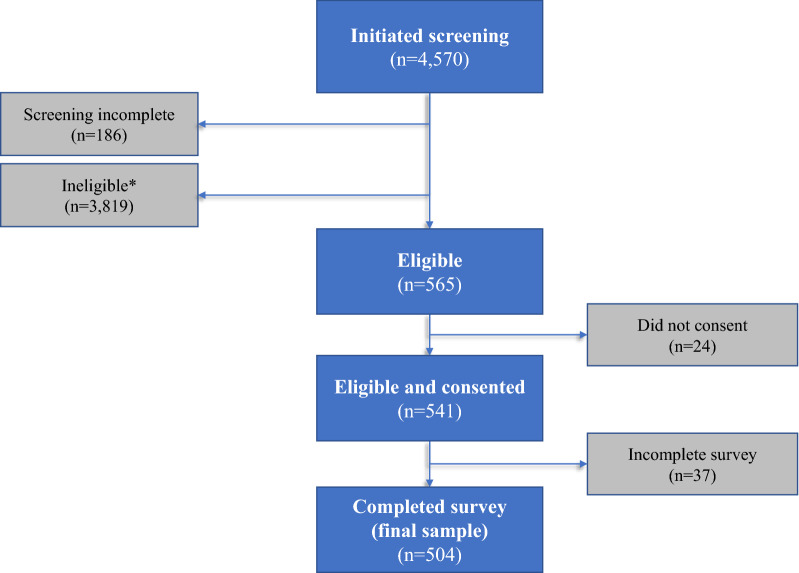
Forty participants completed the revised survey in phase 2. No problems with the survey or data were identified in phase 2, and therefore, no changes were made to the survey prior to beginning phase 3. Therefore, data from phase 2 (n = 40) and 3 (n = 4530) were combined for the final analysis data set. In phases 2 and 3, a total of 4570 individuals who were invited to complete the survey initiated the screening procedures (Fig. 1). Of those who began the screening, 186 did not complete the screening form, and 3819 were found to be ineligible, resulting in a potential sample of 565 eligible individuals. Common reasons for not being eligible included not currently being treated with injectable medication (n = 2239) and having type 1 instead of type 2 diabetes (n = 433).
Fig. 1.
Sample selection. *Reasons for ineligibility: age or geography (n = 50) (e.g., under 18, did not reside in UK or US); diagnosis (n = 833) (e.g., diagnosed with type 1 diabetes, diagnosis of T2D less than 6 months prior to the survey); medication (n = 2905) (e.g., not currently using injectable medication for T2D); and other less common reasons (n = 31) (e.g., unwilling to provide informed consent, conflict of interest due to employment at a pharmaceutical company)
Twenty-four of the individuals did not provide consent, while another 37 did not fully complete their survey. Thus, a total of 504 eligible respondents provided consent and completed the full survey. The analysis data set includes data from these 504 individuals, including 251 patients from the UK and 253 patients from the US, with 254 treated with a GLP-1 receptor agonist and 250 using an insulin pen. Table 1 summarizes the study population by phase, country, and medication type.
Table 1.
Study population by phase of study, country, and medication type
| Medication type | Phase 1: qualitative pilot survey | Phase 2: quantitative pilot survey | Phase 3: main survey | Final analysis samplea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UK | US | Total | UK | US | Total | UK | US | Total | UK | US | Total | |
| Insulin pen | 3 | 2 | 5 | 19 | 13 | 32 | 103 | 115 | 218 | 122 | 128 | 250 |
| GLP-1 RA | 2 | 3 | 5 | 1 | 7 | 8 | 128 | 118 | 246 | 129 | 125 | 254 |
| Total | 5 | 5 | 10 | 20 | 20 | 40 | 231 | 233 | 464 | 251 | 253 | 504 |
UK United Kingdom, US United States, GLP-1 RA glucagon-like peptide 1 receptor agonist
aBecause no changes were made to the survey during or following phase 2, the phase 2 data were combined with phase 3 data for the final analysis sample
Sample Characteristics
There were some demographic differences between the US and UK samples (Table 2). On average, the UK sample was somewhat younger than the US sample (50.9 vs. 59.2 years old; p < 0.001) and more likely to be male (71.3% vs. 55.3%; p < 0.001). Participants in both countries were predominantly White (US, 88.5%; UK, 98.8%). The majority of UK participants were from England (n = 217, 86.5%), while the US participants had greater geographic variation across the four regions of the country (Northeast, Midwest, South, West).
Table 2.
Sociodemographic information
| Sociodemographic questions | UK patients (N = 251) | US patients (N = 253) | Total sample (N = 504) | p valuea |
|---|---|---|---|---|
| Age (mean, SD) (years) | 50.9 (15.5) | 59.2 (13.1) | 55.1 (14.9) | < 0.001 |
| Gender (n, %) | < 0.001 | |||
| Female | 72 (28.7%) | 113 (44.7%) | 185 (36.7%) | |
| Male | 179 (71.3%) | 140 (55.3%) | 319 (63.3%) | |
| Ethnic background (n, %) | ||||
| UK categories (n, %) | – | |||
| Asian/Asian British | 3 (1.2%) | – | – | |
| White | 248 (98.8%) | – | – | |
| US categories (n, %) | – | |||
| American Indian or Alaska Native | – | 1 (0.4%) | – | |
| Asian | – | 7 (2.8%) | – | |
| Black or African American | – | 12 (4.7%) | – | |
| White | – | 224 (88.5%) | – | |
| Multipleb | – | 5 (2.0%) | – | |
| Other | – | 4 (1.6%) | – | |
| Employment status (n, %) | < 0.001 | |||
| Full-time work | 127 (50.6%) | 88 (34.8%) | 215 (42.7%) | |
| Part-time work | 18 (7.2%) | 15 (5.9%) | 33 (6.5%) | |
| Homemaker | 5 (2.0%) | 13 (5.1%) | 18 (3.6%) | |
| Student | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | |
| Unemployed | 16 (6.4%) | 8 (3.2%) | 24 (4.8%) | |
| Retired | 73 (29.1%) | 121 (47.8%) | 194 (38.5%) | |
| Other | 11 (4.4%) | 8 (3.2%) | 19 (3.8%) | |
| Education level (n, %) | 0.926 | |||
| No university degree | 119 (47.4%) | 121 (47.8%) | 240 (47.6%) | |
| University degree | 132 (52.6%) | 132 (52.2%) | 264 (52.4%) | |
| Locations in UK (n, %) | – | |||
| England | 217 (86.5%) | – | – | |
| Northern Ireland | 4 (1.6%) | – | – | |
| Scotland | 22 (8.8%) | – | – | |
| Wales | 8 (3.2%) | – | – | |
| Regions in USc (n, %) | – | |||
| Northeast | – | 56 (22.1%) | – | |
| Midwest | – | 72 (28.5%) | – | |
| South | 85 (33.6%) | – | ||
| West | 40 (15.8%) | – | ||
ap values are for analyses comparing UK patients to US patients. Statistical tests are t tests for continuous variables and chi-square analyses for categorical variables
bMultiple includes American Indian or Alaska Native + White (n = 3); Black or African American + White (n = 1); White + other race (n = 1)
cBased on published Census Bureau Regions listed (https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf)
On average, participants in this study had been living with T2D for 10.9 (± 9.1) years. Participants’ mean body mass index (calculated based on self-reported height and weight) was 31.8 (± 8.9), and the majority (n = 294, 58.3%) reported their most recent HbA1c level to be between 7.1% and 9% (when responding to a question with several categorical response options). More than half of the sample reported no diabetes complications (n = 276, 54.8%). The most commonly reported diabetes complications included nerve complications (e.g., numbness or pain in feet or hands) (n = 128, 25.4%) and eye complications (e.g., diabetic retinopathy) (n = 72, 14.3%). The most commonly reported comorbidities were high blood pressure (n = 251, 49.8%), arthritis (n = 123, 24.4%), depression (n = 113, 22.4%), and anxiety (n = 101, 20.0%).
Most of the GLP-1 receptor agonist-treated participants (n = 163, 64.2%) reported using a single-dose pen rather than a multi-dose pen, and the average length of time on their GLP-1 receptor agonist medication was 2.2 (± 2.8) years. More than half of the GLP-1 receptor agonist-treated participants reported once-weekly dosing (n = 142, 55.9%), while a smaller proportion injected themselves either twice-daily (n = 46, 18.1%) or once-daily (n = 66, 26.0%). Insulin pen users reported that they had been treated with insulin for a mean of 7.7 (± 6.5) years. Almost all participants treated with insulin (n = 223, 89.2%) reported using a prefilled injection pen that is discarded after the medication runs out (as opposed to an injection pen with replaceable medication cartridges). Frequency of insulin use was split almost evenly across the three response categories: once-daily, 38.8%; twice-daily, 30.0%; three or more times daily, 31.2%.
EQ-5D-5L results suggest that this sample was relatively healthy in terms of mobility, self-care, usual activities, and anxiety/depression with half or more (48.8–72.0%) reporting “no problems” in these four areas. However, the majority of participants (57.2%) reported either slight or moderate problems with pain/discomfort. The mean EQ-5D-5L index score was 0.73 (± 0.25), while the mean visual analogue scale score was 67.35 (± 19.58).
Perceptions of Injectable Medication
Patients reported that their doctor first recommended an injectable medication a mean of 5.5 (± 6.7) years after receiving their T2D diagnosis. Most reported that they started using injectable medication to either better control their blood sugar levels (n = 407, 80.8%) and/or because oral medication alone had not been working for them (n = 317, 62.9%) (responses to this question were not mutually exclusive). Respondents tended to start injectable medication soon after it was recommended by their physicians. The majority started their injectable medication regimen either within a week (n = 250, 49.6%) or within the same day (n = 138, 27.4%) that their doctor recommended injectable medication. Participants reported experiencing a wide range of emotions in response to their doctor’s recommendation for injectable medication, as presented in Table 3. The most commonly reported emotional responses were acceptance (n = 214, 42.5%), hopefulness (n = 182, 36.1%), and worry or concern (n = 121, 24.0%).
Table 3.
Emotional reaction to recommendation of injectable medicationa
| Emotional reaction | UK patients (N = 251) | US patients (N = 253) | Total sample (N = 504) |
|---|---|---|---|
| Acceptance | 103 (41.0%) | 111 (43.9%) | 214 (42.5%) |
| Hopeful | 87 (34.7%) | 95 (37.5%) | 182 (36.1%) |
| Worried | 59 (23.5%) | 62 (24.5%) | 121 (24.0%) |
| Scared | 60 (23.9%) | 60 (23.7%) | 120 (23.8%) |
| Unsure | 42 (16.7%) | 61 (24.1%) | 103 (20.4%) |
| Sad | 48 (19.1%) | 52 (20.6%) | 100 (19.8%) |
| Relieved | 33 (13.1%) | 29 (11.5%) | 62 (12.3%) |
| Overwhelmed | 26 (10.4%) | 31 (12.3%) | 57 (11.3%) |
| Angry | 33 (13.1%) | 22 (8.7%) | 55 (10.9%) |
| Indifferent | 40 (15.9%) | 13 (5.1%) | 53 (10.5%) |
| Denial | 31 (12.4%) | 17 (6.7%) | 48 (9.5%) |
| Guilty | 26 (10.4%) | 19 (7.5%) | 45 (8.9%) |
| Empowered | 24 (9.6%) | 18 (7.1%) | 42 (8.3%) |
| None of these | 6 (2.4%) | 4 (1.6%) | 10 (2.0%) |
aParticipants were responding to the following item: “After your doctor first recommended injectable medication, what was your emotional reaction? Please select up to three emotional states below that best describe your reaction.” The emotional reactions listed in this table were presented as options
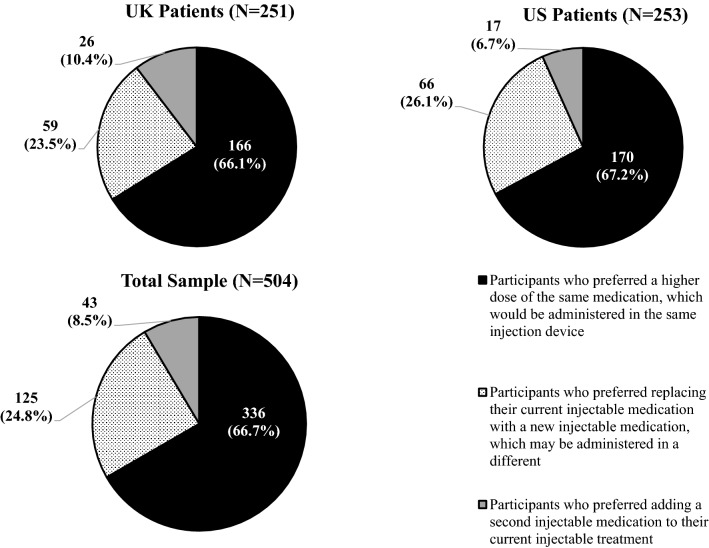
To assess comfort with their current injectable regimens, participants were asked to think about a hypothetical scenario in which they needed to change their injectable medication regimen. They were asked for their preference between three possible new regimens. Two-thirds of participants preferred a higher dose of the same medication (same injection device) (n = 336, 66.7%) over the other two options (Fig. 2). Approximately one-quarter (n = 125, 24.8%) preferred replacing their current injectable medication with a new one (potentially using a different device), while relatively few opted for adding a second injectable medication to their current injectable medication (n = 43, 8.5%).
Fig. 2.
Reaction to hypothetical scenario of needing to adjust injectable treatment regimen: preference between three treatment options. Participants were responding to the following item: “If your doctor says you need to change your injectable treatment, which one of these options would you prefer?”
Most Important Characteristics of Injectable Medication for T2D
Respondents were presented with a list of 17 characteristics of injectable medication and asked to indicate which five characteristics were most important to them when choosing a treatment for T2D (Table 4). Respondents most frequently selected confidence in administering the correct dose (n = 300, 59.5%); ease of selecting the correct dose (n = 268, 53.2%); overall ease of using the injection device (n = 239, 47.4%); frequency of injections (n = 223, 44.2%); and ease of carrying the device when it is necessary to inject away from home (n = 190, 37.7%). The characteristics that were least frequently cited as important included dose escalation (n = 79, 15.7%); handling the needle (n = 74, 14.7%); connectivity to an electronic device (n = 70, 13.9%); and the time required to prepare and inject each dose (n = 62, 12.3%).
Table 4.
Most important characteristics when choosing an injectable treatment for type 2 diabetes
| Characteristics of injectable treatmenta | Frequency (%) of respondents indicating that each treatment characteristic is important | ||
|---|---|---|---|
| UK patients (N = 251) | US patients (N = 253) | Total sample (N = 504) | |
| Confidence that you are administering the correct dose | 155 (61.8%) | 145 (57.3%) | 300 (59.5%) |
| Ease of selecting or giving yourself the correct dose of medication | 131 (52.2%) | 137 (54.2%) | 268 (53.2%) |
| Overall ease of using the injection device | 102 (40.6%) | 137 (54.2%) | 239 (47.4%) |
| Frequency of injections (e.g., daily vs. weekly) | 108 (43.0%) | 115 (45.5%) | 223 (44.2%) |
| Ease of bringing the injection device with you when it is necessary to inject away from home | 93 (37.1%) | 97 (38.3%) | 190 (37.7%) |
| Ease of storing the injection device and medication | 88 (35.1%) | 89 (35.2%) | 177 (35.1%) |
| Ease of preparing the injection device for each injection (some medications require extra steps for preparing the device before injecting) | 84 (33.5%) | 89 (35.2%) | 173 (34.3%) |
| Size of the needle | 76 (30.3%) | 84 (33.2%) | 160 (31.7%) |
| Dose timing (some medications need to be taken with meals or at the same time every day while other medications are more flexible) | 77 (30.7%) | 67 (26.5%) | 144 (28.6%) |
| The need to refrigerate (some medications need to be kept cool and others do not) | 45 (17.9%) | 60 (23.7%) | 105 (20.8%) |
| Size of the injection device (i.e., the injection pen) | 44 (17.5%) | 43 (17.0%) | 87 (17.3%) |
| Dose titration (with some medications like insulin, the dose may be increased or decreased as needed based on your blood sugar level) | 45 (17.9%) | 40 (15.8%) | 85 (16.9%) |
| Single-use vs. multi-use (some injection devices are reusable, whereas others are used once before being disposed) | 34 (13.5%) | 50 (19.8%) | 84 (16.7%) |
| Dose escalation (some medications start at a lower dose and then have one or more increases to get to a higher dose) | 46 (18.3%) | 33 (13.0%) | 79 (15.7%) |
| Handling the needle (some devices require you to attach the needle for each injection while other devices come with a pre-attached needle) | 38 (15.1%) | 36 (14.2%) | 74 (14.7%) |
| Connectivity to your phone or another electronic device (some injection devices transmit information about your injections to an online app) | 58 (23.1%) | 12 (4.7%) | 70 (13.9%) |
| Time it takes to prepare and inject each dose of medication | 31 (12.4%) | 31 (12.3%) | 62 (12.3%) |
aParticipants were responding to the following item: “When choosing an injectable treatment for T2D, you and your doctor would consider the effectiveness and safety of the possible treatments. There are also many other factors you might consider. Some of these characteristics of injectable treatments are listed below. Please select the five characteristics below that are most important to you.” The 17 characteristics of injectable treatment listed in this table were presented as options. Item text and bolding are shown as originally presented in the survey
Discussion
Over time, the process of how injectable T2D medications are taken has evolved. Results of this survey provide insight into patient perspectives of currently available injectable treatment options. Findings suggest that patients prioritize some attributes of injectable treatments over others. Attributes that patients most frequently cited as important include confidence in delivering the correct dose, ease of administering the correct dose, ease of using the injection device, and dose frequency. Attributes that appear to be less important to patients include dose escalation, handling the needle, electronic connectivity, and dose preparation time.
Current results are consistent with previous studies comparing injection devices for treatment of T2D. A range of studies assessing preference among insulin and GLP-1 receptor agonist injection devices have found that patients preferred devices that were perceived as easier to use [48, 67, 68]. As in the current study, previous research has also shown that patients value features that instill greater confidence that they are receiving the correct dose [69, 70]. The current results add to these previous findings by providing insight into preferences across a wider range of injectable treatment attributes.
These results may have implications for clinical practice. Participants reported experiencing a wide range of emotions in response to their doctor’s recommendation for starting an injectable diabetes medication, with acceptance and hopefulness being the most common reactions. Additionally, when patients were asked for their preference between possible new injection regimens, two-thirds of the sample preferred a higher dose of the same medication in the same injection device. This may be because patients are more comfortable with a familiar treatment regimen compared to a new medication with an unfamiliar device. By prescribing treatments and regimens that patients prefer, it may be possible to improve medication adherence and maximize the benefits of diabetes treatment. Physicians can discuss treatment attributes, such as those listed in Table 4, with patients to identify the optimal treatment regimen for each individual. The patient priorities highlighted in this study may also be relevant to the development of injection devices. To maximize adherence and clinical benefit, device developers can focus on attributes that are most important to patients when designing and modifying injection delivery systems.
Study limitations should be noted. First, online surveys with large samples do not allow for detailed assessment through free-text responses, and participants’ responses in this study were limited to the programmed survey choices. For example, participants reported the medication attributes that were important to them by selecting from a list of characteristics. Although this list of 17 characteristics was relatively thorough, there could be other aspects of treatment administration and injection devices that are important to patients but were not included as an option. In addition, these 17 characteristics focused exclusively on the treatment process associated with injectable medications. Efficacy and safety are the primary concerns when selecting medication for patients with T2D. The treatment process issues examined in the current study should always be considered in combination with efficacy, safety, and other potentially important treatment attributes.
Another limitation is that the sample was recruited entirely in the US and UK, and the samples in both countries were predominantly White. Patient preferences and perceptions related to their healthcare can vary by culture, country, and sociodemographics [71–73]. Therefore, generalizability of results outside these two English-speaking countries is not known. In addition, it should be noted that while the sample is not small, it was not recruited to be nationally representative in either country.
Furthermore, results for the total sample of 504 patients should be interpreted with appropriate caution because it is a heterogeneous group. For example, the total sample includes patients from two countries with substantially different healthcare systems. Aspects of the healthcare system within each country could have an impact on treatment preferences, particularly because health insurance status and cost of medication have more impact on patients in the US than in the UK. Despite these differences, however, it does appear that preferences among characteristics of injectable treatment are quite similar in the two countries, as shown in Table 4.
Another source of heterogeneity is the type of treatment that patients were receiving. This study was designed to explore preferences of a broad range of patients receiving injectable treatment for T2D, and results are presented for this combined group receiving a range of insulins and GLP-1 receptor agonists. It is possible that preferences for injection attributes could differ between patients treated with these two different classes of medications. In addition, it is possible that preferences could vary across patients treated with different types of insulin (e.g., basal insulin vs. rapid-acting analogues) or different types of GLP-1 receptor agonists. Future research may examine these preferences separately within various treatment groups.
Conclusions
In recent years, incorporating the patient voice into the drug and medical device development process has been emphasized in treatment guidelines as well as by healthcare providers, the pharmaceutical industry, and patients themselves [57, 74–78]. In part, this has been driven by regulatory agencies such as the US Food and Drug Administration advocating that patients be included as partners and co-developers from early development through post-marketing [77, 79, 80]. Similarly, in Europe, greater emphasis has been placed on bringing the patient voice into the health technology assessment process [81]. Patient surveys, such as the one in the current study, can be useful for identifying and quantifying patients’ priorities and preferences. With a better understanding of patients’ preferences, new injection delivery systems can be designed with features more likely to enhance adherence and maximize treatment benefit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants of this study.
Funding
Funding for this study, including editorial support and the Rapid Service Fee, was provided by Eli Lilly and Company.
Editorial Assistance
The authors thank Haylee Andrews and Global Perspectives (including Diana de la Puente and Paula González) for assistance with data collection; Robyn Cyr and Karen Malley for statistical programming; and Amara Tiebout of Evidera for editorial support.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors collaborated on the study design, analysis plan, interpretation of data, and/or outline of the manuscript. BC and LM drafted the manuscript text, and KB, RM, and JJ provided input and approval.
Disclosures
Louis Matza, Jessica Jordan, and Brooke Currie are employees of Evidera, a company that received funding from Eli Lilly and Company for time spent conducting this research. Kristina Boye and Raleigh Malik are employees of and own stock in Eli Lilly and Company.
Compliance with Ethics Guidelines
Participants were required to provide written informed consent before completing study procedures, and all procedures and materials were approved by an independent institutional review board (Ethical & Independent Review Services; Initial approval on March 31, 2020 [Protocol # 20021–01]; Modification approval on May 24, 2020 [Protocol # 20021-01B]). This study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent amendments. A license agreement was obtained to use the electronic version of the EQ-5D-5L in this study. Other than the EQ-5D-5L, the content of the survey was developed by the authors. Therefore, no external permissions were required to use or implement the survey.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Kristina S. Boye, Email: boye_kristina_secnik@lilly.com
Jessica B. Jordan, Email: jessica.jordan@evidera.com
Raleigh E. Malik, Email: RMalik@lilly.com
Brooke M. Currie, Email: brooke.currie@gmail.com
Louis S. Matza, Email: louis.matza@evidera.com
References
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Prevention and Control (CDC). National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA Services USDoHaH; 2014, 3
- 3.Espelt A, Borrell C, Palencia L, et al. Socioeconomic inequalities in the incidence and prevalence of type 2 diabetes mellitus in Europe. Gac Sanit. 2013;27(6):494–501. doi: 10.1016/j.gaceta.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Lin CC, Li CI, Hsiao CY, et al. Time trend analysis of the prevalence and incidence of diagnosed type 2 diabetes among adults in Taiwan from 2000 to 2007: a population-based study. BMC Public Health. 2013;13:318. doi: 10.1186/1471-2458-13-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 6.Onat A, Hergenc G, Uyarel H, Can G, Ozhan H. Prevalence, incidence, predictors and outcome of type 2 diabetes in Turkey. Anadolu Kardiyol Derg. 2006;6(4):314–21. [PubMed]
- 7.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed]
- 8.Ubink-Veltmaat LJ, Bilo HJ, Groenier KH, Houweling ST, Rischen RO, Meyboom-de JB. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: a prospective population-based study in the Netherlands (ZODIAC-1) Eur J Epidemiol. 2003;18(8):793–800. doi: 10.1023/A:1025369623365. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Prevention and Control (CDC). National Diabetes Statistics Report: 2020 estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed Oct 13, 2020.
- 10.Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab. 2017;19(11):1537–1545. doi: 10.1111/dom.12964. [DOI] [PubMed] [Google Scholar]
- 11.Evans JM, Barnett KN, Ogston SA, Morris AD. Increasing prevalence of type 2 diabetes in a Scottish population: effect of increasing incidence or decreasing mortality? Diabetologia. 2007;50(4):729–732. doi: 10.1007/s00125-006-0585-9. [DOI] [PubMed] [Google Scholar]
- 12.Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open. 2016;6(1):e010210. [DOI] [PMC free article] [PubMed]
- 13.Feingold KR. Oral and injectable (non-Insulin) pharmacological agents for type 2 diabetes. 2000 [Updated 2020 Jul 12]. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK279141/. [PubMed]
- 14.Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. 2016;7(17):354–395. doi: 10.4239/wjd.v7.i17.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giorgino F, Penfornis A, Pechtner V, Gentilella R, Corcos A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Prefer Adherence. 2018;12:707–719. doi: 10.2147/PPA.S151736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesavadev J, Saboo B, Krishna MB, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020;11(6):1251–1269. doi: 10.1007/s13300-020-00831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4(3):522–531. doi: 10.1177/193229681000400304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, Benjamin MM, Srinivasan S, et al. Battle of GLP-1 delivery technologies. Adv Drug Deliv Rev. 2018;130:113–130. doi: 10.1016/j.addr.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou AY, Trujillo JM. Comparison of usability, accuracy, preference, and satisfaction among three once-weekly GLP-1 receptor agonist pen devices. Diabetes Spectr. 2018;31(4):359–366. doi: 10.2337/ds17-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novo Nordisk. NovoLog Mix70/30 Label - 70% insulin aspart protamine suspension and 30% insulin aspart injection (rDNA origin): Highlights/Full Prescribing Information and Instructions for Use. Bagsvaerd, Denmark; Revised April 2015.
- 21.Eli Lilly and Company. Instructions for Use: Humalog KwikPen - insulin lispro injection (rDNA origin). Indianapolis, IN; Revised 2015.
- 22.Novo Nordisk. NovoPen Echo® User Guide. Tianjin, P.R. China; 2013.
- 23.Eli Lilly and Company. A simple guide to using the HumaPen Luxura HD. Basingstoke, Hampshire; February 2014.
- 24.Eli Lilly and Company. Instructions for Use: Humalog® Junior KwikPen® insulin lispro injection 100 units/mL, 3 mL single-patient-use pen. Indianpolis, IN; Revised November 2019.
- 25.Eli Lilly and Company. A simple guide to using the HumaPen Memoir. Basingstoke, Hampshire; April 2014.
- 26.AstraZeneca. Instructions for Use: Once-weekly Bydureon BCISE (exenatide extended-release), injectable suspension. For subcutaneous use only. Single-dose Autoinjector once weekly 2 mg. Revised July 2019.
- 27.Owen Mumford. Instructions for use: Autopen Classic. Oxford: Woodstock; 2011.
- 28.Eli Lilly and Company. Instructions for Use: Trulicity® (TRU-li-si-tee) (dulaglutide) injection, for subcutaneous use 0.75 mg/0.5 mL Single-Dose Pen use 1 time each week (once weekly). Indianpolis, IN; Revised September 2018.
- 29.Novo Nordisk, Food and Drug Administration (FDA). NovoPen® 3 PenMate® instruction manual. Princeton, NJ; 2005.
- 30.BD Medical. BD AutoShield™ Duo Safety Pen Needle. Mt Wellington, Auckland; 2013.
- 31.Novo Nordisk. NovoLog Label (insulin aspart [rDNA origin] injection) solution for subcutaneous use: Highlights/Full Prescribing Information and Instructions for Use. Revised February 2015.
- 32.Novo Nordisk. NovoPen 4 User Guide. Tianjin, P.R. China; 2014.
- 33.Eli Lilly and Company, ClinicalTrials.Gov. A study of LY3209590 in participants with type 2 diabetes mellitus. ClinicalTrials.gov Identifier: NCT03736785, study completion date: February 18, 2020.https://clinicaltrials.gov/ct2/show/NCT03736785?term=NCT03736785&draw=2&rank=1. Accessed Feb 22, 2021.
- 34.Rosenstock J, Bajaj HS, Janez A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107–2116. doi: 10.1056/NEJMoa2022474. [DOI] [PubMed] [Google Scholar]
- 35.Eli Lilly and Company. Full and Highlights of Prescribing Information - LYUMJEV (insulin lispro-aabc) injection, for subcutaneous or intravenous use. Indianapolis, IN; Initial US approval: 2020.
- 36.FDA approves Fiasp® for treatment of children with diabetes [press release]. Plainsboro, NJ; January 6, 2020.
- 37.Novo Nordisk. Ozempic® (semaglutide) injection 0.5 mg or 1 mg Indication and Limitations of Use. Plainsboro, NJ; 2020.
- 38.Brennan VK, Dixon S. Incorporating process utility into quality adjusted life years: a systematic review of empirical studies. Pharmacoeconomics. 2013;31(8):677–691. doi: 10.1007/s40273-013-0066-1. [DOI] [PubMed] [Google Scholar]
- 39.Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611–1622. doi: 10.2147/PPA.S90842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins A, Barnett J, Meads C, Singh J, Longworth L. Does convenience matter in health care delivery? A systematic review of convenience-based aspects of process utility. Value Health. 2014;17(8):877–887. doi: 10.1016/j.jval.2014.08.2670. [DOI] [PubMed] [Google Scholar]
- 41.Matza LS, Boye KS, Jordan JB, et al. Patient preferences in Italy: health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. Patient Prefer Adherence. 2018;12:971–979. doi: 10.2147/PPA.S159620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matza LS, Boye KS, Stewart KD, Davies EW, Paczkowski R. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17(1):774. doi: 10.1186/s12913-017-2648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toscano D, Brice J, Alfaro C. Usage and perceptions of pen injectors for diabetes management: a survey of type 2 diabetes patients in the United States. J Diabetes Sci Technol. 2012;6(3):686–694. doi: 10.1177/193229681200600325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asakura T, Suzuki S, Aranishi T, Cai Z. Comparative usability study of the dulaglutide single-use pen versus the insulin degludec FlexTouch((R)) among self-injection-naive patients with type 2 diabetes mellitus in Japan. Curr Med Res Opin. 2018;34(6):1117–1124. doi: 10.1080/03007995.2018.1448260. [DOI] [PubMed] [Google Scholar]
- 45.Bode B, Shelmet J, Gooch B, et al. Patient perception and use of an insulin injector/glucose monitor combined device. Diabetes Educ. 2004;30(2):301–309. doi: 10.1177/014572170403000223. [DOI] [PubMed] [Google Scholar]
- 46.Matfin G, Van Brunt K, Zimmermann AG, Threlkeld R, Ignaut DA. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naive patients with type 2 diabetes. J Diabetes Sci Technol. 2015;9(5):1071–1079. doi: 10.1177/1932296815583059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34(8):1457–1464. doi: 10.1080/03007995.2018.1465903. [DOI] [PubMed] [Google Scholar]
- 48.Matza LS, Boye KS, Stewart KD, et al. Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: a crossover study (PREFER) Diabetes Obes Metab. 2020;22(3):355–364. doi: 10.1111/dom.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–661. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- 50.Hixson-Wallace JA, Dotson JB, Blakey SA. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost. 2001;7(1):33–37. doi: 10.1177/107602960100700108. [DOI] [PubMed] [Google Scholar]
- 51.Morris LS, Schulz RM. Medication compliance: the patient's perspective. Clin Ther. 1993;15(3):593–606. [PubMed] [Google Scholar]
- 52.Raue PJ, Schulberg HC, Heo M, Klimstra S, Bruce ML. Patients' depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv. 2009;60(3):337–343. doi: 10.1176/ps.2009.60.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaller M, Sigurgeirsson B, Sarkany M. Patient-reported outcomes from two randomised studies comparing once-weekly application of amorolfine 5% nail lacquer to other methods of topical treatment in distal and lateral subungual onychomycosis. Mycoses. 2017;60(12):800–807. doi: 10.1111/myc.12676. [DOI] [PubMed] [Google Scholar]
- 54.Shikiar R, Rentz A, Barone J, Duncanson F, Katz E. Patient satisfaction with ofloxacin (F) and polymyxin B/Neomycin/Hydrocortisone© in the treatment of otitis externa: results from two randomized clinical trials. J Manag Care Med. 2002;6:24–27. [Google Scholar]
- 55.Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. doi: 10.1111/j.1524-4733.2004.72252.x. [DOI] [PubMed] [Google Scholar]
- 56.Shingler SL, Bennett BM, Cramer JA, Towse A, Twelves C, Lloyd AJ. Treatment preference, adherence and outcomes in patients with cancer: literature review and development of a theoretical model. Curr Med Res Opin. 2014;30(11):2329–2341. doi: 10.1185/03007995.2014.952715. [DOI] [PubMed] [Google Scholar]
- 57.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461–98. [DOI] [PubMed]
- 58.Matza LS, Boye KS, Stewart KD, Paczkowski R, Jordan J, Murray LT. Development of the diabetes injection device experience questionnaire (DID-EQ) and diabetes injection device preference questionnaire (DID-PQ) J Patient Rep Outcomes. 2018;2:43. doi: 10.1186/s41687-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rice D, Liska J, Beaudin M, Murray E, del Aguilla M, Beal A, editors. The importance of patient satisfaction with insulin device administration: does a positive patient experience drive improved diabetes self-management and adherence? American Association of Diabetes Educators; August 17–20, 2018; Baltimore, Maryland.
- 60.Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505–513. doi: 10.1177/193229681000400302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thieu VT, Robinson S, Kennedy-Martin T, Boye KS, Garcia-Perez LE. Patient preferences for glucagon-like peptide 1 receptor-agonist treatment attributes. Patient Prefer Adherence. 2019;13:561–576. doi: 10.2147/PPA.S187907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 63.EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 64.Kind P. The EuroQol Instrument: an index of health related quality of life. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials. 2. Philadelphia: Lippincott Raven; 1996. pp. 191–201. [Google Scholar]
- 65.Rabin R, Gudex C, Selai C, Herdman M. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health. 2014;17(1):70–76. doi: 10.1016/j.jval.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 66.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Clark PE, Valentine V, Bodie JN, Sarwat S. Ease of use and patient preference injection simulation study comparing two prefilled insulin pens. Curr Med Res Opin. 2010;26(7):1745–1753. doi: 10.1185/03007995.2010.489028. [DOI] [PubMed] [Google Scholar]
- 68.Wang T, Conrad KA, van Brunt K, Rees TM. Attributes influencing insulin pen preference among caregivers and patients with diabetes who require greater than 20 units of mealtime insulin. J Diabetes Sci Technol. 2016;10(4):923–931. doi: 10.1177/1932296816633232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heron L, Reaney M, Hermanns N, Abetz L, Gregg L. Perceptions of usability and design for prefilled insulin delivery devices for patients with type 2 diabetes. Diabetes Spectr. 2013;26(1):16–28. doi: 10.2337/diaspect.26.1.16. [DOI] [Google Scholar]
- 70.Pfützner A, Schipper C, Niemeyer M, et al. Comparison of patient preference for two insulin injection pen devices in relation to patient dexterity skills. J Diabetes Sci Technol. 2012;6(4):910–916. doi: 10.1177/193229681200600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DeVoe JE, Wallace LS, Fryer GE., Jr Measuring patients’ perceptions of communication with healthcare providers: do differences in demographic and socioeconomic characteristics matter? Health Expect. 2009;12(1):70–80. doi: 10.1111/j.1369-7625.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fissell RB, Fuller DS, Morgenstern H, et al. Hemodialysis patient preference for type of vascular access: variation and predictors across countries in the DOPPS. J Vasc Access. 2013;14(3):264–272. doi: 10.5301/jva.5000140. [DOI] [PubMed] [Google Scholar]
- 73.Lyratzopoulos G, Elliott M, Barbiere JM, et al. Understanding ethnic and other socio-demographic differences in patient experience of primary care: evidence from the English general practice patient survey. BMJ Qual Saf. 2012;21(1):21–29. doi: 10.1136/bmjqs-2011-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caswell N, Kuru K, Ansell D, et al. Patient engagement in medical device design: refining the essential attributes of a wearable, pre-void, ultrasound alarm for nocturnal enuresis. Pharmaceut Med. 2020;34(1):39–48. doi: 10.1007/s40290-019-00324-w. [DOI] [PubMed] [Google Scholar]
- 75.de Bekker-Grob EW, Berlin C, Levitan B, et al. Giving patients’ preferences a voice in medical treatment life cycle: the PREFER public-private project. Patient. 2017;10(3):263–266. doi: 10.1007/s40271-017-0222-3. [DOI] [PubMed] [Google Scholar]
- 76.Getz K. Reflections on the evolution of patient engagement in drug development. Pharm Med. 2019;33(3):179–185. doi: 10.1007/s40290-019-00284-1. [DOI] [PubMed] [Google Scholar]
- 77.Pitts PJ. Towards meaningful engagement for the patient voice. Patient. 2019;12(4):361–363. doi: 10.1007/s40271-019-00366-x. [DOI] [PubMed] [Google Scholar]
- 78.Pushparajah DS. Making patient engagement a reality. Patient. 2018;11(1):1–8. doi: 10.1007/s40271-017-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chalasani M, Vaidya P, Mullin T. Enhancing the incorporation of the patient’s voice in drug development and evaluation. Res Involvement Engag. 2018;4(1):10. doi: 10.1186/s40900-018-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Food and Drug Administration (FDA). FDA patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making. Silver Spring, MD: Center for Drug Evaluation and Research (CDER); June 18, 2020.
- 81.Lee A, Jones J, Brown A, Macfarlane K, Fox JG. Abstract PHP274: increasing transparency and the patient voice in HTA of new medicines. Value Health. 2015;18:A335–A766. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.