Abstract
Carney complex (CNC) is a rare, autosomal dominant multiple neoplasia syndrome. Although cutaneous myxomas commonly occur in CNC patients, intraoral myxomas are extremely rare. We present a case of a palatal myxoma in a 21-year-old female patient with CNC, along with a review of the pertinent literature. She presented with a sessile nodule on the hard palate that microscopically showed a multilobulated and highly vascularized myxomatous tissue composed of loosely-arranged spindle, polygonal, and stellate cells, suggestive of myxoid neurofibroma. Six years after the oral lesion was removed, she presented with a growth hormone (GH)-producing pituitary adenoma, a cardiac myxoma, two cutaneous myxomas on the lower abdomen area, and one myxoma in the vaginal mucosa. Therefore, the final diagnosis of the palatal lesion was of a soft tissue myxoma related to CNC. The patient remains on close follow-up, with no recurrences of the palatal myxoma after 7 years.
Keywords: Carney complex, Myxoma, Oral myxoma, Palatal myxoma, Mouth, Palate
Introduction
Carney complex (CNC, OMIM 160980) is an autosomal dominant syndrome characterized by skin and mucosal pigmentations, cardiac and cutaneous myxomas, and endocrine and non-endocrine tumors [1]. This condition was first described in 1985 as, “the complex of myxomas, spotty pigmentation, and endocrine overactivity”, in patients presenting with cardiac myxomas, cutaneous alterations (pigmented lesions and/or cutaneous myxomas), and endocrine disorders [primary pigmented nodular adrenocortical disease (PPNAD), testicular tumors, and GH-producing pituitary adenoma] [2].
Most cases of CNC are caused by mutations in the protein kinase cAMP-dependent type I regulatory subunit alpha (PRKAR1A) gene, located in the long arm of chromosome 17 (17q24.2), leading to uncontrolled cell proliferation in different parts of the body [3, 4]. Approximately two thirds of CNC cases are familial, and one third are sporadic [5]. Around 80% of patients with CNC carry mutations on the PRKAR1A gene [5, 6], and more rarely in chromosome 2p16 (CNC type 2), but PRKACA or PRKACB copy number gains have been also reported. However, the cause of most PRKAR1A-negative cases remains unknown [7].
Oral manifestations of CNC include spotty pigmentations on the lips and oral mucosa, and more rarely, oral myxomas affecting primarily the palate as well demarcated masses. Microscopically, these myxomas are circumscribed—but not encapsulated—hypocellular tumors with a myxoid and highly vascularized stroma [8]. Herein we report a case of a palatal myxoma in a CNC patient, along with a review of the literature focused on the oral manifestations of CNC.
Case Report
A 21-year-old Guatemalan woman was referred to our service in 2013 for evaluation of an asymptomatic palatal nodule. The patient was in the 29th week of pregnancy; otherwise, her medical history was unremarkable. At oral examination, the patient presented with a nodular lesion on the hard palate, measuring 2.5 × 2 × 1 cm, with a rubbery consistency and a smooth, normally-colored surface (Fig. 1a). The patient also showed small café-au-lait spots on the lower back (Fig. 1b).
Fig. 1.
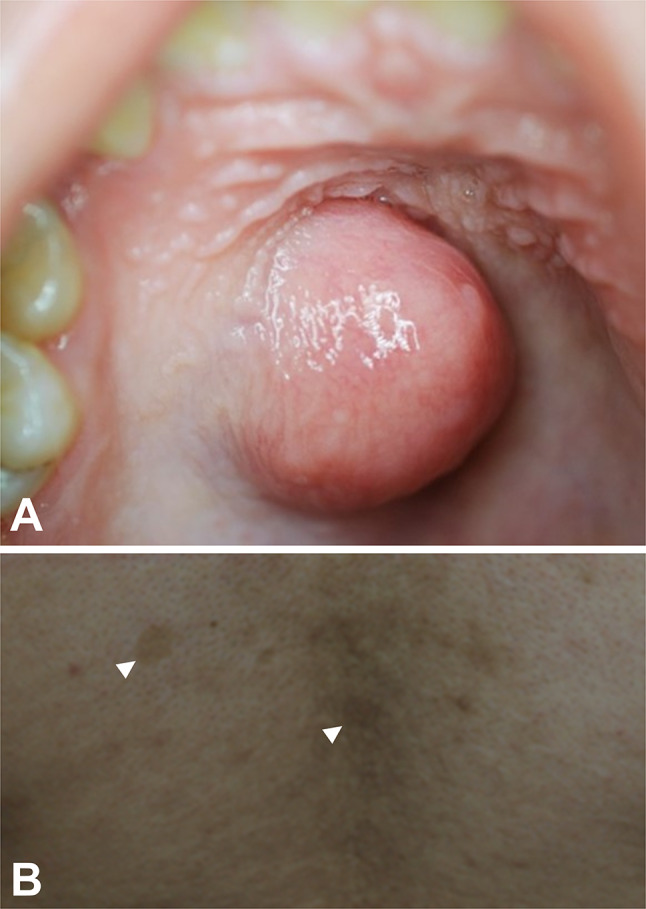
Clinical features of a palatal soft tissue myxoma in a patient with Carney Complex. a Nodular lesion on the right hard palate, presenting a rubbery consistency, normal-colored surface, and measuring 2.5 × 2 × 1 cm. b Small café-au-lait spots on the lower back
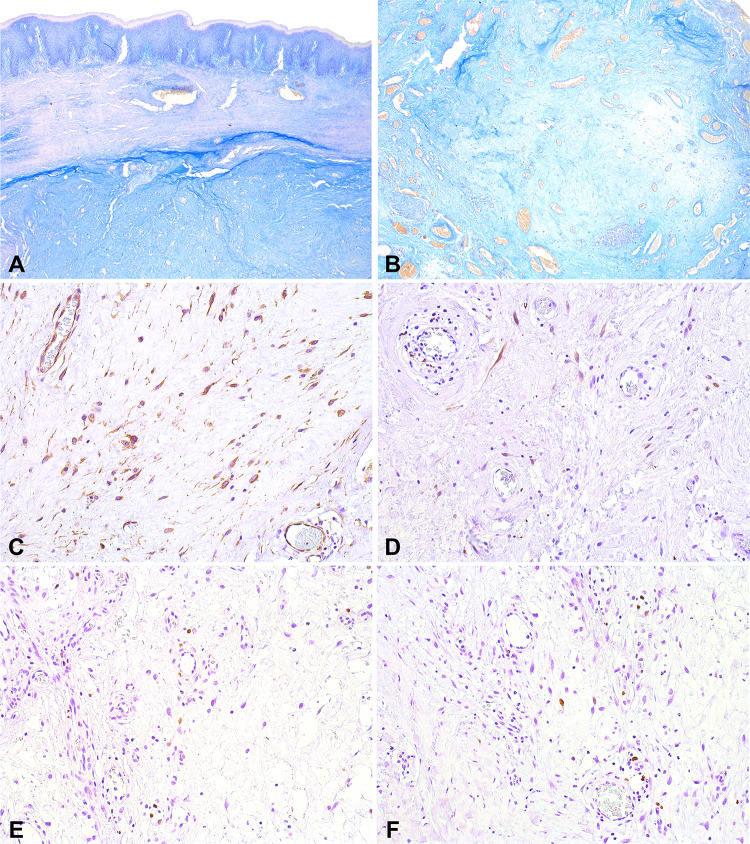
The palatal lesion was surgically removed under the presumed clinical diagnosis of pleomorphic adenoma. Microscopic examination revealed a multilobulated myxomatous tissue composed of loosely-arranged spindle, polygonal, and stellate cells. The lesion was highly vascularized, and a perivascular chronic inflammatory infiltrate was commonly found. (Fig. 2). Lobules of normal minor salivary glands were also present. Alcian Blue (pH 2.7) histochemical reaction revealed mild positivity in the myxoid areas (Fig. 3a, b). Immunohistochemical analysis showed diffuse positivity for vimentin (mouse monoclonal, Vim 3B4, 1:300, DAKO), and scattered positivity for S100 (rabbit polyclonal, 1:10,000, DAKO) (Fig. 3c, d). The proliferation index assessed by Ki67 (mouse monoclonal, MIB-1, 1:100, DAKO) was lower than 5% (Fig. 3e, f). The tumor cells were negative for CD34 (mouse monoclonal, QBEnd-10, 1:50, DAKO), SMA (mouse monoclonal, 1A4, 1:300, DAKO), and calponin (mouse monoclonal, CALP, 1:300, DAKO). At this point, the diagnosis of myxoid neurofibroma was considered, possibly in association with neurofibromatosis type 1 (NF1), but this diagnosis was not confirmed.
Fig. 2.
Microscopic features of a palatal soft tissue myxoma associated with Carney Complex. a Multilobulated, myxomatous lesion composed of loosely-arranged spindle, polygonal, and stellate cells (HE, orig. mag. 25x). b Myxoid areas presenting sparse cells and thin-walled blood vessels (HE, orig. mag. 50x). c Some areas presented with higher cellularity in a more collagenous stroma (HE, orig. mag. 100x). d Perivascular inflammatory infiltrate was a striking feature (HE, orig. mag. 100x). e–f Higher-power view of spindle and stellate cells in a myxoid stroma (HE, orig. mag. 200x)
Fig. 3.
Histochemical and immunohistochemical features of a palatal soft tissue myxoma associated with Carney Complex. a–b Mild Alcian Blue (pH 2.7) positivity in the myxoid areas (AB, orig. mag. 50x). c Diffuse positivity for vimentin (DAB, orig. mag. 200x). d Focal S100 positivity in scattered spindle cells (DAB, orig. mag. 200x). e–f Proliferation index (Ki67) was lower than 5% (DAB, orig. mag. 200x)
After 6 years, the patient returned to our service for reevaluation, but this time her medical history included a GH-producing pituitary adenoma and a cardiac myxoma. Clinical examination revealed acromegaly (Fig. 4a), a chest scar from the removal of the cardiac myxoma in 2018 (Fig. 4b), acanthosis nigricans in both axillae (Fig. 4c), two cutaneous myxomas on the lower abdomen area (Fig. 4d), and one myxoma in the vaginal mucosa (Fig. 4e). Thus, the patient was diagnosed with CNC, and the final diagnosis of the palatal lesion was of an oral soft tissue myxoma related to CNC. After 7 years of follow up, the palatal myxoma has not recurred, and no other oral lesions have developed. The patient remains on close follow-up by medical and dental teams.
Fig. 4.
Clinical features of Carney Complex. a acromegalic features due to GH-producing pituitary adenoma. b Chest scar from the removal of a cardiac myxoma c Acanthosis nigricans in the left axilla. d Two cutaneous myxomas in the lower abdomen and pelvic areas. e Mucosal myxoma in the vaginal region
We reviewed the literature of the oral manifestations of CNC using 3 databases (PubMed/MEDLINE, Scopus, and EMBASE) with the following terms: (“carney complex" OR "carney syndrome") AND ("oral cavity" OR mouth OR palate OR tongue OR lip OR gingiva OR head OR neck). The search was performed on July 29, 2020. Only articles published after the initial description of the syndrome in 1985 were included. A total of 198 articles were found (PubMed/MEDLINE = 59, Scopus = 93, EMBASE = 50). After removal of duplicates (n = 64) using a reference manager (Mendeley Desktop ©, Version 1.19.4, 2008–2019 Mendeley Ltd.), 134 articles were considered for inclusion. After reading titles and abstracts, 114 articles were excluded due to several reasons (e.g. conference abstracts, reviews, patients without CNC, or articles written in languages other than English, Spanish, French or Portuguese). Then, 20 articles were fully read, and only 11 presented sufficient documentation to be included in this review, having reported oral manifestations in 14 CNC patients (Table 1) [8–18].
Table 1.
Oral manifestations in Carney complex patients
| Study (year) | Sex | Age | Systemic alterations | Family history/genetic confirmation | Oral manifestations |
|---|---|---|---|---|---|
| Cook, Lund, and Carney. (1987) | M | 34 | Left atrial myxoma; cutaneous myxomas; lentigines in the face and hands | Mother was heavily freckled; died of mammary carcinoma | Lip pigmentation; Palatal myxoma |
| M | 14 | Pigmented skin lesions; myxoid skin lesions; left atrial myxoma | Parents and brothers heavily “freckled” | Palatal and tongue myxomas | |
| F | 29 | Cardiac myxomas; myxoid fibroadenoma of the breast | Sister with facial lentigines; died of cardiac myxoma | Lip pigmentation; Palatal myxoma | |
| F | 34 | Pigmented spots on facial skin; cardiac myxomas; myxoid fibroadenoma of the breast; cutaneous myxomas; Cushing´s syndrome | Mother and siblings with facial lentigines. One sibling with acromegaly caused by pituitary adenoma | Spots of dark pigmentation on the right buccal mucosa | |
| Reza-Albarrán et al. (1999) | M | 29 | Calyceal and left ureteral urolithiasis; skin pigmentations; Cushing´s syndrome due to PPNAD; adrenal angiomyelolipoma; | Siblings presented Cushing’s syndrome, myxomas, and lentigines | Multiple lentigines on the lips vermilion border |
| Takahashi and Hida (2002) | F | 12 | Angiomyxoma in nasal cavity; cutaneous angiomyxomas; GH-producing pituitary adenoma | No family history | Multiple lentigines on the lower lip mucosa |
| Yoon and Shon. (2003) | F | 27 | Cushing´s syndrome due to PPNAD; spotty skin pigmentation; pituitary adenoma; breast and thyroid nodules | Family history of skin pigmentation | Pigmented spots on the lips; pigmented macula on the left buccal mucosa |
| Hachisuka et al. (2006) | M | 26 | Cardiac myxomas; cutaneous myxomas; calcifications of the testes | No family history | Pigmented macula on the lower lip mucosa |
| Kacerovska et al. (2009) | M | 40 | Multiple cutaneous myxomas; cardiac myxomas; spotty pigmentation at typical sites; blue nevus; lipoma; multiple calcifications in both testes | No family history; heterozygous shift mutation c.796dupA in exon 10 of the PRKAR1A gene | Spotty pigmentation on the lower lip |
| Vandersteen et al. (2009) | M | 15 | Cardiac myxomas; cutaneous angiomyxoma; testicular Sertoli tumor | R288X mutation in the PRKAR1A gene | Lentigines on the lower lip and buccal mucosa |
| Richey et al. (2014) | M | 18 | LCCSCT in the right testicle; multiple lentigines in the face and oral mucosa | No family history | Superficial angiomyxoma in the upper lip |
| Liu et al. (2017) | M | 16 | Spotty skin pigmentations on the face and sclera; Cushing’s features due to PPNAD, microcalcifications of the testes | Heterozygous C > T substitution in PRKAR1A exon 3 | Multiple lentigines on the lips |
| Takigami et al. (2017) | F | 51 | Cardiac myxoma, acromegaly, PPNAD, mammary nodules | Daughter has confirmed CNC; mutation g.106901(c.124) C > T hetero p.Arg 42 Ter in PRKAR1A gene | Large pigmented macule on the lower lip |
| Naito et al. (2019) | F | 14 | Cardiac myxoma, spotty skin pigmentations on the face, GH-producing adenoma, pituitary apoplexy | No mutation in PRKAR1A gene confirmed by genetic examination | Lower lip pigmentations |
GH growth hormone, LCCSCT large cell-calcifying Sertoli cell tumor, PPNAD primary pigmented nodular adrenocortical disease
Discussion
Carney complex is a rare multiple neoplasia syndrome characterized by skin and mucosal pigmentation, cardiac and cutaneous myxomas, and other endocrine and non-endocrine tumors [7]. CNC phenotype varies from pauci-symptomatic to patients presenting multiple manifestations leading to a high risk of morbidity and mortality, especially due to strokes and pulmonary edema caused by cardiac myxomas [6].
According to our literature review, myxomas affecting the oral cavity are extremely rare in CNC patients. Spotty pigmentations on the lips are the most common feature of CNC on the oral region, followed by only 3 cases described in the buccal mucosa [8, 12, 15]. Seventeen out of 40 patients described by Carney et al. (1985) presented pigmented spots on the lips [2]. In 338 CNC patients described by Stratakis et al. (2001), spotty skin pigmentation was the most frequent manifestation of CNC, occurring in 77% of the cases. In the same study, 6 myxomas affecting the oral cavity are mentioned (3 in the tongue and 3 in the hard palate), but without further details [1]. Cook et al. (1987) described 3 patients presenting myxomas in the palate, 2 sessile and 1 pedunculated. Besides the palatal myxoma, one patient also presented with a tongue myxoma. Moreover, one of the palatal lesions recurred 3 years after the initial excision [8].
Interestingly, our patient did not present with the classic skin manifestations of CNC, represented by spotty pigmentation (lentigines) with typical distribution (perioral and periocular zones, conjunctiva, and vaginal mucosa). Instead, small café-au-lait spots and acanthosis nigricans were present. The latter has been described in CNC patients [2], although there is no clear association to the condition itself. Café-au-lait spots are considered suggestive or possibly associated with CNC, but not diagnostic [1]. Conversely, they are associated with several other conditions, including but not limited to McCunne-Albright syndrome, neurofibromatosis (types 1 and 2), and Watson syndrome [7]. This feature was the main reason for our patient being initially considered as having neurofibromatosis and myxoid neurofibroma of the palate. As a matter of fact, CNC had been previously described as NAME syndrome, when the cutaneous myxomas were initially considered as myxoid neurofibromas [19].
Neurofibroma is the most common intraoral neural neoplasm, affecting mainly the gingiva and palate of women, with a wide age range, occurring sporadically or in association to NF1 [20]. Although neurofibromas may present with a variable S100 expression due to its cellular heterogeneity [21], the present case showed S100 expression only in scattered nerve spindle cells. Considering that CNC shares features with several other conditions, diagnostic criteria were proposed in 2001 (Table 2), and must be thoroughly followed to avoid misdiagnoses [1]. In this context, Richey et al. (2014) reported a case of a 18-year-old male with a previous diagnosis of Peutz-Jeghers syndrome due to multiple lentigines on the face and oral mucosa; however, a myxoma in the upper lip along with the history of a large cell-calcifying Sertoli cell tumor (LCCSCT) in the testicle, and absence of intestinal polyps led to the ultimate diagnosis of CNC [16].
Table 2.
Carney Complex (CNC) diagnostic criteria proposed by Stratakis, Kirschner, and Carney (2001)
| Features | Present case |
|---|---|
| Diagnostic criteria | |
| 1. Spotty skin pigmentation with a typical distribution (lips, conjunctiva and inner or outer canthi, vaginal and penile mucosa) | Absent |
| 2. Myxoma (cutaneous and mucosal)a | Present (two cutaneous and two mucosal myxomas) |
| 3. Cardiac myxomaa | Present |
| 4. Breast myxomatosisa or fat-suppressed magnetic resonance imaging findings suggestive of this diagnosis | Absent |
| 5. PPNADa or paradoxical positive response of urinary glucocorticosteroids to dexamethasone administration during Liddle’s test | Absent |
| 6. Acromegaly due to GH-producing adenomaa | Present |
| 7. LCCSCTa or characteristic calcification on testicular ultrasonography | Not applicable |
| 8. Thyroid carcinomaa or multiple, hypoechoic nodules on thyroid ultrasonography, in a young patient | Absent |
| 9. Psammomatous melanotic schwannomaa | Absent |
| 10. Blue nevus, epithelioid blue nevus (multiple)a | Absent |
| 11. Breast ductal adenoma (multiple)a | Absent |
| 12. Osteochondromyxomaa | Absent |
| Supplemental criteria: | |
| 1. Affected first-degree relative | Absent |
| 2. Inactivating mutation of the PRKAR1A gene | Not evaluated |
To confirm a diagnosis of CNC, a patient must either: (1) exhibit two diagnostic features or (2) exhibit one diagnostic feature and one of the supplemental criteria [1]
aMicroscopically confirmed. GH growth hormone, LCCSCT large cell-calcifying Sertoli cell tumor, PPNAD primary pigmented nodular adrenocortical disease
In summary, oral manifestations of CNC are uncommonly considered, although the perioral skin pigmentations are key to the diagnosis. Unfortunately, the oral cavity may have been neglected in the largest studies evaluating CNC patients. Oral lesions may be important for early diagnosis of CNC, including myxomas of the palate and tongue, permitting an earlier and more appropriate treatment of this life-threatening syndrome.
Acknowledgement
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, and by São Paulo Research Foundation (FAPESP)–Grant No. #2018/24715-2.
Compliance with Ethical Standards
Conflict of interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 2.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, et al. Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest. 2000;106:R31–R38. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 5.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase ( PRKAR1A ): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espiard S, Vantyghem M-C, Assié G, Cardot-Bauters C, Raverot G, Brucker-Davis F, et al. Frequency and incidence of carney complex manifestations: a prospective multicenter study with a three-year follow-up. J Clin Endocrinol Metab. 2020;105:e436–e446. doi: 10.1210/clinem/dgaa002. [DOI] [PubMed] [Google Scholar]
- 7.Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015;173:M85–97. doi: 10.1530/EJE-15-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook CA, Lund BA, Carney JA. Mucocutaneous pigmented spots and oral myxomas: the oral manifestations of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Oral Surg Oral Med Oral Pathol. 1987;63:175–183. doi: 10.1016/0030-4220(87)90308-2. [DOI] [PubMed] [Google Scholar]
- 9.Reza-Albarrán AA, Gómez-Pérez FJ, López JC, Herrera M, Gamboa-Dominguez A, Keirns C, et al. Myelolipoma: a new adrenal finding in Carney’s complex? Endocr Pathol. 1999;10:251–257. doi: 10.1007/BF02738887. [DOI] [PubMed] [Google Scholar]
- 10.Naito Y, Mori J, Tazoe J, Tomida A, Yagyu S, Nakajima H, et al. Pituitary apoplexy after cardiac surgery in a 14-year-old girl with Carney complex: a case report. Endocr J. 2019;66:1117–1123. doi: 10.1507/endocrj.EJ19-0183. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Hida T. Carney complex: report of a Japanese case associated with cutaneous superficial angiomyxomas, labial lentigines, and a pituitary adenoma. J Dermatol. 2002;29:790–796. doi: 10.1111/j.1346-8138.2002.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoon HD, Shon HS. A typical Korean case of Carney complex. Korean J Intern Med. 2003;18:260–265. doi: 10.3904/kjim.2003.18.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hachisuka J, Ichikawa M, Moroi Y, Urabe K, Furue M. A case of Carney complex. Int J Dermatol. 2006;45:1406–1407. doi: 10.1111/j.1365-4632.2006.02889.x. [DOI] [PubMed] [Google Scholar]
- 14.Kacerovska D, Sima R, Michal M, Hes O, Roucka P, Zarybnicka M, et al. Carney complex: a clinicopathologic and molecular biological study of a sporadic case, including extracutaneous and cutaneous lesions and a novel mutation of the PRKAR1A gene. J Am Acad Dermatol. 2009;61:80–87. doi: 10.1016/j.jaad.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Vandersteen A, Turnbull J, Jan W, Simpson J, Lucas S, Anderson D, et al. Cutaneous signs are important in the diagnosis of the rare neoplasia syndrome Carney complex. Eur J Pediatr. 2009;168:1401–1404. doi: 10.1007/s00431-009-0935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richey JD, Bradish JR, Lacy SR, Warren S. Carney syndrome in a patient previously considered to have Peutz-Jeghers syndrome. J Am Acad Dermatol. 2014;70:e44–e46. doi: 10.1016/j.jaad.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Tong D, Liu G, Yi Y, Zhang D, Zhang J, et al. Carney complex with PRKAR1A gene mutation. Medicine (Baltimore) 2017;96:e8999. doi: 10.1097/MD.0000000000008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takigami M, Kawata M, Kintsu M, Kodaira M, Sogabe K, Kato Y, et al. Familial Carney complex with biatrial cardiac myxoma. J Cardiol Cases. 2017;15:155–157. doi: 10.1016/j.jccase.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atherton DJ, Pitcher DW, Wells RS, MacDonald DM. A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: the NAME syndrome. Br J Dermatol. 1980;103:421–429. doi: 10.1111/j.1365-2133.1980.tb07266.x. [DOI] [PubMed] [Google Scholar]
- 20.Alotaiby FM, Fitzpatrick S, Upadhyaya J, Islam MN, Cohen D, Bhattacharyya I. Demographic, clinical and histopathological features of oral neural neoplasms: a retrospective study. Head Neck Pathol. 2019;13:208–214. doi: 10.1007/s12105-018-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.do Nascimento GJF, de Pires Rocha DA, Galvão HC, de Lopes Costa AL, de Souza LB. A 38-year review of oral schwannomas and neurofibromas in a Brazilian population: clinical, histopathological and immunohistochemical study. Clin Oral Investig. 2011;15:329–335. doi: 10.1007/s00784-010-0389-7. [DOI] [PubMed] [Google Scholar]