Abstract
Malignant parotid tumors account for approximately 20% of all parotid lesions. In addition to the various primary parotid lesions there are secondary parotid malignancies, such as metastases or lymphomas. Data on histopathological distribution of all malignancies—including secondary parotid lesions—is limited. Recent evidence indicated a rising surgical incidence of secondary parotid malignancies. This study aims to review the distribution of malignancies in parotid resections from a salivary gland center. A retrospective review of prospectively collected data for all patients who had received parotidectomy between 2014 and 2019 was performed. Histopathological distribution was displayed separately for all parotid malignancies and for primary parotid malignancies. Further, patients` characteristics were compared between benign and malignant parotid lesions and between the two most common malignant parotid lesions. Out of 777 patients, 614 (78.9%) patients had a benign and 164 (21.1%) patients had a malignant parotid lesion. The most common parotid malignancy was metastatic cutaneous squamous cell carcinoma (cSCC) accounting for 35.4% of all parotid malignancies. 71.5% of all malignant lesions were secondary malignancies. Patients with metastatic cSCC were significantly older (p < 0.001) and significantly more likely to be male (p < 0.001) than patients with primary parotid malignancies. No significant difference was found when the lesion size of metastatic cSCC was compared to primary parotid malignancies (p = 0.216). The present study shows the high prevalence of secondary parotid malignancies in patients who had received parotidectomy. Furthermore, it confirms a rising surgical incidence of metastatic cSCC to the parotid gland in a series from a salivary gland center. At this time, parotid surgery for malignant lesions is more likely to be performed for metastases than for primary parotid malignancies.
Keywords: Parotid tumor, Parotid malignancy, Cutaneous squamous cell carcinoma, Parotid metastasis, Head and neck surgery
Background
Parotid tumors are rare, as they represent just up to 3% of all tumors in the head and neck. It is estimated that around 12 to 21% of these tumors are malignant. [1–4] Generally, the therapy of choice for parotid masses is parotidectomy with differing extent depending on the tumor’s entity and size.
Preoperative differentiation between benign and malignant lesions can be challenging. It has been shown that primary malignant parotid tumors compared to benign ones are associated with demographic variables such as higher age [1, 5] and with clinical variables such as facial nerve palsy and pain [6]. While up to 95% of benign parotid tumors were shown to be Warthin's tumor or pleomorphic adenoma [4, 5], histological distribution in malignant parotid tumors is more varied. Overall, 22 histopathological entities of primary malignant parotid tumors have been described that are much more evenly distributed than in benign parotid tumors [7]. Mucoepidermoid carcinoma seems to be the most common histopathological subtype of primary parotid malignancies [1, 8–10]. Most studies that describe histopathological epidemiology of parotid malignancies exclusively report primary malignant tumors [1, 5, 8, 9]. Apart from these primary parotid malignancies, there is a various number of secondary parotid malignancies. The parotid gland is the last of salivary glands to become encapsulated and this results in entrapment of lymphoid tissue within the parotid and entrapment of parotid ducts/acini within the periparotid lymph nodes parenchyma. Therefore, the parotid gland is the only salivary gland that contains lymph nodes. Consequently, secondary parotid malignancies can either occur as lymph node metastases or by infiltration of the parotid parenchyma. Particularly for populations with intense sun exposure (especially Australia), secondary parotid malignancies historically account for a high proportion of all parotid malignancies of up to 72–77%. This is mainly due to the high number of parotid metastasis by cutaneous squamous cell carcinomas (cSCC) found in this population [11, 12]. While limited, the data on secondary parotid malignancies from geographical regions other than Australia does indicate that 25% of parotid malignancies are of non-parotid origin (found in a population treated between 1997 and 2004 in Spain) [13]. More current data suggests a rising surgical incidence of secondary parotid malignancies in central Europe [14].
Therefore, this study aims to provide a current overview of histopathology of all malignancies, including secondary parotid malignancies, found in a series of patients that was treated surgically for a parotid mass in a European high-volume center.
Material and Methods
A retrospective review of prospectively collected patient data was performed, with these patients having been treated surgically for a parotid mass at the Department of Otolaryngology of the University Hospital of Augsburg between March 2014 and November 2019. Demographic (age at the time of operation and gender), imaging (size of tumor and side), operation-specific (approach) as well as histopathological data was obtained prospectively for each patient. The tumor’s size was defined by measuring the longest diameter, while the side was noted as right, left or bilateral. The surgical approach for the oncological resection of the lesion was obtained from the surgery protocol. Histopathology was recorded for each tumor by reviewing the pathologist's reports and verified by reviewing the patient’s clinical records. The histopathological standard workup for tumors occurring in the parotid gland included an essential morphological evaluation based on Hematoxylin and Eosin (H&E) and Periodic Acid–Schiff (PAS) staining. In cases where the diagnosis was readily made on conventional staining, an immunohistochemical panel comprised p63, cytokeratin 7, cytokeratin 5/14, and CD117. DOG1 was used in cases where acinic carcinoma was suspected. PanTRK immunohistochemistry was used since 2019 in malignant cases and was not restricted to secretory carcinoma. In cases where a metastatic origin had to be considered, a broad spectrum of further antibodies, including neuroendocrine markers was used. To confirm mucoepidermoid carcinoma in doubtful cases, MAML2-FISH analysis was performed. Approval of the ethics committee of the University Hospital of Augsburg was obtained for the study (BKF: 2018–15) and all patients provided written consent for their participation in this study. Firstly, demographic and imaging data was compared between benign and malignant parotid tumors descriptively and differences were tested for statistical significance. Secondly, histopathological distribution of all parotid malignancies was analyzed and displayed descriptively. Age, gender, mean lesion size, and surgical approach were analyzed for statistical differences between the two most frequent entities metastatic cSCC and primary parotid malignancy. Lastly, the distribution of histopathological entities of primary parotid malignancies was analyzed and displayed descriptively. The Mann–Whitney-U-test was used for metric, non-normally distributed variables, whereas the Chi-square-test was utilized for nominal variables, respectively. Statistical analyzes were performed using SPSS software version 19.0 (IBM, Armonk, NY).
Results
Table 1 displays basic demographic characteristics and sonographic data of patients treated surgically for benign and malignant parotid tumors as well as results of analyses regarding statistical comparison of these data.
Table 1.
Demographic/imaging data of patients with benign and malignant parotid tumors
| Independent variable | Benign tumors | Malignant tumors | Significance p < 0.05 |
|---|---|---|---|
| Number of patients | 613 | 164 | |
| Mean age (years) (SD) | 57.8 (15.2) | 69.7 (17.4) | U = 26,698.0; p < 0.001a |
| Male n (%) | 363 (59.2) | 112 (68.3) | X2 = 4,485; p = 0.034a |
| Female n (%) | 250 (40.8) | 52 (31.7) | |
| Right side n | 277 | 75 | X2 = 3,189; p = 0.203 |
| Left side n | 311 | 87 | |
| Both sides | 25 | 2 | X2 = 3,153; p = 0.076 |
| Size (mm) (SD) | 28.5 (15.4) | 32.2 (20.1) | U = 41,088.0; p = 0.132 |
SD standard deviation, n number of patients
aStatistically significant result
Overall, 777 patients—all of them having been—treated surgically for parotid masses between March 2014 and November 2019 were included in this study. Of these 613 (78.9%) showed a benign and 164 (21.1%) showed a malignant tumor. Patients with malignancies were significantly older than those with benign tumors (p < 0.001; 69.7 ± SD 17.4 vs 57.8 ± SD 15.2 years). Benign and malignant parotid tumors were both found more often in males than in females (59.2% vs 40.8% and 68.3 vs 31.7%). Furthermore, males showed a significantly higher likelihood of parotid malignancy than females and vice versa: females showed a significantly higher likelihood of a benign parotid tumor than males, respectively (p = 0.034). Benign parotid tumors were localized on the right side in 277 cases and on the left side in 311 cases while a malignancy was found on the right side in 75 cases and on the left side in 87 cases. As expected, there was no significant difference concerning the side of lesion when comparing benign and malignant parotid tumors (p = 0.203). 25 patients showed bilateral manifestation of a benign parotid lesion and two patients (one patient with chronic lymphatic leukemia and, one patient with metastases of squamous cell carcinoma) showed bilateral manifestation of parotid malignancy. In terms of bilaterality no significant difference was found between malignant and benign tumors (p = 0.076).
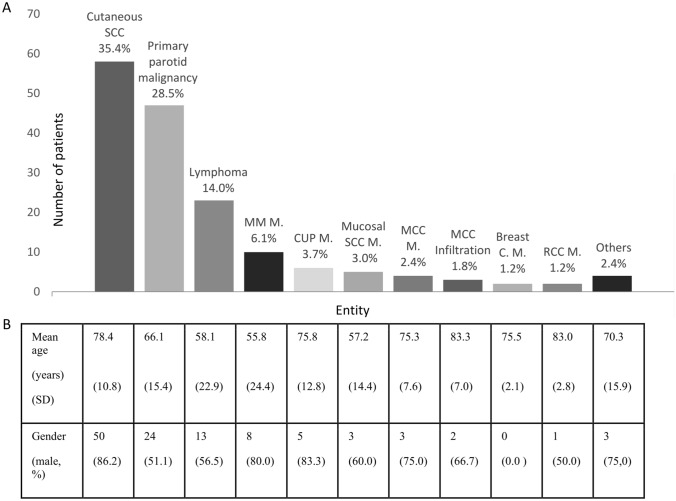
Figure 1 displays the histopathological distribution of parotid malignancies found in all 164 patients as well as their mean age and gender. 71.5% of all malignant parotid lesions were secondary malignancies while 28.5% were primary malignancies. Among the secondary malignancies, the most common entity was infiltration/metastasis of cutaneous squamous cell carcinoma (SCC) (35.4%), followed by lymphomas (14.0%), metastases of malignant melanoma (MM) (6.1%), carcinoma of unknown primary syndrome (CUP syndrome) (3.7%), metastases of mucosal SCC (3.0%), metastases of Merkel cell’s carcinomas (2.4%), infiltration by Merkel cell’s carcinomas (1.8%), metastases of breast cancer (1.2%) and metastases of renal cell carcinomas (1.2%). Rare cases included one infiltration by a basal cell carcinoma, one Langerhans' histiocytosis, one sarcoma, and one metastasis of a sinonasal adeno carcinoma.
Fig. 1.
Descriptive data on distribution of all parotid malignancies and basic demographic variables for each abovementioned subgroup. a All parotid malignancies. b Demographic variables. SCC squamous cell carcinoma, MM malignant melanoma, MCC Merkel cell’s carcinoma, RCC renal cell carcinoma, C carcinoma, M metastases, CUP-syndrome carcinoma of unknown primary syndrome, SD standard deviation
In all six cases of CUP syndrome, an intraparotideal lymph node metastasis of a squamous cell carcinoma was diagnosed. In four cases the diagnosis was made using core needle biopsy, while in two cases parotidectomy revealed the diagnosis after inconclusive fine needle aspiration cytology had been performed. At the time of these two cases, core needle biopsy was not established routinely in the authors’ department. In all cases no prior primary malignancy existed in the medical history. Whole-body FDG-PET-/CT scan and panendoscopy including bilateral tonsillectomy and blind biopsies from the tongue base and nasopharynx could not identify a primary tumor site. Parotidectomy and ipsilateral neck dissection was performed in each case. Figure 2 displays a multipart image of a parotid SCC metastasis in a patient with CUP syndrome. The cervical lymph nodes were tumor-free in four cases, while one patient showed one lymph node metastasis and one patient showed a lymph node metastasis with extracapsular extension (ECE). One patient was diagnosed with pulmonary squamous cell carcinoma 18 months after diagnosis of CUP syndrome.
Fig. 2.
Poorly differentiated squamous cell carcinoma. a H&E Magnification 2.5 ×—Image of a cohesive neoplasm with solid growth pattern. b H&E Magnification 40 ×—the tumor cells show a relatively monomorphic appearance with enlarged atypical nuclei, very scant cytoplasm, and increased mitotic activity. c p63 Magnification 40 ×—strong nuclear expression of p63 in all tumor cells. d Cytokeratin 5 Magnification 40 ×—homogenous cytoplasmic and membranous expression of CK5—CK7, CD56, TTF1 and NUT are not expressed; p16 showed a weak mosaic like expression pattern
Of the 58 patients with infiltration/metastasis of cutaneous SCC, 31 (53.4%) patients showed parotid lymph metastasis and 27 (46.6%) had infiltration of the parotid parenchyma. In all cases of metastatic cutaneous squamous cell carcinoma to the parotid gland there was a known cutaneous squamous cell carcinoma primary of the head and neck. The skin cancer had been removed prior to parotidectomy in 31/58 cases (53.4%). Skin cancer and the parotid were removed simultaneously in 27/58 cases (46.6%). In all 58 patients (100%), unilateral neck dissection was performed.
Patients with infiltration/metastasis of cutaneous SCC were the oldest with the mean age being 78.4 years. Patients showing metastases of MM and lymphoma were the youngest with a mean age of 55.8 and 58.1 years, respectively. Patients with primary parotid malignancies had a mean age of 66.1 years. In terms of gender, there was a male predominance in patients with infiltration/metastasis of cutaneous SCC (86.2%), CUP syndrome (83.3%), mucosal SCC (60.0%), metastases of MM (80.0%), metastases of Merkel cell’s carcinoma (75.0%), and infiltration by Merkel cell’s carcinoma (66.7%). Gender was distributed fairly equally in primary parotid malignancies (51.1% vs 48.9%), lymphomas (56.5% vs 43,5%), and metastases of kidney cell carcinoma (50% vs 50%).
Table 2 illustrates basic demographic data, mean size, surgical approach, as well as histopathological data for the two largest groups of parotid malignancies examined in this study. As mentioned above, patients with parotid infiltration/metastasis of SCC were predominantly male (86.2% vs 13.8%) whereas gender distribution was almost equal in patients with primary malignancy of the parotid gland (51.1% vs 48.9%, p < 0.001). The mean age of patients with parotid infiltration/metastasis of SCC was 78.4 ± SD 10.8 years and the mean age of patients with primary parotid malignancy was 66.1 ± SD 15.4 years (p < 0.001). In term of lesion size, there were no statistically significant differences between primary parotid malignancies and parotid infiltration/metastasis of SCC (31.7 ± SD 19.7 vs 35.5 ± SD 17.9 mm, p = 0.216). Surgical approach was slightly more invasive for parotid infiltration/metastasis of SCC compared to primary parotid malignancy. Partial/superficial parotidectomy was used in 17.0% of patients with primary parotid malignancy and only in 10.3% of patients with parotid infiltration/metastasis of cSCC. 58.6% of patients with parotid infiltration/metastasis of cSCC and 48.9% of patients with primary parotid malignancy received total parotidectomy. Radical parotidectomy was performed on 31.0% of patients with infiltration/metastasis of cSCC and 34.0% of patients with primary parotid malignancy. All patients with parotid infiltration/metastasis of cSCC received ipsilateral neck dissection. 85.1% of patients with primary parotid malignancy received unilateral neck dissection, whereas 14.9% with low-grade carcinoma did not undergo neck dissection. Pathological lymph node status of the neck dissection was classified according to the 8th version of the TNM classification [15]. Pathological cervical lymph nodes were found in 43.1% of patients with metastatic cSCC and in 23.4% of patients with primary parotid malignancy. Pn-, V- and L-status was not available for 39.7% of patients with metastatic cSCC as resection of the primary skin tumor had been performed in other departments than the authors’ in these cases. Among cases in which Pn-, V- and L-status was available, Pn1-, V1- and L1-status was diagnosed in 37.1% (13/35), 17.1 (6/35) and 20.0% (7/35) of patients with metastatic cSCC. In patients with primary parotid malignancy Pn1, V1 and L1-status was diagnosed in 21.3% (10/47), 0% (0/47) and 10.6% (5/47) of these cases, respectively.
Table 2.
Demographic/imaging/operation-specific/histopathological data of patients with primary parotid malignancies and parotid infiltration/metastasis of cutaneous squamous cell carcinoma
| Independent variable | Primary malignancy | Cutaneous SCC (metastasis or infiltration) | Significance p < 0.05 |
|---|---|---|---|
| Number of patients | 47 | 58 | |
| Male n (%) | 24 (51.1) | 50 (86.2) | X2 = 15,410; p < 0.001a |
| Female n (%) | 23 (48.9) | 8 (13.8) | |
| Mean age (years) (SD) | 66.1 (15.4) | 78.4 (10.8) | U = 676.0; p < 0.001a |
| Mean size (mm) (SD) | 31.7 (19.7) | 35.5 (17.9) | U = 959.5; p = 0.216 |
| Approach (Parotidectomy) (%) | Partial/Superficial = 8 (17.0) | Partial/Superficial = 6 (10.3) | |
| Total = 23 (48.9) | Total = 34 (58.6) | ||
| Radical = 16 (34.0) | Radical = 18 (31.0) | ||
| Ipsilateral neck dissection (%) | 40 (85.1) | 58 (100) | |
| No neck dissection (%) | 7 (14.9) | 0 (0) | |
| pNX (%) | 7 (14.9) | 0 (0) | |
| pN0 (%) | 29 (61.7) | 33 (56.9) | |
| pN1 (%) | 4 (8.5) | 10 (17.2) | |
| pN2a (%) | 0 (0) | 0 (0) | |
| pN2b (%) | 6 (12.8) | 12 (20.7) | |
| pN2c (%) | 0 (0) | 0 (0) | |
| pN3a (%) | 0 (0) | 0 (0) | |
| pN3b (%) | 1 (2.1) | 3 (5.2) | |
| PnX (%) | 0 (0) | 23 (39.7) | |
| Pn0 (%) | 37 (78.7) | 22 (37.9) | |
| Pn1 (%) | 10 (21.3) | 13 (22.4) | |
| VX (%) | 0 (0) | 23 (39.7) | |
| V0 (%) | 47 (100) | 29 (50.0) | |
| V1 (%) | 0 (0) | 6 (10.3) | |
| LX (%) | (0) | 23 (39.7) | |
| L0 (%) | 42 (89.4) | 28 (48.3) | |
| L1 (%) | 5 (10.6) | 7 (12.1) |
SD standard deviation, n number of patients, Pn perineural invasion, V vascular invasion, L lymphovascular invasion
aStatistically significant result
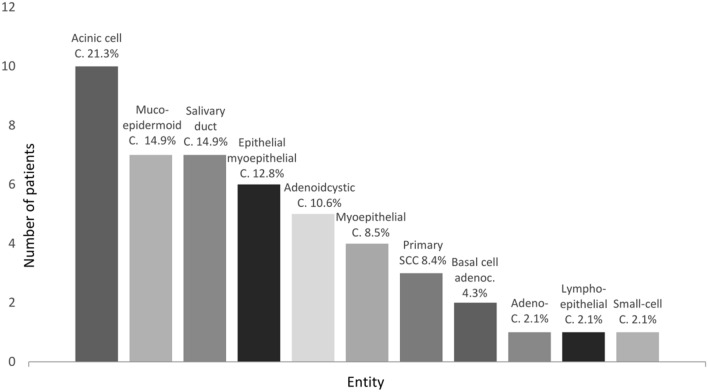
Figure 3 displays the histopathological distribution of primary parotid malignancies found in 47 patients. The most common entity was acinus cell carcinoma (21.3%), followed by mucoepidermoid carcinoma (14.9%), salivary duct carcinoma (14.9%), epithelial-myoepithelial carcinoma (12.8%), adenoidcystic carcinoma (10.6%), myoepithelial carcinoma (8.5%), primary SCC of the parotid gland (8.4%), and, lastly basal cell adenocarcinoma (4.3%). Also, there was one patient with adeno carcinoma, one patient with lymphoepithelial carcinoma and one patient with small cell carcinoma.
Fig. 3.
Descriptive data on distribution of primary parotid malignancies. C carcinoma, SCC squamous cell carcinoma
Discussion
While there is an extensive amount of literature showing the histopathological distribution of primary parotid malignancies, it lacks comprehensive studies displaying all malignant lesions, including secondary malignancies of the parotid gland. Thus, the main objective of this study was to show updated numbers of primary and secondary parotid malignancies and their characteristics among patients with a surgically treated parotid lesion in a center for salivary gland surgery in a short period.
Parotid surgery was performed on a total of 777 patients between March 2014 and November 2019. 613 (78.9%) benign tumors and 164 (21.1%) malignant tumors were found, which is in line with current literature reporting approximately 20% of all parotid masses being of malignant histopathology [1–4]. Also corresponding with the literature, the mean age of patients with benign parotid tumor in this series (57.8 ± SD 15.2 years) was significantly lower than that of patients with malignant parotid tumor (69.7 ± SD 17.4 years; p < 0.001) [1, 5]. While the mean age difference was 9.6 years in a publication by Maahs et al. [1], which exclusively included parotid tumors, and 12 years in a study by De Oliveira et al. [5], (which included all salivary gland tumors), mean age difference in this series was as high as 22.0 years. This was mainly due to the fact that all malignancies found in the parotid gland were included. These contained a high number of relatively old patients (mean age 78.4 ± SD 10.8 years) showing infiltration/metastasis of cutaneous squamous cell carcinoma (cSCC). This means that: the older a patient with a parotid mass is, the higher the likelihood of malignancy. In contrast to many previous studies, in this series there was a male predominance in benign parotid tumors (male:female ratio of 1.45:1) as well as in malignant parotid tumors (male:female ratio of 2.15:1). Male predominance in benign tumors can probably be explained by the fact that in this study 42.7% of all benign tumors were Warthin’s tumors compared to only 8.3% and 16.3% in previous studies [1, 16], as well as the fact that Warthin’s tumor is clearly associated with male gender [4] and is rising in incidence [17]. The male predominance among malignant tumors in this series is likely also due to the fact that all malignancies found in the parotid gland with a high percentage of infiltration/metastasis of cutaneous SCC (86.2% males) were displayed.
As far as the side of the lesion, as expected, there was no statistically significant difference between benign and malignant parotid tumors (p = 0.203). Moreover, although 25 benign parotid lesions (4.1%) and only two (1.2%) malignant tumors were bilateral, this difference was not significant (p = 0.076). Therefore, bilaterality was found not to be associated with benign histopathology. Since all bilateral benign lesions were Warthin’s tumors and frequency of Warthin’s tumor within the group of benign parotid tumors has generally been growing over the last few decades [4], it is possible that bilateral benign parotid lesions will be significantly more common in the future than bilateral malignancies. Furthermore, the lesion’s size was not significantly different when comparing benign and malignant parotid tumor in this series (p = 0.132). This finding supports the results of previous studies [18]. A possible explanation could be that malignant parotid tumors were shown to grow faster than benign tumors, yet become symptomatic earlier through symptoms such as pain or facial nerve palsy [6].
In addition, infiltration/metastasis of cutaneous squamous cell carcinoma (cSCC) was found to be the most frequent histopathological subgroup, which represented 35.4% of all parotid malignancies. This was followed by primary parotid malignancies, which accounted for 28.5% and lymphomas, which represented 14.0% of all parotid malignant lesions. 6.1% of parotid malignancies were metastases of malignant melanomas and 3.7% were metastases of CUP syndrome.
Overall, 71.5% of all malignant parotid lesions were secondary malignancies and 28.5% were primary malignancies. To date, studies displaying all parotid malignancies, including secondary malignancies, are rare. Such studies were mostly conducted in Australia where SCC incidence is traditionally high due to the population’s extensive exposure to ultraviolet radiation [19]. In an Australian series of 71 patients who were treated surgically for a parotid malignancy, 26.8% of them were found to have primary parotid malignancies. Infiltration/metastasis of cSCC accounted for 59.2% of all parotid malignancies in this study [12]. Similar results were found in another Australian study by Bron et al., which included 232 patients with parotid malignancy. 23.3% of these patients showed primary malignancies. In this case, too, the largest group was metastatic cSCC—accounting for 43.5% of all parotid malignancies in this study [11]. Among the limited data concerning secondary parotid malignancies from geographical regions other than Australia there is one abovementioned analysis from Spain that displayed data of 48 patients treated for parotid malignancy between 1997 and 2004. 75% of these were primary parotid malignancies. Metastatic cSCC accounted for only 16.7% of all parotid malignancies compared to 35.4% in this series. Consequently, it seems that the surgical incidence of secondary parotid malignancies has increased in Europe over the last few decades. In part, this may be due to an increasing life expectancy in Europe [20] leading to a rising incidence of cSCC [21] with an increased percentage of metastatic cSCC to the parotid gland. A recent German study supports this observation by describing the surgical incidence of parotid metastases over the last four decades [14]: In a study that included 108 patients with surgically treated parotid malignancies between 1975 and 2015 the incidence of parotid metastasis was reported to have increased from 10% between 1975 and 1985 to 57% between 2006 and 2015. In this series of 164 patients with surgically treated parotid malignancy between 2014 and 2019, 57.5% patients presented metastatic parotid malignancy, which is in line with the cited study. Accounting for 35.2% of all malignant parotid lesions, the most common metastatic parotid malignancy was cSCC, which is also in line with the 35.4% found in this series.
Overall, these results from a large and current series of patients treated surgically for parotid malignancy confirm the increased surgical incidence of secondary parotid malignancies mainly due to metastasis of cSCC. However, it must be stated that this was a hospital-based study and not a population-based study. This means that the presented incidences are incidences of surgical cases and not epidemiological incidences.
As mentioned above, the two most common parotid malignancies in this series were infiltration/metastasis of cSCC (n = 58) and primary parotid malignancy (n = 47). Males had a significantly higher likelihood of showing parotid infiltration/metastasis of cSCC than primary parotid malignancy. This was mainly due to the known high incidence of cSCC found in men compared to women [22, 23] and a rather even distribution of gender in primary parotid malignancies [10]. There was a significant difference between mean age of patients showing infiltration/metastasis of cSCC (78.4 ± SD 10.8 years) and patients with primary parotid malignancy (66.1 ± SD 15.4 years). This is in line with registry data showing the mean age of diagnosis of primary parotid malignancy being in the seventh decade of life [10] and median age of diagnosis of cSCC being 80 years [24]. Interestingly, there was no significant difference between the mean size of parotid primary malignant lesions and parotid infiltration/metastasis of SCC, even though mean values were 31.7 ± SD 19.7 mm and 35.5 ± SD 17.9 mm, respectively. This could indicate that there is a lack of a sufficient parotid staging when head and neck cSCC is first diagnosed. In presence of a sufficient parotid staging, e.g. by B-mode ultrasound (US), metastases to the parotid gland should be detected earlier than primary parotid malignancies and mean size of metastatic lesions would be lower than that of primary parotid lesions. To avoid overtreatment and to plan adequate oncological resection in case of a suspicious parotid lesion, malignancy can be confirmed by US-guided fine-needle aspiration cytology (FNAC). In diagnostic of a malignant versus a benign parotid lesion FNAC shows a sensitivity and specificity of 78–82% and 90–98%, respectively [25, 26]. An even higher sensitivity (94%) and specificity (98%) can be reached by US-guided core needle biopsy [27].
Lastly, the surgical approach was more invasive for parotid infiltration/metastasis of SCC than for primary parotid malignancy in this study. While 89.6% of patients with metastatic SCC to the parotid gland received a total or radical parotidectomy, total or radical parotidectomy was only performed in 82.9% of patients with a primary parotid malignancy. On the other hand, 17.0% of patients with a primary parotid malignancy received a partial or superficial parotidectomy which was only the case for 10.3% of patients with metastatic cSCC to the parotid gland. The explanation for this difference is that all primary malignancies that were resected by partial/superficial parotidectomy were low-grade carcinomas. In these cases, the malignancy had not been expected prior to surgery. Sonographic follow-up was performed in the presence of R0 resection. Pathological cervical lymph nodes were found in 43.1% of patients with metastatic cSCC and in 23.4% of patients with primary parotid malignancy. Pn-, V- and L-status was positive in 37.1%, 17.1, and 20.0% of patients with metastatic cSCC (when available) and in 21.3%, 0% and 10.6% with primary parotid malignancy, respectively. The distribution of these histopathological parameters is most likely due to the fact that metastatic malignancy when compared to primary malignancy is more aggressive and has possibly been developing for a longer time.
In the subgroup of primary parotid malignancies, the most frequent tumor was acinic cell carcinoma (21.3%), followed by mucoepidermoid carcinoma (14.9%), salivary duct carcinoma (14.9%), epithelial-myoepithelial carcinoma (12.8%), and adenoid cystic carcinoma (10.6%). In most previous studies either mucoepidermoid carcinoma [1, 8, 28], adenoid cystic carcinoma [6], or adenocarcinoma [12] were the most frequent primary parotid malignancy. These differences in distribution of primary parotid tumors may be due to populations differing between geographical regions and due to the relatively small sample groups of primary parotid tumors with various histopathological entities. Further research is needed to evaluate the histopathological distribution of primary parotid malignancies and the influence of risk factors.
Conclusion
The present study displays a comprehensive overview of histopathological distribution of parotid malignancies in a current series of patients treated surgically for a parotid malignancy. Results show a high percentage of secondary parotid malignancies among all malignant parotid tumors with infiltration/metastasis of cutaneous squamous cell carcinoma being the most common malignancy in the parotid gland. Patients with parotid infiltration/metastasis of cutaneous squamous cell carcinoma were significantly older and more likely male than patients with a primary parotid tumor. The presented data confirms the recently found increase in surgical incidence of secondary parotid malignancies among the limited data from Europe. Furthermore, it shows that parotid surgery nowadays more frequently means surgery of metastases rather than surgery of primary malignancies. These results of the updated numbers on distribution of parotid malignancies and patients' characteristics can help clinicians with differential diagnosis in presence of a suspicious parotid lesion.
Acknowledgements
The authors thank all the patients for their participation in the study.
Funding
The authors have no funding to declare.
Compliance With Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed Consent
Informed consent was obtained from all individual participants included in this study (no identifying information about participants is available in this article).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maahs GS, Oppermann Pde O, Maahs LG, Machado Filho G, Ronchi AD. Parotid gland tumors: a retrospective study of 154 patients. Braz J Otorhinolaryngol. 2015;81(3):301–306. doi: 10.1016/j.bjorl.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin CC, Tsai MH, Huang CC, Hua CH, Tseng HC, Huang ST. Parotid tumors: a 10-year experience. Am J Otolaryngol. 2008;29(2):94–100. doi: 10.1016/j.amjoto.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Eveson JW, Cawson RA. Salivary gland tumours. A review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol. 1985;146(1):51–58. doi: 10.1002/path.1711460106. [DOI] [PubMed] [Google Scholar]
- 4.Franzen AM, Kaup Franzen C, Guenzel T, Lieder A. Increased incidence of Warthin tumours of the parotid gland: a 42-year evaluation. Eur Arch Otorhinolaryngol. 2018;275(10):2593–2598. doi: 10.1007/s00405-018-5092-3. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira FA, Duarte EC, Taveira CT, Maximo AA, de Aquino EC, Alencar Rde C, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3(4):271–275. doi: 10.1007/s12105-009-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sungur N, Akan IM, Ulusoy MG, Ozdemir R, Kilinc H, Ortak T. Clinicopathological evaluation of parotid gland tumors: a retrospective study. J Craniofac Surg. 2002;13(1):26–30. doi: 10.1097/00001665-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 7.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 8.Shashinder S, Tang IP, Velayutham P, Prepageran N, Gopala KG, Kuljit S, et al. A review of parotid tumours and their management: a ten-year-experience. Med J Malays. 2009;64(1):31–33. [PubMed] [Google Scholar]
- 9.Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomark Prev. 2009;18(11):2899–2906. doi: 10.1158/1055-9965.EPI-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahlberg P, Anderson H, Biorklund A, Moller T, Perfekt R. Carcinoma of the parotid and submandibular glands–a study of survival in 2465 patients. Oral Oncol. 2002;38(7):706–713. doi: 10.1016/S1368-8375(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 11.Bron LP, Traynor SJ, McNeil EB, O'Brien CJ. Primary and metastatic cancer of the parotid: comparison of clinical behavior in 232 cases. Laryngoscope. 2003;113(6):1070–1075. doi: 10.1097/00005537-200306000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74(7):563–568. doi: 10.1111/j.1445-2197.2004.02988.x. [DOI] [PubMed] [Google Scholar]
- 13.Pomar Blanco P, Martin Villares C, San Roman Carbajo J, Tapia Risueno M, Fernandez Pello M. Metastasis to the parotid gland. Acta Otorrinolaringol Esp. 2006;57(1):47–50. doi: 10.1016/S0001-6519(06)78662-9. [DOI] [PubMed] [Google Scholar]
- 14.Franzen A, Buchali A, Lieder A. The rising incidence of parotid metastases: our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol Ital. 2017;37(4):264–269. doi: 10.14639/0392-100X-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Hoboken: Wiley; 2017. [Google Scholar]
- 16.Haldar S, Sinnott JD, Tekeli KM, Turner SS, Howlett DC. Biopsy of parotid masses: review of current techniques. World J Radiol. 2016;8(5):501–505. doi: 10.4329/wjr.v8.i5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Psychogios G, Vlastos I, Tholken R, Zenk J. Warthin's tumour seems to be the most common benign neoplasm of the parotid gland in Germany. Eur Arch Otorhinolaryngol. 2020;277(7):2081–2084. doi: 10.1007/s00405-020-05894-z. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The national cancer data base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(9):951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 19.Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control. 1994;5(4):367–392. doi: 10.1007/BF01804988. [DOI] [PubMed] [Google Scholar]
- 20.Mackenbach JP, Looman CW. Life expectancy and national income in Europe, 1900–2008: an update of Preston's analysis. Int J Epidemiol. 2013;42(4):1100–1110. doi: 10.1093/ije/dyt122. [DOI] [PubMed] [Google Scholar]
- 21.Stang A, Khil L, Kajuter H, Pandeya N, Schmults CD, Ruiz ES, et al. Incidence and mortality for cutaneous squamous cell carcinoma: comparison across three continents. J Eur Acad Dermatol Venereol. 2019;33(Suppl 8):6–10. doi: 10.1111/jdv.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimi A, Clark JR, Ahmadi N, Palme CE, Morgan GJ, Veness MJ. Prognostic significance of disease-free interval in head and neck cutaneous squamous cell carcinoma with nodal metastases. Head Neck. 2013;35(8):1138–1143. doi: 10.1002/hed.23096. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara J, Ferlito A, Takes RP, Rinaldo A, Strojan P, Shaha AR, et al. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland–a review of current recommendations. Head Neck. 2011;33(12):1789–1795. doi: 10.1002/hed.21583. [DOI] [PubMed] [Google Scholar]
- 24.Venables ZC, Autier P, Nijsten T, Wong KF, Langan SM, Rous B, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol. 2019;155(3):298–306. doi: 10.1001/jamadermatol.2018.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CC, Jethwa AR, Khariwala SS, Johnson J, Shin JJ. Sensitivity, specificity, and posttest probability of parotid fine-needle aspiration: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2016;154(1):9–23. doi: 10.1177/0194599815607841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eytan DF, Yin LX, Maleki Z, Koch WM, Tufano RP, Eisele DW, et al. Utility of preoperative fine needle aspiration in parotid lesions. Laryngoscope. 2018;128(2):398–402. doi: 10.1002/lary.26776. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Kim JS. Ultrasound-guided core needle biopsy in salivary glands: a meta-analysis. Laryngoscope. 2018;128(1):118–125. doi: 10.1002/lary.26764. [DOI] [PubMed] [Google Scholar]
- 28.Li LJ, Li Y, Wen YM, Liu H, Zhao HW. Clinical analysis of salivary gland tumor cases in West China in past 50 years. Oral Oncol. 2008;44(2):187–192. doi: 10.1016/j.oraloncology.2007.01.016. [DOI] [PubMed] [Google Scholar]