Abstract
Background
Periprosthetic joint infections (PJI) are challenging complications following arthroplasty. Staphylococci are a frequent cause of PJI and known biofilm producers. Biofilm formation decreases antimicrobial susceptibility, thereby challenging favourable treatment outcomes. The aims of this study were to characterize the biofilm abilities and antimicrobial susceptibilities of staphylococci causing first-time PJI and correlate them to clinical outcome (infection resolution and recurrence).
Methods
Reoperations for PJI of the hip or knee between 1st January 2012 to 30th June 2015 performed at the Sahlgrenska University Hospital were identified in a local database. Medical records were reviewed and clinical parameters recorded for patients whose intraoperative bacterial isolates had been stored at the clinical laboratory. Staphylococcal strains isolated from reoperations due to first-time PJI were characterised by their ability to form biofilms using the microtiter plate test. Antimicrobial susceptibility of the strains was determined by minimum inhibitory concentration (MIC) when grown planktonically, and by minimum biofilm eradication concentration (MBEC) when grown as biofilms. MBEC determination was conducted using the Calgary biofilm device (CBD) and a custom-made antimicrobial susceptibility plate containing eight clinically relevant antimicrobial agents.
Results
The study group included 49 patients (70 bacterial strains) from first-time PJI, whereof 24 (49%) patients had recurrent infection. Strong biofilm production was significantly associated with recurrent infection. Patients infected with strong biofilm producers had a five-fold increased risk for recurrent infection. Strains grown as biofilms were over 8000 times more resistant to antimicrobial agents compared to planktonic cultures. Biofilms were more susceptible to rifampicin compared to other antimicrobials in the assay. Increased biofilm susceptibility (MBEC > MIC) was observed for the majority of the bacterial strains and antimicrobial agents.
Conclusions
Strong biofilm production was significantly associated with increased antimicrobial resistance and PJI recurrence. This underscores the importance of determining biofilm production and susceptibility as part of routine diagnostics in PJI. Strong staphylococcal biofilm production may have implications on therapeutic choices and suggest more extensive surgery. Furthermore, despite the increased biofilm resistance to rifampicin, results from this study support its use in staphylococcal PJI.
The Translational Potential of this Article
Like for many biomaterial-associated infections, staphylococci are a common cause of PJI. Their ability to adhere to surfaces and produce biofilms on medical devices is proposed to play a role. However, clinical studies where biofilm properties are directly linked to patient outcome are scarce. This study demonstrates that the majority of staphylococci isolated from first-time PJI were biofilm producers with increased antimicrobial resistance. Patients suffering an infection caused by a staphylococcal strain with strong biofilm production ability had a five-fold greater risk of recurrent infection. This novel finding suggests the importance of evaluating biofilm production as a diagnostic procedure for the guidance of treatment decisions in PJI.
Keywords: Antimicrobial resistance, Biofilm, Minimum Biofilm Eradication Concentration, Periprosthetic joint infection, Staphylococci
1. Introduction
Periprosthetic joint infection (PJI) threatens the short- and long-term success of total joint arthroplasties. PJI implies pronounced patient suffering, health care and societal costs, and therapeutic challenges [1,2]. Due to an increasing elderly population the demands for arthroplasty surgery is projected to increase, and a rise in associated infections is expected [3,4]. Over 50% of PJI of the hip or knee are caused by staphylococci, mainly Staphylococcus aureus and Staphylococcus epidermidis [4,5]. Biofilm production has been suggested an important virulence property of staphylococci, and it has been associated with disease in animal models of biomaterial-related infection [6]. Biofilms are complex communities of bacterial cells and self-secreted extracellular polymeric substances (EPS) protecting bacteria from immunological and pharmacological eradication [7]. When documented in vivo, biofilms have been found both on implants and surrounding tissues [8,9]. It has been noted that bacteria causing orthopaedic implant-associated infections are relatively strong biofilm producers compared to bacteria causing other type of infections [10,11]. However, there are few clinical studies on how biofilm properties relate to infection outcome.
Surgical treatment options for PJI are implant extracting, such as one- or two-stage revisions of bone-anchored components, or implant preserving procedures such as DAIR (Debridement, Antibiotics and Implant Retention). DAIR is mainly recommended for early onset PJI, and has success rates varying from 37% to 85% [5,12,13]. Implant extraction, in one or two stages, is mainly recommended when DAIR fails or in late onset PJI. Staged revision has success rates at around 90%, but is considered more resource demanding and challenging for the patient [1,14].
Surgery is combined with prolonged antimicrobial treatment, typically ranging from 6 weeks to 6 months [15,16]. Antimicrobial treatment is currently guided by determining the minimum inhibitory concentration (MIC) of relevant antimicrobial agents, such as intravenous vancomycin and oral rifampicin in polytherapy for staphylococcal infections [15]. MIC is commonly determined in broth or agar cultures of free-living bacteria (i.e. planktonic), which does not mirror biofilm antimicrobial susceptibilities where up to 50,000-fold increases in MICs have been demonstrated [[17], [18], [19]]. The MIC assay can often predict treatment outcome of acute infections, however its prognostic ability in chronic biofilm infections is not established [20]. Due to biofilm resistance, choosing antimicrobial agents based on MICs may be insufficient in biofilm-associated infections.
The concentrations needed to eradicate bacterial cells inside biofilms can be determined by in vitro assays. Such an assay is the Calgary biofilm device (CBD), which in combination with a microbroth dilution plate containing antimicrobials, can determine the minimum biofilm eradication concentration (MBEC). MBEC is the lowest concentration at which visible biofilm cell growth is prevented. Zaborowska et al. reported a link between strong biofilm production and worse clinical outcome in infections associated with bone-anchored amputation prostheses [21]. The analysis of biofilm properties as a diagnostic tool for treatment decisions may be a valuable addition in the management of biofilm-associated infections. Currently, there is a general knowledge that biofilm production may be an important pathogenic factor in PJI, however, very few studies evaluate its association to clinical outcome.
Due to the lack of knowledge on the clinical outcomes in PJI with respect to the ability of microorganisms to produce biofilms, the aims of the current study were to characterize and correlate the biofilm formation and susceptibility of staphylococci to clinical outcome in cases of first-time PJI regardless of onset. The use of biofilm ability measurements and susceptibility testing as diagnostic and prognostic methods may be of clinical relevance if a relationship between these factors and clinical outcome can be established.
2. Materials and methods
2.1. Study population and clinical parameters
The study protocol was approved by the Regional Ethical Review Board in Gothenburg, Sweden (entry number 654-16). The local database for surgical procedures (Operätt) at Sahlgrenska University Hospital (Mölndal, Sweden) was used to search for procedures with ICD-10 codes T845.5F and T845.5G (surgery due to infected hip and knee arthroplasties, respectively) between 1st January 2012 to 30th June 2015. Written consent was obtained from patients and medical records were reviewed until 31st December 2018, representing a follow-up of at least 3.5 years for all patients and a mean follow-up time of 5 years. The recorded variables associated to infection are presented in Table 1.
Table 1.
Demography of study cohort including inflammatory markers, surgical treatment characteristics, infection type and antimicrobial treatment. Averages are presented as means with standard deviations (SD) or as medians with interquartile range (IQR).
| All patients n = 49 | Resolution n = 25 | Re-infection n = 24 | p-value | |
|---|---|---|---|---|
| Baseline demographics | ||||
| Sexn (%) | 0.92 | |||
| Female | 16 (32.7) | 8 (32.0) | 8 (66.7) | |
| Male | 33 (67.3) | 17 (68.0) | 16 (33.3) | |
| Agemean years (SD) | 69 (13) | 69 (10) | 68 (15) | 0.76 |
| BMImean (SD)) | 28.5 (5.4) | 27.9 (5.7) | 29.3 (5.2) | 0.38 |
| ASA classn (%) | 0.16 | |||
| 1: Healthy | 6 (12.2) | 1 (4.0) | 5 (20.8) | |
| 2: Mild systemic disease | 33 (67.3) | 19 (76.0) | 14 (58.3) | |
| 3, 4: Severe systemic disease, potential life threat | 10 (20.4) | 5 (20.0) | 5 (20.8) | |
| Arthroplasty typen (%) | 0.31 | |||
| THA | 32 (65.3) | 18 (72.0) | 14 (58.3) | |
| TKA | 17 (34.7) | 7 (28.0) | 10 (41.7) | |
| Diabetesn (%) | 0.66 | |||
| Yes | 9 (18.4) | 4 (16.0) | 5 (20.8) | |
| No |
40 (81.6) |
21 (84.0) |
19 (79.2) |
|
|
Inflammatory markersmedian (IQR) mean (SD) | ||||
| ESR | 52 (21–84) | 58 (31–84) | 46 (20–93) | |
| 55 (31) | 55 (29) | 54 (36) | 0.94 | |
| WBC | 8.0 (6.7–10.2) | 7.9 (6.5-10-2) | 8.1 (6.7–10.1) | |
| 8.7 (3.4) | 8.4 (2.1) | 9.0 (4.4) | 0.55 | |
| CRP | 58 (21–168) | 46 (19–143) | 150 (22–193) | |
| 112 (116) | 83 (85) |
141 (135) |
0.08 |
|
|
Surgical treatment | ||||
| Index surgery to symptoms | 0.28 | |||
| Mean days (SD) | 496 (1026) | 330 (898) | 670 (1141) | |
| Median days (IQR) | 26 (17–405) | 25 (16-297 | 188 (19–868) | |
| Note: missing for 6 patients | ||||
| Symptom onset to reoperation | 0.40 | |||
| Mean days (SD) | 29 (69) | 20 (30) | 38 (94) | |
| Median days (IQR) | 8 (3–22) | 8 (2–17) | 8 (3–27) | |
| Note: missing for 3 patients | ||||
| Type of surgical proceduren (%) | 0.91 | |||
| Implant preserving | 31 (63.3) | 16 (64.0) | 15 (62.5) | |
| DAIR-non exchange | 5 | 1 | 4 | |
| DAIR-exchange | 26 | 15 | 11 | |
| Complete implant extracting | 18 (36.7) | 9 (36.0) | 9 (37.5) | |
| Stage 1 | 15 | 7 | 8 | |
| Stage 2 | 1 | 1 | 0 | |
| Other |
2 |
1 |
1 |
|
|
Infection and antimicrobial treatment | ||||
| Infectionn (%) | 0.94 | |||
| Monomicrobial | 37 (75.5) | 19 (76.0) | 18 (75.0) | |
| Polymicrobial (≥2 different strains isolated) | 12 (24.5) | 6 (24.0) | 6 (25.0) | |
| Antimicrobials prior to reoperationn (%) | 0.79 | |||
| Yes | 10 (20.4) | 5 (20.0) | 5 (20.8) | |
| No | 39 (79.6) | 20 (80.0) | 19 (79.2) | |
| Antimicrobial agent ivn (%) | 0.81 | |||
| VAN | 23 (46.9) | 12 (48.0) | 11 (45.8) | |
| VAN/poly | 12 (24.5) | 5 (20.0) | 7 (14.3) | |
| Other | 14 (28.6) | 8 (32.0) | 6 (12.2) | |
| Antimicrobial agent oraln (%) | 0.21 | |||
| RIF/poly | 34 (69.4) | 20 (80.0) | 14 (58.3) | |
| Other | 11 (22.4) | 3 (12.0) | 8 (33.3) | |
| None | 4 (8.2) | 2 (8.0) | 2 (8.3) | |
| Duration of therapy (iv and oral)mean weeks (SD) | 15.6 (8.5) | 15.2 (9.9) | 15.9 (7.0) | 0.79 |
Note: Independent Samples T-test was used for the comparisons of means. Pearson’s Chi-Square test and Fischer’s exact test were used for the comparisons of categorical variables. Abbreviations: SD = standard deviation, IQR = interquartile range, BMI = body mass index, ASA = American Society of Anesthesiologists Physical status, THA = total hip arthroplasty, TKA = total knee arthroplasty, ESR = erythrocyte sedimentation rate, WBC = white blood cells, CRP=C-reactive protein, DAIR-NE = debridement, antibiotics and implant retention with no exchange of modular components, DAIR-E = debridement, antibiotics and implant retention with exchange of modular components, VAN = vancomycin, VAN/poly = vancomycin in combination with other intravenous antimicrobial agent, RIF/poly = rifampicin in combination with other oral antimicrobial agent.
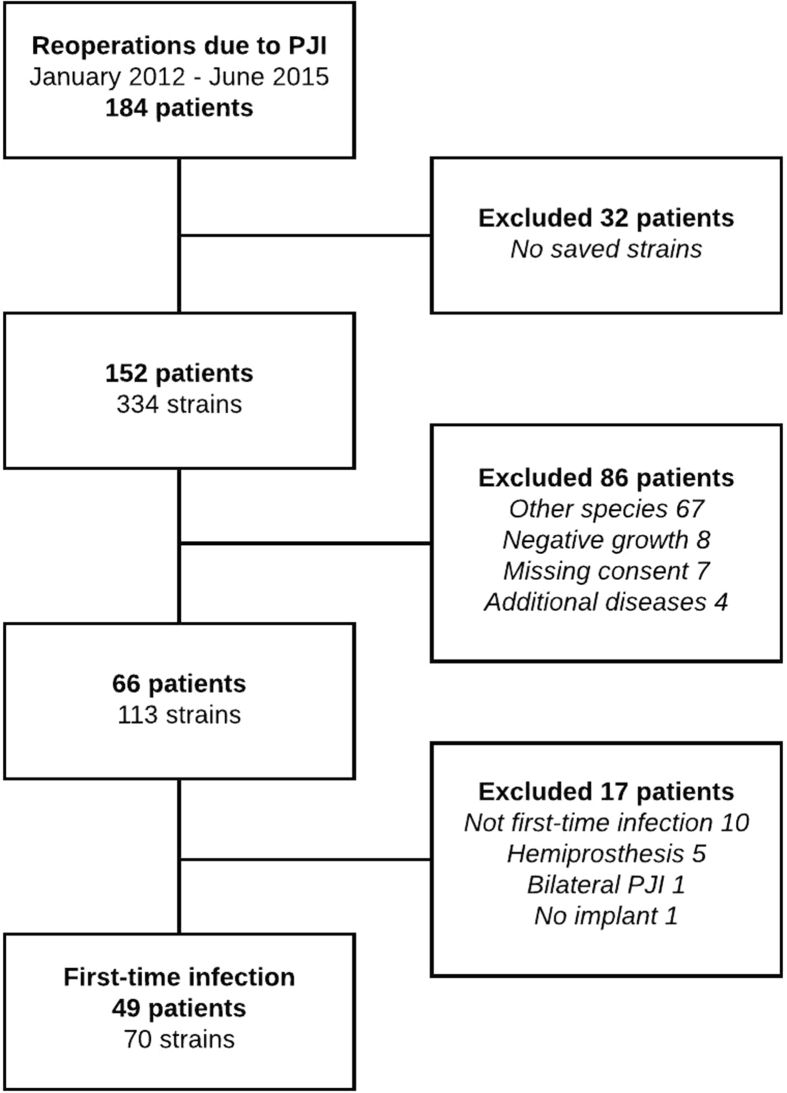
Intraoperative tissue samples were cultured and bacteria isolated, identified and stored at −80 °C at the Clinical Bacteriological Laboratory at Sahlgrenska University Hospital (Gothenburg, Sweden). The isolate inventory was matched to the patient group and compared to medical journal data to determine if the strains were isolated from at least two out of five or more samples. Patients and strains were eligible for analysis if inclusion criteria were met: i) PJI of total hip or knee arthroplasty using the MSIS 2018 criteria [22], ii) first-time PJI, and iii) monomicrobial infection caused by either S. aureus or coagulase negative staphylococci (CoNS), or polymicrobial infection caused by two different species of staphylococci. Cases with negative or polymicrobial (of other species than staphylococci) growth were excluded. Study inclusion is illustrated in Fig. 1.
Fig. 1.
Flow diagram of patient inclusion.
2.2. Outcome
The primary end point was to evaluate the correlation of biofilm production (non/weak or strong) to clinical outcome (infection resolution or recurrence). The secondary outcome measure was to test the relationship between biofilm susceptibility (MBEC, MBEC/MIC-ratio and antibiogram patterns) and clinical outcome.
2.3. Clinical definitions
Infection resolution was defined as no clinical or laboratory suspicion of infection and no further surgical or antimicrobial treatment due to PJI. Onset of symptoms was defined as the first time the patient contacted the health care system with a suspicion of PJI.
End date of antimicrobial treatment was either when the patients had fulfilled the planned treatment course or if they underwent a new surgical procedure. Surgical procedures were categorized either as implant preserving or implant extracting.
Recurrence of PJI was either due to relapse or reinfection. Reinfection was defined as an infection with a strain that was distinct from the strain that caused the original infection. Relapse was defined as an infectious flare due to unsuccessful treatment of the original strain. Relapse was confirmed if the strain belonged to the same species, had the same MIC-susceptibility pattern and similar biofilm production as the strain that caused the primary infection. Additional multi-locus sequence typing (MLST) was conducted to confirm uncertain relapse cases.
Biofilm cultures with MBECs below EUCAST clinical MIC breakpoints [23], were categorized as MBEClow, and categorized as MBEChigh if above. MBEChigh and MBEClow were based on the most potent oral anti-biofilm antimicrobial administered. In cases treated with rifampicin (RIF) in polytherapy, RIF was considered the most potent.
2.4. Species identification of the strains
To further identify the specific species for the strains labelled as coagulase-negative staphylococci (CoNS), the API Staph (bioMérieux SA, Marcy-l’Etoile, France) was used following the manufacturer's instructions.
2.5. Biofilm production abilities of the strains
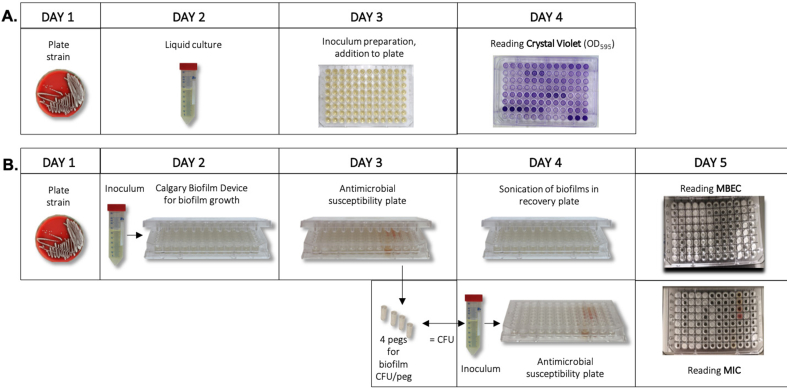
The in vitro biofilm formation ability of a total of 70 strains isolated from the patients was evaluated using the microtiter plate test (crystal violet assay, CV), which allows quantification of the total biofilm biomass, using a previously described procedure described with the following modifications (Fig. 2A) [24]. Strains were cultured overnight (o.n.) at 37 °C on 5% horse blood Columbia agar plates (Media Department, Clinical Microbiology Lab, Sahlgrenska University Hospital). One colony from each strain was inoculated in tryptic soy broth (TSB) (Scharlau, Barcelona, Spain) + 0.25% glucose (for S. aureus) and cultured o.n. at 37 °C and 200 rpm. The cell suspension was adjusted to an OD546 = 1, diluted 1:40 in TSB (+glucose for S. aureus), and 200 μL were added in triplicates to 96-well polystyrene microtiter plates (BioLite Cell Culture Treated Plates, Thermo Scientific™, MA, USA). Plates were incubated for 24 h at 37 °C. Wells were emptied and washed 3x by immersion in water. The remaining adherent cells and EPS were stained with 200 μL crystal violet (2%) (VWR, PA, USA) for 5 min, washed 3x by immersion in water, and air dried. The dye was eluted in 200 μL ethanol-acetone (80:20, vol/vol) for 5 min and 150 μL were transferred to a new plate to determine the OD595 using a plate reader (FLUOstar Omega, BMG Labtech, Offenburg, Germany). Three wells contained sterile TSB to serve as blank and the mean value was subtracted from the experimental readings. Strains were categorized into different biofilm production categories using predefined breakpoints according to Baldassari et al. (Table 2) [25]. For the analyses, biofilm production categories were further dichotomized to i) non/weak producers, and ii) strong producers.
Fig. 2.
Illustration of the methods used to measure biofilm, MBEC and MIC in staphylococcal strains causing periprosthetic joint infection (A) Step-wise description of the microtiter plate test used to quantitate biofilm mass using crystal violet staining (B) Antimicrobial susceptibility testing of the strains when grown planktonically and as biofilms. Biofilms grown on the Calgary biofilm device for 24 h were subjected to the antimicrobial susceptibility plate to determine the minimum biofilm eradication concentration (MBEC). The same number of colony-forming units (CFU) found in biofilms were used to determine the minimum inhibitory concentration of planktonic cultures. MBEC/MIC-ratios were calculated to assess the fold increase in antimicrobial dose needed to inhibit or kill the strain in biofilm compared to planktonic growth. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Classification of biofilm production category using breakpoints according to Baldassarri et al. [25].
| Biofilm category | Optical density (OD) |
|---|---|
| Non-producer | <0.120 |
| Weak producer | 0.120 < OD < 0.240 |
| Strong producer | 0.240 < OD |
For the CoNS strains, S. epidermidis ATCC 35984 and ATCC 12228 were used as reference strains for strong and non-biofilm production, respectively. For S. aureus strains, S. aureus 15981 wild-type and 15981 Δica mutant were used as reference strains for strong and non-biofilm production, respectively.
2.6. Antimicrobial susceptibility testing using the microbroth dilution method
2.6.1. Susceptibility testing of biofilms: Minimum biofilm eradication concentration (MBEC)
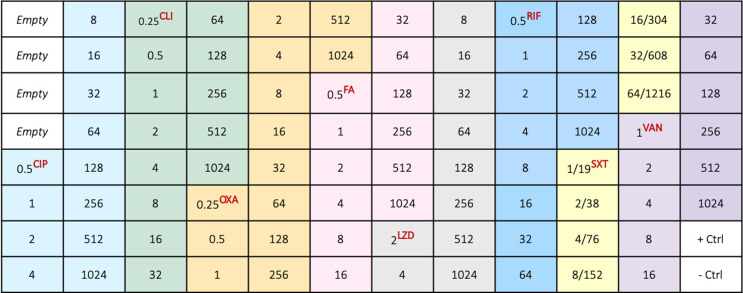
The Calgary Biofilm Device (CBD, MBEC Assay®, Innovotech Inc Edmonton, Canada) allows for the in vitro growth of biofilms [26,27]. A custom-made microbroth dilution plate (CML2FNUN; Sensititre™, Thermo Scientific™, MA, USA) was produced with increasing concentrations of eight common antimicrobials in PJI treatment (Fig. 3). The concentrations ranged from MIC determining levels to above breakpoint values (0.25–1216 μg/mL).
Fig. 3.
Antimicrobial agents and concentrations (μg/mL) included in the custom-made antimicrobial susceptibility plate used for the determination of MIC (minimum inhibitory concentration) and MBEC (minimum biofilm eradication concentration). First four empty wells had no antimicrobial agent. The following antimicrobial agents were included: ciprofloxacin (CIP), clindamycin (CLI), oxacillin + 2% NaCl (OXA), fusidic acid (FA), linezolid (LZD), rifampicin (RIF), trimethoprim/sulfamethoxazole (SXT) and vancomycin (VAN). One well was used as a positive control (+Ctrl) and another as a negative control (-Ctrl).
The CBD and the Sensititre™ antimicrobial plates were combined to determine the MBEC of the strains. The procedure for MBEC determination was done in accordance to Zaborowska et al., summarized in Fig. 2B [21]. In brief, 150 μL of an inoculum (107 CFU/mL) of each strain in Mueller-Hinton Broth 2 (Sigma Aldrich, MO, USA) was added to a CBD and incubated at 37 °C and 125 rpm for 24 h, for the formation of biofilms on the pegs of the CBD lid. Four pegs were removed with sterile pliers and placed in saline, vortexed for 1 min, sonicated for 1 min (40 Hz) and the detached cell solution was 10-fold serially diluted and cultured on blood agar plates o.n. at 37 °C, to quantitate the number of viable bacteria in the biofilms (CFU/peg). To determine the MBEC, biofilms grown on peg lids of the CBD were rinsed in saline and placed in the antimicrobial plate and incubated at 37 °C for approx. 20 h. Each peg lid was rinsed twice, placed in a neutralizing recovery plate, sonicated for 1 min to detach the treated biofilm into each well, and incubated o.n. at 37 °C. MBEC was determined by ocular inspection using the Sensititre™ Manual Viewbox. In cases of growth in the highest concentration, the calculations were based on the next doubling concentration.
2.6.2. Susceptibility testing of planktonic bacterial cells: Minimum inhibitory concentration (MIC)
MIC determinations were performed on planktonic cultures for all strains with equal final concentrations as for the biofilm susceptibility measurements, as described earlier by Zaborowska et al. [21] For each strain, 100 μL of an inoculum concentration equivalent to the same strain's CFU/peg was added to all the wells of the Sensititre® plate and incubated for approx. 20 h at 37 °C. The MIC was determined by ocular inspection as previously described. The S. aureus ATCC 29213 strain was used as control strain.
2.7. Statistical analysis
Descriptive statistics are presented as mean ± standard deviation, standard error of the mean, median and interquartile range (IQR) or mode and range. Chi-square test was used for the analysis of i) biofilm production (non/weak or strong), ii) surgical procedure (implant preservation or extraction) and iii) MBEC category (MBEClow or MBEChigh), in regards of clinical outcome, as well as for the comparison between the susceptibility determination by MIC and MBEC (susceptible or resistant according to the EUCAST MIC breakpoints [23]). Univariate logistic regression was conducted to analyse biofilm production and clinical outcome. One-Sample T-Test was used to compare the absolute CV OD values for biofilm production intra-species. MBEC/MIC-ratios were not normally distributed (Shapiro–Wilk), and Mann–Whitney U-test was used to analyse the relationship between MBEC/MIC-ratios and clinical outcome, as well as to compare the absolute values of MBEC and MIC, for each antimicrobial agent. Biofilm production (absolute values measured by CV OD) and MBEC/MIC-ratios were tested with Independent Samples T-Test. One-way ANOVA was used to analyse MBEC/MIC-ratios for each antimicrobial agent and biofilm production. Two-way ANOVA was conducted to examine the combined effect of biofilm production category and clinical outcome on MBEC/MIC-ratios for each antimicrobial agent. For all tests the significance was defined at p-value < 0.05. The statistical analyses were performed using SPSS Statistics (version 26, IBM corporation, USA) and R software (version 3.6.1, The R project, Vienna, Austria).
3. Results
3.1. Study participants and parameters
In total, 49 patients and 70 isolated strains were eligible for the study (Fig. 1). Of the 49 patients 16 (32.7%) were female and 33 (67.3%) male (Table 1). The majority of patients had a total hip arthroplasty (65.3%), mild systemic disease as defined by ASA (67.3%), monomicrobial infection (69.4%) and were treated for their PJI with implant preserving surgery (63.2%). Although non-significant, the duration from index surgery to symptom onset was longer in patients who had recurrent infection (Table 1). Receiving polytherapy with rifampicin (RIF) was more frequent in the group of resolved infections (n = 20) compared to the group of recurrent infections (n = 14). There was no relationship between surgical treatment (implant extracting or preserving procedures) in regard to clinical outcome (p = 0.806). Pre-operative blood sample parameters (erythrocyte sedimentation rate, white blood cell count and C-reactive protein) were similar in the two groups (Supplementary Table S2).
3.2. Characterization of the strains and biofilm production
Strains of S. aureus (n = 31), S. epidermidis (n = 31) and other CoNS (S. capitis n = 5, S. lugdunensis n = 2 and S. simulans n = 1) were isolated from intraoperative samples. Of the 70 isolates, 51 (72.9%) were categorized as strong biofilm producers and 19 (27.1%) as non/weak biofilm producers, as characterized by the microtiter plate test (Table 3).
Table 3.
The bacteriology of the 70 clinical isolates.
| Bacteriology for 70 strains (n = 70) | All strains | Infection resolution | Recurrent infection | p-value |
|---|---|---|---|---|
| Bacterial speciesn (%) | 0.52 | |||
| S. aureus | 31 (44.3) | 18 (50.0) | 13 (38.2) | |
| S. epidermidis | 31 (44.3) | 15 (41.7) | 16 (47.1) | |
| Other CoNS | 8 (11.4) | 3 (8.3) | 5 (14.7) | |
| Biofilm productionn (%) | 0.01 | |||
| Non/weak | 19 (27.1) | 15 (41.7) | 4 (11.8) | |
| Strong | 51 (72.9) | 21 (58.3) | 30 (88.2) | |
| MBEC degreen (%)∗ | 0.28 | |||
| MBEClow | 28 (46.7) | 18 (50.0) | 10 (29.4) | |
| MBEChigh | 32 (53.3) | 15 (41.7) | 17 (79.4) | |
Note: Pearson's Chi–Square test and Fischer's exact test were used for the comparisons of categorical variables. Abbreviations: CoNS = coagulase-negative staphylococci, MBEC = minimum biofilm eradication concentration.
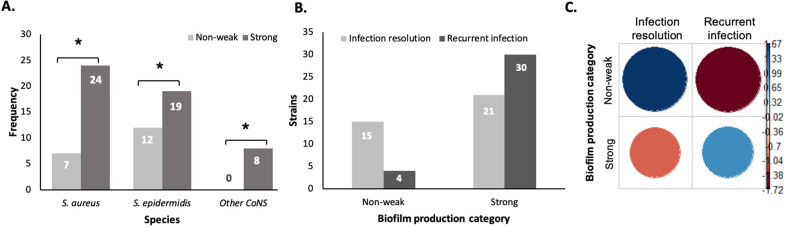
Moreover, the majority of strains within the same species had a higher proportion of strong biofilm producers than non/weak producers (Fig. 4A). The number of viable colony-forming units (CFU) in the biofilms grown on pegs was similar between the biofilm categories (Supplementary Table S1).
Fig. 4.
Distribution of biofilm production in bacterial species and for clinical outcome (A) Biofilm production within each bacterial species (S. aureus (n = 31), S. epidermidis (n = 31) and other CoNS (S. capitis n = 5, S. lugdunensis n = 2 and S. simulans n = 1)); (B) Distribution clinical outcome according to biofilm production category (non/weak producers vs strong producers); (C) Association between biofilm production and infection status (recurrent/resolved infection). The two blue circles indicate a significant association between the variables, and the red circles imply no association. The non/weak producers were significantly associated to infection resolution, whereas strong biofilm producers associated with recurrent infection (p = 0.011). Note: ∗indicates a statistically significant difference between the groups with p < 0.05. Data represent the strain frequencies attributed to bacterial species (A) and biofilm production category (B).
3.3. Clinical outcome in relation to biofilm production
Out of 49 patients, 24 (49%) had recurrent infection (Table 1), and among patients with recurrent infection, 22 (92%) had been infected by strong biofilm producers. Strong biofilm producers were more frequently found in patients with recurrent infections and non/weak producers were more often detected in patients with resolved infections (Fig. 4B): as shown by the significant association between biofilm production and clinical outcome (p = 0.011) (Fig. 4C). Univariate regression analysis showed a greater probability of recurrent infection in the presence of strong biofilm producers, with Odds Ratio 5.5 (95% confidence interval = 1.56–18.44, p = 0.008).
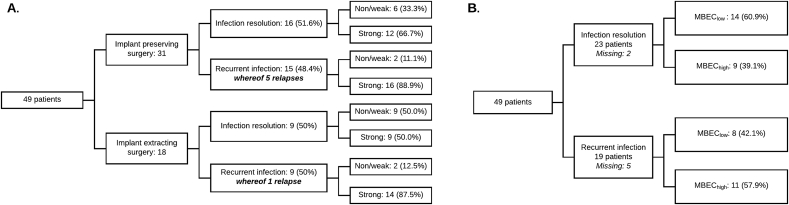
For 12 of the 24 patients with recurrent infection, strains were isolated and saved from subsequent operations. In this material, there were six confirmed infection relapses (Supplementary material, S3). Relapses were more commonly caused by strong biofilm producers (non/weak n = 1, strong n = 5), and were more often found in patients treated with implant preserving surgery (Fig. 5A).
Fig. 5.
Distribution of patients in surgical procedures, clinical outcome and strain properties (A) Distribution of patients according to surgery, clinical outcome and biofilm category. Patients having undergone implant preserving surgery had the most relapses n = 5. Biofilm production for each strain (n = 70) is listed to the right in the figure; (B) Distribution of patients according to clinical outcome and MBEC degree defined as either MBEChigh or MBEClow for each tested antimicrobial and strain. If a strain was defined as susceptible according to MBEC (following EUCAST clinical breakpoint definitions for MIC), the MBEC degree was considered low. If MBEC defined the strain as resistant, MBEC degree was considered high. MBEC degree was only based on the most potent oral antibiotic administered. In cases treated with RIF in polytherapy, RIF was considered the most potent. MBEC degree was defined according to a worst-case model where the highest MBEC degree was used if a patient had been infected by more than one strain. MBEC degree was missing in 7 patients (due to no oral antibiotics n = 5, or that the oral antibiotic received was not part of the antimicrobial susceptibility test panel n = 2).
3.4. MBEC in relation to clinical outcome
By our low threshold definition, although not statistically significant, MBEChigh was more frequent (57.9%) in cases with recurrent infection whereas MBEClow was more frequent (60.9%) in cases with resolved infection (Fig. 5B). No statistically significant association between MBEChigh or MBEClow and clinical outcome was observed (p = 0.275).
3.5. Antimicrobial susceptibility testing according to MIC and MBEC
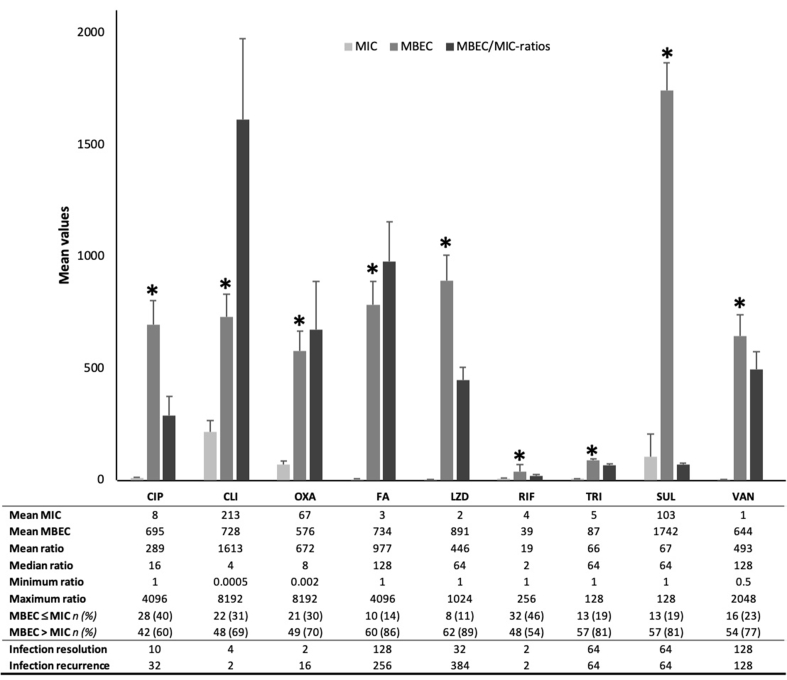
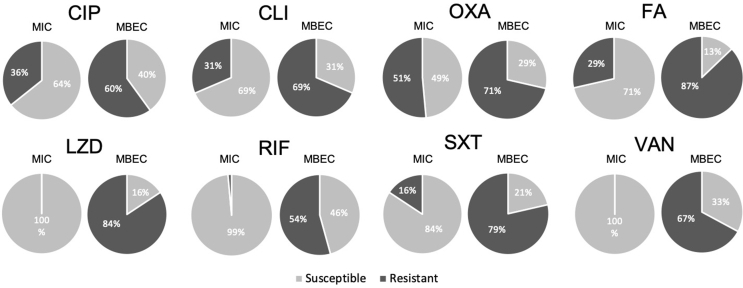
For all tested antimicrobial agents, absolute values of MBEC were significantly higher than MIC (Fig. 6), and for most of them susceptibility testing according to MIC and MBEC showed different antibiograms (Fig. 7). For all antimicrobial agents, a greater proportion of the strains was categorized as resistant according to MBEC (54–87%) compared to MIC (0–51%). Even the most susceptible antibiograms according to MIC (linezolid (LZD), rifampicin (RIF) and vancomycin (VAN)) increased greatly in resistance, by 54–84%, when tested according to MBEC. Oxacillin (OXA) was the antimicrobial with the most similar susceptibility pattern between MIC and MBEC.
Fig. 6.
The average mean values of MIC and MBEC, and mean MBEC/MIC-ratios per antimicrobial agent. MBEC ≤ MIC is the number of strains with MBEC equal to or lower than MIC. MBEC > MIC is the number of strains with MBEC higher than MIC. Both are presented with percentages. The median MBEC/MIC-ratios are presented for infection resolution and infection recurrence for each antimicrobial agent. Note: Data in the figure is presented as means with standard error of the means (SEM). ∗indicates statistical significant difference between the groups with p < 0.001. Abbreviations: ciprofloxacin (CIP), clindamycin (CLI), oxacillin + 2% NaCl (OXA), fusidic acid (FA), linezolid (LZD), rifampicin (RIF), trimethoprim/sulfamethoxazole (SXT) and vancomycin (VAN), minimum inhibitory concentration (MIC) and minimum biofilm eradication concentration (MBEC).
Fig. 7.
Antibiograms for each tested antimicrobial agent. The pie charts indicate the percentage of strains categorized as susceptible or resistant according to MIC and MBEC, using the EUCAST breakpoints. Overall, the staphylococcal strains isolated from PJI showed a greater resistance pattern to all antibiotics when grown as biofilms and the MBEC was determined.; Abbreviations: minimum inhibitory concentration (MIC), minimum biofilm eradication concentration (MBEC), ciprofloxacin (CIP), clindamycin (CLI), oxacillin + 2% NaCl (OXA), fusidic acid (FA), linezolid (LZD), rifampicin (RIF), trimethoprim/sulfamethoxazole (SXT) and vancomycin (VAN).
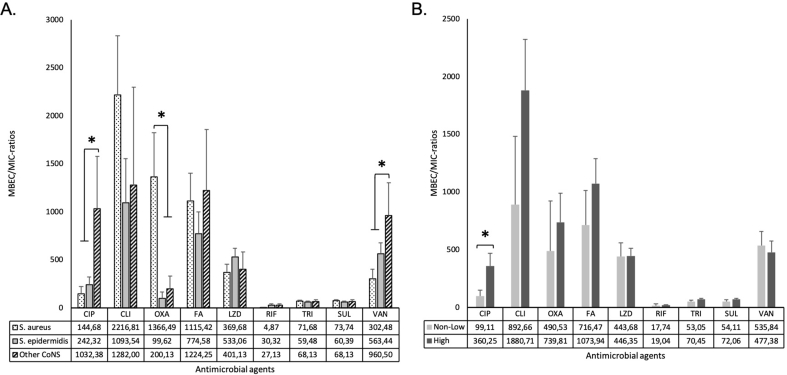
The MBEC/MIC-ratios, i.e. the fold increase in antimicrobial dose needed to inhibit or kill the strain in biofilm compared to planktonic growth, were calculated by dividing the absolute values for MBEC by MIC. The maximum ratios were 8192 (CLI and OXA) and the lowest 0.0005 (CLI) (Fig. 6). The median MBEC/MIC-ratios for the tested antimicrobials were between 2 (RIF) and 128 (VAN and FA). Ratios were compared between the different staphylococcal species. Other CoNS than S. epidermidis required higher MBECCIP and MBECVAN whereas S. aureus required higher MBECOXA (Fig. 8A).
Fig. 8.
MBEC/MIC-ratios in relation to bacterial species and biofilm category (A) Mean MBEC/MIC-ratios for each bacterial species (S. aureus (n = 31), S. epidermidis (n = 31) and other CoNS (S. capitis n = 5, S. lugdunensis n = 2 and S. simulansn = 1)); (B) Mean MBEC/MIC-ratios within each biofilm category. Note: Data is presented as means with standard error of the means (SEM). ∗indicates statistical significant difference between the groups with p < 0.05. Abbreviations: minimum inhibitory concentration (MIC), minimum biofilm eradication concentration (MBEC), ciprofloxacin (CIP), clindamycin (CLI), oxacillin + 2% NaCl (OXA), fusidic acid (FA), linezolid (LZD), rifampicin (RIF), trimethoprim/sulfamethoxazole (SXT) and vancomycin (VAN).
3.6. Relationship between biofilm production, MBEC/MIC-ratios for all antimicrobials and clinical outcome
Biofilm production category was compared to MBEC/MIC-ratios for each antimicrobial agent (Fig. 8B). MBEC/MIC-ratios for CIP showed a statistically significant difference between non/weak (99) and strong producers (360) (p = 0.037). No other statistically significant difference was found between the ratios and biofilm production. However, when biofilm formation was further categorized (non-, weak, moderate, strong), biofilm forming strains showed higher MBEC/MIC-ratios for OXA, TRI and SUL than non-biofilm producers (Supplementary material, S4).
For OXA only, the MBEC/MIC-ratios were significantly higher in recurrent infections compared to resolved infections (p = 0.01). The median MBECOXA was 16 times higher than MICOXA in recurrent infections compared to two times higher than MICOXA in resolved infections (Fig. 6). No statistically significant interaction between the combined effects of biofilm production and MBEC/MIC-ratios in clinical outcome was observed.
Discussion
The present study demonstrates that strong biofilms more likely lead to treatment failure in PJI. Overall, the majority of the staphylococcal strains included in this study showed strong biofilm formation ability as described in previous studies [9,28]. On the other hand, only a few studies report on biofilm production and clinical outcome. Some of these have identified a correlation between strong biofilm production and worse clinical outcome but include other species than staphylococci and other infections than PJI [21,28,29]. In patients with PJI and fracture fixation, Morgenstern et al. observed that cure rates decreased significantly when biofilm-forming ability increased (76–84% cure rates for non/weak producers versus 60% for strong producers); and Post et al. showed that strong biofilm formation was associated with non-resolved infections [29,30]. In contrast, there are studies on non-orthopaedic biofilm infections where no correlation between biofilm production and clinical outcome was found [10,31,32]. However, comparisons of study results are difficult as study protocols, bacterial species, classifications of biofilm production, definitions of clinical outcome and type of infections vary.
In the present study, MBECs were significantly higher compared to MICs, in agreement with previous research [[17], [18], [19],21,[33], [34], [35]]. Strains growing as biofilms required up to 8192 times higher concentrations of antimicrobials than as planktonic. Intravenous VAN as mono- or polytherapy and RIF in polytherapy were the most common antimicrobial agents used in our cohort. It should be noted that VAN coincided with the highest median MBEC/MIC-ratio in our material (128) and 77% of the strains had higher MBECVAN than MICVAN (Fig. 6). Resistance to VAN according to MBEC has been previously reported in biofilm forming strains [34]. For RIF, the median MBEC/MIC-ratio was the lowest of all tested antimicrobials. Yet, 54% of the strains required between 2 and 256 times higher RIF concentrations to eradicate the biofilm than when in planktonic state. RIF is considered among the most effective alternative for treatment of staphylococcal biofilms [10,19]. However, the current study indicates that the prescribed RIF dose based on MICRIF may have been insufficient. The relative inability for eradication of biofilm using RIF has previously been reported and its use may result in treatment failure in about 1/3 of implant retaining regiments [36].
In the current study, cell amounts in planktonic cultures were set up to be equal to biofilm cultures (CFUplanktonic = CFUbiofilm). Hence, differences in MIC and MBEC were mainly due to the formation of biofilm and not the cell number, as further confirmed by similar CFU/peg in non/weak and strong biofilm producers. Increased biofilm biomass has previously not been related to increased cell numbers [21]. In vitro assays aim to mirror in vivo biofilm formation but the total biofilm biomass and maturity formed in the patient is difficult to establish and reproduce. Although some studies have detected biofilms in vivo [8,37], an increased understanding of the phenotypic characteristics and molecular mechanisms of biofilms in humans is urgently required. Further, the presence, distribution and activities of host defence cells in relation to the biofilm would be of great relevance. In this context, the systematic evaluation of retrieved, explanted implants and associated tissue would be interesting.
Furthermore, MIC guided treatment may not reflect the true susceptibility of bacteria grown as biofilm on prostheses [20]. Although a bacterial strain may be reported susceptible according to MIC, clinicians need to be aware that MICs may not reflect the antimicrobial's ability to eradicate the same strain when grown as biofilm. Inadequate antimicrobial doses lead to sub-inhibitory concentrations in vivo, which in turn may induce biofilm formation [38], and the development of antimicrobial resistance. MBEC determination may be a complementary method to optimize the selection of antimicrobials in biofilm-associated infections such as PJI. However, the present results confirm that dosing systemic antimicrobials to surpass MBECs could imply toxicity in patients [19]. Therefore, meticulous surgery including sufficient reduction of avascular tissues and biofilm, is judged as highly important for treatment success [39]. In our study, we found that there was no difference in infection recurrence when the surgical methods were compared. However, a greater number of infection relapses were observed in patients who had been treated with implant preservation surgery. Determining biofilm properties by using methods, like those employed in this study (CV), may help decision-making on which infections are suitable for implant preserving treatment, and which would require more drastic approaches such as implant exchanging surgery.
A limitation of the study is that the selection of patients was dependent on monomicrobial staphylococcal PJI and the number of stored bacterial strains. However, this sample size was enough to address the primary outcome, and a significant association was observed between biofilm production and clinical outcome. Nevertheless, MBEClow and MBEChigh did not show a consistent association to neither biofilm production nor clinical outcome. This could be due to sample size (seven missing cases treated with antimicrobials not included in this study), the distribution of strains (low number of non/weak biofilm producers) or the fact that the MBECs were consistently higher than MICs in both outcome groups. Furthermore, it could be hypothesized that non/weak biofilm producers were intrinsically resistant to several antimicrobial agents, resulting in lower MBEC/MIC-ratios, like those observed for CIP, CLI, OXA and SXT. Interestingly, a previous study also showed that antimicrobial resistance did not influence treatment cure rate of device-related osteomyelitis caused by S. epidermidis [30].
Since it was not possible to attribute which strain was the causative strain, all strains were analysed in cases where several strains were isolated from the same patient. As any synergistic effect between the strains is unknown, it may be more justified to regard the combined strains as the causative agent. Our model of analysis allocated all strains of a polymicrobial infection to the same clinical outcome regardless of their individual biofilm ability. Classifying clinical strains into their different biofilm formation abilities is challenging and there are different procedures and breakpoints for the classification [28,34]. Moreover, for the allocation of strains into susceptible/resistant categories the clinical breakpoints for MIC testing were used [40], since at present there are no standardized definitions of biofilm susceptibility breakpoints. In this study, when a strain was susceptible according to MIC it was also categorized as susceptible according MBEC, and likewise when a strain was resistant according to MIC it was also resistant according to MBEC. This further validates the use of MBEC since it agreed with MIC in detecting the strains with intrinsic antimicrobial resistance.
Although an acknowledged method of studying biofilm susceptibility, the CBD is an in vitro model and cannot be directly translated to in vivo settings [27]. The peg lids of the CBD do not resemble the design, chemistry and topography of the most common prosthetic materials and test media do not exactly reflect the tissue conditions or include components of the host immune response, which bacteria are exposed to in vivo [27]. However, current diagnostic methods, such as MIC, are also based on polystyrene and standardised culture media to ensure reproducibility, easy implementation and long-shelf life, which is why the CBD used in this study followed the same principle. The MBEC method is reproducible, likely to be easy to implement by clinical laboratories, inexpensive, and can provide results within five days from pathogen isolation. Prospective studies on MBEC as a future surrogate marker for antimicrobial potency against biofilm infections are warranted.
The demography of patients in the group of recurrent and resolved infections was similar, so was the distribution of surgical treatment. In regards of antimicrobial treatment, a higher frequency of patients with infection resolution had been treated with RIF in combination therapy. There may be remaining factors contributing to infections that we have not been able to address, but the similarity of demography in the clinical outcome groups supports that the main difference may be biofilm production.
Conclusions
Strong biofilm production was associated with worse clinical outcome and represented a five-fold increased risk for developing recurrent infection. This novel finding, suggests the importance of evaluating biofilm production as part of the underpinning basis for diagnostic decisions in PJI. In agreement with previous recommendations, methods to determine biofilm production, such as the microtiter plate test, are suggested to be part of the clinical routine [33,34]. Biofilms were more susceptible to rifampicin than the other antimicrobial agents evaluated. The effects of biofilm susceptibility on clinical outcome need to be further elucidated in a prospectively followed patient cohort of PJI.
Funding
This work was sponsored by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 754412 [MoRE2020 - Region Västra Götaland], CARe - Centre for Antibiotic Resistance Research at University of Gothenburg, Swedish Research Council [2018–02891], the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement [ALFGBG-725641; ALFGBG-719961], the Inga-Britt and Arne Lundberg Foundation, the Hjalmar Svensson Foundation, Doctor Felix Neuberghs Foundation, the Adlerbertska Foundation, the Sylvan Foundation, Göteborgs Läkarsällskap/The Gothenburg Medical Society research grants [for PhD-studies and Svea Bäcksins grant GLS-780551], and the Area of Advance Materials of Chalmers/GU Biomaterials within the Strategic Research Area initiative launched by the Swedish government.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank Dr. Bodil Jönsson (Department of Infectious Diseases, Institute for Biomedicine, Sahlgrenska Academy, University of Gothenburg, Sweden) for helping us recover the stored bacterial isolates. We would also like to thank Björn Andersson at the Bioinformatics Core Facility (University of Gothenburg, Sweden) for help with the statistical analysis of data.
Footnotes
The material contained in this manuscript has not been published elsewhere and is not being currently submitted elsewhere. Karin Svensson Malchau and Margarita Trobos.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2021.05.008.
Contributor Information
Karin Svensson Malchau, Email: karin.am.svensson@vgregion.se.
Margarita Trobos, Email: margarita.trobos@biomaterials.gu.se.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Moore A.J. Deep prosthetic joint infection: a qualitative study of the impact on patients and their experiences of revision surgery. BMJ Open. 2015;5(12) doi: 10.1136/bmjopen-2015-009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi T.J., Konopka J.F., Bedair H.S. What is the long-term economic societal effect of periprosthetic infections after THA? A markov analysis. Clin Orthop Relat Res. 2017;475(7):1891–1900. doi: 10.1007/s11999-017-5333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemes S. Projections of total hip replacement in Sweden from 2013 to 2030. Acta Orthop. 2014;85(3):238–243. doi: 10.3109/17453674.2014.913224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campoccia D., Montanaro L., Arciola C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Lowik C.A.M., Parvizi J., Jutte P.C., Zijlstra W.P., Knobben B.A.S., Xu C. Debridement, antibiotics and implant retention is a viable treatment option for early periprosthetic joint infection presenting more than four weeks after index arthroplasty. Clin Infect Dis. 2020;71(3):630–636. doi: 10.1093/cid/ciz867. [DOI] [PubMed] [Google Scholar]
- 6.Fey P.D., Olson M.E. Current concepts in biofilm formation of Staphylococcus epidermidis. Future Microbiol. 2010;5(6):917–933. doi: 10.2217/fmb.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flemming H.C., Neu T.R., Wozniak D.J. The EPS matrix: the "house of biofilm cells". J Bacteriol. 2007;189(22):7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoodley P. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008;90(8):1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson S. A novel soft tissue model for biomaterial-associated infection and inflammation - bacteriological, morphological and molecular observations. Biomaterials. 2015;41:106–121. doi: 10.1016/j.biomaterials.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Kwiecinski J.M. Biofilm formation by Staphylococcus aureus clinical isolates correlates with the infection type. Inf Disp. 2019;51(6):446–451. doi: 10.1080/23744235.2019.1593499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura H. Quantitative analysis of biofilm formation of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients with orthopaedic device-related infections. FEMS Immunol Med Microbiol. 2011;63(1):10–15. doi: 10.1111/j.1574-695X.2011.00821.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs A.M.E. Evaluation one year after DAIR treatment in 91 suspected early prosthetic joint infections in primary knee and hip arthroplasty. J Bone Jt Infect. 2019;4(5):238–244. doi: 10.7150/jbji.37757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehring T.K. Failure of irrigation and debridement for early postoperative periprosthetic infection. Clin Orthop Relat Res. 2013;471(1):250–257. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim M.S. A multidisciplinary team approach to two-stage revision for the infected hip replacement: a minimum five-year follow-up study. Bone Joint Lett J. 2014;96-B(10):1312–1318. doi: 10.1302/0301-620X.96B10.32875. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerli W., Trampuz A., Ochsner P.E. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 16.Osmon D.R. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):1–10. doi: 10.1093/cid/cis966. [DOI] [PubMed] [Google Scholar]
- 17.Donlan R.M. Role of biofilms in antimicrobial resistance. Am Soc Artif Intern Organs J. 2000;46(6):S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Marques C. Effects of antibiotics on biofilm and unattached cells of a clinical Staphylococcus aureus isolate from bone and joint infection. J Med Microbiol. 2015;64(9):1021–1026. doi: 10.1099/jmm.0.000125. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen N.P. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis. 2016;74(4):ftw019. doi: 10.1093/femspd/ftw019. [DOI] [PubMed] [Google Scholar]
- 20.Moriarty T.F. Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Rev. 2016;1(4):89–99. doi: 10.1302/2058-5241.1.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaborowska M. Biofilm formation and antimicrobial susceptibility of staphylococci and enterococci from osteomyelitis associated with percutaneous orthopaedic implants. J Biomed Mater Res Part B. 2017;105B:2630–2640. doi: 10.1002/jbm.b.33803. [DOI] [PubMed] [Google Scholar]
- 22.Parvizi J. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309–1314 e2. doi: 10.1016/j.arth.2018.02.078. [DOI] [PubMed] [Google Scholar]
- 23.Diseases, E.S.o.C.M.a.I . 2020. European commitee on antimicrobial susceptibility testing.http://www.eucast.org/clinical_breakpoints/ Available from: [Google Scholar]
- 24.Valle J., Echeverz M., Lasa I. sigma(B) inhibits poly-N-acetylglucosamine exopolysaccharide synthesis and biofilm formation in Staphylococcus aureus. J Bacteriol. 2019;201(11) doi: 10.1128/JB.00098-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldassarri L. Effect of iron limitation on slime production by Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2001;20(5):343–345. doi: 10.1007/pl00011274. [DOI] [PubMed] [Google Scholar]
- 26.Ceri H. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37(6):1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison J.J. Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat Protoc. 2010;5(7):1236–1254. doi: 10.1038/nprot.2010.71. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez C.J., Jr. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Post V. Comparative genomics study of Staphylococcus epidermidis isolates from orthopedic-device-related infections correlated with patient outcome. J Clin Microbiol. 2017;55(10):3089–3103. doi: 10.1128/JCM.00881-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgenstern M. Biofilm formation increases treatment failure in Staphylococcus epidermidis device-related osteomyelitis of the lower extremity in human patients. J Orthop Res. 2016;34(11):1905–1913. doi: 10.1002/jor.23218. [DOI] [PubMed] [Google Scholar]
- 31.Cha J.O. Investigation of biofilm formation and its association with the molecular and clinical characteristics of methicillin-resistant Staphylococcus aureus. Osong Public Health Res Perspect. 2013;4(5):225–232. doi: 10.1016/j.phrp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guembe M. Biofilm production is not associated with poor clinical outcome in 485 patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2018;24(6):659 e1–659 e3. doi: 10.1016/j.cmi.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Brady A.J. Antibiotic susceptibility of planktonic- and biofilm-grown staphylococci isolated from implant-associated infections: should MBEC and nature of biofilm formation replace MIC? J Med Microbiol. 2017;66(4):461–469. doi: 10.1099/jmm.0.000466. [DOI] [PubMed] [Google Scholar]
- 34.Antunes A.L. Application of a feasible method for determination of biofilm antimicrobial susceptibility in staphylococci. APMIS. 2010;118(11):873–877. doi: 10.1111/j.1600-0463.2010.02681.x. [DOI] [PubMed] [Google Scholar]
- 35.Molina-Manso D. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents. 2013;41(6):521–523. doi: 10.1016/j.ijantimicag.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Lora-Tamayo J. Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents. 2016;48(3):310–316. doi: 10.1016/j.ijantimicag.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Naumenko Z.S. Challenging diagnostics of biofilm associated periprosthetic infection in immunocompromised patient: a clinical case. Open Access Maced J Med Sci. 2019;7(5):786–790. doi: 10.3889/oamjms.2019.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majidpour A. Dose-dependent effects of common antibiotics used to treat Staphylococcus aureus on biofilm formation. Iran J Pathol. 2017;12(4):362–370. [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell J.B. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37(7):1604–1609. doi: 10.1002/jor.24291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The European Committee on Antimicrobial Susceptibility Testing . 2020. Breakpoint tables for interpretation of MICs and zone diameters.http://www.eucast.org Version 10.0. Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.