Abstract
Introduction
Dirofilaria repens is a vector-borne filaroid helminth of carnivorous animals, primarily domesticated dogs. Humans are considered to be accidental hosts in which D. repens rarely reach sexual maturity but induce local inflammation, mainly in subcutaneous and ocular tissues.
Methods
In the current study, we present the detection of multiple adults of D. repens, endosymbiont Wolbachia sp. and microfilariae by molecular analysis in peripheral tissues and bloodstream of a human host. A subsequent meta-analysis of published literature identified 21 cases of human infection with adult D. repens producing microfilariae.
Results
Within the study population, there were 13 (59.09%) males, eight (36.36%) females and, in one (4.55%) case, sex was not reported. A total of 11 (50.00%) cases had subcutaneous dirofilariasis, six (27.27%) had ocular dirofiliariasis, with single cases (4.55% each) of genital, mammary, lymphatic and a combination of subcutaneous and pulmonary dirofilariasis described. In one (4.55%) case, the primary anatomical site of adult D. repens could not be found. D. repens microfilariae were detected in the local tissue (local microfilariasis) in 11 (50.00%) cases and the peripheral blood (microfilaremia) in 11 (50.50%) cases. Final identification of D. repens microfilariae was based on morphological detection in 14 (63.64%) cases, and molecular detection in eight (36.36%) cases.
Conclusion
The results of this study suggest that humans may act as a final host for D. repens, however its role as a source of D. repens infection is less clear.
Keywords: Dirofilaria repens, Microfilaremia, Human, Host
Highlights
-
•
Humans may act as a final host for D. repens
-
•
Immunodeficiency is not a risk factor for human D. repens microfilaremia.
-
•
Surgical extirpation of adult D. repens is a mainstay of treatment.
1. Introduction
Dirofilaria repens is a vector-borne filaroid helminth of canids with dogs representing the major reservoirs of infestation. The full life-cycle of D. repens comprises five larval stages with a prepatent period of approximately 6–9 months [1]. The development of the parasite depends on the availability of competent mosquito species, suitable hosts, adult male and female D. repens helminths and the presence of the bacterial endosymbiont, Wolbachia sp. [2], the latter required for successful molting and embryogenesis of filariae. Humans acquire D. repens infestation in the same manner as dogs after the bite of a mosquito species from the Culicidae family [3]. In most cases, however, infective larvae are detected by the body's immune system, leading to destruction of the parasite prior to the infestation being recognised [4]. In some cases, a single larva can survive and molt into a preadult and adult worm. D. repens infestation is manifested with local inflammation, mainly in subcutaneous and ocular tissues. Symptoms are usually mild and resolve shortly after surgical extraction of the worm [5]. The development of D. repens into a sexually mature worm in humans appears to be uncommon, with antigen sets from both D. repens and their endosymbiont Wolbachia sp. stimulating specific immunologic reactions that block complete development of the helminth [1]. For this reason, humans were considered to be dead-end hosts for these helminths [6]. In rare cases, D. repens can avoid the host's defence mechanisms and reach sexual maturity [7].
In the literature, there are currently 10 case reports of human D. repens microfilaremia, and only a few are confirmed with molecular analysis [8]. In the currently study, we report on the detection of multiple adults of D. repens, Wolbachia sp. and microfilariae in peripheral blood in a human case of D. repens filariasis. Additional meta-analysis of the available literature was also conducted identifying additional evidence to support humans as a definitive host for this helminth.
2. Case description
Full details of the patient's clinical history are provided in Supplmentary Material 1. Briefly, a 17-year old adolescent athlete presented at an emergency room on 10th December 2019 due to an acute onset of burning pain in the left inguinal region, followed by formation of a shallow subcutaneous nodule measuring 5 × 3 cm in size. The patient was subfebrile (37.2 °C), in a good general condition without any previous relevant medical history. He denied allergies to food and drugs with a skin prick test confirming negative results. Blood tests showed elevation of eosinophil to 16%, but all other parameters were unremarkable. A solution of 80 mL methylprednisolone was administrated intramuscularly with a recommendation of daily use of betamethasone cream locally on the skin lesion.
On 7th January 2020, examination of a nodule in the left inguinal region noted a linear plaque measuring 2 × 7 cm (Supplementary Figs. 1A and 1B). The laboratory test showed leukocyte counts of 12.5 cells/μL and 27% eosinophils. All other blood parameters were unremarkable. The patient denied international travel during the previous year but confirmed daily contact with a neighbour's three dogs. Coprological and serologic tests for intestinal and systemic parasitic diseases were subsequently ordered. On 14th January 2020, control examination revealed an increase in leukocyte count of 15.4 cells/μL with 35% eosinophils. All serological assays and coprological tests, repeated three times, were negative.
On 20th January 2020, the plaque in the left inguinal region spontaneously resolved, however, two additional painless, subcutaneous nodules were detected. One oval nodule in the left hypochondrium measured 1 × 2 cm (Supplemental figs. 1C and 1D), and a round nodule in the left axilla measured 1.5 × 1.5 cm. Ultrasonography imaging of the abdominal wall nodule showed a fusiform and elongated hyperechoic structure within the left rectus abdominis muscle. Radiological findings suggested parasitic myositis, so fine needle aspiration (FNA) of the nodule was performed. Eosinophil counts in peripheral blood were raised to 44% corresponding to FNA findings of numerous mature eosinophils, moderate numbers of mature lymphocytes, several macrophages and plasma cells. No parasitic structures were found.
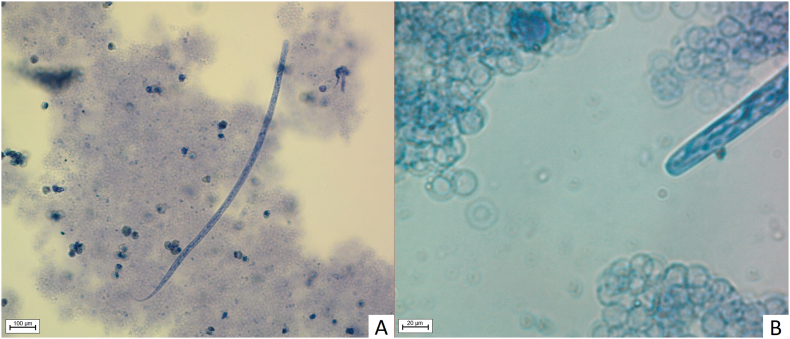
Blood samples were collected for dirofilariasis screening on 23rd January 2020. Modified Knott's test performed on 6 mL of EDTA blood (6 × 1 mL) revealed the presence of 2 microfilaria/mL that morphologically corresponded to D. repens (Fig. 1). The suspected presence of D. repens was confirmed using species-specific PCR that amplifies a portion of the cytochrome oxidase subunit 1 (COI) gene [9].
Fig. 1.
Microfilaria of D. repens stained with Diff Quick. 1A: Whole microfilaria; 1B: Anterior part (AxioImager. M2, Zeiss, Jena, Germany).
At the same time, skin inspection revealed a new nodule, measuring 1 cm in diameter, located under the intact skin of the neck (Supplementary Figs. 1E and 1F). Surgical excision of all three subcutaneous nodules was performed on 27th January 2020. In preoperative laboratory findings, eosinophilia was evaluated, reaching 48% of white blood cells. A transversal cut surface of two extracted nodules had centrally placed white, slender, filarial worms.
All symptoms resolved within 48 h after surgical removal of parasites. No additional treatment was applied. Over the next three weeks, the patient was regularly monitored for skin lesions, complete and differential blood count and peripheral blood microfilaremia (Table 1). Additionally, blood samples from all three dogs in contact with the patient were collected for dirofilariasis screening.
Table 1.
Laboratory results of complete leukocyte count, relative lymphocyte and eosinophil count, Knott's test and molecular detection of Dirofilaria repens and Wolbachia sp. prior and after surgical extraction of nematodes. Date of operative procedure is marked with underline. Wbc – white blood count; Ref – reference; PCR – polymerase chain reaction; Jan – January, Feb – February, Mar – March.
| Parameter | Jan 7 | Jan 14 | Jan 22 | Jan 24 | Jan 27 | Feb 3 | Feb 13 | Mar 9 | May 29 |
|---|---|---|---|---|---|---|---|---|---|
| Wbc (x 109/L) Ref range: 4.4–11.6 |
12.5 | 15.4 | 14.8 | 14.8 | 13.6 | ⁎ | ⁎ | ⁎ | ⁎ |
| Lymphocytes (%) Ref range: 19–52 |
31 | 22 | 20.1 | 18 | 19 | ⁎ | ⁎ | ⁎ | ⁎ |
| Eosinophils (%) Ref range: 0–9 |
27 | 35 | 44 | 48 | 40 | 20 | 28 | 12 | 4.5 |
| Knott's test (microfilariae/mL) |
12/4.5 | 0/4 | 0/6 | 0/4 | 0/4 | 0/4 | |||
| PCR D. repens | + | − | − | − | − | − | |||
| PCR Wolbachia sp. | + | − | − | − | − | − |
Within reference value.
3. Materials and methods
3.1. Morphological and molecular examinations
The modified concentration procedure by Knott was performed on EDTA blood samples collected from the human patient and dogs for detection of microfilariae (L1) [1] during the study period.
For species confirmation, parasites or tissue samples were cut in pieces and DNA was extracted using the DNA ‘Blood and tissue kit’ (Qiagen, Hilden, Germany) in the automatic extraction system Qiacube (Qiagen, Hilden, Germany). The same kit was used for extraction of nucleic acids from 200 μL blood samples. Species-specific PCRs that amplify a fragment of approximately 200 bp specific to the COI gene for D. immitis (DI COI —F1 AGT GTA GAG GGT CAG CCT GAG TTA and DI COI-R1 ACA GGC ACT GAC AAT ACC AAT) and for D. repens (DR COI-F1 AGT GTT GAT GGT CAA CCT GAA TTA and DR COI-R1 GCC AAA ACA GGA ACA GAT AAA ACT) were used in the study [9]. For sequencing, the protocol described by Casiraghi et al. [10] was applied to amplify a 667-bp region of the COI gene. All samples were also screened by conventional PCR for Anaplasma/Ehrlichia species based on amplification of a 345-bp 16S rRNA gene fragment [11]. The amplified products were analyzed by capillary electrophoresis (QIAxcel System®, QIAGEN) with size markers in the range of 100–2500 bp. Samples were purified with ExoSAP-IT® (USB Corp., Cleveland, United States) and sequenced in both directions by Macrogen Inc. (The Netherlands). Sequences were assembled using the SeqMan Pro software, edited with EditSeq of the Lasergene software (DNASTAR, Madison WI, USA) and compared with available sequences using BLAST.
3.2. Literature review
Metadata on cases of human infection with D. repens in adult (L5) and microfilarial (L1) stage were analyzed. The studies and their analysis examined in the present work are the result of extensive text mining and searching through electronically available databases (Medline/PubMed, Web Of Science, Embase, Scopus), individual journals and proceedings papers for all results retrieved by searches of any of the keywords: “Zoonosis”, “Vector-borne disease”, “Parasite”, “Helminths”, “Nematode”, “Human”, “Dirofilariasis”, “Dirofilaria repens“, “Microfilaria”, “Microfilaremia”, “Microfilariasis”, “Blood”, “Subcutaneous”, “Ocular”, “Eosinophils”, “Eosinophilia”, “Knott”, “PCR”; as well as their combinations. Both cases with D. repens microfilariae detected in peripheral blood and/or local tissues were considered. The cross-referenced list of articles included in the review was manually checked for relevant studies. All studies written in English and other than English language were analyzed. After the screening of all identified articles, only those that met the criteria for eligibility were included in the study.
The search retrieved a total number of 19 articles [5,7,8,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. One article was excluded because it was not available [28]. The review contained articles published until May 2021.
4. Results
4.1. Molecular detection
D. repens adults were detected in two surgically removed nodules. Microscopic examination revealed the presence of female-producing microfilariae in the nodule from the hypogastic region (Fig. 2), while females isolated from the axillar region were free from microfilariae (Fig. 3). No male parasites were detected. Both amplified sequences (MT847642) were identical to each other but showed two nucleotide difference from Croatian D. repens sequences previously detected in a sample obtained from a human scrotum (KX265049). Blood samples were collected from all three pet dogs owned by the patient's neighbour. Microscopic analysis of blood smears after Knott's concentration test revealed 7500 and 8200 microfilariae/mL in two of the dogs, respectively. Identical sequencing results were obtained from both dogs and human patient.
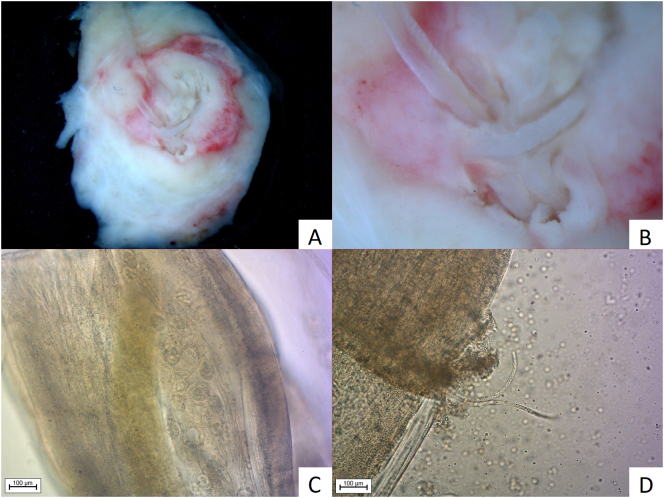
Fig. 2.
Nodule removed from hypogastric region immersed in physiological saline solution. 2A, 2B: Nodule (5 × 1 cm) with centrally placed D. repens; 2C: Microfilariae in uterus of female; 2D: Microfilariae releasing from female (AxioImager. M2, Zeiss, Jena, Germany).
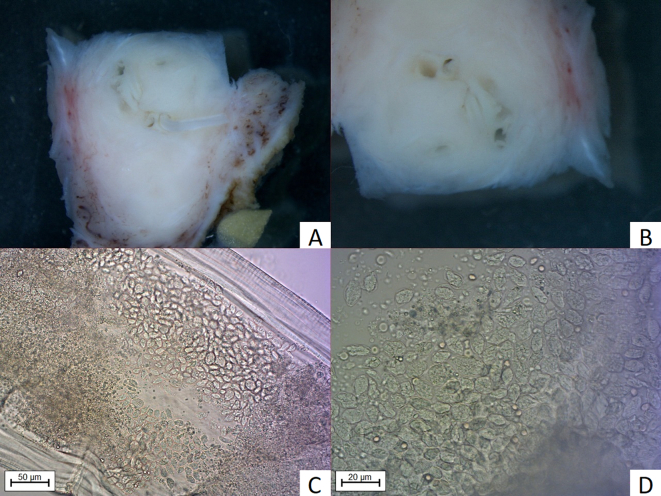
Fig. 3.
Nodule removed from the axillar region. 3A, 3B: Nodule (2.5 × 1 cm) with centrally placed D. repens (StereoDiscovery.V20, Zeiss, Jena, Germany); 3C, 3D: Eggs within uterus of female visible after enlightening with lactophenol (AxioImager. M2, Zeiss, Jena, Germany).
Partial sequences amplified from microfilaria from blood and adult parasites were identical to a Wolbachia sp. endosymbiont of D. repens previously detected in blood from Croatian dogs [29].
4.2. Histopathology
Macroscopically, the tissues removed from the axillar and hypogastric regions were of a soft consistency, measuring 2.5 × 1 cm and 5 × 1 cm and contained centrally placed curled, thin white “structures” 3 mm thick within a narrow canal-like cavity (Fig. 2A and B, Fig. 3A and B). The surrounding panniculus, muscular fibers and dermis showed marked irregular thickening. The samples had a firm consistency with a greyish-red to yellow appearance. The nodular structure removed from the nuchal region on the cut surface revealed a lymph node embedded in edematous subcutaneous tissue.
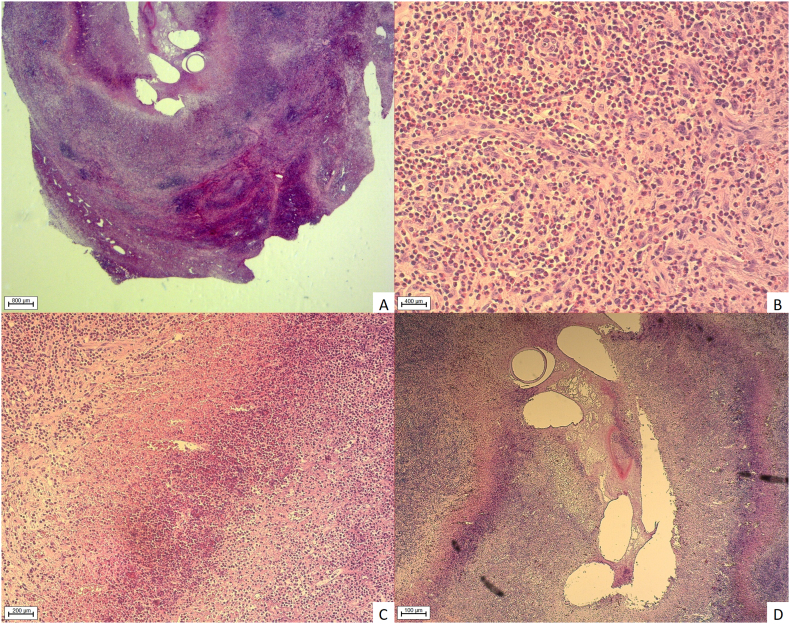
Histology of both nodules containing nematodes revealed severe, poorly demarcated infiltration of the subcutaneous and/or skeletal muscle tissue by numerous eosinophilic granulocytes, scattered lymphocytic follicular agglomerations and fewer macrophages, plasma cells, and rare mast cells (Fig. 4A). These infiltrates replaced and extended adipose and/or rectus muscle tissue circumferentially, extending numerous newly formed blood vessels on the lesion periphery (Fig. 4B and C). Many of the newly formed blood vessels showed endothelial hypertrophy embedded within eosinophilic granulocytes cuffs, rare fibroblasts and few lymphoid follicles (Fig. 4C). Massive areas of liquefactive necrosis (Fig. 4D) centrally within both lesions were found harbouring multiple cross and a few longitudinal sections of metazoan parasites, measuring approximately 200–300 μm in diameter. Nematodes showed a 4 μm thick, smooth, eosinophilic cuticle (Fig. 4D). Cuticular ridges were not preserved on longitudinal sections. Coelomyarian musculature was not preserved in the sections, as well as organs of the body cavity.
Fig. 4.
Histopathological examination. 4A: Complete replacement of subcutaneous and muscular tissue by inflammatory cells. 4B: Numerous transversal, tangential and longitudinal sections of blood vessels with pronounced endothelial hyperplasia surrounded by myriad eosinophilic granulocytes. 4C: Border between degenerated inflammatory cells and vital eosinophilic granulocytes. 4D: Transversal cuts through nematode cuticle, artificial empty spaces (loss of nematodes elements) embedded in fibrin, cellular and nuclear debris (AxioImager. M2, Zeiss, Jena, Germany).
4.3. Literature review
The bibliography search retrieved 19 results between 1992 and 2021, reporting a further 21 human infestations with adult D. repens producing microfilariae beyond the case described in this study. A total of 20 (90.91%) cases were from Europe and two (9.09%) from Asian countries. Only four (18.18%) patients had medical record of chronic immune disorder while others were immunocompetent (63.64%) or data were not described (18.18%) (Table 2).
Table 2.
Epidemiological characteristics of human infection with D. repens in adult (L5) and microfilarial (L1) stage (chart review of the world literature). Ref – reference.
| Ref | Year | Age | Sex | Country | Travelling history |
Immunological status |
|---|---|---|---|---|---|---|
| [17] | 1992 | 53 | Female | Italy | Unknown | Malignancy |
| [18] | 1994 | 50 | Male | France | Corsica | Malignancy |
| [16] | 1998 | 70 | Male | Greece | None | Immunocompetent |
| [12] | 2004 | 60 | Male | Russia | None | Immunocompetent |
| [13] | 2005 | 62 | Male | Hungary | None | Malignancy |
| [19] | 2007 | 40 | Male | Iran | None | Immunocompetent |
| [23] | 2009 | 40 | Male | Serbia | None | Immunocompetent |
| [23] | 2009 | 21 | Male | Serbia | None | Immunocompetent |
| [15] | 2009 | 45 | Male | Germany | India; Sri Lanka | Immunocompetent |
| [22] | 2013 | 63 | Female | Russia | Southeast Asia | Immunocompetent |
| [14] | 2013 | 28 | Female | Ukraine | Unknown | Unknown |
| [14] | 2013 | 65 | Female | Ukraine | Unknown | Unknown |
| [24] | 2014 | 62 | Male | India | Unknown | Immunocompetent |
| [26] | 2015 | 38 | Female | France | Tunisia | Immunocompetent |
| [21] | 2016 | 30 | Female | Italy | India; Australia | Immunocompetent |
| [5] | 2016 | 17–61 | Unknown | Czech Republic | Hungary; Slovakia; Croatia | Unknown |
| [25] | 2017 | 70 | Female | France | New Caledonia | Immunocompetent |
| [8] | 2018 | 28 | Male | Poland | None | Immunocompetent |
| [7] | 2018 | 76 | Male | Belgium | Senegal | Diabetes mellitus |
| [20] | 2020 | 56 | Female | Austria | Greece; India | Immunocompetent |
| [27] | 2021 | 79 | Male | Germany | Sri Lanka | Unknown |
| ⁎ | 2021 | 17 | Male | Croatia | None | Immunocompetent |
current study.
Within the study population, there were 13 (59.09%) males, eight (36.36%) females and, in one (4.55%) case, sex was not reported. The mean age at presentation was 50.14 years, ranging from 17 to 79 years. A total of 11 (50.00%) cases had subcutaneous dirofilariasis, six (27.27%) had ocular dirofiliariasis, with single cases (4.55% each) of genital, mammary, lymphatic and a combination of subcutaneous and pulmonary dirofilariasis described. In one (4.55%) case, the primary anatomical site of adult D. repens could not be found. D. repens microfilariae were detected in the local tissue (local microfilariasis) in 11 (50.00%) cases and the peripheral blood (microfilaremia) in 11 (50.50%) cases. Samples were obtained by venipuncture in 11 (50.00%) cases, excisional biopsy in eight (36.36%) cases and FNAC in three (13.64%) cases. In cases of microfilaremia, adult D. repens were located in subcutaneous tissue in six (54.55%) cases, the eye in four (36.36%) cases and an unknown primary site in one (9.09%) case. The mean value of microfilariae detected on Knott's test was 5.56/mL (range, 1–12/ml). The mean eosinophil count was 2356 cells/μL (range, 1100–6900 cells/μL). Final identification of D. repens microfilariae was based on morphological detection (microscopy) in 14 (63.64%) cases, and molecular detection (PCR) in eight (36.36%) cases. Treatment with surgical extirpation of adult D. repens was performed in 18 (81.82%) cases and medications were administered in 11 (50.00%) cases, the latter consisting of antiparasitic drugs in nine (40.91%) cases, antibiotics in four (18.18%) cases and corticosteroids in one (4.55%) case. In one (4.55%) case, treatment modality was unknown (Table 3).
Table 3.
Clinical characteristics of human infection with D. repens in adult (L5) and microfilarial (L1) stage (chart review of the world literature). Ref – reference; KT – Knott's test; N – number; FNAC – fine needle aspiration cytology; HPE – histopathological examination; PCR – Polymerase chain reaction.
| Ref | D. repens L5 location | D. repens L1 location |
D. repens L1 detection |
KT (N/ml) |
Eosinophils cells/μL |
Treatment modality |
|---|---|---|---|---|---|---|
| [17] | Mammary | Local tissue | HPE | / | Eosinophilia | Surgical |
| [18] | Subcutaneous | Blood | KT | 11 | Unknown | Unknown |
| [16] | Unknown | Blood | KT | Several | 3000 | Diethylcarbamazine |
| [12] | Subcutaneous | Local tissue | FNAC | / | Unknown | None |
| [13] | Ocular | Blood | KT | 1 | Unknown | Surgical; Mebendazol; Levamisolum; Albendazol; Ivermectin |
| [19] | Subcutaneous | Local tissue | FNAC | / | Eosinophilia | Surgical |
| [23] | Genital | Local tissue | HPE | / | Unknown | Surgical |
| [23] | Subcutaneous | Local tissue | HPE | / | Unknown | Surgical |
| [15] | Subcutaneous | Local tissue | HPE | / | 1100 | Surgical; Albendazol; Methyl-prednisolone |
| [22] | Subcutaneous | Blood | KT; PCR | 2 | 1300 | Surgical; Doxycycline |
| [14] | Ocular | Local tissue | HPE | / | Unknown | Surgical |
| [14] | Ocular | Local tissue | HPE | / | Unknown | Surgical |
| [24] | Subcutaneous | Local tissue | HPE | / | Unknown | Surgical |
| [26] | Pulmonary, Subcutaneous |
Blood | KT; PCR | 1 | 2300 | Surgical; Ivermectin, Albendazole; Diethylcarbamazine |
| [21] | Subcutaneous | Local tissue | FNAC; PCR | / | 1100 | Doxycycline; Ivermectin |
| [5] | Lymphatic | Local tissue | HPE | / | Unknown | Surgical |
| [25] | Ocular | Blood | KT; PCR | 4 | Unknown | Surgical; Doxycycline; Ivermectin |
| [8] | Subcutaneous | Blood | KT, PCR | 12 | 1400 | Surgical; Ivermectin; Albendazole |
| [7] | Ocular | Blood | KT | 6 | 2400 | Surgical; Ivermectin |
| [20] | Subcutaneous | Blood | KT; PCR | 4 | 1700 | Surgical; Doxycycline |
| [27] | Ocular | Blood | PCR | / | Eosinophilia | Surgical; Ivermectin |
| ⁎ | Subcutaneous | Blood | KT; PCR | 9 | 6900 | Surgical |
Current study.
5. Discussion
We report a new case of D. repens microfilariemia in the circulatory system of a young and healthy male patient with severe eosinophilia. Clinical manifestation commenced with rash erythema. Differential diagnosis included allergic reaction, cutaneous larvae migrans and malignant neoplasia due to increased leukocyte count and eosinophilia, but with normal IgE titres. Although all tests for parasitic infestation were negative, a constant increase in eosinophils and highly specific findings on ultrasonography of formed subcutaneous nodules raised the suspicion of filarial infection [30]. D. repens infection was finally confirmed with morphological and molecular identification of microfilariae from the blood stream [31]. This case also represented, to the best of our knowledge, the first molecular confirmation of Wolbachia sp., endosymbionts of D. repens from blood, raising questions about the potential use of this approach in the diagnosis of D. repens infections.
It is generally considered that human hosts are unsuitable for completion of the D. repens life cycle. Based on analysis of 266 human cases, Ermakova et al. concluded that humans are a biological ‘dead-end’ for this helminth [32]. The usual findings involve the detection of a single subadult/adult worm but, on occasion, they may develop to mature adults, mate and produce microfilariae. In rare cases, the microfilariae may even reach the bloodstream [1,3,6]. In the current study, both helminths from skin nodules were morphologically identified as adult females. The worm from the skin nodule in the patient's abdominal wall had developed to maturity and contained microfilariae (Fig. 2) while the female from the axilla did not contain microfilariae (Fig. 3). Detection of a female producing microfilariae indicates the likely presence of a male worm even if it was not specifically detected [1]. In addition to our case, meta-analysis revealed 21 published cases of human infestation with females producing microfilarae since 1992. In half of the cases, microfilariae were detected in the bloodstream with the modified Knott's test while, in others, fertility was confirmed after their detection in worms or tissue. On this basis, and combined with the fact that Ermakova et al. described sexually matured parasites in 10.4% of nodules, the presumption that humans are a ‘dead-end’ for this helminth does not appear to be correct [32]. Although detection of D. repens microfilariae in the circulation indicates the likely presence of adult worms of both sexes within the human host, the definitive presence of both adult male and female helminths in human cases remains to be demonstrated. Such evidence will be required to put this question to bed. As to whether this human and other human cases could act as reservoirs of infection, this is less clear. In the current case, the presence of microfilariae in the blood appears to have only been short-lived. Combined with the fact that only a low number of parasites (1–12/mL) could be detected in the blood in this case and in other 10 patients previously described (Table 3), its our assumption that the risk of humans acting as reservoirs of D. repens infection is relatively low, certainly at least compared to other hosts such as dogs.
Previously described cases in human patients suggested immunodeficiency as a risk factor for D. repens microfilaremia [13,17,18], however, in most cases described in the literature, as well as the current case, the patients were immunocompetent (Table 2). Knowledge of the immunopathogenic mechanisms of dirofilariosis in humans is poorly understood. In the most comprehensive study, eosinophilia appeared in 16.4% patients and was attributed to migration of helminths [32]. In our case, both parasites were settled within necrotic tissue, demarcated but not encapsulated, with massive inflammation and neoangiogenesis of the subcutaneous and/or skeletal muscle tissue. Systemic eosinophilia continued to increase. It is possible that the very high levels of peripheral blood eosinophilia described in this case may have been stimulated by biomediators released from necrotic tissue [33] surrounding the adults delivering microfilaria. The support for this thesis lies in the fact that all previously reported cases with microfilariae developed eosinophilia (Table 3). In terms of the host serological response, in contrast to a study reported by Lechner et al. and in agreement with work by Potters et al., levels of IgE antibodies were within reference ranges in our case [7,20].
Unfortunately, as illustrated in Table 3, the modified Knott's test has been rarely used for detection of microfilariaemia despite their presence within tissue after excisional biopsy or FNA. In order to provide a more accurate insight into the number of patients with circulating microfilariae infected with D. repens, the modified Knott's test should be used regularly and in larger blood volumes than 1 mL.
In addition to the surgical removal of both nodules that contained the adult helminths, systemic anti-helminthic or doxycycline therapy was recommended given the presence of microfilariaemia, however, was not actioned by the patient. In order to prevent recurrence of disease, two dogs identified as potential sources of infection were treated and protected with repellent collars [34]. Contrary to previous reports, this is the first microfilaretic human patient treated only surgically without any additional anti-helminthic or antibiotic treatment (Table 3). During follow-up, all blood samples were free from microfilariae 12 months after adults of the D. repens extirpation. The extremely high levels of eosinophilia may be potentially responsible for the short duration of microfilariemia. Given the scarce knowledge of a persistence of microfilaria in the human blood stream, the findings from this case suggest transient microfilariaemia and short survival. All previous patients with microfilariemia received medicamentous treatment and it was impossible to otherwise evaluate the duration of microfilariemia.
As already mentioned, the heavily infected dogs with 7500 and 8200 microfilariae/mL are the likely source of infection with continuous exposure to multiple infections probably crucial for development of adult parasites rather than the immune status of patient (Table 2).
6. Conclusion
To our knowledge, this is a first report on the molecular identification of multiple D. repens adults, Wolbachia sp. and microfilariae in a human patient, with suspected transmission from infected dogs. Despite the fact that dirofilariasis is one of the most important parasitic diseases emerging in humans and dogs across Europe, it is still neglected and rarely considered as cause of human ocular and dermatological manifestations as described in the current case. The evidence of the current case provides evidence to support a role for humans as definitive hosts of D. repens. From a diagnostic perspective, regular use of modified Knott's test in humans and dogs should be adopted when investigating human clinical presentations with symptoms that may be consistent with filariasis [35]. More broadly, this study highlights the crucial value of a One Health approach to further dissecting the host range and transmission of this neglected vector-borne filaroid helminth.
Supplementary data to this article can be found online at [doi].
Supplementary Fig. S1.
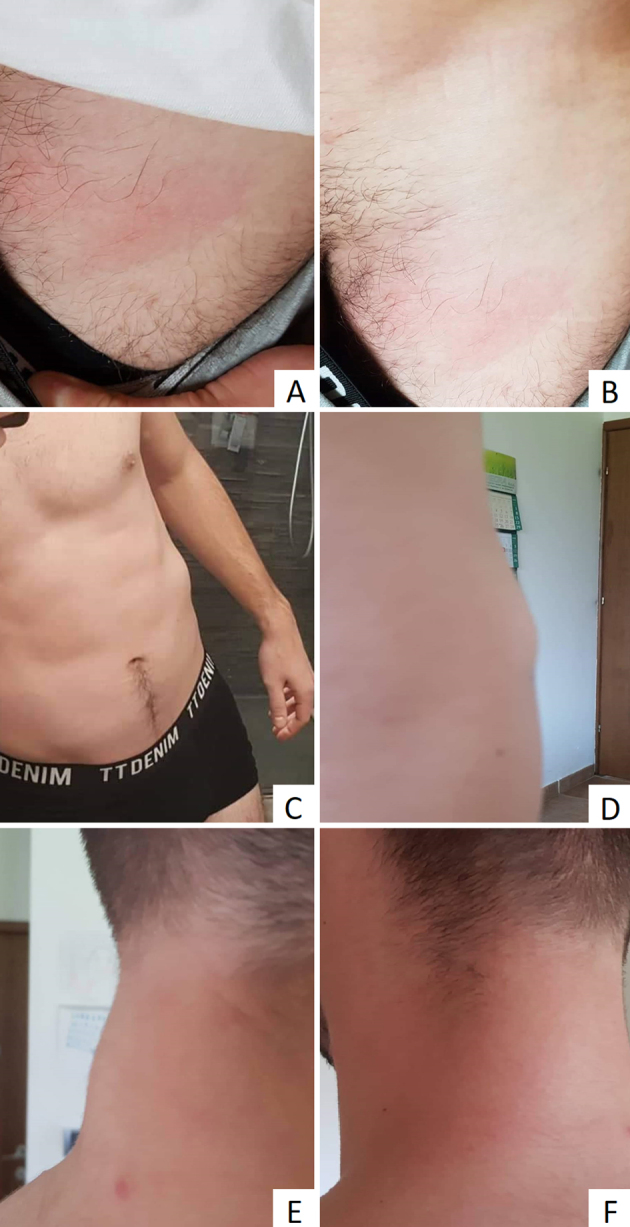
A detailed description of the case and the course of the disease in the patient.
Supplementary material
Ethics approval and patient consent
The authors assert that all procedures contributing to this work were approved and complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Declaration of Helsinki 1964 (revised in 2013).
Written consent was provided by the patient and his parents to enable anonymized reporting of the results of this study.
Availability of data and materials
The data generated during this study are included within this manuscript or are available upon request from the corresponding author.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Conceptualization, R.B. and A.P·B.; methodology, R.B., A.B. and J.P.B.; software, D.J.; validation, R.B., A.B. and A.P.; formal analysis, R.B. and D.J.; investigation, A.P.B. and A.B.; resources, R.B.; data curation, J.P.B.; writing—original draft preparation, A.P.B., J.P.B, R.B. and A.B.; writing—review and editing, A.P.; visualization, J.P.B.; supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
None to declare.
Contributor Information
Adam Polkinghorne, Email: adam.polkinghorne@health.nsw.gov.au.
Relja Beck, Email: beck@veinst.hr.
References
- 1.Simon F., Siles-Lucas M., Morchon R., Gonzalez-Miguel J., Mellado L., Carreton E., Montoya-Alonso J.A. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012;25:507–544. doi: 10.1128/CMR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabunas V., Radzijevskaja J., Sakalauskas P., Petkevicius S., Karveliene B., Ziliukiene J., Lipatova I., Paulauskas A. Dirofilaria repens in dogs and humans in Lithuania. Parasit. Vectors. 2019;12:177. doi: 10.1186/s13071-019-3406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuehrer H.P., Auer H., Leschnik M., Silbermayr K., Duscher G., Joachim A. Dirofilaria in Humans, Dogs, and Vectors in Austria (1978–2014)-From Imported Pathogens to the Endemicity of Dirofilaria repens. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004547. Article e0004547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon F., Morchon R., Gonzalez-Miguel J., Maecos-Atxutegi M., Siles-Lucas F.M. What is new about animal and human dirofilariosis? Trends in Parasitology. 2009;25:404–509. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Mateju J., Chanova M., Modry D., Mitkova B., Hrazdilova K., Zampachova V., Kolarova L. Dirofilaria repens: emergence of autochthonous human infections in the Czech Republic (case reports) BMC Infect. Dis. 2016;16:171. doi: 10.1186/s12879-016-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capelli G., Genchi C., Baneth G., Bourdeau P., Brianti E., Cardoso L., Danesi P., Fuehrer H.P., Giannelli A., Ionica A.M., Maia C., Modry D., Montarsi F., Krucken J., Papadopoulos E., Petric D., Pfeffer M., Savic S., Otranto D., Poppert S., Silaghi C. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit. Vectors. 2018;11 doi: 10.1186/s13071-018-3205-x. Article 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potters I., Vanfraechem G., Bottieau E. Dirofilaria repens Nematode Infection with Microfilaremia in Traveler Returning to Belgium from Senegal. Emerg. Infect. Dis. 2018;24:1761–1763. doi: 10.3201/eid2409.180462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kludkowska M., Pielok L., Frackowiak K., Masny A., Golab E., Paul M. Dirofilaria repens infection as a cause of intensive peripheral microfilariemia in a Polish patient: process description and cases review. Acta Parasitol. 2018;63:657–663. doi: 10.1515/ap-2018-0077. [DOI] [PubMed] [Google Scholar]
- 9.Rishniw M., Barr S.C., Simpson K.W., Frongillo M.F., Franz M., Alpizar J.L. Dominguez. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet. Parasitol. 2006;135:303–314. doi: 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Casiraghi M., Anderson T.J., Bandi C., Bazzocchi C., Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122(Pt 1):93–103. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- 11.Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P., Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94:707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- 12.Supriaga V.G., Tsybina T.N., Denisova T.N., Morozov E.N., Romanenko N.A., Starkova T.V. The first case of diagnosis of dirofilariasis from the microfilariae detected in the human subcutaneous tumor punctate. Med Parazitol (Mosk) 2004:6–8. [PubMed] [Google Scholar]
- 13.Salomváry B., Korányi K., Kucsera I., Szénási Z., Czirják S. A new case of ocular dirofilariosis in Hungary. Szemészet. 2005;142:31–35. [Google Scholar]
- 14.Salamatin R.V., Pavlikovska T.M., Sagach O.S., Nikolayenko S.M., Kornyushin V.V., Kharchenko V.O., Masny A., Cielecka D., Konieczna-Sałamatin J., Conn D.B., Golab E. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitol. 2013;58:592–598. doi: 10.2478/s11686-013-0187-x. [DOI] [PubMed] [Google Scholar]
- 15.Poppert S., Hodapp M., Krueger A., Hegasy G., Niesen W.D., Kern W.T., Tannich E. Dirofilaria repens infection and concomitant meningoencephalitis. Emerg. Infect. Dis. 2009;15:1844–1846. doi: 10.3201/eid1511.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrocheilou V., Theodorakis M., Williams J., Prifti H., Georgilis K., Apostolopoulou I., Mavrikakis Microfilaremia M. From a Dirofilaria-like parasite in Greece. Case report. APMIS. 1998;106:315–318. [PubMed] [Google Scholar]
- 17.Pampiglione S., Schmid C., Montaperto Human C. Dirofilariasis: discovery of a gravid female of Dirofilaria repens in a subcutaneous nodule. Pathologica. 1992;84:77–81. [PubMed] [Google Scholar]
- 18.Nozais J.P., Bain O., Gentilini M. A case of subcutaneous dirofilaria (Nochtiella) repens with microfilaremia originating in Corsica. Bull. Soc. Pathol. Exot. 1994;87:183–185. [PubMed] [Google Scholar]
- 19.Negahban S., Daneshbod Y., Atefi S., Daneshbod K., Sadjjadi S.M., Hosseini S.V., Bedayat G.R., Abidi H. Dirofilaria repens diagnosed by the presence of microfilariae in fine needle aspirates: a case report. Acta Cytol. 2007;51:567–570. doi: 10.1159/000325796. [DOI] [PubMed] [Google Scholar]
- 20.Lechner A.M., Gastager H., Kern J.M., Wagner B., Tappe D. Case report: successful treatment of a patient with microfilaremic dirofilariasis using doxycycline. Am J Trop Med Hyg. 2020;102:844–846. doi: 10.4269/ajtmh.19-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontanelli Sulekova L., Gabrielli S., De Angelis M., Milardi G.L., Magnani C., Di Marco B., Taliani G., Cancrini G. Dirofilaria repens microfilariae from a human node fine-needle aspirate: a case report. BMC Infect. Dis. 2016;16:248. doi: 10.1186/s12879-016-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedianina L.V., Shatova S.M., Rakova V.M., Shaitanov V.M., Lebedeva M.N., Frolova A.A., Morozov E.N., Morozova L.F. Microfilaraemia in human dirofilariasis caused by Dirofilaria repens Raiet et Henry, 1911, A case report. Med Parazitol (Mosk) 2013:3–7. [PubMed] [Google Scholar]
- 23.Dzamic A.M., Colovic I.V., Arsic-Arsenijevic V.S., Stepanovic S., Boricic I., Dzamic Z., Mitrovic S.M., Rasic D.M., Stefanovic I., Latkovic Z., Kranjcić-Zec I.F. Human Dirofilaria repens infection in Serbia. J. Helminthol. 2009;83:129–137. doi: 10.1017/S0022149X09341346. [DOI] [PubMed] [Google Scholar]
- 24.Damle A.S., Iravane Bajaj J.A., Khaparkhuntikar M.N., Maher G.T., Patil R.V. Microfilaria in human subcutaneous dirofilariasis: a case report. J. Clin. Diagn. Res. 2014;8:113–114. doi: 10.7860/JCDR/2013/6886.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaizot R., Receveur M.C., Millet P., Otranto D., Malvy D.J.M. Systemic Infection With Dirofilaria repens in Southwestern France. Ann. Intern. Med. 2018;168:228–229. doi: 10.7326/L17-0426. [DOI] [PubMed] [Google Scholar]
- 26.Benzaquen M., Brajon D., Delord M., Yin N., Bittar F., Toga I., Berbis P., Parola P. Cutaneous and pulmonary dirofilariasis due to Dirofilaria repens. Br. J. Dermatol. 2015;173:788–791. doi: 10.1111/bjd.13859. [DOI] [PubMed] [Google Scholar]
- 27.Frenzen F.S., Loewe I., Muller G., Schoenlebe J., Tappe D., Teichmann D. Dirofilaria repens infection of the eye with concomitant microfilaremia in a traveller. J Travel Med. 2021;28 doi: 10.1093/jtm/taaa119. [DOI] [PubMed] [Google Scholar]
- 28.Stevanović R., Savić-Cvetojević M., Petrović Z. Dirofilariasis in a 10-year-old boy: A case report. Acta Parasitologica Iugoslavica. 1971;2:93–97. [Google Scholar]
- 29.Huber D., Reil I., Duvnjak S., Jurkovic D., Lukacevic D., Pilat M., Beck A., Mihaljevic Z., Vojta L., Polkinghorne A., Beck R. Molecular detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia sp. but not Ehrlichia canis in Croatian dogs. Parasitol. Res. 2017;116:3019–3026. doi: 10.1007/s00436-017-5611-y. [DOI] [PubMed] [Google Scholar]
- 30.Giori L., Garbagnoli V., Venco L., Genchi M., Bazzocchi C., Bertazzolo W. What is your diagnosis? Fine-needle aspirate from a subcutaneous mass in a dog. Mixed neutrophilic-eosinophilic inflammation with Dirofilaria fragments. Vet. Clin. Pathol. 2010;39:255–256. doi: 10.1111/j.1939-165X.2009.00212.x. [DOI] [PubMed] [Google Scholar]
- 31.Di Cesare A., Otranto D., Di Giulio E., Simonato G., Latrofa M.S., La Torre F., Coccia G., Traversa D. Microfilarial periodicity of Dirofilaria repens in naturally infested dogs. Parasitol. Res. 2013;112:4273–4279. doi: 10.1007/s00436-013-3619-5. [DOI] [PubMed] [Google Scholar]
- 32.Ermakova L., Nagorny S., Pshenichnaya N., Ambalov Y., Boltachiev K. Clinical and laboratory features of human dirofilariasis in Russia. IDCases. 2017;9:112–115. doi: 10.1016/j.idcr.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell R.N. In: Robbins & Cotran Pathologic Basis of Disease. Kumar V., Abbas A., Aster J., editors. Elsevier; Philadelphia, US: 2014. Inflammation and Repair; pp. 69–113. [Google Scholar]
- 34.Genchi C., Kramer L. Subcutaneous dirofilariosis (Dirofilaria repens): an infection spreading throughout the old world. Parasit. Vectors. 2017;10:517. doi: 10.1186/s13071-017-2434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pupić-Bakrač A., Pupić-Bakrač J., Jurković D., Capar M., Lazarić Stefanović L., Antunović Ćelović I., Kučinar J., Polkinghorne A., Beck R. The trends of human dirofilariasis in Croatia: Yesterday – Today – Tomorrow. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100153. Article 100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data generated during this study are included within this manuscript or are available upon request from the corresponding author.