Abstract
Background
Cancer-related cognitive impairment (CRCI) is a prevalent source of comprised quality of life in cancer survivors. This study evaluated the efficacy of Emotional Freedom Techniques (EFT) on self-reported CRCI (sr-CRCI).
Methods
In this prospective multicentre randomised wait-list controlled study (ClinicalTrials.gov Identifier: NCT02771028), eligible cancer survivors had completed curative treatment, were 18 years or older and screened positive for sr-CRCI with ≥ 43 on the Cognitive Failures Questionnaire (CFQ). Participants were randomised to the immediate treatment group (ITG) or wait-list control (WLC) group, based on age (< or ≥ 65 years), gender, treatment (chemotherapy or not), and centre. The ITG started to apply EFT after inclusion and performed this for 16 weeks. The WLC group could only start the application of EFT after 8 weeks of waiting. Evaluations took place at baseline (T0), 8 weeks (T1) and 16 weeks (T2). The primary outcome was the proportion of patients with sr-CRCI according to the CFQ score.
Findings
Between October 2016 and March 2020, 121 patients were recruited with CFQ ≥ 43 indicating sr-CRCI. At T1, the number of patients scoring positive on the CFQ was significantly reduced in the ITG compared to the WLC group (40.8% vs. 87.3% respectively; p<0.01). For the WLC group, a reduction in CFQ scores was observed at T2, comparable to the effect of the ITG at T1. Linear mixed model analyses indicated a statistically significant reduction in the CFQ score, distress, depressive symptoms, fatigue and also an improvement in quality of life.
Interpretation
This study provides evidence for the application of EFT for sr-CRCI in cancer survivors and suggests that EFT may be useful for other symptoms in cancer survivors.
Keywords: Subjective cognitive complaints, Emotional freedom techniques, Cancer-related cognitive impairment, Cancer survivorship
Research in context.
Evidence before this study
Cancer-related cognitive impairment (CRCI) refers to changes or impairments in cognitive function associated with a cancer diagnosis and/or its treatment. There is a growing recognition of the multifactorial, complex nature of CRCI which has led to research on its aetiology and underlying mechanisms. Distress has been identified as one of the factors causing subjective or self-reported CRCI (sr-CRCI). An approach that has received increasing attention over the past years to deal with several psychological symptoms such as distress is emotional freedom techniques (EFT). Clinical EFT is an evidence-based method that combines elements of cognitive and somatic therapies with acupressure. This manual stimulation of acupuncture points is performed while using a spoken affirmation to target a psychological issue. Before undertaking this study, a literature search was done in PubMed. An independent EFT practitioner and clinical expert was consulted for the trial development. This approach has been validated in more than 100 clinical trials that have shown that EFT is an efficacious and safe self-help tool to improve both physiological and psychological symptoms in non-cancer patients.
Added value of this study
The present study aimed to identify EFT as an effective treatment to deal with sr-CRCI by acknowledging it as an effective self-help stress-reduction technique for cancer survivors who suffer from sr-CRCI. This is the first study to acknowledge EFT as an active treatment for sr-CRCI and highlights the potential of EFT to improve quality of life, distress levels, depressive symptoms, and fatigue in cancer survivors.
Implication of all the available evidence
As a safe and reliable self-help method, EFT demonstrates clinical utility as a low-cost non-drug treatment for sr-CRCI in cancer survivors, easy to implement in clinical practice.
Alt-text: Unlabelled box
1. Introduction
Advances in cancer treatments have significantly increased the number of cancer survivors with an estimated number of 16.9 million in the United States (2019) [1]. Despite this record number, some adverse effects of cancer therapies remain present after treatment. Cancer-related cognitive impairment (CRCI) refers to changes or impairments in cognitive function associated with a cancer diagnosis and/or its treatment. This important clinical problem may persist for months or years after the cancer treatment has ended [2].
These cognitive problems have been defined with terms as chemobrain and chemofog. Although widely accepted, the term chemobrain is somewhat unfavourable as longitudinal trials have documented that CRCI occurs in up to 25% of patients with cancer prior to chemotherapy [3], [4], [5], [6]. Recent investigations established that other treatments such as targeted therapies can also induce cognitive deficits [7,8]. CRCI is one of the most feared long-term adverse effects of a cancer treatment and has been associated with comprised health-related quality of life (HRQoL) and impaired social and occupational functioning [9]. Reducing those long-term consequences after cancer treatment, such as cognitive impairment, was recently described as one of the research priorities by the ASCO [2].
There is a growing recognition of the multifactorial, complex nature of CRCI which has led to research on its aetiology and underlying mechanisms. Research has shown that neuropsychological disorders in cancer patients can evolve out of psychosocial risk factors such as distress [10,11]. Distress is defined as an unpleasant experience of a mental, physical, social, or spiritual nature that may interfere with the ability to cope with cancer [12]. Research by our group reported that higher levels of distress are associated with subjective or self-reported CRCI (sr-CRCI) [6,9,13]. Furthermore, distress may lead to compromised HRQoL if lEFT untreated [12,13]. Overall, anxiety, depression, and fatigue are frequent in cancer patients and have been associated with CRCI as well [11,[14], [15], [16], [17], [18], [19], [20], [21], [22]].
Over the past few years, psychosocial interventions, such as cognitive behaviour therapy (CBT) and mindfulness, are encouraged to help patients cope with emotional stress, anxiety and depression during and after cancer [16,[23], [24], [25]]. Long-term trends in the use of these complementary and alternative medical therapies have led to the development of novel approaches for treating psychological symptoms. An approach that has received increasing attention is emotional freedom techniques (EFT). EFT is a mental or emotional version of acupressure that can be self-applied for a wide range of emotional, health, and performance issues [26].
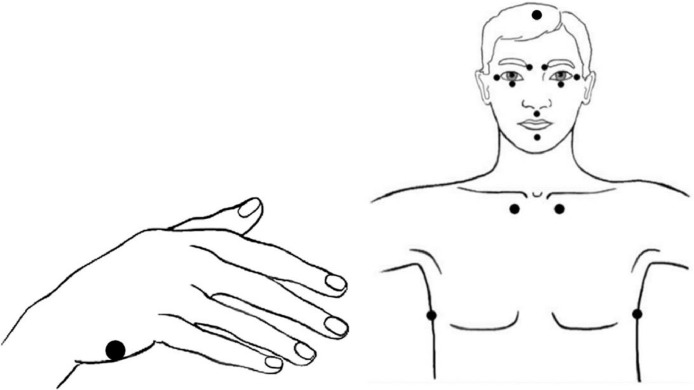
EFT utilizes established techniques including cognitive restructuring, exposure, and systematic desensitization, but it adds the novel component of stimulating acupuncture points (acupoints) whilst disturbing memories or triggers are mentally activated [27]. The process involves a ‘Setup Statement’ that consists of a reference to the traumatic event or related feelings combined with a self-acceptance statement and simultaneous acupoint tapping on the side of the hand. For the remainder of the process, patients gently tap with two fingers on head and torso points (Fig. 2). During this part the patient repeats a shortened version of the Setup Statement, usually one or two words, referred to as the ‘Reminder Phrase’. By tapping the acupoints, the distress related to the emotional or physical triggering memory should be reduced.
Fig. 2.
Head, torso and hand acupressure points involved in the application of EFT (Figure adapted from Church et al. [27]). The process starts with a ‘Setup Statement’ while performing acupoint tapping on the side of the hand. For the remainder of the process, patients gently tap with two fingers on head and torso points while repeating the ‘Reminder Phrase’.
This approach has been validated in over 100 clinical trials that have shown that EFT is an efficacious and safe self-help tool that can be used both on a self-help basis and as a primary evidence-based treatment to improve both physiological and psychological symptoms in a variety of populations [28], [29], [30], [31], [32], [33], [34], [35], [36]. A first meta-analysis comprised 18 randomised controlled trials (RCTs) (n = 921) and found moderate treatment effect of EFT for psychological conditions [36]. Another meta-analysis included 14 studies (n = 658) and concluded that EFT therapy is associated with a significant treatment effect for anxiety (d = 1.23) [30]. Another large treatment effect was detected in RCTs examining EFT for depression (d = 1.31) [31]. A last meta-analysis showed that a series of 4–10 EFT sessions creates a large treatment effect for posttraumatic stress disorder (PTSD) (d = 2.96) [28].
Combining brief psychological exposure with the manual stimulation of acupoints is an intervention strategy that integrates established clinical principles with methods derived from healing traditions of Eastern cultures. Several RCTs identified the acupoint stimulation as an active ingredient of EFT [37].
Moreover, Swingle et al. demonstrated neurophysiological evidence of the efficacy of EFT using electroencephalogram (EEG) imaging recording brain waves of car accident victims suffering from PTSD [38]. Another trial concerning PTSD suggested that the stimulation of acupoints directly sends deactivating signals to the amygdala, resulting in the rapid attenuation of threat responses to innocuous stimuli [39]. Studies with functional MRI suggest that acupoint tapping is able to regulate the autonomic nervous system and fear responses [39].
Furthermore, the manual stimulation of acupoints appears to have a positive effect on cortisol levels(33), but also induces neurochemical changes which generate a relaxation response which reciprocally inhibits anxiety and creates a rapid desensitization to traumatic stimuli, confirming the mechanism of EFT [40]. The application of EFT also appears to result in a differential gene expression with an upregulation of immune markers and downregulation of inflammation and stress markers [41], [42], [43], [44].
While there are many traditional psychotherapy techniques such as CBT, EFT may offer significant practical advantages to these well-known interventions that often include personal guidance and specific training which generally entail a time-intensive programme. What makes EFT in particular appealing is that is a low-threshold, easily accessible tool that is proven to be easily learned, rapidly effective, safe to use on one's own outside the therapist's office and can be instructed in groups. Except for a study where EFT was found effective to reduce the side effects of tamoxifen and aromatase inhibitors (45) and a trial with spiritual EFT for cancer pain (46), evidence in cancer patients remains scarce.
The wide range of conditions for which EFT is effective are usually attributed to the technique's ability to deal with mild to severe distress [[32], [33], [34],40]. Distress, a component of many emotional and physical disorders, was also found to be a predictor for sr-CRCI [13]. We hypothesize that the application of EFT may relieve sr-CRCI in cancer survivors through a reduction of the level of distress.
2. Methods
2.1. Study design
This prospective multicentre randomised wait-list controlled study was coordinated by AZ Groeninge Kortrijk (Belgium) and took place in three Belgian centres: AZ Groeninge Kortrijk, AZ Klina Brasschaat, and UZ Brussel (ClinicalTrials.gov Identifier: NCT02771028). The study was conducted on behalf of the Belgian Society for Medical Oncology (BSMO) Cancer Survivorship Task Force. Human ethics approval was obtained by the leading ethics committee of UZ Brussel (registration number: B143201628822) and the local ethics committees of AZ Groeninge and AZ Klina. The study period started in October 2016 and patient inclusion was ended in March 2020. The study was conducted according to good clinical practice guidelines and reported conform to CONSORT 2010 guidelines. The study protocol is available (online only).
2.2. Participants
Patients eligible for this study were diagnosed with a solid tumour or haematologic malignancy and had completed curative cancer treatment including chemotherapy, radiotherapy, surgery or targeted therapy. They could continue anti-hormonal treatment. There was no limitation on time since treatment end. All participants had to be 18 years or older, had an expected life expectancy of at least 5 years, and suffered from sr-CRCI (CFQ ≥ 43). Patients with mental deterioration, organic brain syndrome, alcohol or drug dependency, or patients coping with a major psychiatric or neurological disorder that could potentially invalidate assessment were excluded from participation. Patients were locally recruited by their treating physicians and oncopsychologists (MSc). In addition, information brochures and flyers were distributed at the day clinic and outpatient department at the three centres. Written informed consent was obtained from all participants.
2.3. Randomisation
After baseline interview (T0) including the completion of the CFQ, eligible patients were randomised into one of two groups: an immediate treatment group (ITG) or wait-list control (WLC) group. Immediately after allocation, patients were informed to which group they were assigned. The ITG started the application of EFT after allocation and applied this technique for 16 weeks: the first eight weeks they applied the technique under supervision of the EFT instructor (until time point T1), while the next eight weeks they were asked to continue the application of EFT without intervention (‘observation 8 weeks’ until T2). The WLC group could only start the application of EFT at T1, after eight weeks of waiting. Thereafter, they cross over to the intervention arm and applied EFT for 8 weeks under supervision of the EFT instructor, until T2.
Participants were randomly assigned to the ITG or WLC group by the principle of minimization. Stratification was based on (1) age (< 65 or ≥ 65 years); (2) gender (male or female); (3) chemotherapy (yes or no) and (4) centre of inclusion (AZ Groeninge, AZ Klina and UZ Brussel). Allocation was performed by the central study coordinator (AZ Groeninge) by computer software developed for this trial. Subsequently, they were referred to a physician, oncopsychologist or study trial coordinator (MSc or PhD) qualified and trained to conduct the trial (Association for the Advancement of Meridian Energy Techniques (AAMET) EFT level 1 certificate), present at one of the three centres (M.L., T.L., C.K., L.T.).
2.4. Procedures
Together with 2 certified EFT practitioners (M.L.,T.L.) and an independent EFT practitioner and clinical expert (K.M.), the principal investigator developed the programme which was based on the standardized protocol by Craig [45]. The key goals of the EFT intervention in this study were: to teach a strategy for controlling worry and excessive distress, to address existential thoughts and worries and to educate survivors with the self-help stress reduction technique in order to reduce sr-CRCI.
Following the baseline interview, a first session “how to apply EFT” took place with the local EFT practitioner. This first session lasted between 40 and 90 min. A second follow-up session usually took place one week later and lasted about 10 to 20 min. Patients were individually guided during study participation and when desired or needed, sequential sessions were planned in order to stimulate patients and ensure adequate follow-up. Participants were asked to apply EFT at least once a day and report this in a patient diary they received upon participation. This diary was not obligatory to complete, but it could stimulate participants to apply EFT and help them to monitor their own progress.
Assessments were done at baseline (before randomisation) (time point 0 (T0)), after eight weeks (T1), and after 16 weeks (T2). Participants could continue the application of EFT after the period of eight or 16 weeks. Follow-up evaluation six and 12 months after study participation consisted of the EuroQol EQ-5D-3 L and the question if patients continued the application of EFT.
2.5. Outcomes
The primary outcome was the efficacy of EFT over the course of eight weeks, assessed by a reduction in sr-CRCI at T1. Presence of sr-CRCI was defined as presenting with a CFQ score ≥ 43. The CFQ rates subjective cognitive complaints on a Likert scale from 0 to 4 [46]. Total scores range from 0 to 100, with cut-off < 43. Where a normal score ranges between 21 and 43, significant cognitive complaints start from a CFQ total score > 42.9 [47]. Other patient-reported outcome measures (PROMs) evaluated at T0, T1 and T2 included the distress thermometer (DT) and 38-item problem list (PL)(12, 50); the Beck Depression Inventory-II (BDI-II))(35, 51) and the Functional Assessment of Chronic Illness Therapy-Fatigue Subscale (FACIT-Fatigue)(52). HRQoL was measured using the cancer-specific European Organization for Research and Treatment for Cancer QoL Questionnaire (EORTC QLQ-C30)(53) and the EuroQol EQ-5D-3L(54). For the EORTC QLQ-C30, a higher score indicates a better health, except for the symptom scales where a high score mirrors a high level of symptomatology. A difference of ≥10 points on each HRQoL scale or item is defined as clinically relevant [48]. Demographic details were self-reported at T0, while clinical details were looked up in the electronic patient record by the trial coordinator.
2.6. Statistical analyses
Based on a two-sided test for independent proportions and the assumption to find a difference in proportion of 25% between the ITG and WLC group at T1, achieving a power of 80% at the 5% significance level, a sample size of maximum 140 patients and expected dropout of 15% was considered appropriate. Thus, by including 118 evaluable patients, the goal was to establish that 25% more patients showed a reduction in sr-CRCI after EFT intervention in the ITG compared to the WLC group.
Descriptive statistics were shown for demographic and clinical characteristics. To assess the efficacy of EFT at T1, a χ2 (or Fisher exact) was used and two-sample t-tests were used to compare baseline characteristics between the ITG and WLC group. Linear mixed-effects regression models were used to assess whether the improvement in CFQ scores and secondary PROMs were significantly different over time. Models included the stratification variables (age, gender, treatment and centre) as fixed effects and time x group as interaction effect to account for correlation amongst repeated observations per participant. Estimated marginal means with 95% confidence interval (CI) were calculated for each time point for both groups. Within-group differences were obtained by pairwise comparisons, enabling us to detect differences over time within the ITG and WLC group. Between-group differences on the other hand, resulted from the estimates of the interaction term time x group with T0 as reference time point. This outcome represents the difference in slope and measures the effect of the intervention with EFT.
A 5% significance level was used. All statistical analyses were conducted using IBM SPSS v.26 (SPSS, Inc., Chicago, IL) software.
2.7. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. Results
3.1. Patient demographics
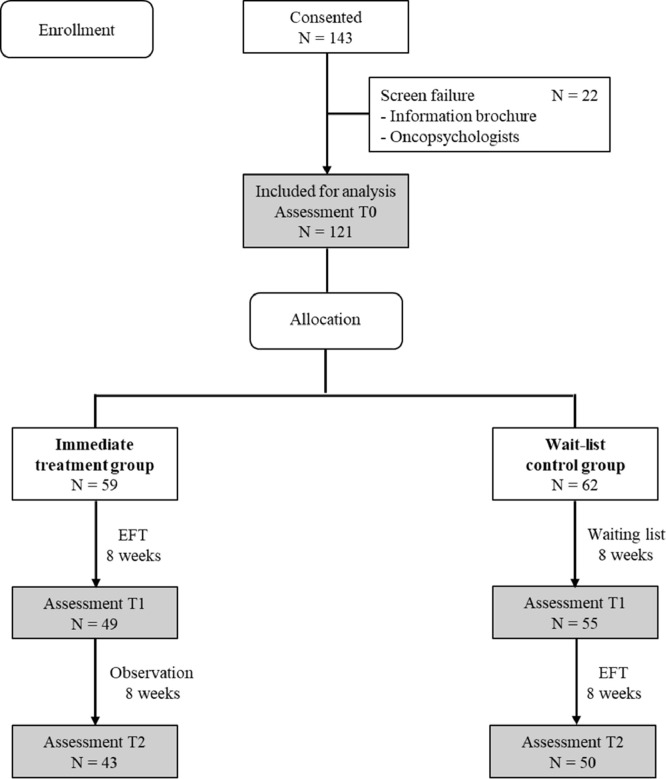
Between October 2016 and March 2020, 143 patients gave informed consent for cognitive screening with the CFQ (Fig. 1). There were 22 patients excluded from participation because they did not have sr-CRCI (CFQ < 43). Finally, 121 patients were eligible for analysis and randomised to the ITG or WLC group. At T1 and T2, respectively 104 and 93 patients completed all questionnaires. The main reason for drop-out was an emotional unstable situation due to which cancer survivors were not able to apply EFT adequately, while a minority (n = 6) dropped out when acceptance was hampered by the disbelief that the application of EFT could help to overcome serious sr-CRCI and emotional problems..
Fig. 1.
CONSORT diagram.
Participants had an average age of 52.2 years (range 28–78 years). Women were overrepresented compared to male individuals (93.4% and 6.6%, respectively). A previous diagnosis of breast cancer was the main malignant condition (77.7%). Most patients had received systemic treatment (86.8%) and/or surgery (87.6%). Baseline patient characteristics are presented in Table 1 (online only).
Table 1.
Demographic and clinical characteristics (online only).
| Characteristics | Immediate treatment group | Wait-list control group | Overall | Significance* |
|---|---|---|---|---|
| Number of Patients | 59 | 62 | 121 | NA |
| Age (average ± SD) | 52.0 ± 7.7 | 52.4 ± 8.4 | 52.2 ± 8.0 | p = 0.78 |
| Age category | ||||
| < 65 years | 56 (94.9%) | 58 (93.5%) | 114 (94.2%) | p = 0.75 |
| ≥ 65 years | 3 (5.1%) | 4 (6.5%) | 7 (5.8%) | |
| Gender | ||||
| Female | 55 (93.2%) | 58 (93.5%) | 113 (93.4%) | p = 1.00 |
| Male | 4 (6.8%) | 4 (6.5%) | 8 (6.6%) | |
| Social status | ||||
| Married/Living together | 43 (72.9%) | 44 (71.0%) | 87 (71.9%) | p = 0.71 |
| Single | 3 (5.1%) | 4 (6.5%) | 7 (5.8%) | |
| Divorced | 13 (22.0%) | 12 (19.4%) | 25 (20.7%) | |
| Missing | 0 (0%) | 1 (1.6%) | 1 (0.8%) | |
| Living situation | ||||
| Home with partner | 48 (81.4%) | 53 (85.5%) | 101 (83.5%) | p = 0.75 |
| Home alone | 9 (15.3%) | 8 (12.9%) | 17 (14.0%) | |
| Home with family member | 1 (1.7%) | 0 (0%) | 1 (0.8%) | |
| Missing | 1 (1.7%) | 1 (1.6%) | 2 (1.7%) | |
| Education level | ||||
| Lower high school | 6 (10.2%) | 6 (9.7%) | 12 (9.9%) | p = 0.70 |
| Higher high school | 17 (28.8%) | 18 (29.0%) | 35 (28.9%) | |
| Bachelor degree | 22 (37.3%) | 30 (48.4%) | 52 (43.0%) | |
| Master degree | 11 (18.6%) | 6 (9.7%) | 17 (14.0%) | |
| Other | 1 (1.7%) | 1 (1.6%) | 2 (1.7%) | |
| Missing | 2 (3.4%) | 1 (1.6%) | 3 (2.5%) | |
| Cancer type | ||||
| Non Hodgkin Lymphoma | 2 (3.4%) | 5 (8.1%) | 7 (5.8%) | p = 0.28 |
| Breast | 46 (78.0%) | 48 (77.4%) | 94 (77.7%) | |
| Ovary | 4 (6.8%) | 2 (3.2%) | 6 (5.0%) | |
| Other** | 7 (11.9%) | 7 (11.3%) | 14 (11.6%) | |
| Type of therapy | ||||
| Surgery | 54 (91.5%) | 52 (83.9%) | 106 (87.6%) | p = 0.18 |
| Radiotherapy | 46 (78.0%) | 45 (72.6%) | 91 (75.2%) | |
| Systemic treatment | 51 (86.4%) | 54 (87.1%) | 105 (86.8%) | |
| Hormonal treatment | 34 (57.6%) | 36 (58.1%) | 70 (57.9%) | |
| Other*** | 24 (40.7%) | 17 (27.4%) | 41 (33.9%) | |
*Baseline characteristics were compared using Exact Pearson Chi-square Tests and two-sample t-tests.
⁎⁎Other malignancies include Hodgkin Lymphoma, Acute Myeloid Leukaemia, Acute Lymphocytic Leukaemia, Colon, Cervix uteri, Prostate, Kidney, Melanoma.
⁎⁎⁎Other types of therapy include targeted therapy and immune therapy.
Abbreviations: SD = standard deviation.
3.2. The efficacy of EFT for sr-CRCI
At T1, 59.2% of the ITG and 12.7% of the WLC group scored <43 on the CFQ. These data illustrate that an intervention with EFT significantly reduced subjective cognitive complaints with a difference in proportion of 46.5% between the ITG and WLC group at T1 (p<0.01).
In a linear mixed model for CFQ scores over time, the time x group interaction effect was found to be significant (p<0.001) as were time and group separately (p<0.001). Gender was also a significant main effect (p<0.005), but treatment, centre and age were not.
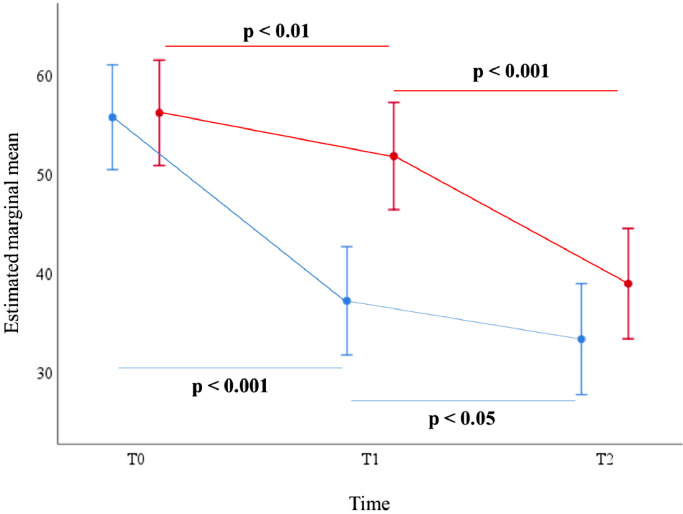
In the ITG group, there was a significant reduction in CFQ score at all time points (Fig. 3). We observed an estimated mean difference of 18.5 points at T1 (95% CI: −21.73, −15.28; p<0.001) which continued at T2 (−3.85; 95% CI: −7.32, −0.38; p<0.05). For the WLC group, there was already a significant reduction in sr-CRCI at T1 (−4.35; 95% CI: −7.42, −1.28; p<0.01). Further reduction in CFQ score was observed at T2 (−12.86; 95% CI: −16.11, −9.62; p<0.001). Detailed information on these pairwise comparisons is summarized in Table 2.
Fig. 3.
CFQ scores over time for the immediate treatment group (ITG) and wait-list control (WLC) group, based on results of the linear mixed model analyses. CFQ scores may vary between 0 and 100, with ≥43 indicating CRCI. In the ITG, there is an estimated mean difference of 18.5 points at T1 (95% CI: −21.73, −15.28; p<0.001) which continued at T2 (−3.85; 95% CI: −7.32, −0.38; p<0.05). For the WLC group, there was already a significant reduction in sr-CRCI at T1 (−4.35; 95% CI: −7.42, −1.28; p<0.01) which continued at T2 (−12.86; 95% CI: −16.11, −9.62; p<0.001).
Legend: blue = immediate treatment group; red = wait-list control group. Error bars present 95% confidence interval for estimated marginal mean (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Table 2.
Within-group differences and between-group differences.
| Pairwise comparison of the immediate treatment group |
Pairwise comparison of the wait-list control group |
Between-group differences |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T1 vs T0 | T2 vs T0 | T2 vs T1 | T0 | T1 | T2 | T1 vs T0 | T2 vs T0 | T2 vs T1 | T1 vs T0 | T2 vs T0 | |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean difference (95%CI) | Mean difference (95%CI) | Mean difference (95%CI) | Mean (SE) | Mean (SE) | Mean (SE) | Mean difference (95%CI) | Mean difference (95%CI) | Mean difference (95%CI) | Mean difference (95%CI) | Mean difference (95%CI) | |
| CFQ | 24.79 (2.27) | 31.95 (2.33) | 33.87 (2.38) | −18.50 (−21.73,−15.28) |
−22.36 (−26.43,−18.28) |
−3.85 (−7.32,−0.38) |
27.51 (2,28) | 27.65 (2.32) | 33,16 (2,36) | −4.35 (−7.42,−1,28) |
−17.21 (−21. 08, −13.34) |
−12.86 (−16.11,−9.62) | −14.16 (−18.61,−9.70) |
−5.15 (−10.76,0.47) |
| p<0.001 | p<0.001 | p = 0.03 | p = 0.006 | p<0.001 | p<0.001 | p<0.001 | p = 0.073 | |||||||
| Distress Thermometer | 5.89 (0.39) | 4.27 (0.41) | 4.37 (0.42) | −1.63 (−2.21,−1.04) |
−1.53 (−2.22,−0.84) |
0.10 (−0.53,0.73) |
5.73 (0.39) | 5.98 (0.40) | 3.85 (0.41) | 0.25 (−0.31,0.81) |
−1.878 (−2.53,−1,23) |
−2.13 (−2.72,−1.54) |
−1.88 (−2.69,−1.07) |
0.35 (−0.60,1.30) |
| p<0.001 | p<0.001 | p = 0.760 | p = 0.375 | p<0.001 | p<0.001 | p<0.001 | p = 0.468 | |||||||
| Distress 38-item PL | 12.53 (1.13) | 8.03 (1.16) | 6.83 (1.19) | −4.50 (−5.71,−3.30) |
−5.71 (−7.27,−4.14) |
−1.20 (−2.49,0.09) |
12.65 (1.14) | 11.09 (1.16) | 8.22 (1.18) | −1.57 (−2.71,−0.42) |
−4.428 (−5.92,−2.93) |
−2.86 (−4.08,−1.65) |
−2.94 (−4.61,−1.27) |
−1.28 (−3.44,0.89) |
| p<0.001 | p<0.001 | p = 0.068 | p = 0.008 | p<0.001 | p<0.001 | p = 0.001 | p = 0.247 | |||||||
| BDI-II | 19.03 (1.76) | 10.46 (1.80) | 9.96 (1.84) | −8.58 (−10.26,−6.89) |
−9.07 (−11.31,−6.84) |
−0.47 (−2.30,1.31) |
19.38 (1.77) | 18.21 (1.79) | 11.74 (1.82) | −1.17 (−2.77,0.43) |
−7.64 (−9.75,−5.53) |
−6.47 (−8.15,−4.80) |
−7.41 (−9.73,−5.09) |
−1.43 (−4.50,1.64) |
| p<0.001 | p<0.001 | p = 0.589 | p = 0.152 | p<0.001 | p<0.001 | p<0.001 | p = 0.360 | |||||||
| FACIT-Fatigue | 24.79 (2.27) | 31.95 (2.33) | 33.87 (2.38) | 7.16 (4.81,9.50) |
9.07 (6.00,12.14) | 1.92 (−0.61,4.40) |
27.51 (2.28) | 27.65 (2.32) | 33.16 (2.36) | 0.14 (−2.09,2.38) |
5.65 (2.75,8.55) | 5.51 (3.17,7.85) | 7.01 (3.78,10.25) | 3.42 (−0.80,7.65) |
| p<0.001 | p<0.001 | p = 0.136 | p = 0.900 | p<0.001 | p<0.001 | p<0.001 | p = 0.112 | |||||||
| EORTC QLQ-C30 | ||||||||||||||
| Summary Score* | 68.47 (3.24) | 77.56 (3.31) | 77.81 (3.38) | 9.10 (6.26,11.93) | 9.34 (5.52,13.16) | 0.24 (−2.80,3.29) |
70.98 (3.27) | 70.31 (3.30) | 78.79 (3.35) | −0.67 (−3.36,2.03) |
7.81 (4.21,11.41) | 8.47 (5.66,11.29) | 9.76 (5.86,13.67) | 1.53 (−3.72,6.78) |
| p<0.001 | p<0.001 | p = 0.875 | p = 0.626 | p<0.001 | p<0.001 | p<0.001 | p = 0.566 | |||||||
| Global Health Status* | 51.22 (3.90) | 59.79 (4.03) | 60.21 (4.13) | 8.56 (4.00,13.12) | 8.99 (3.18,14.79) | 0.42 (−4.48,5.33) |
55.34 (3.93) | 53,78 (3.99) | 63.39 (4.08) | −1.55 (−5.89,2.79) |
8.06 (2.57,13.54) | 9.61 (5.06,14.16) | 10.12 (3.82,16.41) | 0.93 (−7.06,8.92) |
| p<0.001 | p = 0.003 | p = 0.865 | p = 0.481 | p = 0.004 | p<0.001 | p = 0.002 | p = 0.819 | |||||||
| Physical functioning | 73.72 (3.97) | 80.65 (4.04) | 82.64 (4.13) | 6.93 (3.45,10.42) | 8.92 (4.23,13.62) | 1.99 (−1.75,5.73) |
77.89 (4.00) | 79.80 (4.04) | 84.58 (4.10) | 1.92 (−1.39,5.23) |
6.70 (2.27,11.12) | 4.78 (1.31,8.25) | 5.02 (0.21,9.82) | 2.23 (−4.23,8.68) |
| p<0.001 | p<0.001 | p = 0.296 | p = 0.255 | p = 0.003 | p = 0.007 | p = 0.041 | p = 0.497 | |||||||
| Role functioning | 45.69 (5.95) | 68.19 (6.04) | 77.21 (6.18) | 22.50 (17.86,27.14) | 31.52 (25.10,37.95) | 9.02 (3.98,14.06) |
47.46 (6.00) | 63.93 (6.05) | 80.44 (6.13) | 16.47 (12.03, 20.90) |
32.98 (26.96, 39.00) |
16.52 (11.87, 21.16) |
6.04 (−0.38,12.46) | −1.46 (−10.27,7.35) |
| p<0.001 | p<0.001 | p = 0.001 | p<0.001 | p<0.001 | p<0.001 | p = 0.065 | p = 0.745 | |||||||
| Emotional functioning* | 55.62 (5.32) | 71.69 (5.50) | 75.16 (5.64) | 16.07 (9.76,22.38) |
19.55 (11.55,27.55) |
3.48 (−3.31,10.26) |
60.15 (5.36) | 55.92 (5.45) | 76.18 (5.57) | −4.23 (−10.23,1.77) |
16.03 (8.47,23.60) |
20.26 (13.97,26.56) |
20.30 (11.59 ,29.00) |
3.51 (−7.50, 14.53) |
| p<0.001 | p<0.001 | p = 0.313 | p = 0.166 | p<0.001 | p<0.001 | p<0.001 | p = 0.531 | |||||||
| Cognitive functioning* | 48.24 (4.80) | 69.93 (4.99) | 74.21 (5.13) | 21.69 (15.30,28.09) | 25.98 (18.13,33.83) | 4.29 (−2.60,11.17) |
49.16 (4.83) | 51.32 (4.93) | 66.10 (5.04) | 2.17 (−3.92,8.26) |
16.94 (9.51,24.37) | 14.77 (8.38,21.17) | 19.53 (10.70,28.36) |
9.04 (−1.77, 19.85) |
| p<0.001 | p<0.001 | p = 0.221 | p = 0.484 | p<0.001 | p<0.001 | p<0.001 | p = 0.101 | |||||||
| Social functioning | 59.17 (5.52) | 71.47 (5.72) | 70.06 (5.88) | 12.30 (5.36,19.24) | 10.89 (2.22,19.55) | −1.41 (−8.88,6.05) |
57.69 (5.55) | 61.36 (5.66) | 68.71 (5.79) | 3.67 (−2.93,10.28) |
11.02 (2.82,19.21) | 7.34 (0.41,14.27) | 8.63 (- 0.96,18.21) |
−0.13 (−12.06,11.80) |
| p = 0.001 | p = 0.014 | p = 0.710 | p = 0.274 | p = 0.009 | p = 0.038 | p = 0.077 | p = 0.983 | |||||||
| Fatigue* | 60.40 (4.99) | 47.28 (5.18) | 43.42 (5.32) | −13.11 (−19.52,−6.71) | −16.97 (−24.93,−9.02) |
−3.86 (−10.75,3.03) | 48.98 (5.02) | 54.90 (5.12) | 44.00 (5.24) | 5.92 (−0.19,12.02) |
−4.98 (−12.51,2.55) |
−10.90 (−17.30,−4.49) | −19.03 (- 27.87,−10.18) |
−11.99 (−22.95,−1.04) |
| p<0.001 | p<0.001 | p = 0.271 | p = 0.057 | p = 0.194 | p = 0.001 | p<0.001 | p = 0.032 | |||||||
| Nausea and vomiting | 8.14 (2.63) | 5.14 (2.75) | 4.75 (2.83) | −3.00 (−6.88,0.88) |
−3.39 (−7.99,1.21) |
−0.39 (−4.57,3.79) |
7.82 (2.65) | 8.09 (2.71) | 4.55 (2.78) | 0.27 (−3.43,3.97) |
−3.28 (−7.64,1.08) |
−3.55 (−7.44,0.34) |
−3.27 (−8.63,2.09) |
−0.11 (−6.46,6.23) |
| p = 0.129 | p = 0.148 | p = 0.853 | p = 0.855 | p = 0.140 | p = 0.073 | p = 0.230 | p = 0.972 | |||||||
| Pain | 36.90 (6.91) | 30.17 (7.07) | 31.03 (7.23) | −6.74 (−13.46,−0.01) | −5.87 (−14.78,3.03) |
0.86 (−6.37,8.09) |
34.19 (6.96) | 32.52 (7.04) | 28.16 (7.17) | −1.67 (−8.07,4.72) |
−6.03 (−14.44,2.38) |
−4.35 (−11.05,2.35) |
−5.06 (−14.35,4.22) |
0.16 (−12.09, 12.41) |
| p = 0.050 | p = 0.195 | p = 0.814 | p = 0.606 | p = 0.159 | p = 0.201 | p = 0.283 | p = 0.980 | |||||||
| Dyspnoea | 8.14 (2.63) | 5.14 (2.75) | 4.75 (2.83) | −3.00 (−6.88,0.88) |
−3.39 (−8.00,1.21) |
−0.39 (−4.57,3.79) |
7.82 (2.65) | 8.09 (2.71) | 4.55 (2.78) | 0.27 (−3.43,3.97) | −3.28 (−7.64,1.08) | −3.55 (−7.44,0.34) | −3.27 (−8.63,2.09) |
−0.11 (−6.46,6.23) |
| p = 0.129 | p = 0.148 | p = 0.853 | p = 0.885 | p = 0.140 | p = 0.073 | p = 0.230 | p = 0.972 | |||||||
| Insomnia | 40.98 (7.34) | 30.62 (7.63) | 32.73 (7.84) | −10.36 (−20.09,−0.63) | −8.25 (−20.21,3.71) |
2.11 (−8.36,12.58) |
52.26 (7.38) | 48.69 (7.54) | 35.87 (7.71) | −3.57 (−12.84,5.70) | −16.39 (−27.71,−5.06) | −12.81 (−22.55,−3.08) | −6.79 (−20.23,6.65) | 8.14 (−8.34, 24.62) |
| p = 0.037 | p = 0.176 | p = 0.691 | p = 0.448 | p = 0.005 | p = 0.010 | p = 0.320 | p = 0.332 | |||||||
| Appetite loss | 11.53 (4.31) | 11.59 (4.46) | 6.89 (4.58) | 0.06 (−5.17,5.28) |
−4.64 (−11.22,1.95) |
−4.69 (−10.31,0.92) |
8.44 (4.34) | 11.04 (4.42) | 8.94 (4.51) | 2.59 (−2.38,7.56) | 0.50 (−5.73,6.73) | −2.09 (−7.31,3.12) | −2.54 (−9.74,4.67) | −5.14 (−14.20,3.93) |
| p = 0.983 | p = 0.167 | p = 0.101 | p = 0.305 | p = 0.875 | p = 0.429 | p = 0.489 | p = 0.266 | |||||||
| Constipation | 12.99 (6.24) | 7.47 (6.40) | 10.94 (6.55) | −5.53 (−11.91,0.86) |
−2.05 (−10.42,6.32) |
3.48 (−3.39,10.34) |
10.70 (6.28) | 16.04 (6.37) | 7.97 (6.48) | 5.34 (−0.73,11.42) |
−2.72 (−10.63,5.18) |
−8.07 (−14.43,−1.71) | −10.87 (−19.68,−2.06) |
0.67 (−10.85,12.19) |
| p = 0.089 | p = 0.630 | p = 0.319 | p = 0.084 | p = 0.498 | p = 0.013 | p = 0.016 | p = 0.909 | |||||||
| Diarrhoea | 9.88 (3.95) | 9.93 (4.10) | 7.98 (4.21) | 0.05 (−5.02,5.10) |
−1.90 (−8.18,4.39) |
−1.94 (−7.39,3.50) |
11.01 (3.97) | 11.54 (4.05) | 8.72 (4.14) | 0.53 (−4.29,5.35) |
−2.29 (−8.24,3.66) |
−2.82 (−7.88,2.24) |
−0.49 (−7.48,6.50) |
0.39 (−8.26,9.05) |
| p = 0.985 | p = 0.553 | p = 0.482 | p = 0.828 | p = 0.449 | p = 0.272 | p = 0.891 | p = 0.929 | |||||||
| Financial difficulties | 23.62 (6.71) | 14.98 (6.85) | 14.19 (7.00) | −8.65 (−14.70,−2.60) | −9.44 (−17.55,−1.33) | −0.79 (−7.29,5.71) | 20.59 (6.76) | 16.82 (6.84) | 16.91 (6.95) | −3.77 (−9.52,1.98) |
−3.68 (−11.34,3.98) |
0.09 (−5.93,6.11) |
−4.88 (−13.22,3.47) | −5.76 (−16.92,5.40) |
| p = 0.005 | p = 0.023 | p = 0.810 | p = 0.197 | p = 0.345 | p = 0.976 | p = 0.251 | p = 0.310 | |||||||
| EuroQOL EQ-5D-3L | 0.63 (0.05) | 0.73 (0.05) | 0.77 (0.05) | 0.09 (0.04,0.14) | 0.13 (0.07,0.20) | 0.04 (−0.01,0.09) |
0.65 (0.05) | 0.68 (0.05) | 0.75 (0.05) | 0.03 (−0.02,0.08) |
0.10 (0.04,0.16) | 0.07 (0.02,0.12) | 0.06 (−0.01,0.13) |
0.03 (−0.05,0.12) |
| p<0.001 | p<0.001 | p = 0.121 | p = 0.196 | p = 0.001 | p = 0.005 | p = 0.074 | p = 0.461 | |||||||
Abbreviations: CFQ: Cognitive Failure Questionnaire; PL: Problem List; BDI-II: Beck Depression Inventory II; FACIT: Functional Assessment of Chronic Illness Therapy; EORTC: European Organization for Research and Treatment for Cancer Quality of Life Questionnaire; EuroQOL: European Quality of Life Scale.
Statistically significant differences are indicated in bold.
Clinically significant differences in EORTC QLQ-C30 scales at the time of the primary endpoint T1 are indicated with *.
The effect of the intervention is measured by the difference in estimated mean CFQ scores between the ITG and WLC group at T1, with T0 as reference time point. This between-group difference was statistically significant at T1 (−14.16; 95% CI: −18.61,−9.70; p<0.001), but no longer at T2 (−5.15; 95% CI: −10.76,0.47; p = 0.07). Detailed information on these between-group differences is summarized in Table 2.
3.3. Effect of EFT on secondary PROMs
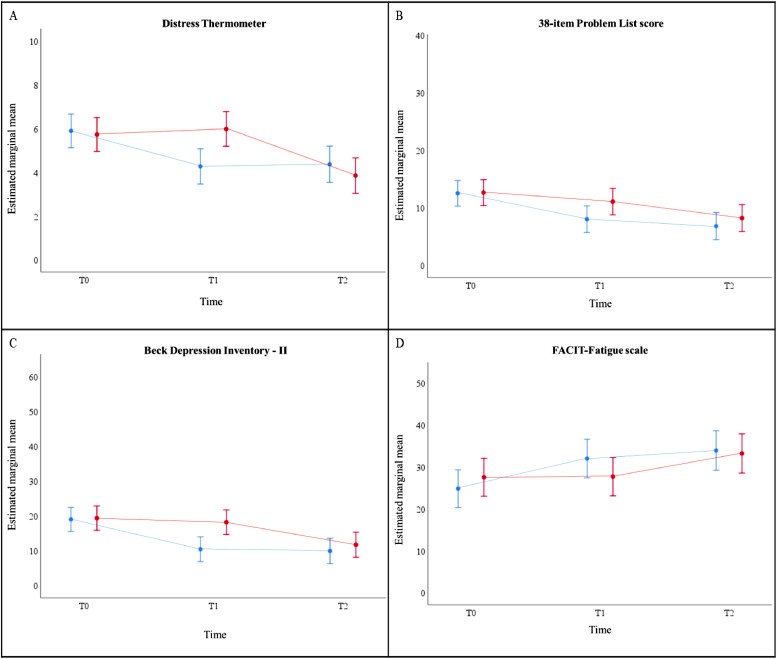
In line with the primary outcome, the time x group interaction effect was found to be significant in all secondary PROMs (DT, 38-item PL, BDI-II and FACIT-fatigue scale) (p<0.005). The results of the PROMs are illustrated in Fig. 4, and detailed information on the pairwise comparisons is summarized in Table 2. The DT, 38-item PL, BDI-II and FACIT-fatigue scale significantly improved from T0 to T1 in the ITG group (p<0.001). In the WLC group, the 38-item PL was already statistically significant improved from T0 to T1 (p<0.01), while the DT, BDI-II and FACIT-fatigue scale improved as from T1 (p<0.001) (Table 2).
Fig. 4.
Estimated marginal mean scores over time for secondary PROMs: (A) DT (scores ranging from 0 to 10; high score indicates more distress); (B) 38-item PL (39 items, scores ranging from 0 to 38; high score indicates more distress); (C) BDI-II (21 items, scores are classified as minimal (0–13), mild (14-19), moderate (20-28), and severe (29-63)); (D) FACIT-Fatigue (13 items, scores ranging from 0 to 52; high score indicates less fatigue), separately for both treatment arms in the total study population, based on results of the linear mixed model analyses.
Results of the linear mixed model analyses indicated that the DT, 38-item PL, BDI-II and FACIT-fatigue scale significantly improved from T0 to T1 in the immediate treatment group (ITG) (p<0.001). This did not continue at T2 (p>0.05). In the wait-list control (WLC) group, the 38-item PL was already statistically significant improved from T0 to T1 (p<0.01) which continued at T2 (p<0.001). The DT, BDI-II and FACIT-fatigue scale only improved as from T1 in the WLC group (p<0.001).
Legend: blue = immediate treatment group; red = wait-list control group. Error bars present 95% confidence interval for estimated marginal mean.
Abbreviations: PROMs: patient-reported outcome measures; DT: distress thermometer; PL: 38-item problem list (PL); BDI-II: Beck Depression Inventory-II; FACIT-Fatigue: Functional Assessment of Chronic Illness Therapy-Fatigue Subscale (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Similar to the primary outcome CFQ score, the between-group difference for all secondary endpoints showed a statistically significant difference at T1 (p<0.001). At T2, the effect of the intervention was no longer significantly different between both arms (p>0.05; Table 2).
3.4. Effect of EFT on HRQoL
Estimated mean quality of life scores of the EORTC QLQ-C30 scales are presented in Fig. 5. The scales of EORTC QLQ-C30 were analysed over time with linear mixed models, as were the primary and other secondary outcomes. The time x group interaction effect was found statistically significant for Summary score, GHS, Role functioning, Emotional functioning, Cognitive functioning and Fatigue and Constipation symptom scale (p<0.05).
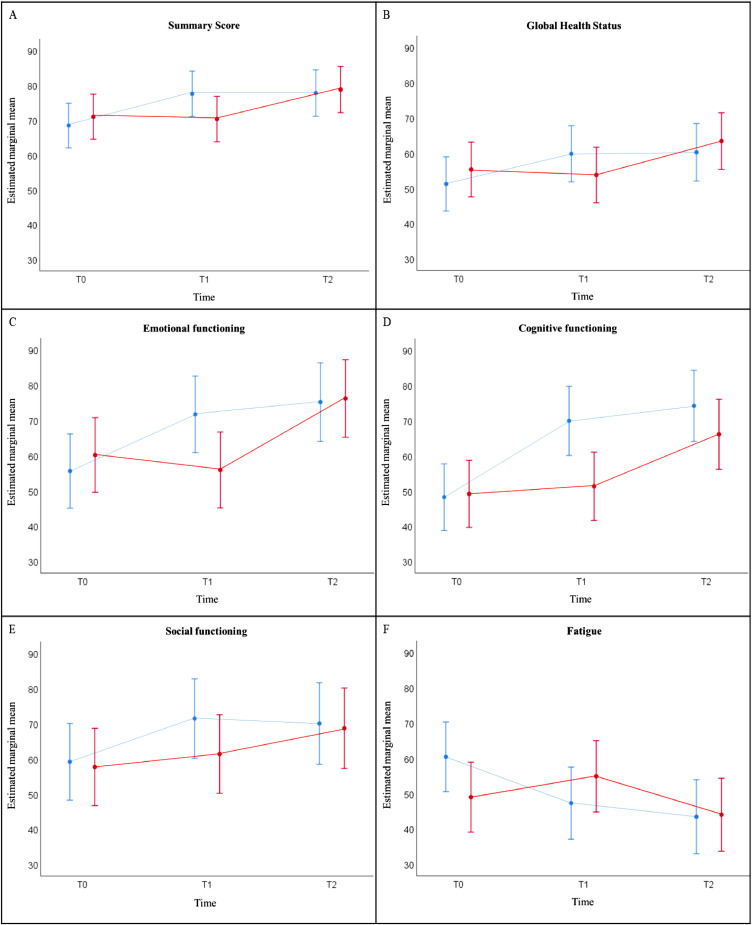
Fig. 5.
Estimated marginal mean scores over time for HRQoL measures, derived from the EORTC QLQ-C30: (A) Summary Score; (B) Global Health Status; (C) Emotional functioning; (D) Cognitive functioning; (E) Social functioning; (F) Fatigue, separately for both treatment arms in the total study population, based on results of the linear mixed model analyses. All of the scales range in score from 0 to 100.
Results of the linear mixed model analyses indicated that the Summary Score, Global Health Status, Emotional functioning, Cognitive functioning, Social functioning and Fatigue significantly improved from T0 to T1 in the immediate treatment group (ITG) (p<0.001). This did not continue at T2 (p>0.05). For the wait-list control (WLC) group, there was no significant improvement observed at T1 (p>0.05). Improvement in the WLC for Summary Score (p<0.001), Global Health Status (p<0.001), Emotional functioning (p<0.001) Cognitive functioning (p<0.001), Social functioning (p<0.05) and Fatigue (p = 0.001) was observed at T2.
Legend: blue = immediate treatment group; red = wait-list control group. Error bars present 95% confidence interval for estimated marginal mean.
Abbreviations: HRQoL: health-related quality of life; EORTC QLQ-C30: European Organization for Research and Treatment for Cancer QoL Questionnaire (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Analyses by pairwise comparisons indicated that the EORTC QLQ-C30 Summary score, the GHS and all functional scales statistically significantly improved over time in both arms (p<0.05) (Table 2).
Furthermore, the difference on HRQoL scores of the EORTC QLQ-C30 between both groups were analysed over time with T0 as reference time point (Table 2). Summary score, GHS, Physical, Emotional and Cognitive functioning indicated there was a significant difference between the ITG and WLC group at T1 vs T0 (p<0.05), and no longer at T2 vs T0 (p>0.05). The scores on the Fatigue symptom scale on the other hand, were significantly different between the ITG and WLC group at both time points T1 (p<0.001) and T2 (p<0.05) versus reference time point T0.
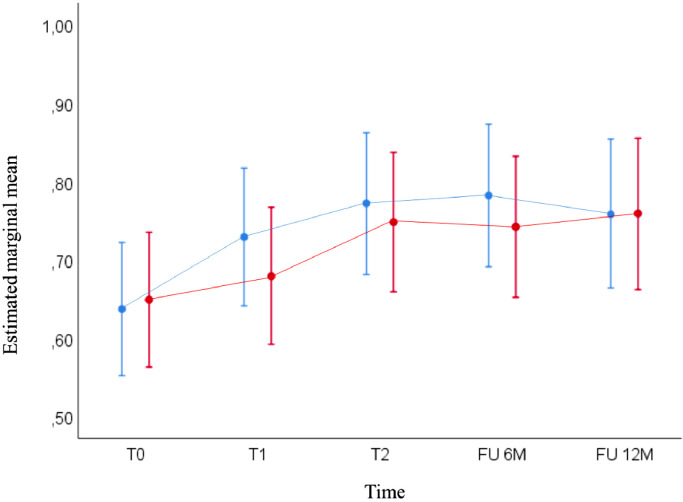
Besides the quality of life, the EuroQoL EQ-5D-3 L score or health state index was measured over the three time periods (T0, T1, T2; Table 2) and at 6 and 12 months after baseline interview (T0) (Fig. 6).
Fig. 6.
EuroQoL EQ-5D-3L: Estimated marginal mean scores over time for the immediate treatment group (ITG) and wait-list control (WLC) group, based on results of the linear mixed model analyses. These analyses indicated that the EuroQol EQ-5D-3 L scores significantly improved at all time points when compared to baseline result at T0 in the ITG (p<0.005). In the WLC group, this significant improvement was observed as from T1 and was maintained until 12 months of follow-up when compared to baseline result at T0 (p<0.005).
Legend: blue = immediate treatment group; red = wait-list control group. Error bars present 95% confidence interval for estimated marginal mean.
Abbreviations: EuroQol EQ-5D-3L: instrument including 5 dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) with 3 levels of severity (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Six and 12 months after T0, response rate to the follow-up questionnaires was 77.8% and 75.6% in the ITG group and 84.0% and 68.0% in the WLC group, respectively. At six months, 60.0% of the ITG group and 45.2% of the WLC group responded they continued the application of EFT. At 12 months follow-up, 55.9% of the ITG group and 35.3% of the WLC group still applied EFT.
In the ITG group, there was a significant improvement of the health state index scores at all time points when compared to baseline result at T0 (p<0.005; data not shown). In the WLC group, this significant improvement was observed as from T1 and was maintained until 12 months of follow-up (p<0.005; data not shown). Pairwise comparisons indicated that the strongest improvement in health state index scores took place after the EFT intervention: T1 vs T0 for the ITG (p<0.001) and T2 vs T1 for the WLC group (p = 0.005) (Fig. 6; Table 2).
The estimates of fixed effects show that the EuroQoL EQ-5D-3 L scores between the ITG and WLC group were not statistically significantly different over time, with T0 as reference time point and measured until follow-up of 12 months (p>0.05; Table 2 for T1 and T2 vs T0; data not shown for follow-up).
4. Discussion
We aimed to establish EFT as an effective treatment to help cancer survivors manage sr-CRCI. To the best of our knowledge, this multicentre randomised wait-list controlled trial is the first to evaluate the application of EFT as a tool for improving sr-CRCI. These data showed that an intervention with EFT reduced subjective cognitive complaints with a difference in proportion of 46.5% between the ITG and WLC group at T1 (p<0.01), almost twice the assumed difference. The linear mixed model analysis established the potential of this intervention by a significant difference in estimated mean CFQ scores at T1 between both groups (p<0.001). At T2, this between-group difference was no longer statistically significant as the application of EFT resulted in a similar reduction in the WLC group, who caught up with the effect in the ITG.
Perceived cognitive problems are often associated with psychological factors such as anxiety, depression, fatigue or insomnia [49]. As mentioned recently by Ganz and Van Dyk in their paper on the impact of chemotherapy and endocrine therapy, patient reported outcomes on depressive symptoms and fatigue are valuable covariates in their evaluation of changes in CRCI over time because these common treatment-related symptoms may affect cognitive function [50]. In this trial, EFT was shown to improve the PROMs such as distress, depressive symptoms/emotional status and fatigue. These results are in line with those of previous RCTs where EFT appeared effective in individuals without cancer for psychological conditions such as anxiety, depression, phobias and PTSD [27,28,31,34,51].
Cognitive difficulties can have a detrimental effect on a patient's HRQoL (autonomy, return to work, social relationships, and self-confidence) in the context of long-term cancer care [49]. When verifying the clinically relevant outcomes regarding HRQoL after EFT intervention, determined by a difference of ≥10 points on the EORTC QLQ-C30 scales, we may conclude that the overall health (EORTC QLQ-C30 Summary Score and GHS), Emotional functioning, Cognitive functioning and Fatigue were clinically improved in the ITG compared to the WLC group at T1. These outcomes support the hypothesis that subjective cognitive complaints impact HRQoL in cancer patients, which was stated earlier by our research group [9]. The fact that we can detect significant improvement in HRQoL underlines the importance of sr-CRCI as opposed to objective cognitive complaints. In this trial, we decided not to implement a neuropsychological test battery, as neuropsychological test scores were found insufficiently correlated with subjectively assessed cognitive symptoms [52,53].
When comparing the EuroQoL EQ-5D-3 L scores between both groups, we did not observe a difference at any time point. Still, the health state was improved in both groups when the intervention with EFT started. Interestingly, 60.0% and 45.2% in the ITG and WLC group, respectively, responded to continue the application of EFT at six months follow-up. Moreover, one year after participating in the EMOTICON trial, 55.9% and 35.3% in the ITG and WLC group, respectively, still applied EFT. We believe this result points out the feasibility of EFT.
These outcomes support the association of sr-CRCI with psychological risk factors such as distress, and the efficacy of EFT to improve quality of life. The results from the EMOTICON trial are in line with a biopsychosocial model where the occurrence of sr-CRCI does not only depend on biological or physical factors, but is also affected by psychological factors and social or environmental factors [54].
There are different other strengths of this study. This is the first trial to examine the efficacy of EFT to reduce sr-CRCI in a large group of cancer survivors. EFT is a novel therapy in oncology, but more than 100 studies already acknowledged this technique as a well-established evidence-based practice for both physiological and psychological symptoms [32,34,55,56]. Second, we believe that the promising results are very valuable: PROMs are considered the gold standard for quantifying any possible symptomatic treatment effect in non-cancer patients. Despite self-report measures may cause (recall) bias, they are important to understand the patient's perspective on the experienced problem. Third, adequate care was taken to ensure proper conduct of EFT. Furthermore, information sessions were adjusted to the needs of the patients individually. When necessary, additional one-on-one practice was foreseen. By only a few contact moments, patients stated they felt EFT had helped them with their emotional and physical symptoms. During the planned evaluation moments, patients could reflect on personal progress. Such statements support the hypothesis that sr-CRCI might have an emotional cause such as distress. Therapy by EFT could be a low cost and low threshold treatment. Research has shown that EFT requires fewer sessions than CBT and that the effects of an EFT intervention last longer [57]. Last, the randomised controlled design of the study ensured a correct distribution of participants over the ITG and WLC group. An explorative analysis on stratification by age suggested that the cognitive decline experienced by participants was not age-related (data not shown). Although participants older than 65 years were equally divided over the ITG and WLC group, the majority is younger than 65 years old. Stratification by cancer type did not occur, which resulted in a heterogeneous sample. In general, we believe the population who benefits the most of these trial results could be female breast cancer survivors younger than 65 years old.
Due to the COVID-19 pandemic, leading to a government-issued halt of including new patients in clinical trials in April 2020, we could only include 121 patients of whom 104 were evaluable at T1. As the effect observed was much larger than the estimated effect of 25%, the smaller sample size had no effect on the statistical analysis. Furthermore, by performing linear mixed model analyses, we allowed inclusion of all 121 patients even though some patients had missing outcomes at T1 and/or T2. Linear mixed model analyses will yield valid results when these missing outcomes are missing at random. Analyses were performed on the full analysis set with preservation of the initial randomization. The small number of males and patients older than 65 years included in the study may be considered to be a limitation, as well as the fact that stress response biomarkers were not measured. Another limitation is the use of the CFQ cut-off score as both the screener and outcome measure. Also, the use of the CFQ cut-off as main outcome instead of the continuous score is a limitation. A last limitation is the exploratory nature of the analysis for T2 as there was no pre-defined sample size for this time point.
Future studies will no doubt offer a focused perspective in which to view the impressive and efficient effects of EFT for use within this broad array of psychological symptoms. The promising results of both primary and secondary outcomes, although explorative for T2, lead us to plan a subsequent trial with the focus on distress and fear of cancer recurrence. We also plan a post-hoc explorative analysis of the patient diaries to evaluate participant engagement throughout the trial. These participant notes may inform us about the problems EFT was addressed for, which factors lead to continuous application of EFT measured at follow-up and how to avoid drop-out in future trials because of disbelief of the power of the application of EFT. Furthermore, we will examine if hormonal treatment and the time since treatment end affect the potential success of EFT as both factors are known to influence sr-CRCI.
This multicentre randomised wait-list controlled trial demonstrated that EFT is an effective strategy for patients suffering from sr-CRCI. Furthermore, EFT is associated with multidimensional improvements in mental well-being and quality of life. These data highlight the importance of monitoring for sr-CRCI and to implement strategies to manage sr-CRCI. This trial highlights EFT as a safe, effective, low cost and low threshold intervention, easy to implement in clinical practice.
Declaration of Competing Interest
All authors have no conflicts of interest to declare, except for co-author dr. Christel Fontaine who received financial support for attending online ESMO 2020, ICOS 2020, SABCS 2020, EBCC 2021, ASCO 2021, MASCC 2021, ECHNO 2021.
Acknowledgments
Funding
Stand up to Cancer (Kom op tegen Kanker), the King Baudouin Foundation (J1121310) and the AZ Groeninge clinical trials fund.
Contributors
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content as listed below. All authors had full access to all the data and accept responsibility to submit for publication. Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke and Hans Pottel verified the data.
Conceptualisation: Philip R. Debruyne, Tessa Lefebvre, Michelle Lycke, Kathleen Meryck
Data curation: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Marleen Borms, Marianne Hanssens, Christel Knops, Hans Pottel
Formal analysis: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Hans Pottel
Funding acquisition: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Marleen Borms, Marianne Hanssens, Christel Knops, Kathleen Meryck, Tom Boterberg, Hans Pottel, Patricia Schofield
Investigation: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Marleen Borms, Marianne Hanssens, Christel Knops
Methodology: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Kathleen Meryck, Tom Boterberg, Hans Pottel, Patricia Schofield
Project administration: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke
Resources: Philip R. Debruyne, Hans Pottel
Software: Philip R. Debruyne, Hans Pottel
Supervision: Philip R. Debruyne
Validation: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Marleen Borms, Marianne Hanssens, Christel Knops, Kathleen Meryck, Tom Boterberg, Hans Pottel, Patricia Schofield
Visualisation: Philip R. Debruyne, Laura Tack
Writing– original draft: Philip R. Debruyne, Laura Tack
Writing– review & editing: Philip R. Debruyne, Laura Tack, Tessa Lefebvre, Michelle Lycke, Chistine Langenaeken, Christel Fontaine, Marleen Borms, Marianne Hanssens, Christel Knops, Kathleen Meryck, Tom Boterberg, Hans Pottel, Patricia Schofield
Data sharing statement
Study data will not be made available. Study related documents such as the study protocol will be available as supplementary material with publication.
Acknowledgements
The study was developed and analysed by following sponsors: Stand up to Cancer (Kom op tegen Kanker), the King Baudouin Foundation (J1121310) and the AZ Groeninge clinical trials fund. We are grateful for all investigators, physicians and onco-psychologists who contributed to this trial. We thank all the participants in the trial for their interest in the application of EFT and dedication to the trial.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101081.
Appendix. Supplementary materials
References
- 1.American Cancer Society . American Cancer Society; Atlanta: 2019. Cancer treatment & survivorship facts & figures 2019-2021. [Google Scholar]
- 2.Pal S.K., Miller M.J., Agarwal N., Chang S.M., Chavez-MacGregor M., Cohen E. Clinical cancer advances 2019: annual report on progress against cancer from the american society of clinical oncology. J Clin Oncol. 2019;37(10):834–849. doi: 10.1200/JCO.18.02037. [DOI] [PubMed] [Google Scholar]
- 3.Ahles T.A., Root J.C., Ryan E.L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janelsins M.C., Heckler C.E., Peppone L.J., Ahles T.A., Mohile S.G., Mustian K.M. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol. 2018;36(32) doi: 10.1200/JCO.2018.78.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurria A., Somlo G., Ahles T. Renaming "chemobrain". Cancer Investig. 2007;25(6):373–377. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 6.Lycke M., Pottel L., Pottel H., Ketelaars L., Stellamans K., Van Eygen K. Predictors of baseline cancer-related cognitive impairment in cancer patients scheduled for a curative treatment. Psychooncology. 2017;26(5):632–639. doi: 10.1002/pon.4200. [DOI] [PubMed] [Google Scholar]
- 7.Joly F., Heutte N., Duclos B., Noal S., Léger-Hardy I., Dauchy S. Prospective evaluation of the impact of antiangiogenic treatment on cognitive functions in metastatic renal cancer. Eur Urol Focus. 2016;2(6):642–649. doi: 10.1016/j.euf.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Joly F., Castel H., Tron L., Lange M., Vardy J. Potential effect of immunotherapy agents on cognitive function in cancer patients. J Natl Cancer Inst. 2020;112(2):123–127. doi: 10.1093/jnci/djz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lycke M., Lefebvre T., Pottel L., Pottel H., Ketelaars L., Stellamans K. Subjective, but not objective, cognitive complaints impact long-term quality of life in cancer patients. J Psychosoc Oncol. 2019;37(4):427–440. doi: 10.1080/07347332.2018.1504154. [DOI] [PubMed] [Google Scholar]
- 10.Moore H.C. An overview of chemotherapy-related cognitive dysfunction, or 'chemobrain'. Oncol (Williston Park) 2014;28(9):797–804. [PubMed] [Google Scholar]
- 11.Danhauer S.C., Legault C., Bandos H., Kidwell K., Costantino J., Vaughan L. Positive and negative affect, depression, and cognitive processes in the cognition in the study of tamoxifen and raloxifene (Co-STAR) trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20(5):532–552. doi: 10.1080/13825585.2012.747671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. Distress during cancer care 2020 [cited 2020 14th November] [Available from: https://www.nccn.org/patients/guidelines/content/PDF/distress-patient.pdf.
- 13.Lycke M., Lefebvre T., Pottel L., Pottel H., Ketelaars L., Stellamans K. The distress thermometer predicts subjective, but not objective, cognitive complaints six months after treatment initiation in cancer patients. J Psychosoc Oncol. 2017;35(6):741–757. doi: 10.1080/07347332.2017.1365798. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon H.M., Tannock I.F., Pond G.R., Renton C., Rourke S.B., Vardy J.L. Perceived cognitive impairment in people with colorectal cancer who do and do not receive chemotherapy. J Cancer Surviv. 2018;12(2):178–185. doi: 10.1007/s11764-017-0656-6. [DOI] [PubMed] [Google Scholar]
- 15.Pullens M.J., De Vries J., Van Warmerdam L.J., Van De Wal M.A., Roukema J.A. Chemotherapy and cognitive complaints in women with breast cancer. Psychooncology. 2013;22(8):1783–1789. doi: 10.1002/pon.3214. [DOI] [PubMed] [Google Scholar]
- 16.Janelsins M.C., Heckler C.E., Peppone L.J., Kamen C., Mustian K.M., Mohile S.G. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017;35(5):506–514. doi: 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz P.A., Kwan L., Castellon S.A., Oppenheim A., Bower J.E., Silverman D.H. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seliktar N., Polek C., Brooks A., Hardie T. Cognition in breast cancer survivors: hormones versus depression. Psychooncology. 2015;24(4):402–407. doi: 10.1002/pon.3602. [DOI] [PubMed] [Google Scholar]
- 19.Vardy J.L., Dhillon H.M., Pond G.R., Rourke S.B., Bekele T., Renton C. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. doi: 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardy J.L., Stouten-Kemperman M.M., Pond G., Booth C.M., Rourke S.B., Dhillon H.M. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2019;13(1):15–26. doi: 10.1007/s11682-017-9728-5. [DOI] [PubMed] [Google Scholar]
- 21.Schilder C.M., Seynaeve C., Linn S.C., Boogerd W., Beex L.V., Gundy C.M. Self-reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21(5):479–487. doi: 10.1002/pon.1928. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Yu L., Long Z., Li Y., Cao F. Perceived cognitive impairment in Chinese patients with breast cancer and its relationship with post-traumatic stress disorder symptoms and fatigue. Psychooncology. 2015;24(6):676–682. doi: 10.1002/pon.3710. [DOI] [PubMed] [Google Scholar]
- 23.Bray V.J., Dhillon H.M., Bell M.L., Kabourakis M., Fiero M.H., Yip D. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol. 2017;35(2):217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 24.King S., Green H.J. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol. 2015;5:72. doi: 10.3389/fonc.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ercoli L.M., Petersen L., Hunter A.M., Castellon S.A., Kwan L., Kahn-Mills B.A. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015;24(11):1360–1367. doi: 10.1002/pon.3769. [DOI] [PubMed] [Google Scholar]
- 26.Bach D., Groesbeck G., Stapleton P., Sims R., Blickheuser K., Church D. Clinical EFT (Emotional Freedom Techniques) improves multiple physiological markers of health. J Evid Based Integr Med. 2019;24 doi: 10.1177/2515690X18823691. 2515690x18823691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Church D., Feinstein D. The manual stimulation of acupuncture points in the treatment of post-traumatic stress disorder: a review of clinical emotional freedom techniques. Med Acupunct. 2017;29(4):194–205. doi: 10.1089/acu.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebastian B., Nelms J. The effectiveness of emotional freedom techniques in the treatment of posttraumatic stress disorder: a meta-analysis. Explore (NY) 2017;13(1):16–25. doi: 10.1016/j.explore.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Chatwin H., Stapleton P., Porter B., Devine S., Sheldon T. The effectiveness of cognitive behavioral therapy and emotional freedom techniques in reducing depression and anxiety among adults: a pilot study. Integr Med (Encinitas) 2016;15(2):27–34. [PMC free article] [PubMed] [Google Scholar]
- 30.Clond M. Emotional freedom techniques for anxiety: a systematic review with meta-analysis. J Nerv Ment Dis. 2016;204(5):388–395. doi: 10.1097/NMD.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 31.Nelms J.A., Castel L. A systematic review and meta-analysis of randomized and nonrandomized trials of clinical emotional freedom techniques (EFT) for the treatment of depression. Explor (NY) 2016;12(6):416–426. doi: 10.1016/j.explore.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Church D. Clinical EFT as an evidence-based practice for the treatment of psychological and physiological conditions. Psychology. 2013;4(8):645–654. doi: 10.4236/psych.2013.48092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Church D., Yount G., Brooks A.J. The effect of emotional freedom techniques on stress biochemistry: a randomized controlled trial. J Nerv Ment Dis. 2012;200(10):891–896. doi: 10.1097/NMD.0b013e31826b9fc1. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein D. Acupoint stimulation in treating psychological disorders: evidence of efficacy. Rev Gen Psychol. 2012;16(4):364–380. [Google Scholar]
- 35.Church D., De Asis M.A., Brooks A.J. Brief group intervention using emotional freedom techniques for depression in college students: a randomized controlled trial. Depress Res Treat. 2012;2012 doi: 10.1155/2012/257172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilomen S.A., Lee C.W. The efficacy of acupoint stimulation in the treatment of psychological distress: a meta-analysis. J Behav Ther Exp Psychiatry. 2015;48:140–148. doi: 10.1016/j.jbtep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Church D., Stapleton P., Yang A., Gallo F. Is tapping on acupuncture points an active ingredient in emotional freedom techniques? a systematic review and meta-analysis of comparative studies. J Nerv Ment Dis. 2018;206(10):783–793. doi: 10.1097/NMD.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 38.Swingle P.G., Pulos L., Swingle M.K. Neurophysiological indicators of EFT treatment of posttraumatic stress. Subtle Energies Energy Med. 2004;15(1):75–86. [Google Scholar]
- 39.Feinstein D. Rapid treatment of ptsd: why psychological exposure with acupoint tapping may be effective. Psychotherapy. 2010;47(3):385–402. doi: 10.1037/a0021171. [DOI] [PubMed] [Google Scholar]
- 40.Lane J. The neurochemistry of counterconditioning: acupressure desensitization in psychotherapy. Energy Psychol. 2009;1(1):31–44. [Google Scholar]
- 41.Church D., Yount G., Rachlin K., Fox L., Nelms J. Epigenetic effects of PTSD remediation in veterans using clinical emotional freedom techniques: a randomized controlled pilot study. Am J Health Promot. 2018;32(1):112–122. doi: 10.1177/0890117116661154. [DOI] [PubMed] [Google Scholar]
- 42.Church D., Nelms J., editors. Psychological change in a population with frozen shoulder: A randomized controlled dismantling study of clinical EFT (emotional freedom techniques) Archives of Scientific Psychology; 2015. [Google Scholar]
- 43.Church D., Nelms J. Pain, range of motion, and psychological symptoms in a population with frozen shoulder: a randomized controlled dismantling study of clinical EFT (emotional freedom techniques) Arch Sci Psychol. 2016;4(1):38–48. [Google Scholar]
- 44.Maharaj M.E. Differential gene expression after emotional freedom techniques (EFT) treatment: a novel pilot protocol for salivary mRNA assessment. Energy Psychol Theory Res Treat. 2016;8(1):17–32. [Google Scholar]
- 45.Craig G. Elite Books; 2011. The EFT manual. [Google Scholar]
- 46.Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 47.Ponds R., Van Boxtel M., Jolles J. De cognitive failure questionnaire als maat voor subjectief cognitief functioneren. Tijdschr voor Neuropsychol. 2006;2:37–45. [Google Scholar]
- 48.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 49.Lange M., Joly F., Vardy J., Ahles T., Dubois M., Tron L. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019;30(12):1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganz P.A., Van Dyk K. Cognitive impairment in patients with breast cancer: understanding the impact of chemotherapy and endocrine therapy. J Clin Oncol. 2020;38(17):1871–1874. doi: 10.1200/JCO.20.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boath E., Stewart A., Carryer A., Walton I., Hill L. Can emotional freedom techniques (EFT) be effective in the treatment of emotional conditions? Results of a service evaluation in Sandwell. Eur J Integr Med. 2014;6(5):614. [Google Scholar]
- 52.Horowitz T.S., Suls J., Treviño M. A call for a neuroscience approach to cancer-related cognitive impairment. Trends Neurosci. 2018;41(8):493–496. doi: 10.1016/j.tins.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Bray V.J., Dhillon H.M., Vardy J.L. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J Cancer Surviv. 2018;12(4):537–559. doi: 10.1007/s11764-018-0692-x. [DOI] [PubMed] [Google Scholar]
- 54.Loh K.P., Janelsins M.C., Mohile S.G., Holmes H.M., Hsu T., Inouye S.K. Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol. 2016;7(4):270–280. doi: 10.1016/j.jgo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Church D., Hawk C., Brooks A.J., Toukolehto O., Wren M., Dinter I. Psychological trauma symptom improvement in veterans using emotional freedom techniques: a randomized controlled trial. J Nerv Ment Dis. 2013;201(2):153–160. doi: 10.1097/NMD.0b013e31827f6351. [DOI] [PubMed] [Google Scholar]
- 56.Church D., Feinstein D., Palmer-Hoffman J., Stein P.K., Tranguch A. Empirically supported psychological treatments: the challenge of evaluating clinical innovations. J Nerv Ment Dis. 2014;202(10):699–709. doi: 10.1097/NMD.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 57.Feinstein D. Energy psychology interactive: rapid interventions for lasting change: Innersource; 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.