Highlights
-
•
TAC in segments of the ascending and descending thoracic aorta can be assessed by routine CAC scanning.
-
•
TAC assessment with the threshold of 300 improved risk prediction and reclassification for CVD mortality when added to the ASCVD risk score and CAC.
-
•
TAC >300 may improve patient selection for those who would benefit more strongly from statin use, from intermediate ASCVD risk patients who should consider a statin (CAC=1-100), and those where a statin is not recommended (CAC=0).
Keywords: Thoracic aortic calcification, Coronary artery calcium, Prognosis, Cardiovascular mortality, Computed tomography
Abstract
Objective
TAC is associated with an increased atherosclerotic cardiovascular disease (ASCVD) risk, but it is unclear how to interpret thoracic aortic calcification (TAC) findings in conjunction with ASCVD risk and coronary artery calcium (CAC) score according to 2018 ACC/AHA Multisociety cholesterol guidelines. We evaluate the incremental value of thoracic aortic calcification TAC over CAC for predicting and reclassifying ASCVD mortality risk.
Method
The study included 30,630 asymptomatic individuals (mean age: 55 ± 8 years, male: 64%) from the CAC Consortium. TAC was categorized as TAC 0, 1-300, and >300. Patients were categorized as low (<5%), borderline (5–7.5%), intermediate (7.5–20%), or high (≥20%) 10-year ASCVD risk according to the Pooled Cohorts Equation. In the intermediate risk group, the utility of TAC beyond CAC for statin eligibility was assessed according to the guideline. CAC was categorized as CAC=0 (no statin), CAC 1-100 (favors statin), or CAC>100 (initiate stain).
Results
During the median 11.2 years (IQR 9.2–12.4) follow-up, 345 (1.1%) CVD deaths occurred. TAC>300 was associated with increased CVD mortality after adjusting for ASCVD risk and CAC (HR:4.72, 95% CI: 3.39–6.57, p<0.001). In borderline and intermediate risk groups, TAC improved discrimination when added to a model included ASCVD risk and CAC (C-statistic: 0.77 vs. 0.68 in borderline group; 0.67 vs. 0.63 in intermediate group, both p < 0.05). The addition of TAC over CAC improved risk reclassification in borderline, intermediate and high-risk groups (categorical net reclassification index: 0.40, 0.29, and 0.49, respectively, all p < 0.001). Of intermediate risk participants for whom consideration of CAC was recommended based on the guideline, TAC >300 was associated with an increased CVD mortality risk across each statin eligibility group (all p < 0.001, compared to TAC 0).
Conclusion
TAC was independently associated with CVD death. Among individuals with borderline or intermediate ASCVD risk, a TAC threshold of 300 may provide added prognostic and reclassification value beyond the current guideline-based approach.
1. Introduction
Cardiovascular disease (CVD) death is one of the major causes of death, with more than 850,000 deaths annually, approximately one-third of all-cause deaths, attributed by CVD in the United States [1]. Coronary artery calcium (CAC), by ECG-gated non-contrast computed tomography imaging, is associated with atherosclerotic burden and adverse CVD clinical outcomes [2], [3], [4]. The CAC score effectively reclassifies CVD risk of asymptomatic patients that may facilitate treatment decision-making in clinical settings [5], [6], [7]. The 2018 ACC/AHA Multisociety guidelines recommend CAC scanning to guide preventive treatment strategies among asymptomatic individuals with intermediate 10-year atherosclerotic cardiovascular disease (ASCVD) risk if a decision about statin therapy is uncertain [8].
Thoracic aortic calcification (TAC) is a marker of aortic atherosclerosis and has been associated with CVD risk factors such as arterial stiffening and systemic arterial hypertension [9], [10], [11]. TAC in segments of the ascending and descending thoracic aorta can be assessed by routine CAC scanning without additional radiation. Several population-based studies have shown that the presence and extent of TAC is associated with adverse CVD outcomes [12], [13], [14]. However, less is known about the additional prognostic benefit of TAC beyond CAC. Further, in clinical practice, it is unclear how to interpret TAC findings in conjunction with patients’ ASCVD risk and CAC score. We examined the prognostic significance of TAC beyond ASCVD risk score and CAC in asymptomatic adults. Further, we explored the clinical implications of TAC over CAC in reclassifying risk for statin eligibility in patients with intermediate ASCVD risk according to 2018 ACC/AHA Multisociety cholesterol lowering guidelines.
2. Methods
2.1. Study population
The CAC Consortium is a large cohort of primarily asymptomatic patients who underwent non-contrast cardiac-gated CAC testing in four clinical sites; it was designed to study the relationship between CAC scoring and long-term cause-specific mortality. Details of the rationale and design of the CAC Consortium have been described [15]. A total of 66,636 patients were enrolled in the CAC Consortium with baseline CAC testing from 1991 to 2010. All study participants provided informed consent at the time of enrollment and CAC scanning. The study protocol was approved by the Institutional Review Board at all participating sites. We included 30,630 patients with age 40–75 years, from two of the four CAC Consortium sites (Cedars-Sinai Medical Center and UCLA Harbor) in whom TAC quantification was performed.
2.2. Computed tomography data
Non-contrast ECG-gated computed tomography scans for CAC scoring were performed at each site. A common standard protocol was used for each scanner. CAC, TAC, aortic valve calcification (AVC), and mitral valve calcification (MVC) were computed using the sum of individual area-density products according to the Agatston method [2]. TAC included calcium scored from segments of the ascending and descending portion of the thoracic aorta visible in the CAC scan. Calcification in the aortic annulus and root was considered as TAC. Calcium deposits in the aortic valve leaflets and mitral valve leaflet/annulus were computed as AVC and MVC, respectively. AVC and MVC information are available in 8713 patients from one of the four CAC Consortium centers (Cedars-Sinai Medical Center).
2.3. Clinical data and adjudication of events
Hypertension, diabetes, and dyslipidemia were defined as either a prior diagnosis or current treatment with medical therapy for these conditions. Dyslipidemia was also considered if patients had concomitant lipid panel showing LDL-C >160 mg/dL, HDL-C <40 mg/dL in men or <50 mg/dL in women, or fasting triglycerides >150 mg/dL. Smoking status was defined as current smoker or not. More detailed descriptions of risk factor definitions in the CAC Consortium have been previously described [15]. A 10-year risk for ASCVD was calculated using baseline data according to the Pooled Cohort Equations [16].
Adjudication of mortality was performed by interrogation of the Social Security Death Index (SSDI) Death Master File, using unique patient identifiers, such as name, date of birth, and social security number (SSN). Cause of death was determined based on ICD-9 and ICD-10 codes on the death certificate. CVD death was defined as mortality from coronary heart disease, stroke, heart failure or other cardiovascular conditions. Follow-up of the cohort occurred through June 2014 for this report.
2.4. Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are reported as counts with proportions. A 10-year risk predictor score for ASCVD was calculated using baseline data according to the Pooled Cohort Equations, then categorized as low (<5%), borderline (5 to <7.5%), intermediate (7.5 to <20%), and high risk (>20%) group. Patients with LDL-C>190 or diabetes were included in the high risk group according to guidelines. CAC score was categorized as 0, 1 to 99, 100-399 and ≥ 400.
To develop and validate an optimal TAC cutoff for CVD mortality prediction, the study population was divided into derivation and validation cohorts according to participating sites (derivation cohort: UCLA Harbor, n=18,349; validation cohort: Cedars-Sinai, n=12,281). In the derivation cohort, TAC 300 cutoff was derived by receiver-operating characteristic curves for CVD death prediction using the maximum Youden index. The TAC 300 cutpoint remained significantly associated with increased CVD death risk in the validation cohort after adjustment for ASCVD and CAC (HR: 4.03, 95% CI: 2.63-6.18, p<0.001). TAC score was categorized using the cutoff, as TAC=0 (no TAC), TAC=1-300 (low TAC), or TAC>300 (high TAC). Supplemental Figs. 1 and 2 displayed example of scans showing TAC >300 and <300.
Comparisons between TAC categories were performed by one-way analysis of variance for continuous variables (ANOVA) and by Pearson's χ2 test for categorical measures. The incidence of CVD death (events per 1,000 person-years at risk) was calculated to determine the risk of CVD death across TAC and CAC categories. Cox proportional hazard regression was performed to estimate risks associated with TAC, AVC, and MVC. When assessing the incremental prognostic value of TAC over risk factors and CAC, we employed the following models: ASCVD alone, ASCVD + CAC category, and ASCVD + CAC category + TAC category. In addition, we developed a model including AVC and MVC (ASCVD + CAC category + TAC category + presence of AVC + presence of MVC) in 8713 patients who had AVC/MVC quantification information. Harrell's C-index was used to assess discrimination of CVD death events for each model and C-indexes were compared using the method described by DeLong et al. [17]. Additionally, we assessed the incremental value of TAC over risk factors and CAC for all-cause, coronary heart disease (CHD) and stroke mortality outcomes. We calculated categorical net reclassification improvement (NRI), which was used to estimate reclassification performance of each model wherein TAC=0 reclassifies downward risk, and TAC>300 reclassifies upward risk. C-index and NRI analyses were performed after the sample was stratified by ASCVD categories.
The 2018 AHA/ACC Guideline on Cholesterol management states that the CAC score can help guide statin therapy in people at intermediate ASCVD risk [8]. Specifically, the guideline suggests (1) to consider no statin therapy with a CAC score of 0 (no statin), (2) to favor statin therapy with a CAC score 1 to 99 (favors statin), and (3) statin therapy is indicated if a CAC score ≥100 (initiate statin). We tested the implication of TAC categories in risk reclassification in intermediate risk group according to the guidelines. Incidence of CVD death per 1000 person-years was compared across CAC and TAC categories in intermediate risk group. All statistical analyses were performed using STATA (version 14; StataCorp, College Station, TX, USA), and a P-value <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics according to TAC score category
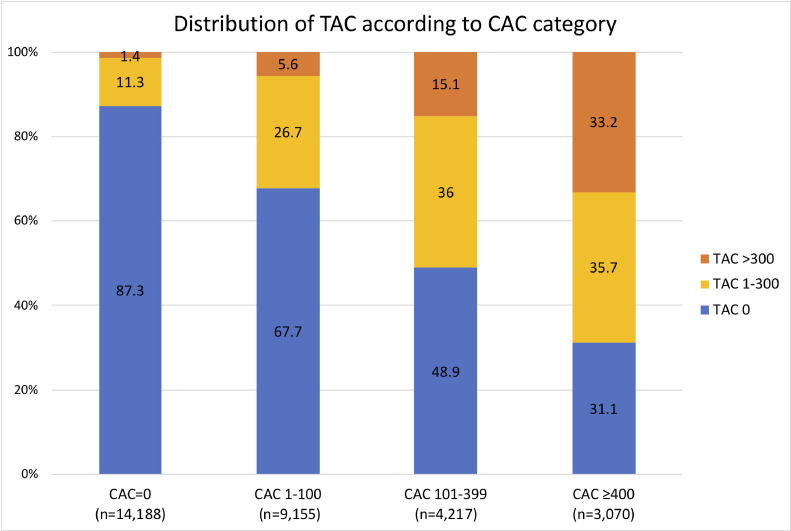
Baseline characteristics of the study population according to TAC are shown in Table 1. The mean age was 55±8 years and 64% (n=19,747) were male. Age and the prevalence of hypertension, diabetes, dyslipidemia, and current smoking increased with increasing TAC scores. The proportion of males and patients with a family history of CAD did not differ among TAC groups. Regarding ASCVD risk, 15,226 (49.7%), 4,787 (15.6%), 7,424 (24.2%) and 3,193 (10.4%) patients were in the low, borderline, intermediate, and higher risk groups, respectively. The prevalence of TAC was 29.5% (9,036) and CAC was 53.7% (16,442) in the overall population. The proportion of subjects in the borderline, intermediate and high ASCVD categories increased with increasing TAC (all p<0.001). The presence and severity of TAC were associated with CAC (Fig. 1). The presence and magnitude of TAC increased with higher CAC scores. Among patients with CAC=0, 12.7% of patients had TAC while in patients with CAC ≥400, 68.9% had TAC.
Table 1.
Baseline characteristics according to TAC.
| TAC=0 (n=21,596, no TAC) | TAC= 1–300 (n=6,666, low TAC) | TAC>300 (n=2,368, high TAC) | P-value | |
|---|---|---|---|---|
| Age | 52.1±7.3 | 58.9±7.5 | 64.9±6.7 | <0.001 |
| Male | 13,979 (64.7) | 4287 (64.3) | 1481 (62.5) | 0.103 |
| Hypertension | 5378 (24.9) | 2563 (38.5) | 1301 (54.9) | <0.001 |
| Hyperlipidemia | 12,538 (58.1) | 4710 (70.7) | 1722 (72.7) | <0.001 |
| Diabetes | 959 (4.4) | 521 (7.8) | 345 (14.6) | <0.001 |
| Family history of CHD | 9760 (45.2) | 3029 (45.4) | 1047 (44.2) | 0.585 |
| Current smoker | 1999 (9.3) | 690 (10.4) | 336 (14.2) | <0.001 |
| ASCVD risk score | 5.3±5.2 | 9.8±7.4 | 16.0±10.3 | <0.001 |
| low risk | 13,082 (60.6) | 1928 (28.9) | 216 (9.1) | <0.001 |
| Borderline risk | 3358 (15.6) | 1173 (17.6) | 256 (10.8) | <0.001 |
| Intermediate risk | 3754 (17.4) | 2569 (38.5) | 1101 (46.5) | <0.001 |
| High risk | 1402 (6.5) | 996 (14.9) | 795 (33.6) | <0.001 |
| Any CAC | 9213 (42.7) | 5057 (75.9) | 2172 (91.7) | <0.001 |
| Any MVC* | 76 (1.3) | 143 (7.0) | 157 (18.8) | <0.001 |
| Any AVC* | 297 (5.1) | 428 (21.1) | 330 (39.5) | <0.001 |
MVC and AVC available in 28% (8,713 patients) of the study population.
TAC, thoracic aortic calcification; CHD, coronary heart disease; ASCVD atherosclerotic cardiovascular disease; MVC, mitral valve calcification; AVC, aortic valve calcification
Fig. 1.
Distribution of TAC according to CAC category (p for trend <0.001)
TAC, thoracic aortic calcification; CAC, coronary artery calcium.
3.2. CVD death during study follow-up
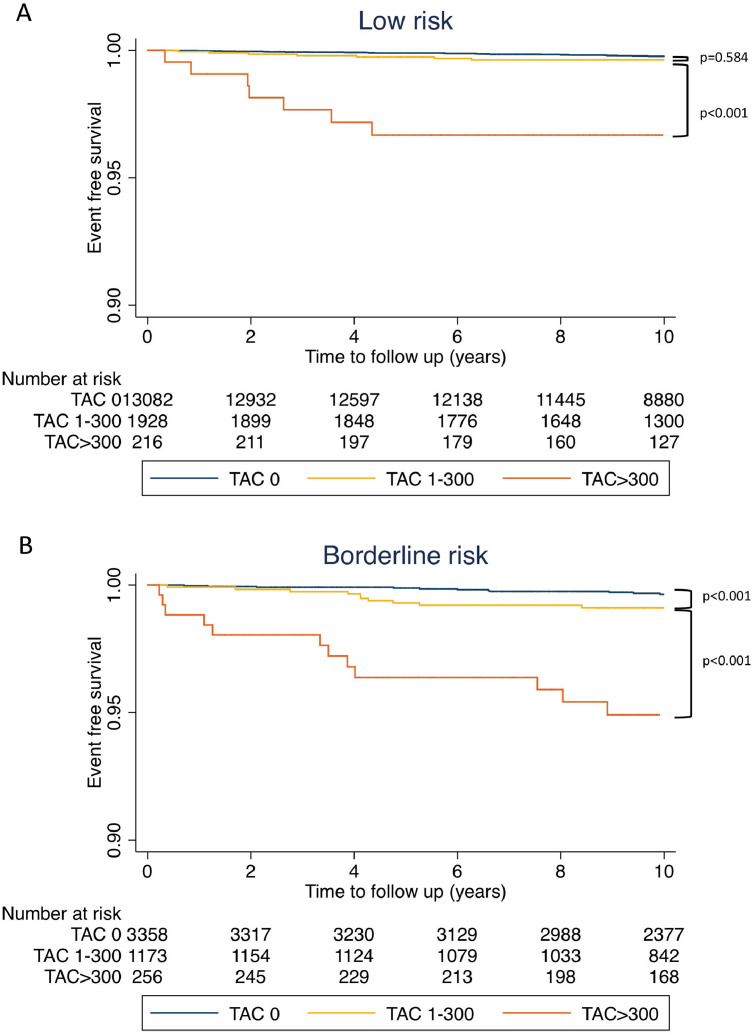
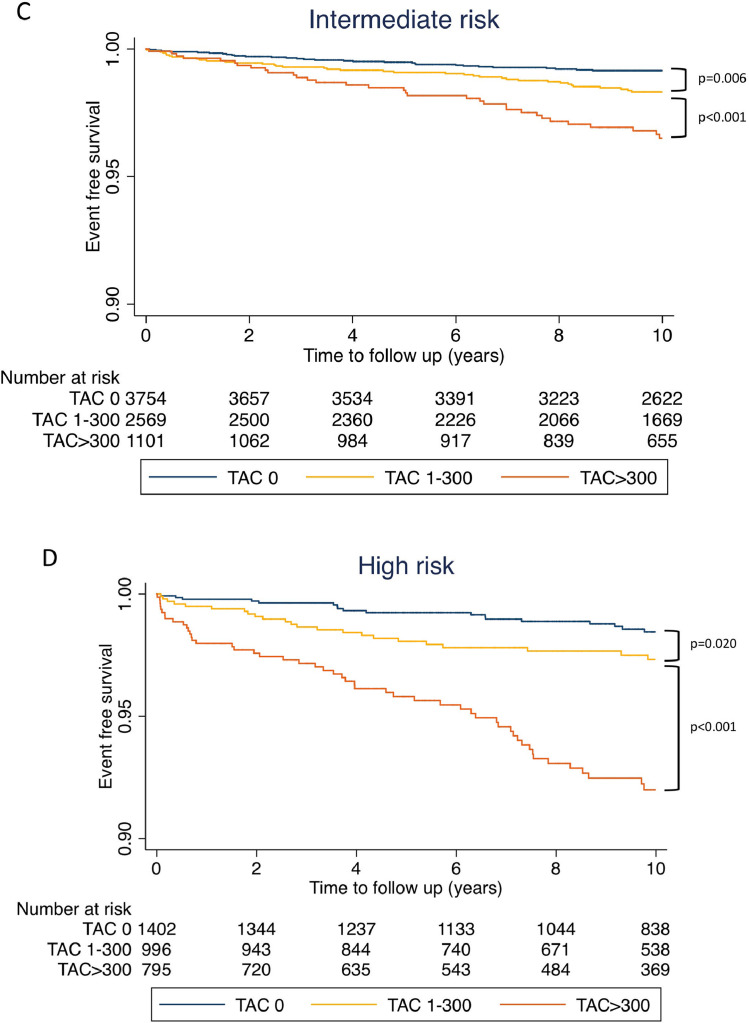
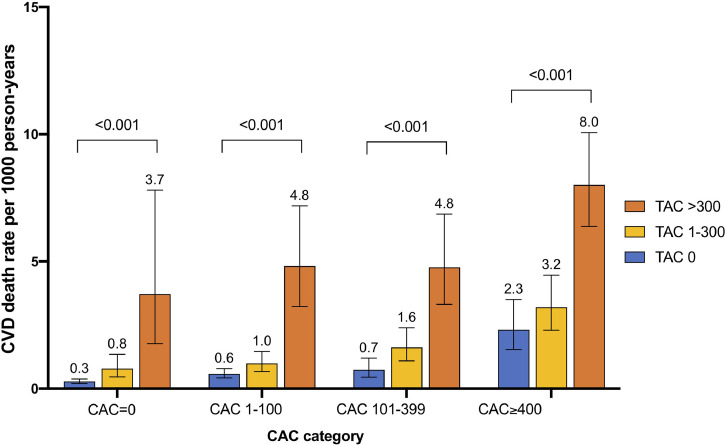
During the median 11.2 years (Interquartile range: 9.2-12.4) of study follow-up, 345 (1.1%) CVD deaths occurred. The incidence of CVD death events per 1000 person-years was 0.5 [95% confidence interval (95% CI): 0.4–0.6], 1.4 (95% CI: 1.2–1.7), and 6.0 (95% CI: 5.1–7.2) for the patients with TAC=0, TAC=1-300, and TAC >300, respectively. On Kaplan-Meier survival curves, increasing TAC was associated with a significantly higher incidence of CVD death events in each of the ASCVD risk categories (Fig. 2 A–D). The incidence of CVD mortality per 1000 person-year according to TAC and CAC categories is shown in Fig. 3. Participants with TAC=0 and CAC=0 had the lowest incidence of CVD mortality, whereas participants with TAC>300 and CAC ≥ 400 had the highest CVD mortality rate. Participants with CAC=0 but with TAC>300 had a high CVD mortality rate of 3.7 deaths per 1000 person-years compared with 2.3 CVD deaths per 1,000 person-years among participants with CAC≥400 and TAC=0. TAC>300 was associated with increased CVD mortality incidence across all CAC categories (all p<0.001). In the Cox regression analysis, the risk of CVD death increased at higher ASCVD risk score, CAC, and TAC (Table 2). After adjustment for ASCVD and CAC, TAC remained significantly associated with CVD mortality (HR: 1.72, 95% CI: 1.29-2.28, p<0.001 for TAC=1-300; HR: 4.23, 95% CI: 3.11–5.76, p<0.001 for TAC>300)
Fig. 2.
Kaplan-Meier curve for CVD death according to TAC categories. (A) low risk, (B) borderline risk, (C) intermediate risk and (D) high risk group
TAC, thoracic aortic calcification; CVD, cardiovascular disease.
Fig. 3.
CVD death rate per 1000 person-year according to CAC and TAC categories
TAC, thoracic aortic calcification; CAC, coronary artery calcium; CVD, cardiovascular disease.
Table 2.
Cox regression analysis for CVD death risk.
| Variable | Crude |
Adjusted (by CAC and ASCVD risk score) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| CAC category | ||||||
| CAC=0 | 1 (ref) | - | - | - | - | - |
| CAC 1-100 | 2.38 | 1.70–3.33 | <0.001 | - | - | - |
| CAC 101-399 | 4.26 | 2.99–6.05 | <0.001 | - | - | - |
| CAC ≥400 | 11.5 | 8.44–15.77 | <0.001 | - | - | - |
| ASCVD risk category | ||||||
| Low risk | 1 (ref) | - | - | - | - | - |
| Borderline risk | 2.38 | 1.59–3.55 | <0.001 | - | - | - |
| Intermediate risk | 5.19 | 3.80–7.10 | <0.001 | - | - | - |
| High risk | 11.12 | 8.05–15.36 | <0.001 | - | - | - |
| Extra-coronary calcification (ECC) | ||||||
| TAC category | ||||||
| TAC=0 (No TAC) | 1 (ref) | - | - | 1 (ref) | - | - |
| TAC=1-300 (Low TAC) | 2.88 | 2.19–3.77 | <0.001 | 1.72 | 1.29–2.28 | <0.001 |
| TAC>300 (High TAC) | 12.10 | 9.42–15.55 | <0.001 | 4.23 | 3.11–5.76 | <0.001 |
| Ln (TAC+1) | 1.43 | 1.38–1.49 | <0.001 | 1.24 | 1.18–1.29 | <0.001 |
| Ln (MVC+1)a | 1.22 | 1.08–1.38 | 0.001 | 1.01 | 0.89–1.15 | 0.822 |
| Ln (AVC+1) a | 1.38 | 1.27–1.49 | <0.001 | 1.14 | 1.04–1.24 | 0.004 |
MVC and AVC available in 29% (10,007 patient) of study population
TAC, thoracic aortic calcification; CAC, coronary artery calcium; CVD, cardiovascular disease; ASCVD atherosclerotic cardiovascular disease; MVC, mitral valve calcification; AVC, aortic valve calcification
3.3. Incremental value of TAC over CAC and ASCVD risk group
In the overall study population, TAC displayed incremental benefits over the ASCVD+CAC model for predicting CVD death (C-statistic for ASCVD+CAC = 0.776 vs. for ASCVD + CAC+TAC= 0.796; p = 0.001, Table 3). When stratified by ASCVD risk categories, the addition of TAC improved discrimination beyond ASCVD+CAC for those belonging to the borderline and intermediate risk (C-statistic for ASCVD + CAC = 0.683 and 0.631 vs. for ASCVD + CAC + TAC = 0.772 and 0.668, respectively; both p<0.05). The incremental benefit of TAC was not significant in patients with low ASCVD risk (C-statistic for ASCVD + CAC = 0.704 vs. ASCVD + CAC + TAC = 0.713; p=0.635) and high risk (ASCVD + CAC = 0.714 vs. ASCVD + CAC + TAC = 0.737; p=0.053).
Table 3.
Discriminatory value of TAC for predicting CVD mortality event.
| Model | C-statistic | 95% CI | P-value vs. ASCVD | P-value vs. ASCVD+CAC |
|---|---|---|---|---|
| Overall (n=30,630) | ||||
| ASCVD | 0.757 | 0.732–0.781 | - | - |
| ASCVD + CAC | 0.776 | 0.752–0.800 | 0.034 | - |
| ASCVD + CAC + TAC | 0.796 | 0.773–0.820 | <0.001 | 0.001 |
| Low risk (<5%, n=15,226) | ||||
| ASCVD | 0.661 | 0.593–0.728 | - | - |
| ASCVD + CAC | 0.704 | 0.638–0.769 | 0.241 | - |
| ASCVD + CAC + TAC | 0.713 | 0.647–0.779 | 0.172 | 0.635 |
| Borderline risk (5 to <7.5%, n=4,787) | ||||
| ASCVD | 0.520 | 0.433–0.608 | - | - |
| ASCVD + CAC | 0.683 | 0.608–0.759 | <0.001 | - |
| ASCVD + CAC + TAC | 0.772 | 0.697–0.847 | <0.001 | 0.004 |
| Intermediate risk (7.5 to <20%, n=7,424) | ||||
| ASCVD | 0.593 | 0.544–0.642 | - | - |
| ASCVD + CAC | 0.631 | 0.584–0.678 | 0.143 | - |
| ASCVD + CAC + TAC | 0.668 | 0.624–0.713 | 0.003 | 0.037 |
| High risk (≥20%, n=3,193) | ||||
| ASCVD | 0.678 | 0.630–0.726 | - | - |
| ASCVD + CAC | 0.714 | 0.668–0.760 | 0.032 | - |
| ASCVD + CAC + TAC | 0.737 | 0.690–0.783 | 0.003 | 0.053 |
TAC, thoracic aortic calcification; CAC, coronary artery calcium; CVD, cardiovascular disease; ASCVD atherosclerotic cardiovascular disease
3.4. TAC and All-cause, CHD, and stroke related mortality outcomes
1,246 (4.1%) all-cause, 160 (0.5%) CHD and 75 (0.2%) stroke death occurred over the follow-up period. In Cox regression analysis, TAC>300 was associated with all-cause, CHD and stroke death after adjustment for ASCVD and CAC (all-cause death: HR: 3.15, 95% CI: 2.68-3.69, p<0.001; CHD death: HR: 2.79, 95% CI: 1.81–4.31, p<0.001; stroke death: HR: 4.88, 95% CI: 0.56–9.32, p<0.001). Addition of TAC over ASCVD and CAC improved prediction for all-cause death and stroke death in the overall study population (Supplemental Table 2, both p<0.05). The incremental benefit of TAC was not significant for CHD death prediction (p=0.067). When stratified by ASCVD risk category, the addition of TAC improved prediction for all-cause death over ASCVD+CAC for patients with low, borderline and intermediate risk (all p<0.05). There was no improvement of discrimination for all-cause death in patients with high risk (p=0.528).
3.5. Risk reclassification
The addition of TAC improved CVD death risk reclassification in patients with borderline, intermediate, and high-risk group (NRI: 0.399, 0.290, and 0.492, respectively, all p<0.001; Table 4). There was no significant improvement in reclassification in the low risk, with NRI of 0.085 (p = 0.386).
Table 4.
Addition of TAC over CAC for reclassifying CVD mortality events according to ASCVD risk categories.
| NRI | 95% CI | p-value | % Event reclassified | % Non-events reclassified | |
|---|---|---|---|---|---|
| Low risk (n=15,226) | 0.085 | -0.102–0.272 | 0.386 | -16.4% | 24.9% |
| Borderline risk (n=4,787) | 0.399 | 0.165–0.632 | <0.001 | 7.1% | 32.7% |
| Intermediate risk (n=7,424) | 0.290 | 0.162–0.418 | <0.001 | 8.8% | 20.3% |
| High risk (n=3,193) | 0.492 | 0.361–0.624 | <0.001 | 45.9% | 3.3% |
TAC, thoracic aortic calcification; CAC, coronary artery calcium; CVD, cardiovascular disease; ASCVD atherosclerotic cardiovascular disease; NRI, net reclassification index
3.6. CVD mortality rate by TAC and CAC in intermediate ASCVD risk patients regarding statin recommendations
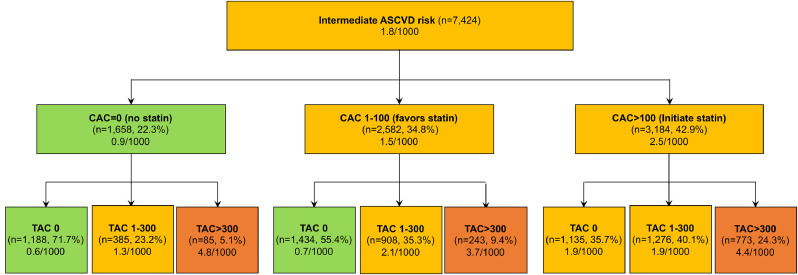
2018 ACC/AHA Multisociety guidelines for cholesterol management suggest if a decision about statin therapy is uncertain in patients with intermediate ASCVD risk, CAC measurement should be considered to guide statin therapy as follows: CAC=0 (no statin), CAC=1-100 (favors statin), and CAC>100 (initiate statin) [8]. The CVD mortality rates per 1000 person-years across TAC and CAC categories are shown in Fig. 4. Of the intermediate ASCVD risk patients, 15% had TAC>300. The CVD death rate in patients with intermediate risk and CAC=0, CAC=1–100, and CAC>100 was 0.9, 1.5, and 2.5 events per 1000 person-years, respectively. Incidence of CVD death in each CAC groups was heterogenous when further stratified by TAC categories (p for trend<0.001). TAC>300 was associated with a concurrent higher incidence of CVD death per 1000 person-years compared to TAC=0 across each CAC categories (TAC>300 vs. TAC=0: 4.8 vs. 0.6 in CAC 0; 3.7 vs. 0.7 in CAC 1-100; 4.4 vs. 1.9 in CAC>100, all p < 0.001).
Fig. 4.
Implication of TAC over CAC in reclassifying risk in intermediate risk group regarding statin use
In intermediate risk patients whom the current guidelines recommend considering CAC measuring to guide statin use, TAC provided better risk reclassification over guideline-based approach with CAC. Incidence of CVD death was stratified according to events rate (<1 (green color), 1 to <3 (yellow) and ≥3 (red) per 1000 person-years).
TAC, thoracic aortic calcification; CAC, coronary artery calcium; CVD, cardiovascular disease; ASCVD atherosclerotic cardiovascular disease (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
3.7. AVC and MVC over TAC for CVD mortality
AVC and MVC quantification information was available in 28% (n=8,713) of the study population. AVC and MVC were observed in 12% and 4% of patients, respectively. The prevalence of MVC and AVC were increased with a higher TAC (Table 1). In the multivariate Cox analysis, AVC was an independent predictor of CVD mortality after adjusting for ASCVD risk score and CAC (ln (AVC+1), HR: 1.14, 95% CI: 1.04-1.24, p=0.004, Table 2). However, the prognostic significance of MVC was attenuated after accounting for ASCVD risk score and CAC (ln (MVC+1): 1.01, 95% CI: 0.89-1.15, p=0.822). The addition of AVC and MVC displayed no further incremental benefit beyond TAC for the prediction of CVD death across all ASCVD risk groups (all p>0.05, Supplementary Table 1).
4. Discussion
In this large cohort with TAC quantification from non-contrast cardiac CT scan, we demonstrate that TAC is independently associated with increased risk of CVD mortality events. TAC assessment with the threshold of 300 improved risk prediction and reclassification for CVD mortality when added to the ASCVD risk score and CAC, particularly in patients with borderline and intermediate ASCVD risk group. Of the intermediate ASCVD risk patients whom current guidelines recommend considering CAC measuring to guide statin treatment, TAC>300 provided further risk reclassification over guideline-based CAC categories. TAC was uncommon (12.7% for TAC 1-300, 1.4% for TAC >300) and assessment did not provide significant improvements among low ASCVD risk patients. There were no further benefits when adding MVC and AVC over ASCVD risk score, CAC, and TAC.
4.1. Comparison to prior studies
The current study confirms prior findings of the association between TAC and increased risk of adverse CVD outcomes. Wong et al. examined predictive value of TAC for CVD events in 2303 asymptomatic adults with 4.4 years follow up and found that increasing TAC was significantly associated with the incidence of CVD events [12]. In another study, Santos et al. reported that the presence of TAC was associated with a 60% increase in all-cause mortality [13]. In the Multi-Ethnic Study of Atherosclerosis (MESA) study, the presence of TAC was a significant predictor of future coronary events independent of traditional risk factors [14]. However, the incremental value of TAC when added to a model containing risk factors and CAC was not significant [12] or only significant in women [14]. In the current study, the addition of TAC over the ASCVD risk score and CAC displayed significant improvement for CVD death prediction in the overall population. The potential explanation of the discrepancies between prior findings vs. our results is previous studies included a relatively small sample size with few CVD-related hard outcomes. For example, the study by Wong et al. [12] observed only 47 total CVD events, of which more than half were soft CHD events (i.e. coronary revascularization). The TAC study in the MESA population considered only CHD events as a primary endpoint [14]. Our results were similar to the previous findings that the addition of TAC over the ASCVD risk score and CAC displayed no significant improvement for CHD death prediction. However, the addition of TAC over the ASCVD+CAC model significantly improved all-cause, CVD, and stroke-related death prediction in the over study population. The large sample size with median 11.2 years long term follow-up of the CAC consortium allowed to have enough power for the cause-specific mortality analysis.
4.2. Clinical significance
Although prior studies indicated that the TAC is an independent predictive marker for adverse CVD outcomes, it has been unclear how to interpret and utilize TAC findings from non-contrast CAC scans in clinical practice. Previous studies used a binary assessment (presence/absence) (11) or arbitrary thresholds (0, 100, and 400) from CAC score (9) in interpretation. We suggested a TAC threshold of 300, based on the threshold estimation analysis for CVD mortality outcomes. Our findings indicated that CVD mortality risk increased with increasing TAC and was particularly high with TAC >300. This suggests a defining of a high TAC group might be superior to a simple binary approach of presence or absence for identifying patients at high CVD risk.
We assessed the benefit of TAC over CAC after stratifying the study population according to guideline based ASCVD risk groups. The maximal benefit of the TAC assessment over CAC was overserved in borderline to intermediate ASCVD risk patients. Further, our findings suggest TAC assessment with a threshold of 300 may have clinical utility for risk reclassification over CAC for shared decision making in cholesterol lowering treatment: TAC >300 may improve patient selection for those who would benefit more strongly from statin use, from intermediate ASCVD risk patients who should consider a statin (CAC=1-100), and those where a statin is not recommended (CAC=0).
4.3. MVC and AVC over ASCVD risk, CAC and TAC
Other sites of extra coronary calcification (ECC) such as AVC and MVC can also be assessed by routine CAC scan. In a sub-study of the MESA population, multi-site ECC, defined as TAC, AVC, MVC, and aortic root calcification, showed that an increasing number of ECC sites incrementally improves prediction for CHD event, CHD mortality, and all-cause mortality [18]. However, the addition of ECC had only minimal improvement for the prediction of adverse events over CAC and traditional risk factors [18,19]. Consistent with previous findings, while AVC was associated with CVD deaths independent of traditional risk factors, there is no added benefit when adding AVC and MVC in a model with the ASCVD risk score, CAC and TAC. However, AVC and MVC have been closely associated with valvular heart disease [20,21] or cerebrovascular events [22], [23], [24]. Evaluation of AVC or MVC may be useful for the early detection and assessment of its specific disease entities.
4.4. Limitations
This study has several limitations. The retrospective and observational nature of the current study may be prone to potential unmeasured confounding factors. The CAC consortium participants are predominantly Caucasian and comprised of patients referred for a clinically indicated CAC scanning. Thus, the study sample did not represent a random sample of the population thus was prone to potential selection bias. In addition, due to self-reporting and binary nature of risk factor information, the possibility remains that residual confounding could have influenced the association of TAC with mortality outcomes. The Agatston scoring method was used for quantification of TAC, AVC and MVC in this study. However, this score was not designed for non-coronary calcification quantification. Despite this, our findings demonstrated robust prognostic significance of TAC score based on the Agatston scoring method. Routine CAC scanning typically excludes the aortic arch. Hence, the TAC from CAC scan cannot reflect the atherosclerosis burden in the aortic arch, which has been shown to be significantly associated with cerebrovascular disease [25,26]. The outcome only included CVD mortality events; non-fatal CVD or CHD events were not evaluable in this study.
5. Conclusion
TAC demonstrated improved risk prediction and reclassification over the ASCVD risk score and CAC score. The benefits of TAC were significant in patients with borderline to intermediate ASCVD risk. TAC quantification using the threshold of 300 on routine CAC scans provides additional prognostic and reclassification values beyond the current guideline-based approach.
CRediT authorship contribution statement
Donghee Han: Conceptualization, Writing – original draft. Keiichiro Kuronuma: Conceptualization, Writing – original draft. Alan Rozanski: Data curation, Writing – review & editing. Matthew J Budoff: Data curation, Writing – review & editing. Michael D Miedema: Data curation, Writing – review & editing. Khurram Nasir: Data curation, Writing – review & editing. Leslee J Shaw: Data curation, Writing – review & editing. John A Rumberger: Data curation, Writing – review & editing. Heidi Gransar: Formal analysis. Roger S Blumenthal: Data curation, Writing – review & editing. Michael J Blaha: Data curation, Writing – review & editing. Daniel S Berman: Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
NIH Grant L30 HL110027 and Miriam and Sheldon G. Adelson Medical Research Foundation
Acknowledgements
None
Disclosures
Dr. Budoff receives modest grant support from General Electric. All other authors have no conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100232.
Appendix. Supplementary materials
References
- 1.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P., LaBree L., Azen S.P., Doherty T.M., Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 4.Budoff M.J., Shaw L.J., Liu S.T. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 5.Blaha M.J., Budoff M.J., DeFilippis A.P. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684–692. doi: 10.1016/S0140-6736(11)60784-8. London, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miedema M.D., Duprez D.A., Misialek J.R. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7(3):453–460. doi: 10.1161/CIRCOUTCOMES.113.000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasir K., Bittencourt M.S., Blaha M.J. Implications of coronary artery calcium testing among statin candidates according to American college of cardiology/american heart association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2015;66(15):1657–1668. doi: 10.1016/j.jacc.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 8.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Takasu J., Katz R., Nasir K. Relationships of thoracic aortic wall calcification to cardiovascular risk factors: the multi-ethnic study of atherosclerosis (MESA) Am Heart J. 2008;155(4):765–771. doi: 10.1016/j.ahj.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEniery C.M., McDonnell B.J., So A. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53(3):524–531. doi: 10.1161/HYPERTENSIONAHA.108.126615. (Dallas, Tex: 1979) [DOI] [PubMed] [Google Scholar]
- 11.Jensky N.E., Criqui M.H., Wright M.C., Wassel C.L., Brody S.A., Allison MA. Blood pressure and vascular calcification. Hypertension. 2010;55(4):990–997. doi: 10.1161/HYPERTENSIONAHA.109.147520. (Dallas, Tex: 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong N.D., Gransar H., Shaw L. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc Imaging. 2009;2(3):319–326. doi: 10.1016/j.jcmg.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Santos R.D., Rumberger J.A., Budoff M.J. Thoracic aorta calcification detected by electron beam tomography predicts all-cause mortality. Atherosclerosis. 2010;209(1):131–135. doi: 10.1016/j.atherosclerosis.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Budoff M.J., Nasir K., Katz R. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2011;215(1):196–202. doi: 10.1016/j.atherosclerosis.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaha M.J., Whelton S.P., Al Rifai M. Rationale and design of the coronary artery calcium consortium: a multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11(1):54–61. doi: 10.1016/j.jcct.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff D.C., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 17.DeLong E.R., DeLong D.M., Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 18.Tison G.H., Guo M., Blaha M.J. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: the multi-ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9(5):406–414. doi: 10.1016/j.jcct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kianoush S., Al Rifai M., Cainzos-Achirica M. Thoracic extra-coronary calcification for the prediction of stroke: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2017;267:61–67. doi: 10.1016/j.atherosclerosis.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavel M.A., Pibarot P., Messika-Zeitoun D. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64(12):1202–1213. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawade T., Clavel M.A., Tribouilloy C. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11(3) doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- 22.Kizer J.R., Wiebers D.O., Whisnant J.P. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the strong heart study. Stroke. 2005;36(12):2533–2537. doi: 10.1161/01.STR.0000190005.09442.ad. [DOI] [PubMed] [Google Scholar]
- 23.Kohsaka S., Jin Z., Rundek T. Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc Imaging. 2008;1(5):617–623. doi: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin E.J., Plehn J.F., D'Agostino R.B. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327(6):374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 25.Amarenco P., Cohen A., Hommel M., Moulin T., Leys D., Bousser MG. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334(19):1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 26.Elias-Smale S.E., Odink A.E., Wieberdink R.G. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam Study. Atherosclerosis. 2010;212(2):656–660. doi: 10.1016/j.atherosclerosis.2010.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.