Abstract
Vaccination with ChAdOx1 nCov‐19 can result in vaccine‐induced immune thrombotic thrombocytopenia (VITT). This phenomenon mimics heparin‐induced thrombocytopenia (HIT), yet it does not require heparin as a trigger. HIT screen/ELISA along with optical density and functional assay are useful in diagnosis. A 64‐year‐old man presented to the emergency department with intermittent fever and persistent, dull, nonspecific abdominal pain 7 days after the first dose of ChAdOx1 nCov‐19 vaccine. Laboratory results showed significantly reduced platelet count, acute kidney injury, and low basal cortisol. He underwent investigations including computed tomography angiography, which revealed multiple sites of arterial and venous thrombosis. We present the first reported case of VITT at our institution and in Oman. This case highlights the potentially life‐threatening complication associated with ChAdOx1 nCov‐19 vaccine, clinical presentation, diagnostic approach, and treatment.
Keywords: COVID‐19, heparin, thrombocytopenia, vaccine, venous thromboembolism
Essentials.
Vaccine‐induced immune thrombotic thrombocytopenia is associated with the ChAdOx1 nCov‐19 vaccine.
A man presented with thrombocytopenia and multiple thrombosis after the ChAdOx1 nCov‐19 vaccine.
The patient had a very high D‐dimer and strongly positive heparin‐induced thrombocytopenia ELISA.
A combination of nonheparin anticoagulation and intravenous immunoglobulin resulted in a full recovery.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a newly emerged disease since December 2019, and has been a pandemic since March 2020. Moreover, it has severely affected public health on a day‐to‐day basis, with over 140 million people affected globally and fatalities reaching almost 3.1 million to date.1 Vaccines against SARS‐CoV‐2 are vital in the fight against the COVID‐19 pandemic.2 From December 2020 to March 2021, the European Medicines Agency has approved four vaccines, BNT162b2 (Pfizer–BioNTech), mRNA‐1273 (Moderna), ChAdOx1 nCov‐19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen), based on multiple randomized/blinded controlled trials.2
As of June 2021, more than 2.8 billion doses of COVID‐19 vaccines have been administered worldwide, and approximately one‐quarter of recipients were given the ChAdOx1 nCoV‐19 (AstraZeneca/Oxford) vaccine. Beginning in February 2021, several reports of unusual cases of thrombotic events were reported in combination with thrombocytopenia in patients who had received ChAdOx1 nCov‐19.2, 3, 4 The terms vaccine‐induced immune thrombotic thrombocytopenia (VITT) and vaccine‐induced prothrombotic immune thrombocytopenia have been coined in light of recently published cases series and case reports in Norway, Germany, and the United Kingdom.3, 4, 5 The majority of affected patients thus far are women under the age of 55 years, and cerebral venous thrombosis or other thrombosis seems to occur 4 to 16 days after vaccination.2, 3, 4, 5 This phenomenon mimics autoimmune heparin‐induced thrombocytopenia (HIT), yet it does not require heparin as a trigger. The Paul Ehrlich Institute demonstrated that affected individuals in Germany have antibodies against platelet factor 4 (PF4)/heparin complex that induces massive platelet activation, reducing the platelet count and causing thrombosis.3, 4, 5, 6, 7
In the following case, we present the first reported VITT case from our institution and in Oman. This case highlights the potentially life‐threatening complication associated with ChAdOx1 nCov‐19 vaccine, clinical presentation, diagnostic approach, and treatment.
2. CASE PRESENTATION
A 64‐year‐old man with hypertension and hyperlipidemia presented to our emergency department at Sultan Qaboos University Hospital in March 2021 with intermittent subjective fever, lethargy, malaise, and persistent nonspecific vague abdominal pain. There was no nausea, vomiting, diarrhea, constipation, or urinary tract symptoms. The patient’s relatives noted altered mental status with increased lethargy and excessive somnolence. There were no other cardiac, respiratory, or neurological symptoms. There was no history suggestive of a bleeding diathesis, for example, bruising, hemoptysis, or upper or lower gastrointestinal bleeding symptoms. He had started no new medications, herbal products, steroids, or anticoagulants. Finally, there was no history suggestive of any familial bleeding or thrombotic disorder either in the patient or his immediate family members. His symptoms started 7 days after he received the first dose of the ChAdOx1 nCov‐19 vaccine.
On presentation to the emergency department, he was alert but disoriented to time and person, with no obvious hemodynamic distress. His vital signs were as follows: temperature, 37.3℃; heart rate, 90 bpm; respiration rate, 18 breaths per minute; blood pressure, 140/70 mm Hg; and oxygen saturation of 97% on room air. There were no petechiae, ecchymoses, or rashes. Abdominal examination revealed bilateral flank and left renal angle tenderness; there were no signs of peritonitis and no palpable organomegaly. Cardiovascular, respiratory, and musculoskeletal examination were unremarkable. Finally, his neurological examination was intact with no meningeal signs.
Initial laboratory investigation showed severe thrombocytopenia (20 000 per cubic millimeter [150‐450]), acute kidney injury (serum creatinine 104 μmol/L [59‐104; estimated glomerular filtration rate 62 mL/min/1.73m2), slightly deranged coagulation (prothrombin time 13 seconds [9.8‐12.0]), international normalized ratio 1.21 [0.9‐1.10]), activated partial thromboplastin time [aPTT] 49.2 seconds [25.0‐36.4], Thrombin time [TT] 19.8 seconds [12.8‐17.6] with normal fibrinogen, 4 g/L [1.7‐3.6), high D‐dimer (36.9 mg/L fibrinogen equivalent units [0.2‐0.7]), and exceptionally low basal cortisol [6 nmol/L (133‐537)]. There was no evidence of idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome on initial screening investigations. Cultures including blood (aerobic and anaerobic), urine, and stool were negative. Transthoracic echocardiogram was normal with no obvious vegetations; hence, in view of normal echocardiogram and negative culture, possibility of endovascular infection with septic emboli was ruled out.
Abdominal and pelvic computed tomography (CT) angiogram showed multiple sites of arterial and venous thrombosis: descending aortic thrombi (Figure 1A), pulmonary embolism (Figure 1B), left renal vein thrombosis with renal infarction (Figure 1C), and bilateral adrenal gland hemorrhage (likely hemorrhagic infarction secondary to bilateral adrenal venous thrombosis) (Figure 1D). His initial electrocardiogram and chest X‐ray were normal. CT brain with CT arteriogram and CT venogram were normal with no evidence of arterial or venous occlusion.
FIGURE 1.
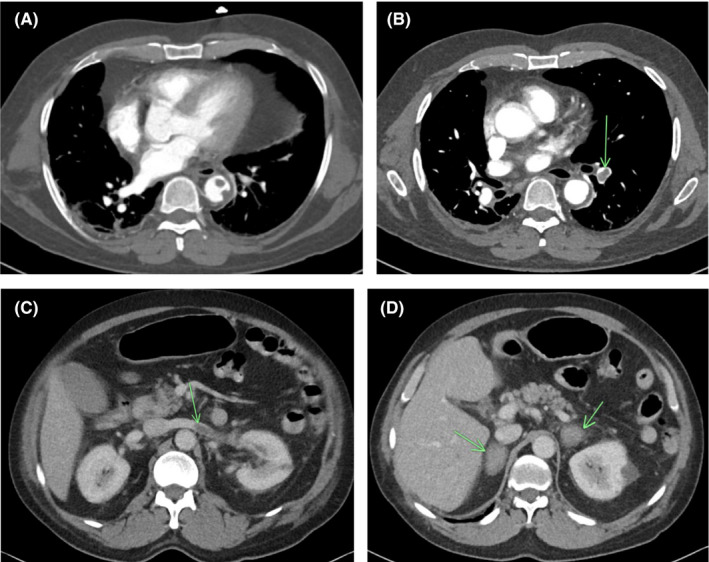
(A) Multiple small filling defects with the descending thoracic aorta adherent to the lateral and posterior wall. (B) Multiple filling defects within the segmental branches of the pulmonary artery of the lower lobe of the left lung. (C) Long filling defect with the left renal vein representing renal vein thrombus, also with wedge shape infarct in left kidney. (D) Bilateral adrenal gland masses with ill‐defined margins representing adrenal hematomas
During the course of admission, his platelet count declined to 7000/mm2 requiring multiple random donor platelet transfusions (before VITT diagnosis). There was no clinical deterioration noted after platelet transfusion. The initial HIT screening test using rapid lateral flow immunoassay (STic Expert HIT; Diagnostica Stago SAS, Asniéres‐sur‐Seine, France) was negative. However, because of the high suspicion, we proceeded with a HIT ELISA test (Asserachrom HPIA IgG assay; Diagnostica Stago), which resulted strongly positive, with optical density (OD) of 2.11. Lupus anticoagulant and antiphospholipid antibody screening were both negative. Unfortunately, HIT functional assays (serotonin release assay or heparin‐induced platelet activation) are not available in Oman and therefore were not performed.
For management, he was initiated on an argatroban infusion (to target aPTT ratio between 1.5 and 2.5 of normal) as a nonheparin anticoagulation for 3 days as his renal function further deteriorated and did not permit for fondaparinux or direct oral anticoagulants. Anticoagulation was started despite the adrenal hemorrhages. As the renal function improved significantly, anticoagulation was changed to fondaparinux for 4 days until the patient’s platelet count improved, then shifted to rivaroxaban at the time of discharge. We also treated him with intravenous immunoglobulin 0.5 gm/kg for 4 days (total of 2 gm given over 4 days). The platelets were 114 000 per cubic millimeter at the day of discharge (Figure 2). The management was tailored according to the case at hand and recommended interim guidelines.8, 9, 10 Two week later, repeated blood work showed complete recovery of the platelet count (216 000/mm2), reduction in D‐dimer (ie, 5.6 mg/L and a decline in the HIT ELISA OD to 0.99. As far as his hypoadrenalism was concerned, he was initially given stress doses of hydrocortisone during the acute phase (ie, the first 3 days) followed by a maintenance regimen. There were no bleeding complications to anticoagulation during admission or follow‐up.
FIGURE 2.
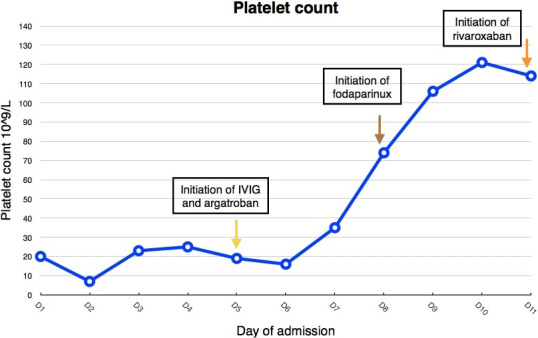
Trend of platelet count during the course of admission shows improvement after initiation of treatment
In summary, our patient presented 7 days after ChAdOx1 nCov‐19 vaccine with severe thrombocytopenia and multiple arterial and venous thromboses. Therefore, the main differential was VITT caused by the AstraZeneca vaccine. This was confirmed with a strongly positive HIT ELISA highly suggestive of spontaneous HIT (without heparin exposure). He had the full spectrum of VITT complicated by hypoadrenalism. Prompt and adequate intervention was established, and he recovered with a platelet count improved to normal within 3 weeks.
3. DISCUSSION
VITT is a new emerging phenomenon with different COVID‐19 vaccines. However, further studies are required to understand whether these antibodies are autoantibodies against PF4 induced by the strong inflammatory stimulus of vaccination or antibodies induced by the vaccine that cross‐reacts with PF4 and platelets. In addition, mRNA vaccines have been linked with severe thrombocytopenia and bleeding.10, 11
Our patient had a similar presentation to previously reported cases of VITT. He presented 7 days after receiving the vaccine and had severe thrombocytopenia, a high D‐dimer level, hypofibrinogenemia over course (this developed later but not at presentation), a negative rapid HIT screen, and a positive HIT ELISA with high OD.3, 4, 5, 8
Our patient’s course differed from previously reported cases in several ways. He is an elderly male as opposed to young women. Reports of VITT are much less frequent in older men.4 He did not have cerebral vein thrombosis, which is the most commonly reported thrombosis site.3, 4, 5, 6 He presented with thrombosis at several sites, including arterial and venous sites. To our knowledge, bilateral adrenal hemorrhage secondary to bilateral adrenal vein thrombosis has not been reported before in VITT. In addition, to our knowledge, this is the first case of VITT outside of the United States, Canada, and Europe.
4. CONCLUSION
The exact pathogenesis and risk factors for VITT are still unknown, that is, who is at higher risk in terms of age, sex, or preexisting comorbidities. This underlines the necessity that more data and further studies are needed to understand the pathogenesis of this unusual clotting disorder.
RELATIONSHIP DISCLOSURE
A written, well‐informed consent for case publication was obtained from the patient. The authors of this case report vouch for accuracy and integrity of the data provided. There is no conflict of interest.
AUTHOR CONTRIBUTIONS
BA designed, performed research, helped in writing, and was the main reviewer of the manuscript. HB provided vital reviews of the manuscript. ZJ, AB, AM, and NB collected, analyzed, interpreted data, and wrote the manuscript. All authors read and approved the final manuscript.
Al Rawahi B, BaTaher H, Jaffer Z, Al‐Balushi A, Al‐Mazrouqi A, Al‐Balushi N. Vaccine‐induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine–A case report. Res Pract Thromb Haemost. 2021;5:e12578. 10.1002/rth2.12578
Handling Editor: Dr Lana Castellucci.
Funding information
This study had no funding
REFERENCES
- 1.Zhao J, Zhao S, Ou J, et al. COVID‐19: coronavirus vaccine development updates. Front Immunol. 2020;23(11):602256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cines DB, Bussel JB. SARS‐CoV‐2 vaccine‐induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384(23):2254‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai M, Grill A, Ivers N, Maltsev A, Miller KJ & Razak F et al. Vaccine Induced Prothrombotic Immune Thrombocytopenia (VIPIT) Following AstraZeneca COVID‐19 Vaccination [Internet]. Ontario COVID‐19 Science Advisory Table; 2021 Mar [cited 2021 Apr 21]. Available from: https://covid19‐sciencetable.ca/sciencebrief/vaccine‐induced‐prothrombotic‐immune‐thrombocytopenia‐vipit‐following‐astrazeneca‐covid‐19‐vaccination
- 7.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41(3):184‐189. [DOI] [PubMed] [Google Scholar]
- 8.Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost. 2021;19(7):1819‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karnam A, Lacroix‐Desmazes S, Kaveri SV, Bayry J. Vaccine‐induced prothrombotic immune thrombocytopenia (VIPIT): Consider IVIG batch in the treatment. J Thromb Haemost. 2021;19(7):1838‐1839. 10.1111/jth.15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of vaccine‐induced immune thrombotic thrombocytopenia (VITT) for SARS‐CoV‐2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585‐1588. doi: 10.1111/jth.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021. doi: 10.1002/ajh.26132 [DOI] [PMC free article] [PubMed] [Google Scholar]


