Abstract
Background
The plasma colloid osmotic pressure (COP) plays a major role in transcapillary fluid balance. There is no information on plasma COP of healthy infants beyond the first post-natal week. The normal COP in healthy adult subjects (25 mmHg) is currently also applied as a reference value for healthy infants. This study was designed to test whether plasma COP values in healthy infants are the same as those in normal adults.
Methods
Plasma COP was measured in 37 male and female healthy infants from 1 to 11 months old. For this purpose, 1 ml blood was collected during the patient's regularly scheduled visit if the patient required any type of blood test for routine laboratory analyses.
Results
Plasma COP levels correlated slightly with increasing age from 1 to 9 months old (linear regression analysis; r2 = 0.1, P < 0.049). We found no correlation between plasma COP and body weight at the same age (r2 = 0.05, P = 0.155). The mean and standard deviation of COP in all infants was 25.1 ± 2.6 mmHg, which is almost identical to an average COP of 25 mmHg in healthy adult subjects. Arbitrary division of the infants into three different age groups (1–3 months [n = 11], 5–8 months [n = 13] and 9–11 months [n = 13]) showed an average increase of approximately 2 mmHg in COP of 9-month-old to 11-month-old infants, compared with 1-month-old to 3-month-old infants (one-way analysis of variance; P = 0.26). There was no gender difference in the COP level (unpaired t-test), with an average of 25.1 ± 2.4 mmHg in 19 male infants compared with 25.2 ± 2.9 in 18 female infants. The 95% confidence interval for COP in both male and female infants (n = 37) was between 24.3 to 26.0 mmHg, ranging from 19.5 to 30.3 mmHg, with a median value of 25.2 mmHg.
Conclusions
The data accept the null hypothesis that the COP range in infants younger than 1 year old is similar to those observed in adult subjects. Our observations, compared with previously reported neonatal COP values, suggest that there is a sharp increase in COP within the first months after birth.
Keywords: colloid osmotic pressure, colloid therapy, fluid balance, infant patients, neonate patients
Introduction
Plasma COP is generated by the plasma proteins, particularly albumin, and is known to vary during various neonatal diseases [1,2,3,4]. Lower plasma COP favors a fluid shift from intravascular space into interstitial space, with subsequent formation of peripheral and pulmonary edema [3,4,5,6,7,8]. To stabilize the intravascular volume and prevent or reverse the events leading to peripheral and pulmonary edema, albumin or other colloid solutions are frequently administered, to maintain the COP in the 'normal' range [7,8,9,10,11,12]. Albumin administration for various conditions, however, is controversial [13,14,15]. Plasma COP in healthy neonates, sick neonates, and prematurely born infants are reported to be much lower than in adults [16,17,18]. The normal range of COP for healthy adults is reported to be between 22 and 28 mmHg, with a mean of 25 mmHg [19,20]. There is, however, no information in the literature on normal COP range beyond the early neonatal age. The adult COP values in our intensive care unit are also considered to be normal for infants. The excessive use of albumin and its potential complications [15,21,22] may be avoided if the normal range of COP in infants is significantly less than in adults. The present study was therefore designed to determine the normal range of COP in infants younger than 1 year of age and to establish whether there is any correlation between post-natal age, gender, or weight and COP. The null hypothesis is that the COP range in the first months of life is in fact similar to those observed in adult subjects.
Patients and methods
The protocol for this study was approved by the Miami Children's Hospital Institutional Review Board and conducted in the Continuing Care Center of Miami Children's Hospital. The COP was determined in infants from birth to 12 months of age. Blood samples were taken during the patient's regularly scheduled clinic visit for 'well baby visits'. An additional 1 ml blood was collected for our research if the patient required any type of blood test for routine laboratory analyses. The COP was measured using the Model 4400 Colloid Osmometer (Wescor Inc., Logan, UT, USA) on serum that was separated from the sample after centrifugation.
The study was designed as a non-blinded, sequential, descriptive study. Patients were included in the study, after informed consent was obtained from the parent or guardian. Patients older than 1 year, those with an underlying illness, such as nephropathy, liver disease, cardiac anomalies, malnutrition, viral illness with resulting gastrointestinal disturbances or acute febrile illness, or history of any disease state that may interfere with protein metabolism were excluded.
Statistical analysis
A simple linear regression test was used to determine whether there is any correlation between age and COP, or body weight and COP. An unpaired t-test was used to evaluate gender differences in COP. We also used one-way analysis of variance (ANOVA) to evaluate whether the average COP levels were different among infants that were categorized into three different age groups of 1–3, 5–8, and 9–11 months old. All values are presented as mean ± standard deviation (SD), and P < 5% were considered significant.
Results
The study was concluded after completion of a single COP measurement in 19 male infants and 18 female infants from 1 to 11 months old. The study population consisted of 32 infants out of 37 infants with Hispanic origin. A relatively weak but significant correlation (r2 = 0.10, P < 0.049) was found between the COP values and age within the first year of life in healthy infants (Fig. 1). There was, however, no correlation between COP and body weight (Fig. 2). No gender differences were found between the male and female infants in this age group (Table 1). An arbitrary division of the all infants into three different age categories (1–3 months old [n = 11], 5–8 months old [n = 13] and 9–11 months old [n = 13]) showed an average increase of approximately 2 mmHg in COP in 9-month-old to 11-month-old infants, compared with 1-month-old to 3-month-old infants (one-way ANOVA). This increase from 24 to 25.8 mmHg was not statistically significant (one-way ANOVA; P = 0.26). The median value of COP for either male or female infants separately, or combined, was 25.1–25.2 mmHg, with a 95% confidence interval of 24.3–26.0 mmHg for the combined male and female infants (Table 1).
Figure 1.
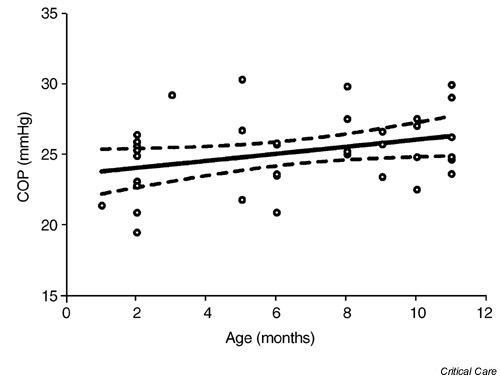
A weak association was found between colloid osmotic pressure (COP) levels and age in healthy infants within the first post-natal year (r2 = 0.10, n = 37, P < 0.049).
Figure 2.
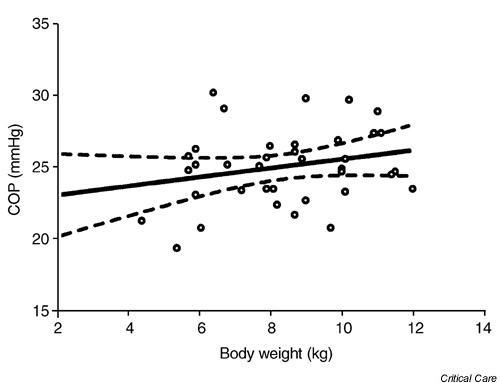
There is no significant correlation between colloid osmotic pressure (COP) levels and body weight in healthy infants within the first post-natal year (r2 = 0.05, n = 37, P = 0.15).
Table 1.
Colloid osmotic pressure (COP) values in 1-month-old to 11-month-old healthy infants
| Male and female | Males | Females | |
| COP (mmHg) | (n = 37) | (n = 19) | (n = 18) |
| Mean ± SD | 25.1 ± 2.6 | 25.1 ± 2.4 | 25.2 ± 2.9 |
| Median | 25.2 | 25.2 | 25.1 |
| Range | 19.5–30.3 | 20.9–29.8 | 19.5–30.3 |
| 95% Confidence interval | 24.3–26.0 | 23.9–26.3 | 23.7–26.6 |
SD, standard deviation.
Discussion
Sepsis, acute respiratory distress syndrome, hemorrhage and renal failure cause a decrease in the total plasma proteins with consequent reduction in COP, either because of the loss of blood proteins or owing to capillary leakage [4,5,6,7,8,9]. There are no standards for volume resuscitation and COP restoration of intensive care patients [22]. Because of the controversial debate on colloid versus crystalloid solutions for volume resuscitation [9,12,13,14,15,21,22], definition of the normal range of COP in different age groups is crucial. The present study was therefore designed to test whether the normal COP in adults does indeed represent the normal COP values in infants that are younger than 1 year old. The results of the study confirm that plasma COP in humans can reach its peak level as early as 1–3 months after birth. The results, therefore, justify the use of the adult COP range as a reference point for either colloid or crystalloid therapy for restoration of COP during various post-natal diseases that occur within the first year of life.
The plasma COP in the pre-term and full-term healthy neonates and in pre-term and full-term sick neonates [16,17,18] is much lower than those reported in adults [19,20]. For example, the average COP ± SD in five healthy pre-term neonates was reported to be 15.4 ± 1.3 mmHg [16], compared with an average of 25 mmHg in adults [19,20]. The COP in 40 sick pre-term neonates was also significantly lower (12.4 ± 1.6 mmHg) compared with healthy pre-term neonates [16]. Solo and Gregory [17] have also reported a similar range of COP (12.5 ± 2.5 mmHg) in 362 sick pre-term infants. Healthy full-term neonates (n = 99), delivered vaginally, had a higher COP of 19.4 ± 2.2 mmHg, compared with 40 healthy full-term neonates who were delivered by Cesarean section [17]. Zimmermann et al [18] found a strong relationship between water homeostasis and neonatal respiratory distress. They showed a significantly lower COP of 15 ± 2.2 mmHg in 1-day-old neonates with respiratory distress, compared with 17.9 ± 2.5 mmHg in healthy neonates [18].
The lack of a significant gender difference in COP of our healthy infants allowed us to combine the COP of the male and female subjects in a larger group. The demographic cross-section of the population used in this study was mostly of Hispanic origin (32 of 37 infants). Although the Hispanic population in South Florida is not ethnically a homogeneous group, the question is whether our sample can truly represent infants from other ethnic groups. Our infant's population included only one 9-month-old black female with a COP of 23.4 mmHg. This value falls slightly below the 95% confidence interval, calculated for the entire female infants of our sample (Table 1). This COP level, however, was within the observed range, and was only one unit of SD away from the sample mean (equivalent to the 33rd percentile). The mean ± SD of four non-Hispanic white infants (three females and one male) in our sample was 25.5 ± 3.7 mmHg, compared with an average of 25.1 ± 2.5 mmHg in 32 Hispanic infants. This small sample suggests that the observed COP values in the larger Hispanic group are indeed representative of COP levels in healthy infants of non-Hispanic children. In conclusion, our data extend the age spectrum for the normal COP levels to also include the first post-natal year. Our results compared with those observed during the early neonatal period in healthy full-term and pre-term infants [15,16,17,18] suggest a sharp increase in COP within the first months after birth.
Competing interests
None declared.
Abbreviations
ANOVA = analysis of variance; COP = colloid osmotic pressure; SD = standard deviation.
Acknowledgments
Acknowledgement
The technical assistance of Miss Danielle Katz is gratefully acknowledged.
References
- Wiederhielm CA. Dynamics of transcapillary fluid exchange. J Gen Physiol. 1968;52:29–61. [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Haupt MT. Colloid-hydrostatic gradients and pulmonary edema. Chest. 1982;82:398–399. [Google Scholar]
- Vincent JL. Fluids for resuscitation. Br J Anaesth. 1991;67:185–193. doi: 10.1093/bja/67.2.185. [DOI] [PubMed] [Google Scholar]
- Weil MH, Henning RJ, Puri VK. Colloid oncotic pressure: clinical significance. Crit Care Med. 1997;7:113–116. doi: 10.1097/00003246-197903000-00006. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Lindsey AW. Effect of left atrial and decreased plasma protein on the development of pulmonary edema. Circ Res. 1959;7:649. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- Rackow EC, Fein IA, Leppo J. Colloid osmotic pressure as a prognostic indicator of pulmonary edema and mortality in the critically ill. Chest. 1997;72:709–713. doi: 10.1378/chest.72.6.709. [DOI] [PubMed] [Google Scholar]
- Haupt MT, Kaufman BS, Carlson RW. Fluid resuscitation in patients with increased vascular permeability. Crit Care Clin. 1992;8:341–353. [PubMed] [Google Scholar]
- Joles JA, Rabelink TJ, Braam B, Koomans HA. Plasma volume regulation: Defences against edema formation (with special emphasis on hypoproteinemia). Am J Nephrol. 1993;13:399–412. doi: 10.1159/000168654. [DOI] [PubMed] [Google Scholar]
- Waiker SS, Chertow GM. Crystalloids versus colloids for resuscitation in shock. Curr Opin Nephrol Hypertens. 2000;9:501–504. doi: 10.1097/00041552-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Kaminski MV, Jr, Haase TJ. Albumin and colloid osmotic pressure: implications for fluid resuscitation. Crit Care Clin. 1992;8:311–321. [PubMed] [Google Scholar]
- Roberts JS, Bratton SL. Colloid volume expanders. Problems, pitfalls and possibilities. Drugs. 1998;55:621–630. doi: 10.2165/00003495-199855050-00002. [DOI] [PubMed] [Google Scholar]
- Vincent JL. Strategies in body fluid replacement. Minerva Anesthesiol. 2000;66:278–284. [PubMed] [Google Scholar]
- Camacho MT, Totapally BR, Torbati D, Wolfsdorf J. Pulmonary and extrapulmonary effects of increased colloid osmotic pressure during endotoxemia in rats. Chest doi: 10.1378/chest.120.5.1655. [DOI] [PubMed] [Google Scholar]
- Allison SP, Lobo DN. Debate: Albumin administration should not be avoided. Crit Care. 2000;4:147–150. doi: 10.1186/cc687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulimood TB, Park GR. Debate: Albumin administration should be avoided in the critically ill. Crit Care. 2000;4:151–155. doi: 10.1186/cc688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Javed S, Malalis L, Vidyasagar D. Critical care problems in neonates: colloid osmotic pressure in healthy and sick neonates. Crit Care Med. 1981;9:563–567. doi: 10.1097/00003246-198108000-00001. [DOI] [PubMed] [Google Scholar]
- Solo A, Gregory GA. Colloid osmotic pressure of normal newborns and premature infants. Crit Care Med. 1981;9:568–572. doi: 10.1097/00003246-198108000-00002. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Francoise M, Germain JF, Lallemant C, Gouyon JB. Colloid osmotic pressure in neonateal respiratory distress syndrome. Arch Petiatr (Paris) 1997;4:952–958. doi: 10.1016/s0929-693x(97)86090-3. [DOI] [PubMed] [Google Scholar]
- Weil MH, Morissette M, Michaels S. Routine plasma colloid osmotic pressure measurements. Crit Care Med. 1974;2:229–234. doi: 10.1097/00003246-197409000-00001. [DOI] [PubMed] [Google Scholar]
- Morissette MP. Colloid osmotic pressure: its measurement and critical value. CMAJ. 1977;116:897–900. [PMC free article] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients. a systematic review of randomized trials. BMJ. 1998;316:961–964. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt J, Lenz M, Kumle B, Papsdorf M. Volume replacement strategies of intensive care units: results from a postal survey. Intensive Care Med. 1998;24:147–151. doi: 10.1007/s001340050536. [DOI] [PubMed] [Google Scholar]


